Abstract
Reduction of residual albuminuria during single–agent renin-angiotensin-aldosterone blockade is accompanied by improved cardiorenal outcomes in CKD. We studied the individual and combined effects of the vitamin D receptor activator paricalcitol (PARI) and dietary sodium restriction on residual albuminuria in CKD. In a multicenter, randomized, placebo (PLAC)–controlled, crossover trial, 45 patients with nondiabetic CKD stages 1–3 and albuminuria >300 mg/24 h despite ramipril at 10 mg/d and BP<140/90 mmHg were treated for four 8-week periods with PARI (2 μg/d) or PLAC, each combined with a low-sodium (LS) or regular sodium (RS) diet. We analyzed the treatment effect by linear mixed effect models for repeated measurements. In the intention-to-treat analysis, albuminuria (geometric mean) was 1060 (95% confidence interval, 778 to 1443) mg/24 h during RS + PLAC and 990 (95% confidence interval, 755 to 1299) mg/24 h during RS + PARI (P=0.20 versus RS + PLAC). LS + PLAC reduced albuminuria to 717 (95% confidence interval, 512 to 1005) mg/24 h (P<0.001 versus RS + PLAC), and LS + PARI reduced albuminuria to 683 (95% confidence interval, 502 to 929) mg/24 h (P<0.001 versus RS + PLAC). The reduction by PARI beyond the effect of LS was nonsignificant (P=0.60). In the per-protocol analysis restricted to participants with ≥95% compliance with study medication, PARI did provide further albuminuria reduction (P=0.04 LS + PARI versus LS + PLAC). Dietary adherence was good as reflected by urinary excretion of 174±64 mmol Na+ per day in the combined RS groups and 108±61 mmol Na+ per day in the LS groups (P<0.001). In conclusion, moderate dietary sodium restriction substantially reduced residual albuminuria during fixed dose angiotensin–converting enzyme inhibition. The additional effect of PARI was small and nonsignificant.
Keywords: randomized-controlled trial, paricalcitol, VDRA, dietary sodium restriction, albuminuria, chronic kidney disease
Pharmacologic renin-angiotensin-aldosterone system (RAAS) blockade reduces albuminuria and BP, subsequently retarding renal function loss and lowering the risk of cardiovascular morbidity and mortality in CKD.1–5 However, in a considerable proportion of patients, RAAS blockade is unable to halt the progression of CKD, despite BP control. Residual albuminuria (or proteinuria), persisting despite optimally dosed RAAS blockade, is strongly associated with adverse long–term renal and cardiovascular outcomes6,7 and therefore, considered a target for additional intervention.
Dietary sodium restriction potentiates the albuminuria-lowering efficacy of RAAS blockade in nondiabetic and diabetic patients with CKD,8–10 which has been associated with improved long–term cardiorenal protection.11,12 In addition, vitamin D receptor activator (VDRA) therapy may lower residual albuminuria as suggested by preclinical studies13,14 and several small– to medium–scale randomized, controlled trials in patients with CKD.15,16 The renoprotective effect of VDRA therapy may at least in part be mediated by a direct inhibitory effect on the RAAS.17,18 Given the consistent finding that dietary sodium restriction potentiates the albuminuria-lowering efficacy of conventional RAAS blockade including angiotensin–converting enzyme inhibition (ACEi)9 and angiotensin receptor blockade,8 it seems plausible that sodium restriction would also potentiate the capacity of VDRA treatment to lower residual albuminuria. In line with this assumption, we recently found that dietary sodium restriction potentiates the antiproteinuric and renoprotective efficacy of VDRA treatment in a rat model of proteinuric nephropathy19 and that sodium intake modulates the inverse association between plasma vitamin D levels and the risk of developing increased albuminuria in the general population.20 At variance, however, a post hoc analysis of the VITamin D receptor activator for Albuminuria Lowering (VITAL) trial16 as well as an observational study in nondiabetic CKD21 suggested that patients with albuminuria and higher baseline dietary sodium intake had a stronger antiproteinuric response to VDRA treatment than those with lower baseline sodium intake.
In the Vitamin D Receptor activator and sodium restriction for Treatment of Urinary albumin Excretion in chronic kidney disease (ViRTUE-CKD) trial, therefore, we prospectively studied the separate and combined albuminuria–lowering effects of the VDRA paricalcitol and dietary sodium restriction during fixed dose RAAS blockade, the current standard treatment, in nondiabetic patients with CKD. The trial compares residual albuminuria during four subsequent study periods in random order: paricalcitol or placebo combined with either dietary sodium restriction (target 50 mmol Na+ per day) or a regular sodium (RS) diet (target 200 mmol Na+ per day) all during fixed dose ACEi.
Results
Study Population
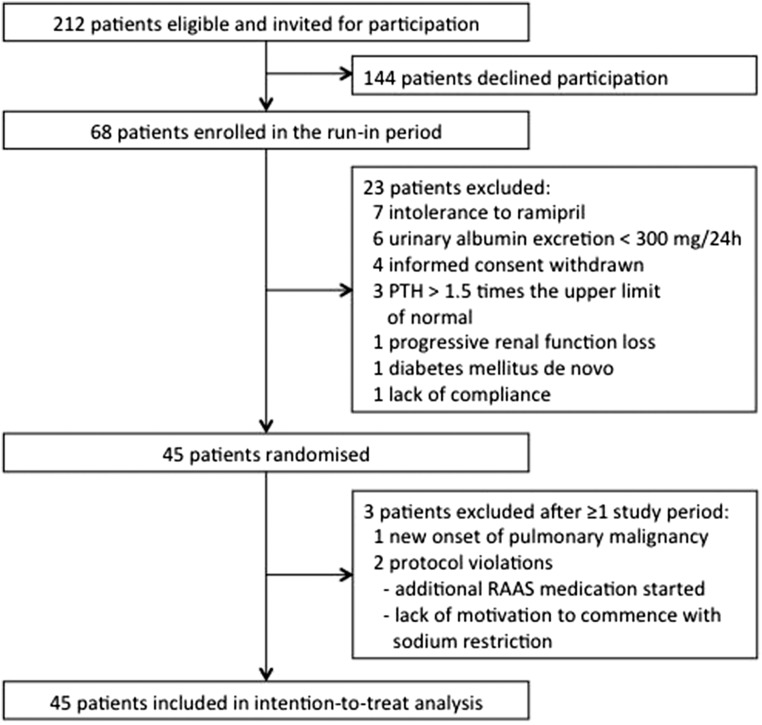
Of 212 eligible patients, 68 patients gave written informed consent and were subsequently enrolled in the run-in period, in which BP was targeted to <140/90 mmHg using a standardized regimen (Figure 1). During the run-in period, 23 patients discontinued the study. Of the 45 patients subsequently randomized, three patients were excluded during the study after completion of at least one study period. Supplemental Table 1 shows the baseline characteristics of the 45 study participants after randomization according to the sequence of the study periods. All patients received background ACEi in a fixed dose throughout the study (ramipril at 10 mg/d).
Figure 1.
Trial profile of the ViRTUE-CKD Study. Diagram indicating the disposition of study participants during screening, enrollment, randomization, and participation in the trial.
Primary Efficacy Analyses
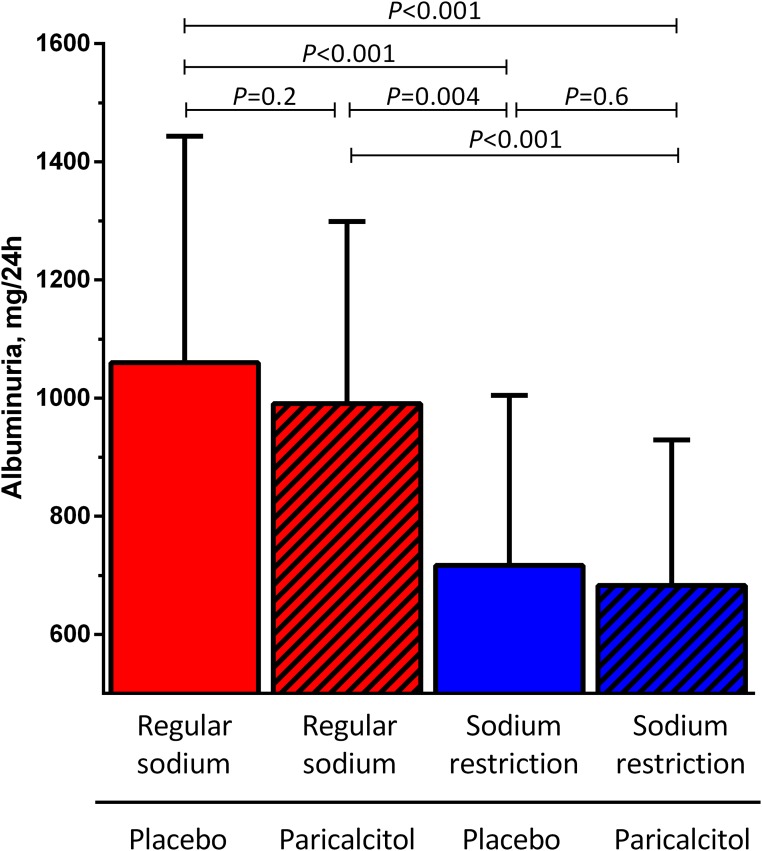
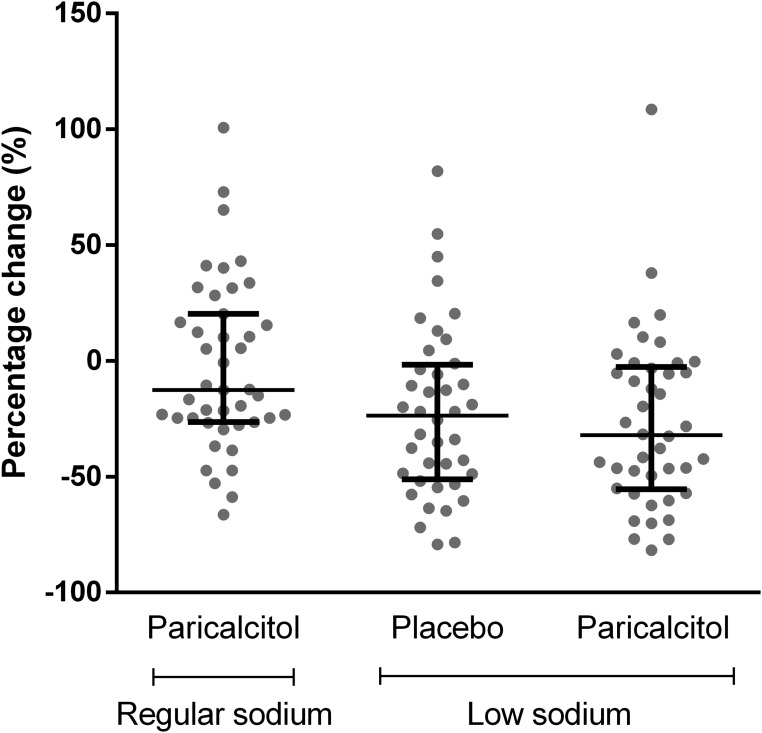
During RS diet combined with placebo treatment, residual albuminuria was 1060 (95% confidence interval [95% CI], 778 to 1443) mg/24 h (Figure 2). In the intention-to-treat analysis, paricalcitol provided a weak and nonsignificant albuminuria reduction to 990 (95% CI, 755 to 1299) mg/24 h (−12.5%; 95% CI, −26.0% to 26.3% versus RS + placebo; P=0.20) (Figure 3). During a low-sodium (LS) diet combined with placebo treatment, albuminuria was reduced to 717 (95% CI, 512 to 1005) mg/24 h (−25.4%; 95% CI, −52.6% to −2.3% versus RS + placebo; P<0.001). The strongest albuminuria reduction was reached by LS + paricalcitol: 683; 95% CI, 502 to 929 mg/24 h (−31.7%; 95% CI, −55.0% to −0.9%; P<0.001 versus RS + placebo). Paricalcitol did not reduce albuminuria further than the effect of the LS diet in itself (P=0.60). Adjustment for BP did not change the results. Results were similar for the urinary albumin-to-creatinine ratio (Table 1). Linear mixed effect model analysis indicated no carryover effects (center P=0.70, treatment P<0.001, sequence P=0.90, and treatment × sequence P=0.40). Linear mixed effect model analysis also indicated no interaction between the two interventions (center P=0.60, period P=0.30, sequence P=0.70, medication P=0.30, diet P<0.001, and medication × diet P=0.80). During RS + placebo, albuminuria and 25-hydroxyvitaminvitamin D3 [25(OH)D] were not significantly correlated (linear regression β = −0.02; P=0.10). The albuminuria-lowering effect of dietary sodium restriction and paricalcitol was not influenced by the level of 25(OH)D (linear mixed effect model analysis P=0.50).
Figure 2.
Effects of sodium restriction and paricalcitol on albuminuria in the intention-to-treat analysis. Albuminuria during RS diet or dietary sodium restriction in combination with paricalcitol (2 μg/d) or placebo. Data are shown as geometric mean (95% CI). P value shows treatment effect by linear mixed modeling with center, treatment, sequence, and the interaction treatment × sequence as fixed factors.
Figure 3.
Relative change in residual albuminuria compared with RS + placebo in the intention-to-treat analysis. The percentage change is shown as individual data with median and interquartile range; data for one participant with extreme values (+259%, −61%, and +165%, respectively) are not shown.
Table 1.
Clinical parameters during the four treatment periods: Intention-to-treat analysis
| Parameters | RS Diet | Sodium Restriction Diet | ||
|---|---|---|---|---|
| Placebo, n=44 | Paricalcitol, n=44 | Placebo, n=43 | Paricalcitol, n=43 | |
| Plasma/serum | ||||
| Hemoglobin, mmol/L | 9.0±0.9 | 9.0±0.8 | 9.1±0.9 | 9.0±0.8 |
| Sodium, mmol/L | 140.6±2.3 | 140.1±2.0 | 139.8±2.4a | 140.4±2.4b |
| Potassium, mmol/L | 4.3±0.4 | 4.2±0.4 | 4.3±0.4c | 4.4±0.5c |
| Calcium, mmol/L | 2.35±0.11 | 2.37±0.10 | 2.37±0.13 | 2.41±0.15a,b |
| Phosphate, mmol/L | 0.94±0.17 | 0.98±0.16a | 0.94±0.14 | 1.00±0.15a,b |
| Creatinine, μmol/L | 110±32 | 112±32 | 113±31 | 120±35a,b,c |
| eGFR, ml/min per 1.73 m2 | 68±25 | 67±24 | 67±24 | 63±25a,b,c |
| Albumin, g/L | 38±5 | 39±5 | 40±4a | 40±4a |
| Total cholesterol, mmol/L | 5.2±1.2 | 5.2±1.2 | 4.9±1.0a,c | 5.1±1.2b |
| HDL cholesterol, mmol/L | 1.4±0.4 | 1.4±0.4 | 1.3±0.4a,c | 1.3±0.4 |
| LDL cholesterol, mmol/L | 3.1±0.9 | 3.0±1.0 | 2.9±0.7a | 3.1±0.90 |
| Renin, pg/ml | 42.9 [30.9 to 59.5] | 45.3 [32.5 to 63.1] | 61.3 [44.6 to 84.2]a,c | 66.5 [48.4 to 91.4]a,c |
| PTH, pmol/L | 5.0 [4.4 to 5.7] | 3.5 [3.0 to 4.1]a | 5.5 [4.8 to 6.2]c | 3.4 [3.0 to 4.0]a,b |
| 25(OH)D, nmol/L | 50.4±22.8 | 50.6±23.4 | 52.7±22.6 | 56.4±24.2 |
| FGF-23, RU/ml | 114 [102 to 128] | 139 [122 to 158]a | 120 [106 to 135]a,c | 152 [130 to 178]a,b,c |
| Urine | ||||
| Creatinine, mmol/24 h | 14.7±3.9 | 14.5±3.8 | 13.8±3.7a | 14.4±3.4b |
| Sodium, mmol/24 h | 170±61 | 178±68 | 104±59a,c | 111±63a,c |
| Urea, mmol/24 h | 419±128 | 416±132 | 383±120a | 404±118 |
| Potassium, mmol/24 h | 78±25 | 80±25 | 81±26 | 82±24 |
| Calcium, mmol/24 h | 2.4±2.0 | 4.5±3.3a | 2.2±2.4c | 3.9±2.9a,b |
| Phosphate, mmol/24 h | 32.4±9.5 | 33.8±13.3 | 30.4±13.2 | 31.5±9.9 |
| Albuminuria, mg/24 h | 1060 [778 to 1443] | 990 [755 to 1299] | 717 [512 to 1005] | 683 [502 to 929] |
| Proteinuria, g/24 h | 1.4 [1.0 to 1.8] | 1.3 [1.0 to 1.6] | 1.0 [0.7 to 1.3]a,c | 0.9 [0.7 to 1.2]a,c |
| Albumin-to-creatinine ratio | 75 [55 to 101] | 71 [53 to 94] | 54 [39 to 75]a,c | 49 [36 to 66]a,c |
| Creatinine clearance, ml/min | 101±41 | 97±38 | 91±38a | 90±35a |
| Other | ||||
| Systolic BP, mmHg | 129±14 | 128±14 | 123±12a,c | 122±12a,c |
| Diastolic BP, mmHg | 77±9 | 78±11 | 74±9a,c | 74±9a,c |
| MAP, mmHg | 95±10 | 95±11 | 90±9a,c | 90±9a,c |
| Heart rate, bpm | 65±10 | 66±10 | 65±10 | 65±10 |
| Body weight, kg | 90±17 | 89±17 | 88±18a,c | 87±17a,c |
| Compliance, % | 95±7 | 97±6 | 97±5 | 97±4 |
Data are presented as mean±SD or geometric mean [95% CI] for normally or skewed distributed data, respectively. P value shows treatment effect by linear mixed modeling with center, treatment, sequence, and the interaction treatment × sequence as fixed factors.
P<0.05 versus placebo on RS diet.
P<0.05 versus placebo on sodium restriction diet.
P<0.05 versus paricalcitol on RS diet.
Secondary and Exploratory Outcomes
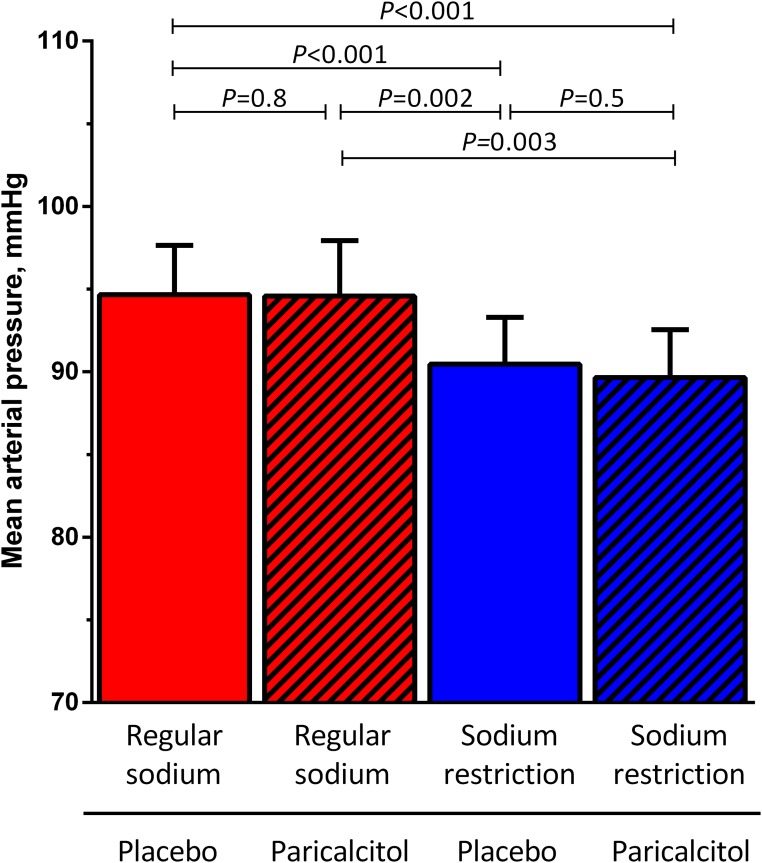
Mean arterial pressure (MAP) was 95 (95% CI, 92 to 98) mmHg during RS + placebo (Figure 4). Paricalcitol did not affect MAP during RS diet (95 mmHg; 95% CI, 91 to 98 mmHg; P=0.80 versus RS + placebo) or LS diet (90 mmHg; 95% CI, 87 to 93 mmHg; P=0.50 versus LS + placebo). Dietary sodium restriction in itself reduced MAP to 90 (95% CI, 88 to 93) mmHg (P<0.001 LS + placebo versus RS + placebo). Treatment effects were similar for systolic and diastolic BPs (Table 1).
Figure 4.
Effect of sodium restriction and paricalcitol on BP in the intention-to-treat analysis. MAP during RS diet or dietary sodium restriction in combination with paricalcitol (2 μg/d) or placebo. Data are shown as mean (95% CI). P value shows treatment effect by linear mixed modeling with center, treatment, sequence, and the interaction treatment × sequence as fixed factors.
Creatinine clearance was 101±41 ml/min during RS + placebo (Table 1) and was not significantly changed by paricalcitol (97±38 ml/min; P=0.20). Sodium restriction induced a reduction in creatinine clearance during both placebo (91±38 ml/min; P=0.01 versus RS + placebo) and paricalcitol (90±35 ml/min; P=0.004). Paricalcitol did not influence creatinine clearance beyond the effect of dietary sodium restriction (P=0.70 versus LS + placebo).
During both RS and LS diet, paricalcitol increased serum phosphate and urinary calcium excretion and reduced parathyroid hormone (PTH), consistent with the known effects of paricalcitol on calcium and phosphate metabolism (Table 1). During dietary sodium restriction, paricalcitol also increased serum calcium. Dietary sodium restriction decreased body weight and plasma sodium and increased plasma renin and albumin concentrations, consistent with a reduction of extracellular volume. Paricalcitol did not affect these parameters (Table 1). Both LS diet and paricalcitol increased plasma carboxy–terminal fibroblast growth factor 23 (FGF-23) (Table 1). Serum 25(OH)D was not affected by LS diet or paricalcitol (Table 1).
Adverse Effects
Nine patients developed hypercalcemia during a paricalcitol treatment period, and in five of these patients, hypercalcemia was also present during a placebo treatment period. From these five patients, two developed hypercalcemia during a placebo treatment period before having received paricalcitol treatment, and the other three had at least one normal calcium measurement between hypercalcemia during a paricalcitol treatment period and hypercalcemia during a placebo treatment. There was no persisting hypercalcemia when paricalcitol was ceased. Hypercalcemia during a safety control visit led to a dose reduction in five patients: two during RS + paricalcitol and three during LS + paricalcitol. Severe orthostatic complaints required tapering of antihypertensive medication in one patient during LS + paricalcitol. Mild orthostatic complaints, not necessitating drug withdrawal, occurred in two patients on RS + placebo, one patient on paricalcitol + RS, 10 patients on LS + placebo, and four patients on LS + paricalcitol. These and all other reported adverse effects possibly or probably related to treatment are listed in Supplemental Table 2.
Compliance
We assessed compliance with the diet by 24-hour urinary sodium excretion and compliance with study medication by counting returned capsules. Mean urinary sodium excretion was 174±64 mmol Na+ per day (approximately 4000 mg Na+ per day or 10 g NaCl per day) during the two study periods on the RS diet and 108±61 mmol Na+ per day (approximately 2500 mg Na+ per day or 6.2 g NaCl per day; P<0.001 versus RS diet) during the two LS periods. Compliance with the pharmacologic intervention was similar among the four treatment periods (Table 1).
Per-Protocol Analyses
The primary end point was reanalyzed in participants with ≥95% compliance with the study medication assessed per study period. For each study period, data from 31 to 34 participants were available for this analysis. Compliance of the excluded participants during the excluded study periods was 88%±7% and unknown for five patients (during 10 study periods). Supplemental Table 3 shows the main clinical parameters during the four treatment periods of participants in the per-protocol analysis. Here, estimated albuminuria was 1177 (95% CI, 823 to 1682) mg/24 h during RS + placebo. During RS diet, paricalcitol provided a nonsignificant albuminuria reduction to 1082 (95% CI, 772 to 1516) mg/24 h (−18.0%; 95% CI, −27.0% to 29.1%; P=0.30 versus RS + placebo). In contrast, dietary sodium restriction in itself reduced albuminuria to 804 (95% CI, 564 to 1146) mg/24 h (−35.1%; 95% CI, −53.9% to −6.8%; P<0.001 versus RS + placebo), and the combination of paricalcitol and dietary sodium restriction further reduced albuminuria to 690 (95% CI, 480 to 993) mg/24 h (−42.0%; 95% CI, −59.6% to −5.9%; P=0.04 versus RS + placebo). In this analysis, paricalcitol significantly reduced albuminuria beyond the effect of sodium restriction (P=0.04 LS + paricalcitol versus LS + placebo). Similar results were observed when considering the urinary albumin-to-creatinine ratio (Supplemental Table 3). There was no interaction between the two interventions on the primary end point (center P=0.80, period P=0.20, sequence P=0.90, medication P=0.03, diet P<0.001, and medication × diet P=0.30).
During RS diet but not during sodium restriction, paricalcitol treatment resulted in a small but significant reduction in MAP (P=0.05) (Supplemental Table 3). Additional adjustment for urinary sodium excretion did not materially influence the results on residual albuminuria, but the effect of paricalcitol during RS intake on MAP was no longer significant (P=0.07).
Discussion
The main aim of this trial was to prospectively study the separate and combined effects of paricalcitol and dietary sodium restriction to lower residual albuminuria during fixed dose single–agent RAAS blockade in nondiabetic patients with CKD. Moderate dietary sodium restriction substantially reduced residual albuminuria, whereas the effect of paricalcitol was nonsignificant. There was no interaction between the dietary sodium intake and paricalcitol on albuminuria reduction. Our prospective data did not confirm the previously raised suggestion that albuminuria reduction by paricalcitol is optimal during high-sodium intake.16,21
The capacity of paricalcitol to reduce albuminuria or proteinuria has been suggested in several clinical studies in different CKD populations, predominantly albeit not exclusively in patients with diabetes.16,21–26 Two previously published reports on the basis of post hoc analyses from clinical studies suggested that paricalcitol provides stronger albuminuria reduction in patients with higher baseline sodium intake.16,21 This was interpreted as related to suboptimal RAAS blockade efficacy during high-sodium intake,21 and consequently, paricalcitol was suggested to be a suitable add on to RAAS blockade for patients on high-sodium intake.16 Our prospective intervention is at variance with the latter suggestion.
Our results are consistent with several clinical studies showing that sodium intake potentiates RAAS blockade8–10,27,28 as well as recent data from a prospective study in a rat model of proteinuric nephropathy.19 In this study, combined treatment with paricalcitol and an ACEi reduced proteinuria, renal interstitial inflammation, glomulerosclerosis, and interstitial prefibrotic changes during LS but not during high-sodium intake.19 Dietary sodium restriction reduced residual albuminuria and BP during single–agent RAAS blockade, in line with previous studies.8–10 Paricalcitol in itself provided only a mild further reduction of residual albuminuria beyond dietary sodium restriction, in contrast with prior findings with hydrochlorothiazide, which further reduced residual proteinuria beyond the effect of sodium restriction and angiotensin receptor blockade in a previous study.8 The effect of paricalcitol added to sodium restriction was stronger and reached statistical significance in a per-protocol analysis restricted to patients with >95% compliance with study medication. A possible explanation for the relatively small effect of paricalcitol on albuminuria during ACEi and sodium restriction could be the substantially lower albuminuria elicited by sodium restriction in itself. Residual proteinuria during sodium restriction was relatively low compared with that in other trials in nondiabetic patients with CKD treated with paricalcitol,21,24 suggesting that the efficacy of ACEi combined with LS diet may have diluted the residual treatment effect of paricalcitol. Furthermore, it should also be taken into consideration that our study had a run-in period to optimize RAAS blockade and antihypertensive treatment, because we were interested in the effect of add-on paricalcitol on residual albuminuria during optimal treatment. The albuminuria-lowering effect of paricalcitol was not influenced by the baseline 25(OH)D level; therefore, preexistent vitamin D status is unlikely to explain the nonsignificant effect of paricalcitol.
The renoprotective effects of moderate sodium restriction during single RAAS blockade, lowering albuminuria and BP, are likely multifactorial. The capacity of sodium restriction to reduce residual albuminuria is probably mediated by not only BP8 but additionally, anti-inflammatory and antifibrotic pathways29–31 and local tissue RAAS activity in kidney, vasculature, and brain.32
Experimental studies have shown that VDRA treatment exerts direct protective effects on podocytes,33 negatively regulates the RAAS by suppressing renin production,17,34,35 and has anti-inflammatory and antifibrotic effects.13,36,37 These effects could either alone or most likely, in combination explain the antialbuminuric effect of VDRA in addition to RAAS blockade, which was also supported by our recent preclinical data showing renal tissue protection during ACEi, paricalcitol, and dietary sodium restriction in experimental proteinuric nephropathy.19 In our trial in nondiabetic patients with CKD, the intention-to-treat analysis showed no additional albuminuria–lowering effect of paricalcitol on top of the dietary sodium restriction. Our results are in line with recent studies in nondiabetic CKD,25,38,39 where the antialbuminuric effect of paricalcitol was less than expected on the basis of studies in diabetic CKD or even absent. In the absence of head-to-head comparisons between diabetic and nondiabetic CKD, however, it remains unclear whether there is a consistent difference in responsiveness to paricalcitol between patients who are diabetic and patients who are not diabetic. The absence of an antihypertensive effect of paricalcitol is in accordance with a recent meta-analysis showing that neither paricalcitol nor other vitamin D analogs are effective in lowering BP.40
Both sodium restriction and paricalcitol were well tolerated. The most common adverse effects were mildly symptomatic hypotension (sodium restriction) and hypercalcemia (paricalcitol). Creatinine clearance was significantly reduced by the dietary sodium restriction. This decline was reversible and therefore, probably reflects a reduction of glomerular pressure. It has been shown that a reduction in renal function during initiation of RAAS blockade predicts a slower rate of long–term renal function decline.41,42 These data suggest that the initial fall in renal function in response to antihypertensive therapy reflects renal protection, but whether this is also true for the effect of dietary sodium restriction on top of RAAS blockade has not been established. Paricalcitol also increased serum creatinine and (consequently) decreased creatinine-based eGFR, and creatinine clearance was not influenced by paricalcitol treatment on either sodium intake. An increase in serum creatinine without altering the true GFR has been reported previously for paricalcitol43 and may be related to an effect on muscle metabolism.
Whether the combination of paricalcitol and dietary sodium restriction translates into beneficial long–term outcomes remains to be addressed, but caution is warranted in extrapolation from antialbuminuric effects only, because potential beneficial effects of the lower albuminuria could be counterbalanced by unfavorable effects of the rise in serum phosphate and the phosphate–regulating hormone FGF-23 triggered by paricalcitol.44 To investigate the overall effect of VDRA treatment combined with moderate sodium restriction on long–term clinical outcomes in CKD, a large randomized, controlled clinical trial would be needed.
A limitation of our study is the limited exposure time to paricalcitol, precluding conclusions on the effect of paricalcitol and dietary sodium restriction on long–term clinical outcomes. The length of treatment periods was on the basis of previous studies with paricalcitol showing maximum albuminuria reduction at 4–6 weeks after treatment initiation.16,23 No washout periods were included in the study design; the 8-week period was long enough to minimize potential carryover. Furthermore, the sample size is relatively small, which increases the chance of a false negative finding; however, the crossover design increased statistical power, because subjects served as their own internal control, and the within-patient variability is smaller than the variability between patients. Also, our study was performed in a selection of highly motivated patients under well controlled and intensive treatment, limiting the external validity of our findings. Because we aimed to study the effect of sodium restriction in a clinically relevant setup, we applied sodium intervention by dietary counseling rather than in a blinded design with add-on placebo or sodium supplement. Lastly, BP was evaluated during outpatient clinic visits for 15 minutes by an automatic device and was not evaluated by 24-hour ambulatory BP monitoring. However, major strengths of our study include the crossover design with participants serving as their own internal controls, the documentation of sodium intake by 24-hour urinary excretion, and the prospective intervention design to investigate the influence of sodium intake on the renoprotective efficacy of add-on paricalcitol.
In conclusion, moderate dietary sodium restriction strongly and significantly reduced residual albuminuria during single–agent RAAS blockade. Furthermore, paricalcitol had a small, nonsignificant effect on reducing residual albuminuria in nondiabetic patients with CKD. In this prospective study, we did not confirm that albuminuria reduction by paricalcitol is optimal during high-sodium intake; oppositely, there was a trend toward optimal albuminuria reduction of paricalcitol during sodium restriction. The capacity of moderate sodium restriction to potentiate the antiproteinuric effect of conventional RAAS blockade has been associated with cardiorenal protection in both diabetic12 and nondiabetic11 CKD. Future studies should address whether the combination of paricalcitol and dietary sodium restriction may further enhance cardiorenal protection in addition to conventional RAAS blockade.
Concise Methods
Study Design
We performed an investigator–initiated, multicenter, randomized, double–blind, placebo–controlled, crossover trial in five Dutch hospitals. Patients were included between January of 2012 and May of 2014. Inclusion was concluded on reaching the predefined sample size (see below); the last follow-up visit of the last patient took place in March of 2015. The study was conducted according to the principles of the Declaration of Helsinki; the study protocol has been approved by the Medical Ethical Committee of the University Medical Centre Groningen (METc 2009.272) and registered in the Dutch Clinical Trial Register (NTR2898). The rationale and study protocol of the ViRTUE-CKD Trial have been published previously.45
Participants
We recruited nondiabetic patients with stages 1–3 CKD (creatinine clearance >30 ml/min) and residual albuminuria. Inclusion criteria were residual albuminuria >300 mg/d despite single–agent RAAS blockade, stable renal function (<6-ml/min decline in the previous year) with a creatinine clearance >30 ml/min, PTH values <1.5 times the upper limit of normal, serum calcium (adjusted for serum albumin) between 2.0 and 2.6 mmol/L, serum phosphate ≤1.5 mmol/L, and age over 18 years old. Exclusion criteria were diabetes mellitus, uncontrolled hypertension, hyperkalemia (potassium >6.0 mmol/L), a cardiovascular event in the previous 6 months, heart failure New York Heart Association class III–IV, epilepsy, liver disease, active malignancy, a bowel disorder resulting in fat malabsorption, treatment with vitamin D analog in the previous 3 months, regular use (more than two doses per week) of nonsteroidal anti–inflammatory drugs, use of immunosuppressive treatment, digoxin or psychiatric medication, drug or alcohol abuse, incompliance with the study diet or study medication, pregnancy, or breastfeeding.
Study Design
Detailed information regarding the study protocol has been published previously.45 During a run-in period, patients received standardized RAAS blockade (10 mg ramipril per day). Existing treatment with other RAAS–blocking agents and diuretics (except for furosemide) was discontinued. If the target BP of <140/90 mmHg was not reached within 6 weeks after the initiation of ramipril, additional antihypertensive therapy (metoprolol, doxazosin, and/or amlodipine) was added to the treatment regimen with 4-week intervals. When the target BP was reached, patients were allowed to enter the study protocol. After a maximum wash–in/washout period of 18 weeks, patients with a BP value <180/100 mmHg were able to enroll in the study, whereas patients with a BP>180/100 mmHg were not included in the study.45
Patients were subjected to four subsequent treatment periods of 8 weeks each. These study periods consisted of (1) the VDRA paricalcitol (19-nor-1,25[OH]2–vitamin D2; 2 μg/d) combined with an RS diet (target sodium intake 200 mmol Na+ per day [approximately 4.8 g]; i.e., the average sodium intake in the general population), (2) paricalcitol (2 μg/d) combined with dietary sodium restriction (target sodium intake 50 mmol Na+ per day [approximately 1.2 g]), (3) placebo combined with an RS diet, or (4) placebo combined with dietary sodium restriction. To prevent systematic errors resulting from the crossover design, the order of the treatment periods was randomized (1:1:1:1) for each patient. Four different treatment sequences were defined.45 The study medication (paricalcitol or placebo) was provided by AbbVie. Placebo capsules had a similar appearance, smell, and taste compared with paricalcitol capsules. Computer-generated randomization was performed by AbbVie. The investigators (C.A.K. and G.F.v.B.) enrolled participants. Patients received study medication containers labeled with a unique number representing the randomly allocated sequence, whereby all participants and involved investigators and care providers remained blinded to the study medication type (paricalcitol or placebo) throughout the entire study. Assignment of the treatment order was not disclosed until the study database was locked. The dietary intervention was open label.
At the start of the first dietary sodium restriction study period, patients received personal dietary advice from a dietician. Sodium restriction was achieved by replacing sodium-rich products with an LS product of the same product group, aiming for isocaloric intake with a similar balance among protein, carbohydrate, and fat. Compliance with the sodium diet was monitored by measuring 24-hour urinary sodium excretion every 4 weeks, and patients were counseled to use this information.
At the start of the run-in period, medication use of the participants was verified. Use of (self–initiated) vitamin supplementation was specifically inquired. Any form of vitamin D supplementation was discontinued. Participants were instructed not to use supplemental vitamin D (calciferol) and report all changes in prescribed and self–initiated medication use during the entire study.
Four weeks after the start of each treatment period, serum albumin, calcium, and PTH were measured for a safety analysis. In case of hypercalcemia (corrected serum calcium >2.60 mmol/L) or hypoparathyroidism (PTH<1.5 pmol/L), the dose of the study medication (paricalcitol or placebo) was reduced from two capsules to one capsule per day for the remaining study period(s). All patient–reported or observed adverse effects were recorded.
Primary and Secondary End Points
The primary end point of our study was albuminuria measured in a 24-hour urine sample collected at the end of each study period. Secondary study end points were BP, creatinine clearance, eGFR, urinary sodium excretion, and plasma renin concentration measured at the end of each study period.
Measurements
At the end of each 8-week treatment period, patients collected 24-hour urine samples, BP was measured, and a blood sample was taken after overnight fasting. Albuminuria was measured using a turbidimetric assay using benzethonium chloride (Modular; Roche Diagnostics, Mannheim, Germany). BP was evaluated during every outpatient clinic visit under constant conditions at 1-minute intervals for 15 minutes by an automatic device (Dinamap; DE Medical Systems, Milwaukee, WI) with the patient in a semisupine position. The mean of three readings was used for further analysis.45 Blood electrolytes, lipids, proteins, and urinary electrolytes were determined by using an automated multianalyzer (Modular; Roche Diagnostics). Plasma renin concentration was measured using a two–site immunoradiometric assay (Beckman Coulter; Immunotech, Prague, Czech Republic). PTH concentrations were assessed with the Roche Cobas Electrochemoluminescent Immunometric Assay (Roche Diagnostics); 25(OH)D levels were determined by liquid chromatography tandem mass spectrometry. Carboxy–terminal FGF-23 was determined in duplicate using a human FGF-23 ELISA (Immutopics, San Clemente, CA).
Dietary sodium intake was assessed from urinary sodium excretion in 24-hour urine samples. Creatinine clearance was calculated from creatinine concentrations in plasma and 24-hour urine collections, and eGFR was calculated using the creatinine–based Chronic Kidney Disease Epidemiology Collaboration formula.46 Serum calcium was adjusted for hypoalbuminemia as follows: corrected calcium = serum calcium (millimoles per liter) +0.023×(40− serum albumin [grams per liter]) if serum albumin is <35 g/L. Peripheral pitting edema was assessed at the pretibia area of both legs by visual and manual examination and scored dichotomously (absent or present).
Statistical Analyses
On the basis of data from a previous study,8 a sample size of 39 patients was calculated to detect a difference of 23% in albuminuria (log Δalbuminuria of −0.26) between high sodium + placebo and LS + paricalcitol with 90% power, considering an SD of 0.5 for the log Δalbuminuria.45 Assuming a dropout rate of 15%, we aimed to include 45 patients. The sample size calculation took into account that each patient serves as his/her own internal control, increasing statistical power.
Data are presented as mean±SD in case of normally distributed data, geometric mean (95% CI) for non–normally distributed data, and number (percentage) for nominal data unless stated otherwise. The relative change in albuminuria between study periods is presented as median (interquartile range). Variable distribution was tested with histograms and probability plots. P values for differences between the four treatment sequences were assessed with ANOVA for normally distributed continuous data, the Kruskal–Wallis test for non–normally distributed data, and the chi-squared test for nominal data. Data at the end of the run-in period were considered baseline values. To determine the effect of treatment, we used linear mixed effect models for repeated measurements using the unstructured covariance structure with random intercept and center, treatment, and sequence as well as their interaction (treatment × sequence) as fixed factors. Non–normally distributed variables were 2 log transformed before entering the model. Linear mixed model analysis was used to investigate possible carryover effects: nonsignificant (P>0.05) effects of sequence and treatment × sequence were interpreted as indicating that carryover effects were absent.
To investigate a possible interaction between the interventions on the primary end point, we also analyzed the primary outcome (2 log-transformed albuminuria) by linear mixed effect models for repeated measurements using the unstructured covariance structure with random intercept and center, period, sequence, medication (placebo or paricalcitol), and diet (normal sodium or LS diet) as well as their interaction (medication × diet) as fixed factors.
For the primary analysis, all available data from all 45 patients were included (intention-to-treat analysis). As a per-protocol analysis, we reanalyzed the primary end point in a study population restricted to those participants with ≥95% compliance with the study medication (assessed by counting the returned paricalcitol capsules) for each treatment period. There were 31–34 participants available for this analysis. To account for missing data, we report the estimated (geometric) means obtained from the linear mixed modeling for this analysis. In another secondary analysis, we addressed the compliance with the dietary sodium restriction by adding 24-hour urinary Na+ excretion (as a continuous variable) to the linear mixed model analysis.
A two-tailed P<0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS 22.0 for Windows (IBM SPSS, Chicago, IL) and GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
Disclosures
AbbVie (Chicago, IL) funded the study medication (paricalcitol and placebo).
Supplementary Material
Acknowledgments
We appreciate the willingness of patients to participate in this trial. We also appreciate the skillful assistance of Carla Wassenaar, research nurse at the Medical Centre Leeuwarden; Hiske Wellink and Marjon van Vliet, research nurses at the Vrije Universiteit (VU) Medical Centre; Ineke Knot, nurse practitioner at Martini Hospital Groningen; and all participating dieticians. We thank AbbVie for providing the study medication (paricalcitol and placebo). We also thank AML, Sonic Healthcare Benelux (Antwerp, Belgium) for performing the renin analyses.
This trial was supported by a consortium grant from the Dutch Kidney Foundation (NIGRAM Consortium grant CP10.11). H.J.L.H. is supported by a grant from The Netherlands Organization for Scientific Research (Veni and Vidi grants). M.H.d.B. is supported by personal grants from the Dutch Kidney Foundation (grant no. KJPB.08.07) and the Netherlands Organization for Scientific Research (Veni grant).
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
The NIGRAM Consortium consists of the following principal investigators: Piet ter Wee and M.G.V. (VU University Medical Centre); René J. Bindels and Joost G. Hoenderop (Radboud University Medical Centre, Nijmegen, The Netherlands); and G.N., Jan-Luuk Hillebrands, and M.H.d.B. (University Medical Centre Groningen).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Low Sodium Diet, Vitamin D, or Both for RAASi-Resistant, Residual, Proteinuria in CKD? The ViRTUE Trial Points the Way Forward but Is Not the Last Word,” on pages 1016–1019.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016040407/-/DCSupplemental.
Contributor Information
Collaborators: Charlotte A. Keyzer, G. Fenna van Breda, Marc G. Vervloet, Maarten A. de Jong, Gozewijn D. Laverman, Marc H. Hemmelder, Wilbert M.T. Janssen, Hiddo J. Lambers Heerspink, Arjan J. Kwakernaak, Stephan J.L. Bakker, Gerjan Navis, and Martin H. de Borst
References
- 1.Zucchelli P, Zuccalà A, Borghi M, Fusaroli M, Sasdelli M, Stallone C, Sanna G, Gaggi R: Long-term comparison between captopril and nifedipine in the progression of renal insufficiency. Kidney Int 42: 452–458, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P: Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The angiotensin-converting-enzyme inhibition in progressive renal insufficiency study group. N Engl J Med 334: 939–945, 1996 [DOI] [PubMed] [Google Scholar]
- 3.GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia): Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 4.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G: Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med 342: 145–153, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Ruggenenti P, Perna A, Remuzzi G; GISEN Group Investigators : Retarding progression of chronic renal disease: The neglected issue of residual proteinuria. Kidney Int 63: 2254–2261, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Holtkamp FA, de Zeeuw D, de Graeff PA, Laverman GD, Berl T, Remuzzi G, Packham D, Lewis JB, Parving HH, Lambers Heerspink HJ: Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: A post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J 32: 1493–1499, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G: Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 19: 999–1007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slagman MC, Waanders F, Hemmelder MH, Woittiez AJ, Janssen WM, Lambers Heerspink HJ, Navis G, Laverman GD; HOlland NEphrology STudy Group : Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: Randomised controlled trial. BMJ 343: d4366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwakernaak AJ, Krikken JA, Binnenmars SH, Visser FW, Hemmelder MH, Woittiez AJ, Groen H, Laverman GD, Navis G; Holland Nephrology Study (HONEST) Group : Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: A randomised clinical trial. Lancet Diabetes Endocrinol 2: 385–395, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P: Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol 23: 165–173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambers Heerspink HJ, Holtkamp FA, Parving HH, Navis GJ, Lewis JB, Ritz E, de Graeff PA, de Zeeuw D: Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int 82: 330–337, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E: Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol 18: 1796–1806, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC: Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: Blockade of compensatory renin increase. Proc Natl Acad Sci U S A 105: 15896–15901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Borst MH, Hajhosseiny R, Tamez H, Wenger J, Thadhani R, Goldsmith DJ: Active vitamin D treatment for reduction of residual proteinuria: A systematic review. J Am Soc Nephrol 24: 1863–1871, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D: Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP: 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaidya A, Sun B, Larson C, Forman JP, Williams JS: Vitamin D3 therapy corrects the tissue sensitivity to angiotensin ii akin to the action of a converting enzyme inhibitor in obese hypertensives: An interventional study. J Clin Endocrinol Metab 97: 2456–2465, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirkovic K, Frenay AS, van den Born J, van Goor H, Navis G, de Borst MH; NIGRAM consortium : Sodium restriction potentiates the renoprotective effects of combined vitamin D receptor activation and angiotensin-converting enzyme inhibition in established proteinuric nephropathy [published online ahead of print August 25, 2015]. Nephrol Dial Transplant doi: 10.1093/ndt/gfv304 [DOI] [PubMed] [Google Scholar]
- 20.Keyzer CA, Lambers-Heerspink HJ, Joosten MM, Deetman PE, Gansevoort RT, Navis G, Kema IP, de Zeeuw D, Bakker SJ, de Borst MH; PREVEND Study Group : Plasma vitamin D level and change in albuminuria and eGFR according to sodium intake. Clin J Am Soc Nephrol 10: 2119–2127, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Nicola L, Conte G, Russo D, Gorini A, Minutolo R: Antiproteinuric effect of add-on paricalcitol in CKD patients under maximal tolerated inhibition of renin-angiotensin system: A prospective observational study. BMC Nephrol 13: 150, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D: Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int 68: 2823–2828, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R: Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: A randomized double-blind pilot trial. Hypertension 52: 249–255, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Fishbane S, Chittineni H, Packman M, Dutka P, Ali N, Durie N: Oral paricalcitol in the treatment of patients with CKD and proteinuria: A randomized trial. Am J Kidney Dis 54: 647–652, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Larsen T, Mose FH, Bech JN, Pedersen EB: Effect of paricalcitol on renin and albuminuria in non-diabetic stage III-IV chronic kidney disease: A randomized placebo-controlled trial. BMC Nephrol 14: 163, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hojs N, Bevc S, Balon BP, Hojs R, Ekart R: Paricalcitol reduces proteinuria in non-dialysis chronic kidney disease patients. Ther Apher Dial 17: 368–372, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Heeg JE, de Jong PE, van der Hem GK, de Zeeuw D: Efficacy and variability of the antiproteinuric effect of ACE inhibition by lisinopril. Kidney Int 36: 272–279, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Fabris B, Jackson B, Johnston CI: Salt blocks the renal benefits of ramipril in diabetic hypertensive rats. Hypertension 17: 497–503, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Ying WZ, Sanders PW: Dietary salt modulates renal production of transforming growth factor-beta in rats. Am J Physiol 274: F635–F641, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Kwakernaak AJ, Waanders F, Slagman MC, Dokter MM, Laverman GD, de Boer RA, Navis G: Sodium restriction on top of renin-angiotensin-aldosterone system blockade increases circulating levels of N-acetyl-seryl-aspartyl-lysyl-proline in chronic kidney disease patients. J Hypertens 31: 2425–2432, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Slagman MC, Nguyen TQ, Waanders F, Vogt L, Hemmelder MH, Laverman GD, Goldschmeding R, Navis G: Effects of antiproteinuric intervention on elevated connective tissue growth factor (CTGF/CCN-2) plasma and urine levels in nondiabetic nephropathy. Clin J Am Soc Nephrol 6: 1845–1850, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Borst MH, Navis G: Sodium intake, RAAS-blockade and progressive renal disease. Pharmacol Res 107: 344–351, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K: 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol 286: F526–F533, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Kong J, Deb DK, Chang A, Li YC: Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol 21: 966–973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC: 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem 282: 29821–29830, 2007 [DOI] [PubMed] [Google Scholar]
- 36.He W, Kang YS, Dai C, Liu Y: Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E: Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int 53: 1696–1705, 1998 [DOI] [PubMed] [Google Scholar]
- 38.de Boer IH, Sachs M, Hoofnagle AN, Utzschneider KM, Kahn SE, Kestenbaum B, Himmelfarb J: Paricalcitol does not improve glucose metabolism in patients with stage 3-4 chronic kidney disease. Kidney Int 83: 323–330, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundwall K, Jörneskog G, Jacobson SH, Spaak J: Paricalcitol, microvascular and endothelial function in non-diabetic chronic kidney disease: A randomized trial. Am J Nephrol 42: 265–273, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, Alvarez JA, Boxer RS, Dalbeni A, Gepner AD, Isbel NM, Larsen T, Nagpal J, Petchey WG, Stricker H, Strobel F, Tangpricha V, Toxqui L, Vaquero MP, Wamberg L, Zittermann A, Witham MD; D-PRESSURE Collaboration : Effect of vitamin D supplementation on blood pressure: A systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med 175: 745–754, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apperloo AJ, de Zeeuw D, de Jong PE: A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney Int 51: 793–797, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, Parving HH, Brenner BM, Shahinfar S, Lambers Heerspink HJ: An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80: 282–287, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Agarwal R, Hynson JE, Hecht TJ, Light RP, Sinha AD: Short-term vitamin D receptor activation increases serum creatinine due to increased production with no effect on the glomerular filtration rate. Kidney Int 80: 1073–1079, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Scialla JJ, Wolf M: Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol 10: 268–278, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Keyzer CA, de Jong MA, Fenna van Breda G, Vervloet MG, Laverman GD, Hemmelder M, Janssen WM, Lambers Heerspink HJ, Navis G, de Borst MH; Holland Nephrology Study (HONEST) Network : Vitamin D receptor activator and dietary sodium restriction to reduce residual urinary albumin excretion in chronic kidney disease (ViRTUE study): Rationale and study protocol. Nephrol Dial Transplant 31: 1081–1087, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III , Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.