Abstract
The demonstration of impaired C regulation in the thrombotic microangiopathy (TMA) atypical hemolytic uremic syndrome (aHUS) resulted in the successful introduction of the C inhibitor eculizumab into clinical practice. C abnormalities account for approximately 50% of aHUS cases; however, mutations in the non-C gene diacylglycerol kinase-ε have been described recently in individuals not responsive to eculizumab. We report here a family in which the proposita presented with aHUS but did not respond to eculizumab. Her mother had previously presented with a post–renal transplant TMA. Both the proposita and her mother also had Charcot–Marie–Tooth disease. Using whole-exome sequencing, we identified a mutation in the inverted formin 2 gene (INF2) in the mutational hotspot for FSGS. Subsequent analysis of the Newcastle aHUS cohort identified another family with a functionally-significant mutation in INF2. In this family, renal transplantation was associated with post-transplant TMA. All individuals with INF2 mutations presenting with a TMA also had aHUS risk haplotypes, potentially accounting for the genetic pleiotropy. Identifying individuals with TMAs who may not respond to eculizumab will avoid prolonged exposure of such individuals to the infectious complications of terminal pathway C blockade.
Keywords: complement, hemolytic uremic syndrome, focal segmental glomerulosclerosis
Genetic abnormalities in the alternative pathway of C have been demonstrated to account for many cases of the thrombotic microangiopathy (TMA) atypical hemolytic uremic syndrome (aHUS) (MIM 235400).1 Understanding the role of C in the pathogenesis of aHUS has resulted in the successful introduction of the C inhibitor eculizumab into clinical practice.2
Recently, mutations in the non-C gene DGKE have been demonstrated to be associated with aHUS (MIM 615008).3 Individuals with DGKE mutations display phenotypic variability with some patients presenting with membranoproliferative GN.4 As might be expected with a genetic cause which does not appear to be C-mediated, individuals have not responded to treatment with eculizumab.3
Despite recent advances, the genetic basis of many cases of familial aHUS remain unsolved. In this study, we describe the finding of inverted formin 2 gene (INF2) mutations in two families with TMA.
Our index case, patient III:2, presented aged seven with pex cavus and difficulty walking and was diagnosed with Charcot–Marie–Tooth (CMT) (Figure 1). Aged 15 she presented 5 days after a sore throat with microangiopathic hemolytic anemia on blood film (Hb, 9.4 g/dl), thrombocytopenia (platelets 119 × 109/L), and renal failure (creatinine 879 µmol/L). Haptoglobins were undetectable, lactate dehydrogenase was 844 U/L, and there was proteinuria (4.8 g/L). BP on admission was 185/110 mmHg. Hemodialysis was commenced at presentation and plasma exchange was undertaken before commencement of eculizumab. Initially, there was an improvement in the platelet count to 173 × 109/L, but subsequently this fell to 100 × 109/L. A bone marrow biopsy was unremarkable and trough eculizumab concentration was adequate with a completely suppressed CH50. Three months after presentation a renal biopsy was undertaken demonstrating characteristic changes of a thrombotic microangiopathy (Figure 2A). There were also features of a distinct glomerulosclerosis with small glomeruli and arteriosclerosis (Figure 2B). After 9 months with no renal recovery eculizumab was withdrawn.
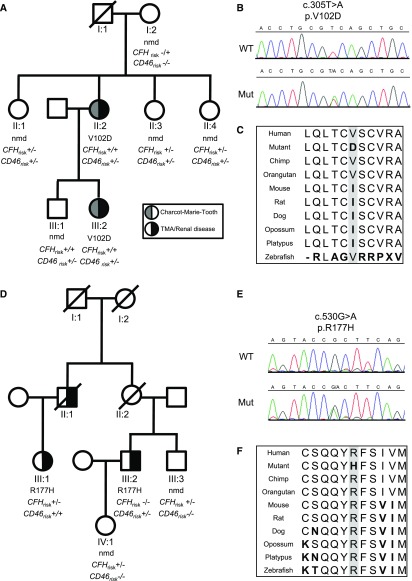
Figure 1.
Pedigrees of families with INF2 genetic variants. The pedigrees demonstrate the segregation of the renal/neurologic phenotype with the rare genetic variant, c.305T>A (p.V102D) in family 1 (A), and c.530G>A (p.R177H) in family 2 (D). Individuals tested but not carrying the mutation are shown (nmd, no mutation detected). The number of alleles carrying the aHUS risk haplotype CFH-H3 (CFHrisk) and CD46GGAAC (CD46risk) are shown on the pedigree. Sanger sequencing trace of wild type (WT) and mutant (Mut) for c.305T>A (p.V102D) (B) and c.530G>A (p.R177H) (E). Alignment of human, chimpanzee, orangutan, mouse, rat, dog, opossum, platypus, and zebrafish INF2 demonstrating amino acid conservation (C and F) (performed using http://genome.ucsc.edu/cgibin/hgTrackUi?hgsid=309786867&c=chr21&g=cons46way#a_cfg_phyloP).
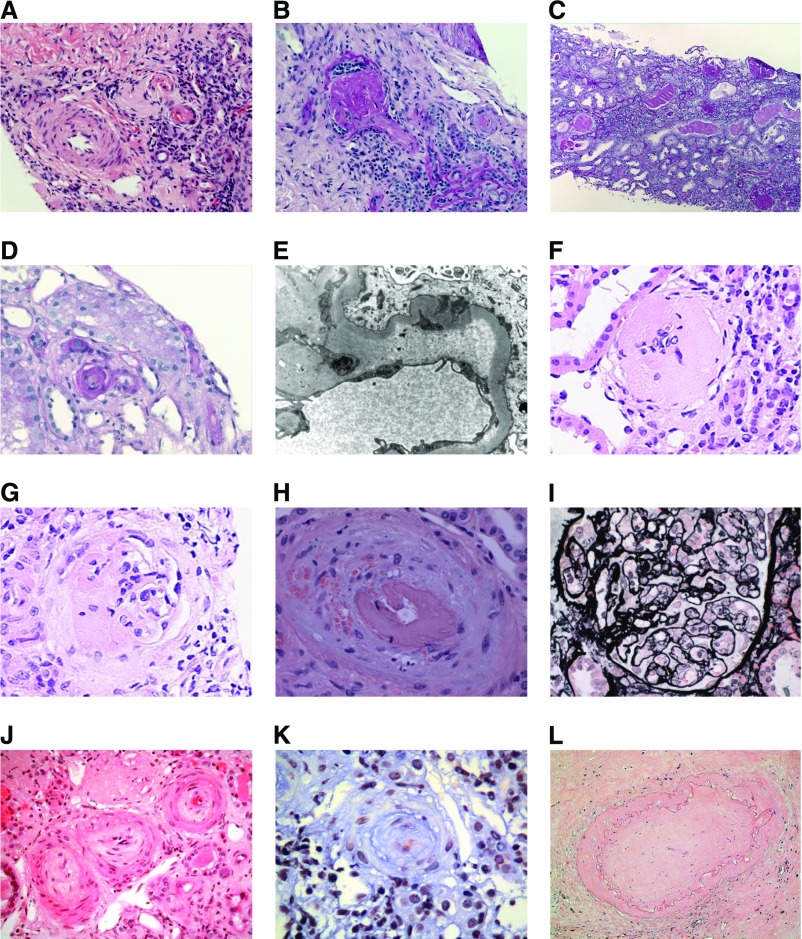
Figure 2.
Thrombotic microangiopathy in renal biopsy from patients with INF2 mutations. Renal biopsies. Native renal biopsy from family 1, patient III:2. (A) Two arterioles (right) showing features of active thrombosis and a small artery (left) with relatively slight intimal edema with fibrosis (hematoxylin and eosin stain [H&E]). (B) Glomerulus (left) showing global sclerosis and occluded arteriole (right) (periodic acid–Schiff [PAS]). Native renal biopsy from family 1, patient II:2 demonstrating end-stage changes with diffuse global sclerosis (C). Renal transplant biopsy from family 1, patient II:2 demonstrating (D) an occluded arteriole (PAS) and (E) a capillary loop with abundant subendothelial fluffy material (electron microscopy). Native renal biopsy from family 2, patient III:2 demonstrating (F) a sclerosed glomeruli and (G) a segmental sclerosing lesion. Renal transplant biopsy from family 2, patient III:1. Mucoid intimal thickening is seen in an interlobular artery with red cell fragmentation in the wall and luminal thrombus (H). Mesangiolysis is also seen 3–6 o’clock (I) (silver stain). Native renal biopsy from family 2, patient III:2 showing a subacute/chronic arterial TMA with fibroproliferative obliteration of small arteries and arterioles (J) (H&E) and (K) (trichrome). Renal transplant biopsy from family 2, patient III:2 demonstrating end stage change with fibrous obliteration of arteries. (L) (H&E).
Screening for known inherited and acquired causes of aHUS did not reveal any abnormality.1 The C5 variant c.2654G>A (p.R885H), which impairs eculizumab efficacy, was not present.5
Family history revealed that the proposita’s mother, patient II:2 (Figure 1), also had CMT, had presented with ESRD aged 17, and had a post-transplant TMA (Figure 2, D and E) (case history, Supplemental Material).
Because of the absence of an abnormality in a known aHUS-associated gene we sequenced the exomes of the two affected individuals in this family.
This revealed a rare variant in INF2, c.305T>A (p.V102D) (Figure 1). Mutations in INF2 are the commonest cause of familial autosomal dominant nephrotic syndrome.6–8 In a minority of these cases the mutations cause a syndromic form of FSGS associated with the demyelinating peripheral neuropathy, CMT9,10 (Figure 3).
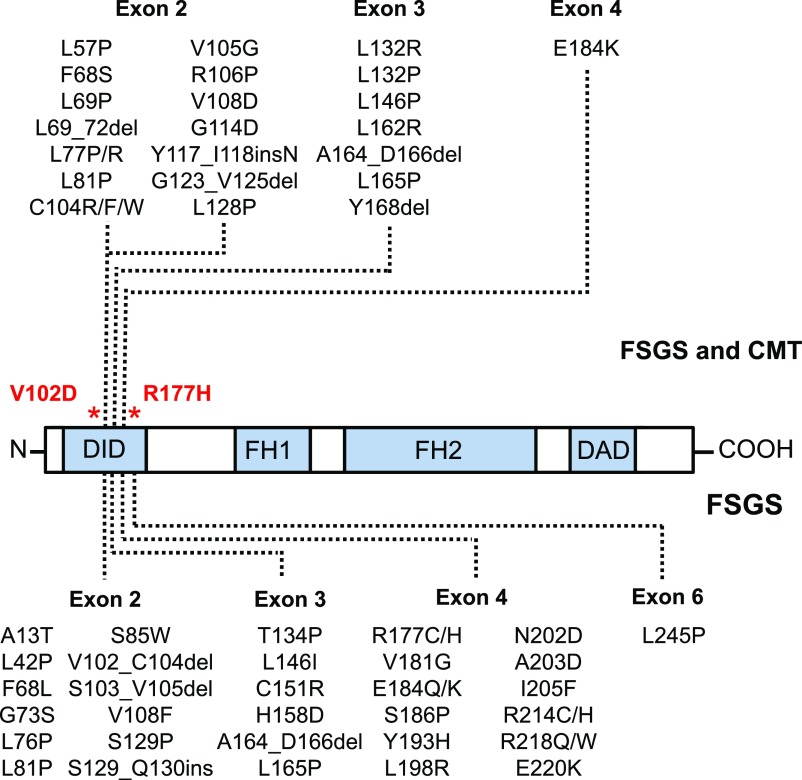
Figure 3.
Hotspots for inverted formin 2 (INF2) variants in FSGS, CMT, and aHUS. A representation of the domain structure of INF2 showing the DID domain, formin homology domains (FH1, FH2), and the DAD domain. Genetic variants associated with isolated FSGS are shown below the domain structure. Genetic variants with a combined FSGS/CMT phenotype are shown above the domain structure. Variants from Mademan et al.57 The locations of the aHUS-associated mutations demonstrated in the study are shown in red.
To assess whether mutations in INF2 account for other cases of familial or sporadic TMA previously referred to the Newcastle aHUS center,11 we analyzed 28 familial cases by exome sequencing and undertook Sanger sequencing on an additional 161 sporadic aHUS cases. In one family, in which no known genetic risk factors had been found, we identified a functionally-significant mutation in INF2, c.530G>A (p.R177H), which segregated with the disease (Figures 1 and 2, Supplemental Material). No INF2 variants were identified in sporadic aHUS cases.
INF2 is a ubiquitously-expressed formin protein12 which accelerates actin polymerization and depolymerization, thus regulating a range of cytoskeleton-dependent cellular functions including the secretory pathway.13,14 INF2 comprises formin homology 1 and 2 domains, an N-terminal diaphanous inhibitory domain (DID), and a C-terminal diaphanous autoregulatory domain (DAD).15 Mutations in INF2 predominate in the DID domain.6,7,10 Functional analysis of INF2 mutations in disease has demonstrated disorganized cytoskeletal functions7,9 although the precise mechanism of disease remains elusive.
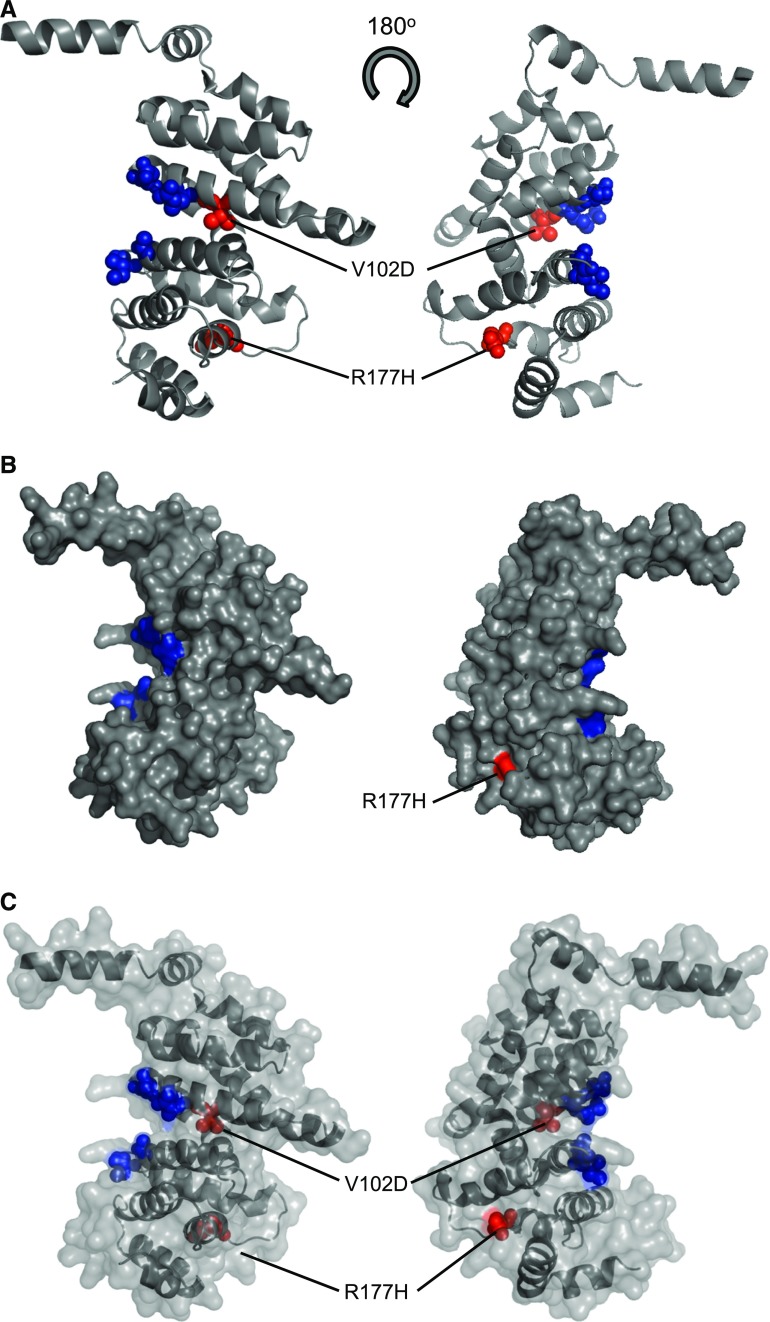
The two INF2 variants we describe here reside in the mutational hotspot for disease.6–10 The c.305T>A (p.V102D) variant resides in exon 2 whereas c.530G>A (p.R177H) resides in exon 4 (Figures 3 and 4). Structural modeling reveals that the p.V102D variant is in close proximity to the DAD binding region. Modeling does not predict a surface-exposed residue but instead the variant may be expected to disrupt the architecture of the eighth α-helix of the DID domain (Figure 4). The p.R177H variant resides before the 13th α-helix of the DID domain and is surface-exposed (Figure 4). Amino acids at both these positions are conserved across species with GERP++ scores of 4.76 (p.V102D) and 4.48 (p.R177H) (Figure 1).
Figure 4.
Predicted structure of DID domain of inverted formin 2 (INF2). A structural model of the DID domain (amino acids 1–234) was generated using Phyre2 (A).58 Blue spheres represent the amino acids involved in binding to DAD domain: (R106, N110, A149, I152). The position of the p.V102D and R177H mutations are highlighted in red. The p.V102D lies in the eighth α-helix of the DID (amino acids) domain. (B) Surface representation of the modeled structure highlighting the surface-exposed amino acids responsible for DAD binding (R106, N110, A149, I152). The mutant p.V102D is not buried but may be expected to disrupt the architecture of the eighth α-helix. The p.R177H variant resides before the 13th α-helix of the DID domain and is surface-exposed. (C) Semi transparent representation of the surface of the INF2 model highlighting the variants.
Disease-causing mutations in FSGS have mainly been found to occur in the DID domain, in exons 2–4, with only one report of a mutation in exon 6 (Figure 3).6–8,16 Within this hot-spot there is a cluster between nucleotides 300 and 500 which accounts for those with FSGS and CMT.9,10 The p.V102D mutation resides in this region and this family have CMT, whereas the p.R177H mutation resides downstream of this region and this family has no neurologic phenotype. The p.R177H mutation has previously been reported in three unrelated pedigrees and in all cases had the nonsyndromic form of FSGS.6,8 Functional analysis of this mutation demonstrated altered INF2 localization and disruption of the actin cytoskeleton.9
It is well reported that even in individual families with the same INF2 mutation there is phenotypic variability. Most commonly, individuals present with disease in adolescence with mild proteinuria, developing ESRD in the third or fourth decade, although individuals have been reported to be unaffected into their sixth or seventh decade.16 Variable intrafamilial penetrance has also been reported for the neurologic phenotype.16 The clinical and pathologic disease pleiotropism we describe with INF2 mutations is also seen in individuals with recessive DGKE mutations, where some individuals present with proteinuria and progressive renal failure whereas others present with aHUS. Likewise, the biopsy findings in DGKE-associated disease are also heterogenous ranging from a membranoproliferative pattern to a TMA. These findings can vary according to the time of presentation. It is only with genetic analysis that the underlying pathologic process can be identified and a therapeutic intervention sought. It has been suggested that genetic background or environmental factors modify the penetrance and phenotype of disease.16,17 It is interesting to note that in family 1 both affected members, II:2 and III:2, were homozygous for the aHUS at-risk CFH-H3 haplotype,18 and both carried one risk CD46 allele.19 In family 2, patient III:1 was homozygous for the CD46GGAAC risk haplotype whereas III:2 was heterozygous. III:1 also carried one copy of the CFH-H3 haplotype (Figure 1).
Although INF2 mutations have not previously been associated with a renal thrombotic microangiopathy, aHUS has been reported in patients with primary FSGS,20–23 and FSGS is a frequent pathologic sequalae of sporadic Stx-HUS.24 A thrombotic microangiopathy has also been associated with other causes of nephrotic syndrome25,26 and primary GN, including IgA nephropathy,27–29 Henoch Schonlein Purpura,23 ANCA-associated vasculitis,22,23 and anti-GBM nephropathy.23
It has been hypothesized that either direct or indirect (via impaired VEGF secretion from podocytes) endothelial injury leads to a constricted microvasculature with perturbed hemodynamic flow, leading to the formation of platelet microthrombi and a thrombotic microangiopathy. Loss of coagulation regulators with upregulation of procoagulation factors has also been suggested as a contributory factor in those individuals with coexistent nephrotic syndrome.30–33
It is intriguing that all three patients who had renal transplants had biopsy-proven evidence of a thrombotic microangiopathy in their renal allografts. The risk of FSGS recurrence post-transplant in those with genetic defects of the glomerular filtration barrier is low due to correction of the underlying defect.34–36 An exception to this is in those individuals with complete deficiency of NPHS1 due to the presumed generation of antibodies against this immunologically novel protein in the allograft. Such a scenario would not be expected in a dominantly-inherited condition.
Currently, there is little information available as to the recurrence of FSGS post-transplantation in individuals carrying INF2 mutations. However, in one small study recurrence was seen in one of three individuals.8 INF2 is expressed ubiquitously12 and recurrence of FSGS in an allograft suggests that a circulating factor or cell type is predisposing to recurrent disease. Such a factor may account for post-transplant TMA. It should be noted that INF2 has been demonstrated to complex with and alter the intracellular transport of the C regulators CD55 and CD59, which are present on all circulating cells including platelets. We cannot, however, rule out the possibility that the TMA was a consequence of the post-transplant milieu (e.g., viral diseases, ischemia reperfusion injury, donor-specific antibodies, immunosuppressive drugs).
In summary, we describe two families with mutations in INF2 in addition to common aHUS risk haplotypes who present with aHUS or a post-transplant TMA. Eculizumab was unsuccessful in preventing either ongoing TMA or ESRD as is seen with other non–C-mediated causes of aHUS. Identifying individuals who will not respond to eculizumab will avoid exposing these individuals to the infectious risks of terminal pathway C blockade. This study represents an initial application of whole-exome sequencing in personalized management of TMA.
Concise Methods
The study was approved by North East–York Research Ethics Committee, and informed consent was obtained in accordance with the Declaration of Helsinki.
C Assays
C3 and C4 levels were measured by rate nephelometry (Beckman Coulter Array 360). Factor H levels were measured by radial immunodiffusion (Binding Site). Screening for C autoantibodies was undertaken using ELISA as described previously.37,38
Genetic Analysis and Multiplex Ligation–Dependent Probe Amplification
Mutation screening of CFH,39 CFI,40 CFB,41 MCP,42 C3,43 and DGKE3 was undertaken using Sanger sequencing as previously described. Screening for genomic disorders affecting CFH, CFHR1, CFHR2, CFHR3, CFHR5, CFI, and CD46 was undertaken using multiplex ligation-dependent probe amplification.44,45 Mutation screening of INF2 was undertaken using Sanger sequencing using the primer conditions in Supplemental Table 5.
Whole-Exome Sequencing
Enrichment from isolated DNA was performed using either Illumina Nextera Rapid Capture Exome by AROS AB (family 1) or Agilent SureSelectXT Human All Exon V5 by GATC Biotech, Konstanz (family 2, III:1) as described previously.46 Library preparation was performed postcapture, with adaptor sequences and indexing incorporated using proprietary methods of AROS AB and GATC Biotech, compatible for Illumina sequencing technology. Illumina sequencing was performed on the HiSeq2000 instrument (v3 chemistry) (Supplemental Table 2).
The quality of sequencing reads was firstly checked with FastQC.47 Duplicated reads were removed with FastUniq.48 The remaining reads were mapped to the human reference genome GRCh37 with BWA.49 The alignments were refined with tools of the GATK suite.50 Variants were called according to GATK Best Practice recommendations,51,52 including recalibration. Freebayes was also used to call variants from the same set of samples.53 The variants called by Freebayes with total coverage ≥5, minor allele coverage ≥5, and variants call quality ≥20 were added to those identified by GATK. Annovar was used for annotations and prediction of functional consequences.54 Variants identified in family 1 were filtered as detailed (Supplemental Table 2). First, we selected for variants in high-effect regions and selected variants at a minor allele frequency <5% in 1000G and ESP6500. We then selected those variants segregating in a dominant fashion with disease. Variants predicted to be deleterious by Polyphen-2 HDIV and HVAR, Mutation Taster, Mutation Assessor, FATHMM, or RadialSVM were selected for further analysis. A more stringent minor allele frequency cut-off of <0.1% in 1000G and ESP6500 was applied and nonconserved variants (<2 by GERP++ and <0.5 by PhyloP) were discarded (Supplemental Table 3). Phenotypic data were then used to interrogate the remaining 34 genes providing only one candidate gene known to have both renal and neurologic conditions inherited in an autosomal dominant pattern (Supplemental Table 4).
Protein Modeling
Phyre2 was used to generate an approximate protein structure using the inputted amino acid sequence of INF2 (NP 071934.3, amino acids 1–250) using the intensive modeling mode. Protein domain boundaries for INF2 were taken from Pfam.55 Three-dimensional protein structures were manipulated using PyMOL.56
Disclosures
Newcastle University has received funding from Alexion Pharmaceuticals, Cheshire, UK, for consultancy work undertaken by T.H.J.G and D.K. D.K. is scientific advisor to Gyroscope Therapeutics, London.
Supplementary Material
Acknowledgments
The research leading to these results has received funding from the European Union’s Seventh Framework Programme (FP7/2007–2013) under Grant 305608 (EURenOmics). V.B. is funded by Northern Counties Kidney Research Fund. Funding for this study was provided by the UK Medical Research Council (G0701325). E.K.S.W. is a Medical Research Council clinical training fellow. D.K. is a Wellcome Trust intermediate clinical fellow.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015101189/-/DCSupplemental.
References
- 1.Kavanagh D, Goodship TH, Richards A: Atypical hemolytic uremic syndrome. Semin Nephrol 33: 508–530, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C: Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368: 2169–2181, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Lemaire M, Frémeaux-Bacchi V, Schaefer F, Choi M, Tang WH, Le Quintrec M, Fakhouri F, Taque S, Nobili F, Martinez F, Ji W, Overton JD, Mane SM, Nürnberg G, Altmüller J, Thiele H, Morin D, Deschenes G, Baudouin V, Llanas B, Collard L, Majid MA, Simkova E, Nürnberg P, Rioux-Leclerc N, Moeckel GW, Gubler MC, Hwa J, Loirat C, Lifton RP: Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet 45: 531–536, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozaltin F, Li B, Rauhauser A, An SW, Soylemezoglu O, Gonul II, Taskiran EZ, Ibsirlioglu T, Korkmaz E, Bilginer Y, Duzova A, Ozen S, Topaloglu R, Besbas N, Ashraf S, Du Y, Liang C, Chen P, Lu D, Vadnagara K, Arbuckle S, Lewis D, Wakeland B, Quigg RJ, Ransom RF, Wakeland EK, Topham MK, Bazan NG, Mohan C, Hildebrandt F, Bakkaloglu A, Huang CL, Attanasio M: DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN. J Am Soc Nephrol 24: 377–384, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimura J, Yamamoto M, Hayashi S, Ohyashiki K, Ando K, Brodsky AL, Noji H, Kitamura K, Eto T, Takahashi T, Masuko M, Matsumoto T, Wano Y, Shichishima T, Shibayama H, Hase M, Li L, Johnson K, Lazarowski A, Tamburini P, Inazawa J, Kinoshita T, Kanakura Y: Genetic variants in C5 and poor response to eculizumab. N Engl J Med 370: 632–639, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Boyer O, Benoit G, Gribouval O, Nevo F, Tête MJ, Dantal J, Gilbert-Dussardier B, Touchard G, Karras A, Presne C, Grunfeld JP, Legendre C, Joly D, Rieu P, Mohsin N, Hannedouche T, Moal V, Gubler MC, Broutin I, Mollet G, Antignac C: Mutations in INF2 are a major cause of autosomal dominant focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 239–245, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown EJ, Schlöndorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, Higgs HN, Henderson JM, Pollak MR: Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet 42: 72–76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gbadegesin RA, Lavin PJ, Hall G, Bartkowiak B, Homstad A, Jiang R, Wu G, Byrd A, Lynn K, Wolfish N, Ottati C, Stevens P, Howell D, Conlon P, Winn MP: Inverted formin 2 mutations with variable expression in patients with sporadic and hereditary focal and segmental glomerulosclerosis. Kidney Int 81: 94–99, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer O, Nevo F, Plaisier E, Funalot B, Gribouval O, Benoit G, Huynh Cong E, Arrondel C, Tête MJ, Montjean R, Richard L, Karras A, Pouteil-Noble C, Balafrej L, Bonnardeaux A, Canaud G, Charasse C, Dantal J, Deschenes G, Deteix P, Dubourg O, Petiot P, Pouthier D, Leguern E, Guiochon-Mantel A, Broutin I, Gubler MC, Saunier S, Ronco P, Vallat JM, Alonso MA, Antignac C, Mollet G: INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N Engl J Med 365: 2377–2388, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Caridi G, Lugani F, Dagnino M, Gigante M, Iolascon A, Falco M, Graziano C, Benetti E, Dugo M, Del Prete D, Granata A, Borracelli D, Moggia E, Quaglia M, Rinaldi R, Gesualdo L, Ghiggeri GM: Novel INF2 mutations in an Italian cohort of patients with focal segmental glomerulosclerosis, renal failure and Charcot-Marie-Tooth neuropathy. Nephrol Dial Transplant 29[Suppl 4]: iv80–iv86, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Sheerin N, Kavanagh D, Goodship TH, Johnson S: A national specialised service in England for atypical haemolytic uraemic syndrome - the first year’s experience. QJM 109: 27–33, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Madrid R, Aranda JF, Rodríguez-Fraticelli AE, Ventimiglia L, Andrés-Delgado L, Shehata M, Fanayan S, Shahheydari H, Gómez S, Jiménez A, Martín-Belmonte F, Byrne JA, Alonso MA: The formin INF2 regulates basolateral-to-apical transcytosis and lumen formation in association with Cdc42 and MAL2. Dev Cell 18: 814–827, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Chhabra ES, Ramabhadran V, Gerber SA, Higgs HN: INF2 is an endoplasmic reticulum-associated formin protein. J Cell Sci 122: 1430–1440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramabhadran V, Gurel PS, Higgs HN: Mutations to the formin homology 2 domain of INF2 protein have unexpected effects on actin polymerization and severing. J Biol Chem 287: 34234–34245, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhabra ES, Higgs HN: INF2 is a WASP homology 2 motif-containing formin that severs actin filaments and accelerates both polymerization and depolymerization. J Biol Chem 281: 26754–26767, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Barua M, Brown EJ, Charoonratana VT, Genovese G, Sun H, Pollak MR: Mutations in the INF2 gene account for a significant proportion of familial but not sporadic focal and segmental glomerulosclerosis. Kidney Int 83: 316–322, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bullich G, Trujillano D, Santin S, Ossowski S, Mendizabal S, Fraga G, Madrid A, Ariceta G, Ballarin J, Torra R, Estivill X, Ars E: Targeted next-generation sequencing in steroid-resistant nephrotic syndrome: Mutations in multiple glomerular genes may influence disease severity. Eur J Hum Genet 23: 1192–1199, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickering MC, de Jorge EG, Martinez-Barricarte R, Recalde S, Garcia-Layana A, Rose KL, Moss J, Walport MJ, Cook HT, de Córdoba SR, Botto M: Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med 204: 1249–1256, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esparza-Gordillo J, Goicoechea de Jorge E, Buil A, Carreras Berges L, López-Trascasa M, Sánchez-Corral P, Rodríguez de Córdoba S: Predisposition to atypical hemolytic uremic syndrome involves the concurrence of different susceptibility alleles in the regulators of complement activation gene cluster in 1q32. Hum Mol Genet 14: 703–712, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Benz K, Amann K, Dittrich K, Dötsch J: Thrombotic microangiopathy as a complication in a patient with focal segmental glomerulosclerosis. Pediatr Nephrol 22: 2125–2128, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Bökenkamp A, Hoyer PF, Offner G, Helmchen U, Brodehl J: Recurrent haemolytic uraemic syndrome in a boy with focal and segmental glomerulosclerosis. Eur J Pediatr 151: 791–792, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Noris M, Mele C, Remuzzi G: Podocyte dysfunction in atypical haemolytic uraemic syndrome. Nat Rev Nephrol 11: 245–252, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Manenti L, Gnappi E, Vaglio A, Allegri L, Noris M, Bresin E, Pilato FP, Valoti E, Pasquali S, Buzio C: Atypical haemolytic uraemic syndrome with underlying glomerulopathies. A case series and a review of the literature. Nephrol Dial Transplant 28: 2246–2259, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Caletti MG, Gallo G, Gianantonio CA: Development of focal segmental sclerosis and hyalinosis in hemolytic uremic syndrome. Pediatr Nephrol 10: 687–692, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Siegler RL, Brewer ED, Pysher TJ: Hemolytic uremic syndrome associated with glomerular disease. Am J Kidney Dis 13: 144–147, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Sherbotie JR, van Heyningen V, Axton R, Williamson K, Finn LS, Kaplan BS: Hemolytic uremic syndrome associated with Denys-Drash syndrome. Pediatr Nephrol 14: 1092–1097, 2000 [DOI] [PubMed] [Google Scholar]
- 27.El Karoui K, Hill GS, Karras A, Jacquot C, Moulonguet L, Kourilsky O, Frémeaux-Bacchi V, Delahousse M, Duong Van Huyen JP, Loupy A, Bruneval P, Nochy D: A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol 23: 137–148, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita S, Sakai T, Okamoto N, Funabiki A, Okada Y, Hasegawa Y, Amano K, Yoshikawa N, Kasuga M: Hemolytic uremic syndrome associated with immunoglobulin A nephropathy: A case report and review of cases of hemolytic uremic syndrome with glomerular disease. Intern Med 38: 495–499, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Chang A, Kowalewska J, Smith KD, Nicosia RF, Alpers CE: A clinicopathologic study of thrombotic microangiopathy in the setting of IgA nephropathy. Clin Nephrol 66: 397–404, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Chen G, Liu H, Liu F: A glimpse of the glomerular milieu: From endothelial cell to thrombotic disease in nephrotic syndrome. Microvasc Res 89: 1–6, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Gao C, Xie R, Yu C, Wang Q, Shi F, Yao C, Xie R, Zhou J, Gilbert GE, Shi J: Procoagulant activity of erythrocytes and platelets through phosphatidylserine exposure and microparticles release in patients with nephrotic syndrome. Thromb Haemost 107: 681–689, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Isome M, Fujinaka H, Yaoita E, Feng L, Adhikary LP, Abe A, Tsuchida S, Kawasaki K, Suzuki H, Kihara I, Wilson CB, Yamamoto T: Involvement of endothelial cell adhesion molecules in the development of anti-Thy-1 nephritis. Exp Nephrol 10: 338–347, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Tkaczyk M, Czupryniak A, Owczarek D, Lukamowicz J, Nowicki M: Markers of endothelial dysfunction in children with idiopathic nephrotic syndrome. Am J Nephrol 28: 197–202, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Rood IM, Deegens JK, Wetzels JF: Genetic causes of focal segmental glomerulosclerosis: Implications for clinical practice. Nephrol Dial Transplant 27: 882–890, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Patrakka J, Ruotsalainen V, Reponen P, Qvist E, Laine J, Holmberg C, Tryggvason K, Jalanko H: Recurrence of nephrotic syndrome in kidney grafts of patients with congenital nephrotic syndrome of the Finnish type: Role of nephrin. Transplantation 73: 394–403, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Maas RJ, Deegens JK, van den Brand JA, Cornelissen EA, Wetzels JF: A retrospective study of focal segmental glomerulosclerosis: Clinical criteria can identify patients at high risk for recurrent disease after first renal transplantation. BMC Nephrol 14: 47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore I, Strain L, Pappworth I, Kavanagh D, Barlow PN, Herbert AP, Schmidt CQ, Staniforth SJ, Holmes LV, Ward R, Morgan L, Goodship TH, Marchbank KJ: Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood 115: 379–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kavanagh D, Pappworth IY, Anderson H, Hayes CM, Moore I, Hunze EM, Bennaceur K, Roversi P, Lea S, Strain L, Ward R, Plant N, Nailescu C, Goodship TH, Marchbank KJ: Factor I autoantibodies in patients with atypical hemolytic uremic syndrome: Disease-associated or an epiphenomenon? Clin J Am Soc Nephrol 7: 417–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richards A, Buddles MR, Donne RL, Kaplan BS, Kirk E, Venning MC, Tielemans CL, Goodship JA, Goodship TH: Factor H mutations in hemolytic uremic syndrome cluster in exons 18-20, a domain important for host cell recognition. Am J Hum Genet 68: 485–490, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kavanagh D, Kemp EJ, Mayland E, Winney RJ, Duffield JS, Warwick G, Richards A, Ward R, Goodship JA, Goodship TH: Mutations in complement factor I predispose to development of atypical hemolytic uremic syndrome. J Am Soc Nephrol 16: 2150–2155, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Kavanagh D, Kemp EJ, Richards A, Burgess RM, Mayland E, Goodship JA, Goodship TH: Does complement factor B have a role in the pathogenesis of atypical HUS? Mol Immunol 43: 856–859, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Richards A, Kemp EJ, Liszewski MK, Goodship JA, Lampe AK, Decorte R, Müslümanoğlu MH, Kavukcu S, Filler G, Pirson Y, Wen LS, Atkinson JP, Goodship TH: Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci USA 100: 12966–12971, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frémeaux-Bacchi V, Miller EC, Liszewski MK, Strain L, Blouin J, Brown AL, Moghal N, Kaplan BS, Weiss RA, Lhotta K, Kapur G, Mattoo T, Nivet H, Wong W, Gie S, Hurault de Ligny B, Fischbach M, Gupta R, Hauhart R, Meunier V, Loirat C, Dragon-Durey MA, Fridman WH, Janssen BJ, Goodship TH, Atkinson JP: Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood 112: 4948–4952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Challis RC, Araujo GSR, Wong EKS, Anderson HE, Awan A, Dorman AM, Waldron M, Wilson V, Brocklebank V, Strain L, Morgan BP, Harris CL, Marchbank KJ, Goodship THJ, Kavanagh D: A de novo deletion in the RCA cluster resulting in a novel hybrid CFH/CFHR3 gene causing impaired cell surface complement regulation in atypical haemolytic uraemic syndrome. J Am Soc Nephrol 27: 1617–1624, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gleeson PJ, Wilson V, Cox TE, Sharma SD, Smith-Jackson K, Strain L, Lappin D, McHale T, Kavanagh D, Goodship TH: Chromosomal rearrangement-A rare cause of complement factor I associated atypical haemolytic uraemic syndrome. Immunobiology 221: 1124–1130, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Wong EK, Anderson HE, Herbert AP, Challis RC, Brown P, Reis GS, Tellez JO, Strain L, Fluck N, Humphrey A, Macleod A, Richards A, Ahlert D, Santibanez-Koref M, Barlow PN, Marchbank KJ, Harris CL, Goodship TH, Kavanagh D: Characterization of a factor H mutation that perturbs the alternative pathway of complement in a family with membranoproliferative GN. J Am Soc Nephrol 25: 2425–2433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrews S: FastQC: A Quality Control Tool for High Throughput Sequence Data. Available at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc. Accessed February 2, 2015
- 48.Xu H, Luo X, Qian J, Pang X, Song J, Qian G, Chen J, Chen S: FastUniq: A fast de novo duplicates removal tool for paired short reads. PLoS One 7: e52249, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, Durbin R: Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26: 589–595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA: The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20: 1297–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J, Banks E, Garimella KV, Altshuler D, Gabriel S, DePristo MA: From FastQ data to high-confidence variant calls: The genome analysis toolkit best practices pipeline. In: Current Protocols in Bioinformatics, NY, John Wiley & Sons, Inc., 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ: A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43: 491–498, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrison E, Marth G: Haplotype-Based Variant Detection from Short-Read Sequencing. arXiv.org. Available at https://arxiv.org/abs/1207.3907. Published July 20, 2012. Accessed February 2, 2015
- 54.Wang K, Li M, Hakonarson H: ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38: e164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, Heger A, Hetherington K, Holm L, Mistry J, Sonnhammer ELL, Tate J, Punta M: Pfam: The protein families database. Nucleic Acids Res 42: D222–D230, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schrödinger, LLC: The PyMOL Molecular Graphics System. Version1.5.0.4 ed., New York, Schrödinger, LLC, 2010
- 57.Mademan I, Deconinck T, Dinopoulos A, Voit T, Schara U, Devriendt K, Meijers B, Lerut E, De Jonghe P, Baets J: De novo INF2 mutations expand the genetic spectrum of hereditary neuropathy with glomerulopathy. Neurology 81: 1953–1958, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Kelley LA, Sternberg MJ: Protein structure prediction on the web: A case study using the Phyre server. Nat Protoc 4: 363–371, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.