Abstract
Introduction
Previous investigations have demonstrated that major depression is associated with particular patterns of cytokine signalling. The primary aim of this study was to examine peripheral pro-inflammatory and anti-inflammatory cytokines and immune balance in generalised anxiety disorder (GAD).
Methods
A case-controlled cross-sectional study design was employed: 54 patients with GAD and 64 healthy controls were recruited. Participants completed self-report measures of anxiety and depression. Two pro-inflammatory and two anti-inflammatory cytokines were measured using multiplex technology.
Results
Case-control logistic regression analyses revealed significant differences in serum levels of IL-10, TNF-α, and IFN-γ between GAD and control groups after adjusting for age, gender, body mass index, smoking and alcohol consumption: these group differences were independent of the presence or degree of depression. Comparison of pro-inflammatory to anti-inflammatory cytokine ratios indicated that there were significantly higher ratios of TNF-α /IL10, TNF-α /IL4, IFN-γ /IL10, and IFN-γ /IL4 in the GAD group compared to the control group.
Conclusions
This study is the first to investigate both pro- and anti-inflammatory cytokines and their balance in patients with GAD in comparison to healthy controls. The findings indicate a relatively increased pro-inflammatory response and decreased anti-inflammatory response and provide the first demonstration of an altered cytokine balance in GAD. Serum cytokine levels in GAD were independent of the presence of depression.
Keywords: Generalised Anxiety Disorder, inflammation, pro-inflammatory cytokine, anti-inflammatory cytokine, cytokine ratios
1. Introduction
Research into psychoneuroimmunology (PNI) has led to substantial advances in understanding of the reciprocal interactions between the central nervous system and the immune system in neuropsychiatric disorders (1–5). Evidence from experimental and clinical research shows the pivotal roles of cytokine signalling to the brain to produce neurochemical, neuroendocrine, neuroimmune, and behavioural changes (6–11). The presence of inflammatory responses, and in particular, the role of cytokines in major depression has been addressed in numerous studies. However, neuroinflammatory markers in anxiety disorders have been studied less extensively. There is a need for better understanding of both the heterogeneous role of specific cytokines and immune balance in anxious states and in different anxiety disorders (12).
Cytokines are soluble bioactive mediators released by various cell types both at the periphery (such as monocytes and macrophages) and in the brain (such as microglia, astrocytes, and endothelial cells), which operate within a complex network and can act synergistically or antagonistically. Based on the functional profile of an immune response, cytokine production is broadly orchestrated by T helper 1 cells (Th1) which generally mediate a pro-inflammatory cellular immune response, and T helper 2 cells (Th2) which enhance anti-inflammatory and humoral immune reactions. The pro-inflammatory cytokines, tumour necrosis factor-alpha (TNF-α) and interferon-γ (IFN-γ), prime a Th1 response, and enhance the elimination of intracellular pathogens, while the anti-inflammatory cytokines, interleukin (IL)-4 and IL-10, enhance a Th2 response, enabling phagocytosis of extracellular pathogens, tissue repair and dampening the synthesis of pro-inflammatory cytokines (13). The balance between Th1 and Th2 cytokines is an important determinant in regulating the inflammatory response, and a delicate balance of pro- and anti-inflammatory cytokines is required for normal neuropsychological functioning (8,10).
Since signs of immune disturbances in depression were first reported in early 1990s (14–17) the presence of inflammatory responses and the role of cytokines in major depression have been extensively studied. The high comorbidity of anxiety disorders and major depression and similar effects of antidepressants suggest common neurobiological substrates. In addition, the pronounced response of central and peripheral cytokines to stress has prompted further interest in the role of cytokines in the pathogenesis of anxiety disorders.
Generalized anxiety disorder (GAD), the most common impairing anxiety disorder, is a common and frequently chronic condition characterized by excessive, uncontrollable and often irrational worry about everyday things. GAD affects approximately 1.9-5.1% of the general population, and 8% of patients in primary care (28). Impairments of GAD are similar in magnitude to those of major depression (29). Camacho (30) proposed that anxious-depression should be considered as a chronic inflammatory phenomenon but the only longitudinal study found the association between GAD and increased C-reactive protein (CRP) level to be attributable to body mass index (BMI) and medication use (31). A large cohort study examined the association between anxiety disorders (including GAD, social phobia, PD, and agoraphobia) and inflammation (32), and the authors reported elevated CRP levels in male patients with current anxiety disorders and immune dysregulation in patients with a late-onset anxiety disorder. In addition, an integrated specificity model emphasizes specific patterns of biological responses to specific psychological states (33,34) and an anxiety-specific effect on inflammatory activity in clinically anxious individuals has been reported (35). It is uncertain whether anxiety is associated with inflammatory activity in GAD either through a specific anxiety pathway or through a more general negative affective pathway.
The primary aims of our study, therefore, were to (1) examine pro- and anti- inflammatory cytokine levels and ratios in patients with GAD in comparison to healthy controls; and (2) determine whether peripheral inflammatory cytokine levels in GAD are independent of depression. The main predictions were as follows: Hypothesis 1: There will be differences of serum cytokine levels as well as pro-inflammatory to anti-inflammatory cytokine ratios between the GAD group and the control group; Hypothesis 2: The effect of GAD on cytokine levels will be independent of depression.
2. Methods
2.1. Participants
A total of 54 patients (aged between 18-65 years with BMI between 18.5-29.9) with a primary diagnosis of GAD were recruited from community mental health team outpatient clinics and general practice surgeries. All patients met DSM-IV and ICD-10 diagnostic criteria for GAD. All patients completed a pre-test screening interview comprising a structured diagnostic Mini International Neuropsychiatric Interview - MINI (47) and the 7-item Generalised Anxiety Disorder Questionnaire (GAD-7) with a threshold score of 10 points (48). Due to high comorbidity of anxiety and depression in GAD, and in order to explore the influence of depression, patients with coexisting depressive symptoms were not excluded. 64 healthy controls were recruited from the community by advertising on posters and internet during the same period. All participants were able to understand both spoken and written English. Participants were excluded if they reported intake of any medication with known immune-modulating effects (such as glucocorticoids), had acute or chronic organic illnesses, or met criteria for additional mental disorders. Participants who had experienced any inflammatory event within the 2 weeks before the assessment were excluded. Patients taking anxiolytic drugs were not excluded.
The study was approved by the National Research Ethics Service Committee Health Research Authority South Central - Portsmouth (Reference Number 11/SC/0484).
2.2. Self-reported questionnaire measures
The following self-reported questionnaire measures were used:
-
1)
The Hospital Anxiety Depression Scale (HADS), a well-validated measure of depression and anxiety, which consists of two 7-item subscales (49).
-
2)
The Perceived Stress Scale (PSS), a well-validated and widely used measure of subjective stress (50). Participants rated the degree to which they perceived their lives to be unpredictable, uncontrollable, and overwhelming. Total scores range from 0 to 40, with higher scores indicating greater stress.
-
3)
The Anxiety Sensitivity Index (ASI), a self-report measure of fears of arousal-reactive bodily symptoms (51), which is extensively used in clinical and health psychology research and has acceptable psychometric properties.
A structured general information questionnaire determining the socio-demographic and clinical features of participants was also employed.
2.3. Measure of peripheral inflammatory cytokines
A sample of 10ml venous blood was taken from all participants at the same time of day (9:00-10:00AM) and centrifuged for 15 min at 2500rpm. The cell free-serum was collected and aliquoted in freezer vials and stored at -80° C until further analysis. Serum levels of two pro-inflammatory cytokines (TNF-α and IFN-γ) and two anti-inflammatory cytokines (IL-4 and IL-10) were measured using a multiplex ultra-sensitive immunoassay - Meso Scale Discovery (MSD, USA). Calibrators were run in duplicate to generate a stand curve which was modelled using least squares fitting algorithms so that signals from samples with known levels of the analyte can be used to calculate the concentration of analyte in the sample. The sensitivity was indicated by the lower limit of detection (LLOD) of these cytokines (IL-4: 0.31 pg/ml, IL-10: 0.36 pg/ml, TNF-α: 0.48 pg/ml and IFN-γ: 0.39 pg/ml). Processing of blood samples was based on a protocol provided for human multiplex assays and recommendations for clinical trials (52).
2.4. Study design and procedure
A case-controlled cross-sectional cohort design was employed. All potential participants were given detailed information sheets regarding the study and all participants provided written informed consent before taking part in the study. Participants who attended the laboratory were asked to rest for 5 minutes before the assessment. After blood samples were taken, they completed a questionnaire booklet. All data were analysed with Statistical Package for the Social Sciences (SPSS version 21).
2.5. Data analysis
All variables were tested for normality, and transformation to symmetry using normal scores was undertaken when necessary. Group differences in sociodemographic characteristics between the GAD and healthy control groups were assessed by independent t-tests for continuous measures, and by Chi-square tests for categorical variables. Regression analysis was used to test group differences across the following primary outcome measures: anxiety depression, two pro-inflammatory cytokines including TNF-α and IFN-γ, two anti-inflammatory cytokines including IL-4 and IL-10, and pro-inflammatory to anti-inflammatory cytokine ratios after controlling for age, gender, BMI, smoking, and alcohol consumption. The strength of associations between state anxiety and cytokine levels were examined using multiple linear regression analysis with anxiety score as the dependent variable. To test hypothesis 2 we constructed a set of multiple logistic regression analyses in which GAD (the outcome) was modelled with predictor variables age, sex, BMI and depression score and with each cytokine included in turn.
3. Results
3.1. Comparison of demographic and clinical characteristics of study groups
Demographic and clinical characteristics of the GAD and healthy control groups are shown in Table 1. There were more males in the GAD group than the healthy control group (X2=8.771, p=0.03). They were also older (t=4.264, p<0.05) and had higher BMIs (t=2.817, p<0.05). We therefore controlled for gender, age and BMI when comparing variables between groups using logistic regression analysis (see Table 2). There were no significant differences between groups in terms of smoking, alcohol consumption, hours of sleep and exercise per day (p>0.05 in all cases). The GAD group had significantly higher stress, anxiety and depression (P<0.001, in all cases) than the control group, consistent with clinical features of patients with GAD. Within the GAD group, there were 36 who were on anxiolytic medication and 18 who were medication free.
Table 1. Demographic and clinical characteristics of GAD and healthy control groups.
| GAD (n=54) | Control (n=64) | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Age (years) | 35.06 ± 14.45 | 25.75 ± 8.87 |
| BMI (kg/m2) | 24.84 ±5.70 | 22.45 ± 3.27 |
| Sleep (hrs) | 7.22 ±1.48 | 7.44 ±0.84 |
| Median (LQ, UQ) | Median (LQ, UQ) | |
| HADS - Anxiety | 14 (11, 16) | 5 (3, 8) |
| HADS - Depression | 8.5 (6, 11) | 1 (0, 4) |
| Perceived Stress Scale | 10 (7.25, 11) | 4 (2, 6) |
| Anxiety Sensitivity Index | 35 (27.5, 42) | 16 (11, 21.75) |
| Exercise (hrs) | 1 (0.88, 2) | 1 (0.5, 1) |
| n (%) | n (%) | |
| Gender - male | 41 (76%) | 32 (50%) |
| Smoking | ||
| Smoker | 12 (22%) | 22 (34%) |
| Non-smoker | 42 (78%) | 42 (66%) |
| Alcohol consumption | ||
| Frequent user (more than 3 times/week) | 13 (24%) | 14 (22%) |
| Non-frequent users ( less than 2 times/week) | 41(76%) | 50 (78%) |
| Use of anxiolytic medication | ||
| Yes | 36 (67%) | ¯ |
| No | 18 (33%) | ¯ |
Demographic and clinical characteristics of the GAD group and the healthy control group were presented in Table 1. GAD, generalised anxiety disorder; BMI, body mass index; SD, standard deviation; LQ, lower quartile; UQ, upper quartile; n, number of participants.
Table 2. Comparison of anxiety and depression between GAD and healthy control groups.
| Predictor score Outcome = GAD (1=yes, 0=no) |
OR† | 95%CI | P |
|---|---|---|---|
| HADS - Anxiety | 1.60 | 1.35-1.89 | <0.001 |
| HADS - Depression | 1.55 | 1.31-1.83 | <0.001 |
| Perceived Stress Scale | 1.73 | 1.41-2.11 | <0.001 |
| Anxiety Sensitivity Index | 1.19 | 1.12-1.27 | <0.001 |
There were significant group differences in terms of HADS-anxiety, HADS-depression, perceived stress, and anxiety sensitivity index (p<0.001 in all cases). †Odds ratios adjusted for gender, age and BMI. GAD, generalised anxiety disorder; HADS, the Hospital Anxiety Depression Scale.
3.2. Comparison of serum inflammatory cytokines between the GAD and control groups
Serum cytokine levels in the GAD and healthy control groups are shown in Table 3. After controlling for age, gender, and BMI, the GAD group had lower levels of IL-10 but higher levels of IFN-γ and TNF-α (p<0.001 in all cases). These differences remained significant after adjustment for co-morbid depression (see Table 3).
Table 3. Comparison of cytokine profile between GAD and healthy control groups.
|
Cytokines (pg/ml) |
GAD Median (LQ, UQ) |
Control Median (LQ, UQ) |
Controlling for age, gender, BMI, smoking, and alcohol consumption + controlling for depression |
|||||
|---|---|---|---|---|---|---|---|---|
|
OR† Per SD |
95%CI | P |
OR† Per SD |
95%CI | P | |||
| IL-4 | 0.04 (0.02, 0.06) | 0.04 (0.00, 0.14) | 0.96 | 0.60, 1.54 | 0.866 | 1.24 | 0.69, 2.23 | 0.481 |
| IL-10 | 0.22 (0.16, 0.31) | 0.80 (0.46, 1.25) | 0.27 | 0.14, 0.51 | <0.001 | 0.35 | 0.17, 0.70 | 0.003 |
| TNF-α | 1.71 (1.30, 1.99) | 1.11 (0.72, 1.69) | 0.17 | 0.09, 0.32 | <0.001 | 0.22 | 0.19, 0.47 | <0.001 |
| IFN-γ | 3.02 (2.07, 5.16) | 1.07 (0.69, 1.63) | 6.83 | 2.94, 15.90 | <0.001 | 7.46 | 2.50, 22.22 | <0.001 |
After controlling for age, gender, BMI, smoking and alcohol consumption, the GAD group had lower levels of IL-10 but higher levels of IFN-γ and TNF-α (p<0.001 in all cases). These differences remained significant after adjustment for co-morbid depression. GAD, generalised anxiety disorder. † Odds ratios adjusted for gender, age, BMI, smoking, and alcohol consumption. LQ, lower quartile; UQ, upper quartile; OR, odds ratio; CI, confidence interval.
We assessed whether there was any medication effect on cytokines levels within the GAD group between the 36 GAD patients who were taking anxiolytic medication and the 18 who were not (Table 4). As shown in Table 4, there were no differences between subgroups based on whether they were on anxiolytic medication or not.
Table 4. Associations between cytokine levels and GAD according to use of medication.
| Cytokine (pg/ml) |
Whether on medication | OR | 95%CI | P | P for difference |
|---|---|---|---|---|---|
| IL4 | Yes | 0.92 | 0.56, 1.53 | 0.76 | 0.88 |
| No | 1.07 | 0.59, 1.93 | 0.83 | ||
| IL10 | Yes | 0.34 | 0.18, 0.64 | 0.001 | 0.59 |
| No | 0.25 | 0.11, 0.57 | 0.001 | ||
| TNF-α | Yes | 0.19 | 0.08, 0.43 | <0.001 | 0.64 |
| No | 0.20 | 0.08, 0.52 | 0.001 | ||
| IFN-γ | Yes | 6.58 | 2.65, 16.33 | <0.001 | 0.30 |
| No | 6.14 | 2.34, 16.13 | <0.001 |
There were no differences between subgroups based on whether they were on anxiolytic medication or not.
3.3. Comparison of pro- to anti-inflammatory cytokine ratios between groups
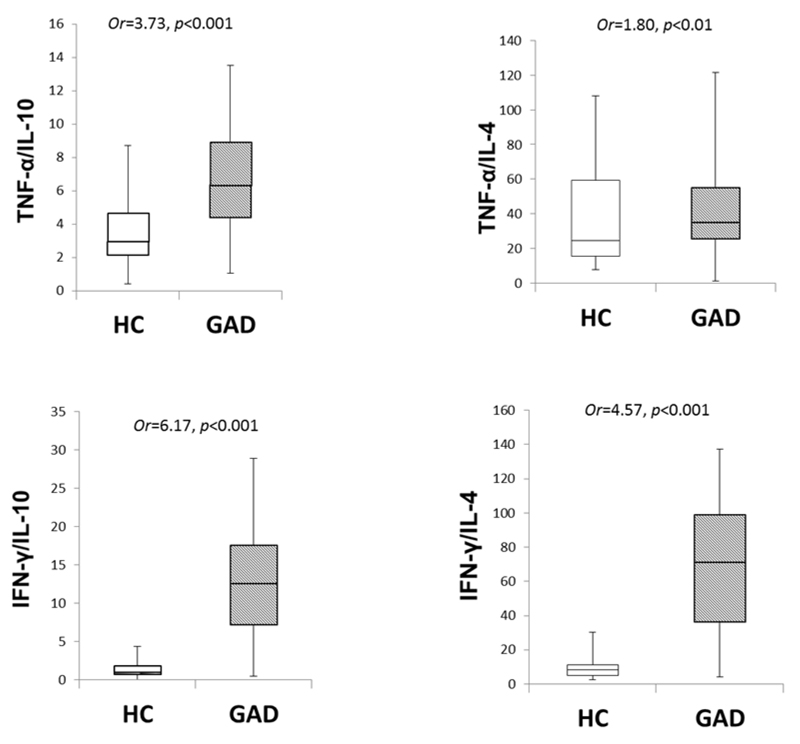
We further analysed pro- to anti-inflammatory cytokine ratios controlling for age, gender, BMI, smoking, and alcohol consumption. In comparison to the control group, the GAD group had significantly higher ratios of TNF-α/IL10, TNF-α /IL4, IFN-γ/IL10, and IFN-γ/IL4 (see Figure 1). These differences remained significant after adjustment for co-morbid depression (p<0.05 in all cases). Further multiple linear regression analyses with total anxiety score as the dependent variable were conducted within the GAD group to examine the associations between pro-and anti-inflammatory cytokine ratios and state anxiety. State anxiety was significantly correlated with IFN-γ /IL4 ratio (B=1.44, p=0.02), but not with TNF-α /IL10 (B=0.72, p=0.25), TNF-α /IL4 (B=0.83, p=0.20), or IFN-γ /IL10 (B=0.63, p=0.34).
Figure 1. Comparison of pro-and anti-inflammatory cytokine ratios between groups.
Four box- and- whisker plots were used to depict the distribution of pro-to anti-inflammatory cytokine ratios between the healthy control group and the patients group with generalised anxiety disorder. In comparison to the heathy control group, the patients group had significantly increased pro- to anti-inflammatory cytokine ratios, which suggests a distinct cytokine imbalance. HC, healthy control; GAD, generalised anxiety disorder.
4. Discussion
To our knowledge this is the first case-controlled investigation of pro- and anti-inflammatory cytokines and their balance in patients with GAD. Our data suggest a more pronounced pro-inflammatory response in association with a reduced anti-inflammatory compensation in GAD. Group differences in serum levels of IL-10, TNF-α, and IFN-γ, were independent of depression suggesting a specific inflammatory response in GAD. Ratios of TNF-α /IL10, TNF-α /IL4, IFN-γ /IL10, and IFN-γ /IL4 in the GAD group were significantly higher than the control group. State anxiety of GAD patients was significantly correlated with the IFN-γ/IL4 ratio. Combining analysis of both serum cytokine levels and cytokine ratios, the findings from this study revealed an increased pro-inflammatory response, a decreased anti-inflammatory response, and an altered cytokine balance in patients with GAD.
Due to a reliance on the measure of single cytokines, small sample sizes, the lack of standardized laboratory measurements, high co-morbidity with depression and other confounding factors, findings from research into anxiety disorders are not consistently observed. A recent systematic review of 20 studies revealed that post-traumatic stress disorder (PTSD) is associated with increased IL-6, IL-1β, TNF- α, and IFN-γ (18), whereas mixed findings have been reported in panic disorder (PD) (19–22). Hoge et al. (22) examined a broad spectrum of peripheral cytokines in 20 PTSD and 20 PD and found elevated median cytokine levels for 18 of 20 different cytokines compared to healthy controls. A recent Swiss population-based study found evidence of chronic low-grade systemic inflammation in individuals with agoraphobia (23). Lipopolysaccharide (LPS) - stimulated cytokine profile has been examined in patients with obsessive compulsive disorder (OCD) and generalized social anxiety disorder (GSAD) (24). Leukocytes of OCD patients produced less IL-6 compared with matched controls, whereas no cytokine differences were found between GSAD patients and matched controls. Studies in OCD have reported either increased or decreased TNF-α level (24–26). Meta-analysis of six studies involving 150 OCD subjects and 196 controls found no overall difference in TNF-α and IL-6 levels (27).
Previous studies of inflammation and anxiety were either limited to non-clinical samples (53,54), confined to specific anxiety disorders in small clinical samples (55,56,22), or in populations with heart disease (57). We found patients with GAD showed higher levels of pro-inflammatory cytokines and lower levels of anti-inflammatory cytokines than healthy controls. This extends evidence of general low grade pro-inflammatory serum profile in acute stress (58) and of a chronic, low-grade inflammatory response and activation of the compensatory anti-inflammatory reflex system in depression (59–61). Maes et al. (62) were the first to report that inflammatory responses were significantly correlated with stress-induced anxiety, and, in particular, subjects with anxiety showed significantly higher levels of IFN-γ and lower IL-10 than those without anxiety. Our results are in line with these findings - group differences were largest for IFN-γ suggesting a pro-inflammatory response in GAD. This pro-inflammatory cytokine is mainly produced by NK cells and CD4+ T cells, but macrophages have also been reported to secrete IFN-γ; the cellular source of this cytokine in our study remains to be elucidated. The finding of lower levels of serum anti-inflammatory cytokines IL-4 and IL-10 in GAD is similar to findings in patients with chronic pain where low IL-4 and IL-10 were found to be associated with increased pain perception (63), which may indicate a possible mechanism underlying the high association between pain and anxiety (64,65).
Anxiety and depression are highly comorbid and characterised by negative affect, consequently the observed associations between anxiety and inflammation could be due to general negative emotionality. Therefore, we conducted a further analysis to control for the presence and degree of depression, the significant differences of cytokine levels between groups remained, which indicates that the inflammatory phenotype in GAD is independent of depression. This finding supports anxiety-specific effects on inflammatory responses and is consistent with the integrated specificity model (34) and evidence of anxiety-specific inflammatory response pathway (35).
Tryptophan metabolism can alter immune activation by modulating Th1/Th2 balance (36, 37). Current evidence suggests a Th1 predominant immunophenotype in major depressive disorder, which shifts kynurenine catabolism towards microglial quinolinic acid; and a Th2 predominant immunophenotype in schizophrenia (38, 39, 40, 41), which shifts kynurenine catabolism towards astroglial kynurenic acid. One hypothesis is that the pro-inflammatory signature observed in our study is related to altered enzyme activity in tryptophan metabolism leading to serotonin degradation in GAD, through similar mechanisms to those in major depressive disorder. Vieira et al (46) revealed Th1 and Th2 cytokine deficiencies following T-cell activation in 20 GAD patients in comparison to 20 healthy controls, but ratios of Th1 to Th2 cytokines were not examined. Findings from our study revealed larger increase of all studied pro- to anti-inflammatory cytokine ratios in GAD in comparison to healthy controls. This provides what we believe to be the first demonstration of peripheral cytokine imbalance which appears to conform to the hypothesis that might incorporate an imbalance of Th1 and Th2 in GAD, although further studies are required to identify the cellular source of the pro-inflammatory cytokines and the underlying mechanisms that drive these changes. Significant increases in IFN-γ/IL10, IFN-γ/IL4, TNF-α/IL4 and TNF-α/IL10 ratios, reflect a distinct cytokine imbalance with a predominant pro-inflammatory response in GAD which is different from any acute or chronic stress response, during which a shift in balance in favour of an anti-inflammatory response (66–68). IFN-γ and IL-4 play major roles in the generation and regulation of immune responses and central in this respect is their mutually antagonistic functions. IFN-γ/IL-4 ratio is the most established ratio used to reflect Th1/Th2 patterns and balance in cytokine production (42–45). In the current study, the IFN-γ/IL-4 ratio showed the largest increase in GAD comparing to other ratios. The higher ratio of IFN-γ/IL-4 suggests a pro-inflammatory immune signature, possibly due to an imbalance of Th1/Th2 balance, which is further emphasized by increased TNF-a and decreased IL-10, but further studies are required to dissect the underlying mechanisms, including functional assays on immune effector cells. If replicated in subsequent studies pro- and anti-inflammatory cytokine ratios could be examined as potential trait markers for GAD, with IFN-γ/IL4 as a state marker for anxiety in GAD, as well as potential biomarkers for treatment response. Kubera et al. (69) reported that the anti-inflammatory effects of antidepressants may be related to altered immune balance through suppression of the IFN-γ/IL10 ratio.
The strengths of the current study include the measurement of multiple pro- and anti-inflammatory cytokines and consideration of a number of potential confounding factors in the study design, such as age, gender, BMI, smoking, alcohol consumption, medication, sleep, exercise, and standard culture procedure. In addition, any systemic influence of culture or variation of technical procedures can be adjusted for by using cytokine ratios. However, the findings of the study must be interpreted in light of several limitations. First, the cross-sectional design of the current study does not allow for definite conclusions on causal directions in the observed associations. Second, serum cytokine levels are unstable and can be affected by biological circadian rhythms, although we tried to control for this by taking blood between 8am and 10am. Third, our study was limited to a panel of 4 cytokines and does not exclude the possibility that other cytokines or chemokines may be important. Fourth, we were unable to adequately record disease severity and stages of GAD which may be associated with different inflammatory responses. As we investigated patients with established GAD, it is possible that we overlooked significant etiopathogenetic alterations of inflammatory markers. Finally, there are a number of cellular sources for the cytokines we measured e.g. natural killer cells, mast cells, monocytes and macrophages in addition to Th1 and Th2 cells. Thus, further studies are required to identify the cellular source of inflammatory cytokines and the underlying mechanisms that drive these changes. There is a persistent need to develop new treatment approaches for anxiety disorders due to sub-optimal short-term and long-term effectiveness and significant acceptability concerns. Enhanced understanding of altered cytokine balance, in particular, how cytokines mediate interactions between the central nervous system and the immune system could reveal biomarkers for treatment resistance and predictors for treatment response, identify new targets for the development of novel anxiolytic agents, and help improve clinical outcomes in anxiety disorders. Cytokine patterns have shown promise in predicting treatment response in depression, which could provide the key to treatment resistance (70, 71). Anti-inflammatory drugs demonstrate anti-depressant effects and can enhance responsiveness to antidepressants in a subgroup who show evidence of increased inflammation (72–75). With supporting data accumulating in depression (76), more evidence is needed to investigate whether dysregulated immune systems may contribute to treatment resistance in anxiety disorders, and can provide new treatment targets, in particular, for anxiety patients with increased systemic inflammation.
5. Conclusion
We demonstrate a distinct cytokine anxiety phenotype independent of the presence and degree of depression as well as an altered cytokine balance in patients with GAD. Growing understanding of cytokines in GAD may elucidate a unique inflammatory signature for diagnosis and treatment response, and guide our search for new drugs that selectively target and modulate specific immune phenotypes. Future work will benefit from applying the spectrum of immune phenotypes characterized in the periphery to the diversity of the immune process in the brain and making distinctions between different types of inflammatory states, as proposed by Brothers and Wilcock (77), as this may offer the best opportunity to individual treatment approaches.
Acknowledgement
The study was funded by the University of Southampton. We would like to thank Dr David Culliford from Statistical Sciences Research Institute for his statistical advice on the initial design of the study. We would also like to thank Ms Yvonne Clements, a MSD senior field applications scientist, and Mr Ben Coles, a senior laboratory manager, for providing training on blood sample handling process and cytokine analysis. Data from this study have been presented as posters at the British Association for Psychopharmacology meeting, the European College of Neuropsychopharmacology congress, and American Psychoneuroimmunology Research Society conference (2016).
Footnotes
Financial Disclosures
In the past two years, Professor David S. Baldwin has received financial support from H. Lundbeck A/S (advisory board attendance), AstraZeneca, Janssen and Pfizer (lecture fees), and the UK Ministry of Defence (research ethics committee membership). The other authors including Dr Ruihua Hou, Professor Clive Holmes, Dr Matthew Garner, Professor Clive Osmond, Professor Jessica Teeling, and Dr Laurie Lau report no biomedical financial interests or potential conflicts of interest.
References
- 1.Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosomatic Medicine. 1975;37:333–340. doi: 10.1097/00006842-197507000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Ader R, Cohen N, Felten D. Psychoneuroimmunology: interactions between the nervous system and the immune system. The Lancet. 1995;345:99–103. doi: 10.1016/s0140-6736(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 3.Miller AH, Maletic V, Raison CL. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard BE, Myint A. The psychoneuroimmunology of depression. Human psychopharmacology. 2009;24:165–175. doi: 10.1002/hup.1011. [DOI] [PubMed] [Google Scholar]
- 6.Kronfol Z, Remick DG. Cytokines and the Brain: Implications for Clinical Psychiatry. Am J Psychiatry. 2000;157:683–694. doi: 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- 7.Maier SF. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain, Behavior, and Immunity. 2003;17:69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 8.Loftis JM, Huckans M, Morasco BJ. Neuroimmune mechanisms of cytokine-induced depression: Current theories and novel treatment strategies. Neurobiol Dis. 2010;37:519–533. doi: 10.1016/j.nbd.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Molecular Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 12.Hou R, Tang Z, Baldwin DS. Potential neuroimmunological targets in the treatment of anxiety disorders. Mod Trends Pharmacopsychiatri. 2013;29:67–84. doi: 10.1159/000351965. [DOI] [PubMed] [Google Scholar]
- 13.Kronfol Z, Remick DG. Cytokines and the Brain: Implications for Clinical Psychiatry. Am J Psychiatry. 2000;157:683–694. doi: 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- 14.Maes M, Bosmans E, Suy E, Vandervorst C, De Jonckheere C, Raus J. Immune disturbances during major depression: upregulated expression of interleukin-2 receptors. Neuropsychobiology. 1990;24:115–120. doi: 10.1159/000119472. [DOI] [PubMed] [Google Scholar]
- 15.Maes M, Bosmans E, Suy E, Vandervorst C, DeJonckheere C, Raus J. Depression-related disturbances in mitogen-induced lymphocyte responses and interleukin-1 beta and soluble interleukin-2 receptor production. Acta Psychiatrica Scandinavica. 1991;84:379–386. doi: 10.1111/j.1600-0447.1991.tb03163.x. [DOI] [PubMed] [Google Scholar]
- 16.Maes M, Scharpe S, Bosmans E, Vandewoude M, Suy E, Uyttenbroeck W, et al. Disturbances in acute phase plasma proteins during melancholia: Additional evidence for the presence of an inflammatory process during that illness. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1992;16:501–515. doi: 10.1016/0278-5846(92)90056-k. [DOI] [PubMed] [Google Scholar]
- 17.Maes M, Stevens W, DeClerck L, Bridts C, Peeters D, Schotte C, et al. Immune disorders in depression: higher T helper/T suppressor-cytotoxic cell ratio. Acta Psychiatr Scand. 1992;86:423–31. doi: 10.1111/j.1600-0447.1992.tb03292.x. [DOI] [PubMed] [Google Scholar]
- 18.Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2:1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- 19.Weizman R, Laor N, Wiener Z, Wolmer L, Bessler H. Cytokine production in panic disorder patients. Clin Neuropharmacol. 1999;22:107–109. doi: 10.1097/00002826-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Brambilla F, Bellodi L, Perna G, Bertani A, Panerai A, Sacerdote P. Plasma interleukin-1 beta concentrations in panic disorder. Psychiatry Res. 1994;54:135–42. doi: 10.1016/0165-1781(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 21.van Duinen MA, Schruers KR, Griez EJ, Maes M. Neuroimmunological parameters in panic disorder. Acta Neuropsychiatr. 2004;16:94–100. doi: 10.1111/j.0924-2708.2004.0079.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depression and Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 23.Wagner EN, Wagner JT, Glaus J, Vandeleur CL, Castelao E, Strippoli MF, et al. Evidence for chronic low-grade systemic inflammation in individuals with agoraphobia from a population-based prospective study. PLoS ONE. 2015;10(4):e0123757. doi: 10.1371/journal.pone.0123757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fluitman S, Denys D, Vulink N, Schutters S, Heijnen C, Westenberg H. Lipopolysaccharide-induced cytokine production in obsessive-compulsive disorder and generalized social anxiety disorder. Psychiatry Research. 2010;178:313–316. doi: 10.1016/j.psychres.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Konuk N, Tekın IO, Ozturk U, Atik L, Atasoy N, Bektas S, et al. Plasma levels of tumor necrosis factor-alpha and interleukin-6 in obsessive compulsive disorder. Mediators of Inflammation. 2007;2007:65704. doi: 10.1155/2007/65704. Epub 2007 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monteleone P, Catapano F, Fabrazzo M, Tortorella A, Maj M. Decreased blood levels of tumor necrosis factor-alpha in patients with obsessive-compulsive disorder. Neuropsychobiology. 1998;37:182–185. doi: 10.1159/000026500. [DOI] [PubMed] [Google Scholar]
- 27.Gray SM, Bloch MH. Systematic review of proinflammatory cytokines in obsessive-compulsive disorder. Curr Psychiatry Rep. 2012;14:220–228. doi: 10.1007/s11920-012-0272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. European Neuropsychopharmacology. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman DL, Dukes EM, Wittchen HU. Human and economic burden of generalized anxiety disorder. Depression and Anxiety. 2008;25:72–90. doi: 10.1002/da.20257. [DOI] [PubMed] [Google Scholar]
- 30.Camacho A. Is anxious-depression an inflammatory state? Med Hypotheses. 2013;81:577–581. doi: 10.1016/j.mehy.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Generalized anxiety and C-reactive protein levels: a prospective, longitudinal analysis. Psychol Med. 2012;42:2641–2650. doi: 10.1017/S0033291712000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013 Apr 23;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemeny ME. An interdisciplinary research model to investigate psychosocial cofactors in disease: Application to HIV-1 pathogenesis. Brain, Behavior, and Immunity. 2003;17:62–72. doi: 10.1016/s0889-1591(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 34.Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stress have different biological profiles. Brain, Behavior, and Immunity. 2010;24:215–219. doi: 10.1016/j.bbi.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 35.O'Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O'Farrelly C, et al. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain, Behavior, and Immunity. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steiner J, Bogerts B, Sarnyai Z, Walter M, Gos T, Bernstein HG, et al. Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World J Biol Psychiatry. 2012;13:482–492. doi: 10.3109/15622975.2011.583941. [DOI] [PubMed] [Google Scholar]
- 37.Müller N, Schwarz MJ. Immune system and schizophrenia. Curr Immunol Rev. 2010;6:213–220. doi: 10.2174/157339510791823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 39.Mándi Y, Vécsei L. The kynurenine system and immune regulation. Neural Transm (Vienna) 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- 40.Müller N, Schwarz MJ. Immunological aspects of depressive disorders. Der Nervenarzt. 2007;78:1261–1273. doi: 10.1007/s00115-007-2311-3. [DOI] [PubMed] [Google Scholar]
- 41.Müller N, Schwarz MJ. The immunological basis of glutamatergic disturbance in schizophrenia: towards an integrated view. J Neural Transm Suppl. 2007;72:269–280. doi: 10.1007/978-3-211-73574-9_33. [DOI] [PubMed] [Google Scholar]
- 42.Wagner B, Burton A, Ainsworth D. Interferon-gamma, interleukin-4 and interleukin-10 production by T helper cells reveals intact Th1 and regulatory TR1 cell activation and a delay of the Th2 cell response in equine neonates and foals. Vet Res. 2010;41:47. doi: 10.1051/vetres/2010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halonen M, Lohman IC, Stern DA, Spangenberg A, Anderson D, Mobley S, et al. Th1/Th2 patterns and balance in cytokine production in the parents and infants of a large birth cohort. J Immunol. 2009;182:3285–3293. doi: 10.4049/jimmunol.0711996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scola MP, Thompson SD, Brunner HI, Tsoras MK, Witte D, Van Dijk MA, et al. Interferon-gamma:interleukin 4 ratios and associated type 1 cytokine expression in juvenile rheumatoid arthritis synovial tissue. J Rheumatol. 2002;29:369–78. [PubMed] [Google Scholar]
- 45.Yin Z, Siegert S, Neure L, Grolms M, Liu L, Eggens U, et al. The elevated ratio of interferon gamma-/interleukin-4-positive T cells found in synovial fluid and synovial membrane of rheumatoid arthritis patients can be changed by interleukin-4 but not by interleukin-10 or transforming growth factor beta. Rheumatology (Oxford) 1999;38:1058–67. doi: 10.1093/rheumatology/38.11.1058. [DOI] [PubMed] [Google Scholar]
- 46.Vieira MM, Ferreira TB, Pacheco PA, Barros PO, Almeida CR, Araújo-Lima CF, et al. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol. 2010;229:212–218. doi: 10.1016/j.jneuroim.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 48.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 49.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 50.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 51.Blais MA, Otto MW, Zucker BG, McNally RJ, Schmidt NB, Fava M, et al. The anxiety sensitivity index: item analysis and suggestions for refinement. J Pers Assess. 2001;77:272–294. doi: 10.1207/S15327752JPA7702_10. [DOI] [PubMed] [Google Scholar]
- 52.de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pitsavos C, Panagiotakos DB, Papageorgiou C, Tsetsekou E, Soldatos C, Stefanadis C. Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA study. Atherosclerosis. 2006;185:320–326. doi: 10.1016/j.atherosclerosis.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Liukkonen T, Räsänen P, Jokelainen J, Leinonen M, Järvelin MR, Meyer-Rochow VB, et al. The association between anxiety and C-reactive protein (CRP) levels: results from the Northern Finland 1966 birth cohort study. European Psychiatry. 2011;26:363–369. doi: 10.1016/j.eurpsy.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspectives in Psychiatric Care. 2009;45:262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- 56.Spitzer C, Barnow S, Völzke H, Wallaschofski H, John U, Freyberger HJ, et al. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. Journal of Psychiatric Research. 2010;44:15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Bankier B, Barajas J, Martinez-Rumayor A, Januzzi JL. Association between C-reactive protein and generalized anxiety disorder in stable coronary heart disease patients. European Heart Journal. 2008;29:2212–2217. doi: 10.1093/eurheartj/ehn326. [DOI] [PubMed] [Google Scholar]
- 58.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behavior, and Immunity. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 59.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 60.Maes M, Berk M, Goehler L, Song C, Anderson G, Gałecki P, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berk M, Williams LJ, Jacka FN, O'Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 63.Uçeyler N, Valenza R, Stock M, Schedel R, Sprotte G, Sommer C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis & Rheumatism. 2006;54:2656–2664. doi: 10.1002/art.22026. [DOI] [PubMed] [Google Scholar]
- 64.Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, et al. Prevalence, severity and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291:2581–2590. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- 65.Scott K, McGee MA, Schaaf D, Baxter J. Mental-physical comorbidity in an ethnically diverse population. Social Science & Medicine. 2008;66:1165–1173. doi: 10.1016/j.socscimed.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 66.Decker D, Schondorf M, Bidlingmaier F, Hirner A, von Ruecker AA. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery. 1996;119:316–325. doi: 10.1016/s0039-6060(96)80118-8. [DOI] [PubMed] [Google Scholar]
- 67.Nakano Y, Nakamura S, Hirata M, Harada K, Ando K, Tabuchi T, et al. Immune function and lifestyle of taxi drivers in Japan. Industrial Health. 1998;36:32–39. doi: 10.2486/indhealth.36.32. [DOI] [PubMed] [Google Scholar]
- 68.Marshall GD, Jr, Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ. Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav Immun. 1998;12:297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- 69.Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, Maes M. Anti-Inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol. 2001;21:199–206. doi: 10.1097/00004714-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 70.Gimeno D, Kivimäki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39:413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Au B, Smith KJ, Gariepy G, Schmitz N. The longitudinal associations between C-reactive protein and depressive symptoms: evidence from the English Longitudinal Study of Ageing (ELSA) Int J Geriatr Psychiatry. 2015;30:976–984. doi: 10.1002/gps.4250. [DOI] [PubMed] [Google Scholar]
- 72.Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, et al. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70:812–820. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- 73.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 75.Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.167. doi: 10.1038/mp. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Reviews Immunology. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brothers HM, Wilcock DM. Are inflammatory profiles the key to personalized Alzheimer's treatment? Neurodegener Dis Manag. 2013;3:343–351. doi: 10.2217/nmt.13.40. [DOI] [PMC free article] [PubMed] [Google Scholar]