Abstract
Previous studies have suggested the benefits of physical exercise for patients on dialysis. We conducted the Exercise Introduction to Enhance Performance in Dialysis trial, a 6-month randomized, multicenter trial to test whether a simple, personalized walking exercise program at home, managed by dialysis staff, improves functional status in adult patients on dialysis. The main study outcomes included change in physical performance at 6 months, assessed by the 6-minute walking test and the five times sit-to-stand test, and in quality of life, assessed by the Kidney Disease Quality of Life Short Form (KDQOL-SF) questionnaire. We randomized 296 patients to normal physical activity (control; n=145) or walking exercise (n=151); 227 patients (exercise n=104; control n=123) repeated the 6-month evaluations. The distance covered during the 6-minute walking test improved in the exercise group (mean distance±SD: baseline, 328±96 m; 6 months, 367±113 m) but not in the control group (baseline, 321±107 m; 6 months, 324±116 m; P<0.001 between groups). Similarly, the five times sit-to-stand test time improved in the exercise group (mean time±SD: baseline, 20.5±6.0 seconds; 6 months, 18.2±5.7 seconds) but not in the control group (baseline, 20.9±5.8 seconds; 6 months, 20.2±6.4 seconds; P=0.001 between groups). The cognitive function score (P=0.04) and quality of social interaction score (P=0.01) in the kidney disease component of the KDQOL-SF improved significantly in the exercise arm compared with the control arm. Hence, a simple, personalized, home-based, low-intensity exercise program managed by dialysis staff may improve physical performance and quality of life in patients on dialysis.
Keywords: dialysis, CKD, exercise, physical functioning, rehabilitation, six minute walking test
Poor physical functioning is perhaps the most pervasive and disabling disturbance in patients with stage G5 CKD maintained on chronic dialysis (CKD-5D).1–3 National Kidney Foundation Kidney Disease Outcomes Quality Initiative Guidelines formally recommend that patients with CKD-5D be “counseled and regularly encouraged by nephrology and dialysis staff to increase their level of physical activity.”4 However, the evidentiary basis for recommending exercise training in CKD-5D is still limited. Although the effect of regular physical exercise training on physical performance in selected patients with CKD-5D studied in standardized experimental settings in the laboratory is well documented,5 how exercise training should be articulated (intradialysis or off-dialysis, in-center only, daily versus other schedules) and implemented (duration and intensity) still remains an open problem. In studies performed so far, physical exercise was mainly proposed under supervision during the dialysis session or between two dialysis sessions.5–7 However, organization and cost problems mainly related with instruments, personnel, and intensification of visits to the dialysis center may hinder patients’ acceptability of exercise programs and, ultimately, the diffusion of such programs.
On the basis of a model developed for peripheral arterial disease rehabilitation,8 we designed an easy-to-implement home program of physical exercise for patients with CKD-5D and documented the feasibility of such a program in a pilot study.9 After this pilot experience, we further simplified this program into a format whereby a home-based, individualized, low-intensity exercise program could be managed by the dialysis staff, without extra visits to the dialysis center (see video at: https://www.youtube.com/watch?v=ki8YX_t-0jA). We have now tested in a multicenter, randomized clinical trial, the EXerCise Introduction To Enhance performance in dialysis patients trial (EXCITE), whether this home exercise program improves the degree of fitness and quality of life in patients with CKD-5D.
Results
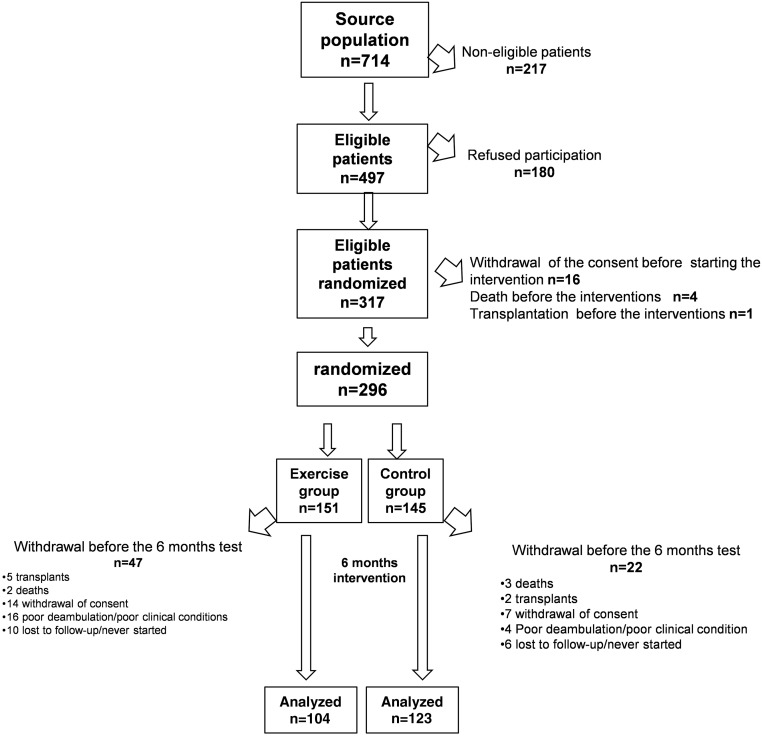
All eligible patients were recruited between November of 2009 and February of 2011. The Consolidated Standards of Reporting Trials (CONSORT) diagram describing the flow of patients through this open, parallel, randomized, two-group trial is shown in Figure 1.
Figure 1.
CONSORT diagram of the flow of patients across the various phases of the trial. All baseline measurements (including 6MWT and 5STS) were done after randomization. Baseline demographic data, main cardiovascular comorbidities, and results of the 6MWT and 5STS in patients who completed the 6-month training program and those who dropped out in the two study arms are detailed in Supplemental Table 4.
The source population in the 13 Nephrology Units participating in the trial was composed of 714 patients receiving dialysis. Of these, 296 patients in nine centers (59.6% eligible patients, 41.5% of total population) were randomized to walking exercise (n=151; hemodialysis, n=127; continuous ambulatory peritoneal dialysis [CAPD], n=24) or usual care and normal physical activity (n=145; hemodialysis, n=120; CAPD, n=25). As depicted in Figure 1, 227 of 296 patients (77%) could be retested after 6 months (104 in the active arm [hemodialysis, n=90; CAPD, n=14] and 123 in the control arm [hemodialysis, n=102; CAPD, n=21]). The reasons for nonparticipation and exit from the study are detailed in Figure 1. The two study groups did not differ for demographic, clinical, and biochemical data (Table 1) but did for systolic BP (mean±SD; 138±18 versus 127±18 mmHg), which tended to be higher (P=0.06) in patients in the active arm than in those in the control arm.
Table 1.
Demographic, clinical, and biochemical data of patients that completed the study
| Active Arm (n=104) | Control Arm (n=123) | P Value | |
|---|---|---|---|
| Age, yr | 63±13 | 64±14 | 0.60 |
| Men, % | 64 | 68 | 0.54 |
| Hemodialysis/CAPD, n | 90/14 | 102/21 | 0.45 |
| BMI, kg/m2 | 26±4 | 27±6 | 0.32 |
| Smoking, % (0=no; 1=yes) | 18 | 19 | 0.93 |
| Diabetes, % (0=no; 1=yes) | 18 | 18 | 0.88 |
| Systolic BP, mmHg | 132±18 | 127±18 | 0.06 |
| Diastolic BP, mmHg | 72±10 | 71±12 | 0.43 |
| HR, beats/min | 75±9 | 74±8 | 0.51 |
| Total cholesterol, mg/dl | 164±39 | 166±39 | 0.67 |
| Triglycerides, mg/dl | 166±116 | 160±86 | 0.68 |
| Hemoglobin, g/dl | 11±1 | 11±2 | 0.22 |
| Albumin, g/dl | 3.9±0.4 | 3.8±0.5 | 0.44 |
| Calcium, mg/dl | 8.8±0.7 | 8.9±0.7 | 0.42 |
| Phosphate, mg/dl | 4.9±1.5 | 4.8±1.4 | 0.35 |
| PTH, pg/ml | 280 (179–456) | 283 (156–396) | 0.55 |
| Creatinine, md/dl | 10.5±2.7 | 9.8±2.6 | 0.41 |
| Glycemia, mg/dl | 111±64 | 102±36 | 0.23 |
| Urea, mg/dl | 153±42 | 148±40 | 0.33 |
| CRP, mg/L | 5.0 (3.1–9.0) | 4.6 (3.0–8.0) | 0.60 |
| Kt/V hemodialysis | 1.42±0.25 | 1.43±0.30 | 0.68 |
| Kt/V CAPD | 1.96±0.29 | 1.80±0.60 | 0.36 |
| Myocardial infarction, % | 15 | 17 | 0.73 |
| Stroke/transient ischemic attack, % | 8 | 14 | 0.14 |
| Anginal episodes, % | 11 | 13 | 0.74 |
| Arrhythmia, % | 12 | 7 | 0.19 |
| Heart failure, % | 17 | 24 | 0.24 |
| Peripheral vascular disease, % | 7 | 12 | 0.16 |
| History of neoplasia, % | 22 | 18 | 0.52 |
| Antihypertensive therapy, % | 77 | 70 | 0.27 |
| NYHA class, % | |||
| I | 38 | 34 | 0.46 |
| II | 14 | 16 | |
| III–IV | 4 | 10 | |
| Mobility, % | |||
| Assisted | 4 | 3 | 0.56 |
| Independent | 96 | 97 | |
BMI, body mass index; HR, heart rate; PTH, paratohormone; CRP, C-reactive protein; NYHA, New York Heart Association.
Adherence to the Exercise Program in the Active Arm
Out of 104 patients in the exercise arm who were re-evaluated after 6 months, 76 correctly filled the study diary and 81 returned the metronome for battery verification. Overall, 91 patients (87.5%) documented their degree of compliance to the exercise program with at least one of these two instruments. As reported in the personal diaries, the average number of sessions performed was 119±103 (range, 7–336), corresponding to 83% of the 144 prescribed sessions. Forty-six patients exceeded the number of prescribed sessions because they did extra sessions on the dialysis days, whereas 29 performed just a minimal amount (<10%) of the prescribed sessions. The level of adherence to the exercise program was high for 55 patients and low for 49 patients. The main determinants of low adherence were scarce interest (n=22), orthopedic problems (n=7), intercurrent nonorthopedic problems (n=10), and problems related with work (n=10). The residual battery charge was significantly higher in patients with poor adherence compared with those with high adherence (2.982 versus 2.961 mV; P<0.05), but this indicator had very modest discriminatory ability to identify patients with a high degree of adherence from those with a low degree, and was not applied for the stratification of patients.
Effect of the Home-Based Training Program on Functional Capacity and Other Parameters
BP and heart rate remained unchanged after the 6-month exercise program in the exercise group (6-month BP, 133±15/73±9 mmHg; heart rate, 74±9 beats/min). Similarly, serum creatinine (9.9±2.7 mg/dl), urea (150±41 mg/dl), Kt/V (hemodialysis, 1.46±0.30; CAPD, 1.93±0.66), albumin (3.7±0.4 g/dl), phosphate (5.0±1.5 mg/dl), calcium (8.8±1.0 mg/dl), parathyroid hormone (269 pg/ml [range, 167–429]), cholesterol (166±38 mg/dl), triglycerides (182±112 mg/dl), and glucose (115±70 mg/dl) remained the same in the active group. No change in the same parameters was observed in the control group. Drug therapy at baseline was similar in the two groups and did not change across the trial (see Supplemental Table 1).
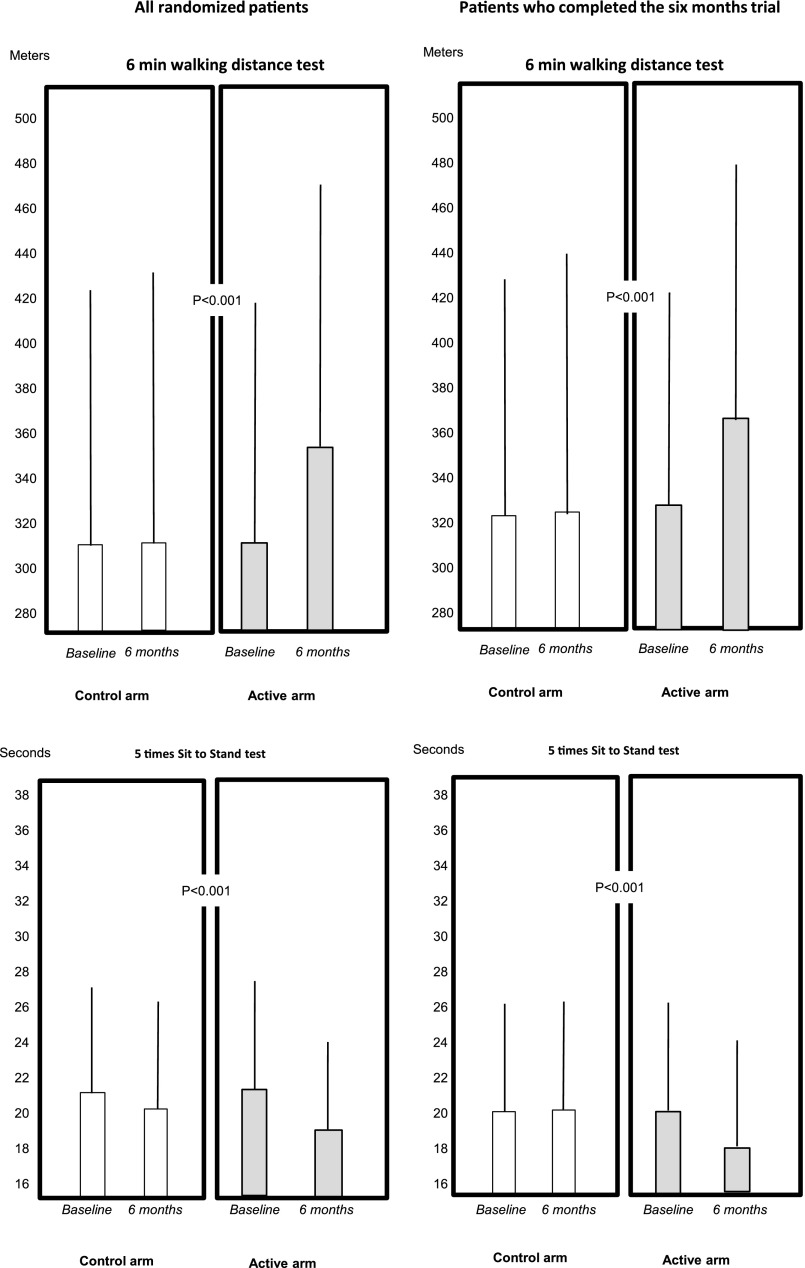
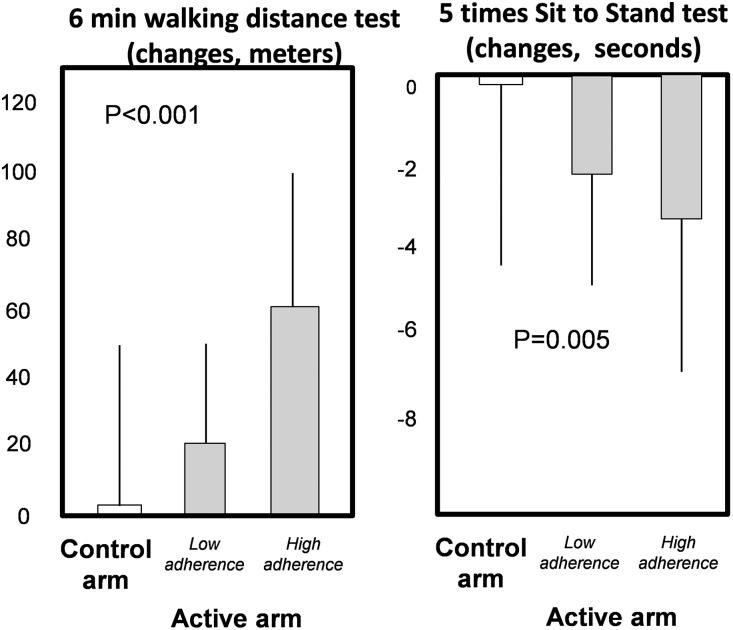
The 6-minute walking distance (6MWD) improved in the exercise group both in analysis made in all randomized patients who started the trial (6 months versus baseline: exercise group, +39 m [95% confidence interval (95% CI), 33 to 46 m], control group, +2 m [95% CI, −5 to 10]; P<0.001) and in analysis restricted to patients who completed the trial (exercise group: +41 m, 95% CI, 31 to 51 m; control group +3 m, 95% CI, −7 to 12 m; P<0.001), (Figure 2, Supplemental Table 2). Among patients who completed the trial there was a dose–response relationship between the adherence to exercise program and 6MWD changes across the trial (Figure 3) (P<0.001). Among the same patients at the 6-month testing session, the average rate of the patient’s perceived exertion by the Borg CR10 Scale was unmodified both in the active group (2.6; 95% CI, 2.2 to 2.9 at baseline, and 2.8; 95% CI, 2.5 to 3.1 after 6 months) and in the control group (2.7; 95% CI, 2.4 to 3.0 at baseline, and 3.2; 95% CI, 2.8 to 3.5 after 6 months). No effect modification by dialysis treatment modality (hemodialysis versus CAPD) was found on the relationship between allocation arm and changes in 6MWD (P=0.86) and five times sit-to-stand test (5STS; P=0.26).
Figure 2.
Effect of the study interventions on walking capacity (6MWT) and lower limb strength (5STS) in the two arms of the study. The figures overlying the columns are SD of the corresponding mean values. The P value compares changes (6 months versus baseline) between the two groups (exercise versus control).
Figure 3.
Dose–response relationship between achieved physical performance by the 6MWT and 5STS across the control arm and the low adherence and high adherence to the exercise program (active arm). The bars are SD of the mean. Low and high adherence were defined as performance of <60% and ≥60% of the prescribed sessions, respectively. See also adherence to exercise program in Supplemental Material.
At baseline, 13 patients in the exercise arm and eight in the control arm were unable to perform any repetition in the 5STS. In addition, 25 patients in the active arm and 21 in the control arm were unable to complete the test for asthenia and peripheral fatigue. All these patients were maintained in the trial and after 6 months, the corresponding figures of patients unable to perform any repetition were four (active arm) and seven (control arm), and of those unable to complete the test were nine and 15, respectively. Overall, across the trial the proportion of patients completely or partially unable to perform the 5STS was significantly reduced in the first group compared with the second group (P<0.001, chi-squared test).
Among patients who completed the test, 5STS time improved in the exercise group but not in the control group (Figure 2) (between-group difference, P=0.001) and there was a dose–response relationship between the 5STS time changes (6 months versus baseline) and adherence to the exercise program (P=0.01) (Figure 3).
Quality of Life
Overall, the global score (Table 2, last line) of the 19 items composing the Kidney Disease Quality of Life, Short Form, version 1.3 (KDQOL-SF) on average changed more favorably in the exercise than in the control arm, but the difference largely failed to achieve statistical significance (P=0.17). When compared with changes in the control arm only two items, both in the kidney disease component (cognitive function [P=0.04] and quality of social interaction [P=0.01]), achieved formal statistical significance.
Table 2.
Differences in quality of life indicators within and between study groups
| Active Group | Control Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 mo | Change (6 mo versus Baseline) | P Value (6 mo versus Baseline) | Baseline | 6 mo | Change (6 mo versus Baseline) | P Value (6 mo versus Baseline) | Changes Between Two Groups | P Value (Active versus Control) | |
| Kidney disease component | ||||||||||
| Symptom/problem list | 74.0 (70.3 to 77.6) | 73.4 (69.7 to 77.0) | −0.6 (from −3.1 to 1.8) | 0.62 | 73.4 (69.9 to 76.8) | 72.5 (69.0 to 76.0) | −0.8 (from −3.8 to 2.0) | 0.56 | −0.2 (from −3.9 to 3.5) | 0.91 |
| Effects of kidney disease | 57.6 (52.1 to 63.1) | 59.1 (54.1 to 64.1) | 1.5 (from −2.7 to 5.7) | 0.49 | 61.9 (57.7 to 66.1) | 61.6 (57.4 to 65.7) | −0.3 (from −4.0 to 3.4) | 0.86 | −1.8 (from −7.4 to 3.8) | 0.52 |
| Burden of kidney disease | 40.1 (33.8 to 46.4) | 42.7 (37.2 to 48.1) | 2.6 (from -2.2 to 7.3) | 0.28 | 41.6 (36.4 to 46.8) | 42.0 (36.5 to 47.4) | 0.4 (from −4.6 to 5.4) | 0.88 | −2.2 (from −9.0 to 4.7) | 0.53 |
| Work status | 29.3 (20.8 to 37.8) | 29.5 (21.0 to 38.1) | 0.3 (from −4 to 4.6) | 0.90 | 26.3 (19.3 to 33.3) | 25.4 (17.9 to 33.0) | −0.9 (from −6.5 to 4.7) | 0.75 | −1.2 (from −8.1 to 5.8) | 0.74 |
| Cognitive function | 68.7 (63.5 to 73.8) | 69.0 (64.1 to 73.8) | 0.3 (from −3.2 to 3.8) | 0.87 | 72.0 (66.8 to 77.2) | 65.6 (60.0 to 71.2) | −6.4 (from −11.9 to −0.9) | 0.02 | −6.7 (from −13.2 to −0.2) | 0.04 |
| Quality of social interaction | 76.8 (72.9 to 80.7) | 78.9 (75.7 to 82.1) | 2.1 (from −1.1 to 5.4) | 0.19 | 80.2 (76.4 to 84.0) | 75.6 (71.4 to 79.7) | −4.6 (from −8.3 to −0.9) | 0.02 | −6.7 (from −11.6 to −1.8) | 0.01 |
| Sexual function | 73.7 (65.6 to 81.8) | 68.8 (60.1 to 77.5) | −4.9 (from −12.6 to 2.7) | 0.20 | 66.3 (57.6 to 75.1) | 64.2 (55.5 to 72.9) | −2.1 (from −10.5 to 6.2) | 0.61 | 2.8 (from −8.4 to 14.0) | 0.62 |
| Sleep | 60.6 (56.6 to 64.6) | 64.2 (60.6 to 67.9) | 3.7 (from 0.8 to 6.6) | 0.01 | 58.3 (54.5 to 62.1) | 59.0 (55.4 to 62.5) | 0.7 (from −2.7 to 4.1) | 0.69 | −3.0 (from −7.4 to 1.4) | 0.19 |
| Social support | 72.4 (67.8 to 76.9) | 70.9 (65.8 to 75.9) | −1.5 (from −5.9 to 2.8) | 0.49 | 73.9 (69.6 to 78.1) | 71.9 (67.6 to 76.2) | −2.0 (from −6.2 to 2.3) | 0.36 | −0.5 (from −6.5 to 5.6) | 0.88 |
| Dialysis staff encouragement | 72.3 (69.5 to 75.1) | 71.3 (68.0 to 74.6) | 1.1 (from −4 to 1.9) | 0.48 | 75.4 (72.6 to 78.2) | 73.8 (71.0 to 76.6) | −1.6 (from −4.8 to 1.6) | 0.33 | −0.5 (from −4.9 to 3.8) | 0.80 |
| Patient satisfaction | 74.8 (69.7 to 79.9) | 73.2 (68.4 to 78.0) | −1.6 (from −5.1 to 2) | 0.39 | 75.9 (71.5 to 80.4) | 71.3 (66.7 to 75.9) | −4.6 (from −9.2 to −0.1) | 0.05 | −3.1 (from −8.8 to 2.7) | 0.29 |
| Physical functioning component | ||||||||||
| Physical functioning | 56.5 (49.7 to 63.3) | 57.9 (51.0 to 65.0) | 1.5 (from −2.7 to 5.6) | 0.49 | 55.5 (49.7 to 61.3) | 52.7 (46.4 to 59.0) | −2.7 (from −7.7 to 2.2) | 0.27 | −4.2 (from −10.6 to 2.2) | 0.20 |
| Role physical | 40.5 (30.9 to 50.2) | 40.8 (30.7 to 50.8) | 0.2 (from −9 to 9.5) | 0.96 | 41.7 (32.3 to 51.0) | 32.5 (23.7 to 41.3) | −9.2 (from −18.7 to 0.3) | 0.06 | −9.4 (from −22.6 to 3.8) | 0.16 |
| Pain | 57.9 (51.4 to 64.3) | 56.7 (50.7 to 62.7) | −1.1 (from −7.2 to 4.9) | 0.71 | 61.3 (54.7 to 67.9) | 58.1 (51.8 to 64.5) | −3.2 (from −9.3 to 2.9) | 0.30 | −2.0 (from −10.6 to 6.5) | 0.64 |
| General health | 35.7 (31.2 to 40.2) | 36.5 (31.8 to 41.1) | 0.8 (from −3 to 4.6) | 0.68 | 32.8 (29.1 to 36.4) | 30.3 (26.8 to 33.9) | −2.5 (from −6 to 1.1) | 0.17 | −3.2 (from −8.4 to 1.9) | 0.21 |
| Mental functioning component | ||||||||||
| Emotional wellbeing | 58.9 (53.7 to 64.2) | 60.1 (54.9 to 65.3) | 1.2 (from −2.6 to 5) | 0.53 | 62.9 (58.3 to 67.5) | 59.0 (54.2 to 63.7) | −3.9 (from −8.2 to 0.4) | 0.07 | −5.1 (from −10.79 to 0.6) | 0.08 |
| Role emotional | 54.9 (44.7 to 65.2) | 53.1 (42.9 to 63.4) | −1.8 (from -11.9 to 8.3) | 0.72 | 53.6 (44.4 to 62.9) | 46.2 (37.0 to 55.4) | −7.5 (from −17.6 to 2.7) | 0.15 | −5.7 (from −19.85 to 8.5) | 0.43 |
| Social function | 80.8 (75.4 to 86.1) | 78.3 (73.1 to 83.5) | −2.5 (from −8.2 to 3.3) | 0.40 | 80.0 (75.3 to 84.7) | 79.2 (74.2 to 84.2) | −0.8 (from −5 to 3.3) | 0.69 | 1.6 (from −5.44 to 8.7) | 0.65 |
| Energy/fatigue | 47.9 (42.2 to 53.0) | 47.1 (41.8 to 52.3) | 0.8 (from −5.1 to 3.4) | 0.70 | 49.8 (44.9 to 54.7) | 46.1 (41.0 to 51.1) | −3.7 (from −8.2 to 0.8) | 0.10 | −2.9 (from −9.0 to 3.3) | 0.35 |
| Total score | 62.3 (59.0 to 65.7) | 62.8 (59.5 to 66.2) | 0.5 (from −1.7 to 2.6) | 0.66 | 63.0 (59.9 to 66.0) | 61.2 (58.1 to 64.4) | −1.7 (from −4.1 to 0.6) | 0.15 | −2.2 (from −5.3 to 1.0) | 0.17 |
Data are mean and 95% CI.
Safety of the Exercise Program
Symptoms of moderate intensity, not limiting the program execution, were reported by 44 patients and included moderate fatigue (n=31), “heavy legs” or leg pain (n=35), moderate dyspnea (n=29), or other symptoms, including joint pain (n=17). Five patients reported four symptoms, 22 reported three symptoms, nine reported at least two symptoms, and eight only reported one symptom during the exercise sessions. Overall, the training program was well tolerated and only five telephone calls were received by the rehabilitation team across the trial. No angina episode or other major symptoms/complications during exercise were reported in the active arm of the trial. No systematic symptoms collection was undertaken in the control arm.
Secondary Outcomes: Death, Cardiovascular Events, and Hospitalizations
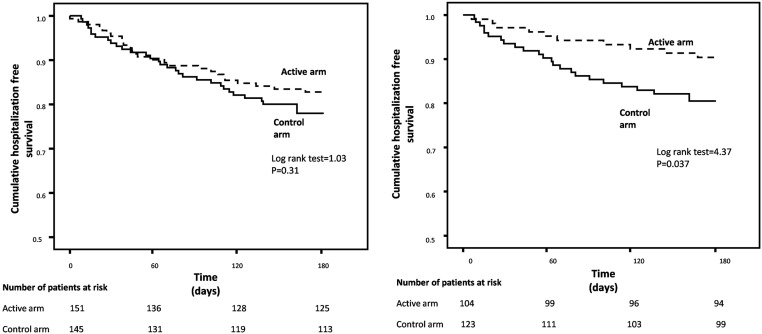
In a Kaplan–Meier analysis including randomized patients who started the trial (151 in the exercise and 145 in the control arm), there was a largely nonsignificant reduction in the risk of hospitalization. However, in an analysis restricted to patients who completed the trial, the hospitalization-free survival was lower (P=0.04) in patients in the active group than in the control group (Figure 4). As detailed in Supplemental Table 3, among these patients there were 18 hospitalizations in ten patients in the exercise group (35 hospitalizations per 100 person-years; 95% CI, 21 to 55) and 35 hospitalizations in 24 patients in the control group (57 hospitalizations per 100 person-years; 95% CI, 40 to 79).
Figure 4.
Kaplan–Meier survival curves of hospitalizations in the active and control arms of the trial. The left panel shows analysis of all randomized patients. The right panel shows analysis of patients who completed the 6-month trial.
An analysis of AV-fistula events in randomized patients who started the trial showed that the incidence rate of these outcomes did not significantly differ (P=0.22) between the exercise (22 events in six patients, 35 events per 100 person-years; 95% CI, 22 to 53) and control (13 events in six patients, 23 events per 100 person-years; 95% CI, 12 to 39) group. A parallel analysis in patients who completed the trial provided similar results (ten events in six patients [19 events per 100 person-years; 95% CI, 9 to 35] in the exercise group and nine events in seven patients [15 events per 100 person-years; 95% CI, 7 to 28] in the control group).
Discussion
In this multicenter, randomized trial in patients with stage 5D-CKD a simple, personalized, low-intensity, home-based walking program managed by the dialysis staff improved the functional status compared with usual care in these patients. Two items in the kidney disease component of the KDQOL-SF, namely cognitive function and quality of social interaction, showed a statistically significant improvement in the exercise group compared with the control group.
A comprehensive meta-analysis published in 2014 identified 29 randomized trials, all focused on a single center, testing physical exercise programs in patients on dialysis.5 In the majority of these trials (14 trials) exercise was performed in-center, during the hemodialysis session. Overall, these trials documented an improvement in muscular strength and aerobic capacity. The number of patients in these trials ranged from 13 to 96, and the vast majority had <40 patients. In most of these trials the outcome measure was aerobic capacity and/or cardiovascular/hemodynamic biomarkers (BP, heart rate, left ventricular mass) or muscle strength. Walking capacity was a main outcome measure in just one of these trials. The most recent meta-analysis, published in late 2015,7 added 11 trials to the 29 analyzed in the previous meta-analysis. Again, these trials were relatively small (from eight to 26 patients), almost all in-center (during hemodialysis, n=9), and looked at disparate outcome measures. A global analysis of the scientific quality of trials performed so far according to the Cochrane collaboration recommendations10 showed that the random sequence generation and the allocation concealment in these trials was unclear. Furthermore, the flow diagram of the progress of patients through the phases of the trial and the outcome data in these trials were largely incomplete, and reporting was selective in most trials.7 These limitations notwithstanding, it is indisputable that research on physical exercise performed so far in ESRD forms a solid basis for considering physical exercise as a potentially valuable intervention to improve health outcomes in this population.
The majority of physical exercise trials performed so far tested interventions performed during the hemodialysis session and consisted either of aerobic exercise (cycling) or resistance training of some muscular groups against elastic bands, or a combination thereof.5,7 These in-center programs allow effective, supervised exercise training. On the other hand, promoting therapeutic programs embedded in the actual familial and social context of individual patients,11 like the home-based exercise program tested in this trial, is an important opportunity for expanding the application of these programs and for patients empowerment.
The walking capacity encompasses cardiorespiratory and muscle endurance, muscle strength, and balance and coordination, which is fundamental in daily living in patients with chronic disease and in the elderly.12 In the systematic review and meta-analysis by Heiwe and Jacobson,5 only one physical exercise program was specifically focused on walking exercise,13 and just four13–16 contemplated walking capacity as an outcome measure. Of note, the intervention being tested in did not improve walking capacity in any of these studies. In a study of 26 patients, subsequent to Hewei meta-analysis, a combined resistance and aerobic intervention17 was more effective than isolated resistance training to improve walking capacity.
This multicenter trial tested a low-intensity (20 minutes of walking at low-to-moderate speed every second day) exercise program of gradually increasing intensity. The CONSORT diagram (Figure 1) shows that 32% (227 of 714) of the whole population in participating centers actually completed the training program, which is a significant proportion of the dialysis population. This program was well accepted by patients and among the 151 patients randomized to the exercise arm, 104 (69%) completed the 6-month training. Such an intervention allowed a meaningful increase in walking distance (41 m). At least in theory, such an increase may favorably affect clinical outcomes because, beyond the actual duration of the trial (6 months), in a separate, long-term observational analysis of the EXCITE cohort extended to 3.3 years, we found that a 40 m increase in walking capacity entails an 23% reduction in risk of mortality and an 8% reduction in risk of hospitalization.18 This possibility needs to be tested in long-term trials because the effect of interventions may wane with a reduction in the intensity of staff involvement.19 Along with walking capacity, the exercise program increased muscle strength in the lower limbs as measured by the 5STS test, a test predicting the risk of falls,20 which is pervasive in ESRD.21 The response to this test at baseline was very poor in both study arms (average time 19 versus 13 seconds in age- and sex-matched individuals). The improvement (about 3 seconds) registered in this trial is of potential clinical relevance because in elderly men it entails a 25% risk reduction for all-cause mortality.20
In Barcellos et al.7 meta-analysis quality of life was tested in 21 trials in patients on hemodialysis. Small improvements in the physical component score of the Short Form 36 were noted in four studies,22–27 two of which combined the exercise program to a pharmacologic intervention, i.e., erythropoietin to normalize hematocrit24 or nandrolone.25 Only two trials applied the instrument specific to CKD, the KDQOL-SF,26,27 which we adopted in this trial. We found a favorable trend for quality of life to improve in the exercise group compared with the control group, with a significant improvement restricted to two items from the kidney disease component of the KDQOL-SF, namely cognitive function and quality of social interaction. Overall, our data are in keeping with the concept that exercise may contribute to improve quality of life even though in our study the effect of this intervention per se was mild. Improving quality of life in this population is a challenging goal and perhaps multiple interventions represent the best approach to the problem.28
The study has limitations. First, given the nature of the intervention, the trial was designed as a randomized, unblinded trial. However, physical performance testing and help in quality of life questionnaire compilation was done by operators not involved in the daily care of patients participating in the study. Second, the duration of the trial was limited to 6 months. Further study is needed to assess the long-term adherence to this home-based physical exercise program. Third, even though improvement in physical performance and quality of life are relevant outcomes, our study was not powered to assess the effect of physical exercise on major clinical outcomes like death and cardiovascular events. In this respect, analysis of hospitalizations in patients who completed the trial was of borderline significance, suggesting a potential beneficial effect of exercise on hospitalization. However, the number of events was small. The important question of whether exercise programs may translate into a reduction of hospitalizations needs to be tested in a well powered, long-term trial.
In conclusion, this trial shows that a simple, home-based exercise program delivered by dialysis staff safely improves physical capacity and two relevant items in the kidney disease component of KDQOL-SF in patients who can complete this program. This trial further highlights the potential of exercise for improving physical performance in ESRD, and represents a stimulus to the nephrology community for undertaking long-term trials testing simple, sustainable exercise programs looking at clinical end points, including death and cardiovascular disease, in this very high-risk population.
Concise Methods
The protocol of the EXCITE trial (Clinicaltrials.gov identifier: NCT01255969) was approved by the hospital ethics committees of the nine renal units participating in the study, and written informed consent was obtained from all patients.
Study Design
Exclusion criteria included physical (e.g., amputation) or clinical (severe effort angina or stage 4 NYHA heart failure, any intercurrent illness requiring hospitalization) limitations to mobility or a high degree of fitness, that is the ability to walk a distance of >550 m in 6 minutes during the standard walking test (see below).
The study is a randomized controlled trial aimed at testing the effect of an individualized, home-based, low-intensity personalized program of walking exercise (exercise group) versus nonexposure (control group) on functional capacity and other clinical end points in patients with stage G5 CKD. To ensure balanced allocation of patients with heart disease across the two treatments groups, randomization was stratified by NYHA class. Allocation concealment was ensured by central randomization and nominal communication of patient allocation in the two arms of the study across the whole recruitment phase of the trial. Patients in the control group received usual care and generic advice to maintain an active lifestyle. The description of the 6-minute walking test (6MWT), i.e., the test adopted as a basis for stepping up the home-based exercise program, is described in Table 3, alongside the 5STS. Both tests, together with quality of life, were the main outcome measures of the trial. The whole program was supervised by the rehabilitation team (University of Ferrara, Italy) who ensured the education of the dialysis personnel, as well as exercise performance testing. Training was gradually stepped up as described in Table 4. The dialysis personnel received detailed information about the home-based exercise program and about the physical performance tests, and closely assisted the rehabilitation team during the training phase of the trial. Furthermore, dialysis nurses throughout the whole trial encouraged patients randomized to the exercise arm to keep an adequate adherence to the exercise prescription, and provided daily feedback to patients, consulting the rehabilitation team whenever required. Even though members of this team were extraneous to the daily management of patients on hemodialysis and were in contact with patients and nurses only during the initial training and in the testing sessions, no special measure was adopted to keep them blind to patient allocation. The walking cadence (steps per minute) to be maintained at home was focused on the use of a low cost (about $25), easily available metronome (Seiko DM50; Seiko Ltd., Japan), distributed to all patients, who were specifically instructed to walk in rhythm with it. The residual battery charge in the metronome at the end of the trial was measured and considered as corollary information to estimate the adherence to the exercise program.
Table 3.
Tests of walking capacity (6MWT29), lower extremity strength (5STS30) and quality of life (KDQOL-SF31)
| Test | Description | Indicator(s) |
|---|---|---|
| 6MWT, m | To walk back and forth along a 22 m course (two 10-m straight lines connected by two 1-m curves) in a corridor as quickly as possible for 6 min. Subjects are allowed to rest in case of fatigue or pain, and to resume when possible | Total distance covered in 6 min |
| 5STS, s | To move from a sitting position to a standing position on a 42-cm high chair as quick as possible, for five times | Time to complete the five repetitions |
| For patients unable to complete the test: number of standing positions reached and related time | ||
| KDQOL-SF | Questionnaire measuring quality of life in the version translated into Italian and specifically validated in Italian patients with CKD | Score domains |
| Self-administered | ||
| Assistance by nurses unaware of the patients’ treatment allocation is allowed |
Assessment was always performed on a nondialysis day by members of the rehabilitation team, i.e., by professionals not involved in the care of patients on hemodialysis. No encouragement was allowed during the testing sessions.
Table 4.
Stepping up of the exercise program
| Functioning Capacity Level | Normal | Moderate | Low | Very Low |
|---|---|---|---|---|
| 6 min distance walked at baseline, m | >300 to ≤550 | <300 to >200 | <200 | <200 +severe symptoms |
| Number of training sessions per d (always on nondialysis days) | 2 | 2 | 2 | 2 |
| Duration of training sessions, min | 10 | 10 | 10 | 10 |
| Frequency, times per wk | 3 | 3 | 3 | 3 |
| Training speed | ||||
| Baseline, km/h | 2.8 | 2.0 | 1.4 | 1.4 |
| Miles per h | 1.7 | 1.2 | 0.9 | 0.9 |
| wk 1–14, steps/min | 72–120 | 66–100 | 56–80 | 56–80 |
| wk 15–24, steps/min | 90–120 | 80–100 | 60–80 | 60–80 |
| wk 1–14 | ||||
| Work/rest time, min | 5:1 | 5:1 | 5:1 | 2:1 |
| No. of repetitions | 2 | 2 | 2 | 5 |
| wk 15–24 | ||||
| Work/rest time, min | 10:0 | 10:0 | 10:0 | 5:1 |
| No. of repetitions | 1 | 1 | 1 | 2 |
Functional Capacity Tests
The testing sessions were always arranged on a nondialysis day (see also Supplemental Material), 24 hours after the dialysis session, either in the morning (between 7 a.m. and 1 p.m.) or in the afternoon (between 2 p.m. and 6 p.m.). Functional capacity testing in both study arms (exercise and control groups) was performed at baseline and after 6 months, using the 6MWT29 and the 5STS.30
These tests were repeated in both study arms after 6 months. Patients in the control group were given no recommendation about physical activity, just generic advice to maintain an active lifestyle.
Quality of Life
Quality of life was measured by the KDQOL-SF in the version translated into Italian and specifically validated in a sample of Italian patients with CKD.31 Whenever needed, the compilation of the replies to the KDQOL-SF was helped by nurses unaware of the treatment allocation of patients.
Statistical Analyses
The primary outcome of the EXCITE study was functional capacity changes (from baseline to 6 months) assessed by the 6MWT29 and the 5STS,30 induced by the home-based exercise program and changes in quality of life as measured by the KDQOL-SF.32–34 The secondary study outcomes were mortality (all causes and cardiovascular only), non-fatal cardiovascular events, all-causes hospitalizations and dialysis access survival.
The power of this trial was calculated on the basis of our previous pilot study.9 In this pilot trial, the same 6-month walking exercise program tested in the present trial induced a +43±118 m increase in walking distance in 17 patients on hemodialysis, whereas the walking distance reduced −4±76 m in a control group of 14 patients on hemodialysis who did not exercise during the same period. On the basis of these pilot data, we calculated that a study enrolling 180 patients (90 per study arm) with a 20% attrition rate would have an 80% power to detect statistical significance (P<0.05, two-tailed), defined as a 40-m difference between groups in the 6MWD across the trial. Data are expressed as mean and SD (normally distributed data), median and interquartile range (non-normally distributed data), or as percentage frequency (binary data), and comparisons between groups were performed by t-test (normally distributed data), Mann–Whitney U test (non-normally distributed data), or chi-squared test (binary data), as appropriate. Within-group comparisons were done by paired t-test (normally distributed data) or Wilcoxon rank test (non-normally distributed data), as appropriate. Between- and within-group differences are expressed as mean change and 95% CIs.
We used intention-to-treat analyses for both the primary study outcomes (the 6MWT and the 5STS), as well as for hospitalizations. Missing longitudinal 6MWT and 5STS data were imputed along with recommendations made for the analysis of longitudinal data.35
Data analysis was performed using a standard statistical package (SPSS for Windows, version 9.01 and 21; IBM SPSS, Chicago, IL) as well as with R 3.0.1. and STATA 11.0.
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016030378/-/DCSupplemental.
References
- 1.Painter P: Implementing exercise: What do we know? Where do we go? Adv Chronic Kidney Dis 16: 536–544, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Johansen KL, Chertow GM, Ng AV, Mulligan K, Carey S, Schoenfeld PY, Kent-Braun JA: Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int 57: 2564–2570, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Manfredini F, Lamberti N, Malagoni AM, Felisatti M, Zuccalà A, Torino C, Tripepi G, Catizone L, Mallamaci F, Zoccali C: The role of deconditioning in the end-stage renal disease myopathy: Physical exercise improves altered resting muscle oxygen consumption. Am J Nephrol 41: 329–336, 2015 [DOI] [PubMed] [Google Scholar]
- 4.KDOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45: 16–153, 2005 [PubMed] [Google Scholar]
- 5.Heiwe S, Jacobson SH: Exercise training in adults with CKD: A systematic review and meta-analysis. Am J Kidney Dis 64: 383–393, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Cheema BS, Singh MA: Exercise training in patients receiving maintenance hemodialysis: A systematic review of clinical trials. Am J Nephrol 25: 352–364, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Barcellos FC, Santos IS, Umpierre D, Bohlke M, Hallal PC: Effects of exercise in the whole spectrum of chronic kidney disease: A systematic review. Clin Kidney J 8: 753–765, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manfredini F, Malagoni AM, Mascoli F, Mandini S, Taddia MC, Basaglia N, Manfredini R, Conconi F, Zamboni P: Training rather than walking: The test in -train out program for home-based rehabilitation in peripheral arteriopathy. Circ J 72: 946–952, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Malagoni AM, Catizone L, Mandini S, Soffritti S, Manfredini R, Boari B, Russo G, Basaglia N, Zamboni P, Manfredini F: Acute and long-term effects of an exercise program for dialysis patients prescribed in hospital and performed at home. J Nephrol 21: 871–878, 2008 [PubMed] [Google Scholar]
- 10.Higging J, Green S, editors: Cochrane Handbook of Systematic Reviews of Interventions 5.0.1. The Cochrane Collaboration, 2008, Chapter 8, Cochrane Database Systematic Reviews, 2016 [Google Scholar]
- 11.Shearer NB: Health empowerment theory as a guide for practice. Geriatr Nurs 30[Suppl]: 4–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck P: The elderly patient. In: Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd Ed., edited by Walker HK, Hall WD, Hurst JW, Boston, MA, Butterworths, 1990 [PubMed] [Google Scholar]
- 13.Koh KP, Fassett RG, Sharman JE, Coombes JS, Williams AD: Intradialytic versus home-based exercise training in hemodialysis patients: A randomised controlled trial. BMC Nephrol 10: 2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DePaul V, Moreland J, Eager T, Clase CM: The effectiveness of aerobic and muscle strength training in patients receiving hemodialysis and EPO: A randomized controlled trial. Am J Kidney Dis 40: 1219–1229, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Koufaki P, Mercer TH, Naish PF: Effects of exercise training on aerobic and functional capacity of end-stage renal disease patients. Clin Physiol Funct Imaging 22: 115–124, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Segura-Ortí E, Kouidi E, Lisón JF: Effect of resistance exercise during hemodialysis on physical function and quality of life: Randomized controlled trial. Clin Nephrol 71: 527–537, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Orcy RB, Dias PS, Seus TL, Barcellos FC, Bohlke M: Combined resistance and aerobic exercise is better than resistance training alone to improve functional performance of haemodialysis patients--results of a randomized controlled trial. Physiother Res Int 17: 235–243, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Torino C, Manfredini F, Bolignano D, Aucella F, Baggetta R, Barillà A, Battaglia Y, Bertoli S, Bonanno G, Castellino P, Ciurlino D, Cupisti A, D’Arrigo G, De Paola L, Fabrizi F, Fatuzzo P, Fuiano G, Lombardi L, Lucisano G, Messa P, Rapanà R, Rapisarda F, Rastelli S, Rocca-Rey L, Summaria C, Zuccalà A, Tripepi G, Catizone L, Zoccali C, Mallamaci F; EXCITE Working Group : Physical performance and clinical outcomes in dialysis patients: A secondary analysis of the EXCITE trial. Kidney Blood Press Res 39: 205–211, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ; Look AHEAD Research Group : Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 369: 145–154, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Buyser SL, Petrovic M, Taes YE, Toye KR, Kaufman JM, Goemaere S: Physical function measurements predict mortality in ambulatory older men. Eur J Clin Invest 43: 379–386, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Cook WL, Tomlinson G, Donaldson M, Markowitz SN, Naglie G, Sobolev B, Jassal SV: Falls and fall-related injuries in older dialysis patients. Clin J Am Soc Nephrol 1: 1197–1204, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Molsted S, Eidemak I, Sorensen HT, Kristensen JH: Five months of physical exercise in hemodialysis patients: Effects on aerobic capacity, physical function and self-rated health. Nephron Clin Pract 96: c76–c81, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Ouzouni S, Kouidi E, Sioulis A, Grekas D, Deligiannis A: Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil 23: 53–63, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Painter P, Moore G, Carlson L, Paul S, Myll J, Phillips W, Haskell W: Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T: Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J Am Soc Nephrol 17: 2307–2314, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Pellizzaro CO, Thomé FS, Veronese FV: Effect of peripheral and respiratory muscle training on the functional capacity of hemodialysis patients. Ren Fail 35: 189–197, 2013 [DOI] [PubMed] [Google Scholar]
- 27.de Lima MC, Cicotoste CL, Cardoso KS, Forgiarini LA Jr , Monteiro MB, Dias AS: Effect of exercise performed during hemodialysis: Strength versus aerobic. Ren Fail 35: 697–704, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Mitema D, Jaar BG: How can we improve the quality of life of dialysis patients? Semin Dial 29: 93–102, 2016 [DOI] [PubMed] [Google Scholar]
- 29.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories : ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 166: 111–117, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Mong Y, Teo TW, Ng SS: 5-repetition sit-to-stand test in subjects with chronic stroke: Reliability and validity. Arch Phys Med Rehabil 91: 407–413, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Klersy C, Callegari A, Giorgi I, Sepe V, Efficace E, Politi P; Pavia Working Group on QoL in Organ Transplant : Italian translation, cultural adaptation and validation of KDQOL-SF, version 1.3, in patients with severe renal failure. J Nephrol 20: 43–51, 2007 [PubMed] [Google Scholar]
- 32.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB: Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 3: 329–338, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Leaf DE, Goldfarb DS: Interpretation and review of health-related quality of life data in CKD patients receiving treatment for anemia. Kidney Int 75: 15–24, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, Fukuhara S, Young EW, Kurokawa K, Saito A, Bommer J, Wolfe RA, Held PJ, Port FK: Health-related quality of life as a predictor of mortality and hospitalization: The dialysis outcomes and practice atterns study (DOPPS). Kidney Int 64: 339–349, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Twisk J, de Vente W: Attrition in longitudinal studies. How to deal with missing data. J Clin Epidemiol 55: 329–337, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.