Abstract
CKD associates with a 1.5- to 3.5-fold increased risk for cardiovascular disease. Both diseases are characterized by increased inflammation, and in patients with CKD, elevated C-reactive protein level predicts cardiovascular risk. In addition to systemic inflammation, local arterial inflammation, driven by monocyte-derived macrophages, predicts future cardiovascular events in the general population. We hypothesized that subjects with CKD have increased arterial and cellular inflammation, reflected by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography computed tomography (PET/CT) of the arterial wall and a migratory phenotype of monocytes. We assessed 18F-FDG uptake in the arterial wall in 14 patients with CKD (mean±SD age: 59±5 years, mean±SD eGFR: 37±12 ml/min per 1.73 m2) but without cardiovascular diseases, diabetes, or inflammatory conditions and in 14 control subjects (mean age: 60±11 years, mean eGFR: 86±16 ml/min per 1.73 m2). Compared with controls, patients with CKD showed increased arterial inflammation, quantified as target-to-background ratio (TBR) in the aorta (TBRmax: CKD, 3.14±0.70 versus control, 2.12±0.27; P=0.001) and the carotid arteries (TBRmax: CKD, 2.45±0.65 versus control, 1.66±0.27; P<0.001). Characterization of circulating monocytes using flow cytometry revealed increased chemokine receptor expression and enhanced transendothelial migration capacity in patients with CKD compared with controls. In conclusion, this increased arterial wall inflammation, observed in patients with CKD but without overt atherosclerotic disease and with few traditional risk factors, may contribute to the increased cardiovascular risk associated with CKD. The concomitant elevation of monocyte activity may provide novel therapeutic targets for attenuating this inflammation and thereby preventing CKD-associated cardiovascular disease.
Keywords: cardiovascular disease, chronic kidney disease, Chronic inflammation, chemokine receptor
CKD is a major risk factor for developing cardiovascular (CV) events,1 with an overall estimated 20%–30% increase in CV risk for each 30% reduction in eGFR.2 In fact, subjects with early stages of CKD are more likely to die from heart disease than to actually ever reach ESRD.3 As CKD is highly prevalent (estimated 8%–16% prevalence worldwide),4 these numbers underscore the importance of adequate CV prevention in patients with CKD.
Traditional cardiovascular disease (CVD) risk factors underestimate CVD risk by as much as 50% in the CKD population,5 suggesting other disease-causing mechanisms in CKD-associated CVD. Over the past several decades, inflammation has gained increasing interest as a contributing factor to the increased CVD risk in patients with CKD. Serum biomarkers of inflammation, such as C-reactive protein (CRP) and IL-6, are associated with increased CVD risk, not only in the general population, but also in patients with CKD.6,7 Furthermore increased CRP levels were known to correlate with eGFR decline.8 The prevailing mechanisms behind this relationship are still unclear. In addition to systemic inflammation, local inflammatory activity of the arterial wall can be assessed measuring 18F-fluorodeoxyglucose (18F-FDG) uptake with positron emission tomography computed tomography (PET/CT).9,10 18F-FDG uptake, representing increased metabolic activity, has been previously linked to plaque macrophage content,11 and is an independent predictor for future CV events.12 Data on arterial inflammation in patients with CKD are not available to date.
The enhanced inflammatory activity in the arterial wall in CVD patients is thought to be primarily driven by continuous influx of circulating monocytes,13 eventually leading to subendothelial inflammatory macrophages. In CVD, data supports that systemic stimuli can polarize circulating monocytes toward more inflammatory phenotypes, rendering them more prone to enter the arterial wall and promote local inflammation.14,15 Interestingly, decreased kidney function is associated with a shift toward a more adhesive phenotype in circulating monocytes,16 which was shown to predict CV events in the CKD population.17
In this study, we set out to evaluate arterial wall inflammation in patients with CKD using 18F-FDG PET/CT. We also addressed activation and function of monocytes ex vivo, using flow cytometry and an endothelial migration assay.
Results
We included 14 patients with CKD, (59±5 years, 50% men) and selected 14 age- and sex-matched controls (age 60±11 years, 50% men). Clinical characteristics are outlined in Table 1. Patients with CKD had an eGFR of 37±12 ml/min per 1.73 m2 versus 86±16 ml/min per 1.73 m2 (P<0.001) with median plasma creatinine levels of 183 (interquartile range [IQR], 123–197) umol/L versus 77 (IQR, 68–84) umol/L in controls (P<0.001). Creatinine clearance was 48 (IQR, 29–65) ml/min and 24-hour protein excretion was low (0.17 [IQR, 0.1–0.3] g/24 h). Plasma urea levels were elevated compared with reference ranges (11.2 [IQR, 8–17] mmol/L; reference range, 2.5–6.4).
Table 1.
Clinical characteristics of patients with CKD and healthy controls
| Characteristic | Control (n=14) | CKD (n=14) | P Value |
|---|---|---|---|
| Sex, men/women | 7/7 | 7/7 | 1.00 |
| Age, yr | 60.4±11 | 58.6±5 | 0.58 |
| BMI, kg/m2 | 22.6±2 | 25.2±4 | 0.04 |
| Smoking, yes/no | 0/14 | 3/11 | 0.07 |
| SBP, mmHg | 131±10 | 135±18 | 0.51 |
| DPB, mmHg | 77±7 | 80±8 | 0.29 |
| Creatinine, umol/L | 77[68–84] | 183 [123–197] | <0.001 |
| eGFR (CKD-EPI), ml/min per 1.73 m2 | 86±16 | 37±12 | <0.001 |
| Total cholesterol, mmol/L | 5.6±0.8 | 5.7±1.3 | 0.92 |
| LDL cholesterol, mmol/L | 3.5±0.88 | 3.5±1.0 | 0.92 |
| HDL cholesterol, mmol/L | 1.8±0.34 | 1.4±0.31 | 0.02 |
| Triglycerides, mmol/L | 0.69 [0.50–0.98] | 1.34 [0.98–1.81] | 0.01 |
| Coronary calcium score | 0[0–0] | 0[0–1.5] | 0.83 |
| CRP, mg/dl | 1.1 [0.7–1.5] | 2.2 [0.7–3.8] | 0.16 |
| WBC, 10E9/L | 5.6±1.5 | 5.3±1.6 | 0.61 |
| Lymphocytes | 1.9±0.5 | 1.5±0.5 | 0.93 |
| Neutrophils | 3.2±1.4 | 3.2±1.4 | 0.06 |
| Monocytes | 0.4±0.07 | 0.4±0.1 | 0.60 |
Values are n, mean±SD, or median [interquartile range] for skewed data. SBP, systolic BP; DBP, diastolic BP; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; WBC, white blood cell count.
CV risk factors, including body mass index (BMI), BP, and plasma cholesterol levels, were comparable between patients and control subjects, although there were slightly more smokers in the CKD group. Plasma triglycerides were slightly increased in CKD subjects (1.34 [IQR, 0.98–1.81] mmol/L versus 0.69 [0.50–0.98] mmol/L in controls; P=0.01) and plasma HDL cholesterol was decreased (1.4±0.31 mmol/L versus 1.8±0.34 mmol/L in controls; P=0.02). Further characterization of the patients with CKD is outlined in Table 2.
Table 2.
Detailed CKD-related parameters
| Characteristic | All (n=14) | Reference Rangesa |
|---|---|---|
| Diagnosis | ||
| ADPKD | 10 | |
| Hypertensive | 3 | |
| Monokidney | 1 | |
| Plasma sodium, mmol/L | 141±1.7 | 135–145 |
| Plasma potassium, mmol/L | 4.2±0.43 | 3.5–5.0 |
| Plasma phosphate, mmol/L | 1.0±0.11 | 0.9–1.5 |
| Plasma PTH, pmol/L | 7.5 [5.4–8.3] | 0.6–6.7 |
| Plasma urea, mmol/L | 11.2 [8–17] | 2.5–6.4 |
| Plasma uric acid, mmol/L | 0.42±0.06 | Men: 0.20–0.42 Women: 0.12–0.34 |
| Plasma albumin, g/L | 43±2.4 | 35–55 |
| Plasma venous HCO3−, mmol/L | 24.7±2.6 | 21–27 |
| Plasma calcium, mmol/L | 2.5±0.11 | 2.10–2.55 |
| Medication use | N/A | |
| Statins | 2 | |
| Antihypertensive | 12 | |
| ACI or ARB | 9 | |
| Diuretic | 3 | |
| β Blocker/calcium antagonist | 3 |
Values are n, mean±SD, or median [interquartile range] for skewed data. ADPKD, adult dominant polycystic kidney disease; PTH, parathyroid hormone; HCO3−, bicarbonate; N/A, not applicable; ACI, angiotensin-converting-enzyme inhibitor; ARB, Angiotensin II receptor antagonists.
Reference ranges are derived from reference values clinical chemistry (referentiewaarden klinische chemie, www.farmacotherapeutischkompas.nl).
Increased Arterial Wall Inflammation in CKD Patients
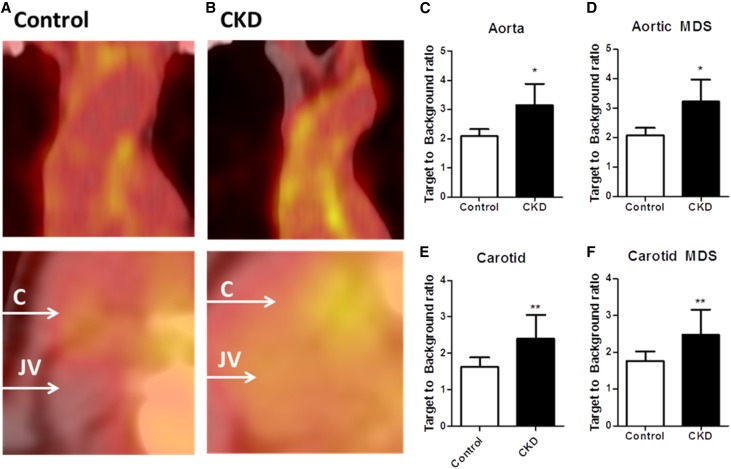
We performed 18F-FDG PET/CT imaging to assess aortic and carotid arterial wall inflammation. 18F-FDG uptake in patients with CKD, quantified as target-to-background ratio (TBR), was increased in the arterial wall of both the aorta and the carotid arteries (aortamax TBR: CKD, 3.14±0.70 versus control, 2.12±0.27, adjusted P=0.001; carotidmax TBR: CKD, 2.45±0.65 versus control, 1.66±0.27, adjusted P<0.001; P values adjusted for CV risk factors which differed at baseline: BMI, current smoking status, and statin use) (Figure 1, C and E). Uptake in the most diseased segment (MDS) was also elevated in both arterial beds in patients with CKD (aortaMDS: CKD, 3.21±0.71 versus control, 2.20±0.26, adjusted P=0.001; carotidMDS: CKD, 2.55±0.68 versus control, 0.79±0.26, adjusted P=0.001) (Figure 1, D and F). Coronary calcium scores were also derived from the computed tomography scans, but were low for both groups (CKD, 0 [0–0] versus control, 0 [0–1.5]; P=0.83), and thus showed no relation to 18F-FDG uptake.
Figure 1.
Increased arterial wall inflammation in patients with CKD. 18F-FDG uptake in the aortic arch (top) and the carotid arteries (bottom) was quantified as the TBR in controls (n=14, represented in A) and patients with CKD (n=14, represented in B). In patients with CKD uptake in the whole vessel (TBRmax) (C, E) as well as the MDS (D, F). Data are mean±SD; *P<0.05; **P<0.01. C, carotid; JV, jugular vein.
To explore which factors could potentially explain the TBR differences, we performed univariate and multivariate (with BMI, current smoking status, and statin used as covariates, none of which showed a correlation with TBR; Supplemental Tables 1 and 2) linear regression with aortic TBRmax as the outcome variable. We found eGFR and plasma urea levels significantly correlated with 18F-FDG uptake, with a concomitant trend for plasma creatinine levels and systolic BP. Plasma HDL cholesterol and triglyceride levels, which differed from controls at baseline, showed no correlation with arterial wall inflammation, and neither did LDL cholesterol or CRP levels (Table 3). None of the other baseline parameters showed correlation with TBR (Supplemental Table 3). As hypertension was found to be a potential confounder in CKD-associated arterial wall inflammation, we subsequently selected a cohort of hypertensive, non-CKD subjects (n=8) from a contemporary PET/CT study performed at our center.18 This revealed that although the selected hypertensive patients had a worse CV risk profile compared with patients with CKD, based on traditional risk assessment (men only, higher BMI, higher diastolic BP, and mean arterial pressure), aortic TBR was lower compared with CKD subjects, whereas carotid TBR was nonsignificantly increased in CKD subjects compared with hypertensive subjects (baseline and TBR values outlined in Supplemental Tables 4 and 5). Finally, we assessed the contribution of systolic BP to the arterial inflammation in CKD by adding systolic BP to the covariance analysis used to assess differences in TBR. If systolic BP was added to the adjusted model, the TBR difference between patients with CKD and healthy controls remained highly significant (P<0.001 for aortamax; P=0.001 for aortaMDS; P<0.001 for carotidmax; and P=0.001 for carotidMDS). However, when adding eGFR to the model, significance was lost (P=0.25 for aortamax; P=0.23 for aortaMDS; P=0.17 for carotidmax; and P=0.28 for carotidMDS).
Table 3.
Univariate and multivariate linear regression analysis with aortic TBRmax as the dependent variable
| Characteristic | Univariate Analyses | Multivariate Analysesa | ||
|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | |
| Urea | 0.51 (−0.01 to 0.18) | 0.08 | 0.82 (0.01 to 0.26) | 0.04 |
| Creatinine | 0.36 (−0.01 to 0.01) | 0.23 | 0.85 (0.00 to 0.02) | 0.05 |
| eGFR (CKD-EPI) | −0.40 (−0.06 to 0.01) | 0.18 | −0.08 (−0.09 to 0.00) | 0.05 |
| SBP | 0.61 (0.01 to 0.05) | 0.04 | 0.86 (−0.01 to 0.07) | 0.06 |
| LDL cholesterol | −0.01 (−0.45 to 0.44) | 1.00 | −0.09 (−0.69 to 0.57) | 0.84 |
| HDL cholesterol | −0.10 (−1.84 to 1.35) | 0.75 | −0.13 (−2.25 to 1.63) | 0.73 |
| TG | −0.16 (−1.34 to 0.82) | 0.61 | −0.22 (−1.73 to 1.01) | 0.56 |
| CRP | −0.10 (−0.14 to 0.10) | 0.74 | −0.01 (−0.21 to 0.21) | 0.98 |
Data are standardized coefficient (β) with 95% confidence intervals (95% CI). CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; SBP, systolic BP; TG, triglycerides.
BMI, current smoking status, and statin use were used as covariates.
Cellular Activation in Patients with CKD
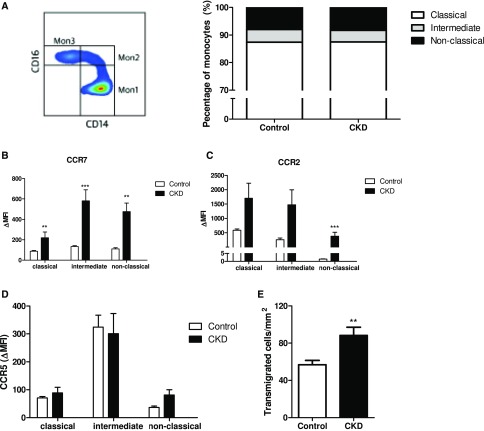
Finally, we assessed phenotype and function of freshly isolated monocytes. To this end, we included 14 controls, matched for age and sex to the CKD cohort, and the clinical characteristics were comparable to the control cohort used for the PET/CT (Supplemental Table 6). Monocytes were classified according to HLA-DR, CD14, and CD16 expression19: The distribution across the classic (Mon1: CD14++CD16−), intermediate (Mon2: CD14++CD16+), and nonclassic (Mon3: CD14(+)CD16+) subsets did not differ between patients with CKD and controls (Figure 2A). However, CCR7 expression was increased on all monocyte subsets in CKD subjects compared with controls, with a similar pattern for CCR2 (Figure 2, B and C), whereas no significant effect was found for CCR5 expression (Figure 2D). In a transendothelial migration assay, we found a significant increase in monocytes that crossed the endothelial barrier (transmigrated cells per millimeter2: CKD, 88±27 versus control, 57±15; P=0.01).
Figure 2.
Monocytes of CKD subjects show increased expression of chemokine receptors and enhanced migratory capacity. Flow cytometry on whole blood was performed to study expression of monocyte surface markers. Monocytes were divided into classic (CD14++CD16−), intermediate (CD14++CD16+), or nonclassic (CD14(+)CD16+) monocytes. (A) Surface expression of monocyte CCR7, CCR2, and CCR5 represented as Δ median fluorescence intensity (MFI), in CKD subjects (n=14) versus controls (n=14). (B, C, D) Transendothelial migratory capacity was quantified as the number of transmigrated cells per millimeter2. (E) For each subject, transmigrated cells are calculated of independent counts of five frames of view. Data represent mean±SEM. *P<0.05; **P<0.01; ***P<0.001.
Discussion
Here we show that patients with CKD are characterized by an increased 18F-FDG uptake of the arterial wall on PET/CT, compatible with an elevated inflammatory state and increased CVD risk. Furthermore, monocytes from patients with CKD display a proadhesive phenotype on flow cytometry, as well as enhanced migratory capacity ex vivo. In multifactorial analysis, eGFR and urea plasma levels were independent predictors of arterial wall inflammation. These data imply that CKD-associated inflammatory activation may contribute to the elevated CVD risk in CKD-patients.
18F-FDG-PET/CT imaging is a validated technique to quantify arterial wall inflammation and used as an independent predictor of CVD risk.12,20,21 Increased 18F-FDG arterial wall uptake was found in active culprit lesions,22,23 primarily in macrophage rich areas,22 and correlated to macrophage content11 as well as inflammatory gene expression.24 Hence, 18F-FDG PET/CT imaging is increasingly used to monitor therapeutic efficacy of antiatherosclerotic and anti-inflammatory strategies.25–29 Here we show that CKD subjects without overt atherosclerotic disease (represented by the low coronary calcium scores in our study) or comorbidity, have increased inflammatory activity in the arterial wall in comparison to age- and sex-matched controls. The correlation with eGFR and plasma urea levels, and to a lesser extent with creatinine and systolic BP, suggests a ‘dose-dependent’ effect of (the consequences of) renal insufficiency on arterial wall inflammation. Loss of kidney function reflects the accumulation of uremic retention solutes: a diverse group of compounds that adversely affect different organs and cells.30 These uremic toxins have been proposed to play an important role in development and progression of CKD-associated CVD through diverse mechanisms, including activation of leukocytes and enhancing monocyte-endothelial interactions.31 The elevated systolic BP observed in our CKD cohort can contribute to endothelial activation32 but cannot exclusively explain the arterial inflammation, evidenced by the lower levels in hypertensive subjects without CKD, thereby further augmenting this process.
Monocyte trafficking across the arterial wall is an important contributor to arterial wall inflammation.13 In contrast to previous data33 we did not find differences in monocyte subsets in our study, likely due to the limited number of patients. We did observe increased monocyte CCR7 and (to an extent) CCR2 expression. CCR7 and its ligands, Chemokine (CC-motif) ligand 19 (CCL19) and 21 (CCL21) have been associated with increased monocyte migration and are associated with increased CVD risk in humans.34 CCR2, the receptor for monocyte chemo-attractant protein-1 (MCP-1), also enhances chemotaxis35 and is important in the process of plaque formation in mice.36 In our study, monocytes of patients with CKD also showed enhanced migratory capacity compared with healthy controls, suggesting that expression of these chemokine receptors in humans may also contribute to the inflammatory status of the arterial wall. Thus, our data supports a role for activation of circulating monocytes contributing to the enhanced CVD risk in patients with CKD.
On the basis of the significant reduction in CV events after lipid-lowering treatment in patients with CKD,37 the Kidney Disease Improving Global Outcomes (KDIGO) guidelines recently proposed that for those >50 years, an eGFR <60 ml/min per 1.73 m2 is an indication for primary prevention using statins, independent of baseline cholesterol levels and other risk factors,38 and Schneider et al. showed that implementation of this guideline requires a substantial increase in prescription rates.39 Our study provides a potential explanation for the benefits of statin therapy in patients with CKD37 in the absence of elevated cholesterol, as several studies have shown direct effects of statin therapy on arterial inflammation.29,40,41 However, arterial wall inflammation in our study correlated with measures of kidney decline, and not with lipids. Post hoc analyses of the Treating to New Targets (TNT) and Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trials also suggest beneficial effects of statins on eGFR,42,43 and this proposes an additional mechanism by which statins may reduce CV risk in patients with CKD. Combined, these data imply that the reduction in arterial inflammation after statin therapy may contribute to lowering of CVD risk in patients with CKD. Future studies are necessary to investigate if statin therapy fully suffices to lower CKD driven inflammation and subsequent CV risk, or if more specific therapies are warranted that target the direct inflammatory effects of decreased kidney function.
Potential Limitations
Several limitations of this study merit closer consideration. First, because of the limited sample size and cross-sectional design, we cannot draw definite conclusions on causality of our findings. Traditional risk factors are also increased in patients with CKD,44 and distinguishing which particular factors dominate CVD risk is challenging. However, in this study, baseline characteristics were comparable between CKD subjects and controls and, where applicable, outcomes were corrected for any (non-CKD–related) parameter that differed at baseline. However, as has been widely recognized, virtually all forms of kidney disease coincide with hypertension.45 Therefore, full dissection of the impact of kidney disease versus BP increase is challenging. Second, due to ethical restraints considering radiation exposure, and the need of fresh monocytes for the ex vivo studies, different control groups were used for the imaging and monocyte studies. Both control groups were comparable regarding baseline characteristics, minimizing the chance that this would significantly affect the assessed parameters. Finally, although 18F-FDG uptake has been shown to predict subsequent events in the general population,12 whether this also holds true for patients with CKD requires long-term outcome studies.
Summary and Conclusions
This is the first study to show increased arterial wall inflammation in patients with CKD, without known atherosclerotic or inflammatory disease, and with few traditional risk factors and comorbidity. Arterial wall inflammation correlates directly to measures of kidney function, implying a potentially causal relationship between these observations. As arterial inflammation is directly associated with CVD risk, these data support the need for early CVD prevention, as was recently incorporated in the new KDIGO guidelines on statin use. Alternatively, specific anti-inflammatory therapies may be required to further reduce the large CVD burden in patients with CKD, as LDL cholesterol is not the dominant risk factor in these patients.
Concise Methods
Study Population
We performed a cross-sectional cohort study in subjects >50 years old with a diagnosis of CKD stages 3 or 4, defined as eGFR <60 but >15 ml/min per 1.73 m2. Exclusion criteria comprised any chronic inflammatory disease (including GN), gout, diabetes, history of a CV event, uncontrolled hypertension, proteinuria >1 g/L, overt lipid disorders, and use of anti-inflammatory drugs. 18F-FDG PET/CT scans were performed as published previously.9,12 Images were analyzed with dedicated software (OsiriX, Geneva, Switzerland; http://www.osirix-viewer.com). Standardized uptake values were averaged for each artery, and divided by the average venous background activity to obtain the TBR.10 Also, computed tomography scans were used to calculate coronary calcium scores as described previously.12 Because of ethical constraints relating to radiation exposure, for the imaging studies, healthy controls were selected from a contemporaneous study using identical imaging protocols and performed on the same scanner. For the ex vivo monocyte studies, healthy controls matched on age and sex were included on the same study days as the patients with CKD. Monocyte phenotype was determined ex vivo using flow cytometry, classifying monocytes on the basis of HLA-DR, CD14, and CD16 expression, and subsequently assessing surface marker chemokine (CCR2, CCR7, and CCR5) expression. Migratory capacity was assessed using a transendothelial migration assay, as described previously.13 CD14 bead-isolated monocytes were added to a confluent layer of human aortic endothelial cells (HAECs) for 30 minutes and then fixed with formaldehyde. Multiple images were recorded and adhered (bright morphology), and transmigrated monocytes (dark morphology) were quantified. The study protocol was approved by the Institutional Review Board of the Academic Medical Center in Amsterdam, The Netherlands. Written informed consent was obtained from each participant. Full details on the methods are available in the Supplemental Material.
Statistical Analyses
All data were analyzed using Prism version 5.0 (GraphPad Software, La Jolla, CA) and SPSS version 21.0 (SPSS Inc., Chicago, IL). Data are presented as mean±SD for normally distributed data, or medians with interquartile range for non-normally distributed data, unless stated otherwise. Depending on distribution, all comparisons of subgroups were performed using t test or Mann–Whitney U test. Differences in TBR were assessed with covariance analysis, correcting for possible confounders which were different at baseline. Univariate and multivariate linear regression analysis was used to assess the influence of clinical parameters on arterial wall inflammation.
Disclosures
E.S.S. has received lecturing fees from Merck (Kenilworth, NJ), Novartis (Basel, Switzerland), Ionis Pharmaceuticals (Carlsbad, CA), Amgen (Thousand Oaks, CA), and Sanofi-Aventis (Paris, France), none of which are related to the contents of this manuscript. All other authors have no disclosures.
Supplementary Material
Acknowledgments
The authors would like to thank M.F. Lam, M.E. Hemayat, and E. Poel for their assistance with 18F-fluorodeoxyglucose positron emission tomography computed tomography.
This work was supported by a grant from the Netherlands Heart Foundation (The Netherlands Cardiovascular Research Committee [CVON] 2011/B019: Generating the best evidence-based pharmaceutical targets for atherosclerosis) and a European Horizon-2020 grant (PHC-03-2015: 667837, REPROGRAM).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016030317/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Mafham M, Emberson J, Landray MJ, Wen C-P, Baigent C: Estimated glomerular filtration rate and the risk of major vascular events and all-cause mortality: a meta-analysis. PLoS One 6: e25920, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY-M, Yang C-W: Chronic kidney disease: global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ: The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol 50: 217–224, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Zebrack JS, Anderson JL, Beddhu S, Horne BD, Bair TL, Cheung A, Muhlestein JB; Intermountain Heart Collaborative Study Group : Do associations with C-reactive protein and extent of coronary artery disease account for the increased cardiovascular risk of renal insufficiency? J Am Coll Cardiol 42: 57–63, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Knight EL, Rimm EB, Pai JK, Rexrode KM, Cannuscio CC, Manson JE, Stampfer MJ, Curhan GC: Kidney dysfunction, inflammation, and coronary events: a prospective study. J Am Soc Nephrol 15: 1897–1903, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Stuveling EM, Hillege HL, Bakker SJL, Gans ROB, De Jong PE, De Zeeuw D: C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int 63: 654–661, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Rudd JHF, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, Fuster V, Fayad ZA: (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol 50: 892–896, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Rudd JHF, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, Rafique A, Hargeaves R, Farkouh M, Fuster V, Fayad ZA: Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med 49: 871–878, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ: In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 48: 1818–1824, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, Lawler MA, Grinspoon SK, Brady TJ, Nasir K, Hoffmann U, Tawakol A: Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging 6: 1250–1259, 2013 [DOI] [PubMed] [Google Scholar]
- 13.van der Valk FM, Kroon J, Potters WV, Thurlings RM, Bennink RJ, Verberne HJ, Nederveen AJ, Nieuwdorp M, Mulder WJM, Fayad ZA, van Buul JD, Stroes ESG: In vivo imaging of enhanced leukocyte accumulation in atherosclerotic lesions in humans. J Am Coll Cardiol 64: 1019–1029, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Ghattas A, Griffiths HR, Devitt A, Lip GYH, Shantsila E: Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol 62: 1541–1551, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JHF, Fayad ZA, Lehrer-Graiwer J, Korsgren M, Figueroa AL, Fredrickson J, Rubin B, Hoffmann U, Truong Q a., Min JK, Baruch A, Nasir K, Nahrendorf M, Tawakol A: Splenic metabolic activity predicts risk of future cardiovascular events. JACC Cardiovasc Imaging 8: 121–130, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez R, Carracedo J, Merino A, Soriano S, Ojeda R, Alvarez-Lara MA, Martín-Malo A, Aljama P: CD14+CD16+ monocytes from chronic kidney disease patients exhibit increased adhesion ability to endothelial cells. Contrib Nephrol 171: 57–61, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Große-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Böhm M, Fliser D, Heine GH: CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol 60: 1512–1520, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Van der Valk FM, Verweij SL, Zwinderman KAH, Strang AC, Kaiser Y, Marquering HA, Nederveen AJ, Stroes ESG, Verberne HJ, Rudd JHF: Thresholds for Arterial Wall Inflammation Quantified by (18)F-FDG PET Imaging: Implications for Vascular Interventional Studies [published online ahead of print September 7, 2016]. JACC Cardiovasc Imaging doi:10.1016/j.jcmg.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passlick B, Flieger D, Ziegler-Heitbrock HW: Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74: 2527–2534, 1989 [PubMed] [Google Scholar]
- 20.Marnane M, Merwick A, Sheehan OC, Hannon N, Foran P, Grant T, Dolan E, Moroney J, Murphy S, O’Rourke K, O’Malley K, O’Donohoe M, McDonnell C, Noone I, Barry M, Crowe M, Kavanagh E, O’Connell M, Kelly PJ: Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol 71: 709–718, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, Nikolaou K, Reiser MF, Bartenstein P, Hacker M: 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med 50: 1611–1620, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnström P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD, Weissberg PL: Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 105: 2708–2711, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Davies JR, Rudd JHF, Fryer TD, Graves MJ, Clark JC, Kirkpatrick PJ, Gillard JH, Warburton EA, Weissberg PL: Identification of culprit lesions after transient ischemic attack by combined 18F fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke 36: 2642–2647, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Pedersen SF, Graebe M, Fisker Hag AM, Højgaard L, Sillesen H, Kjaer A: Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun 31: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC, Rudd JH, Farkouh ME, Tawakol A; dal-PLAQUE Investigators : Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 378: 1547–1559, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JHF, Farkouh ME, Nunes IO, Beals CR, Shankar SS: Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multi-center FDG-PET/CT feasibility study. J Am Coll Cardiol 62: 909–917, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Tawakol A, Singh P, Rudd JHF, Soffer J, Cai G, Vucic E, Brannan SP, Tarka EA, Shaddinger BC, Sarov-Blat L, Matthews P, Subramanian S, Farkouh M, Fayad ZA: Effect of treatment for 12 weeks with rilapladib, a lipoprotein-associated phospholipase A2 inhibitor, on arterial inflammation as assessed with 18F-fluorodeoxyglucose-positron emission tomography imaging. J Am Coll Cardiol 63: 86–88, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Van Wijk DF, Sjouke B, Figueroa A, Emami H, van der Valk FM, MacNabb MH, Hemphill LC, Schulte DM, Koopman MG, Lobatto ME, Verberne HJ, Fayad ZA, Kastelein JJP, Mulder WJM, Hovingh GK, Tawakol A, Stroes ESG: Nonpharmacological lipoprotein apheresis reduces arterial inflammation in familial hypercholesterolemia. J Am Coll Cardiol 64: 1418–1426, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, Hayabuchi N, Imaizumi T: Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 48: 1825–1831, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Vanholder R, Baurmeister U, Brunet P, Cohen G, Glorieux G, Jankowski J; European Uremic Toxin Work Group : A bench to bedside view of uremic toxins. J Am Soc Nephrol 19: 863–870, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Moradi H, Sica DA, Kalantar-Zadeh K: Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am J Nephrol 38: 136–148, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Endemann DH, Schiffrin EL: Endothelial dysfunction. J Am Soc Nephrol 15: 1983–1992, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH: CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J 32: 84–92, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Cai W, Tao J, Zhang X, Tian X, Liu T, Feng X, Bai J, Yan C, Han Y: Contribution of homeostatic chemokines CCL19 and CCL21 and their receptor CCR7 to coronary artery disease. Arterioscler Thromb Vasc Biol 34: 1933–1941, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Han KH, Tangirala RK, Green SR, Quehenberger O: Chemokine receptor CCR2 expression and monocyte chemoattractant protein-1-mediated chemotaxis in human monocytes. A regulatory role for plasma LDL. Arterioscler Thromb Vasc Biol 18: 1983–1991, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Boring L, Gosling J, Cleary M, Charo IF: Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394: 894–897, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R; SHARP Investigators : The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377: 2181–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members : Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158: 825–830, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Schneider MP, Hübner S, Titze SI, Schmid M, Nadal J, Schlieper G, Busch M, Baid-Agrawal S, Krane V, Wanner C, Kronenberg F, Eckardt K-U: Implementation of the KDIGO guideline on lipid management requires a substantial increase in statin prescription rates. Kidney Int 88: 1411–1418, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Ishii H, Nishio M, Takahashi H, Aoyama T, Tanaka M, Toriyama T, Tamaki T, Yoshikawa D, Hayashi M, Amano T, Matsubara T, Murohara T: Comparison of atorvastatin 5 and 20 mg/d for reducing F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: a randomized, investigator-blinded, open-label, 6-month study in Japanese adults scheduled for percutaneous coronary intervention. Clin Ther 32: 2337–2347, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Wu Y-W, Kao H-L, Huang C-L, Chen M-F, Lin L-Y, Wang Y-C, Lin Y-H, Lin H-J, Tzen K-Y, Yen R-F, Chi Y-C, Huang P-J, Yang W-S: The effects of 3-month atorvastatin therapy on arterial inflammation, calcification, abdominal adipose tissue and circulating biomarkers. Eur J Nucl Med Mol Imaging 39: 399–407, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Amarenco P, Callahan A 3rd, Campese VM, Goldstein LB, Hennerici MG, Messig M, Sillesen H, Welch KMA, Wilson DJ, Zivin JA: Effect of high-dose atorvastatin on renal function in subjects with stroke or transient ischemic attack in the SPARCL trial. Stroke 45: 2974–2982, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Shepherd J, Kastelein JJP, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK; Treating to New Targets Investigators : Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2: 1131–1139, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, Eckardt K-U, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E: Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80: 572–586, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.