Abstract
Vascular endothelial growth factor (VEGF) is implicated in the peritoneal membrane remodeling that limits ultrafiltration in patients on peritoneal dialysis (PD). Although the exact mechanism of VEGF induction in PD is unclear, VEGF concentrations in drained dialysate correlate with IL-6 levels, suggesting a link between these cytokines. Human peritoneal mesothelial cells (HPMCs), the main source of IL-6 and VEGF in the peritoneum, do not bear the cognate IL-6 receptor and are thus unable to respond to classic IL-6 receptor signaling. Here, we investigated whether VEGF release by HPMCs is controlled by IL-6 in combination with its soluble receptor (IL-6 trans–signaling). Although treatment with either IL-6 or soluble IL-6 receptor (sIL-6R) alone had no effect on VEGF production, stimulation of HPMCs with IL-6 in combination with sIL-6R promoted VEGF expression and secretion through a transcriptional mechanism involving STAT3 and SP4. Conditioned medium from HPMCs cultured with IL-6 and sIL-6R promoted angiogenic endothelial tube formation, which could be blocked by silencing SP4. In vivo, induction of peritoneal inflammation in wild-type and IL-6–deficient mice showed IL-6 involvement in the control of Sp4 and Vegf expression and new vessel formation, confirming the role of IL-6 trans–signaling in these processes. Taken together, these findings identify a novel mechanism linking IL-6 trans–signaling and angiogenesis in the peritoneal membrane.
Keywords: peritoneal dialysis, peritoneal membrane, vascular endothelial growth factor, Cell Signaling
The efficacy of peritoneal dialysis (PD) as a treatment modality largely depends on maintaining peritoneal membrane integrity. Dysfunction of the peritoneum as a dialysis organ may result from progressive membrane injury occurring over time on PD. The underlying pathophysiologic mechanisms involve a gradual rise in small solute transport due to an increase in peritoneal perfusion and a decrease in peritoneal hydraulic conductance due to tissue fibrosis.1 Both of these processes are related to peritoneal angiogenesis. On the one hand, an increase in peritoneal vascularity increases the surface area available for solute diffusion and leads to rapid dissipation of the osmotic gradient that drives ultrafiltration. On the other hand, angiogenesis is a prominent feature of tissue repair, scar formation, and fibrosis. Indeed, it has been estimated that up to 75% of patients with ultrafiltration failure may have increased vascular surface area.2,3 Moreover, peritoneal biopsies taken from patients on PD show that fibrosis occurs significantly more often in the presence of vasculopathy,4 and the density of peritoneal blood vessels and submesothelial and perivascular fibrosis are significantly greater in patients with membrane failure.4–6 Animal models of PD confirm the existence of an inverse correlation between increased vascularization and ultrafiltration.7 These studies suggest that a decline in ultrafiltration could be partially abrogated by antiangiogenic therapy.7
Vascular endothelial growth factor (VEGF) is the most important proangiogenic mediator.8 The effect of VEGF on peritoneal vascularity is shown by the association of genetic polymorphisms determining increased VEGF production with increased peritoneal solute transport.9 Moreover, the rates of VEGF appearance in the dialysate are elevated in patients with high peritoneal transport status.10,11
Several mechanisms are implicated in peritoneal VEGF induction in PD.12 One of the factors involved has been suspected to be IL-6, because IL-6 concentrations in the drained dialysate correlate with peritoneal solute transport rates13–15 and dialysate levels of VEGF.10,14 Interestingly, it has been shown that the peritoneal mesothelium is the main source of both VEGF16,17 and IL-6.18,19 Although VEGF can be induced by several inflammatory cytokines,20 IL-6 has not been classically viewed as a driver of VEGF production in mesothelial cells, because these do not express the cognate IL-6 receptor (IL-6R).21 They do, however, express gp130, a signal transduction element for IL-6, that allows them to respond to IL-6 in the presence of soluble IL-6 receptor (sIL-6R). Indeed, this process of so-called IL-6 trans–signaling plays a critical role in controlling chemokine production and contributes to successful resolution of inflammation.21 The mechanism appears to be particularly active in the acute phase of peritonitis when mesothelial cells release large quantities of IL-619 and infiltrating leukocytes shed sufficient levels of sIL-6R.21 Interestingly, viral IL-6 that binds directly to gp130 in a process resembling IL-6 trans–signaling is also capable of inducing VEGF in mesothelial cells.22
We have, therefore, hypothesized that IL-6 together with sIL-6R might be important in controlling VEGF production in human peritoneal mesothelial cells (HPMCs). Here, we characterized the transcriptional regulation of VEGF in HPMCs and identified transcription factors STAT3 and SP4 as forming a novel axis linking IL-6 and VEGF production and new vessel formation in the peritoneum.
Results
Induction of VEGF by IL-6 and sIL-6R in Mesothelial Cells
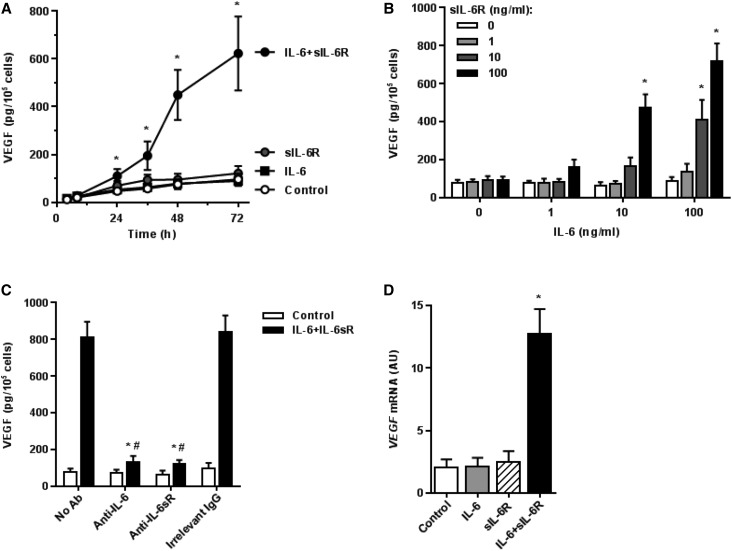
Because mesothelial cells are the main source of peritoneal VEGF, we have examined whether VEGF production by HPMCs can be regulated by IL-6, either directly through classic IL-6R signaling or through IL-6 trans–signaling. Indeed, neither IL-6 nor sIL-6R alone had any significant effect on VEGF protein release. However, simultaneous exposure to IL-6 plus sIL-6R resulted in a significant time– and dose–dependent increase in VEGF secretion (Figure 1, A and B). The greatest effect was achieved with both IL-6 and sIL-6R at a dose of 100 ng/ml. The specificity of this effect was confirmed by specific blocking experiments. Here, specific antibodies against either IL-6 or sIL-6R but not control IgG inhibited the induction of VEGF (Figure 1C). The effect exerted by a combination of IL-6 and sIL-6R on VEGF secretion was accompanied by a corresponding increase in VEGF mRNA (Figure 1D). These observations prompted us to examine in more detail the signaling events leading to VEGF gene induction.
Figure 1.
Induction of VEGF in HPMCs by a combination of IL-6 and sIL-6R. (A) Kinetics of VEGF secretion by HPMCs treated with IL-6 (10 ng/ml) and sIL-6R (25 ng/ml) either singly or in combination. *P<0.05 versus control cells at each time point (n=6). (B) Dose effect of IL-6 and sIL-6R on VEGF release over the period of 48 hours. *P<0.05 versus untreated cells. (C) Blockade of the effect of the IL-6 + sIL-6R complex. HPMCs were treated with IL-6 + sIL-6R (both at 100 ng/ml) for 48 hours in the presence of 10 μg/ml antibodies against either IL-6 (MAB206; R&D Systems) or sIL-6R (MAB227; R&D Systems). An additional group received an irrelevant antibody of the same class and at the same dose. *P<0.05 versus cells not exposed to antibodies (n=5); #P<0.05 versus cells treated with an irrelevant antibody (n=5). (D) Induction of VEGF mRNA by IL-6 and/or sIL-6R. HPMCs were treated with IL-6 and sIL-6R (both at 100 ng/ml) either singly or in combination for 48 hours. *P<0.05 versus untreated cells (n=4).
Activation of VEGF Gene Promoter by IL-6 and sIL-6R
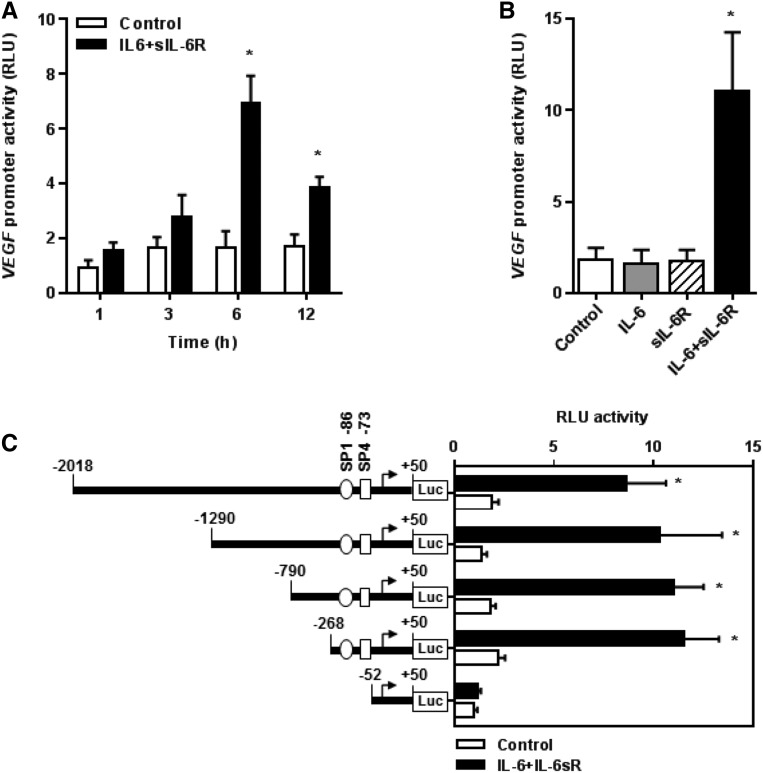
To investigate how the IL-6 + sIL-6R complex affects the activity of the VEGF gene promoter, HPMCs were transiently transfected with VEGF luciferase reporter gene constructs and stimulated with combinations of IL-6 and sIL-6R. This resulted in a time-dependent increase in VEGF promoter activity, which peaked at 6 hours (Figure 2A). Exposure for 6 hours to IL-6 or sIL-6R alone had no stimulatory effect on the full–length VEGF promoter. In contrast, the combination of IL-6 and sIL-6R strongly increased the activity of the VEGF promoter (Figure 2B). To identify VEGF promoter regions mediating the response to IL-6 trans–signaling, functional 5′ deletions of the VEGF promoter were generated. Truncation of the promoter region spanning positions −268 to −53 resulted in a loss of VEGF promoter activity to respond to stimulation with IL-6/sIL-6R combinations (Figure 2C). This result suggested that the region identified comprised essential regulatory elements for the VEGF promoter activity. The in silico analysis predicted that the region contained high–affinity binding sites for the transcription factors SP1 and SP4. To determine which of these transcription factors was regulated by IL-6 + sIL-6R, electrophoretic mobility shift assays (EMSAs) were performed.
Figure 2.
Identification of sequences in the human VEGF promoter responsive to stimulation with the IL-6 + sIL-6R complex. Cells were transiently transfected with VEGF promoter constructs and stimulated with IL-6 and/or sIL-6R (both at 100 ng/ml) as indicated. Luciferase activity was determined as described in Concise Methods and expressed as relative light units (RLU). (A) Time effect of the IL-6 + sIL-6R complex on the full-length VEGF promoter activity. *P<0.05 versus unstimulated control (n=3). (B) Full-length VEGF promoter activity after 6 hours of stimulation with IL-6 and/or sIL-6R. *P<0.05 versus unstimulated control (n=4). (C) Effect of progressive 5′ deletions of the VEGF promoter on its activity on stimulation with IL-6 + sIL-6R for 6 hours. *P<0.05 versus unstimulated controls (n=4).
Activation of the Transcription Factor SP4 by IL-6 and sIL-6R
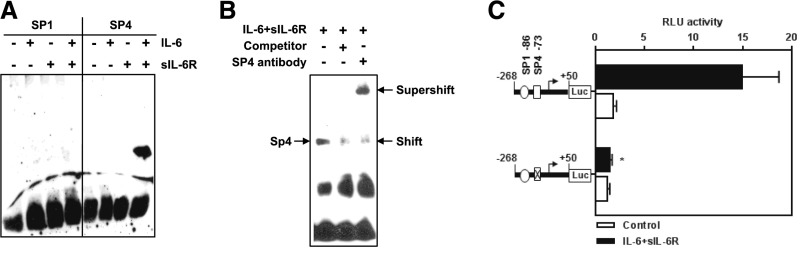
EMSA was performed using biotin–labeled double–stranded oligonucleotides corresponding to positions −59 to −82 (SP4) and −80 to −103 (SP1) of the VEGF promoter. Analysis of nuclear extracts from cells stimulated with IL-6 + sIL-6R showed formation of a prominent DNA-protein complex with a consensus oligonucleotide for SP4 binding (Figure 3A). No binding was, however, seen with a consensus motif for SP1. Nuclear extracts from cells stimulated singly with IL-6 and sIL-6R failed to produce any effect.
Figure 3.
Identification of SP4 as a transcription factor mediating human VEGF promoter induction by the IL-6 + sIL-6R complex. (A and B) Cells were stimulated with IL-6 + sIL-6R at 100 ng/ml for 6 hours, and the nuclear fractions were obtained and analyzed with EMSA. In A, EMSA was performed with consensus oligonucleotide probes for SP1 and SP4. In B, formation of nuclear complexes with SP4 probe was assessed in the presence of either 100-fold molar excess of unlabeled VEGF DNA (resulting in a reduced shift) or SP4-specific antibody (resulting in supershift). (C) Effect of site-directed mutagenesis in the SP4 binding site within the VEGF promoter on its activity after stimulation with IL-6 + sIL-6R. Luciferase activity was expressed as relative light units (RLU). *P<0.05 versus relevant control (n=4).
To verify the specificity of SP4 binding, EMSA was performed with a 100-fold molar excess of an unlabeled SP4 consensus oligonucleotide (Figure 3B). This competition assay inhibited detection of the SP4-DNA complex. In turn, EMSA with a specific anti–SP4 antibody led to a supershift of the DNA-protein complex (Figure 3B). Furthermore, transfection of a mutant construct specific to the SP4 binding site completely eliminated VEGF promoter activation after IL-6 + sIL-6R stimulation (Figure 3C).
Effect of STAT3 Blockade on VEGF Production
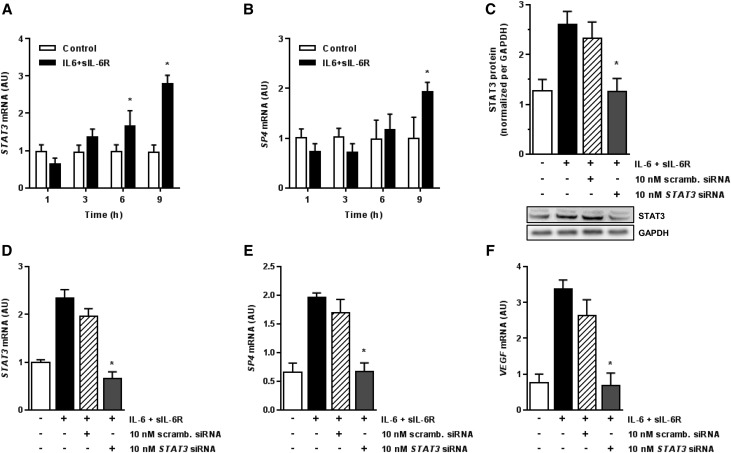
Because SP4 has not been typically associated with IL-6 signal transduction, we examined the activation of STAT3, a key regulator of IL-6 signaling.23 Indeed, stimulation of HPMCs with IL-6 + sIL-6R resulted in a time-dependent induction of STAT3 but also, SP4 mRNA (Figure 4, A and B). To determine if there exists a link between STAT3, SP4, and VEGF, the expression of STAT3 gene was blocked by RNA interference. These experiments showed that STAT3-targeting siRNA, but not a scrambled siRNA control, inhibited STAT3 itself at a protein and mRNA level (Figure 4, C and D). It also inhibited SP4 and VEGF induction by IL-6 + sIL-6R (Figure 4, E and F).
Figure 4.
The role of STAT3 in SP4–mediated VEGF induction by the IL-6 + sIL-6R complex. (A and B) Kinetics of STAT3 and SP4 mRNA induction in HPMCs treated with IL-6 + sIL-6R (both at 100 ng/ml). *P<0.05 versus control cells at each time point (n=4). (C–F) Effect of STAT3 silencing on STAT3, SP4, and VEGF expression. Cells were transiently transfected with either STAT3 siRNA or scrambled (scramb.) siRNA and then, stimulated with IL-6 + sIL-6R (both at 100 ng/ml) for 9 hours. Cells were assessed for (C) STAT3 protein expression by Western blotting and mRNA expression by quantitative PCR for (D) STAT3, (E) SP4, and (F) VEGF. In C, a representative immunoblot is presented together with quantified data from four independent experiments. STAT3 protein expression was normalized per that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). *P<0.05 versus cells stimulated with IL-6 + sIL-6R in the absence of siRNA (n=4).
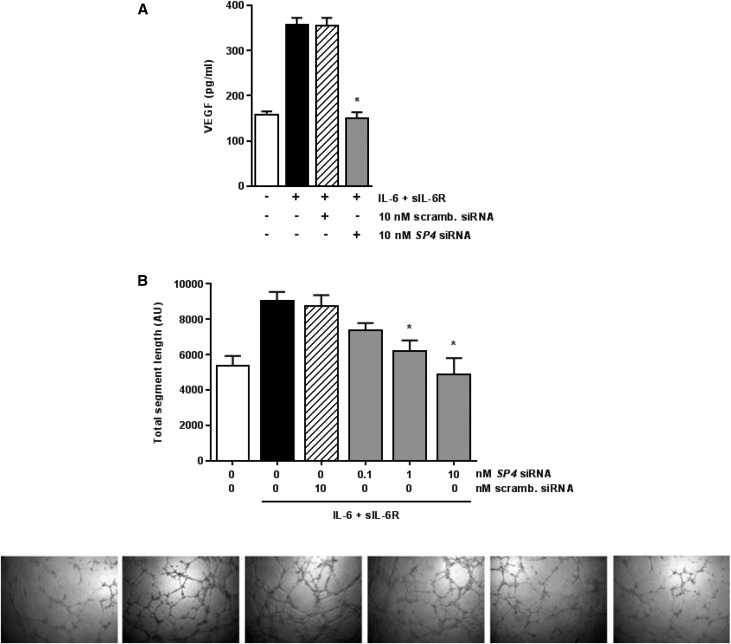
Effect of SP4 Blockade on VEGF Production and Angiogenesis
To further confirm the involvement of SP4 in VEGF production and biologic activity, HPMCs were stimulated with IL-6 + sIL-6R in the presence of either SP4-targeting siRNA or scrambled siRNA. The SP4 blockade resulted in a significant inhibition of VEGF protein release (Figure 5A). To test the functional properties of the VEGF production, conditioned medium from stimulated HPMCs was transferred to endothelial cell cultures, and the formation of capillaries was assessed. Incubation of human umbilical vein endothelial cells (of the EA.hy926 line) in the presence of conditioned medium from IL-6 + sIL-6R–stimulated HPMCs significantly increased endothelial cell tube formation. A similar degree of stimulation was observed when conditioned medium from HPMCs treated with scrambled siRNA was used. However, endothelial cell angiogenesis was significantly less in response to conditioned medium from HPMCs treated with siRNA against SP4 (Figure 5B). This effect was not unique to EA.hy926 endothelial cells, because similar effects on tube formation were also observed in cultures of human dermal microvascular endothelial cells (Supplemental Figure 1).
Figure 5.
Effect of SP4 on VEGF–mediated endothelial cell tube formation. (A) Cells were transiently transfected with either SP4 siRNA or scrambled (scramb.) siRNA, stimulated with IL-6 + sIL-6R (both at 100 ng/ml) for 24 hours, and then, assessed for VEGF secretion. *P<0.05 versus cells stimulated with IL-6 + sIL-6R in the absence of siRNA (n=5). (B) Effect of conditioned medium (10% vol/vol) from HPMCs treated as in A on endothelial cell tube formation within 16 hours. *P<0.05 versus cells stimulated with IL-6 + sIL-6R in the absence of siRNA (n=4). Representative phase contrast images are presented in order corresponding to experimental groups as shown in graph from left to right. Original magnification, ×100.
Effect of IL-6 Signaling on Peritoneal Sp4 and Vegf Expression in Mice
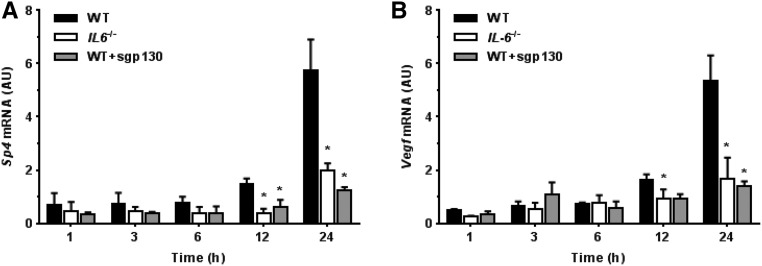
To test whether IL-6 signaling modulates Vegf expression during peritoneal inflammation, wild-type (WT) and IL-6–deficient (IL6−/−) mice were challenged (intraperitoneally) with a cellfree supernatant prepared from of a clinical isolate of Staphylococcus epidermidis (SES).21 At designated time points, Sp4 and Vegf expression in the samples of parietal peritoneum was analyzed by quantitative PCR. As illustrated in Figure 6A, SES-induced inflammation in WT mice was associated with a time-dependent increase in Vegf mRNA expression. In contrast, Vegf expression was significantly less in IL6−/− mice. To assess whether this effect was related to sIL-6R activity, WT mice were treated with SES together with soluble gp130 (sgp130), which inhibits IL-6 trans–signaling by the IL-6 + sIL-6R complex but does not inhibit classic IL-6 signaling through the cognate IL-6R.24 Addition of sgp130 significantly decreased Vegf expression in WT mice, which resembled the effect seen in IL6−/− mice and indicated that sIL-6R–mediated effects were involved in regulation of VEGF expression in the inflamed peritoneum.
Figure 6.
Effect of IL-6 signaling on peritoneal Sp4 and Vegf expression in mice. WT and IL6−/− mice were intraperitoneally administered with SES and then analyzed at defined time points for (A) Sp4 and (B) Vegf expression in the parietal peritoneum. An additional group of WT animals received SES together with sgp130 (150 ng/mouse). *P<0.05 versus WT mice at the same time point (n=4 mice per condition).
Having identified SP4 as a mediator of IL-6 trans–signaling in human cells, we next examined peritoneal expression of Sp4 in mice. Induction of peritoneal inflammation with SES led to a time-dependent increase in Sp4 expression in WT mice but not in IL6−/− animals. In WT mice administered with sgp130, the expression of Sp4 followed the pattern seen in IL6−/− mice (Figure 6B).
Effect of IL-6 Signaling in Mice on Peritoneal Expression of Genes Essential for VEGF Activity
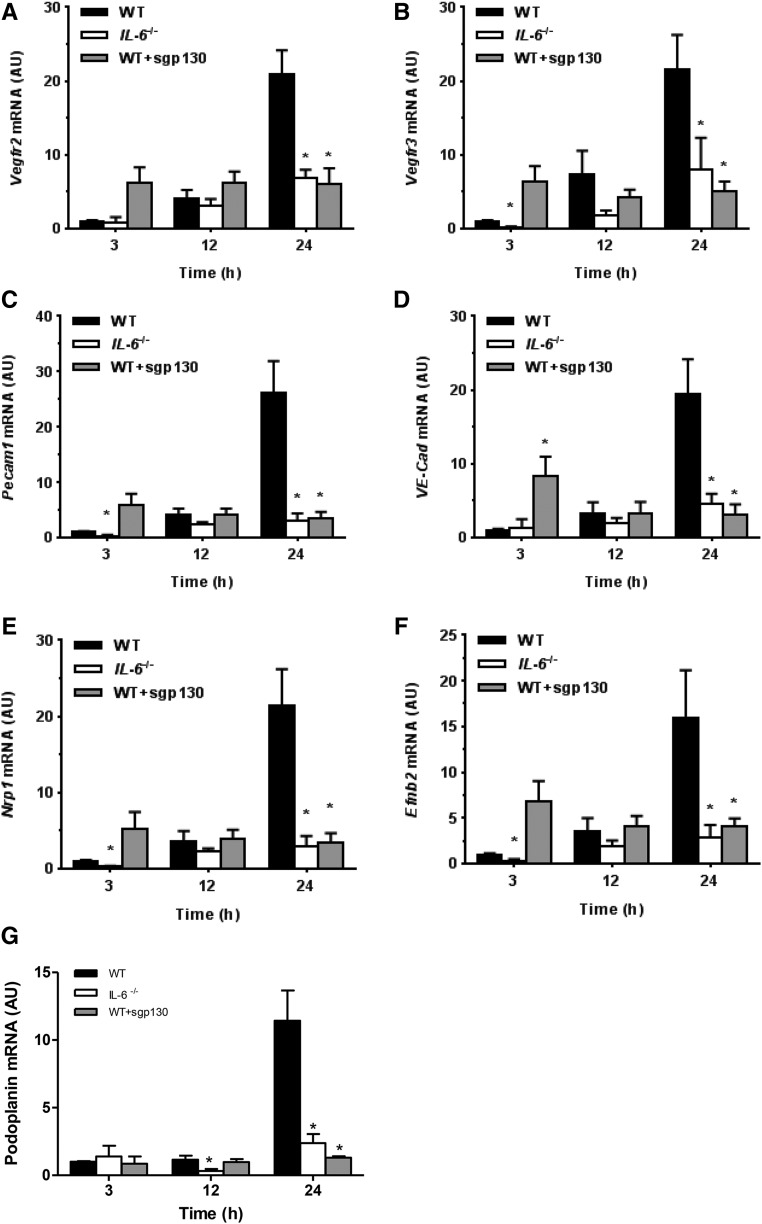
To determine whether VEGF induced in the peritoneum by IL-6 signaling can initiate events leading to increased vascular permeability and angiogenesis, we examined expression of several key endothelial–specific targets involved in these processes.25 These included vascular endothelial growth factor receptors (Vegfrs; Vegfr2 and Vegfr3), junctional proteins (vascular endothelial cadherin [VE-Cad] and platelet/endothelial cell adhesion molecule 1 [Pecam1]), markers of arterial commitment neuropilin 1 and ephrinB2, and a marker of lymphatic commitment podoplanin. Expression of all of these angiogenesis-related targets increased significantly within 24 hours of SES-induced inflammation in WT mice but not in IL6−/− mice and not in WT mice receiving sgp130 (Figure 7).
Figure 7.
Effect of IL-6 signaling on peritoneal expression of endothelial-specific targets. Mice were treated as in Figure 6 and analyzed for peritoneal expression of Vegfr2 (A), Vegfr3 (B), Pecam1 (C), VE-Cad (D), neuropilin 1 (Nrp1, E), ephrinB2 (Efnb2, F), and podoplanin (Pdpn, G). *P<0.05 versus WT mice at the same time point (n=4 mice per condition).
Effect of IL-6 Signaling on Peritoneal Vasculature during Recurrent Inflammation in Mice
To test whether these acute consequences of increased IL-6 signaling are associated with changes in peritoneal vasculature in the long term, we analyzed the parietal peritoneum of WT and IL6−/− mice challenged repeatedly with SES as described previously.26 After four sequential rounds of acute inflammation, at the time point where parietal peritoneum shows increased fibrosis,26 the peritoneum of WT animals appeared only modestly vascularized but consistently showed increased numbers of vessels staining positively for PECAM1 (CD31) and podoplanin (Gp38) compared with IL6−/− mice (Supplemental Figure 2).
Effect of STAT3/SP4–Mediated IL-6 Signaling on Dialysate–Induced VEGF Expression in Peritonitis
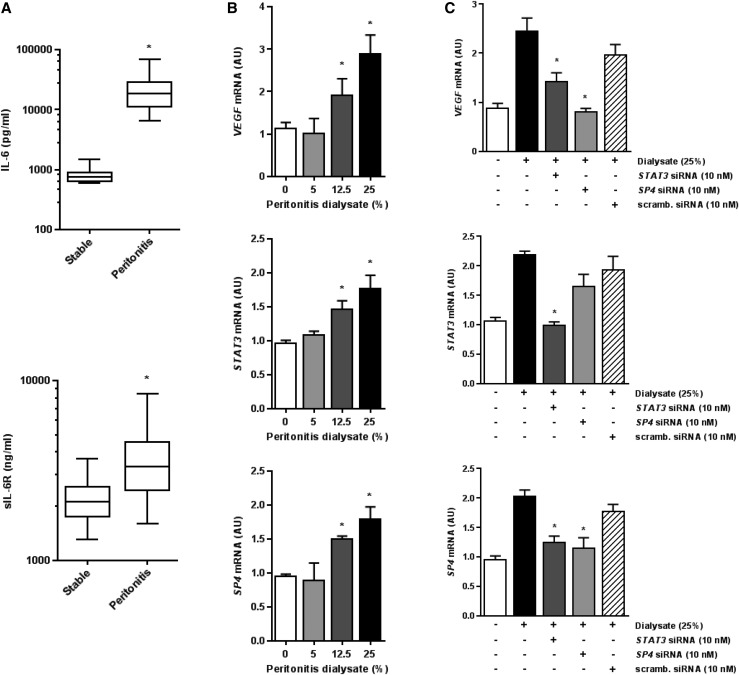
Because peritoneal effluents may contain high levels of IL-6 and sIL-6R during peritonitis, we have asked whether such effluents could induce VEGF in HPMCs through a signaling pathway identified. Confirming earlier reports,21,27 we have detected significantly elevated concentrations of IL-6 and sIL-6R in the dialysate drained from patients on PD during an acute phase of peritonitis (Figure 8A). When an exemplary PD effluent from a patient with peritonitis was added to the culture medium, it stimulated VEGF, STAT3, and SP4 mRNA expression in mesothelial cells in a concentration-dependent manner (Figure 8B). The increase in VEGF mRNA was reduced to control levels in the presence of either STAT3 siRNA or SP4 siRNA but not in the presence of the scrambled siRNA (Figure 8C). Interestingly, the inhibition of STAT3 signaling resulted also in decreased expression of SP4 and STAT3 itself. In contrast, the SP4 blockade reduced the expression of SP4 but not STAT3.
Figure 8.
IL-6 signaling contributes to VEGF release by HPMCs during peritonitis. (A) IL-6 and sIL-6R levels in dialysates drained from either stable patients on PD after a routine 4-hour dwell (n=20) or patients with peritonitis at first presentation (n=11); the data are presented as box and whiskers plots, with the median, 25th and 75th percentiles, and range of data indicated. *P<0.05 versus stable patients. (B) The dose effect of exemplary PD effluent drained during peritonitis on (top panel) VEGF, (middle panel) STAT3, and (bottom panel) SP4 mRNA expression in HPMCs. Cells were treated with increasing doses of effluent for 24 hours. *P<0.05 versus untreated controls (n=4). (C) Cells were transiently transfected with 10 μM STAT3 siRNA, SP4 siRNA, or scrambled (scramb.) siRNA as indicated. After that, cells were exposed for 24 hours to peritoneal effluent (25% vol/vol) from a patient on PD with peritonitis and assessed for mRNA expression of (top panel) VEGF, (middle panel) STAT3, and (bottom panel) SP4. *P<0.05 versus cells treated with the dialysate in the absence of siRNAs (n=4).
Discussion
The diversity of IL-6 functions is now well appreciated.28 These pleiotropic activities are partly related to the complexity of IL-6 signaling, which allows even cells without the cognate IL-6R to respond to IL-6. Here, we show through a series of in vitro and in vivo experiments and the analysis of clinical samples from patients on PD that IL-6 trans–signaling can contribute significantly to peritoneal VEGF production. In this respect, we have found that the complex of IL-6 and sIL-6R but not IL-6 alone is capable of inducing de novo VEGF synthesis in HPMCs. In the clinical setting, this is most likely to occur during peritonitis, because sIL-6R can then be delivered into the environment by shedding from infiltrating neutrophils.21,29 Indeed, we have observed that peritoneal inflammation induced in mice by S. epidermidis,21 a major causative microorganism of PD-associated peritonitis, resulted in an upregulation of peritoneal Vegf expression. In contrast, the animals deficient in IL-6 failed to produce such a response. A similar lack of effect could be observed in WT mice treated with sgp130. These findings indicated that the induction of Vegf was controlled through sIL-6R rather than by the membrane–bound IL-6R. This mechanism has previously been shown to be pivotal in coordinating leukocyte trafficking during peritonitis.21
Because STAT3 is the major signal transducer downstream of gp13023,30 and its involvement in VEGF gene regulation has already been postulated,31–33 we have examined the consequences of STAT3 inhibition in HPMCs. Indeed, the blockade of STAT3 resulted in an inability of the IL-6 + sIL-6R complex to induce VEGF. Surprisingly, however, the progressive 5′-deletion analysis mapped the IL-6 + sIL-6R response element of the VEGF promoter in HPMCs to a region that did not contain STAT3 binding elements. Instead, it did contain high–affinity binding sites for SP4. Subsequent experiments using EMSA and site-directed mutagenesis confirmed that it was SP4 driving VEGF gene expression in response to IL-6 trans–signaling. Moreover, the blockade of STAT3 inhibited SP4 expression, which positioned STAT3 upstream of SP4 in the signaling cascade. Although the targeting of other transcription factors by STAT3 is recognized,34 the induction of SP4 has not been reported before. The existence of such a signaling axis in vivo was further supported by the observation that S. epidermidis–induced peritoneal inflammation in either IL6−/− mice or WT mice administered with sgp130 did not produce an increase in peritoneal Sp4 expression.
The potential of IL-6 to drive VEGF expression has been observed in several cancer cells31,32,35–37 and implicated in tumor-associated angiogenesis.38,39 Interestingly, it has been shown that anti–IL-6 antibody siltuximab reduced STAT3 activation and angiogenesis in IL-6–producing intraperitoneal ovarian cancer xenografts and reduced VEGF levels in patients with ovarian cancer.40 The exact role of SP4 in these processes remains to be established. The experiments using RNA interference suggested that SP4 might contribute to the regulation of basal VEGF expression in some pancreatic cancer cells lines.41 The involvement of SP4 could also be inferred from the observation that the downregulation of VEGF expression by cyclooxygenase inhibitors in human colon cancer cells was associated with proteasomal degradation of SP4.42
Ablation of STAT3 has long been considered an attractive therapeutic strategy for IL-6–mediated inflammation and cancer.43,44 Our findings suggest that SP4 activity is downstream of STAT3 activation in response to IL-6 trans–signaling. Thus, a therapeutic targeting of SP4 may block specific aspects of IL-6 signaling, while leaving STAT3 activity intact. The fact that blocking SP4 can indeed produce viable biologic effects could be shown by experiments that showed a decrease in angiogenesis induced by conditioned media from HPMCs exposed to the IL-6 + sIL-6R complex in the presence of SP4-silencing RNA.
The consequences of VEGF upregulation by IL-6 trans–signaling in the setting of PD can be twofold. Because VEGF is a potent agent increasing vascular permeability, its rise during acute peritonitis will cause extravasation of fluid rich in plasma proteins, which together with migrating leukocytes, will form an inflammatory infiltrate essential for the clearance of infection. The process would be self-limiting given the massive but short-lived increase in intraperitoneal IL-6 levels21,27 and the role of IL-6 trans–signaling in suppressing neutrophil-specific chemokines21 and promoting neutrophil apoptosis.45 Indeed, it has previously been shown that the IL-6 + sIL-6R complex can induce STAT3– and VEGF–mediated vascular leakage through microvascular endothelial cells.46
However, repeated episodes of peritonitis and the persistent IL-6 + sIL-6R–mediated VEGF stimulation may lead to formation of new hyperpermeable blood vessels, which was found in many chronic inflammatory diseases.47 The accumulation of fibrin in tissues favors fibroblast migration and subsequent extracellular matrix synthesis. In this respect, it has recently been shown that recurrent peritoneal inflammation in mice leads to tissue fibrosis through a process that is strictly dependent on IL-6, albeit in this particular model related to STAT1 and IFN-γ signaling.26 Moreover, it has been shown that experimental peritoneal fibrosis in mice could be reduced by antagonizing VEGF through soluble VEGF type 1 receptor.48 We observed that SES-induced inflammation in WT mice was associated with increased expression of key mediators of VEGF signaling, new vessel formation,25 and vascular remodeling.49 In this respect, increased expression of Pecam1 and VE-Cad likely reflects endothelial cell expansion in vivo but might also be a secondary response to either transient rearrangement of intercellular junctions that underlie increased permeability or degradation of junctional proteins by inflammation-induced proteases.50 Importantly, these increases in endothelial markers were mediated directly by IL-6 trans–signaling, because they did not occur in IL6−/− mice and could be reduced in WT mice by specific blockade with sgp130.
Moreover, these early events in IL-6 signaling during acute inflammation may also contribute to chronic changes in the peritoneal vasculature. We have observed increased immunostaining for PECAM1 and podoplanin in WT mice subjected to repeated episodes of peritonitis compared with IL6−/− mice. Of particular interest is the increased number of podoplanin-positive cells in this setting, because increased expression of podoplanin was consistently detected in patients on PD with encapsulating peritoneal sclerosis.51,52 Furthermore, recent data from the GLOBAL Study showed that patients who developed encapsulating peritoneal sclerosis had earlier higher dialysate levels of IL-6 during PD.53
These two scenarios would fit well into the concept that, depending on a pathophysiologic context, IL-6 can either be crucial for host defense or promote chronic disease.28 Here, we show that dialysate IL-6 and sIL-6R can act together through the trans-signaling pathway controlled by the STAT3-SP4 axis to upregulate mesothelial VEGF production during peritonitis. The identification of this novel mechanism that controls peritoneal VEGF expression at the transcriptional level may help us to understand better how peritoneal inflammation and angiogenesis contribute to adverse peritoneal membrane remodeling during PD.
Concise Methods
Materials
Unless stated otherwise, all chemicals were from Sigma-Aldrich (St Louis, MO), and all culture plastics were Falcon from Becton Dickinson (Franklin Lakes, NJ). Cell culture media and buffers were from Biochrom AG (Berlin, Germany), and FCS was from Invitrogen (Darmstadt, Germany). Human recombinant IL-6 and sIL-6R were from R&D Systems (Wiesbaden, Germany). Antibodies against STAT3 and SP4 were from Santa Cruz Biotechnology (Heidelberg, Germany).
Mesothelial Cell Culture
HPMCs were isolated from the specimens of omentum obtained from consenting patients undergoing elective abdominal surgery. Cells were cultured and characterized as described in detail elsewhere.20 For the experiments, cells were rendered quiescent by serum deprivation for 48 hours and then stimulated with IL-6 and/or sIL-6R as specified in the figures. All experiments were performed with cells no older than from the third passage to minimize the number of senescent cells, because this may affect the level of VEGF released.54
Endothelial Cell Culture and Tube Formation Assay
Human umbilical vein endothelial cells of the EA.hy926 line55 were donated by C.J. Edgell (University of North Carolina, Chapel Hill, NC). For the tube formation assay, Matrigel (Corning, Tewksbury, MA) was poured onto a 96-well plate (50 μl per well) and solidified at 37°C for 30 minutes. Endothelial cells (2×104 cells per well) were seeded onto the Matrigel and cultured in MCDB131 medium (Thermo Fisher Scientific, Waltham, MA) with or without 10% (vol/vol) conditioned medium from HPMCs treated as described in the figures. Capillary networks of tubes formed were photographed under the microscope (Zeiss Axiovert 40 CFL, Zeiss, Oberkochen, Germany), and five randomly selected fields from each well were analyzed for total capillary length using ImageJ 1.43 software.56
Immunoassays
Concentrations of VEGF, IL-6, and sIL-6R were measured using DuoSet Immunoassay Kits (R&D Systems). All assays were designed and performed as per manufacturer’s instructions.
Gene Expression Analyses
The expression of target genes was assessed with quantitative RT-PCR essentially as described previously.20 PCR conditions and primer sequences were as specified in Supplemental Material.
DNA Constructs and Reporter Plasmids
Progressive VEGF 5′-deletion luciferase plasmids (pLuc 2068, pLuc 1340, pLuc 840, pLuc 318, and pLuc 102) were provided by A. Scholz (Charité-Universitätsmedizin, Berlin, Germany). The constructs were generated as reported previously57 and checked for the correct length by restriction digest. To target the SP4 binding site at positions −73 to –75 (GGG) within the VEGF promoter, the desired sequence (AAA) was inserted into the pLuc VEGF 318 construct using the Q5 Site–Directed Mutagenesis Kit (New England BioLabs, Frankfurt, Germany) with forward primer 5′-GGGGCGGGCCAAAGGCGGGGTCCC-3′ and reverse primer 5′-GGGGGGCGGGGACAGGCG-3′.
Transfection Studies
Transient transfection and luciferase assays were performed as previously described in detail.20 Transfections with siRNAs were performed with the siRNA Transfection Reagent and siRNAs for STAT3 (sc-44275) and SP4 (sc-36545) or with scrambled siRNA control (sc-37007) as per the manufacturer’s instructions (all materials from Santa Cruz Biotechnology). Luciferase activity was expressed as relative light units.
Computational Analyses of the VEGF Promoter
The human VEGF promoter region −268 to −51 (GenBank NT_007592.15) was analyzed by PROMO virtual laboratory (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3) for the presence and location of potential transcription factor binding sites.
Nuclear Extracts and EMSA
Nuclear extracts were prepared using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Darmstadt, Germany) according to the manufacturer’s instructions. The extracts obtained were aliquoted and stored at −80°C. Oligonucleotide probes were labeled with the Biotin 3′ End DNA Labeling Kit (Thermo Scientific). For the EMSA, the following probes were used (the corresponding region of the VEGF promoter is given in parentheses): SP4, 5′-GCGGGCCGGGGGCGGGGTCCCGGC-3′ (–59 to –82); and Sp1, 5′-TCGCCTGTCCCCGCCCCCCGGGGC-3′ (–81 to –104). Each binding mixture (20 μl) for EMSA contained 5 μg nuclear extract, 20 fmol labeled double–stranded probe, 1 μg poly-dI/dC, and 2 μl 10× reaction buffer and was incubated at room temperature for 30 minutes. The protein-DNA complexes were then analyzed by electrophoresis in 6% nondenaturing polyacrylamide gels and visualized using a LightShift Chemiluminescent EMSA Kit (Thermo Scientific).
Western Blotting
Cell extracts were prepared as described,58 electrophoresed on SDS-PAGE, and Western blotted using antibodies against STAT3 and SP4 (Santa Cruz Biotechnology), GAPDH (Hytest, Turku, Finland), and appropriate secondary peroxidase–conjugated IgG (Dianova, Hamburg, Germany). The bands obtained were visualized and analyzed using the Enhanced Chemiluminescence Detection System (Thermo Scientific) and ImageJ 1.43 software (National Institutes of Health).
PD Effluent
Peritoneal effluent was obtained from consenting patients undergoing continuous ambulatory PD. Dialysate was collected from either stable patients on PD after a routine 4-hour dwell or the first bag drained from patients presenting with an episode of peritonitis. Samples were processed as described previously.59
Animal Experiments
All animal procedures were performed under appropriate licenses and according to institutional animal care guidelines. The experiments were performed in weight–matched 7- to 12-week-old inbred C57BL/6J WT and IL6−/− mice as previously described.45 Peritoneal inflammation was established through intraperitoneal administration of 500 μl cellfree supernatant (SES) prepared from S. epidermidis isolated from a patient on PD as detailed elsewhere.60 Repeated challenge with SES was used to model recurrent peritoneal inflammation, essentially as described previously.26 Animals were euthanized at designated time points, and specimens of peritoneal peritoneum were collected and snap frozen in liquid nitrogen. The tissue samples were then homogenized in Tri Reagent (Invitrogen), and total RNA was prepared according to the manufacturer’s instructions. Additional biopsy samples were processed for immunohistochemistry as described26 and subsequently stained with antibodies against anti-CD31 (eBioscience) and podaplanin (BioLegend). Antibody labeling was detected using biotinylated secondary antibodies (Dako), the Vectastain ABC Kit Vector Laboratories), and diaminobenzidine chromagen (Vector Laboratories). Sections were counterstained with hematoxylin. Quantification of antibody staining was performed using the Leica QWin microscope imaging software.61
Statistical Analyses
Statistical analysis was performed using GraphPad Prism 6.05 software (GraphPad Software). The data were analyzed with the t test or repeated measures ANOVA for paired data (in vitro experiments) or unpaired data (animal experiments) as appropriate. Results were expressed as means±SEM. Findings with a P value <0.05 were considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
J.W., A.R., N.T., and A.J. were supported by the European Training and Research in Peritoneal Dialysis Programme, a project funded by the European Union within Marie Curie scheme 287813. J.U.F. is the recipient of a la Caixa Studentship awarded through the British Council. S.A.J. has grant funding from Arthritis Research UK and Kidney Research UK.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015101169/-/DCSupplemental.
References
- 1.Davies SJ, Mushahar L, Yu Z, Lambie M: Determinants of peritoneal membrane function over time. Semin Nephrol 31: 172–182, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Heimbürger O, Waniewski J, Werynski A, Tranaeus A, Lindholm B: Peritoneal transport in CAPD patients with permanent loss of ultrafiltration capacity. Kidney Int 38: 495–506, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Ho-dac-Pannekeet MM, Atasever B, Struijk DG, Krediet RT: Analysis of ultrafiltration failure in peritoneal dialysis patients by means of standard peritoneal permeability analysis. Perit Dial Int 17: 144–150, 1997 [PubMed] [Google Scholar]
- 4.Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, Mackenzie RK, Williams GT; Peritoneal Biopsy Study Group : Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 13: 470–479, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Williams JD, Craig KJ, von Ruhland C, Topley N, Williams GT; Biopsy Registry Study Group : The natural course of peritoneal membrane biology during peritoneal dialysis. Kidney Int Suppl 88: S43–S49, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Mateijsen MA, van der Wal AC, Hendriks PM, Zweers MM, Mulder J, Struijk DG, Krediet RT: Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int 19: 517–525, 1999 [PubMed] [Google Scholar]
- 7.Margetts PJ, Gyorffy S, Kolb M, Yu L, Hoff CM, Holmes CJ, Gauldie J: Antiangiogenic and antifibrotic gene therapy in a chronic infusion model of peritoneal dialysis in rats. J Am Soc Nephrol 13: 721–728, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Nagy JA, Dvorak AM, Dvorak HF: VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol 2: 251–275, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Szeto CC, Chow KM, Poon P, Szeto CY, Wong TY, Li PK: Genetic polymorphism of VEGF: Impact on longitudinal change of peritoneal transport and survival of peritoneal dialysis patients. Kidney Int 65: 1947–1955, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Pecoits-Filho R, Araújo MR, Lindholm B, Stenvinkel P, Abensur H, Romão JE Jr., Marcondes M, De Oliveira AH, Noronha IL: Plasma and dialysate IL-6 and VEGF concentrations are associated with high peritoneal solute transport rate. Nephrol Dial Transplant 17: 1480–1486, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues AS, Martins M, Korevaar JC, Silva S, Oliveira JC, Cabrita A, Castro e Melo J, Krediet RT: Evaluation of peritoneal transport and membrane status in peritoneal dialysis: Focus on incident fast transporters. Am J Nephrol 27: 84–91, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Witowski J, Jörres A: Angiogenic activity of the peritoneal mesothelium: Implications for peritoneal dialysis. In: Progress in Peritoneal Dialysis, edited by Krediet RT, Rijeka, Montenegro, In-Tech, 2011, pp 61–74 [Google Scholar]
- 13.Pecoits-Filho R, Carvalho MJ, Stenvinkel P, Lindholm B, Heimbürger O: Systemic and intraperitoneal interleukin-6 system during the first year of peritoneal dialysis. Perit Dial Int 26: 53–63, 2006 [PubMed] [Google Scholar]
- 14.Oh KH, Jung JY, Yoon MO, Song A, Lee H, Ro H, Hwang YH, Kim DK, Margetts P, Ahn C: Intra-peritoneal interleukin-6 system is a potent determinant of the baseline peritoneal solute transport in incident peritoneal dialysis patients. Nephrol Dial Transplant 25: 1639–1646, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Lambie M, Chess J, Donovan KL, Kim YL, Do JY, Lee HB, Noh H, Williams PF, Williams AJ, Davison S, Dorval M, Summers A, Williams JD, Bankart J, Davies SJ, Topley N; Global Fluid Study Investigators : Independent effects of systemic and peritoneal inflammation on peritoneal dialysis survival. J Am Soc Nephrol 24: 2071–2080, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aroeira LS, Aguilera A, Selgas R, Ramírez-Huesca M, Pérez-Lozano ML, Cirugeda A, Bajo MA, del Peso G, Sánchez-Tomero JA, Jiménez-Heffernan JA, López-Cabrera M: Mesenchymal conversion of mesothelial cells as a mechanism responsible for high solute transport rate in peritoneal dialysis: Role of vascular endothelial growth factor. Am J Kidney Dis 46: 938–948, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Gerber SA, Rybalko VY, Bigelow CE, Lugade AA, Foster TH, Frelinger JG, Lord EM: Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathol 169: 1739–1752, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topley N, Jörres A, Luttmann W, Petersen MM, Lang MJ, Thierauch KH, Müller C, Coles GA, Davies M, Williams JD: Human peritoneal mesothelial cells synthesize interleukin-6: Induction by IL-1 beta and TNF alpha. Kidney Int 43: 226–233, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Witowski J, Jörres A, Coles GA, Williams JD, Topley N: Superinduction of IL-6 synthesis in human peritoneal mesothelial cells is related to the induction and stabilization of IL-6 mRNA. Kidney Int 50: 1212–1223, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Catar R, Witowski J, Wagner P, Annett Schramm I, Kawka E, Philippe A, Dragun D, Jörres A: The proto-oncogene c-Fos transcriptionally regulates VEGF production during peritoneal inflammation. Kidney Int 84: 1119–1128, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA: Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 14: 705–714, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Fielding CA, McLoughlin RM, Colmont CS, Kovaleva M, Harris DA, Rose-John S, Topley N, Jones SA: Viral IL-6 blocks neutrophil infiltration during acute inflammation. J Immunol 175: 4024–4029, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F: Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374: 1–20, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jostock T, Müllberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S: Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem 268: 160–167, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Udan RS, Culver JC, Dickinson ME: Understanding vascular development. Wiley Interdiscip Rev Dev Biol 2: 327–346, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fielding CA, Jones GW, McLoughlin RM, McLeod L, Hammond VJ, Uceda J, Williams AS, Lambie M, Foster TL, Liao CT, Rice CM, Greenhill CJ, Colmont CS, Hams E, Coles B, Kift-Morgan A, Newton Z, Craig KJ, Williams JD, Williams GT, Davies SJ, Humphreys IR, O’Donnell VB, Taylor PR, Jenkins BJ, Topley N, Jones SA: Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity 40: 40–50, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman M, Vandenabeele P, Moulart J, Amraoui Z, Abramowicz D, Nortier J, Vanherweghem JL, Fiers W: Intraperitoneal secretion of interleukin-6 during continuous ambulatory peritoneal dialysis. Nephron 56: 277–280, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Hunter CA, Jones SA: IL-6 as a keystone cytokine in health and disease. Nat Immunol 16: 448–457, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Chalaris A, Rabe B, Paliga K, Lange H, Laskay T, Fielding CA, Jones SA, Rose-John S, Scheller J: Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood 110: 1748–1755, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Ernst M, Thiem S, Nguyen PM, Eissmann M, Putoczki TL: Epithelial gp130/Stat3 functions: An intestinal signaling node in health and disease. Semin Immunol 26: 29–37, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL, Xie K: Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene 22: 319–329, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Loeffler S, Fayard B, Weis J, Weissenberger J: Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer 115: 202–213, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, Inglese M, McLoughlin RM, Jones SA, Topley N, Baumann H, Judd LM, Giraud AS, Boussioutas A, Zhu HJ, Ernst M: Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med 11: 845–852, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Carpenter RL, Lo HW: STAT3 target genes relevant to human cancers. Cancers (Basel) 6: 897–925, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su JL, Lai KP, Chen CA, Yang CY, Chen PS, Chang CC, Chou CH, Hu CL, Kuo ML, Hsieh CY, Wei LH: A novel peptide specifically binding to interleukin-6 receptor (gp80) inhibits angiogenesis and tumor growth. Cancer Res 65: 4827–4835, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Adachi Y, Aoki C, Yoshio-Hoshino N, Takayama K, Curiel DT, Nishimoto N: Interleukin-6 induces both cell growth and VEGF production in malignant mesotheliomas. Int J Cancer 119: 1303–1311, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML, Lin JT: Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci 11: 517–527, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, Yao Y: Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther 141: 125–139, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Middleton K, Jones J, Lwin Z, Coward JI: Interleukin-6: An angiogenic target in solid tumours. Crit Rev Oncol Hematol 89: 129–139, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, Thompson R, Schioppa T, Nemeth J, Vermeulen J, Singh N, Avril N, Cummings J, Rexhepaj E, Jirström K, Gallagher WM, Brennan DJ, McNeish IA, Balkwill FR: Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res 17: 6083–6096, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdelrahim M, Smith R 3rd, Burghardt R, Safe S: Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res 64: 6740–6749, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Abdelrahim M, Safe S: Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expression in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Mol Pharmacol 68: 317–329, 2005 [DOI] [PubMed] [Google Scholar]
- 43.O’Shea JJ, Holland SM, Staudt LM: JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med 368: 161–170, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taniguchi K, Karin M: IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol 26: 54–74, 2014 [DOI] [PubMed] [Google Scholar]
- 45.McLoughlin RM, Witowski J, Robson RL, Wilkinson TS, Hurst SM, Williams AS, Williams JD, Rose-John S, Jones SA, Topley N: Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest 112: 598–607, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei LH, Chou CH, Chen MW, Rose-John S, Kuo ML, Chen SU, Yang YS: The role of IL-6 trans-signaling in vascular leakage: Implications for ovarian hyperstimulation syndrome in a murine model. J Clin Endocrinol Metab 98: E472–E484, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Nagy JA, Dvorak AM, Dvorak HF: Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med 2: a006544, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motomura Y, Kanbayashi H, Khan WI, Deng Y, Blennerhassett PA, Margetts PJ, Gauldie J, Egashira K, Collins SM: The gene transfer of soluble VEGF type I receptor (Flt-1) attenuates peritoneal fibrosis formation in mice but not soluble TGF-beta type II receptor gene transfer. Am J Physiol Gastrointest Liver Physiol 288: G143–G150, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA: Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest 126: 821–828, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallez Y, Huber P: Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta 1778: 794–809, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Yaginuma T, Yamamoto I, Yamamoto H, Mitome J, Tanno Y, Yokoyama K, Hayashi T, Kobayashi T, Watanabe M, Yamaguchi Y, Hosoya T: Increased lymphatic vessels in patients with encapsulating peritoneal sclerosis. Perit Dial Int 32: 617–627, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braun N, Alscher DM, Fritz P, Edenhofer I, Kimmel M, Gaspert A, Reimold F, Bode-Lesniewska B, Ziegler U, Biegger D, Wüthrich RP, Segerer S: Podoplanin-positive cells are a hallmark of encapsulating peritoneal sclerosis. Nephrol Dial Transplant 26: 1033–1041, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Lambie MR, Chess J, Summers AM, Williams PF, Topley N, Davies SJ; GLOBAL Fluid Study Investigators : Peritoneal inflammation precedes encapsulating peritoneal sclerosis: Results from the GLOBAL fluid study. Nephrol Dial Transplant 31: 480–486, 2016 [DOI] [PubMed] [Google Scholar]
- 54.Ksiazek K, Jörres A, Witowski J: Senescence induces a proangiogenic switch in human peritoneal mesothelial cells. Rejuvenation Res 11: 681–683, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Edgell CJ, McDonald CC, Graham JB: Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA 80: 3734–3737, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Marco GS, König M, Stock C, Wiesinger A, Hillebrand U, Reiermann S, Reuter S, Amler S, Köhler G, Buck F, Fobker M, Kümpers P, Oberleithner H, Hausberg M, Lang D, Pavenstädt H, Brand M: High phosphate directly affects endothelial function by downregulating annexin II. Kidney Int 83: 213–222, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Finkenzeller G, Sparacio A, Technau A, Marmé D, Siemeister G: Sp1 recognition sites in the proximal promoter of the human vascular endothelial growth factor gene are essential for platelet-derived growth factor-induced gene expression. Oncogene 15: 669–676, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Hegner B, Weber M, Dragun D, Schulze-Lohoff E: Differential regulation of smooth muscle markers in human bone marrow-derived mesenchymal stem cells. J Hypertens 23: 1191–1202, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Witowski J, Tayama H, Ksiazek K, Wanic-Kossowska M, Bender TO, Jörres A: Human peritoneal fibroblasts are a potent source of neutrophil-targeting cytokines: A key role of IL-1beta stimulation. Lab Invest 89: 414–424, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Mackenzie RK, Topley N, Neubauer A, Coles GA, Williams JD: Staphylococcal exoproducts down-regulate cyclooxygenase 1 and 2 in peritoneal macrophages. J Lab Clin Med 129: 23–34, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Jones GW, Bombardieri M, Greenhill CJ, McLeod L, Nerviani A, Rocher-Ros V, Cardus A, Williams AS, Pitzalis C, Jenkins BJ, Jones SA: Interleukin-27 inhibits ectopic lymphoid-like structure development in early inflammatory arthritis. J Exp Med 212: 1793–1802, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.