Abstract
Pregnancy is a state of high metabolic demand. Fasting diverts metabolism to fatty acid oxidation, and the fasted response occurs much more rapidly in pregnant women than in the non-pregnant state. The product of the imprinted Delta-like homologue 1 gene (DLK1) is an endocrine signaling molecule that reaches a high concentration in the maternal circulation during late pregnancy. By utilising murine models with deleted Dlk1 we show that the fetus is the source of maternal circulating DLK1. In the absence of fetally-derived DLK1, the maternal fasting response is impaired. Furthermore, we found that maternal circulating DLK1 levels predict embryonic mass in mice and can differentiate healthy small for gestational age (SGA) from pathologically small infants in a human cohort. Therefore measurement of DLK1 in maternal blood may be a valuable method for diagnosing human disorders associated with impaired DLK1 expression, and to predict poor intrauterine growth and complications of pregnancy.
Introduction
Adaptations to maternal carbohydrate and lipid metabolism occur during pregnancy to ensure a continuous supply of nutrients to the fetus [1]. In late gestation fasting rapidly diverts maternal metabolism to fatty acid (FA) oxidation, and reductions in maternal plasma glucose and insulin combined with elevated FAs and ketones are seen in fasted pregnant women hours before these changes are observed in the non-pregnant state -a phenomenon known as ‘accelerated starvation’ [2].
DLK1 is the product of an imprinted gene that is predominantly expressed from the paternally-inherited chromosome during fetal development [3] [4]. It is a single-pass transmembrane protein that can be cleaved by extracellular proteases to give rise to a circulating form [5]. This soluble moiety reaches a high concentration in the maternal circulation during late pregnancy [6] [7] [8]. DLK1, also known as fetal antigen 1 (FA1) and pre-adipocyte factor 1 (PREF1), is known to play a crucial role in adipose homeostasis[9]. Our previous studies with genetically-modified mice showed that DLK1 shifts nutrient metabolism towards FA oxidation, both in the context of the transition from birth to weaning [10], and following high-fat feeding, in part through modulation of the growth hormone axis [11]. Since pregnancy is similarly associated with global shifts in nutrient partitioning, we hypothesised that high DLK1 levels during this period might modulate maternal metabolic adaptations.
Here we show that the fetus is the source of maternal circulating DLK1 during pregnancy. In the absence of fetally-derived DLK1, maternal ketone levels are not elevated during fasting, suggesting that DLK1 is part of the accelerated starvation response. Furthermore, lack of DLK1 during the mother’s development additionally impairs her ability to respond to the metabolic demands of pregnancy. We demonstrate that circulating DLK1 levels are positively associated with embryonic mass in mouse pregnancies and that DLK1 levels are significantly lower in pregnant women who go on to deliver a small for gestational age (SGA) infant. SGA is simply a descriptive term for an infant whose birth weight falls below a statistical threshold, but is of clinical interest because a proportion of these infants are small because of fetal growth restriction (FGR) [12]. We found that there is no significant difference in DLK1 levels between healthy SGA and controls, but there is a highly statistically significant reduction in DLK1 in FGR. These data support our findings in the mouse that reducing DLK1 dosage compromises pregnancy. Moreover, measuring DLK1 has considerable potential utility in the clinic to identify pregnancies that will require additional monitoring and obstetrical intervention.
Results
Maternal circulating DLK1 is derived from the conceptus
To determine the source and function of high maternal DLK1 in pregnancy we utilised genetically modified mice that lack a functional copy of Dlk1[13]. We first explored the effect of loss of a functional Dlk1 gene in breeding-age females. Total body mass did not vary between genotypes (wild-type (WT), Null (Dlkm-/p-) and Mat (maternal heterozygotes, Dlk1m-/p+), Supplementary Fig. 1a) but body composition was markedly different. At 12 weeks, virgin Null females had increased abdominal white adipose tissue (WAT), and reduced muscle mass compared to Dlk1-expressing females (Supplementary Fig. 1b, 1c). These alterations in body composition are likely to be due to loss of Dlk1 during embryogenesis, since Dlk1 Null embryos had reduced size due to a reduction in skeletal length and lean mass from at least as early as E18.5 (Supplementary Fig. 1d-f). These animals caught up in weight in the preweaning period, but body composition was not normalised (Supplementary Fig. 1g, 1b-c). Elevated WAT mass at 12 weeks was accompanied by an increase in serum leptin levels (Supplementary Fig. 1h). In humans high BMI is associated with elevated DLK1[14], suggesting that levels of this protein might be modulated by nutritional status.
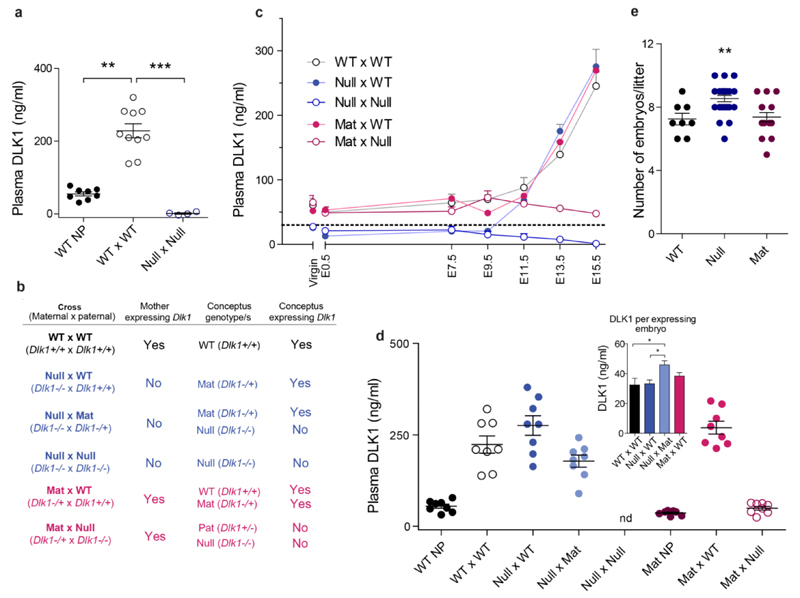
Next, we compared DLK1 in plasma from virgin WT females, with levels at 15 days post conception (E15.5) in WT females that had been mated to WT males. Plasma DLK1 levels increased approximately 5-fold during the first 2 weeks of mouse pregnancy (Fig. 1a). As Dlk1 is expressed predominantly from the paternally-inherited chromosome[3] [4], a cross between a Null mother and a WT father produces offspring that express Dlk1 at WT levels, since they all inherit an intact Dlk1 gene from their father (they are maternal heterozygotes for the deleted allele (Dlk1m-/p+, Mats)). Conversely, a cross between a Dlk1-expressing mother (either a Mat or WT) and a Null father produces offspring that that do not express Dlk1 (they are, respectively, homozygotes or paternal heterozygotes for the deleted allele (Dlk1m-/p- or Dlk1m+/p-, Nulls or Pats)) since maternally inherited Dlk1 is repressed by imprinting. All crosses are summarised in Fig. 1b. In pregnancies where both the mother and the conceptus had inherited the ablated Dlk1 gene (Null x Null), DLK1 could not be detected in the maternal plasma (Fig. 1a and 1c), confirming that there was no residual DLK1 produced from the ablated Dlk1 allele.
Figure 1. The conceptus is the source of elevated maternal plasma DLK1 in late gestation.
(a) Maternal plasma DLK1 levels are elevated during pregnancy. Serum DLK1 levels in non-pregnant WT females (WT NP, n = 8), pregnant WT females crossed to WT males at E15.5 gestation (WT x WT, n = 10), and pregnant Dlk1-/- females crossed to Dlk1-/- males at E15.5 (Null x Null, n = 4). (b) Summary of experimental crosses used in the study. (c) Serial plasma DLK1 measurements over the course of pregnancy (n = 4 females/time–point/group). Groups differ significantly by cross, time–point and the interaction between them (each p < 0.001 by Two-way ANOVA). Dotted line represents the detection threshold of the ELISA. (d) Maternal plasma DLK1 at E15.5 (n = 7–8 females/group). Groups differ significantly by cross and DLK1 levels rise only when the conceptus has a functional copy of Dlk1. nd = not detected (below assay threshold). (Inset) DLK1 in maternal plasma at E15.5 normalised to number of Dlk1–expressing embryos in the litter (n = 8 litters/group). Mat embryos in the Null x Mat cross generate significantly more DLK1 than those in other experimental crosses. (e) Litters from Null mothers have more embryos than litters from WT or Mat mothers. Litter size by maternal genotype in WT (n = 8), Null (n = 24) and Mat (n = 16) mothers. Vertical bars show mean ± s. e. m. Groups were compared by Kruskall-Wallis test, with Dunn’s Multiple comparison post-hoc test as indicated (a, d, e), *p < 0.05, **p < 0.01, ***p < 0.001.
We conducted serial measurements of maternal plasma DLK1 in crosses of mice where the mother, the conceptus, or both were unable to express Dlk1 (Fig. 1b, 1c). DLK1 was detected at high levels in maternal plasma only if the conceptus retained the ability to express Dlk1 (Fig. 1c). Thus, the conceptus is the source of elevated maternal plasma DLK1 in pregnancy. DLK1 levels in maternal plasma began to rise between E9.5 and E11.5 in all crosses that contained Dlk1-expressing conceptuses (Fig. 1c). This rise coincides with the time of formation of the definitive placenta in the mouse[15].
Unexpectedly, Null females crossed with WT males had equal or higher levels of plasma DLK1 compared with WT or Mat females crossed to WT males (Fig. 1d). Since DLK1 levels are known to correlate with the number of conceptuses in the litter[6], we investigated whether litter size was affected by maternal genotype. Consistently, Null mothers had larger litters than mothers with a functional copy of Dlk1 (Fig. 1e and Supplementary Table 1). This was not due to a sampling error caused by controlling our cohort to exclude extremes of litter size and genotype ratio, as when we pooled the data for all crosses generated, Null females consistently produced approximately one additional conceptus compared with WT or Mat females (WT and Mat mean litter size = 7.0 ± 0.3, n = 40 litters; Null mean litter size 8.2 ± 0.3, n = 48 litters, p < 0.001, Mann Whitney test). Appropriate maternal leptin levels are necessary for conception and survival of the early embryo[16]; leptin increases the invasiveness of the early embryo[17] and enhances ovulation[18]. Our finding that Null females had increased fecundity is consistent with their elevated leptin levels (Supplementary Fig. 1h).
When the amount of maternal plasma DLK1 per expressing conceptus was calculated, those in the Null x WT cross produced a similar amount of DLK1 to conceptuses in WT x WT or Mat x WT crosses (Fig. 1e and Supplementary Table 1). However, litters where only half of the conceptuses had an intact Dlk1 allele (Null x Mat cross, Fig. 1b) produced significantly more DLK1 per conceptus than litters where all were able to express Dlk1 (Fig. 1f).
Fetal, not placental, origin of maternal circulating DLK1
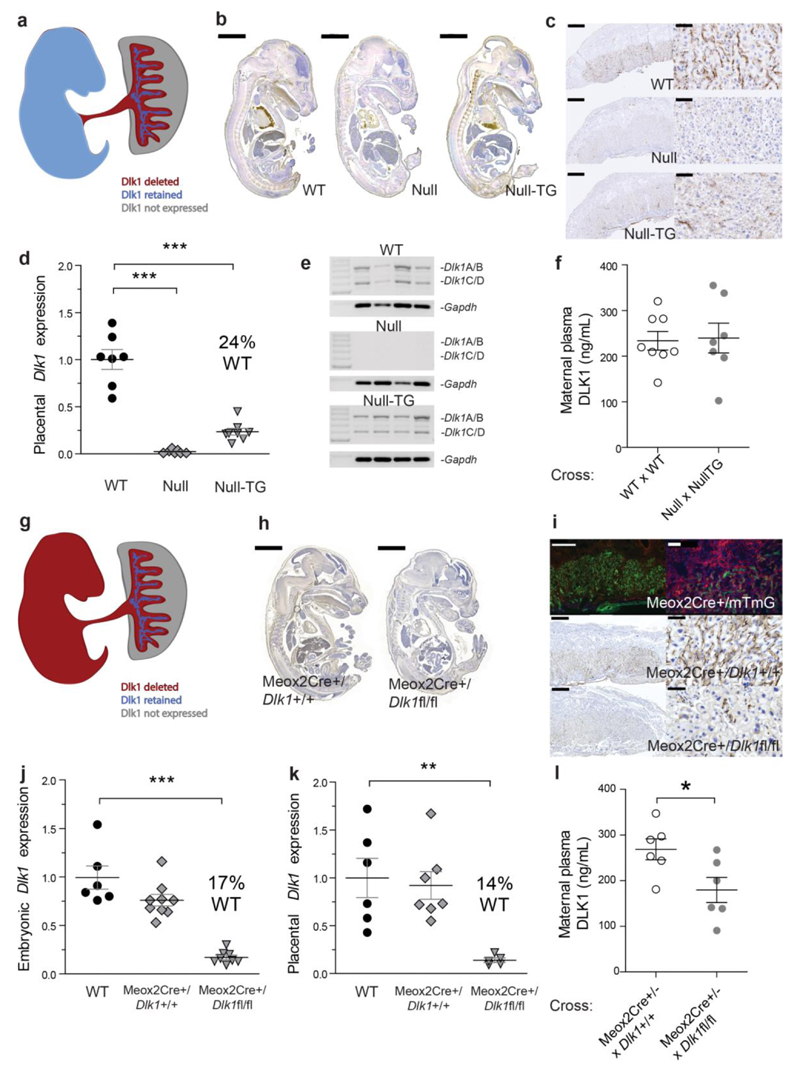
To distinguish whether the placenta or the embryo is the source of maternal plasma DLK1, we utilised additional models of Dlk1 dosage manipulation. We have previously described a transgenic line that recapitulates Dlk1 expression in the embryo, but not in the placenta[19] (Fig. 2a). We crossed the transgene onto a Dlk1-/- background (Null–TG) and measured transgene-specific Dlk1 expression in the embryo and placenta (Fig. 2b, c). As expected, DLK1 expression in the embryo closely resembled the WT, but DLK1 was lost from the fetal endothelial compartment of the Null–TG placenta. However, DLK1 expression was retained in a small population of cells in the placental labyrinth; these cells had large nuclei, consistent with a trophoblast identity, and contributed 24% of the WT Dlk1 expression (Fig. 2d). The placenta is a potential source of circulating DLK1 since, like the embryo, it expresses cleavable isoforms Dlk1A and B. As previously reported, the transgene recapitulated the isoform-specific Dlk1 expression[11] (Fig2e). We crossed Null–TG fathers to Null mothers to generate litters where all Dlk1 was transgene-derived (Supplementary Fig. 2). We found that maternal circulating DLK1 levels were not different from WT x WT crosses (Fig. 2f, Supplementary Table 1), indicating that DLK1 originates from either the embryo or a trophoblast population of the placenta.
Figure 2. Fetus not placenta is the source of maternal circulating DLK1.
(a) Schematic of Dlk1 expression in Null-TG conceptuses. (b) DLK1 expression (brown staining) the WT, Null and Null–TG embryo. Scale bar 2mm. (c) DLK1 is not detected in the fetal endothelium of the Null-TG placenta but is retained in cells with large nuclei. Scale bars left 500um, right 50um. (d) Dlk1 expression in the Null-TG, WT levels, and Null placentae (n = 7–8 per group) *** p < 0.001 by Dunnett’s Multiple comparison post-test (vs WT) following One-Way ANOVA. (e) Expression of cleavable (Dlk1A/B) and membrane-bound isoforms (Dlk1C/D) of Dlk1 in WT and Null-TG placentae. (f) DLK1 levels in maternal plasma in Null x Null-TG litters (n = 7–8 females/cross). (g) Schematic of Dlk1 expression in Meox2Cre/Dlk1fl/fl conceptuses. (h) DLK1 expression in the Meox2Cre/Dlk1fl/fl embryo compared to a Meox2Cre/Dlk1+/+ control. Scale bar 2mm. (i–top) Meox2Cre crossed to the mTmG reporter results in GFP+ cells following Cre excision, and mTomato+ in non–recombined cells. (i–bottom) Dlk1 is not detected the fetal endothelium of the Meox2Cre/Dlk1fl/fl placenta but some labyrinthine expression of DLK1 is retained. Scale bars left 500um, right 50um. (j) and (k) Dlk1 expression in the WT, Meox2Cre/Dlk1+/+ and Meox2Cre/Dlk1fl/fl embryo and placenta (5–8 per group), **p < 0.01, ***p < 0.001 as in (d). (l) Maternal plasma DLK1 in Meox2Cre+/- females crossed with Dlk1+/+ (control) or Dlk1fl/fl males (n = 6 females/cross), *p < 0.05 compared by Students’ t–test. All measurements performed at E15.5. Vertical bars show mean ± s. e. m.
We used a conditional allele of Dlk1[20] to ablate expression in all of the cells of the embryo and the fetal endothelium of the placenta, using the Meox2Cre[21] (Fig. 2g). DLK1 was largely absent in the embryo when the conditional allele (Dlk1fl/fl) was crossed to the Meox2Cre (Fig. 2h). Confirmation that the Meox2Cre was active in the fetal endothelium of the placenta was achieved by crossing to a mTmG reporter line[22] (Fig. 2i). As predicted, DLK1 expression in the placenta was absent from the fetal endothelial compartment in the conditional knock-out line (Fig 2i). In the embryo and placenta residual Dlk1 expression was 14–17% of WT levels following conditional targeting (Fig 2j, k). We crossed Meox2Cre+/- mothers to either WT or Dlk1fl/fl fathers to generate litters where Dlk1 was undeleted or deleted in half of the litter (Supplementary Fig. 2 – since the Meox2Cre is lethal in the homozygous state we were unable to generate a fully deleted litter). We found that maternal circulating DLK1 levels in Dlk1–deleted litters were approximately 50% of those where DLK1 was not deleted (Fig. 2l and Supplementary Table 1), indicating that maternal circulating DLK1 does not originate from the trophoblast cells of the placenta. Taken together these data show that the source of maternal circulating DLK1 is the embryo, not the placenta.
Pregnancies lacking DLK1 have compromised lipid metabolism
We next investigated whether Dlk1 expression dosage was relevant to the metabolic adaptations of pregnancy. Our experimental design allowed us to assess the contribution of both maternal Dlk1 genotype, and the presence or absence of circulating DLK1 from the conceptus (Fig. 1b). During pregnancy, total body weight and food intake did not significantly differ between the groups (Supplementary Table 2) despite the larger litters in Null females (Fig. 1e, Supplementary Table 1). When weight gain was normalised for number of conceptuses in the litter or total litter mass, Null females gained relatively less weight over the course of pregnancy (Fig. 3a, b). WAT, brown adipose tissue (BAT) and liver mass increased during pregnancy (Supplementary Table 3). Virgin and pregnant Null females had enlarged abdominal WAT deposits compared to WT and Mat (Supplementary Table 3), but consistent with their reduced overall weight gain, Null females gained less WAT during pregnancy compared with Dlk1–expressing females (Fig. 3c). This gain was depot–specific, since the Null females had a similar retroperitoneal WAT mass to Mat and WT females when virgins and when pregnant at E15.5. As in virgins, muscle mass was reduced in the pregnant Null females, and brain mass did not differ between the groups (Supplementary Table 3). In summary, females lacking Dlk1 during their own development had altered body composition as virgins, and gained less adipose tissue during pregnancy, suggesting that maternal loss of Dlk1 function limits adipose plasticity during pregnancy. Conceptus–derived circulating DLK1 did not modify any of these body composition parameters (Supplementary Tables 2 and 3).
Figure 3. Maternal genotype and conceptus-derived DLK1 alters maternal metabolism.
Female weight at E15.5 minus weight on the day of conception (E0.5), divided by the number of live fetuses (a), or total litter mass (sum of all placental and embryonic masses within the litter), (b). Null females gain significantly less weight. (c) Derived abdominal WAT gain during pregnancy –pregnant WAT weight for each cross minus average non–pregnant WAT weight for each genotype, expressed as % WT weight gain. Null females ‘gain’ significantly less abdominal WAT. Fasted 3-hydroxybutyrate, 3-OH (d), glucose (e), insulin (f), total cholesterol (h) and HDL-cholesterol (i) in maternal plasma from Null females crossed to WT or Null males, at E15.5. (g) Relative expression of Hmgcs2 in maternal liver at E15.5, in Null females crossed to WT or Null males, Growth Hormone (GH, j), Estradiol (E2, k) and Corticosterone (l) levels in all groups. Vertical bars show mean ± s. e. m (n = 6–8 females per group). Comparisons (a-c, and j-l) were compared by One-way ANOVA, with Bonferroni's Multiple comparison post-hoc test as indicated, *p < 0.05, **p < 0.01. Pregnant and NP GH and corticosterone samples did not have equal variance, so only pregnant samples were compared. WT NP vs WT x WT were compared to each other using a Student’s t-test with Welch’s correction, ***p < 0.001. (d-i) were compared by Student’s t-test; *p < 0.05, **p < 0.01, ***p < 0.001.
In contrast, circulating DLK1 did modify levels of circulating maternal metabolites. While Null x WT responded to pregnancy with a reduction in total cholesterol and HDL–C, pregnant Null females lacking conceptus-derived DLK1 (Null x Null) had a much less marked reduction in HDL–C (Supplementary Table 4). In addition, while Mat females with a normal pregnancy–induced rise in DLK1 had elevated levels of circulating ketones, Mat females without pregnancy-associated DLK1 did not. These changes occurred without alterations to circulating insulin levels (Supplementary Table 4) in free–fed females.
Upon fasting, Null females without pregnancy–induced DLK1 production failed to elevate their circulating ketones and were relatively hyperglycaemic, suggesting that the switch from glucose to fatty–acid fuel utilisation had not occurred (Fig. 3d, e), despite normal insulin levels (Fig. 3f). This was supported by the finding that Null mothers with circulating DLK1 had elevated hepatic expression of Hmgcs2 (encoding a rate limiting enzyme in the ketogenesis pathway, Fig. 3g) compared to Null mothers without circulating DLK1. As in non–fasted animals, Null mothers without conceptus–derived DLK1 did not experience the same magnitude of decrease in circulating HDL–C compared to Null females with circulating DLK1 (Fig. 3h, i). Combined, these data suggest that failure to elevate DLK1 during pregnancy prevents normal maternal metabolic adaptations – specifically reduced HDL–C and an accelerated response to starvation by the induction of ketogenesis.
We previously reported that non–pregnant transgenic mice with DLK1 levels elevated to a similar level as those during pregnancy (DLK1 levels in Dlk1-transgenic females at 6 months 343 ± 37ng/mL compared to WT (x WT) females at 12 weeks, E15.5, 223 ± 24ng/mL) have reduced circulating cholesterol levels and increased peripheral FA utilisation, in part due to increased growth hormone (GH) production[11]. GH levels are elevated in pregnancy in both humans and rodents (although the source of rodent GH in pregnancy is not clear[23], as they lack the placental GH gene variant found in primates[24]). Since GH-like molecules promote maternal adaptations to pregnancy[24], we asked if circulating GH was affected by Dlk1 during gestation. GH levels were elevated 13–fold by E15.5 of pregnancy in WT mice, but pregnancies entirely lacking Dlk1 had much less marked elevation in GH levels (approximately 3–fold in the Null x Null cross, Fig. 3j). These changes occurred in the absence of alterations to endocrine regulators of GH; estradiol and corticosterone (Fig. 3k, l), and pituitary Gh mRNA levels were unaffected (Supplementary Fig. 3). We concluded that pregnancies without a conceptus–derived rise in maternal plasma DLK1 have altered fuel metabolism, which may in part be due to impaired GH release.
Maternal circulating DLK1 reports fetal outcome
We compared embryo and placental weights of conceptuses with similar genotype in different maternal and litter contexts (Fig. 1b, Supplementary Table 5). Null embryos and placentae were smaller than WT or Mats, and neither the maternal genotype (and associated litter size) nor the presence/absence of circulating DLK1 affected weight at this stage (E15.5, Supplementary Table 5). However, Mat embryos in mixed litters with Null littermates (Null x Mat crosses) were significantly larger than Mat or WT embryos in any other context. Interestingly, since this group of conceptuses generated a greater amount of DLK1 per conceptus (Fig. 1e), this demonstrates a direct link between embryonic size and maternal circulating DLK1 levels. Placental size was not similarly increased at this gestational stage (Supplementary Table 5).
We therefore sought to determine whether there was any relationship between maternal plasma DLK1 levels and fetal growth in pregnant women. We used data and samples from a previously described prospective cohort, the Pregnancy Outcome Prediction study[25, 26]. A total of 4,512 women having first pregnancies were recruited and outcome data were available from 4,200. We studied a sample of 45 women who delivered a baby with a customized birth weight percentile less than the 5th (SGA) at term and had a plasma sample available which had been obtained around 36 weeks of gestational age (wkGA). We then identified two comparison groups: (1) matched controls (available for 43 out of the 45 cases), (2) a random sample from the cohort. In the former comparison group, women were one–to–one matched on the basis of maternal age, body mass index, smoking, fetal sex and mode of delivery to controls delivering a normally grown infant, i.e. samples were analysed as a matched case control study [27]. The maternal and outcome characteristics of these three groups are tabulated (Supplementary Table 6). Analysis of the matched cases and controls indicates no significant differences between the groups in either the matching characteristics or the gestational age of measurement of DLK1 (Supplementary Table 7). However, compared with the matched controls, the birth weights of their infants were 820 g lower on average.
Different phenotypes of SGA were classified according to research ultrasonographic examination, and the details of this are described elsewhere[26]. Ultrasonic assessment included serial Doppler flow velocimetry of the uterine and umbilical arteries. Both women and clinicians were blinded to the results of these research scans. High resistance uterine artery flow was defined as a mean pulsatility index in the top decile at 20 wkGA, high resistance umbilical artery flow was defined as a pulsatility index in the top decile at 36 wkGA, and low abdominal circumference growth velocity was defined as a difference in abdominal circumference z score between the 36 and 20 wkGA scans falling in the lowest decile (i.e. this group includes the fetuses which showed the greatest reduction in the relative size of the abdominal circumference at the time of the 36 wkGA scan). Among the 43 SGA pregnancies with matched controls, 20 (47%) had none of these features and were called healthy SGA and 23 (53%) had one or more of these features and were defined as FGR (birth outcomes of these 2 groups are presented in Supplementary Table 8).
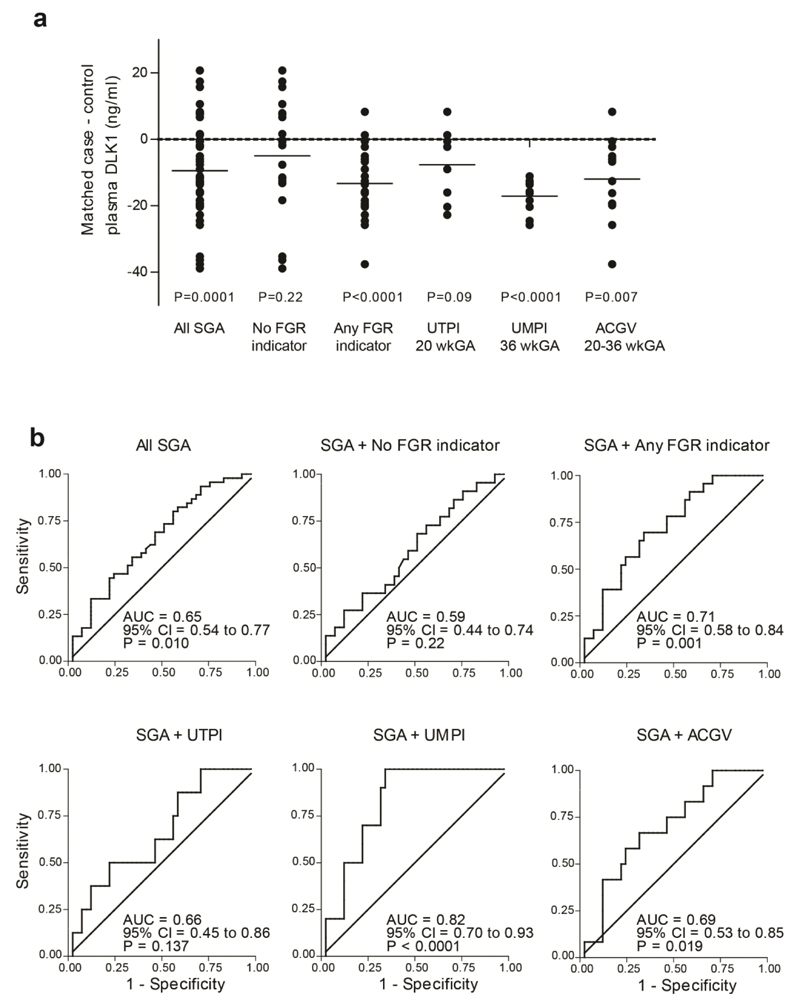
DLK1 levels were measured in maternal plasma obtained at a mean of 36 weeks and 1 day of gestational age (SD 2.7 days), (Fig. 4a). When compared with matched controls (Supplementary Table 9), DLK1 was lower overall in women with SGA infants (mean difference = –9.44, P=0.0001). However, when this population was divided into healthy SGA and FGR, there was no significant difference between healthy SGA and controls (mean difference –4.97, P=0.22), but there was a highly statistically significant reduction in DLK1 in FGR (mean difference = –13.33, P<0.0001). When analyzed by the different phenotypes of FGR, there were very strong associations between DLK1 concentration and SGA in the presence of either high resistance umbilical artery Doppler (mean difference = –17.13, P<0.0001) or low abdominal circumference growth velocity (mean difference = –11.96, P=0.007).
Figure 4. Low DLK1 in human pregnancy is associated with pathological SGA.
(a) Scatter plot of differences in DLK1 levels in maternal plasma of 43 pairs of matched pregnant women, differing by SGA outcome. Pathological = any of the fetal growth restriction (FGR) indicators (UTPI 20wk decile 10, n = 8 pairs; UMPI 36wk decile 10, n = 10 pairs; ACGV 20-36wk decile 1, n = 12 pairs). The short horizontal lines represent means of the differences. (b) Receiver Operating Characteristic (ROC) curve analysis showing the strength of association of maternal plasma DLK1 and SGA comparing cases (n = 45) with the random sample of the cohort (n = 41). AUC = area under curve, 95% confidence interval (CI) and p values for testing the null hypothesis (AUC = 0.5) are shown on respective graphs.
The strength of the association was then further evaluated by comparing cases with the random sample of the cohort using Receiver Operating Characteristic (ROC) curve analysis (Fig. 4b). Overall, DLK1 was moderately predictive of SGA: area under the ROC curve (AUROCC) 0.65 (95% CI 0.54 to 0.77, P=0.01). Similarly to the comparison with matched controls, there was no significant association between DLK1 levels and healthy SGA (AUROCC=0.59; 95% CI=0.44 to 0.74; P=0.22), but there was a highly statistically significant association with FGR (AUROCC=0.71; 95% CI=0.58 to 0.84; P=0.001). Again, the strongest association was with SGA combined with high resistance umbilical artery Doppler (AUROCC=0.82; 95% CI=0.70 to 0.93; P<0.0001).
Discussion
During late pregnancy the mother must be able to adapt her metabolism to changes in food availability in order to maintain a constant supply of energy to the fetus. Here we have shown in the mouse that both DLK1 expression in maternal tissues, and circulating DLK1 derived from the fetus are necessary for appropriate metabolic adaptations to pregnancy; specifically to provide metabolic plasticity by allowing the switch to FA utilisation when resources are limited during fasting.
Moreover, since it is derived from the fetus, maternal circulating DLK1 levels provide a non–invasive read–out of embryonic state. Our data indicate that maternal plasma DLK1 levels are lower in pregnancies complicated by FGR, and suggest that DLK1 measurements in women may be clinically useful to differentiate healthy SGA infants from those which are pathologically small, an area of intense clinical interest. Interestingly, lower DLK1 levels were most strongly associated with high resistance patterns of flow in the umbilical artery and slow abdominal circumference growth velocity. The potential clinical significance of clinical measurement of DLK1 is underlined by the fact that we have previously reported that these two measures were the most effective predictors of neonatal morbidity in pregnancies where the baby was suspected to be small on the basis of ultrasonic fetal biometry[26]. Previous studies have reported contradictory associations between cord blood DLK1 levels and birth weight [28], as well as in complications of pregnancy[29]. Those studies used a commercial ELISA which reports DLK1 levels that were ˜100x lower than values reported in both the original pioneering work on DLK1 as a soluble factor in pregnancy[7] and the values measured in this study. This suggests that further assay development may be required before DLK1 is a suitable biomarker for clinical use. However, our data is consistent with a recent study that reported a genomic variant at the imprinted DLK1–GTL2 locus in humans that segregates with birthweight[30], and with a study where elevated DLK1 expression was associated with LGA pregnancies[31].
The analysis of human plasma samples focused on blood samples obtained at 36 wkGA. The gestational age of analysis was purposeful, as at this stage of pregnancy delivery is a safe and effective intervention to mitigate the risks of FGR where it is clinically suspected. We have previously outlined a case for screening women for adverse pregnancy outcome in late gestation[32]; around one third of all intrauterine fetal deaths (IUFDs) occur at or after 37 weeks and about 30% of all IUFDs at term are thought to be related to poor fetal growth[12]. However, detection of FGR at earlier gestational ages would also be valuable, and further work could address whether these associations are present at earlier gestational ages, and whether DLK1 is also predictive of other adverse pregnancy outcomes.
In conclusion our findings highlight DLK1 measurement as a valuable prenatal diagnostic for known disorders of impaired fetal DLK1 production (such as Temple syndrome[33]), and indicate that measuring maternal plasma DLK1 may have more general utility to differentiate healthy SGA from pathological complications of pregnancy requiring obstetric intervention.
Methods
Animal work
Mice
All animal work was carried out in accordance with UK Government Home Office licencing procedures. Mice were housed in a temperature and humidity controlled room (21 °C, 55 % humidity) with a 12 hour / 12 hour light-dark cycle. All mice were fed standard RM3 (E) diet (Special Diets Services) ad libitum, given fresh tap water daily and re-housed in clean cages weekly. Mice were weaned at 21 days postnatum, or a few days later if particularly small. Thereafter, they were housed in single-sex groups (5 per cage maximum) or occasionally singly housed, except when breeding.
The Dlk1 knock-out line (Dlk1tm1Srba) results in the replacement of 3.8 kb of the endogenous Dlk1 allele, including the Dlk1 promoter and its first three exons, with a neomycin resistance cassette[13]. The Dlk1 knock-out line was backcrossed onto the C57BL6/J background for at least 10 generations, and then routinely maintained by homozygous crosses (Dlk1 tm1Srba / tm1Srba x Dlk1 tm1Srba / tm1Srba). For experimental purposes, maternal heterozygotes (Dlk1 tm1Srba /+, Mats) and wild-types (Dlk1+/+, WTs) were generated. In all cases experimental WT mothers were the age-matched littermates of Mat females, both arising from an intercross between a Mat female and a stock C57BL6/J male.
Conditional Dlk1 deletion (Dlk1tm1.1Jvs) mice on a C57BL6/J background were obtained from JAX, backcrossed to generate homozygotes, and genotyped as described previously[20]. Meox2Cre heterozygous (Meox2tm1(Cre)Sor/+) females on a C57BL6/J background were maintained as heterozygotes as described[21]. Conditional mutants were generated by crossing Meox2Cre+/- females to Dlk1fl/fl males, and the recombination event was detected by PCR as described previously[20]. Meox2Cre-induced recombination in the fetal endothelium of the placenta was validated by crossing to the mTmG dual reporter line (Gt(ROSA)26Sortm4(ACTB-tdTomato, -EGFP)Luo/J), obtained from JAX and genotyped as described previously[22]. These mice ubiquitously express a red fluorescent protein, except following recombination by Cre, which causes GFP to be expressed instead.
Experimental cohort
At eight weeks postnatum, female mice were weighed and excluded from the study if <15g. The blood glucose level was measured in study females and a 20-30 μl blood sample was taken from the tail vein for plasma extraction. Female mice were then mated with stud males in the crosses described in Fig 1b. Females were monitored once daily between 08:00 and 10:00 for the presence of a vaginal plug. The day of plug discovery was designated embryonic day 0.5 (E0.5). Additional eight-week-old female WT, Mat and Null mice were not mated, but remained in their home cage until the experimental start day (E0.5-equivalent). Blood sampling was repeated on E0.5, E7.5, E9.5, E11.5, E13.5 and E15.5, between 10:45 and 12:30. From E0.5, the female mice were singly housed and provided with a non-limiting, weighed quantity of standard RM3 (E) diet. The remaining food weight was recorded at E7.5 and E15.5 as an indicator of food intake. On E15.5, the female mice were killed by terminal anaesthesia using ˜0.8 mg pentobarbitol (Dolethal; Vetoquinol) per gram body weight, injected intra-abdominally. Upon cessation of the twitch reflex, mice were exsanguinated by cardiac puncture. Maternal and conceptus tissues were weighed and collected for processing. All dissections were carried out between 12:00 and 17:00. The following exclusion criteria were applied to all mice: (1) if the female mouse did not show evidence of a vaginal plug after 11 days spent with a stud male; (2) if a pregnant mouse carried <5 or >12 live conceptuses at E15.5; (3) if a pregnant mouse with a mixed-genotype litter had an unbalanced conceptus genotype ratio (>3:<1 genotype ratios, when the predicted Mendelian ratio was 1:1); (3) if upon dissection the female mouse was found to have a confounding anatomical abnormality; (4) if the female mouse died during the experiment.
Fasted cohort
On E15.5, the female mice were fasted for 4 hours then their tail blood glucose was measured. They were immediately killed by terminal anaesthesia and exsanguinated as above. All fasts were started between 09:15 and 11:00 and all dissections were carried out between 13:15 and 16:00.
Null-TG mice were generated by crossing Dlk1 70kb BAC transgenic mice (TgDlk1-70C) on a C57BL6/J background with the Dlk1tm1Srba knock-out line, and genotyped as described above and as previously described[19].
Immunohistochemistry
Immunostaining was carried out on wax-embedded material as described previously[19], using an anti-DLK1 antibody(R&D AF8277).
Serum and tissue biochemistry
Enzymatic assay kits were used for determination of plasma FFAs (Roche), TAGs and glycerol (Sigma-Aldrich), and Cholesterol (Dade-Behring). ELISA kits were used for measurements of Insulin (Crystalchem), Estradiol (Calbiotech), Leptin and Growth Hormone (Millipore), all according to manufacturers’ instructions. Blood glucose levels were measured using a glucose meter (One Touch Ultra, LifeSpan, UK). The methods for the mouse DLK1 ELISA and tissue determination of TAG were described previously [10]. The human DLK1 ELISA was used for measurement on maternal plasma according to the manufacturers’ instructions (Adipogen).
Real-time quantitative PCR
mRNAs were analysed by RT-PCR as described [10]. Quantification was performed using the relative standard curve method, and target gene expression was normalised to the expression of a reporter gene (mean of Hprt and α tubulin for liver samples, β actin for pituitary samples, and mean of all 3 for e15.5 placenta and embryo samples), the expression of which did not differ between the groups. All primers (sequences in Supplementary Table 10) amplified with efficiency greater than or equal to 85%.
Statistical analysis of animal data
All statistical tests were performed using the GraphPad Prism Software version 4.00 for Windows, GraphPad Software, San Diego California USA. Data was tested for Normal distribution (Kolmogorov-Smirnov test) and statistical evaluation applied accordingly. Tests, significance values and number of samples analysed are indicated in the respective figure/table legends, and all error bars represent the standard error of the mean (s.e.m).
Human data
Study design and participants
All samples and data were obtained from the Pregnancy Outcome Prediction (POP) study as described previously[25, 26]. Ethical approval for the study was obtained from the Cambridgeshire Research Ethics Committee (reference 07/H0308/163). Participants provided written consent. The plasma had been frozen on the day of collection and stored at -80°C. Measurement of DLK1 was performed blind to both the ultrasonic and outcome data.
Maternal characteristics of the SGA infants included in this analysis were representative of the whole POP study population (Supplementary Table 11). Since the analysis required a 36 week sample, all SGA infants included were born at term whereas 15% of the SGA cases not included in the analysis were born preterm. Therefore, the SGA infants not included in the analysis weighed less at birth. However, the distribution of the birth weight percentile was not markedly different between the groups. The distribution of DLK1 concentration was fairly normal in cases and unmatched controls, and also the difference in DLK1 concentration between cases and matched controls was normally distributed.
Analysis
The association between DLK1 and SGA was assessed by the ROC curve analysis using random controls as a comparison group and this analysis was repeated for each SGA phenotype. Group means of DLK1 were compared using independent two-sample t tests between SGA cases and random controls. Paired t tests were used to compare SGA cases and their matched controls. Power analyses were performed for both types of tests. Sample size calculation demonstrated that the paired analyses were well powered. We had 90% power to detect a 7.3 ng/mL within-pair difference in DLK1 measurements (n=43 pairs), assuming the observed standard deviation of the difference (14.5 ng/mL) and alpha=0.05 (two sided). The power to detect the observed difference of 9.44 ng/mL was 98.6%. For the analysis of the different phenotypes of FGR we attained 80% power to detect a difference of a 9.6 ng/mL, assuming the average observed standard deviation of the difference within the extreme decile (9.6 ng/mL), n=10 pairs and alpha=0.05. In our unpaired overall analysis, we had 80% power to detect a 7.8 ng/mL difference in DLK1 between the groups (sample sizes 45 and 41), assuming the observed combined standard deviation of the measurement (12.7 ng/mL) and alpha=0.05. All analyses were performed using Stata 14.1 (StataCorp, College Station, TX, USA).
Supplementary Material
Acknowledgements
MAMC was supported by a PhD studentship from the Cambridge Centre for Trophoblast Research. Research was supported by grants from the MRC (MR/J001597/1; MR/L002345/1), The Medical College of Saint Bartholomew's Hospital Trust, Wellcome Trust Investigator Award, EpigeneSys (FP7 Health - 257082), EpiHealth (FP7 Health – 278414), a Herchel Smith Fellowship (NT), NIH RO1 DK89989. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. We thank G Burton for invaluable support, M Constancia and I Sandovici (University of Cambridge, UK) for the Meox2Cre mice. We are extremely grateful to all of the participants in the Pregnancy Outcome Prediction study. This work was supported by the National Institute for Health Research (NIHR) Cambridge Comprehensive Biomedical Research Centre (Women's Health theme), and project grants from the MRC (G1100221) and Sands (Stillbirth and neonatal death charity). The study was also supported by GE Healthcare (donation of two Voluson i ultrasound systems for this study), and by the NIHR Cambridge Clinical Research Facility, where all research visits took place.
Footnotes
Author Contributions
M.C., M.A.M.C., A.C.F–S., G.C.S.S. conceived and designed the experiments. M.C., M.A.M.C., J.A.C., M.H., I.G., N.T., C.L.D., D.S.C–J. performed the experiments. M.C., M.A.M.C., A.C.F–S., C.L.D. F.G., G.C.S.S. analysed the data. M.C. U.S. performed statistical analysis. S.R.B., T.L.P., A.C.F–S., G.C.S.S. contributed reagents. M.C., M.A.M.C., A.C.F–S., G.C.S.S. wrote the manuscript. M.C., A.C.F–S., G.C.S.S. provided supervision.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;71(5 Suppl):1256S–61S. doi: 10.1093/ajcn/71.5.1256s. [DOI] [PubMed] [Google Scholar]
- 2.Metzger BE, et al. “Accelerated starvation” and the skipped breakfast in late normal pregnancy. Lancet. 1982;1(8272):588–92. doi: 10.1016/s0140-6736(82)91750-0. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt JV, et al. The Dlk1 and Gtl2 genes are linked and reciprocally imprinted. Genes Dev. 2000;14(16):1997–2002. [PMC free article] [PubMed] [Google Scholar]
- 4.Takada S, et al. Delta-like and Gtl2 are reciprocally expressed, differentially methylated linked imprinted genes on mouse chromosome 12. Curr Biol. 2000;10(18):1135–8. doi: 10.1016/s0960-9822(00)00704-1. [DOI] [PubMed] [Google Scholar]
- 5.Smas CM, Chen L, Sul HS. Cleavage of membrane-associated pref-1 generates a soluble inhibitor of adipocyte differentiation. Mol Cell Biol. 1997;17(2):977–88. doi: 10.1128/mcb.17.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmann E, et al. Mouse fetal antigen 1 (mFA1), the circulating gene product of mdlk, pref-1 and SCP-1: isolation, characterization and biology. J Reprod Fertil. 1996;107(2):279–85. doi: 10.1530/jrf.0.1070279. [DOI] [PubMed] [Google Scholar]
- 7.Floridon C, et al. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation. 2000;66(1):49–59. doi: 10.1046/j.1432-0436.2000.066001049.x. [DOI] [PubMed] [Google Scholar]
- 8.Carlsson HE, et al. Purification, characterization, and biological compartmentalization of rat fetal antigen 1. Biol Reprod. 2000;63(1):30–3. doi: 10.1095/biolreprod63.1.30. [DOI] [PubMed] [Google Scholar]
- 9.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73(4):725–34. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 10.Charalambous M, et al. Imprinted gene dosage is critical for the transition to independent life. Cell Metab. 2012;15(2):209–21. doi: 10.1016/j.cmet.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charalambous M, et al. DLK1/PREF1 regulates nutrient metabolism and protects from steatosis. Proc Natl Acad Sci U S A. 2014;111(45):16088–93. doi: 10.1073/pnas.1406119111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moraitis AA, et al. Birth weight percentile and the risk of term perinatal death. Obstet Gynecol. 2014;124(2 Pt 1):274–83. doi: 10.1097/AOG.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 13.Raghunandan R, et al. Dlk1 influences differentiation and function of B lymphocytes. Stem Cells Dev. 2008;17(3):495–507. doi: 10.1089/scd.2007.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chacon MR, et al. Human serum levels of fetal antigen 1 (FA1/Dlk1) increase with obesity, are negatively associated with insulin sensitivity and modulate inflammation in vitro. Int J Obes (Lond) 2008;32(7):1122–9. doi: 10.1038/ijo.2008.40. [DOI] [PubMed] [Google Scholar]
- 15.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2(7):538–48. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 16.Malik NM, et al. Leptin requirement for conception, implantation, and gestation in the mouse. Endocrinology. 2001;142(12):5198–202. doi: 10.1210/endo.142.12.8535. [DOI] [PubMed] [Google Scholar]
- 17.Schulz LC, et al. Effect of leptin on mouse trophoblast giant cells. Biol Reprod. 2009;80(3):415–24. doi: 10.1095/biolreprod.108.073130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roman EA, Ricci AG, Faletti AG. Leptin enhances ovulation and attenuates the effects produced by food restriction. Mol Cell Endocrinol. 2005;242(1–2):33–41. doi: 10.1016/j.mce.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 19.da Rocha ST, et al. Gene dosage effects of the imprinted Delta-like homologue 1 (Dlk1/Pref1) in development: implications for the evolution of imprinting. PLoS Genet. 2009;5(2):e1000392. doi: 10.1371/journal.pgen.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appelbe OK, et al. Conditional deletions refine the embryonic requirement for Dlk1. Mech Dev. 2013;130(2–3):143–59. doi: 10.1016/j.mod.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26(2):113–5. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Muzumdar MD, et al. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 23.El-Kasti MM, et al. The pregnancy-induced increase in baseline circulating growth hormone in rats is not induced by ghrelin. J Neuroendocrinol. 2008;20(3):309–22. doi: 10.1111/j.1365-2826.2008.01650.x. [DOI] [PubMed] [Google Scholar]
- 24.Soares MJ. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol. 2004;2:51. doi: 10.1186/1477-7827-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasupathy D, et al. Study protocol. A prospective cohort study of unselected primiparous women: the pregnancy outcome prediction study. BMC Pregnancy Childbirth. 2008;8:51. doi: 10.1186/1471-2393-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sovio U, et al. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet. 2015;386(10008):2089–97. doi: 10.1016/S0140-6736(15)00131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotham KJ, G S. Modern Epidemiology. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 28.de Zegher F, et al. Abundance of circulating preadipocyte factor 1 in early life. Diabetes Care. 2012;35(4):848–9. doi: 10.2337/dc11-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrey S, et al. The adipokine preadipocyte factor-1 is downregulated in preeclampsia and expressed in placenta. Cytokine. 2015;75(2):338–43. doi: 10.1016/j.cyto.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Moore GE, et al. The role and interaction of imprinted genes in human fetal growth. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663) doi: 10.1098/rstb.2014.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kappil MA, et al. Placental expression profile of imprinted genes impacts birth weight. Epigenetics. 2015;10(9):842–9. doi: 10.1080/15592294.2015.1073881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith GC. Researching new methods of screening for adverse pregnancy outcome: lessons from pre-eclampsia. PLoS Med. 2012;9(7):e1001274. doi: 10.1371/journal.pmed.1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ioannides Y, et al. Temple syndrome: improving the recognition of an underdiagnosed chromosome 14 imprinting disorder: an analysis of 51 published cases. J Med Genet. 2014;51(8):495–501. doi: 10.1136/jmedgenet-2014-102396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.