Abstract
Humoral immune responses against donor antigens are important determinants of long-term transplant outcomes. Reactivation of the polyomavirus BK has been associated with de novo antibodies against mismatched donor HLA antigens in kidney transplantation. The effect of polyomavirus reactivation (BK viremia or JC viruria) on antibodies to kidney-specific self-antigens is unknown. We previously reported excellent 5-year outcomes after minimization of immunosuppression for BK viremia and after no intervention for JC viruria. Here, we report the 10-year results of this trial (n=193) along with a nested case-control study (n=40) to explore associations between polyomavirus reactivation and immune responses to the self-antigens fibronectin (FN) and collagen type-IV (Col-IV). Consistent with 5-year findings, subjects taking tacrolimus, compared with those taking cyclosporin, had less acute rejection (11% versus 22%, P=0.05) and graft loss (9% versus 22%, P=0.01) along with better transplant function (eGFR 65±19 versus 50±24 ml/min per 1.73 m2, P<0.001) at 10 years. Subjects undergoing immunosuppression reduction for BK viremia had 10-year outcomes similar to those without viremia. In the case-control study, antibodies to FN/Col-IV were more prevalent during year 1 in subjects with polyomavirus reactivation than in those without reactivation (48% versus 11%, P=0.04). Subjects with antibodies to FN/Col-IV had more acute rejection than did those without these antibodies (38% versus 8%, P=0.02). These data demonstrate the long-term safety and effectiveness of minimizing immunosuppression to treat BK viremia. Furthermore, these results indicate that polyomavirus reactivation associates with immune responses to kidney-specific self-antigens that may increase the risk for acute rejection through unclear mechanisms.
Keywords: kidney transplantation, transplant outcomes, virology, immunosuppression
Whereas acute rejection, calcineurin inhibitor nephrotoxicity, and nonspecific interstitial fibrosis/tubular atrophy were once accepted as the major causes of late graft loss, recent studies have indicated that antibody-mediated injury, recurrent and de novo glomerular diseases, and specific causes of interstitial fibrosis/tubular atrophy (e.g., BK virus–associated nephropathy [BKVAN] and recurrent acute rejection) are more prevalent than previously thought, underscoring the need to control ongoing immune responses effectively while balancing the risks of over-immunosuppression.1–6 BK viremia is common after kidney transplantation and, if uncontrolled, progresses to BKVAN and graft failure or dysfunction.7 Persistent BK viremia (defined as lasting >140 days) was recently associated with de novo development of antibodies against mismatched donor HLA antigens (donor-specific antibodies [DSA]), although in that study there was no effect of either BK viremia or DSA on 3-year graft outcomes.8 There is increasing evidence that humoral immunity, namely immune responses to mismatched donor HLA antigens (e.g., DSA) and self-antigens (e.g., antibodies to fibronectin [FN] and collagen type-IV [Col-IV]), is a common denominator and the major contributor to chronic allograft injury and late graft loss after solid organ transplantation despite the use of more potent immunosuppressive agents in the modern era.2,3,6
To our knowledge, interactions between polyomavirus reactivation, immune responses to kidney-specific self-antigens, and long-term transplant outcomes (>5 years) have not been explored. We hypothesized that polyomavirus reactivation leads to early immune responses to self-antigens that produce inferior long-term outcomes in kidney transplant recipients. We previously reported that neither the choice of calcineurin inhibitor nor antimetabolite affects the incidence of BK viremia, and that pre-emptive reduction of immunosuppression in response to BK viremia was safe and effective in preventing BKVAN at 1 and 5 years post-transplant.9,10 We subsequently characterized this cohort for reactivation of the polyomavirus JC and interactions with BK reactivation and acute rejection.11 The purpose of this study is two-fold: (1) to examine the 10-year patient and graft outcomes from this unique cohort; and (2) to conduct a nested case-control study (n=40) within this cohort to explore associations between polyomavirus reactivation, de novo antibodies to kidney-specific self-antigens, and long-term outcomes.
Results
Retrospective Cohort Analysis
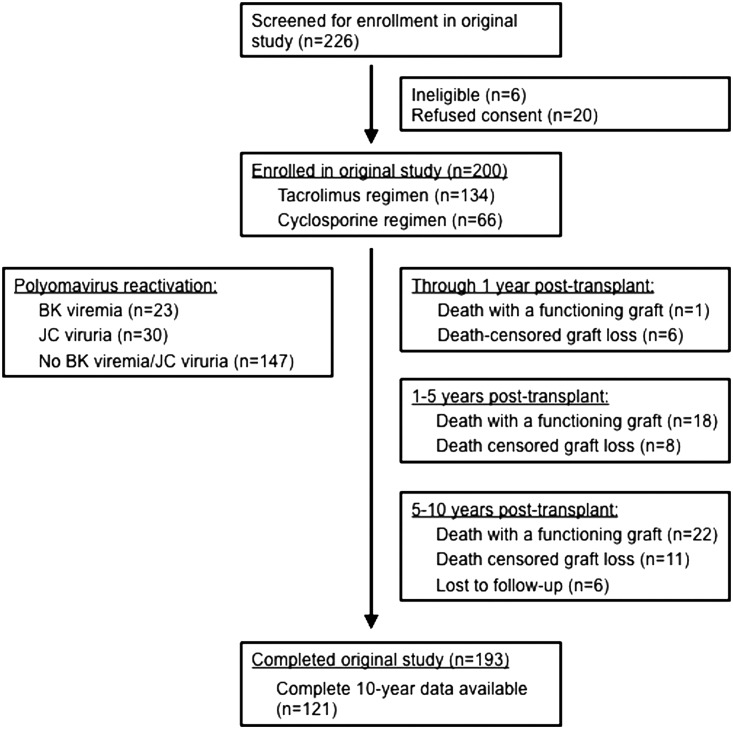
Of the 200 subjects enrolled in the original study between December of 2000 and October of 2002, there were 193 subjects with available follow-up data that were included in the retrospective cohort analysis. Of these, 121 (63%) remained alive with functioning allografts and had available data for the complete 10-year follow-up period (Figure 1). BK viremia resolved in 95% of subjects after a pre-emptive reduction in immunosuppression. No subjects developed evidence of BKVAN on indication biopsies during the 10-year follow-up period. Beyond year 1 post-transplant, clinically driven BK testing was performed at the time of indication biopsies and did not detect episodes of BK viremia. JC viruria resolved spontaneously in 21% of subjects without intervention and none developed JC viremia, JC virus–associated nephropathy, or progressive multifocal leucoencephalopathy. The complete demographic and clinical characteristics of this cohort have been reported previously.9–11 Additional data included for this study are pretransplant diabetes (52 of 193 subjects [27%]) and hepatitis C virus (HCV) positive status (four of 193 subjects [2%]), as well as cytomegalovirus viremia (three of 193 subjects [2%]).
Figure 1.
Flow diagram showing the characteristics of the study cohort at 1, 5, and 10 years post-transplant. Subjects from the original randomized trial (n=200) had clinical data collected for up to 10 years post-transplant. Subjects who were alive with functioning grafts at the end of the original 1-year study (n=193) were included in this retrospective cohort analysis. Polyomavirus reactivation data on the left side of the figure reflects serial testing during year 1 post-transplant.
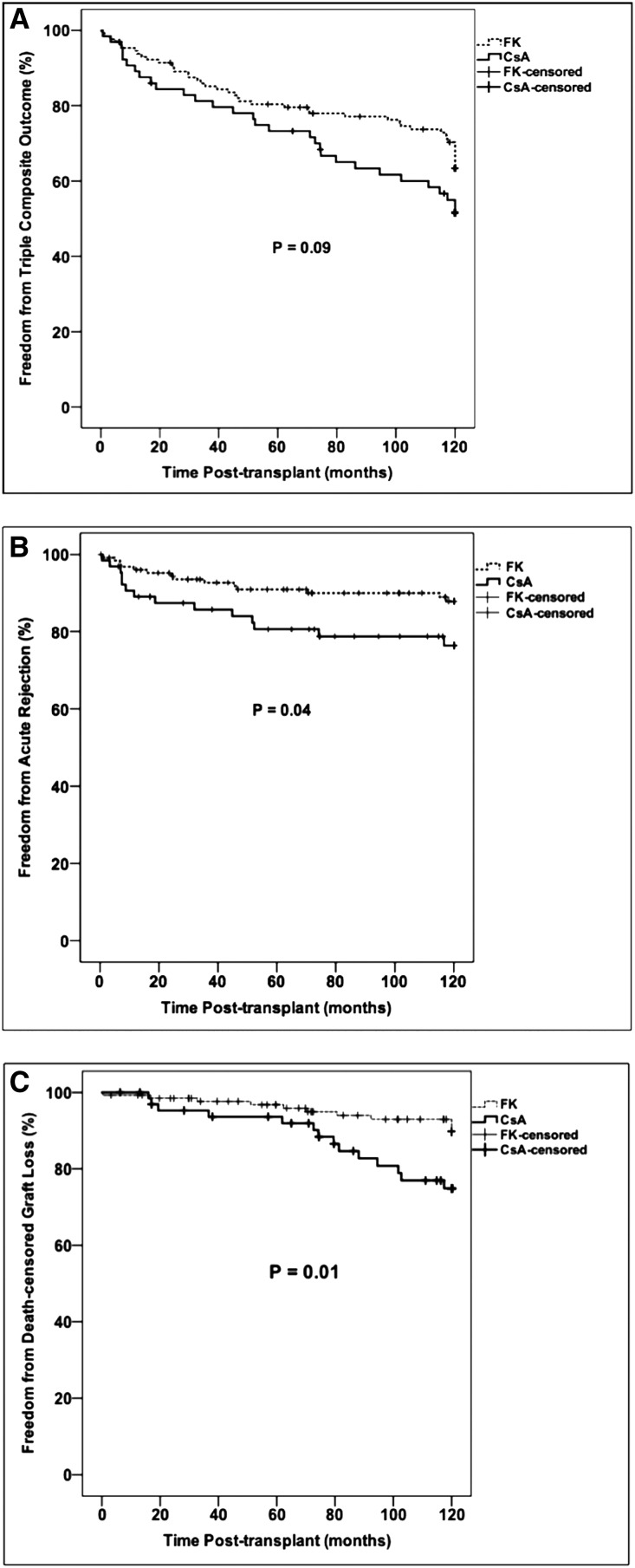
Overall, 75 of 193 subjects (39%) experienced the triple composite end point of acute rejection, death-censored graft loss, or death with a functioning graft during the 10-year follow-up period, including 27 subjects reaching the end point between 5 and 10 years post-transplant. Ten-year rejection-free survival, patient survival, and death-censored graft survival for the entire cohort were 85%, 79%, and 87%, respectively. There was a trend toward fewer subjects reaching the triple composite end point on a tacrolimus-based regimen (Figure 2, Table 1). There were no differences in rates of the triple composite end point according to polyomavirus reactivation status (Table 2).
Figure 2.
Kaplan–Meier analysis of 10-year outcomes according to calcineurin inhibitor. (A) Ten-year incidence of the triple composite outcome, (B) 10-year incidence of acute rejection, and (C) 10-year incidence of death-censored allograft loss. Comparisons between survival curves were made using the log-rank test. CsA, cyclosporin; FK, tacrolimus.
Table 1.
Ten-year outcomes by calcineurin inhibitor
| Outcome | Total (n=193) | FK (n=128) | CsA (n=65) | P Value |
|---|---|---|---|---|
| Triple composite outcome, n (%) | 75 (39) | 45 (35) | 30 (46) | 0.14 |
| Acute rejection, n (%) | 28 (15) | 14 (11) | 14 (22) | 0.05 |
| Early, <1 yr | 12 (6.2) | 5 (4) | 7 (11) | |
| Intermediate, 1–5 yr | 11 (5.7) | 6 (5) | 5 (8) | 0.73 |
| Late, >5 yr | 5 (3) | 3 (2) | 2 (3) | |
| Death with a functioning allograft, n (%) | 41 (21) | 29 (23) | 12 (18) | 0.50 |
| Unknown | 13 (7) | 7 (5) | 6 (9) | |
| Cardiovascular disease | 9 (5) | 6 (5) | 3 (5) | |
| Malignancy | 8 (4) | 7 (5) | 1 (1) | 0.44 |
| Infection | 7 (3) | 6 (5) | 1 (1) | |
| Other | 4 (2) | 3 (2) | 1 (1) | |
| Graft loss, n (%) | 66 (34) | 40 (31) | 26 (40) | 0.23 |
| Death-censored graft loss, n (%) | 25 (13) | 11 (9) | 14 (22) | 0.01 |
| Serum creatinine, mg/dla | 1.4±0.7 | 1.3±0.5 | 1.7±1.0 | <0.01 |
| eGFR, ml/min per 1.73 m2a | 61±22 | 65±19 | 50±24 | <0.001 |
Comparison of 10-year outcomes between subjects in the tacrolimus group (FK) and the cyclosporin group (CsA). The triple composite outcome is acute rejection, graft loss, or death with a functioning allograft. Early, intermediate, and late acute rejection subgroups include biopsy-proven episodes <1 year post-transplant, between 1 and 5 years post-transplant, and >5 years post-transplant, respectively. Numbers and percentages for acute rejection and cause of death subgroups are in reference to the total number of subjects in each column and may include rounding errors. Normally distributed continuous variables were compared using paired t test and are presented as mean±SD. Categoric variables were compared using Pearson chi-squared test or Fisher exact test where appropriate.
Data on serum creatinine and eGFR are from 121 subjects (87 from FK group and 34 from CsA group) who remained alive with functioning allografts and had available data at 10 years post-transplant.
Table 2.
Ten-year outcomes by polyomavirus status
| Outcome | BK Viremia (n=20) | JC Viruria (n=29) | No BK Viremia or JC Viruria (n=144) | P Valuea | BK Viremia or JC Viruria (n=49) | P Valueb |
|---|---|---|---|---|---|---|
| Triple composite outcome, n (%) | 8 (40) | 14 (48) | 53 (37) | 0.51 | 22 (45) | 0.32 |
| Acute rejection, n (%) | 4 (20) | 3 (10) | 21 (15) | 0.64 | 7 (14) | 0.96 |
| Early, <1 yr | 2 (10) | 0 (0) | 10 (7) | 2 (4) | ||
| Intermediate, 1–5 yr | 2 (10) | 2 (7) | 7 (5) | 0.49 | 4 (8) | 0.53 |
| Late, >5 yr | 0 (0) | 1 (3) | 4 (3) | 1 (2) | ||
| Death with a functioning allograft, n (%) | 6 (30) | 10 (35) | 25 (17) | 0.07 | 16 (33) | 0.02 |
| Unknown | 2 (10) | 4 (14) | 7 (5) | 6 (12) | ||
| Cardiovascular disease | 1 (5) | 2 (7) | 6 (4) | 3 (6) | ||
| Malignancy | 2 (10) | 3 (10) | 3 (2) | 0.72 | 5 (10) | 0.27 |
| Infection | 1 (5) | 1 (3) | 5 (3) | 2 (4) | ||
| Other | 0 (0) | 0 (0) | 4 (3) | 0 (0) | ||
| Graft loss, n (%) | 7 (35) | 13 (45) | 46 (32) | 0.41 | 20 (41) | 0.26 |
| Death-censored graft loss, n (%) | 1 (5) | 3 (10) | 21 (15) | 0.44 | 4 (8) | 0.25 |
| Serum creatinine, mg/dlc | 1.3±0.6 | 1.2±0.4 | 1.4±0.8 | 0.40 | 1.2±0.5 | 0.19 |
| eGFR, ml/min per 1.73 m2c | 59±20 | 66±24 | 60±22 | 0.56 | 63±22 | 0.46 |
Comparison of 10-year outcomes between polyomavirus status subgroups: BK viremia (resulting in elimination of antimetabolite +/− reduction in calcineurin inhibitor), JC viruria (no specific intervention), and subjects without BK viremia or JC viruria. The triple composite outcome is acute rejection, graft loss, or death with a functioning allograft. Early, intermediate, and late acute rejection subgroups include biopsy-proven episodes <1 year post-transplant, between 1 and 5 years post-transplant, and >5 years post-transplant, respectively. Numbers and percentages for acute rejection and cause of death subgroups are in reference to the total number of subjects in each column and may include rounding errors. Normally distributed continuous variables are presented as mean±SD.
Comparison of BK viremia, JC viruria, and no BK viremia/JC viruria groups using Pearson chi-squared test for categoric variables and ANOVA for continuous variables.
Comparison of subjects with BK viremia or JC viruria versus subjects without BK viremia or JC viruria using Pearson chi-squared test or Fisher exact test for categoric variables and paired t test for continuous variables.
Data on serum creatinine and eGFR are from 121 subjects (91 from no BK viremia or JC viruria group, 12 from BK viremia group, 18 from JC viruria group) who remained alive with functioning allografts and had available data at 10 years post-transplant.
There was less acute rejection by 10 years post-transplant in the tacrolimus group compared with the cyclosporin group, consistent with our previous findings at 5 years (Table 1). Using Kaplan–Meier analysis, the 10-year incidence of acute rejection was significantly lower in the tacrolimus group versus the cyclosporin group, which was established within the first 2 years post-transplant and remained consistent throughout the follow-up period (Figure 2). Univariable Cox models confirmed that a cyclosporin-based regimen was associated with a more than two-fold greater risk of acute rejection over 10 years (Table 3), although this association was mildly attenuated in multivariable analysis. As in our previous reports, there were no differences in acute rejection rates according to polyomavirus status, including the BK viremia group in whom a reduction in immunosuppression occurred during year 1 post-transplant (Table 2).
Table 3.
Univariable and multivariable Cox proportional hazards modeling of 10-year outcomes in the retrospective cohort analysis (n=193)
| Parameter | Univariable HR (95% CI) | P Value | Multivariable HR (95% CI) | P Value |
|---|---|---|---|---|
| Model A | ||||
| Cyclosporin-based regimen (tacrolimus as reference category) | 2.13 (1.01 to 4.46) | <0.05 | 1.98 (0.93 to 4.24) | 0.08 |
| Polyomavirus status (no BK/JC reactivation as reference category) | 0.96 (0.41 to 2.25) | 0.92 | ||
| Age at transplant, yr | 0.94 (0.92 to 0.97) | <0.001 | 0.94 (0.91 to 0.97) | <0.001 |
| HLA mismatches, number | 1.07 (0.88 to 1.33) | 0.56 | ||
| Black race (nonblack race as reference category) | 2.74 (1.20 to 6.25) | <0.05 | 1.87 (0.78 to 4.50) | 0.16 |
| Deceased donor type (living donor as reference category) | 1.99 (0.88 to 4.52) | 0.10 | 2.30 (0.96 to 5.51) | 0.06 |
| Men (women as reference category) | 1.14 (0.54 to 2.44) | 0.73 | ||
| Pretransplant diabetes (no diabetes as reference category) | 0.46 (0.16 to 1.31) | 0.15 | ||
| HCV positive (negative as reference category) | 4.08 (0.97 to 17.23) | 0.06 | 3.78 (0.83 to 17.14) | 0.09 |
| CMV viremia (no viremia as reference category) | 0.05 (0 to 10,166) | 0.63 | ||
| Model B | ||||
| Cyclosporin-based regimen (tacrolimus as reference category) | 0.88 (0.45 to 1.73) | 0.72 | ||
| Polyomavirus status (no BK/JC reactivation as reference category) | 1.84 (0.98 to 3.45) | 0.06 | 2.22 (1.10 to 4.49) | <0.05 |
| Age at transplant (yr) | 1.05 (1.02 to 1.08) | <0.001 | 1.04 (1.01 to 1.07) | <0.01 |
| HLA mismatches (number) | 1.21 (1.00 to 1.46) | <0.05 | 1.17 (0.96 to 1.44) | 0.13 |
| Black race (nonblack race as reference category) | 2.97 (1.51 to 5.82) | <0.01 | 3.18 (1.45 to 6.93) | <0.01 |
| Deceased donor type (living donor as reference category) | 2.48 (1.22 to 5.06) | 0.01 | 1.17 (0.96 to 1.44) | 0.07 |
| Men (women as reference category) | 0.94 (0.50 to 1.77) | 0.84 | ||
| Pretransplant diabetes (no diabetes as reference category) | 3.09 (1.67 to 5.69) | <0.001 | 2.35 (1.22 to 4.50) | 0.01 |
| HCV positive (negative as reference category) | 1.46 (0.20 to 10.62) | 0.71 | ||
| CMV viremia (no viremia as reference category) | 0.05 (0 to 791) | 0.54 | ||
| Model C | ||||
| Cyclosporin-based regimen (tacrolimus as reference category) | 2.66 (1.21 to 5.86) | <0.05 | 2.58 (1.15 to 5.80) | <0.05 |
| Polyomavirus status (no BK/JC reactivation as reference category) | 0.55 (0.19 to 1.60) | 0.27 | ||
| Age at transplant, yr | 0.93 (0.90 to 0.96) | <0.001 | 0.94 (0.90 to 0.97) | <0.001 |
| HLA mismatches, number | 1.21 (1.00 to 1.46) | <0.05 | 1.14 (0.89 to 1.46) | 0.31 |
| Black race (nonblack race as reference category) | 2.25 (0.90 to 5.64) | 0.09 | 1.15 (0.43 to 3.13) | 0.78 |
| Deceased donor type (living donor as reference category) | 1.19 (0.53 to 2.65) | 0.67 | ||
| Men (women as reference category) | 0.64 (0.27 to 1.53) | 0.31 | ||
| Pretransplant diabetes (no diabetes as reference category) | 0.26 (0.06 to 1.08) | 0.06 | 0.38 (0.08 to 1.68) | 0.20 |
| HCV positive (negative as reference category) | 2.43 (0.33 to 17.97) | 0.39 | ||
| CMV viremia (no viremia as reference category) | 0.05 (0 to 791) | 0.54 |
Univariable and multivariable Cox proportional hazards models were constructed for outcomes that were significant at P<0.05 level by Kaplan–Meier analysis: Model A, 10-year incidence of acute rejection; model B, 10-year incidence of death with a functioning allograft; and model C, 10-year incidence of death-censored graft loss. All multivariable models used forced entry of covariates that were significant at P<0.10 by univariable analysis. HR, hazard ratio; 95% CI, 95% confidence interval; CMV, cytomegalovirus.
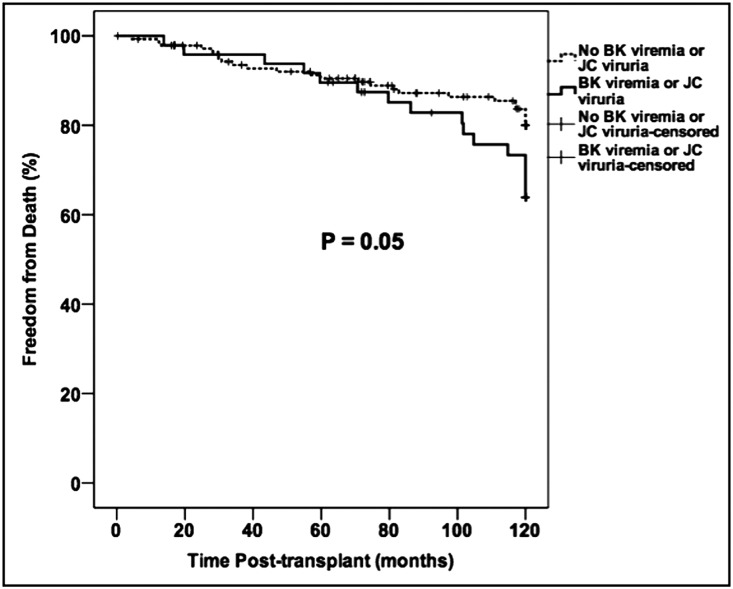
There were 19 deaths reported through 5 years post-transplant, with an additional 22 deaths occurring between 5 and 10 years post-transplant. The leading causes of late mortality in this cohort mirrored those reported at 5 years, namely malignancy, cardiovascular disease, and infections (Tables 1 and 2). There was no difference in overall mortality or cause of death according to the type of calcineurin inhibitor used (Table 1). However, we observed increased 10-year mortality in subjects who had polyomavirus reactivation compared with those who had no reactivation, although there were no differences in overall mortality or cause of death between BK viremia and JC viruria subgroups (Table 2). Using Kaplan–Meier analysis there was a trend toward an increased 10-year mortality in the polyomavirus reactivation group, with diverging patient survival curves after 5 years post-transplant (Figure 3). Univariable and multivariable Cox models confirmed that polyomavirus reactivation was associated with a more than two-fold greater risk of death with a functioning allograft by 10 years post-transplant (Table 3). This association between polyomavirus reactivation and mortality was mildly attenuated in a sensitivity analysis where polyomavirus status was considered as a time-varying covariate (hazard ratio, 1.02; 95% confidence interval, 1.00 to 1.03; P=0.07).
Figure 3.
Kaplan–Meier analysis of the 10-year incidence of death with a functioning graft according to polyomavirus activation status. Comparisons between survival curves were made using the log-rank test.
Death-censored allograft loss was significantly lower in the tacrolimus group compared with the cyclosporin group (Figure 2, Table 1). Univariable and multivariable Cox models showed that a cyclosporin-based regimen was associated with a nearly 2.5-fold increase in death-censored allograft loss by 10 years post-transplant (Table 3). There was no difference in graft loss according to polyomavirus status, and in fact the fewest in number and lowest percentage of graft losses were both in the BK viremia group who underwent a pre-emptive reduction in immunosuppression during year 1 post-transplant (Table 2).
Allograft function was significantly higher at 10 years in the tacrolimus group, but there was no difference in eGFR according to polyomavirus status (Tables 1 and 2), consistent with our previous findings at 1 and 5 years post-transplant.
Nested Case-Control Analysis
Of the 120 possible post-transplant samples (1, 4, and 9 months post-transplant in each of 40 subjects), 117 (98%) were available for testing. Pretransplant samples were available for testing in 36 of 40 subjects (90%). Antibodies to FN/Col-IV were present before transplantation in 11 of 36 (31%) with available pretransplant samples, but all of these subjects tested negative by 1 month post-transplant, such that we considered all post-transplant antibodies to FN/Col-IV to be de novo events (Table 4). Of note, we detected antibodies to FN/Col-IV in three of 12 subjects (25%) with native stage 3 CKD and none of 12 living kidney donor subjects (0%) without CKD that were assessed as additional controls, suggesting that antibodies to FN/Col-IV are present in moderate to severe native CKD in addition to transplant CKD. Demographic and clinical characteristics for the subgroups with and without antibodies to FN/Col-IV are presented in Table 4.
Table 4.
Demographic data for subgroups with antibodies to self-antigens data
| Characteristic | Total (n=40) | FN/Col-IV Abs+ (n=16) | FN/Col-IV Abs− (n=24) | P Value |
|---|---|---|---|---|
| Polyomavirus reactivation, n (%) | 31 (78) | 15 (94) | 16 (67) | 0.04 |
| BK viremia, n (%) | 20 (50) | 8 (50) | 12 (50) | |
| JC viruria, n (%) | 11 (28) | 7 (44) | 4 (17) | 0.09 |
| No BK viremia or JC viruria, n (%) | 9 (23) | 1 (6) | 8 (33) | |
| Age, yr | 47±14 | 47±16 | 48±13 | 0.89 |
| Men, n (%) | 25 (63) | 11 (69) | 14 (58) | 0.51 |
| Black race, n (%) | 6 (15) | 1 (6) | 5 (21) | 0.21 |
| Initial calcineurin inhibitor (tacrolimus/cyclosporin) | 28/12 | 10/6 | 18/6 | 0.40 |
| Initial antimetabolite (MMF/azathioprine) | 17/23 | 10/6 | 7/17 | 0.04 |
| Deceased donor, n (%) | 26 (65) | 11 (69) | 15 (63) | 0.69 |
| Total HLA mismatches, n | 2.8±1.7 | 2.6±1.4 | 3.0±1.9 | 0.47 |
| Pretransplant FN/Col-IV Abs+, n (%)a | 11 of 36 (31) | 3 of 16 (19) | 8 of 20 (40) | 0.17 |
Comparison of baseline demographic and clinical data between subjects who developed antibodies to self-antigens FN and Col-IV (FN/Col-IV Abs+) and those remaining FN/Col-IV antibody negative (FN/Col-IV Abs−) during year 1 post-transplant. Polyomavirus reactivation was considered as detection of BK viremia or JC viruria, because they were mutually exclusive events in this subgroup analysis. Normally distributed continuous variables were compared using paired t test and are presented as mean±SD. Categoric variables were compared using Pearson chi-squared test or Fisher exact test where appropriate. Percentages listed for each category are in reference to the total number of cases within each column. Comparison of FN/Col-IV Abs+ status across subgroups with polyomavirus reactivation (15 of 31 [48%]), no reactivation (one of 9 [11%]), native stage 3 CKD (three of 12 [25%]), and healthy controls (none of 12 [0%]) was significant at P<0.01 using Pearson chi-squared test.
Pretransplant samples were available in 36 out of 40 subjects. Of the three subjects who tested positive for antibodies to FN and Col-IV both pre- and post-transplant, all initially tested negative at 1 month post-transplant so we considered all post-transplant antibodies to be de novo events.
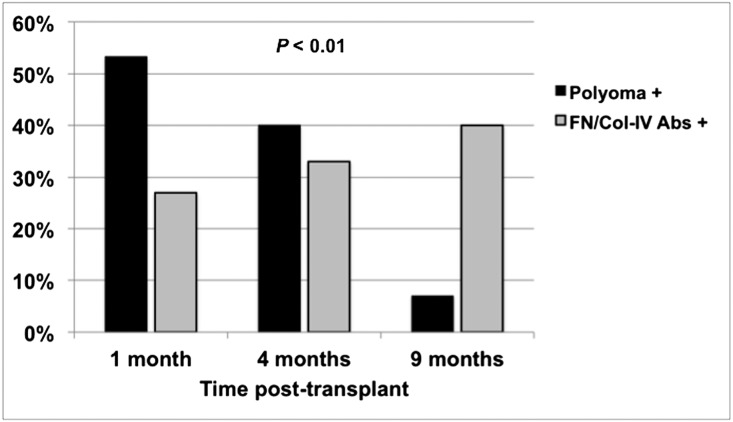
During the first post-transplant year, antibodies to FN/Col-IV developed in 16 of 40 subjects (40%). Polyomavirus status was associated with de novo development of antibodies to FN/Col-IV, with 15 of 31 subjects (48%) with polyomavirus reactivation developing antibodies versus only one of nine BK/JC negative subjects (11%) (P=0.04, Table 4). We observed no associations between intensity (peak viral load) and duration of viral reactivation with the development of antibodies to FN/Col-IV (data not shown), but noted that antibodies developed after polyomavirus reactivation in 12 of 15 subjects (80%) in whom both events occurred (Figure 4). Antibodies to FN/Col-IV developed after BK viremia (treated with immunosuppression reduction) and after JC viruria (no specific intervention), which were mutually exclusive events in our cohort,11 suggesting that the appearance of these antibodies may be associated with polyomavirus reactivation itself and/or a potential untoward effect of pre-emptive immunosuppressive reduction. Additionally, we observed significantly more subjects developing antibodies to FN/Col-IV on a mycophenolate mofetil (MMF) versus azathioprine (AZA)-based regimen, but no other clinical or demographic factors were associated with FN/Col-IV antibody status (Table 4).
Figure 4.
Histograms comparing time to initial polyomavirus reactivation (Polyoma+) and time to initial detection of antibodies to FN/Col-IV (FN/Col-IV Abs+) during year 1 post-transplant within each subject who experienced both end points (n=15). The median (minimum, maximum) time to polyomavirus reactivation (BK viremia or JC viruria) was significantly shorter (1.0 [0.2, 4.5] months) compared with the median time to detection of FN/Col-IV antibodies (4.0 [1.0, 9.0] months) (P<0.01, by Mann–Whitney U test).
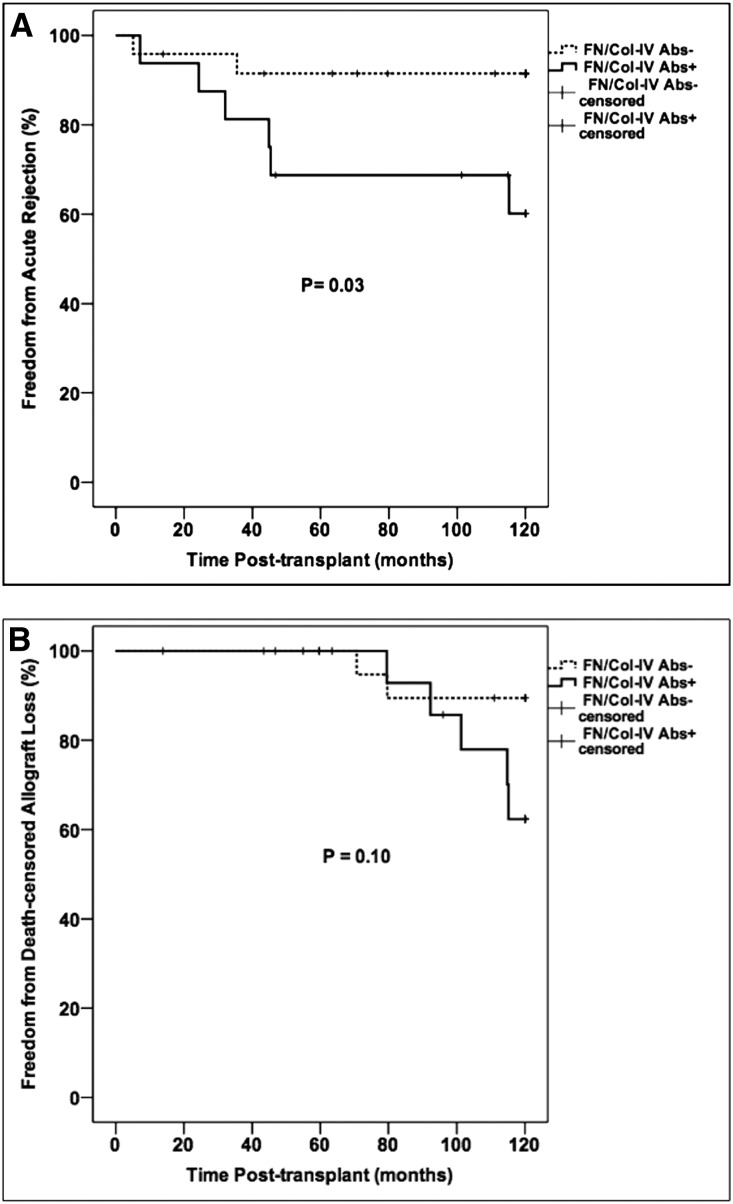
We then examined the effect of early FN/Col-IV antibody status on long-term outcomes. Overall, 15 of 40 subjects (38%) reached the triple composite end point during the 10-year follow-up period, mirroring rates in the larger cohort. There was no difference in the triple composite end point by FN/Col-IV antibody status (Table 5). Combined 10-year acute rejection rates were similar to those of the larger cohort, but were significantly higher in subjects who tested positive for antibodies to FN/Col-IV during year 1 post-transplant compared with those who tested negative (Figure 5, Table 5). Most acute rejection episodes were diagnosed between 1 and 5 years post-transplant and were classified as T cell–mediated rejection with no concomitant antibody-mediated rejection. Subjects with antibodies to FN/Col-IV showed a nonsignificant trend for increased proteinuria (peak urine protein-to-creatinine ratio >0.5), which we used as a surrogate for transplant glomerulopathy and chronic allograft injury in the absence of late biopsies (Table 5).
Table 5.
Ten-year outcomes by antibodies to self-antigens status
| Outcome | FN/Col-IV Abs+ (n=16) | FN/Col-IV Abs− (n=24) | P Value |
|---|---|---|---|
| Triple composite outcome, n (%) | 8 (50) | 7 (29) | 0.18 |
| Acute rejection, n (%) | 6 (38) | 2 (8) | 0.02 |
| Death with a functioning allograft, n (%) | 1 (6) | 5 (21) | 0.21 |
| Graft loss, n (%) | 6 (38) | 7 (29) | 0.58 |
| Death-censored graft loss, n (%) | 5 (31) | 2 (8) | 0.06 |
| Serum creatinine, mg/dla | 1.5±0.3 | 1.4±0.7 | 0.86 |
| eGFR, ml/min per 1.73m2a | 51±15 | 58±21 | 0.37 |
| UPC>0.5, n (%)b | 7 of 15 (47) | 5 of 19 (26) | 0.22 |
Comparison of 10-year outcomes between subjects who developed antibodies to self-antigens FN and Col-IV (FN/Col-IV Abs+) and those who remained FN/Col-IV antibody negative (FN/Col-IV Abs−) during year-1 post-transplant. The triple composite outcome is acute rejection, graft loss, or death with a functioning allograft. Of the six acute rejection episodes in the FN/Col-IV Abs+ group, one was early (<1 year post-transplant), four were intermediate (between 1 and 5 years post-transplant) and one was late (>5 years post-transplant). Of the two acute rejection episodes in the FN/Col-IV Abs− group, one was early and one was intermediate. Normally distributed continuous variables were compared using paired t test and are presented as mean±SD. Categoric variables were compared using Pearson chi-squared test or Fisher exact test where appropriate. UPC, urine protein-to-creatinine ratio.
Data on serum creatinine and eGFR are from 25 subjects (nine from FN/Col-IV Abs+ group and 16 from FN/Col-IV Abs− group) who remained alive with functioning allografts and had available data at 10 years post-transplant.
Data on UPC represent the maximum documented ratio in the medical record up to 10 years post-transplant in the 34 subjects in whom at least one data point was available.
Figure 5.
Kaplan–Meier analysis of (A) 10-year incidence of acute rejection and (B) 10-year incidence of death-censored allograft loss according to FN/Col-IV antibody status. Comparisons between survival curves were made using the log-rank test.
HLA antibody analysis was only performed at the time of indication biopsies. Of the 40 subjects in the nested case-control analysis, ten were evaluated for antibodies against HLA at a median (minimum, maximum) of 96 (36, 120) months post-transplant. Of these, four of ten (40%) tested positive for HLA antibodies between 96 and 120 months post-transplant, including one with a class 2 DSA, one with a class 1 DSA, and two with non-DSA antibodies against HLA. Of these four subjects, one had polyomavirus reactivation, one had antibodies to FN/Col-IV, and two had both polyomavirus reactivation and antibodies to FN/Col-IV during year 1 post-transplant.
Univariable and multivariable Cox models showed that FN/Col-IV antibody status was associated with a nearly six-fold increase in acute rejection by 10 years post-transplant (Table 6). We included age at transplant, race, and calcineurin inhibitor in the multivariable analysis regardless of their performance by univariable analysis because they were significantly associated with acute rejection in the larger cohort. We also performed a sensitivity analysis where FN/Col-IV antibody status was modeled as a time-varying covariate, which attenuated the association between FN/Col-IV antibody status and acute rejection (hazard ratio, 1.06; 95% confidence interval, 0.99 to 1.13; P=0.08). We observed no other significant associations between FN/Col-IV antibody status and long-term outcomes (Table 5).
Table 6.
Univariable and multivariable Cox proportional hazards modeling of 10-year incidence of acute rejection in the nested case-control analysis (n=40)
| Parameter | Univariable HR (95% CI) | P Value | Multivariable HR (95% CI) | P Value |
|---|---|---|---|---|
| FN/Col-IV antibody positive (negative as reference category) | 4.81 (0.97 to 23.89) | 0.05 | 5.79 (1.04 to 32.33) | <0.05 |
| Polyomavirus status (no BK/JC reactivation as reference category) | 0.95 (0.19 to 4.69) | 0.95 | ||
| Cyclosporin-based regimen (tacrolimus as reference category) | 0.74 (0.15 to 3.69) | 0.72 | 0.68 (0.13 to 3.58) | 0.65 |
| Age at transplant, yr | 0.97 (0.92 to 1.02) | 0.22 | 0.97 (0.92 to 1.02) | 0.21 |
| HLA mismatches, number | 0.86 (0.56 to 1.33) | 0.49 | ||
| Black race (nonblack race as reference category) | 0.95 (0.12 to 7.71) | 0.96 | 1.85 (0.19 to 17.66) | 0.60 |
| Deceased donor type (living donor as reference category) | 1.94 (0.39 to 9.63) | 0.42 | ||
| Men (women as reference category) | 0.47 (0.09 to 2.33) | 0.47 |
Univariable and multivariable Cox proportional hazards models were constructed for acute rejection because it was the only outcome that was significant at P<0.05 level by Kaplan–Meier analysis. The final multivariable model used forced entry of FN/Col-IV antibody status, calcineurin inhibitor, age at transplant, and race regardless of their performance in univariable analysis, because they were covariates of interest in the larger cohort analysis. HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
This represents one of the largest studies to simultaneously evaluate 10-year outcomes in three important areas of kidney transplantation: calcineurin inhibitor-based immunosuppression, polyomavirus reactivation, and immune responses to kidney-specific self-antigens. There are four major findings of this study: (1) subjects receiving a tacrolimus- versus cyclosporin-based regimen had lower rates of acute rejection and death-censored graft loss with better kidney function at 10 years; (2) reduction of immunosuppression to control BK viremia was both safe and effective, producing equivalent 10-year outcomes to subjects without BK viremia; (3) polyomavirus reactivation was associated with early de novo antibodies to kidney-specific self-antigens FN and Col-IV; and (4) early immune responses to FN and Col-IV were associated with an increased risk for acute rejection.
With respect to the choice of calcineurin inhibitor, our findings are in agreement with the multicenter Symphony study, which compared four immunosuppressive regimens among 1645 incident kidney transplant recipients including cyclosporin-, tacrolimus-, and sirolimus-based regimens with a nondepleting induction strategy.12 The authors demonstrated that a low-dose tacrolimus-based regimen was associated with better eGFR and lower rates of acute rejection and allograft loss at 1 and 3 years post-transplant compared with other regimens.12,13 Although we had fewer subjects in our single-center study and used a slightly higher target trough for tacrolimus, our 10-year follow-up data extends and agrees with their conclusions. Other studies directly comparing tacrolimus- and cyclosporin-based regimens have showed no differences in patient and graft survival but were limited to 5 years or less of clinical follow-up compared with our study.14–17 Our findings add to these data supporting the long-term superiority of a tacrolimus-based maintenance regimen in kidney transplantation.
Secondly, we analyzed the long-term effect of our approach to BK reactivation. Immediate and permanent discontinuation of the antimetabolite in response to BK viremia prevented all cases of biopsy-proven BKVAN with no increase in acute rejection, death, or death-censored graft loss for up to 10 years post-transplant compared with subjects without BK viremia. These results are in agreement with other studies of pre-emptive immunosuppressive reduction that were limited to follow-up periods of <3 years.18–22 Although we observed a trend toward increased mortality in subjects with BK viremia compared with those without, this is likely not solely attributable to immunosuppressive reduction because we detected similar mortality rates in JC viruric subjects who had no pre-emptive reduction in immunosuppression. The long-term success of our approach endorses immunosuppressive reduction as the primary intervention for preventing the progression of BK viremia to BKVAN.
Thirdly, our findings in the nested case-control analysis augment the growing body of literature supporting the importance of immune responses to self-antigens in determining long-term graft outcomes.23–27 However, the mechanisms involved in developing these immune responses remain unclear. It is possible that early inflammatory events, such as viral reactivation, expose cryptic epitopes on self-antigens that lead to anti–self-immune responses. A similar mechanism has been suggested in lung and kidney transplantation,28,29 although a specific role for polyomaviruses in this mechanism has not been described. Although we were unable to explore similar mechanistic studies, we sought to identify temporal associations between polyomavirus reactivation and the development of antibodies to FN/Col-IV, similar to the association between BK viremia and DSA in Sawinski et al.8 Their cohort was much larger but the clinical characteristics were similar, namely the use of anti-thymocyte globulin induction and steroid-based maintenance.8 Importantly, our cohorts had similar rates of BK viremia during year 1 post-transplant and a similar approach to BK management. Consistent with their findings in de novo DSA, we observed polyomavirus reactivation before the appearance of de novo antibodies to FN/Col-IV in most subjects with BK viremia or JC viruria. This suggests at least a temporal relationship exists between polyomavirus reactivation and immune responses to self-antigens that could be due to pre-emptive immunosuppressive reduction and/or viral reactivation itself. Future prospective studies should attempt to elucidate the mechanisms linking viral reactivation and other inflammatory events to the development of antibodies to self-antigens.
Lastly, to our knowledge this is the first study to demonstrate an association between early de novo antibodies to kidney-specific self-antigens and late acute rejection, an important contributor to allograft loss.30 Giral et al. showed that “presensitization” with antibodies to the angiotensin II type 1 receptor was associated with early acute rejection within 4 months post-transplant.26 We have recently demonstrated an association between antibodies to FN/Col-IV at 4–5 years post-transplant and biopsy-proven transplant glomerulopathy, a form of chronic allograft injury.23 Our current findings add to these data by demonstrating an important link between immune responses to self-antigens during year 1 post-transplant and an increased risk for late acute rejection. Collectively, these data suggest that immune responses to self-antigens play a major role throughout the life of the allograft, similar to immune responses to mismatched donor HLA antigens.31,32 Importantly, previous studies by our group and others reported no association between the development of antibodies to mismatched HLA antigens (either DSA or non-DSA) and the development of antibodies to self-antigens, suggesting they each develop and act via distinct mechanisms and represent complementary risk factors for transplant outcomes.23,26
Strengths of our study include our ability to describe 10-year outcomes using a relevant modern immunosuppressive regimen. Similar long-term studies often include immunosuppressive approaches that may not represent the current standard-of-care.33 In addition, we were able to collect 10-year follow-up data in 97% of subjects from our previous study10 for the intention-to-treat cohort analysis, and were able to analyze 98% of potential samples in our nested case-control analysis. Our single-center design removed any heterogeneity in immunosuppressive approach or BK viral management that is common across many transplant centers. Finally, the BK viremia and JC viruria subgroups were managed with reduction of immunosuppression or no intervention, respectively, allowing us to distinguish between the effects of immunosuppressive reduction and that of viral reactivation alone on the development of antibodies to FN/Col-IV.
Despite these strengths, our study has limitations common to those using available legacy samples to conduct post hoc translational studies. These samples were collected >10 years ago in some subjects, so some antibodies to self-antigens may not have been detected using ELISA methods, especially antibodies circulating in low titers. We attempted to circumvent this possibility by choosing previously unthawed aliquots. We did not perform early surveillance biopsies that would have allowed us to assess the binding of antibodies to FN/Col-IV within the allograft in addition to the circulation. We also performed few late indication biopsies because our cohort had excellent long-term allograft function and relatively few late acute rejection episodes, which did not allow us to correlate antibodies to FN/Col-IV with chronic allograft injury lesions. However, we addressed this by collecting longitudinal data on proteinuria that may be a surrogate for transplant glomerulopathy.23,34 Finally, we cannot exclude the possibility of residual confounding variables from unmeasured factors or uncollected data.
In conclusion, we demonstrated excellent 10-year patient and graft outcomes with both a tacrolimus-based immunosuppressive regimen and a pre-emptive immunosuppression reduction strategy for BK viremia. However, we also detected a temporal relationship between early polyomavirus reactivation and the development of immune responses to kidney-specific self-antigens, which were associated with an increased risk for late acute rejection. Given our increasing knowledge of the importance of immune responses to self-antigens in transplantation, further studies are needed to better understand the interactions between viral immunity and self-immunity that lead to inferior transplant outcomes. Improved understanding of these immune responses will allow for improved treatment strategies that balance the risk of infection, rejection, and sensitization.
Concise Methods
Study Design
Retrospective Cohort Analysis
This was a retrospective review of 10-year outcomes in an open-label, single-center, prospective trial of de novo kidney transplant recipients that were randomized before transplantation in 2:1 block fashion to receive either a tacrolimus- or cyclosporin-based maintenance immunosuppressive regimen.10 Clinical data were prospectively collected on each subject and entered into a research database at least annually for up to 10 years post-transplant, or until a subject experienced death with a functioning graft, death-censored graft loss, or was lost to follow-up. The original study was designed to determine the effect of immunosuppressive regimen on the incidence of BK infection, and to examine the safety and efficacy of a pre-emptive immunosuppression reduction strategy to prevent progression of BK viremia to BKVAN. Subsequent studies in this cohort have described JC reactivation and 1- and 5-year outcomes.9–11 Inclusion and exclusion criteria have been described previously.10 For this study, we also collected data on pretransplant diabetes and HCV status.
Nested Case-Control Analysis
Using available legacy samples and data from the original cohort, we also conducted a nested case-control study to determine associations between polyomavirus reactivation and antibodies to kidney-restricted self-antigens FN and Col-IV during year 1 post-transplant, and assessed their influence on long-term outcomes. All subjects from the original study who developed BK viremia during year 1 post-transplant and had residual unthawed plasma aliquots were included (n=20). These subjects were age- and sex-matched to 20 subjects without BK viruria or viremia that were selected by a research nurse who was blinded to the overall design and purpose of the case-control study, including 11 subjects with JC viruria and nine subjects that were negative for BK and JC viruria/viremia during year 1 post-transplant. We included subjects with and without JC viruria because we previously found that the presence of JC viruria was mutually exclusive for the presence of BK viremia and was associated with favorable 1- and 5-year outcomes that were similar to JC-negative subjects.11 Unlike BK viremic subjects, there was no immunosuppressive reduction in response to JC viruria, which allowed us to explore the effect of polyomavirus reactivation on antibodies to self-antigens in a subgroup without the added confounder of immunosuppressive reduction. Plasma samples were obtained from subjects with native stage 3 CKD (n=12) and approved living kidney donors without kidney disease (n=12) that were used as additional controls. The Human Subjects Protection Office at Washington University approved the study, and all recruitment and consent procedures involving human subjects adhered to guidelines set forth in the Declaration of Helsinki. All clinical and research activities reported here adhered to the principles of the Declaration of Istanbul.
Immunosuppression and Monitoring
All patients except 6-antigen HLA-matched living related allograft recipients were induced with rabbit anti-thymocyte globulin with maintenance tacrolimus or cyclosporin, mycophenolate mofetil or azathioprine, and steroids as previously described.10 No surveillance or protocol biopsies were performed. Subjects were assessed for the presence of DSA at the time of indication biopsies using single antigen bead assays (One Lambda, Canoga Park, CA). At our center, a DSA result was considered positive if the ratio of mean fluorescence intensity in the sample compared with that of a positive control was 0.2 or higher or if an absolute mean fluorescence intensity value was >2000.35 Donor kidneys were accepted with a negative virtual crossmatch and a negative cytotoxicity crossmatch before surgery.
Polyomavirus Monitoring and Management
Plasma and urine samples for BK and JC virus PCR were collected pretransplant, weekly for 16 weeks, and at months 5, 6, 9, and 12. Samples were immediately processed into aliquots and stored frozen at −80°C until ready for use. Details and detection thresholds of the PCR assays developed for BK and JC virus at our center have been described previously.10,11 BK viruria was defined by the presence of BK virus DNA in the urine. BK viremia was defined by detection of BK virus DNA in either whole blood or plasma. All subjects with a rise in creatinine who received indication biopsies were simultaneously assessed for BK viremia, if not already performed as part of the study monitoring protocol. JC viruria was defined by detection of JC virus DNA in the urine. No subjects had detectable JC virus DNA in whole blood or plasma, as previously described.11
Detection of BK viruria triggered a review of the immunosuppressive regimen to confirm that target doses and/or levels were being achieved in accordance with standard-of-care practices at our center. Progression of BK viruria to BK viremia (at any detectable level in whole blood or plasma) triggered immediate and permanent discontinuation of the antimetabolite (AZA or MMF). If BK viremia was sustained for >4 weeks despite withdrawal of the antimetabolite, the calcineurin inhibitor target trough levels were also reduced (tacrolimus 12-hour trough levels of 3–5 ng/ml and cyclosporin 12-hour trough levels of 100–200 ng/ml). Because the incidence of JC viruria was determined in a subsequent study and was mutually exclusive from BK viremia in all subjects, no specific intervention was performed for JC viruria.
Measurement of Antibodies to Kidney-Restricted Self-Antigens FN and Col-IV
Plasma aliquots were obtained from long-term storage at −80°C. We used plasma samples taken pretransplant and at 1, 4, and 9 months post-transplant. Samples were thawed at room temperature on the day the assays were performed. We tested samples for the presence of antibodies to FN and Col-IV using an ELISA developed in our laboratory and described previously.23,36 Briefly, we incubated plasma samples overnight at 4°C on 96-well microplates that were coated with the self-antigen of interest and blocked with 2% BSA. Plasma concentrations of antibodies to FN and Col-IV were determined in triplicate for each sample.
Commercially available anti-human FN and anti-human Col-IV antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were used as positive controls; pooled commercial sera (Atlanta Biologicals, Flowery Branch, GA) and plasma samples from healthy adult controls were used as negative controls for each plate to ensure uniformity across assays. Antibody concentrations were calculated by normalizing the optical density of samples to a curve of known standard concentrations. A sample was considered positive for FN/Col-IV antibodies if the value of each exceeded two SDs above the mean concentration of values obtained from healthy adult volunteers. Investigators performing the assays (M.E.S. and M.G.) were blinded to study group assignment and case-control status.
Exposures and Outcomes
For the retrospective cohort analysis, the primary exposure was the calcineurin inhibitor tacrolimus or cyclosporin. The primary end point was a triple composite outcome composed of acute rejection, death-censored graft loss, or death with a functioning graft by 10 years post-transplant. Secondary exposures included BK viremia or JC viruria at any time during year 1 post-transplant, cytomegalovirus reactivation, pretransplant diabetes, and HCV status, all modeled as categoric variables. Secondary outcomes included each component of the triple composite end point, serum creatinine, and eGFR (calculated by the Modification of Diet in Renal Disease [MDRD] equation)37 by 10 years post-transplant.
For the nested case-control analysis, the primary exposure was polyomavirus reactivation, defined a priori as either BK viremia or JC viruria at any time during year 1 post-transplant and modeled as a categoric variable. We chose this definition because BK viremia has been shown to be more relevant to long-term outcomes than BK viruria, and our previous studies showed no subjects in this cohort developed JC viremia. The primary end points were antibodies to FN/Col-IV status (positive or negative) during year 1 post-transplant and a triple composite outcome composed of acute rejection, death-censored graft loss, or death with a functioning graft by 10 years post-transplant, both modeled as categoric variables. Secondary exposures included age at transplant, sex, race (defined as black or nonblack), calcineurin inhibitor (tacrolimus or cyclosporin), antimetabolite (MMF or AZA), donor type (living or deceased), number of HLA mismatches, and pretransplant FN/Col-IV antibody status (positive or negative). For analysis of 10-year patient and graft outcomes, FN/Col-IV antibody status was also modeled as a secondary exposure. Secondary outcomes included each component of the triple composite end point, serum creatinine, and MDRD eGFR by 10 years post-transplant. Because few subjects in this cohort had late biopsies to evaluate for chronic allograft injury, we also studied proteinuria (defined as a peak post-transplant urine protein-to-creatinine ratio >0.5) as a surrogate for transplant glomerulopathy, which we previously associated with antibodies to FN/Col-IV.23
Statistical Analyses
Continuous variables were assessed for normality using the Kolmogorov–Smirnov test, and comparisons between groups were made using paired t test, Mann–Whitney U test, or one-way ANOVA as appropriate for the normality of the data and the number of comparator groups. Categoric variables were compared between groups using the Pearson chi-squared test or the Fisher exact test where appropriate. Survival distributions for the triple composite end point and each of its components were compared between groups using Kaplan–Meier methods and the log-rank test. For survival analyses with group differences at P<0.05 by the log-rank test, outcomes were also modeled using Cox proportional hazards regression. Any covariates that were associated with the outcome of interest by univariable analysis at P<0.10 or determined to be clinically relevant were entered into a multivariable Cox model. Visual inspection of log-log plots was used to confirm the proportionality of hazards assumption. Polyomavirus status was also modeled as a time-varying covariate in sensitivity analyses, because status changed throughout year 1 post-transplant in many subjects.
All variables were modeled with an intention-to-treat analysis except for serum creatinine and eGFR, which were modeled using only subjects with complete 10-year follow-up data. All statistical tests were two-tailed and α was set at 0.05 for all comparisons. All statistical analyses were performed using SPSS Statistics version 23.0 (IBM, Armonk, NY).
Disclosures
None.
Acknowledgments
This work was supported by the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and KL2 TR000450 from the National Center for Advancing Translational Sciences, and by K23 DK101690-01A1 and L40 DK099748 from the National Institute of Diabetes and Digestive and Kidney Diseases (to M.E.S.). This work was also supported by the Alan A. and Edith L. Wolff Endowment for Renal Diseases, the Eileen Brooks Transplant Nephrology Fund, and the Donald Roach Transplant Nephrology Fund (to D.C.B.).
This work was presented in abstract form on May 20, 2013 at the American Transplant Congress in Seattle, Washington and on July 29, 2014 at the World Transplant Congress in San Francisco, CA.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Stegall MD, Gaston RS, Cosio FG, Matas A: Through a glass darkly: Seeking clarity in preventing late kidney transplant failure. J Am Soc Nephrol 26: 20–29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, Grande J, Halloran P, Hunsicker L, Mannon R, Rush D, Matas AJ: Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation 90: 68–74, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Mannon RB, Matas AJ, Grande J, Leduc R, Connett J, Kasiske B, Cecka JM, Gaston RS, Cosio F, Gourishankar S, Halloran PF, Hunsicker L, Rush D; DeKAF Investigators : Inflammation in areas of tubular atrophy in kidney allograft biopsies: A potent predictor of allograft failure. Am J Transplant 10: 2066–2073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matas AJ, Leduc R, Rush D, Cecka JM, Connett J, Fieberg A, Halloran P, Hunsicker L, Cosio F, Grande J, Mannon R, Gourishankar S, Gaston R, Kasiske B: Histopathologic clusters differentiate subgroups within the nonspecific diagnoses of CAN or CR: Preliminary data from the DeKAF study. Am J Transplant 10: 315–323, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF: Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant 9: 2520–2531, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Hirsch HH, Randhawa P; AST Infectious Diseases Community of Practice : BK polyomavirus in solid organ transplantation. Am J Transplant 13[Suppl 4]: 179–188, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Sawinski D, Forde KA, Trofe-Clark J, Patel P, Olivera B, Goral S, Bloom RD: Persistent BK viremia does not increase intermediate-term graft loss but is associated with de novo donor-specific antibodies. J Am Soc Nephrol 26: 966–975, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardinger KL, Koch MJ, Bohl DJ, Storch GA, Brennan DC: BK-virus and the impact of pre-emptive immunosuppression reduction: 5-year results. Am J Transplant 10: 407–415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, Lockwood M, Torrence S, Schuessler R, Roby T, Gaudreault-Keener M, Storch GA: Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant 5: 582–594, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Cheng XS, Bohl DL, Storch GA, Ryschkewitsch C, Gaudreault-Keener M, Major EO, Randhawa P, Hardinger KL, Brennan DC: Inhibitory interactions between BK and JC virus among kidney transplant recipients. J Am Soc Nephrol 22: 825–831, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF; ELITE-Symphony Study : Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Ekberg H, Bernasconi C, Tedesco-Silva H, Vítko S, Hugo C, Demirbas A, Acevedo RR, Grinyó J, Frei U, Vanrenterghem Y, Daloze P, Halloran P: Calcineurin inhibitor minimization in the Symphony study: Observational results 3 years after transplantation. Am J Transplant 9: 1876–1885, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J: A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: Evidence for improved allograft survival at five years. Transplantation 73: 775–782, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Silva HT Jr, Yang HC, Meier-Kriesche HU, Croy R, Holman J, Fitzsimmons WE, First MR: Long-term follow-up of a phase III clinical trial comparing tacrolimus extended-release/MMF, tacrolimus/MMF, and cyclosporine/MMF in de novo kidney transplant recipients. Transplantation 97: 636–641, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opelz G, Döhler B; Collaborative Transplant Study : Influence of immunosuppressive regimens on graft survival and secondary outcomes after kidney transplantation. Transplantation 87: 795–802, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Kaplan B, Schold JD, Meier-Kriesche HU: Long-term graft survival with neoral and tacrolimus: A paired kidney analysis. J Am Soc Nephrol 14: 2980–2984, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Sood P, Senanayake S, Sujeet K, Medipalli R, Zhu YR, Johnson CP, Hariharan S: Management and outcome of BK viremia in renal transplant recipients: A prospective single-center study. Transplantation 94: 814–821, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Schaub S, Hirsch HH, Dickenmann M, Steiger J, Mihatsch MJ, Hopfer H, Mayr M: Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant 10: 2615–2623, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Saad ER, Bresnahan BA, Cohen EP, Lu N, Orentas RJ, Vasudev B, Hariharan S: Successful treatment of BK viremia using reduction in immunosuppression without antiviral therapy. Transplantation 85: 850–854, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Alméras C, Foulongne V, Garrigue V, Szwarc I, Vetromile F, Segondy M, Mourad G: Does reduction in immunosuppression in viremic patients prevent BK virus nephropathy in de novo renal transplant recipients? A prospective study. Transplantation 85: 1099–1104, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Ginevri F, Azzi A, Hirsch HH, Basso S, Fontana I, Cioni M, Bodaghi S, Salotti V, Rinieri A, Botti G, Perfumo F, Locatelli F, Comoli P: Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am J Transplant 7: 2727–2735, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Angaswamy N, Klein C, Tiriveedhi V, Gaut J, Anwar S, Rossi A, Phelan D, Wellen JR, Shenoy S, Chapman WC, Mohanakumar T: Immune responses to collagen-IV and fibronectin in renal transplant recipients with transplant glomerulopathy. Am J Transplant 14: 685–693, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Nath DS, Tiriveedhi V, Basha HI, Phelan D, Moazami N, Ewald GA, Mohanakumar T: A role for antibodies to human leukocyte antigens, collagen-V, and K-α1-Tubulin in antibody-mediated rejection and cardiac allograft vasculopathy. Transplantation 91: 1036–1043, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M, Steward N, Aloush A, Hachem R, Trulock E, Meyers B, Patterson GA, Mohanakumar T: Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant 30: 624–631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giral M, Foucher Y, Dufay A, Van Huyen JP, Renaudin K, Moreau A, Philippe A, Hegner B, Dechend R, Heidecke H, Brouard S, Cesbron A, Castagnet S, Devys A, Soulillou JP, Dragun D: Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant 13: 2567–2576, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Cardinal H, Dieudé M, Brassard N, Qi S, Patey N, Soulez M, Beillevaire D, Echeverry F, Daniel C, Durocher Y, Madore F, Hébert MJ: Antiperlecan antibodies are novel accelerators of immune-mediated vascular injury. Am J Transplant 13: 861–874, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Bharat A, Kuo E, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T: Respiratory virus-induced dysregulation of T-regulatory cells leads to chronic rejection. Ann Thorac Surg 90: 1637–1644, discussion 1644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heutinck KM, Yong S, Tonneijck L, van den Heuvel H, van der Weerd NC, van der Pant KA, Bemelman FJ, Claas FH, Ten Berge IJ: Virus-specific CD8(+) T-cells cross-reactive to donor-alloantigen are transiently present in the circulation of kidney transplant recipients infected with CMV and/or EBV. Am J Transplant 16: 1480–1491, 2016 [DOI] [PubMed] [Google Scholar]
- 30.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG: Identifying specific causes of kidney allograft loss. Am J Transplant 9: 527–535, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C: Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 21: 1398–1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiebe C, Gibson IW, Blydt-Hansen TD, Pochinco D, Birk PE, Ho J, Karpinski M, Goldberg A, Storsley L, Rush DN, Nickerson PW: Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant 15: 2921–2930, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, Moal MC, Mondragon-Ramirez GA, Kothari J, Polinsky MS, Meier-Kriesche HU, Munier S, Larsen CP: Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 374: 333–343, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Akalin E, Dinavahi R, Dikman S, de Boccardo G, Friedlander R, Schroppel B, Sehgal V, Bromberg JS, Heeger P, Murphy B: Transplant glomerulopathy may occur in the absence of donor-specific antibody and C4d staining. Clin J Am Soc Nephrol 2: 1261–1267, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Morris GP, Phelan DL, Jendrisak MD, Mohanakumar T: Virtual crossmatch by identification of donor-specific anti-human leukocyte antigen antibodies by solid-phase immunoassay: A 30-month analysis in living donor kidney transplantation. Hum Immunol 71: 268–273, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Hesemann LE, Subramanian V, Mohanakumar T, Dharnidharka VR: De novo development of antibodies to kidney-associated self-antigens angiotensin II receptor type I, collagen IV, and fibronectin occurs at early time points after kidney transplantation in children. Pediatr Transplant 19: 499–503, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]