Abstract
AKI is a frequent complication in hospitalized patients. Unfortunately, there is no effective pharmacologic approach for treating or preventing AKI. In rodents, mineralocorticoid receptor (MR) antagonism prevents AKI induced by ischemia-reperfusion (IR). We investigated the specific role of vascular MR in mediating AKI induced by IR. We also assessed the protective effect of MR antagonism in IR-induced AKI in the Large White pig, a model of human AKI. In mice, MR deficiency in smooth muscle cells (SMCs) protected against kidney IR injury. MR blockade by the novel nonsteroidal MR antagonist, finerenone, or genetic deletion of MR in SMCs associated with weaker oxidative stress production. Moreover, ischemic kidneys had higher levels of Rac1-GTP, required for NADPH oxidase activation, than sham control kidneys, and genetic deletion of Rac1 in SMCs protected against AKI. Furthermore, genetic deletion of MR in SMCs blunted the production of Rac1-GTP after IR. Pharmacologic inhibition of MR also prevented AKI induced by IR in the Large White pig. Altogether, we show that MR antagonism, or deletion of the MR gene in SMCs, limited the renal injury induced by IR through effects on Rac1-mediated MR signaling. The benefits of MR antagonism in the pig provide a rational basis for future clinical trials assessing the benefits of this approach in patients with IR-mediated AKI.
Keywords: ischemia-reperfusion, oxidative stress, vascular, aldosterone
AKI is a frequent complication that affects almost 5% of hospitalized patients and 40%–70% of intensive care unit patients.1 It is now recognized as a risk factor for the development of CKD.2 One of the principal features of ischemic AKI is a decrease in renal blood flow, leading to lower levels of oxygen delivery to kidney tissues, resulting in endothelial alterations, tubular cell injury, oxidative stress, and inflammation.3,4 The reduction of renal perfusion is linked to changes in the balance between vasoconstrictors and vasodilators.5 For example, during kidney ischemia-reperfusion (IR), nitric oxide (NO) bioavailability is impaired by the low levels of eNOS activity and NO oxidation by locally produced reactive oxygen species (ROS).6,7
Despite recent advances in our understanding of AKI, there is still no effective treatment. New innovative treatments targeting common mechanisms involved in AKI of different etiologies are therefore required. We have shown that mineralocorticoid receptor (MR) antagonist before, or even just after ischemic damage is beneficial, and can considerably reduce IR injury in rats.8 However, the mechanisms underlying the benefits of MR antagonists (MRAs) remain unclear. The role of MR activation in modulating renal perfusion is highlighted by the normalization of renal blood flow and limitation of renal injury by pharmacologic MR antagonism.9,10 It remains unclear whether the MR expressed in the vasculature is involved in the beneficial effects of MRAs. There is strong evidence to implicate the MR expressed in either endothelial cells (ECs) or smooth muscle cells (SMCs) in the modulation of vascular tone, and thus, BP.11–13 We recently demonstrated that deletion of the MR gene in SMCs prevented the increase in renal vascular resistance in a mouse model of cyclosporin A (CsA) nephrotoxicity, because of increased L-type calcium channel activity.14 This finding suggests that MR activation in renal ECs or SMCs may contribute to IR injury. The goal of this study was to investigate the specific role of the MR in ECs and SMCs during the kidney injury induced by IR, and to understand the mechanisms underlying MR-mediated IR injury. We also assessed the protective effect of MRAs in the Large White pig, an animal model of human AKI, to provide a rational basis for future clinical trials assessing the benefits of MRA in patients with IR-mediated AKI.
Results
MR Antagonism with Finerenone Prevents AKI Induced by IR in Mice
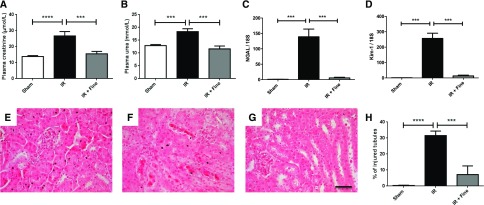
We first investigated whether the novel nonsteroidal MR antagonist finerenone had a beneficial effect on AKI induced by IR in mice, as previously reported for spironolactone9 and BR-42688 in rats. As we planned to address the cell-specific role of MR in the vasculature, we investigated the protective effects of finerenone against IR in mice with a genetic background similar to that of the ECs-MR and SMCs-MR knockout mice used in subsequent experiments. Finerenone prevented the renal dysfunction induced by bilateral ischemia (Figure 1, A and B). Nontreated mice with AKI had high renal mRNA levels of NGAL and Kim-1, two markers of tubular injury. Finerenone blunted the increase in the levels of these two markers (Figure 1, C and D). It also prevented IR-induced tubular injury, as assessed by measuring cast formation and tubular cell detachment, as shown by comparison with the sham treatment (Figure 1, E–H). Plasma potassium concentration was similar and unchanged in the two groups (Supplemental Figure 1).
Figure 1.
Benefits of finerenone (10 mg/kg) for preventing kidney injury induced by IR. Renal function was evaluated by quantifying the plasma levels of (A) creatinine and (B) urea. As markers of tubular injury, (C) levels of mRNA in the kidney for neutrophil gelatinase-associated lipocalin and (D) kidney injury molecule-1 were assessed by RT-PCR. Representative hematoxylin and eosin staining images are shown for the (E) sham, (F) IR, and (G) IR and finerenone (IR+Fine) groups. (H) The percentage of injured tubules was quantified blind, on ten fields per mouse. Scale bar, 20 μm. n=8 per group. One-way ANOVA was performed. ***P<0.001; ****P<0.001.
MR Deficiency in SMCs, but Not in ECs, Protects Mice Against AKI Induced by IR
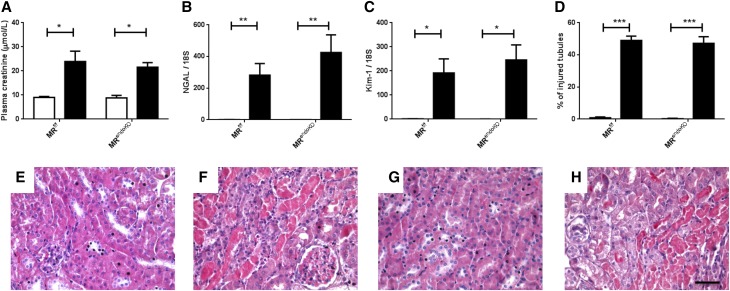
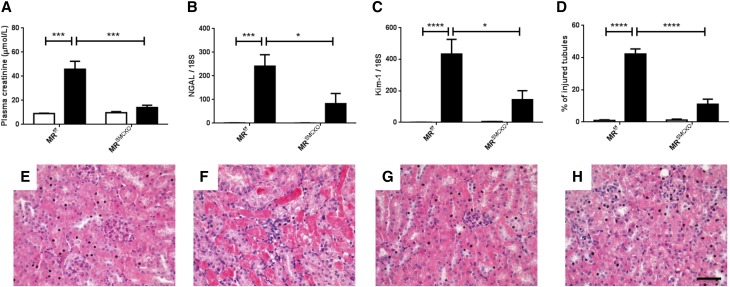
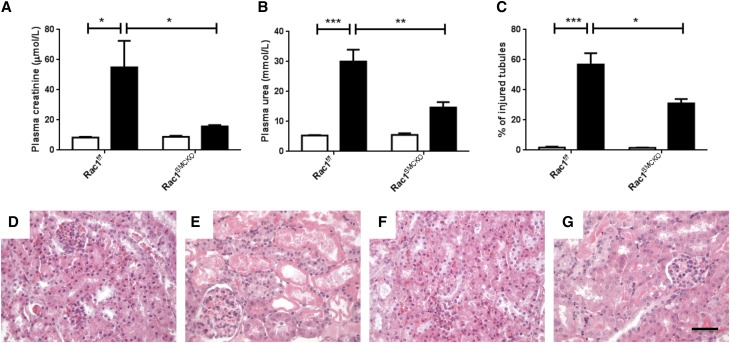
We investigated the role of the MR expressed in ECs or SMCs in kidney IR injury, by generating two knockout mouse models with cell-specific and inducible MR inactivation in ECs (MRendoKO) or SMCs (MRSMCKO). Control MRf/f mice with bilateral renal ischemia developed AKI, as shown by an increase in plasma creatinine (Figure 2A), an increase in mRNA levels for NGAL and Kim-1 (Figure 2, B and C), and the presence of injured tubules (Figure 2, D and F). Similar changes were induced by IR in the MRendoKO mice: plasma creatinine levels increased (Figure 2A), as did NGAL and Kim-1 mRNA levels (Figure 2, B–C), and tubular injury similar to that in control mice was observed (Figure 2, D and H). In contrast, MR deficiency in SMCs protects against IR-induced renal injury, as demonstrated by the lack of increase in plasma creatinine concentration (Figure 3A) and the significantly lower levels of mRNA for NGAL and Kim-1 (Figure 3, B and C). MR knockout in SMCs also prevented the development of tubular lesions (Figure 3, D and H). This suggests that the MR expressed in SMCs, but not in the endothelium, is crucial for the development of IR injury and is a mandatory target for the beneficial effects of the MRA finerenone.
Figure 2.
MR deficiency in ECs does not protect against kidney IR injury. Plasma levels of (A) creatinine were measured as an index of renal function. As markers of tubular injury, we used RT-PCR to determine mRNA levels for (B) neutrophil gelatinase-associated lipocalin and (C) kidney injury molecule-1. (D) The percentage of injured tubules was quantified blind on ten fields per mouse. Representative hematoxylin and eosin staining images are shown for (E) MRf/f sham, (F) MRf/f IR, (G) MRendoKO sham, and (H) MRendoKO IR. Scale bar, 20 μm. White bars represent the sham groups and black bars represent the IR groups. n=8 per group. Two-way ANOVA was performed. *P<0.01; **P<0.001; ***P<0.001.
Figure 3.
MR deficiency in SMCs protects against kidney IR injury. Plasma levels of (A) creatinine, as an index of renal function. As markers of tubular injury, we used RT-PCR to determine the mRNA levels for (B) neutrophil gelatinase-associated lipocalin and (C) kidney injury molecule-1. (D) The percentage of injured tubules was quantified blind on ten fields per mouse. Representative images for hematoxylin and eosin staining are shown for (E) MRf/f sham, (F) MRf/f IR, (G) MRSMCKO sham, and (H) MRSMCKO IR. Scale bar, 20 μm. White bars represent the sham groups and black bars represent the IR groups. n=8 per group. Two-way ANOVA was performed. *P<0.01; ***P<0.0001; ****P<0.00001
Rac1 Activity in SMCs Is Involved in the Deleterious Effects of MR Activation in IR Injury
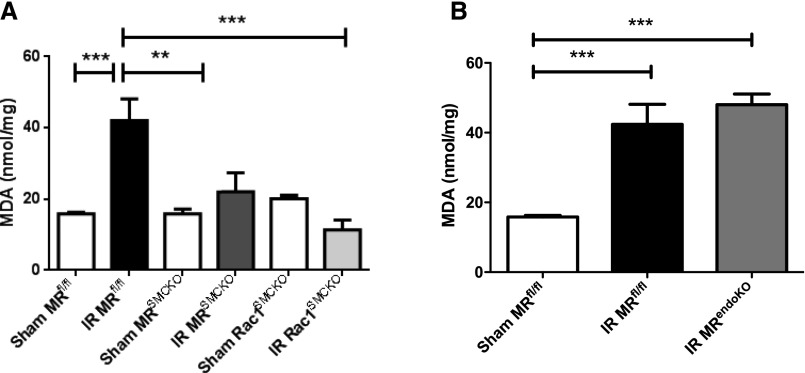
We recently identified an oxidative stress-mediated inactivating post-translational modification of the vasodilator endothelin B receptor (ETB) as a target for MRAs in AKI, improving renal blood flow after MRA treatment.8 We therefore investigated whether the protection yielded in MRSMCKO mice was associated with a decrease in renal oxidative stress, by measuring lipid peroxidation levels. Malondialdehyde (MDA) levels were found to have increased after 24 hours of ischemia in the controls (Figure 4A), as previously reported,15 but this effect was not observed in MRSMCKO mice experiencing renal IR. MRendoKO mice had MDA levels similar to those of MRf/f mice (Figure 4B). Quantification of lipid peroxides confirmed these results (Supplemental Figure 2).
Figure 4.
MR and Rac1 genetic deletion in SMCs prevent oxidative injury induced by IR. (A) Deficiencies of MR and Rac1 in SMCs prevented lipid peroxidation induced by IR. (B) MR deletion in ECs did not prevent lipid peroxidation induced by IR. MDA levels were quantified in kidney lysates. The data were normalized against the protein concentration of the tissue lysate. n=6 per group. Two-way ANOVA was performed. **P<0.001; ***P<0.001.
NADPH oxidase is a major source of ROS during renal IR, and Rac1 is a mandatory subunit of NADPH oxidase for ROS production.16 Pharmacologic inhibition of Rac1 using NSC23766 prevented renal damage after renal ischemia.17 In the ischemic kidney, we observed that Rac1 activity was increased when compared with sham mice (Rac1-GTP/total Rac1 ratio, A.U.: 0.99±0.05 in sham [n=13] versus 2.33±0.6 in IR [n=11]; P<0.05). Because MR activation increases NADPH activity, we hypothesized that SMCs Rac1 may be a direct target of SMC MR involved in the deleterious effects of MR in IR. Mice lacking Rac1 in SMCs (Rac1SMCKO) only displayed lower levels of lipid peroxidation after the induction of renal IR. The elimination of Rac1 from SMCs completely blunted the increase in MDA and lipid hydroperoxide (LPO) levels induced by IR (Figure 4A, Supplemental Figure 2A). We therefore investigated whether Rac1SMCKO mice were protected against renal dysfunction and tubular injury after kidney IR. Renal IR induced an increase in plasma creatinine (Figure 5A) and urea (Figure 5B) concentrations and tubular lesions in control Rac1f/f mice (Figure 5, C and D). Also, Rac1SMCKO mice were protected against the renal failure and tubular injury induced by IR (Figure 5).
Figure 5.
A lack of Rac1 in SMCs protects against kidney IR injury. Plasma levels of (A) creatinine and (B) urea were measured as an index of renal function. (C) The percentage of injured tubules was quantified blind on ten fields per mouse. Representative images for the hematoxylin and eosin staining are shown for (D) Rac1f/f sham, (E) Rac1f/f IR, (F) Rac1SMCKO sham, and (G) Rac1SMCKO IR. Scale bar, 20 μm. White bars represent the sham groups and black bars represent the IR groups. n=5 per group. Mann–Whitney U test analysis was performed. *P<0.01; **P<0.001; ***P<0.001.
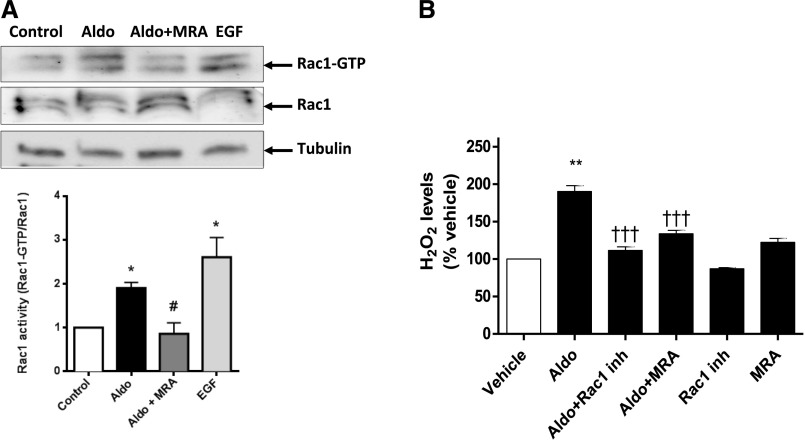
A link between MR and Rac1 activity has been established in the kidney.18,19 A deficiency of MR or Rac1 in SMCs prevented renal injury and oxidative stress after IR. Consequently, we investigated the possible convergence of the MR and Rac1 signaling pathways. In vivo, in basal conditions, Rac1 activity levels were lower in aortas (Rac1-GTP/total Rac1 ratio, A.U.: 0.99±0.02 versus 0.69±0.06; P<0.05) and kidneys (Rac1-GTP/total Rac1 ratio, A.U.: 0.85±0.09 versus 0.41±0.09; P<0.05) from MRSMCKO than in those from control mice. In the ischemic kidney, MRSMCKO mice showed reduced Rac1 activation in response to kidney IR (Rac1-GTP/total Rac1 ratio, A.U.: 1.01±0.19 in MRf/f mice [n=8] versus 0.63±0.11 in MRSMCKO mice [n=8]; P<0.05). In primary cultures of rat SMCs, aldosterone induced a significant increase in Rac1 activity. This effect was prevented by the coincubation of the cells with aldosterone and an MRA, suggesting a direct link between MR activation and Rac1 activity (Figure 6A). We then analyzed the contribution of Rac1 to aldosterone-induced oxidative stress: aldosterone induced a two-fold increase in H2O2 levels, an effect totally abolished by pharmacologic MR or Rac1 antagonism (Figure 6B). Of note, EGF-induced oxidative stress was not affected by MR antagonism (Supplemental Figure 3). This suggests that Rac1 is involved in the oxidative stress observed in SMCs through an MR-dependent mechanism.
Figure 6.
MR activation increases Rac-1 activity and affects oxidative stress. (A) Rac1 activity was determined in rat primary SMCs incubated with aldosterone (Aldo), aldosterone and spironolactone (MRA), or EGF as a positive control. A representative Western blot is shown, together with a densitometric analysis. (B) Hydrogen peroxide production was quantified in rat primary SMCs in the presence of aldosterone, aldosterone and spironolactone, and aldosterone and Rac1 inhibitor (EHT 1864). n=5 per group. One-way ANOVA analysis was performed. *P<0.01 versus control; #P<0.01 versus Aldo; **P<0.01 versus vehicle; †††P<0.01 versus Aldo.
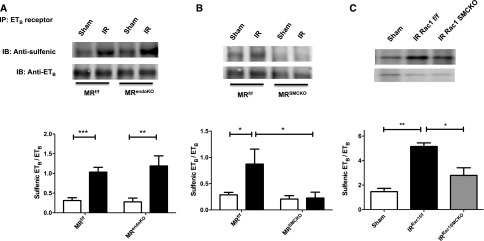
Our past and present results demonstrate strong beneficial effects of the pharmacologic or genetic inhibition of MR in AKI related to IR. We identified an underlying mechanism involving MR-mediated oxidative stress in SMCs. To further explore its molecular effect, we analyzed the effect of MR deletion in ECs and SMCs, as well as Rac1 in SMCs, on the oxidative stress–mediated, inactivating ETB receptor, sulfenic acid modification induced by IR. MR deletion in ECs did not prevent this modification (Figure 7A). However, MR deletion in SMCs (Figure 7B) and Rac1 deletion in SMCs (Figure 7C) both prevented the renal cysteine-sulfenic acid modification on ETB receptor that was present in the ischemic littermate mice.
Figure 7.
MR and Rac1 deficiency in SMCs prevent an inactivating sulfenic acid modification on ETB receptor. The ETB receptor was immunoprecipitated from whole kidneys and a Western blot against cysteine sulfenic acid was performed in (A) MRendoKO mice, (B) MRSMCKO mice, and (C) Rac1SMCKO mice. n=5 per group. Two-way ANOVA was performed. *P<0.01; **P<0.001; ***P<0.001.
MRA Is Beneficial in IR Injury Induced by AKI in the Large White Pig
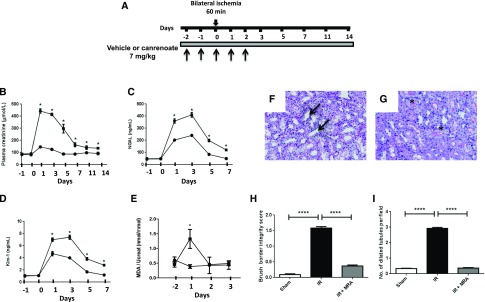
We assessed the effects of MRA on renal IR in a preclinical model, the Large White pig, to provide support for the use of MRA to prevent IR-induced AKI in patients. Soludactone, an injectable form of the MRA potassium, canrenoate (an active metabolite of spironolactone already in use in other clinical settings), was administered to pigs that had been subjected to 60 minutes of bilateral renal ischemia (Figure 8A). The renal dysfunction induced by IR was demonstrated by an increase in plasma creatinine concentration. In contrast, pigs receiving canrenoate were efficiently protected against acute renal failure (Figure 8B), with an impressive prevention of acute renal failure by the MRA canrenoate. Plasma potassium levels were slightly higher in canrenoate-treated animals than in vehicle-treated animals (Supplemental Figure 4), demonstrating the relevance of the pig as a model for assessing the potential hyperkalemic effects of MRAs in this context. Tubular injury was documented by a substantial increase in urinary NGAL and KIM-1 excretion (Figure 8, C and D). The induction of NGAL and KIM-1 was decreased by canrenoate treatment (Figure 8, C and D). Consistent with the evidence obtained for rodents, MR antagonism in the pig was associated with a marked decrease in oxidative stress, as demonstrated by the lower urinary MDA levels in canrenoate-treated pigs (Figure 8E). Moreover, a biopsy on day 7 after IR revealed that untreated pigs displayed a loss of brush border integrity and tubular dilation; canrenoate treatment fully blunted tubular injury (Figure 8, F–I). These findings suggest that MRAs have similar beneficial effects in the pig model of AKI.
Figure 8.
MR antagonism is an effective approach for preventing AKI induced by IR in the Large White pig. (A) Experimental protocol. Arrows indicate the days of vehicle or canrenoate administration. Plasma levels of (B) creatinine were determined as an index of renal function. As markers of tubular injury, (C) urinary NGAL and (D) Kim-1 levels were quantified by ELISA. (E) Urinary MDA levels were determined and normalized against urinary creatinine concentration. Black squares represent pigs subjected to 60 minutes of bilateral ischemia and receiving vehicle, and black circles represent pigs subjected to 60 minutes of bilateral ischemia receiving canrenoate. Day 0 is the day of the ischemia. n=6 per group. Representative hematoxylin and eosin staining for the (F) IR and vehicle and (G) IR and canrenoate groups. Asterisks indicate brush border integrity. Arrows indicate tubular dilation and brush border loss. (H) Quantification of the brush border integrity score and (I) the number of dilated tubules per field. n=6 per group. One-way ANOVA was performed. *P<0.01; ****P<0.00001.
Discussion
AKI is a frequent clinical problem associated with several adverse outcomes. Despite considerable progress in our understanding of the mechanisms involved, no pharmacologic approach has yet been shown to be effective in clinical studies. We therefore need to increase our knowledge of the cellular and molecular players involved in AKI, to make it possible to target them efficiently. MR antagonism has been identified as a promising pharmacologic strategy in a rat model of IR.8–10 However, the mechanisms and cellular targets involved remain unclear, as do the possible beneficial effects in a model of AKI more closely related to humans.
Finerenone is a third-generation nonsteroidal MR antagonist with weaker potassium-sparing effects than previous MRAs, associated with a lower risk of hyperkalemia in patients with renal injury.20,21 We showed that this molecule fully prevented renal dysfunction and tubular injury induced by IR in mice. This finding extends our previous observation that spironolactone and another third-generation MR antagonist (BR-4628) can prevent renal IR injury.8
Renal hemodynamics is strongly affected during IR, as demonstrated by sustained vasoconstriction and a decrease in renal blood flow.22,23 The MR of both ECs and SMCs play a key role in regulating vascular tone.11–13 We therefore hypothesized that the beneficial effects of MRA during renal IR might be because of a blockade of the MR in the vasculature. MR deficiency in ECs had no effect on acute outcome after the induction of IR. In contrast, the targeted deletion of MR in SMCs prevented kidney IR injury, suggesting that the SMC MR plays an essential role in mediating vasoconstriction after IR. These data are consistent with our previous findings indicating that MR deletion in SMCs, but not in ECs, abolishes acute CsA nephrotoxicity by decreasing the vasoconstrictive effect of angiotensin II in the renal microvasculature through a decrease in L-type calcium channel activity.14 Thus, in both acute CsA-induced nephrotoxicity and IR-mediated injury, the MR expressed in ECs is not involved in kidney injury whereas the MR expressed in SMCs is essential for such damage.
Both MR activation24 and renal ischemia16 increase ROS production, but it remains unclear whether ROS play a key role in the deleterious effects of the MR in renal ischemia. The increase in ROS generation plays an important role in tissue injury by directly inducing lipid peroxidation and damage to DNA and proteins, leading to cell death.25 ROS can also contribute to sustained vasoconstriction by oxidizing NO and inducing a sulfenic acid post-translational modification of the vasodilator, ETB receptor. This sulfenic modification has a major consequence as it prevents ETB receptor-mediated eNOS activation and NO release. We have previously shown in a rat model of IR that the prevention of this inactivating sulfenic ETB receptor modification is essential for the protective effects of MRA, and is associated with an improvement in renal blood flow.8 We hypothesized in this study that the increased ROS production was mediated by MR-dependent Rac1 activation in SMCs. Indeed, it has been suggested that there is a link between MR and Rac1 in kidney disease, because MR blockade with eplerenone prevents renal failure in a genetic mouse model with a constitutive activating mutation of Rac1.26 A similar effect has also recently been reported in the heart, with eplerenone preventing cardiac injury because of pressure overload.27 We demonstrated here that Rac1 activity is increased in ischemic kidneys and that this effect is reduced in MRSMCKO mice. Moreover, the elimination of Rac1 in SMCs prevents renal injury associated with IR. This is in accordance with previous finding from Gao et al., showing that in vivo administration of the Rac1 NSC23766 inhibitor prevents IR-mediated AKI in mice.17 The protection conferred by MR or Rac1 deficiency in SMCs was associated with a decrease in lipid peroxidation. The direct link between MR and Rac1 was demonstrated by the increase in Rac1 activity in SMCs after the addition of aldosterone, and the blocking of this effect by MR antagonism. Moreover, ROS production increased with MR activation and was blocked by Rac1 inhibition. The link between oxidative stress induced by MR-Rac1 activation and renal injury during IR is reinforced by the finding that the cysteine-sulfenic acid modification that affects the ETB receptor as a consequence of oxidative stress, was not observed in mice deficient in either MR or Rac1 in SMCs. Our data are suggestive of excessive MR activation in SMCs during renal IR activating Rac1, in turn increasing ROS generation and contributing to a sustained decrease in kidney perfusion, leading to tubular injury. This is consistent with Rac1 acting in a signaling pathway downstream from the MR in SMCs during IR injury. However, our data do not rule out the possibility of Rac1 also acting upstream of MR by modulating its activity, as suggested by Nagase and Fujita,28 because an increase in Rac1 activity led to an increase in the nuclear translocation of MR (which may be considered a surrogate marker of MR activation) in podocytes26 and cardiomyocytes.27
Translating findings in rodent models of AKI to human has proven difficult. We therefore used the Large White pig as a preclinical animal model for assessing the effectiveness of MR antagonism in the context of ischemic AKI. This model is of particular relevance because of the anatomic similarities between pig and human kidneys (characterized by a multilobular structure contrasting with the unilobular structure of rodent and dog kidneys). Moreover, the similarities between human and porcine physiology and immune systems make the Large White pig a useful preclinical model for assessing the efficacy of novel therapeutic approaches.29 Treatment with the injectable MRA canrenoate in pigs subjected to bilateral renal ischemia proved to be a powerful strategy for limiting the deleterious consequences of renal IR, supporting the testing of MR antagonism in patients with AKI in clinical trials. Furthermore, as MRAs are already approved for use in clinical practice, their use in AKI would lead to a repositioning of these drugs for other indications. One of the possible drawbacks of MRAs is hyperkalemia because of the well known potassium-sparing effect reported for first- and second-generation MRAs, particularly in patients with impaired renal function.30 This problem may be overcome using third-generation nonsteroidal antagonists, such as finerenone, that provide similar benefits in AKI as demonstrated here but with potential fewer adverse effects in patients with renal failure. Finerenone has a better safety profile in patients with heart failure and mild renal disease than spironolactone, as concerns the potassium-sparing effect: for a similar benefit in terms of brain natriuretic peptide levels, a smaller increase in plasma potassium concentration because of MR antagonism is observed with finerenone than with spironolactone.21 In patients with diabetic nephropathy, the addition of finerenone to the treatment has been shown to improve the urinary albumin-to-creatinine ratio in a dose-dependent manner, without increasing the incidence of hyperkalemia.20
The benefits of MR antagonism in ischemic AKI may extend to patients undergoing cardiac surgery, major abdominal surgery, or kidney transplantation, in whom AKI development is likely to occur. The increase in plasma potassium was modest and transient in our model of AKI in the Large White pig; however, this might not be the case in animals with preexisting renal dysfunction. Indeed, it is widely acknowledged that the deleterious hyperkalemic effect of MRAs is mostly observed in patients with impaired renal function. Further preclinical studies, in Large White pigs in particular, are required to confirm this possibility.
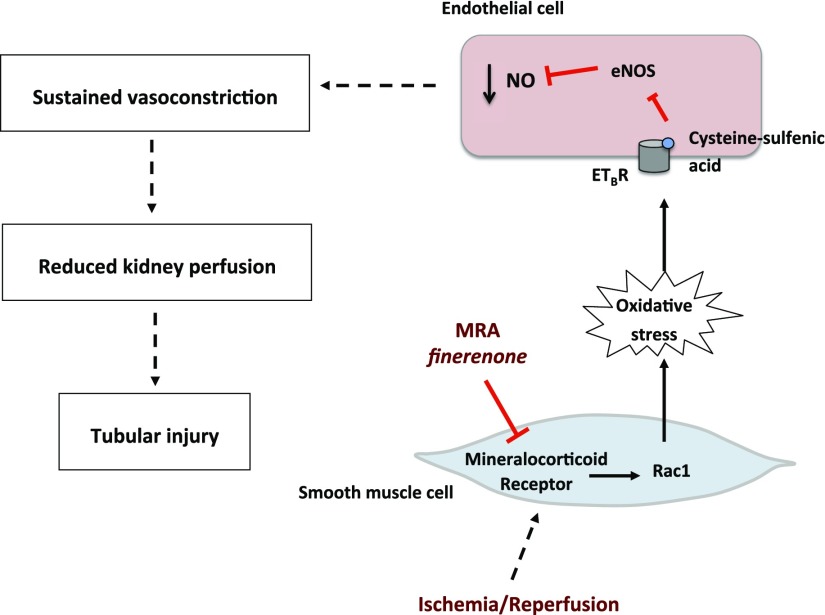
In summary, we show here that pharmacologic or genetic inhibition of the MR in SMCs limits the renal injury induced by IR. This effect was associated with lower levels of oxidative stress because of the blockade of Rac1-mediated MR signaling (Figure 9). MRAs were effective not only in rodents, but also in a preclinical model of AKI in the Large White pig. These findings pave the way for clinical trials testing the potential benefits of MRAs in AKI.
Figure 9.
Proposed mechanism for the benefits of MRAs in renal ischemia. During renal ischemia, MR is activated, signaling to Rac1 and increasing ROS production in the SMCs. The ROS that diffuse to ECs induce a post-translational sulfenic acid modification in endothelin-B receptor (ETBR). When this modification is present, ETBR cannot activate eNOS and therefore NO production is decreased. The decrease in NO generation induces sustained vasoconstriction and reduced kidney perfusion, leading to tubular injury because of decreased oxygen delivery to the cells.
Concise Methods
Mouse Models
All of the experiments involving animal manipulation were performed in accordance with Institut National de la Santé et de la Recherche Médicale guidelines and the European Community directives for the care and use of laboratory animals. The animals were housed in controlled-climate conditions with a 12-hour light/12-hour dark cycle, and were provided with free access to food and water. Male C57Bl/6 mice (Janvier Laboratories, Le Genest Saint Isle, France) were used for pharmacologic experiments with the MRA finerenone (BAY 94–8862). Finerenone (10 mg/kg) was administered by oral gavage (vehicle: 40% Kolliphor HS 15, 10% ethanol, and 50% water) at 48, 24, and 1 hour before the IR procedure. The 10 mg/kg dose was previously identified as an effective dose in preclinical rat models.31 MRendoKO mice were generated by crossing MRf/f floxed mice (kindly provided by Dr. Berger, Heidelberg, Germany)32 with transgenic mice expressing the inducible CreERT2 recombinase under the control of the VE-cadherin promoter [Cdh5(PAC)-CreERT2 line, kindly provided by Prof. Adams, London, UK].33 MRSMCKO mice were generated by crossing MRf/f floxed mice with transgenic mice expressing the inducible CreERT2 recombinase under the control of the SMA promoter (kindly provided by Dr. Metzger, Strasbourg, France).34 In both models, after activation of the Cre recombinase by tamoxifen treatment, mRNA and protein level determinations showed MR inactivation to be efficient in both MRendoKO and MRSMCKO models. Consistent with our previous results for constitutive endothelial or smooth muscle Cre-mediated MR inactivation14 and published findings,12,35 the contribution of ECs or SMCs to total MR expression in the aorta was about 50% (Supplemental Figure 5). MR mRNA expression is decreased by 50% in the MRendoKO mice (Supplemental Figure 5A). The resulting protein expression is mainly of nonendothelial origin because mechanical removal of the endothelium did not change remaining protein expression (Supplemental Figure 5B). MR mRNA expression is decreased by about 70% in the aorta of MRSMCKO mice (Supplemental Figure 5C). Protein expression analysis showed that MR protein expression is virtually absent in whole aortas from MRSMCKO mice when the endothelium is removed (Supplemental Figure 5D). Rac1SMCKO mice were generated by crossing Rac1f/f floxed mice with transgenic mice expressing the inducible CreERT2 recombinase under the control of SMMHC promoter, as previously described.36 The expression of Rac1 in the aorta was reduced at the mRNA and protein level in Rac1SMCKO mice (Supplemental Figure 6, A and B). Further, it was almost absent in the smooth muscle layer of aortas and mesenteric arteries in Rac1SMCKO mice (Supplemental Figure 6, C and D). MRf/f or Rac1f/f littermates lacking the CreERT2 transgene were used as controls. All mice were generated in the C57Bl/6 genetic background (The Jackson Laboratory, Bar Harbor, ME). The sequences of the primers used for genotyping are listed in Supplemental Table 1. All of the animals studied were male and were 2 months old at the time of CreERT2 recombinase induction with tamoxifen (1 mg/d in corn oil for five consecutive days). Renal ischemia was triggered 15 days after the induction of Cre recombinase.
Mouse Kidney IR Injury Model
Male mice were anesthetized by an intraperitoneal injection of sodium pentobarbital (60 mg/kg) and placed on a heating pad with a rectal probe to keep body temperature constant, at about 37°C. Bilateral flank incisions were made to expose the kidneys and the renal pedicle was dissected. Renal ischemia was induced by placing nontraumatic vascular clamps over the pedicles for 20 minutes. The clamps were then released and mice received 1 ml of 0.9% NaCl (37°C). Both incisions were closed in two layers, with 5–0 sutures, and reperfusion was allowed to occur for 24 hours. Sham-treated mice were subjected to the same procedure but without renal pedicle clamping. After the reperfusion period, a blood sample was taken and plasma creatinine and urea concentrations were determined with an automatic analyzer (Konelab 20i; Thermo Fisher Scientific, Vernon Hills, IL). At the end of the experiment, the right kidney was removed and fixed in Bouin fixative solution for histologic studies, and the left kidney was rapidly frozen for molecular studies.
Histologic Analysis
The fixed kidneys were then dehydrated and embedded in paraffin. Sections (4 μm) were cut and stained with hematoxylin and eosin. For each mouse, ten subcortical fields were visualized and analyzed under a Leica DM4000 microscope at a magnification of ×200. The percentage of tubules displaying injury (cast formation, epithelial cell necrosis and detachment, and tubular dilation) was analyzed blind.
RNA Extraction and Real-Time PCR
Total RNA was extracted from the kidneys with TRIZOL reagent (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. Reverse transcription was performed with 1 μg of RNA and the Superscript II Reverse Transcription Kit (Life Technologies). Transcript levels were analyzed by real-time PCR (fluorescence detection of SYBR green) in an iCycler iQ apparatus (Bio-Rad, Hercules, CA), with normalization against 18S as an endogenous control. The primer sequences for the genes analyzed are listed in the Supplemental Table 1.
Lipid Peroxidation
The concentration of MDA was determined as an indicator of lipid peroxidation. This concentration was determined with a commercially available lipid peroxidation assay kit, used according to the manufacturer’s instructions (ab118970; Abcam, Inc., Cambridge, MA). The direct lipid peroxides concentration was determined using the LPO Assay kit (705002; Cayman Chemical). MDA or LPO were normalized relative to the amount of protein in the tissue lysate or urinary creatinine.
SMCs Culture and Treatments for Rac1 Activity
Primary rat aortic SMCs were isolated and cultured as previously described.36 Cells were treated for 24 hours with vehicle, aldosterone (10 nM), aldosterone and spironolactone (1 μM), or epidermal growth factor (as a positive control), and Rac1 activity was then assayed.
Rac1 Activity
Pull-down assays were performed with GST-PBD fusion proteins on fresh SMCs and tissue lysates, to quantify Rac1 activity. The commercial BK035 kit for cells was used according to the manufacturer’s protocol (Cytoskeleton, Denver, CO) and GST beads were used for tissue analysis, as described by Guilluy et al.37 Western blotting was carried out to analyze Rac1 levels in the pull-down fraction and in total cell lysates.
Hydrogen Peroxide Measurement
Hydrogen peroxide was estimated with the Amplex Red assay (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions, on SMCs isolated as previously described.36 Low-passage number cells (passages 4–6) were studied. SMCs were stimulated by incubation for 12 hours with aldosterone (10−8 mol/L), in the presence or absence of a specific inhibitor of Rac1 (EHT 1864, 10−5 mol/L) or the MRA (spironolactone, 10−6 mol/L).
Western Blot Analysis
Total renal proteins were isolated from the kidney or aorta and homogenized in 1% SDS buffer supplemented with protease inhibitor (Roche, Basel, Switzerland). Cells were collected in Cytoskeleton lysis buffer (BK035) kit. Protein samples containing 20–30 μg of total protein were diluted in Laemmli buffer and heated at 95°C. The proteins were resolved by electrophoresis in 4%–15% Mini-PROTEAN TGX precast polyacrylamide gels (Bio-Rad), and rapidly transferred onto nitrocellulose membranes with the TurboBlot kit. (Bio-Rad). Membranes were blocked with 5% blotting-grade nonfat milk powder and then incubated with the appropriate antibodies in 0.1% blotting-grade nonfat milk. Specific antibodies against Rac1 (610650; BD Biosciences, San Jose, CA, and ARC03; Cytoskeleton) and MR (kindly provided by Dr. Gomez-Sanchez; 1:200) were used. After incubation with the primary antibody, membranes were washed and incubated with the appropriate secondary antibody. As a loading control, membranes were incubated overnight at 4°C with goat anti-actin or anti-tubulin antibody (1:5000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). Proteins were detected with an enhanced chemiluminescence kit (Bio-Rad).
ETB Receptor Immunoprecipitation
For the immunoprecipitation analysis, proteins from the whole kidneys were extracted in dimedone lysis buffer. ETB receptor was immunoprecipitated using protein A agarose beads (Thermo Fisher Scientific) and 4 μg of goat-anti ETB antibody (Santa Cruz Biotechnology). The immunoprecipitated proteins were eluted by boiling in Laemmli buffer, blotted, and probed for anti-cysteine sulfenic acid (1:5000 dilution) (EMD Millipore, Billerica, MA) with a rabbit anti-ETB antibody (1:500 dilution).
Pig Kidney IR Injury Model
All experiments involving pigs were carried out in accordance with the Guidelines of the French Agricultural Office and the legislation governing animal studies. Large White male pigs weighing 42–51 kg (INRA, Le Magneraud, Surgères, France) were prepared as previously described.38 A catheter was inserted into the jugular vein for the collection of blood samples. Kidney ischemia was induced by clamping both renal pedicles for 60 minutes with a nontraumatic vascular clamp. All experimental groups were subjected to the same anesthesia protocol. Because the experimental setting necessitates parenteral MRA application, Soludactone, the only available injectable MRA containing potassium canrenoate, was used. Soludactone or vehicle was administered by intravenous injections at a dosage of 7 mg/kg at 48 hours, 24 hours, and 30 minutes before the induction of ischemia. The animals received two additional injections at 24 and 48 hours after reperfusion. Pigs were placed in metabolic cages for 24 hours before the collection of urine and blood samples.
Plasma creatinine concentration was measured on days 1, 3, 5, 7, 11, and 14 with an automatic analyzer (Modular; Roche Diagnostics, Indianapolis, IN). Urinary levels of NGAL and KIM-1 were determined as markers of tubular injury on days 1, 3, 5, and 7 after reperfusion. Urinary MDA levels were determined as described above. Urinary MDA levels were then normalized against urinary creatinine concentration. For histologic analyses in pigs, brush border loss and tubular dilation were assessed on a semiquantitative six-point scale: 0, no abnormality; 1, mild lesions affecting <25% of kidney samples; 2, lesions affecting 25%–50% of kidney samples; 3, lesions affecting 51%–75% of kidney samples; 4, lesions affecting >75% of kidney samples; and 5, extensive necrosis and renal damage.
Statistical Analyses
The results are expressed as means±SEM. In the finerenone studies, the significance of differences between groups was assessed by ANOVA with Bonferroni correction for multiple comparisons, in GraphPad Prism 6 software. For the knockout mouse studies, the significance of differences was determined by two-way ANOVA with Bonferroni correction for multiple comparisons. A nonparametric test (Mann–Whitney U test) was used for small samples. We considered P<0.05 to be statistically significant.
Disclosures
P.K. is an employee of BAYER Pharma AG (Wuppertal, Germany).
Supplementary Material
Acknowledgments
We would like to thank Dr. Berger (Heidelberg, Germany) for donating the MRf/f line, Dr. Metzger (Strasbourg, France) for providing the SMA-CreERT2 line, and Dr. Adams (London, UK) for donating the Cdh5(PAC)-CreERT2 line.
This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Centre de Recherche Industrielle et Technique, the Agence de la Biomédecine (Call offer for research in graft 2014), the French Medical Research Foundation (grant no. DEQ20160334885), and a research grant from BAYER Pharma AG (grant no. 12127a10). J.B.-C. was supported by a postdoctoral fellowship from the French Society of Nephrology (grant no. SPF2012FDR_SN_FRM_Barrera Chimal) and the French Foundation for Medical Research (grant no. SPF20130526725). G.A.-G. was supported by a postdoctoral fellowship from Cardiovascular, Obesity, Kidney and Diabetes research network (Ile-de-France, France).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016040477/-/DCSupplemental.
References
- 1.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A: Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol 18: 1292–1298, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Leung KC, Tonelli M, James MT: Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol 9: 77–85, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Matejovic M, Ince C, Chawla LS, Blantz R, Molitoris BA, Rosner MH, Okusa MD, Kellum JA, Ronco C; ADQI XIII Work Group : Renal hemodynamics in AKI: In search of new treatment targets. J Am Soc Nephrol 27: 49–58, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharfuddin AA, Molitoris BA: Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goligorsky MS, Brodsky SV, Noiri E: NO bioavailability, endothelial dysfunction, and acute renal failure: New insights into pathophysiology. Semin Nephrol 24: 316–323, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Salom MG, Cerón SN, Rodriguez F, Lopez B, Hernández I, Martínez JG, Losa AM, Fenoy FJ: Heme oxygenase-1 induction improves ischemic renal failure: Role of nitric oxide and peroxynitrite. Am J Physiol Heart Circ Physiol 293: H3542–H3549, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Barrera-Chimal J, Prince S, Fadel F, El Moghrabi S, Warnock DG, Kolkhof P, Jaisser F: Sulfenic acid modification of endothelin B receptor is responsible for the benefit of a nonsteroidal mineralocorticoid receptor antagonist in renal ischemia. J Am Soc Nephrol 27: 398–404, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mejía-Vilet JM, Ramírez V, Cruz C, Uribe N, Gamba G, Bobadilla NA: Renal ischemia-reperfusion injury is prevented by the mineralocorticoid receptor blocker spironolactone. Am J Physiol Renal Physiol 293: F78–F86, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Pozos K, Lee-Montiel F, Pérez-Villalva R, Uribe N, Gamba G, Bazan-Perkins B, Bobadilla NA: Polymerized type I collagen reduces chronic cyclosporine nephrotoxicity. Nephrol Dial Transplant 25: 2150–2158, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen Dinh Cat A, Griol-Charhbili V, Loufrani L, Labat C, Benjamin L, Farman N, Lacolley P, Henrion D, Jaisser F: The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J 24: 2454–2463, 2010 [DOI] [PubMed] [Google Scholar]
- 12.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ: Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 18: 1429–1433, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galmiche G, Pizard A, Gueret A, El Moghrabi S, Ouvrard-Pascaud A, Berger S, Challande P, Jaffe IZ, Labat C, Lacolley P, Jaisser F: Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension 63: 520–526, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amador CA, Bertocchio JP, Andre-Gregoire G, Placier S, Duong Van Huyen JP, El Moghrabi S, Berger S, Warnock DG, Chatziantoniou C, Jaffe IZ, Rieu P, Jaisser F: Deletion of mineralocorticoid receptors in smooth muscle cells blunts renal vascular resistance following acute cyclosporine administration. Kidney Int 89: 354–362, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel NS, Sharples EJ, Cuzzocrea S, Chatterjee PK, Britti D, Yaqoob MM, Thiemermann C: Pretreatment with EPO reduces the injury and dysfunction caused by ischemia/reperfusion in the mouse kidney in vivo. Kidney Int 66: 983–989, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Simone S, Rascio F, Castellano G, Divella C, Chieti A, Ditonno P, Battaglia M, Crovace A, Staffieri F, Oortwijn B, Stallone G, Gesualdo L, Pertosa G, Grandaliano G: Complement-dependent NADPH oxidase enzyme activation in renal ischemia/reperfusion injury. Free Radic Biol Med 74: 263–273, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Gao G, Wang W, Tadagavadi RK, Briley NE, Love MI, Miller BA, Reeves WB: TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. J Clin Invest 124: 4989–5001, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawarazaki W, Nagase M, Yoshida S, Takeuchi M, Ishizawa K, Ayuzawa N, Ueda K, Fujita T: Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J Am Soc Nephrol 23: 997–1007, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, Takai Y, Ishikawa A, Shimosawa T, Ando K, Nagase M, Fujita T: Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest 121: 3233–3243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM; Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group : Effect of finerenone on albuminuria in patients with diabetic nephropathy: A randomized clinical trial. JAMA 314: 884–894, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, Nowack C, Kolkhof P, Kim SY, Zannad F: Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur Heart J 34: 2453–2463, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braunagel M, Helck A, Wagner A, Schupp N, Bröcker V, Reiser M, Notohamiprodjo M, Meiser B, Habicht A: Dynamic contrast-enhanced computed tomography: A new diagnostic tool to assess renal perfusion after ischemia-reperfusion injury in mice: Correlation of perfusion deficit to histopathologic damage. Invest Radiol 51: 316–322, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Sutton TA, Fisher CJ, Molitoris BA: Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 1539–1549, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Schäfer N, Lohmann C, Winnik S, van Tits LJ, Miranda MX, Vergopoulos A, Ruschitzka F, Nussberger J, Berger S, Lüscher TF, Verrey F, Matter CM: Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J 34: 3515–3524, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Himmelfarb J, McMonagle E, Freedman S, Klenzak J, McMenamin E, Le P, Pupim LB, Ikizler TA; The PICARD Group : Oxidative stress is increased in critically ill patients with acute renal failure. J Am Soc Nephrol 15: 2449–2456, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T: Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Ayuzawa N, Nagase M, Ueda K, Nishimoto M, Kawarazaki W, Marumo T, Aiba A, Sakurai T, Shindo T, Fujita T: Rac1-mediated activation of mineralocorticoid receptor in pressure overload-induced cardiac injury. Hypertension 67: 99–106, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Nagase M, Fujita T: Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol 9: 86–98, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Giraud S, Favreau F, Chatauret N, Thuillier R, Maiga S, Hauet T: Contribution of large pig for renal ischemia-reperfusion and transplantation studies: The preclinical model. J Biomed Biotechnol 2011: 532127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA: Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med 351: 543–551, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Bärfacker L, Eitner F, Albrecht-Küpper B, Schäfer S: Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol 64: 69–78, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, Chepkova AN, Welzl H, Haas HL, Lipp HP, Schütz G: Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci U S A 103: 195–200, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U, Barberis A, Benjamin LE, Mäkinen T, Nobes CD, Adams RH: Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465: 483–486, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Wendling O, Bornert JM, Chambon P, Metzger D: Efficient temporally-controlled targeted mutagenesis in smooth muscle cells of the adult mouse. Genesis 47: 14–18, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Rickard AJ, Morgan J, Chrissobolis S, Miller AA, Sobey CG, Young MJ: Endothelial cell mineralocorticoid receptors regulate deoxycorticosterone/salt-mediated cardiac remodeling and vascular reactivity but not blood pressure. Hypertension 63: 1033–1040, 2014 [DOI] [PubMed] [Google Scholar]
- 36.André G, Sandoval JE, Retailleau K, Loufrani L, Toumaniantz G, Offermanns S, Rolli-Derkinderen M, Loirand G, Sauzeau V: Smooth muscle specific Rac1 deficiency induces hypertension by preventing p116RIP3-dependent RhoA inhibition. J Am Heart Assoc 3: e000852, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guilluy C, Dubash AD, García-Mata R: Analysis of RhoA and Rho GEF activity in whole cells and the cell nucleus. Nat Protoc 6: 2050–2060, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Jayle C, Milinkevitch S, Favreau F, Doucet C, Richer JP, Deretz S, Mauco G, Rabb H, Hauet T: Protective role of selectin ligand inhibition in a large animal model of kidney ischemia-reperfusion injury. Kidney Int 69: 1749–1755, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.