Abstract
Nephrotoxicity is a major adverse effect in cisplatin chemotherapy, and renoprotective approaches are unavailable. Recent work unveiled a critical role of protein kinase Cδ (PKCδ) in cisplatin nephrotoxicity and further demonstrated that inhibition of PKCδ not only protects kidneys but enhances the chemotherapeutic effect of cisplatin in tumors; however, the underlying mechanisms remain elusive. Here, we show that cisplatin induced rapid activation of autophagy in cultured kidney tubular cells and in the kidneys of injected mice. Cisplatin also induced the phosphorylation of mammalian target of rapamycin (mTOR), p70S6 kinase downstream of mTOR, and serine/threonine-protein kinase ULK1, a component of the autophagy initiating complex. In vitro, pharmacologic inhibition of mTOR, directly or through inhibition of AKT, enhanced autophagy after cisplatin treatment. Notably, in both cells and kidneys, blockade of PKCδ suppressed the cisplatin-induced phosphorylation of AKT, mTOR, p70S6 kinase, and ULK1 resulting in upregulation of autophagy. Furthermore, constitutively active and inactive forms of PKCδ respectively enhanced and suppressed cisplatin-induced apoptosis in cultured cells. In mechanistic studies, we showed coimmunoprecipitation of PKCδ and AKT from lysates of cisplatin-treated cells and direct phosphorylation of AKT at serine-473 by PKCδ in vitro. Finally, administration of the PKCδ inhibitor rottlerin with cisplatin protected against cisplatin nephrotoxicity in wild-type mice, but not in renal autophagy–deficient mice. Together, these results reveal a pathway consisting of PKCδ, AKT, mTOR, and ULK1 that inhibits autophagy in cisplatin nephrotoxicity. PKCδ mediates cisplatin nephrotoxicity at least in part by suppressing autophagy, and accordingly, PKCδ inhibition protects kidneys by upregulating autophagy.
Keywords: cisplatin, apoptosis, nephrotoxicity
Cisplatin is one of the most widely used and most potent chemotherapeutic agents. It is used for the treatment of testicular, ovarian, breast, head and neck, lung, and many other types of cancers.1,2 However, the use of cisplatin is limited by its side effects in normal tissues, especially nephrotoxicity in kidneys.3–7 Despite decades of research, effective renoprotective approaches during cisplatin chemotherapy remain unavailable. Our recent work revealed an important role of protein kinase Cδ (PKCδ) in cisplatin-induced nephrotoxicity.8,9 Notably, inhibiting PKCδ not only protected kidneys but enhanced the chemotherapeutic effects of cisplatin in several tumor models, opening a new avenue for renoprotection during chemotherapy.8,9 However, the mechanism underlying the renoprotective effect of PKCδ inhibition is unclear.
Autophagy is a highly regulated cellular process of catabolism that degrades cytoplasmic constituents via the formation of autophagosome followed by its fusion with lysosome. Originally described as a cellular response to starvation, autophagy is now known to be crucial to the maintenance of cellular homeostasis and play important roles in animal development, physiology, and pathogenesis of a variety of diseases.10–12 In cisplatin nephrotoxicity, autophagy is rapidly activated in kidney tubular cells and tissues.13,14 Using renal tubule–specific Atg-knockout models, recent studies have further demonstrated autophagy as an important kidney protective mechanism.15,16 However, it remains elusive how autophagy is regulated during cisplatin nephrotoxicity.
In view of these findings and questions, we hypothesized that PKCδ may play a regulatory role in autophagy during cisplatin nephrotoxicity and inhibition of PKCδ may protect kidney cells and tissues by upregulating autophagy. In support of this hypothesis, several studies have implicated PKCδ in the regulation of autophagy.17–20 Nonetheless, whether PKCδ promotes or inhibits autophagy remains controversial. For example, Ann and colleagues18 demonstrated that PKCδ mediated autophagy during acute hypoxic stress by phosphorylating/activating JNK1, whereas Ozpolat et al. 17 showed that PKCδ suppressed autophagy in pancreatic cancer cells by inducing tissue transglutaminase.
In this study, we have identified PKCδ as a critical negative regulator of autophagy in both in vitro and in vivo experimental models of cisplatin. Mechanistically, we show that PKCδ may directly bind and phosphorylate AKT at Serine-473, resulting in the activation of mammalian target of rapamycin (mTOR) to suppress ULK1 and autophagy. Moreover, PKCδ inhibitors lost their renoprotective effect in autophagy-deficient mice, supporting a role of autophagy in the effect of PKCδ inhibition.
Results
Autophagy Is Induced during Cisplatin Treatment
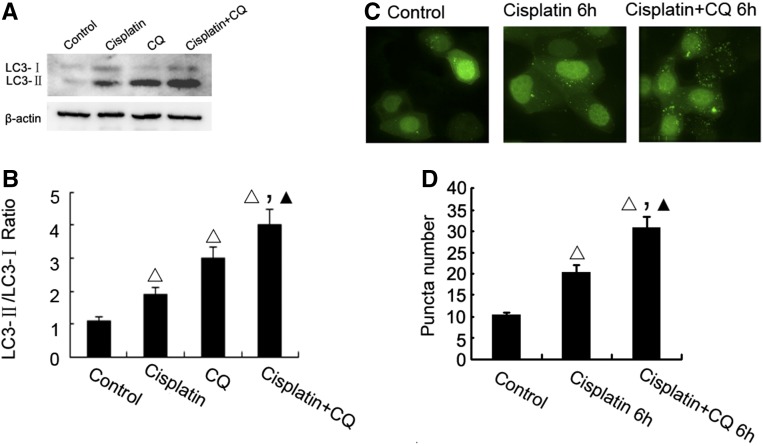
We first verified that cisplatin induced autophagy in vitro in cultured rat proximal tubular cells (RPTC). In this experiment, we also observed the effect of chloroquine (CQ), which accumulates in lysosomes to raise pH resulting in the inhibition of lysosomal enzymes and the suppression of autophagic degradation. By this property, CQ is frequently used to block autolysosomal degradation to reveal upstream autophagic activation upon stimulation. In immunoblot analysis, cisplatin treatment for 6 hours induced the conversion of LC3I to LC3II, which was further enhanced by the presence of CQ (Figure 1, A and B). To visualize autophagsosome formation, the cells were transfected with GFP-LC3 and then treated with cisplatin in the presence or absence of CQ. As shown in Figure 1, C and D, cisplatin treatment increased the number of GFP-LC3 puncta, which was further increased by CQ, confirming autophagy induction in this experimental condition.
Figure 1.
Cisplatin-induced autophagy in RPTC cells. (A) LC3-II formation during cisplatin treatment. RPTC were incubated with 20 μM cisplatin for 6 hours in the absence or presence of 50 μM CQ to collect whole cell lysates for immunoblot analysis of LC3 and also β-actin as a protein loading control. (B) For densitometry, the LC3-II signal was divided by the LC3-I signal of the same sample to determine the ratio. (C) Representative cell images showing punctate GFP-LC3 distribution after cisplatin treatment. RPTC transiently transfected with GFP-LC3 were incubated with 20 μM cisplatin for 6 hours in the absence or presence of CQ and then examined by confocal microscopy. (D) The number of GFP-LC3 puncta per cell in each of the conditions described in (C) was assessed. Data in (B, D) are expressed as mean±SD (n=4); Δ, statistically significantly different from the control group without cisplatin treatment; ▲, statistically significantly different from cisplatin treatment group; P<0.05. Data in panels (A, C) are representative of at least four separate experiments. The results show a rapid induction of autophagy by cisplatin RPTC cells.
mTOR Is Activated to Suppress Autophagy during Prolonged Cisplatin Treatment
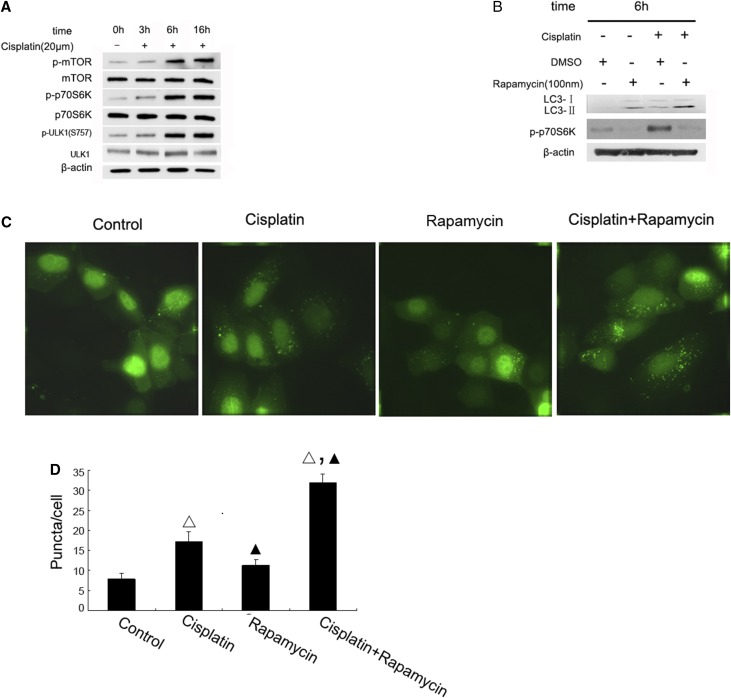
To investigate the regulatory mechanism of autophagy during cisplatin treatment, we initially focused on mTOR, a well documented negative regulator of autophagy. We hypothesized that mTOR might be inactivated during cisplatin treatment to induce autophagy. We first analyzed the status of mTOR activation during cisplatin treatment by monitoring its phosphorylation on Ser2448. As shown in Figure 2A, cisplatin induced significant mTOR-Ser2448 phosphorylation at 6 hours and 16 hours of cisplatin treatment, whereas the phosphorylation was not obvious at 3 hours. Cisplatin also induced a similar pattern of phosphorylation of p70S6 kinase, a well established downstream target of mTOR (Figure 2A). A major mechanism for mTOR regulation of autophagy is through inhibitory phosphorylation of ULK1, a key component of the autophagy initiating complex.21,22 Indeed, we found that cisplatin induced marked ULK1 phosphorylation at Ser757 at 6–16 hours, paralleling with the time course of mTOR activation, whereas total ULK1 was not significantly changed during cisplatin treatment (Figure 2A). To further confirm the role of mTOR in cisplatin-induced autophagy, we examined the effect of rapamycin, a specific pharmacologic inhibitor of mTOR. In immunoblot analysis, rapamycin attenuated p70S6 kinase phosphorylation and, notably, enhanced LC3II accumulation during cisplatin treatment (Figure 2B). Moreover, rapamycin significantly increased autophagic GFP-LC3 puncta formation during cisplatin treatment of GFP-LC3 transfected cells (Figure 2, C and D). These results suggested that mTOR activation may contribute significantly to the decrease of autophagy during the late stage of cisplatin treatment.
Figure 2.
mTOR is activated to suppress autophagy during cisplatin treatment. (A) The time course of cisplatin-induced mTOR activation. RPTC were incubated with 20 μM cisplatin for 0–16 hours to collect whole cell lysates for immunoblot analysis of total and phosphorylated mTOR, p70S6k, and ULK1, and also β-actin as a protein loading control. (B) Inhibition of mTOR by rapamycin increased LC3-II formation during cisplatin treatment. RPTC were incubated with 20 μM cisplatin with or without 0.1 μM rapamycin for 6 hours to collect whole cell lysates for immunoblot analysis of LC-3, p-p70S6k, and β-actin. (C) Representative cell images showing punctate GFP-LC3 distribution after cisplatin with or without rapamycin treatment. RPTC transiently transfected with GFP-LC3 were incubated with 20 μM cisplatin with or without rapamycin for 6 hours and then examined by confocal microscopy. (D) The number of GFP-LC3 puncta per cell in each of the conditions described in (C) was assessed. Data in (D) are expressed as mean±SD (n=4); Δ, statistically significantly different from the control group (P<0.05); ▲, statistically significantly different from cisplatin treatment group (P<0.05). Data are representative of at least four separate experiments. The results show that mTOR is activated during cisplatin treatment to suppress autophagy in RPTC cells.
AKT Acts Upstream of mTOR for Autophagy Suppression during Cisplatin Treatment
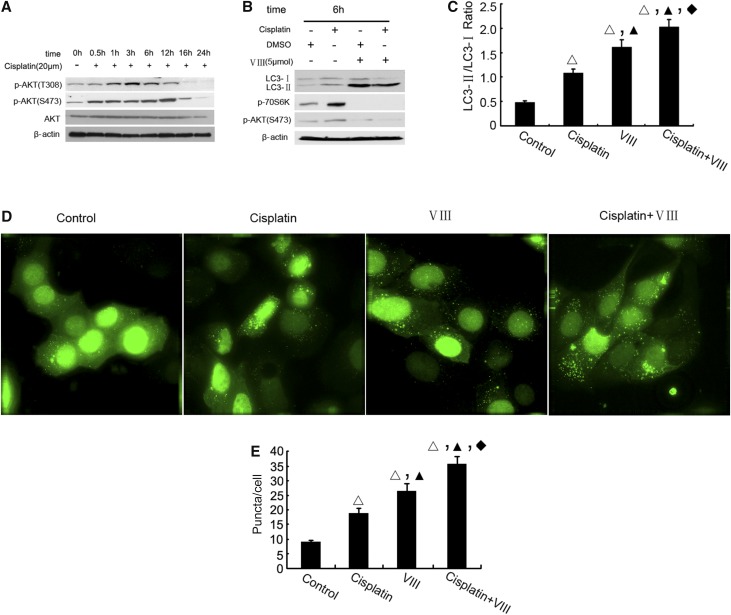
To understand the mechanism of mTOR activation during cisplatin treatment, we focused on AKT, because: (1) AKT may act upstream of mTOR in cell signaling,23 and (2) AKT is activated during cisplatin treatment of kidney tubular cells and tissues.24,25 We verified AKT activation during cisplatin treatment of RPTC by detecting AKT phosphorylation at Ser473 and Thr308 (Figure 3A). Notably, AKT activation started within 0.5 to 1 hours of cisplatin treatment, reached peak levels at 3 hours, and decreased toward basal level by 24 hours. To determine the role of AKT on mTOR and autophagy regulation, we further examined the effects of VIII, a pharmacologic inhibitor of AKT. As shown in Figure 3B, VIII suppressed mTOR as indicated by the attenuation of 70-kDa ribosomal protein S6 kinase (p70S6K) phosphorylation. Concurrently, VIII induced LC3II in control as well as in cisplatin-treated cells (Figure 3, B and C). The autophagy-enhancing effect of VIII was further confirmed by GFP-LC3 puncta formation. Apparently, VIII further increased the formation of GFP-LC3 autophagic puncta (Figure 3, D and E). Thus, inhibition of AKT led to the attenuation of mTOR activation and increased autophagy, suggesting that AKT may act upstream of mTOR to quench autophagy during prolonged cisplatin treatment.
Figure 3.
AKT acts upstream of mTOR for autophagy suppression during cisplatin treatment. (A) The time course of cisplatin-induced AKT activation. RPTC were incubated with 20 μM cisplatin for 0–24 hours to collect whole cell lysates for immunoblot analysis of total and S473 or T308 site phosphorylated AKT. (B) Inhibition of AKT by VIII increased LC3-II formation during cisplatin treatment. RPTC were incubated with 20 μM cisplatin alone or together with 5 μM VIII for 6 hours to collect whole cell lysates for immunoblot analysis of LC-3, p-p70S6k, p-AKT S473, and also β-actin as a protein loading control. (C) For densitometry, the LC3-II signal was divided by the LC3-I signal of the same sample to determine the ratio. (D) Representative cell images from confocal microscopy showing punctate GFP-LC3 distribution. (E) The number of GFP-LC3 puncta per cell in each of the conditions described in (D) was assessed. Data in (C, E) are expressed as mean±SD (n=4); Δ, statistically significantly different from the control group (P<0.05); ▲, statistically significantly different from cisplatin treatment group (P<0.05); ♦, statistically significantly different from VIII group (P<0.05). Data are representative of at least four separate experiments. The results suggest that AKT is upstream of mTOR involved to suppress cisplatin induced autophagy.
AKT is well known for its prosurvival activity and as a result, by inhibiting AKT VIII, is expected to induce cell death. However, our above experiment (Figure 3) demonstrated that VIII could also activate the cytoprotective mechanism of autophagy. Does VIII induce or protect against cell death during cisplatin treatment? As shown in Supplemental Figure 1, VIII further increased apoptosis during 16 hours of cisplatin treatment of RPTC cells. We also analyzed the effect of VIII on cell proliferation (Supplemental Figure 2). Both VIII and cisplatin suppressed cell proliferation, and when added together they had additive effects. The results suggest that, although AKT may suppress cytoprotective autophagy, its overall cell biologic function is for cell survival and proliferation.
PKCδ Is Activated during Cisplatin Treatment to Activate mTOR and Suppress Autophagy
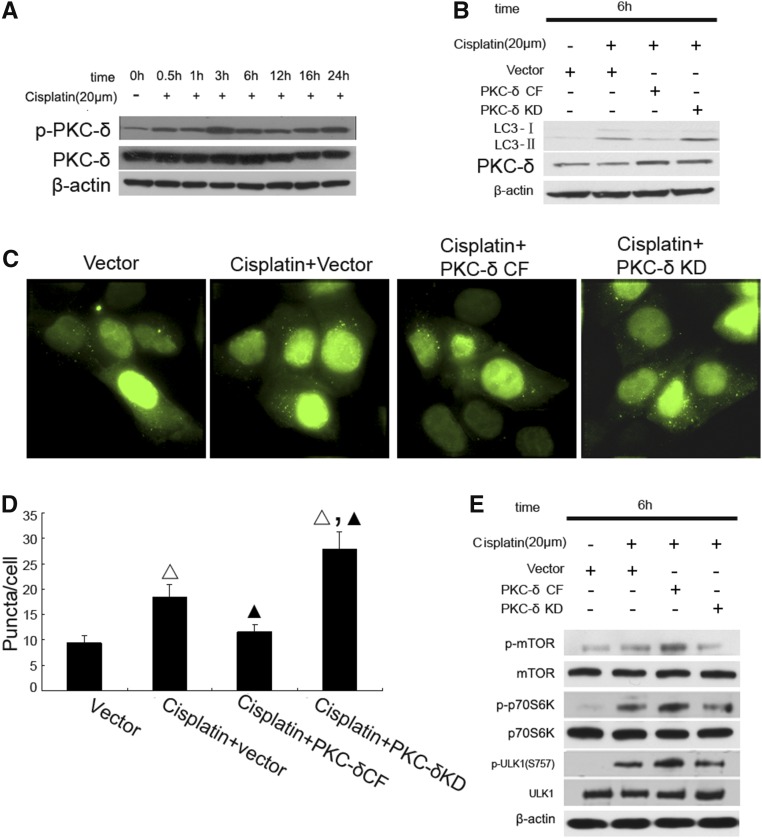
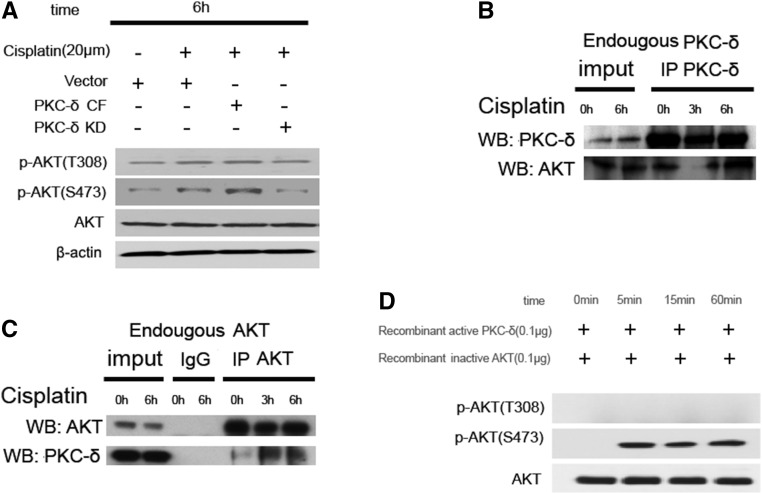
Our recent work demonstrated a rapid activation of PKCδ during cisplatin treatment of RPTC and mice. Moreover, pharmacologic and genetic suppression of PKCδ afforded remarkable renoprotective effects.8 Because autophagy is an important mechanism of renoprotection in kidney injury including cisplatin nephrotoxicity,26 we hypothesized that PKCδ inhibition may protect via autophagy. To test this possibility, we first confirmed PKCδ activation during cisplatin treatment of RPTC by immunoblot analysis of its phosphorylation (Figure 4A). To determine the involvement of PKCδ in cisplatin-induced autophagy, we examined the effects of dominant-negative PKCδ (PKCδ-KD) and catalytically active PKCδ (PKCδ-CF). As shown in Figure 4B, LC3II accumulation during cisplatin treatment was suppressed by PKCδ-CF, but increased by PKCδ-KD. Consistently, in GPF-LC3-transfected cells PKCδ-CF suppressed, whereas PKCδ-KD enhanced, GFP-LC3 puncta formation (Figure 4, C and D). To understand the underlying mechanism, we examined whether PKCδ suppressed autophagy through the activation of mTOR and consequent inhibitory phosphorylation of ULK1. As shown in Figure 4E, cisplatin treatment led to phosphorylation of mTOR, p70S6K, and ULK1, which was further increased by PKCδ-CF, whereas PKCδ-KD was inhibitory. Together, the results suggested that PKCδ might contribute to the activation of mTOR resulting in the suppression of ULK1 and autophagy.
Figure 4.
PKCδ is activated early during cisplatin treatment to induce mTOR for autophagy suppression. (A) The time course of cisplatin-induced PKCδ activation. RPTC were incubated with 20 μM cisplatin for 0–24 hours to collect whole cell lysates for immunoblot analysis of total and phosphorylated PKCδ, and also β-actin as a protein loading control. (B) PKCδ-KD increased, whereas catalytically active PKCδ fragment (PKCδ CF) blocks, cisplatin-induced LC3-II formation. RPTC were transfected with PKCδ-KD or PKCδ-CF, and then treated with 20 μM cisplatin for 6 hours to collect whole cell lysates for immunoblot analysis of LC-3 and PKCδ, and also β-actin as a protein loading control. (C) Representative cell images showing punctate GFP-LC3 distribution. RPTC were cotransfected with GFP-LC3 and another indicated plasmid (empty vector, PKCδ-KD, or PKCδ-CF), and then treated with 20 μM cisplatin for 6 hours. (D) The number of GFP-LC3 puncta per cell in each of the conditions described in (C) was assessed. (E) Regulation of mTOR during cisplatin treatment by PKCδ. RPTC were transfected with PKCδ-KD or PKCδ-CF, and then treated with 20 μM cisplatin for 6 hours to collect whole cell lysates for immunoblot analysis of total and phosphorylated mTOR, p70S6k, and ULK1. Data in (D) are expressed as mean±SD (n=4); Δ, statistically significantly different from the Vector group (P<0.05); ▲, statistically significantly different from Cisplatin+vector group (P<0.05). Data are representative of at least four separate experiments. The results suggest that PKCδ is activated during cisplatin treatment to induce mTOR to suppress autophagy.
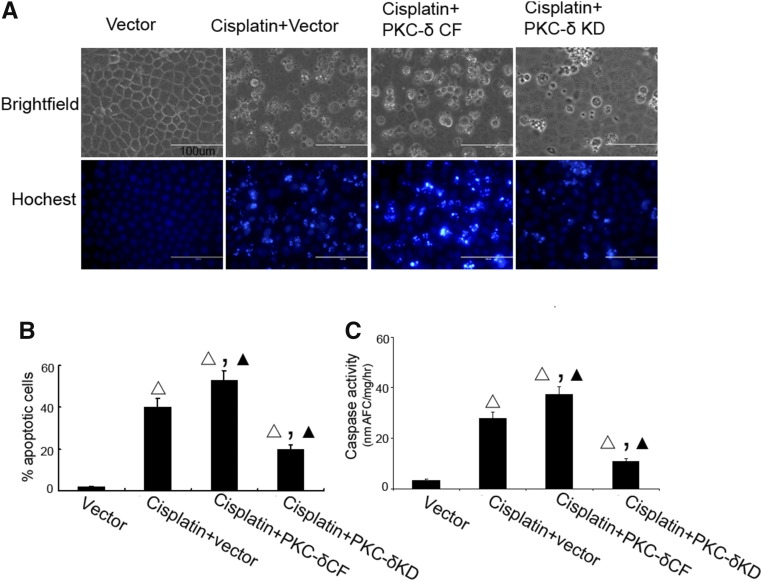
We further monitored the effects of PKCδ-KD and PKCδ-CF on apoptosis during cisplatin treatment. In morphologic analysis, cisplatin induced significant apoptosis in RPTC, which was increased by PKCδ-CF and suppressed by PKCδ-KD (Figure 5, A and B). The morphologic analysis was supported by the results of caspase activity assay (Figure 5C). It was concluded that PKCδ might suppress autophagy to facilitate apoptosis.
Figure 5.
PKCδ mediates AKT phosphorylation on Ser473. (A) AKT phosphorylation at S473 (but not T308) was suppressed by PKCδ-KD. RPTC were transfected with PKCδ-KD or PKCδ-CF, and then treated with 20 μM cisplatin for 6 hours to collect whole cell lysates for immunoblot analysis of p-AKT-S473, p-AKT-T308, and total AKT, and also β-actin as a protein loading control. (B, C) Reciprocal coimmunoprecipitation of AKT and PKCδ. RPTC were incubated with 20 μM cisplatin for 0–6 hours to collect whole cell lysates for immunoprecipitation using anti-PKCδ (B) or anti-AKT (C). The immunoprecipitates were analyzed for AKT and PKCδ by immunoblotting. (D) PKCδ can phosphorylate AKT on Ser473 in vitro. Recombinant active PKCδ (0.1 μg per reaction) were incubated with 0.1 μg recombinant inactive AKT for 0–60 minutes. Reaction products were analyzed by immunoblotting.
PKCδ Binds to and Phosphorylates AKT at Ser473
To investigate the mechanism whereby PKCδ activates mTOR, we first postulated that PKCδ might interact with and directly phosphorylate mTOR. However, coimmunoprecipitation assay did not show the binding of PKCδ to mTOR in control or cisplatin-treated cells (data not shown). We then hypothesized that PKCδ may regulate mTOR indirectly through AKT, because AKT was shown to play a role in mTOR activation during cisplatin treatment (Figure 3). As shown in Figure 6A, AKT phosphorylation at both Ser473 and Thr308 was induced during cisplatin treatment. PKCδ-CF further increased p-AKT(S473), whereas PKCδ-KD had opposite effects. Interestingly, neither PKCδ-CF or PKCδ-KD affected pAKT(T308), suggesting a site specific regulation of AKT by PKCδ. We further assessed whether AKT physically interacted with PKCδ by coimmunoprecipitation. Anti-PKCδ precipitated both PKCδ and AKT (Figure 6B). Anti-AKT also pulled down both AKT and PKCδ (Figure 6C). Reciprocal co-IP of these two proteins was consistently detected at 6 hours of cisplatin treatment (Figure 6, B and C). It was unclear whether PKCδ could directly phosphorylate AKT. To test this possibility, we incubated AKT with recombinant active PKCδ in a kinase reaction buffer. As shown in Figure 6D, this incubation led to evident phosphorylation of AKT at Ser473, but not at Thr308. This test tube experiment, together with the observation of cisplatin-treated cells (Figure 6A), suggested that PKCδ may directly phosphorylate AKT at Ser473 resulting in AKT activation during cisplatin treatment.
Figure 6.
Effects of PKCδ inhibition on cisplatin-induced apoptosis in RPTC. (A–C) RPTC were transfected with PKCδ-KD or PKCδ-CF, and then treated with 20 μM cisplatin for 16 hours. (A) Morphology. After treatment, cells were stained with Hoechst33342 to record cellular and nuclear morphology by phase-contrast and fluorescence microscopy. Bar, 100 μm. (B) Percentage of apoptosis assessed by counting the cells with typical apoptotic morphology. (C) Caspase activity. Cell lysate was collected for enzymatic assay of caspase activity. Δ, statistically significantly different from the Vector group (P<0.05); ▲, statistically significantly different from Cisplatin+vector group (P<0.05). Data are representative of at least four separate experiments.
Restoration of Cisplatin-Induced Kidney Injury in PKCδ-Null Mice by CQ
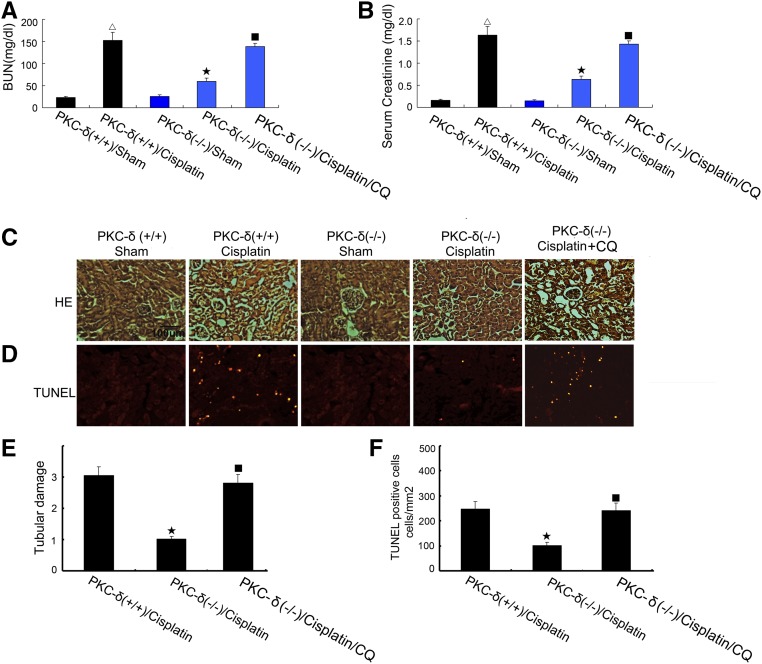
To demonstrate the role of PKCδ in autophagy regulation in vivo, we compared PKCδ-null (PKCδ−/−) mice and their wild-type (PKCδ+/+) littermates for the response to cisplatin treatment. In wild-type mice, cisplatin induced severe renal failure as shown by high levels of blood urea nitrogen (BUN) (152 mg/dl) and serum creatinine (1.6 mg/dl) at day three of treatment. In contrast, PKCδ−/− mice showed significantly better renal function, with 85 mg/dl BUN and 0.9 mg/dl serum creatinine (Figure 7, A and B). Notably, inhibition of autophagy with CQ restored cisplatin injury in PKCδ−/− mice (Figure 7, A and B), supporting a role of autophagy in the observed kidney injury resistance of these mice. Consistently, PKCδ−/− mice showed a better renal histology and less apoptosis than wild-type mice after cisplatin treatment, and again CQ restored kidney tissue damage and apoptosis in PKCδ−/− mice (Figure 7, C and D). At day three of cisplatin treatment, the tissue damage score was 3.1 for wild-type mice, 0.99 for PKCδ−/− mice, and 2.8 for PKCδ−/− mice with CQ, respectively (Figure 7F). This observation was further verified by counting apoptotic cells in kidney cortical and outer medulla regions (Figure 7G).
Figure 7.
Restoration of cisplatin-induced kidney injury in PKCδ-null mice by CQ. (A–D) Wild-type and PKCδ−/− littermate mice (male, 8–10 weeks of age) were injected with 30 mg/kg cisplatin in the absence or presence of 50 mg/kg per day CQ. At day three, the animals were euthanized to collect blood samples to measure BUN (A) and serum creatinine (B). Kidney tissues were collected for H&E staining of histology (C), TUNEL assay of apoptosis (D), and tubular damage and TUNEL-positive apoptosis were also semiquantified (E, F). Δ, statistically significantly different from the Sham group without cisplatin treatment (P<0.05); ★, statistically significantly different from wild-type mice with cisplatin treatment group (P<0.05); ■, statistically significantly different from PKCδ−/− mice with cisplatin treatment group (P<0.05).
PKCδ-Null Mice Show High Renal Autophagy under Control and Cisplatin Treatment Conditions
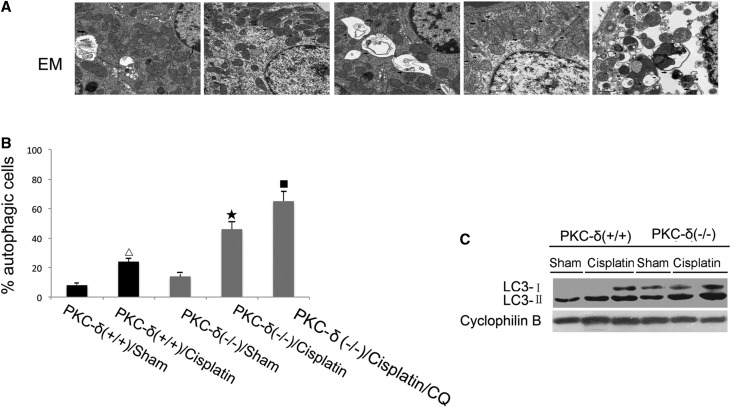
To examine autophagy in kidney tissues, we used electron microscopy. Three days after cisplatin injection, numerous vacuoles appeared in some proximal tubular cells and some of the vacuoles contained cytoplasmic materials, indicative of autophagic vesicles (Figure 8A). For quantification, we counted the cells with autophagic vesicles (Figure 8B). After cisplatin treatment, 23% of renal tubular cells were autophagic in wild-type mice, and 44% in PKCδ−/− mice. CQ further increased it to 66% (Figure 8B). To verify this observation, we collected kidney tissues for immunoblot analysis of LC3 (Figure 8C). PKCδ−/− tissue showed significantly higher LC3 than wild-type mice, and there was a notable increase of LC3-II in renal tissues of wild-type mice after cisplatin injection, which was further increased in PKCδ−/− mice with cisplatin. Together, these results suggested that enhanced autophagy may be responsible for the resistance of PKCδ-null mice to cisplatin nephrotoxicity.
Figure 8.
PKCδ-null mice show higher renal autophagy. Wild-type and PKCδ−/− mice were injected with 30 mg/kg cisplatin. (A, B) Kidney tissues were collected for electron microscopy analysis to determine the percentage of tubular cells with notable autophagic vesicles. (B) Kidney tissues were analyzed for immunoblot of LC3 and cyclophilin B (loading control). Δ, statistically significantly different from the Sham group without cisplatin treatment (P<0.05); ★, statistically significantly different from wild-type mice with cisplatin treatment group (P<0.05); ■, statistically significantly different from PKCδ−/− mice with cisplatin treatment group (P<0.05). Data are representative of at least four separate experiments.
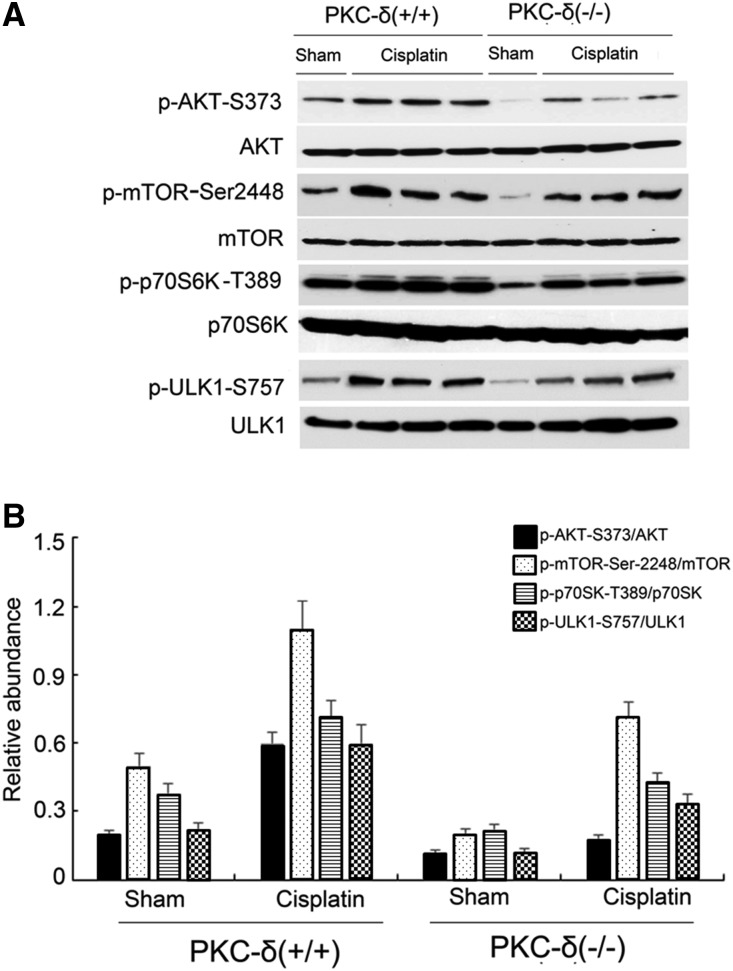
Cisplatin-Induced AKT/mTOR Activation in Kidney Tissues Is Blocked in PKCδ−/− Mice
We further determined the involvement of AKT/mTOR in PKCδ signaling in vivo during cisplatin nephrotoxicity. In wild-type mice, cisplatin induced p-AKT-S473, p-mTOR-Ser2448, p-p70S6K-T389, and p-ULK1-S757 in kidney tissues (Figure 9), indicative of the activation of the AKT/mTOR/ULK1 pathway for autophagy suppression. Notably, this pathway was significantly less active in PKCδ−/− tissues at both control and cisplatin treatment conditions (Figure 9). These in vivo data were consistent with our cell culture results (Figures 4 and 5), suggesting that AKT/mTOR was downstream of PKCδ in autophagic signaling during cisplatin nephrotoxicity.
Figure 9.
Cisplatin-induced AKT/mTOR activation in kidney tissues is blocked in PKCδ−/− mice. Wild-type and PKCδ−/− mice were injected with 30 mg/kg cisplatin to collect whole kidney lysates for immunoblotting analysis of total and phosphorylated AKT, mTOR, p70S6k, and p-ULK1. (A) Representative immunoblots. (B) Densitometry analysis of proteins signals on immunoblots.
The Renoprotective Effect of Rottlerin Is Lost in Kidney Autophagy–Deficient Mice
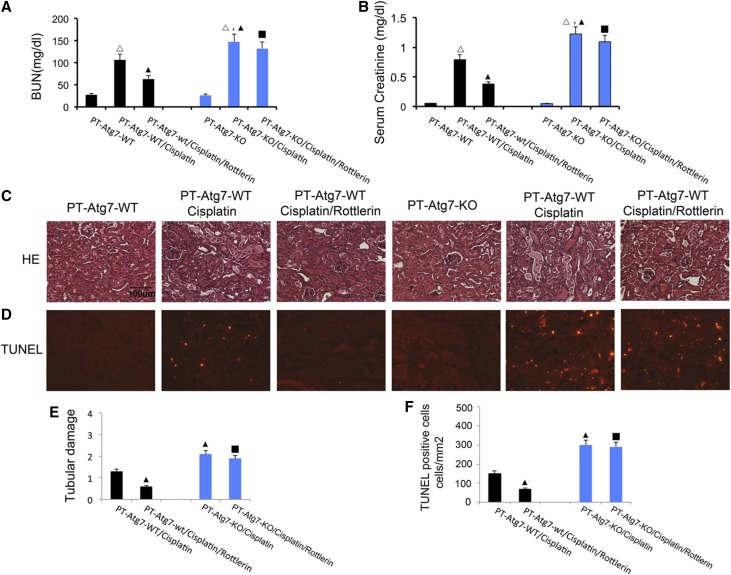
Our recent work demonstrated a critical role of PKCδ in cisplatin nephrotoxicity and inhibitors of PKCδ showed remarkable renoprotective effects.8 The results presented thus far in this study have suggested that PKCδ may activate AKT/mTOR to suppress renal autophagy to contribute to cisplatin-induced injury and cell death. To further investigate this in vivo, we determined whether the renoprotective effect of rottlerin, a pharmacologic PKCδ inhibitor, depended on autophagy in renal tubules. For this purpose, we tested a conditional knockout model in which Atg7 was ablated specifically from kidney proximal tubule cells (PT-Atg7-KO).15 Essentially, the effects of rottlerin on cisplatin-induced kidney injury in PT-Atg7-KO and wild-type littermate mice were examined and compared. Without treatment, PT-Atg7-KO mice and their wild-type littermates showed similarly low levels of BUN and serum creatinine, indicating normal renal function. Three days after cisplatin injection, wild-type mice developed moderate renal failure as indicated by 104 mg/dl BUN and 0.8 mg/dl serum creatinine, which was significantly attenuated by rottlerin (Figure 10). In sharp contrast, cisplatin induced more severe renal failure in PT-Atg7-KO mice with 148 mg/dl BUN and 1.3 mg/dl serum creatinine (Figure 10, A and B). Importantly, rottlerin could not ameliorate cisplatin-induced renal failure in PT-Atg7-KO mice. Histopathologic analysis confirmed that, compared with wild-type, cisplatin induced much severer kidney tissue damage in PT-Atg7-KO mice with widespread, extensively damaged proximal tubules, and notably, rottlerin reduced kidney tissue damage in wild-type mice, but not in PT-Atg7-KO mice (Figure 10C). In quantification, wild-type mice had a tissue damage score of 1.2 that was reduced to 0.5 by rottlerin, whereas PT-Atg7-KO mice had a score of 2.1 that was not affected by rottlerin (Figure 10E). This observation was further verified by counting apoptotic cells in kidney tissues (Figure 10, D and F), showing that rottlerin was effective in reducing apoptosis in wild-type mice, but not in PT-Atg7-KO mice. Together, these results suggested that the renoprotective effect of PKCδ inhibition was at least partly mediated by autophagy.
Figure 10.
The renoprotective effect of rottlerin is lost in kidney autophagy deficient mice. PT-Atg7-WT and PT-Atg7-KO mouse littermates (male, 8–10 weeks of age) were injected with 30 mg/kg cisplatin in the absence or presence of 10 mg/kg/day rottlerin. Blood samples were collected at 3 days to measure BUN (A) and serum creatinine (B). Kidney tissues were collected for H&E staining of histology (C) and TUNEL assay of apoptosis (D). Tubular damage and apoptosis were also semiquantified (E, F). Δ, statistically significantly different from the control group without cisplatin treatment (P<0.05); ▲, statistically significantly different from PT-Atg7-WT/cisplatin group (P<0.05); ■, not different from PT-Atg7-KO/cisplatin group (P>0.05). Data are representative of at least four separate experiments.
Discussion
Nephrotoxicity is a major side effect in cisplatin chemotherapy that limits the use and efficacy of cisplatin in cancer patients. PKCδ was recently identified as a novel regulator of cisplatin nephrotoxicity.8 Remarkably, inhibitors of PKCδ protected against cisplatin-induced kidney tubular cell death and tissue damage while enhancing chemotherapeutic effects in tumors, supporting their potential of clinical applicability.8 However, it was unclear as to how PKCδ mediates cisplatin nephrotoxicity and how PKCδ inhibitors protect kidney cells and tissues. In this study, we have demonstrated that PKCδ suppresses autophagy during cisplatin treatment of kidney cells in vitro and mouse kidneys in vivo, contributing to cisplatin nephrotoxicity. Our results further suggest that PKCδ inhibitors may protect kidneys by upregulating autophagy in renal tubular cells. Mechanistically, the results have, for the first time, demonstrated the evidence of direct phosphorylation and activation of AKT by PKCδ. Upon activation, AKT further activates mTOR to induce the inhibitory phosphorylation of ULK1, resulting in the suppression of autophagy. As a result, this study has unveiled a novel pathway of PKCδ/AKT/mTOR/ULK1 that is responsible for autophagy suppression during cisplatin treatment of kidney cells and tissues, contributing to cisplatin nephrotoxicity during chemotherapy. Accordingly, blockade of this pathway, e.g., by inhibiting PKCδ, maintains autophagy during cisplatin treatment to afford renoprotection.
PKCδ has been implicated in the regulation of a variety of cellular processes, ranging from apoptosis to cell survival, migration, and proliferation.27–31 Notably, the role of PKCδ depends on the cellular context and experimental conditions. As a result, apparently conflicting observations have been reported and the cellular function of PKCδ was described to be “complex and enigmatic”.30 Of much relevance to this study, PKCδ is known to be proapoptotic in some cell types and tissues (e.g., kidneys and HeLa cells8,32), but it is prosurvival in multiple cancer cell lines.33–37 It remains elusive as to how PKCδ regulates apoptosis. This study suggests that PKCδ may contribute to cisplatin-induced kidney cell apoptosis and nephrotoxicity by suppressing autophagy. First, PKCδ activation suppressed autophagy, whereas PKCδ inhibition upregulated autophagy during cisplatin treatment, indicating clearly that PKCδ negatively regulates autophagy under the experimental condition (Figure 4). Second, autophagy regulation by PKCδ was accompanied by changes in apoptosis (Figures 4 and 6). For example, blockade of PKCδ with a dominant negative mutant led to increased autophagy during cisplatin treatment (Figure 4) and a marked decrease in apoptosis (Figure 6). In addition, we and others have demonstrated autophagy as a critical protective mechanism during cisplatin treatment of kidney cells in vitro and nephrotoxicity in vivo. Finally, in this study the protective effect of rottlerin (PKCδ inhibitor) was diminished in kidney tubule–specific autophagy deficient mice (Figure 9), indicating that the effect of PKCδ inhibitors depends on autophagy. Together, these results have identified autophagy suppression as the key mechanism whereby PKCδ mediates cell injury and death.
Regulation of autophagy by PKCδ has been reported recently, but the results from these studies are not consistent. In 2008, Ann and colleagues demonstrated a role of PKCδ in autophagy activation in the model of acute hypoxic stress, where PKCδ was shown to promote JNK1-mediated Bcl-2 phosphorylation leading to the dissociation and release of Beclin-1 from Bcl-2 for autophagy.18,38 Consistently, more recent work by Shahnazari et al. showed that PKCδ was essential to autophagy activation for bacterial clearance from mammalian cells.20 In sharp contrast, PKCδ was suggested to suppress autophagy in pancreatic ductal carcinoma cells by inducing tissue transglutaminase 2.17,39 In addition, inhibition of PKCδ by Safingol (synthetic L-threo–stereoisomer of sphinganine) led to the activation of autophagy in HCT116 colorectal cancer cells.19 The exact cause of the discrepancy between these few studies is unclear. This study demonstrates an autophagy suppressive role of PKCδ. In both in vitro cell culture and in vivo mouse models of cisplatin nephrotoxicity, blockade of PKCδ led to upregulation of autophagy (Figures 4, 7), suggesting that, upon activation, PKCδ suppresses autophagy during cisplatin treatment of kidney cells and tissues. Thus the role of PKCδ depends on, and may change according to, the cellular context and experimental conditions.18
This study provides significant insights into the signaling pathways that contribute to the regulation of cisplatin-induced kidney cell injury and nephrotoxicity. In 2008, we and Kaushal’s group first reported autophagy during cisplatin treatment of kidney tubular cells.13,14 By electron microscopy and immunoblotting of LC3, we further suggested autophagy in vivo in kidneys after cisplatin injection in mice.13 These and subsequent studies, especially those using renal tubule–specific autophagy-deficient mouse models,15,16 have demonstrated convincing evidence for a renoprotective role of tubular autophagy in cisplatin nephrotoxicity. Consistently, autophagy has been demonstrated to be a major renoprotective mechanism in kidney injury induced by renal ischemia-reperfusion.15,40–42 Despite these functional studies, there is a significant lack of understanding of the signaling pathways and/or regulatory mechanisms that govern autophagy in renal tubular cells in AKI conditions. In this study, mTOR did not decrease to account for initial autophagy activation after cisplatin treatment; instead, it was activated as a negative regulation mechanism of autophagy by phosphorylating ULK1 (Figure 2). Upstream of mTOR, our results suggest the signaling relay from AKT (Figure 3). Interestingly, PKCδ was shown to be further upstream of AKT (Figure 6). Therefore, we have elucidated a novel signaling pathway consisting of PKCδ/AKT/mTOR/ULK1 that is activated during cisplatin nephrotoxicity to suppress autophagy. In view of the protective role of autophagy, it is suggested that this signaling pathway is important to the occurrence of tubular cell apoptosis and nephrotoxicity during cisplatin therapy. It is noteworthy that AKT activation occurred earlier than mTOR during cisplatin treatment (Figures 2 and 3). One possibility is that AKT may phosphorylate mTOR at early time-points and p-mTOR may last to later time-points; in this case, later p-mTOR and the associated autophagy decrease still depend on PKCδ-AKT signaling. Nonetheless, autophagy decrease at a late stage of cisplatin treatment may also involve other mechanisms.
Regulation of AKT/mTOR by PKCδ has been reported previously. For example, PKCδ inhibitors suppressed AKT/mTOR and associated signaling in a murine model of allergic asthma.43 The latest work by Dai et al. further suggested that PKCδ might regulate the proliferation and migration of human gastric adenocarcinoma cells via the AKT/mTOR/p70S6 kinase pathway.44 Despite these findings, whether PKCδ can regulate AKT directly via phosphorylation remains unclear. Our current data provide evidence that PKCδ may directly phosphorylate AKT during cisplatin treatment of kidney cells and tissues (Figure 6). In RPTC cells, cisplatin induced AKT phosphorylation at Ser473, which was further increased by active PKCδ and attenuated by dominant negative PKCδ. Our co-IP analysis also suggested the interaction of PKCδ with AKT and, notably, the interaction increased during cisplatin treatment (Figures 5C and 6B). Moreover, incubation of AKT with active PKCδ induced specific AKT phosphorylation at Ser473 (Figure 6D). Thus, PKCδ may regulate AKT by directly phosphorylating AKT at Ser473 under this experimental condition. Obviously, such a conclusion requires further in-depth investigation to substantiate. If proven true, it may have significant implications for the understanding of AKT regulation. It is known that AKT activation depends on its phosphorylation at Thr308 in the T-loop close to the catalytic core of the kinase domain and Ser473 near the carboxyl terminus.45 Phosphoinositide-dependent kinase 1 (PDK1) is responsible for Thr308 phosphorylation, but the molecular basis of Ser473 phosphorylation remains elusive. One possibility is that, after Thr308 phosphorylation and initial activation, AKT may autophosphorylate Ser473. However, the autophosphorylation hypothesis has been seriously challenged. For example, in PDK1-deficient cells, AKT is not phosphorylated at Thr308 and shows low activity, but Ser473 phosphorylation can still be induced by insulin.46 Although this and other studies47 suggest the existence of a distinct kinase-PDK2 that is responsible for Ser473 phosphorylation and full activation of AKT, the identify of PDK2 remains a mystery. This study suggests that PKCδ may be a candidate of PDK2 that is responsible for AKT phosphorylation at Ser473 during cisplatin treatment of kidney cells and tissues. Further investigation needs to extend this finding to verify if PKCδ phosphorylates AKT-Ser473 under other experimental conditions.
In conclusion, this study has delineated a novel signaling pathway consisting of PKCδ, AKT, mTOR, and ULK1, which is activated in experimental models of cisplatin nephrotoxicity and negatively regulates autophagy. In this pathway, PKCδ may directly interact with and phosphorylate AKT at serine-473, resulting in full activation of this kinase. AKT then phosphorylates and activates mTOR to induce inhibitory phosphorylation of ULK1, leading to autophagy suppression. Suppression of autophagy by this pathway contributes to kidney cell death and tissue damage in cisplatin nephrotoxicity. Accordingly, the study further suggests that PKCδ inhibitors protect kidneys during cisplatin treatment at least in part by blocking this pathway to facilitate autophagy, a major protective mechanism in kidney cells.
Concise Methods
Reagents
The following primary antibodies were used: anti-LC3 from Novus Biologicals (Littleton, CO); anti–β-actin and anti-ULK1 from Sigma-Aldrich (St. Louis, MO); anti-cyclophilin B from Abcam Inc. (Cambridge, MA); anti-PKCδ, anti-AKT, anti–phospho-AKT(S473), anti–phospho-AKT(Thr308), anti-mTOR, anti–phospho-mTOR(S2448), anti-p70S6K, anti–phospho-p70S6K (T389), and anti–phospho-ULK1(S757) from Cell Signaling Technology (Danvers, MA). All secondary antibodies for immunoblot analysis were from Thermo Fisher Scientific (Vernon Hills, IL). Carbobenzoxy-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin (DEVD.AFC) and 7-amino-4-trifluoromethyl coumarin (AFC) were from Enzyme Systems Products (Livermore, CA). Recombinant active PKCδ and recombinant inactive AKT were purchased from R&D Systems (Minneapolis, MN) and BD Biosciences (San Jose, CA), respectively. AKT inhibitor VIII was from Calbiochem (San Diego, CA). Cyquant-NF cell proliferation assay kit was from Invitrogen (Carlsbad, CA). Unless indicated, all other reagents (cisplatin, rapamycin, etc.) were from Sigma-Aldrich.
Experimental Models of Cisplatin Nephrotoxicity
PKCδ–/–mice were generated by targeted gene deletion as described previously48 by Dr. Robert Messing’s laboratory at the University of California, San Francisco, CA. The PT-Atg7-KO mouse model was established by breeding Atg7flox/flox mice49 with PEPCK-Cre transgenic mice obtained from Dr. Volker Haase (University of Pennsylvania, Philadelphia, PA).50 The transgenic mouse lines were mainly in C57 background. All animals were housed in the animal facility of Charlie Norwood Veterans Affairs Medical Center. Animal experiments were conducted with the approval of and in accordance with the guidelines established by the Institutional Animal Care and Use Committee of Charlie Norwood Veterans Affairs Medical Center and Medical College of Georgia. The RPTC rat kidney proximal tubule line was described previously.13,40 For in vivo study, mice were injected with a single dose of 30 mg/kg cisplatin to induce kidney injury, as described previously.8,13,40 Control animals were injected with saline. For in vitro study, RPTC were incubated with 20 μM cisplatin, which induced significant apoptosis as indicated previously.8,13,40
Analysis of Renal Function and Histology
Renal failure or loss of renal function was indicated by serum creatinine and BUN using commercial kits as previously described.8,13,40 For histology, kidney tissues were fixed with 4% paraformaldehyde for paraffin embedding and H&E staining. Tissue damage was scored by the percentage of renal tubules with cell lysis, loss of brush border, and cast formation (0, no damage; 1, <25%; 2, 25%–50%; 3, 50%–75%; 4, >75%).
Analysis of Apoptosis
Apoptosis in kidney tissues was analyzed by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay using the In Situ Cell Death Detection Kit from Roche Diagnostics (Indianapolis, IN), as described in our previous work.8,13,40 Apoptosis in cell cultures was analyzed by standard methods, including cell morphology and caspase activity. For morphologic analysis, cells were stained with Hoechst33342. The cells showing typical morphologic features, including cellular and nuclear condensation and fragmentation, were counted to determine the percentage of apoptosis. Caspase activity was measured by an enzymatic assay as previously described.8,13,40 Briefly, cells were lysed with a buffer containing 1% Triton X-100. The lysates of 25 μg protein were added to an enzymatic assay buffer containing 50 μM DEVD.AFC for 60 minutes at 37°C. Fluorescence at excitation 360 nm/emission 535 nm was measured with a GENios plate-reader (Tecan US Inc.). Free DEVD.AFC was used to plot a standard curve, and, using the standard curve, the fluorescence reading from the enzymatic reaction was converted into the nanomolar amount of DEVD.AFC liberated per mg protein per hour as a measure of caspase activity.
Transfection of RPTC
Various PKC plasmids were obtained from Dr. Jae-Won Soh (Inha University, Inchun, Republic of Korea) and Dr. Fushin Yu (Wayne State University, Detroit, MI). The GFP-LC3 plasmid was a generous gift from Dr. Yoshimori (National Institute of Genetics, Mishima, Japan). The RPTC line was obtained from Dr. U. Hopfer (Case Western Reserve University, Cleveland, OH) and maintained for experiments as described previously.51 Cells were plated at 0.5×106 cells per 35 mm dish to reach 50%–60% confluence after overnight growth. The cells were then transfected with 1 μg PKC plasmids (PKCδ-KD, PKCδ-CF) using Lipofectamin 2000 (Invitrogen). To identify the transfected cells for analysis, 0.2 μg pEGFP-C3 was cotransfected. The cells were subjected to experimental treatment after 24 hours of transfection.
Analysis of Autophagy after GFP-LC3 Transfection
GFP-LC3 puncta formation was examined to show autophagy in RPTC cells as described previously.8,13,40 Briefly, cells were plated on a coverslip to reach 50% confluence for transfection with 1 μg of GFP-LC3 by using Lipofectamine reagent (Invitrogen). The cells were then maintained in culture medium for 24 hours to reach 80%–90% confluence for cisplatin treatment. At the end of incubation, cells were fixed with 4% paraformaldehyde and examined by fluorescence microscopy to collect images and evaluate GFP-LC3 puncta.
Coimmunoprecipitation of PKCδ and AKT
Kidney tissue and cell lysates were collected with the immunoprecipitation lysis buffer and subjected to immunoprecipitation using an anti-PKCδ or anti-AKT antibody. The resultant precipitates were analyzed by gel electrophoresis and immunoblotting for AKT and PKCδ.
Test Tube Analysis of AKT Phosphorylation by PKCδ
Recombinant active PKCδ (0.1 μg) and recombinant inactivate AKT1 (0.1 μg) were suspended in the kinase reaction buffer containing 20 μM ATP for 5, 15, and 60 minutes of incubation at 30°C. After the incubation, 2% SDS was added to terminate the reaction. The samples were then subjected to gel electrophoresis and transferred to PVDF membrane for immunoblot analysis of AKT, phospho-AKT(S473), and phospho-AKT(Thr308).
Immunoblot Analysis
Kidney tissue and cell lysates were lysed in 2% SDS buffer containing protease inhibitor cocktail and nuclease. Protein concentration was determined with BCA reagent from Thermo Fisher Scientific. Equal amounts (50–100 μg) of protein were loaded in each lane and separated on SDS-polyacrylamide electrophoresis gel followed by immunoblot analysis by standard method.
Statistical Analyses
Qualitative data shown in this study, including immunoblots and cell and tissue images, are representative of at least three separate experiments. Quantitative data are expressed as mean±SD. t test was used to determine the statistical significance in the differences between two groups. One-way ANOVA followed by Tukey post-hoc test was used to compare multiple treatment groups. Two-way ANOVA was used to assess the statistical significance of the differences between multiple treatment groups at different time points. Statistical analysis was performed using GraphPad Prism version software (GraphPad Software, La Jolla, CA). P<0.05 was considered to reflect significant differences.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Robert Messing at the University of California (San Francisco, CA) for providing PKCδ mice, Dr. Masaaki Komatsu at Tokyo Metropolitan Institute of Medical Science (Tokyo, Japan) for Atg7-floxed mice, Dr. Jae-Won Soh (Inha University, Inchun, Korea) and Dr. Fushin Yu (Wayne State University, Detroit, MI) for PKCδ plasmids, and Dr. Tamotsu Yoshimori (National Institute of Genetics, Mishima, Japan) for GFP-LC3 plasmid, respectively.
The study was supported in part by grants from the National Natural Science Foundation of China (81370791, 81570622, 81570646, 81430017), the National Basic Research Program of China 973 Program No. 2012CB517600 (No. 2012CB517601), and the National Institutes of Health (2R01DK058831, 1R01 DK087843) and the US Department of Veterans Administration (5I01BX000319).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016030337/-/DCSupplemental.
References
- 1.Wang D, Lippard SJ: Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4: 307–320, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Pérez JM: Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem 7: 3–18, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M; European Society of Clinical Pharmacy Special Interest Group on Cancer Care : Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother Pharmacol 61: 903–909, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Pabla N, Dong Z: Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Finkel M, Goldstein A, Steinberg Y, Granowetter L, Trachtman H: Cisplatinum nephrotoxicity in oncology therapeutics: retrospective review of patients treated between 2005 and 2012. Pediatr Nephrol 29: 2421–2424, 2014 [DOI] [PubMed] [Google Scholar]
- 6.dos Santos NA, Carvalho Rodrigues MA, Martins NM, dos Santos AC: Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol 86: 1233–1250, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB: Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel) 2: 2490–2518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, Dong Z: Inhibition of PKCδ reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest 121: 2709–2722, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pabla N, Dong Z: Curtailing side effects in chemotherapy: a tale of PKCδ in cisplatin treatment. Oncotarget 3: 107–111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizushima N, Levine B, Cuervo AM, Klionsky DJ: Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z, Klionsky DJ: Eaten alive: a history of macroautophagy. Nat Cell Biol 12: 814–822, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohsumi Y: Historical landmarks of autophagy research. Cell Res 24: 9–23, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z: Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int 74: 631–640, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Kaushal V, Shah SV, Kaushal GP: Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol 294: F777–F787, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z: Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 82: 1271–1283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi A, Kimura T, Takabatake Y, Namba T, Kaimori J, Kitamura H, Matsui I, Niimura F, Matsusaka T, Fujita N, Yoshimori T, Isaka Y, Rakugi H: Autophagy guards against cisplatin-induced acute kidney injury. Am J Pathol 180: 517–525, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Ozpolat B, Akar U, Mehta K, Lopez-Berestein G: PKC delta and tissue transglutaminase are novel inhibitors of autophagy in pancreatic cancer cells. Autophagy 3: 480–483, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Chen JL, Lin HH, Kim KJ, Lin A, Ou JH, Ann DK: PKC delta signaling: a dual role in regulating hypoxic stress-induced autophagy and apoptosis. Autophagy 5: 244–246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coward J, Ambrosini G, Musi E, Truman JP, Haimovitz-Friedman A, Allegood JC, Wang E, Merrill AH Jr, Schwartz GK: Safingol (L-threo-sphinganine) induces autophagy in solid tumor cells through inhibition of PKC and the PI3-kinase pathway. Autophagy 5: 184–193, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Shahnazari S, Yen WL, Birmingham CL, Shiu J, Namolovan A, Zheng YT, Nakayama K, Klionsky DJ, Brumell JH: A diacylglycerol-dependent signaling pathway contributes to regulation of antibacterial autophagy. Cell Host Microbe 8: 137–146, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Kundu M, Viollet B, Guan KL: AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizushima N: The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 22: 132–139, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Hay N, Sonenberg N: Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kaushal GP, Kaushal V, Hong X, Shah SV: Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int 60: 1726–1736, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Zhou D, Tan RJ, Lin L, Zhou L, Liu Y: Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int 84: 509–520, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livingston MJ, Dong Z: Autophagy in acute kidney injury. Semin Nephrol 34: 17–26, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu A: Involvement of protein kinase C-delta in DNA damage-induced apoptosis. J Cell Mol Med 7: 341–350, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brodie C, Blumberg PM: Regulation of cell apoptosis by protein kinase c delta. Apoptosis 8: 19–27, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Reyland ME: Protein kinase Cdelta and apoptosis. Biochem Soc Trans 35: 1001–1004, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Jackson DN, Foster DA: The enigmatic protein kinase Cdelta: complex roles in cell proliferation and survival. FASEB J 18: 627–636, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Steinberg SF: Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem J 384: 449–459, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu A, Woolard MD, Johnson CL: Involvement of protein kinase C-delta in DNA damage-induced apoptosis. Cell Death Differ 8: 899–908, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Clark AS, West KA, Blumberg PM, Dennis PA: Altered protein kinase C (PKC) isoforms in non-small cell lung cancer cells: PKCdelta promotes cellular survival and chemotherapeutic resistance. Cancer Res 63: 780–786, 2003 [PubMed] [Google Scholar]
- 34.McCracken MA, Miraglia LJ, McKay RA, Strobl JS: Protein kinase C delta is a prosurvival factor in human breast tumor cell lines. Mol Cancer Ther 2: 273–281, 2003 [PubMed] [Google Scholar]
- 35.Wang Q, Wang X, Evers BM: Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J Biol Chem 278: 51091–51099, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Xia S, Chen Z, Forman LW, Faller DV: PKCdelta survival signaling in cells containing an activated p21Ras protein requires PDK1. Cell Signal 21: 502–508, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia S, Forman LW, Faller DV: Protein kinase C delta is required for survival of cells expressing activated p21RAS. J Biol Chem 282: 13199–13210, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakaki K, Wu J, Kaufman RJ: Protein kinase Ctheta is required for autophagy in response to stress in the endoplasmic reticulum. J Biol Chem 283: 15370–15380, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashour AA, Abdel-Aziz AA, Mansour AM, Alpay SN, Huo L, Ozpolat B: Targeting elongation factor-2 kinase (eEF-2K) induces apoptosis in human pancreatic cancer cells. Apoptosis 19: 241–258, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Jiang M, Liu K, Luo J, Dong Z: Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol 176: 1181–1192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N, Walz G, Huber TB: Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 8: 826–837, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T, Kitamura H, Niimura F, Matsusaka T, Soga T, Rakugi H, Isaka Y: Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22: 902–913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi YH, Jin GY, Li LC, Yan GH: Inhibition of protein kinase C delta attenuates allergic airway inflammation through suppression of PI3K/Akt/mTOR/HIF-1 alpha/VEGF pathway. PLoS One 8: e81773, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai L, Zhuang L, Zhang B, Wang F, Chen X, Xia C, Zhang B: DAG/PKCδ and IP3/Ca²⁺/CaMK IIβ Operate in Parallel to Each Other in PLCγ1-Driven Cell Proliferation and Migration of Human Gastric Adenocarcinoma Cells, through Akt/mTOR/S6 Pathway. Int J Mol Sci 16: 28510–28522, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheid MP, Woodgett JR: PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol 2: 760–768, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Williams MR, Arthur JS, Balendran A, van der Kaay J, Poli V, Cohen P, Alessi DR: The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol 10: 439–448, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Hill MM, Andjelkovic M, Brazil DP, Ferrari S, Fabbro D, Hemmings BA: Insulin-stimulated protein kinase B phosphorylation on Ser-473 is independent of its activity and occurs through a staurosporine-insensitive kinase. J Biol Chem 276: 25643–25646, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, Lowell CA, Ferriero DM, Messing RO: Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J Clin Invest 114: 49–56, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T: Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169: 425–434, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rankin EB, Tomaszewski JE, Haase VH: Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woost PG, Orosz DE, Jin W, Frisa PS, Jacobberger JW, Douglas JG, Hopfer U: Immortalization and characterization of proximal tubule cells derived from kidneys of spontaneously hypertensive and normotensive rats. Kidney Int 50: 125–134, 1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.