Abstract
The TNF family member a proliferation-inducing ligand (APRIL; also known as TNFSF13), produced by myeloid cells, participates in the generation and survival of antibody–producing plasma cells. We studied the potential role of APRIL in the pathogenesis of IgA nephropathy (IgAN). We found that a significant proportion of germinal centers (GCs) in tonsils of patients with IgAN contained cells aberrantly producing APRIL, contributing to an overall upregulation of tonsillar APRIL expression compared with that in tonsils of control patients with tonsillitis. In IgAN GC, antigen–experienced IgD−CD38+/−CD19+ B cells expressing a switched IgG/IgA B cell receptor produced APRIL. Notably, these GC B cells expressed mRNA encoding the common cleavable APRIL-α but also, the less frequent APRIL-δ/ζ mRNA, which encodes a protein that lacks a furin cleavage site and is, thus, the uncleavable membrane-bound form. Significant correlation between TLR9 and APRIL expression levels existed in tonsils from patients with IgAN. In vitro, repeated TLR9 stimulation induced APRIL expression in tonsillar B cells from control patients with tonsillitis. Clinically, aberrant APRIL expression in tonsillar GC correlated with greater proteinuria, and patients with IgAN and aberrant APRIL overexpression in tonsillar GC responded well to tonsillectomy, with parallel decreases in serum levels of galactose-deficient IgA1. Taken together, our data indicate that antibody disorders in IgAN associate with TLR9–induced aberrant expression of APRIL in tonsillar GC B cells.
Keywords: IgA nephropathy, immunology, cytokines
IgA nephropathy (IgAN) is the most common form of GN, accounting for 25%–50% of patients with primary GN. Accumulating evidence now suggests that 30%–40% of patients with IgAN progress to ESRD within 20 years from the estimated time of disease onset.1,2 The lack of comprehensive understanding of IgAN development impairs the design of a specific treatment for this disease. The pathogenesis of IgAN may be associated with systemic immune dysregulation from a mucosa-bone marrow axis rather than an abnormality intrinsic to the renal resident cells.3,4 At the mucosal level, infections, particularly in the upper respiratory tract, exacerbate clinical manifestations in patients with IgAN. Recent studies report that aberrantly O–glycosylated IgA1 (i.e., galactose-deficient [Gd] IgA1) and the subsequently formed IgA immune complexes (ICs) with glycan-specific autoantibodies are pivotal to the development of IgAN.5–7
A proliferation-inducing ligand (APRIL) is a member of the TNF superfamily of ligands expressed as a type 2 transmembrane protein.8 APRIL is usually cleaved in the Golgi apparatus by a furin convertase and then, secreted as a soluble ligand.9 Myeloid and mucosal epithelial cells produced APRIL.10–12 APRIL binds to two members of the TNF receptor family: the B cell maturation antigen (BCMA) and the transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI).13 Functionally, APRIL mediates class switch, mostly for IgA.10,14 APRIL is also crucial for long-term survival of plasma cells in the bone marrow and mucosa.11,12,14–17 Recently, high serum level of APRIL in patients with IgAN correlating with urinary proteins was reported.18,19 In addition, a genome–wide association study of patients with IgAN suggested APRIL (TNFSF13) to be a susceptibility gene.20 However, the cellular source of APRIL production and the pathway by which this molecule is upregulated in IgAN remain ill defined.
Toll-like receptors (TLRs) are a family of germline-encoded receptors that recognize a diverse range of conserved molecular motifs commonly found in microbial pathogens. TLR9 recognizes unmethylated DNA sequences in bacterial and viral DNA and is involved in innate immune responses by providing protective immune responses against invading viral and bacterial pathogens.21,22 Although TLR9 has been shown to be implicated in the development of kidney diseases, including IgAN,23–28 the mechanisms by which TLR9 activation contributes to the development of IgAN are poorly understood.
Results
Upregulation of APRIL Expression in Tonsillar Germinal Center of Patients with IgAN
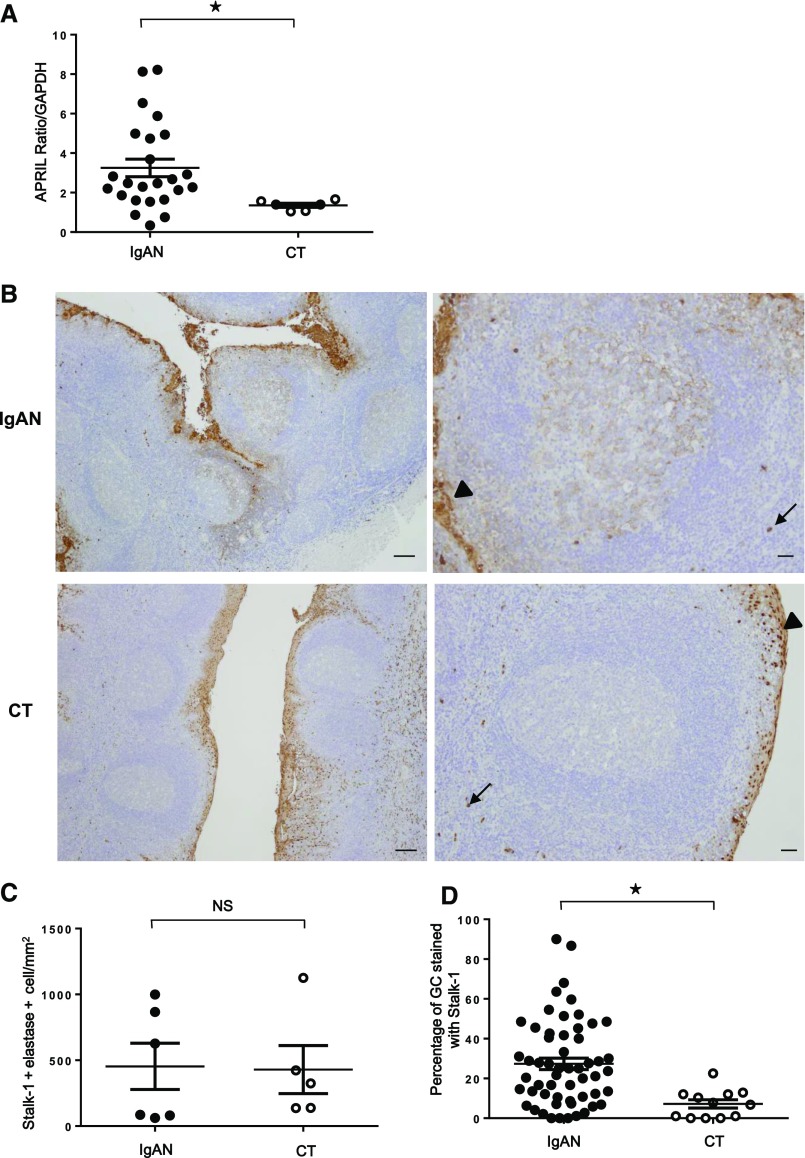
Real–time quantitative PCR (qPCR) analyses in patients with IgAN (n=24) and patients with chronic tonsillitis (CT; n=6) revealed that tonsillar APRIL mRNA expression was significantly higher in IgAN than in CT (Figure 1A) (P<0.01). Immunohistochemistry with Stalk-1, an anti-APRIL antibody that detects APRIL-producing cells, further showed that APRIL-producing cells were present in the tonsillar epithelium and outside in cells identified as neutrophils in both patients with IgAN (n=55) and patients with CT (n=12) (Figure 1B), consistent with our previous report in patients with CT.11 Stalk-1 and elastase (neutrophil-specific) costaining did not reveal a difference in the number of infiltrating neutrophils between patients with IgAN and patients with CT (Figure 1C). The epithelial staining by Stalk-1 was also not different. One obvious difference was the presence of a substantial number of Stalk-1+ APRIL–producing cells in germinal centers (GCs) in patients with IgAN. We observed that the percentage of Stalk-1+GC (27.4%±21.3%) in patients with IgAN was significantly higher than that in patients with CT (7.2%±6.8%; P<0.01) (Figure 1D).
Figure 1.
Increased APRIL expression in tonsils from patients with IgAN. (A) Tonsillar APRIL mRNA expressions in patients with IgAN (n=24) were significantly higher than those in patients with CT (n=6). Bars represent the mean±SEM. *P<0.01. (B) Immunohistochemistry with Stalk-1 (specific for APRIL-producing cells) in patients with IgAN (upper panels) and patients with CT (lower panels). Representative GCs are shown in right panels. Arrows and arrowheads mark Stalk-1–stained neutrophil and epithelial cells, respectively. Pictures shown are representative of 56 tonsils from patients with IgAN and 12 tonsils from patients with CT. Scale bars, 250 μm in left panels; 100 μm in right panels. (C) Quantification of Stalk-1+ elastase+ neutrophils showed no significant difference. (D) However, percentage of GC–containing APRIL–producing cells (Stalk-1+GC) was significantly different in total tonsillar GCs from patients with IgAN and patients with CT. *P<0.01.
Ig–Switched GC B Cells Produce APRIL in Tonsils of Patients with IgAN
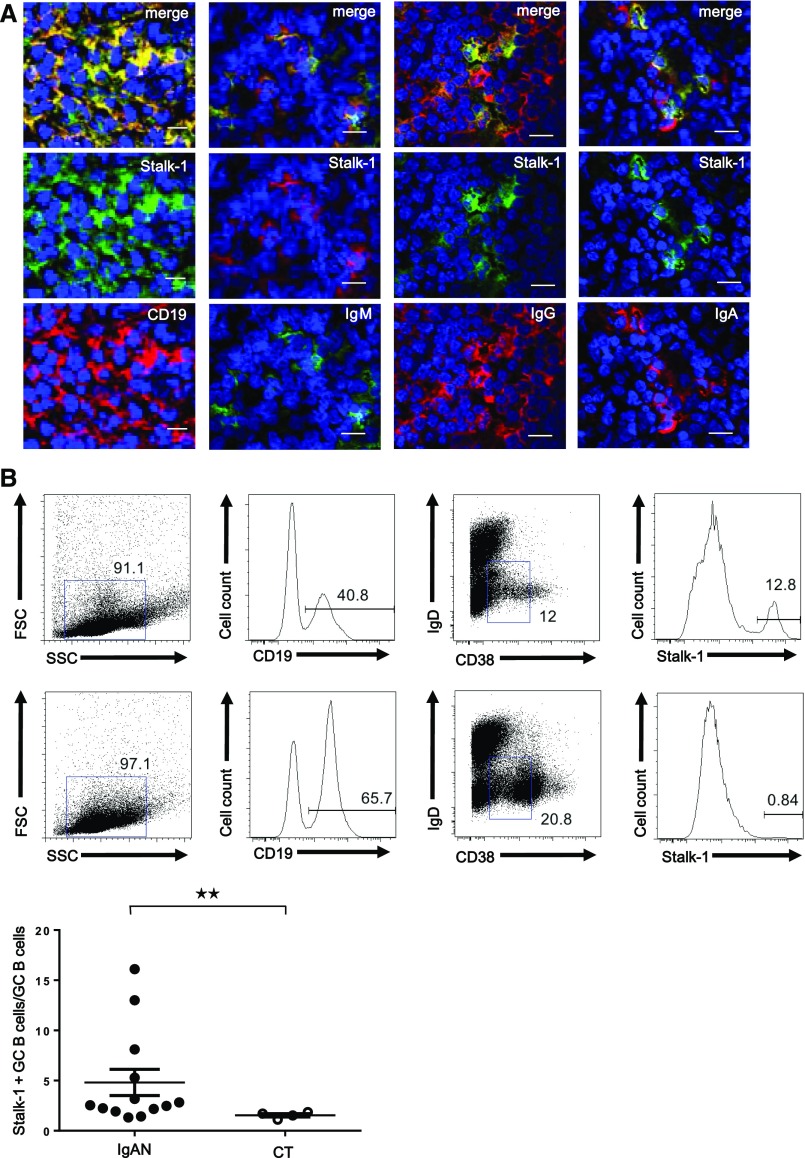
Stalk-1+ cells in tonsillar GCs of patients with IgAN expressed CD19, identifying them as B cells (Figure 2A). We further observed that Stalk-1+ APRIL–producing cells in tonsillar GCs had a nonswitched IgM and switched IgG or IgA phenotypes. Flow cytometric analyses revealed that the Stalk-1+ CD19+ cell population had lost IgD surface expression and variably expressed the GC activation marker CD38 in patients with IgAN (n=13) (Figure 2B, top panel). We also detected this Stalk-1+GC B cell population in CT (n=4) (Figure 2B, middle panel), but this cell population was clearly increased in some patients with IgAN (Figure 2B, bottom panel) (P<0.05).
Figure 2.
CD19+ B cells produce APRIL in tonsillar GCs of patients with IgAN. (A) IgAN tonsils showed costaining for Stalk-1 (green) as well as CD19, IgM, IgG, and IgA (red). A representative GC is shown. Pictures shown are representative of tonsils from patients with IgAN. Scale bars, 20 μm. (B) A cell suspension from IgAN and CT tonsils was surface stained for CD19, CD38, IgD, and after cell permeabilization, Stalk-1 (top and middle panels). Plots for cells gated on CD19 are representative of 13 patients with IgAN and four patients with CT. The percentage of Stalk-1+ cells among CD19+IgD−CD38+/− cells is also shown (bottom panel). **P<0.05.
GC B Cells Express an Uncleavable Form of APRIL in Patients with IgAN
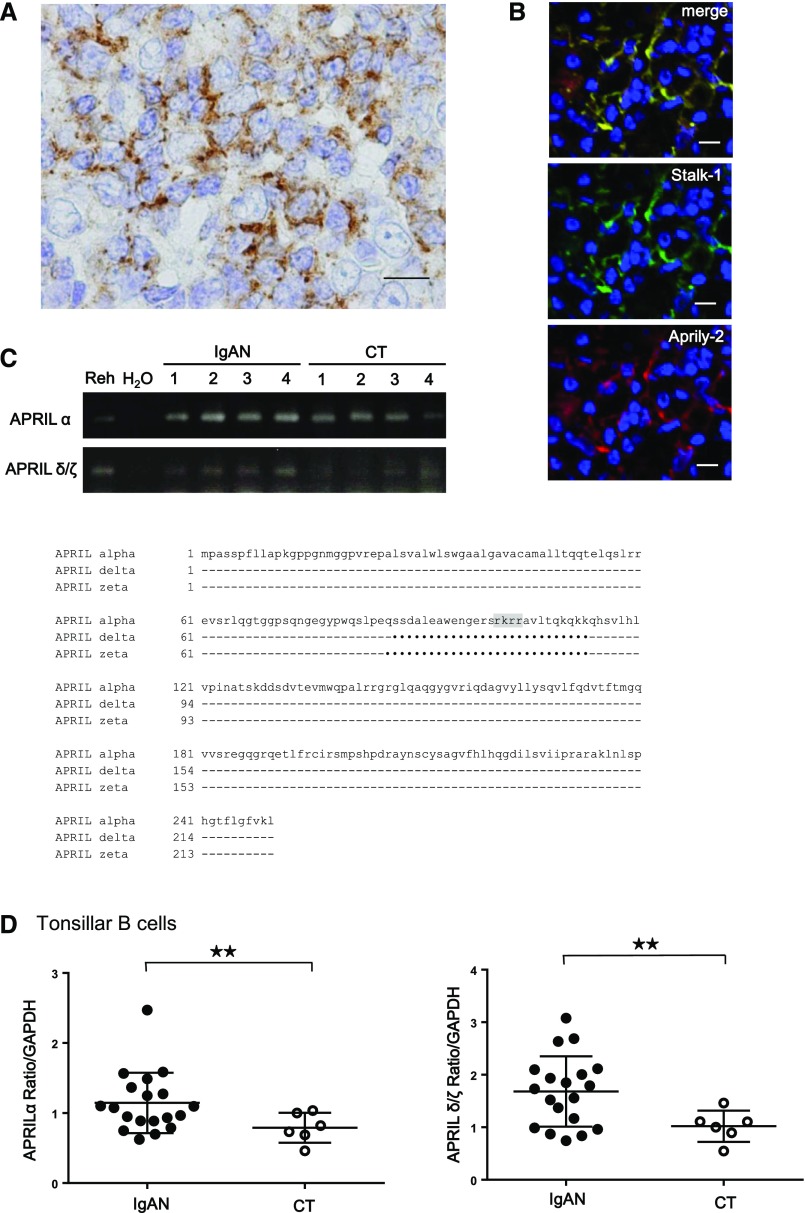
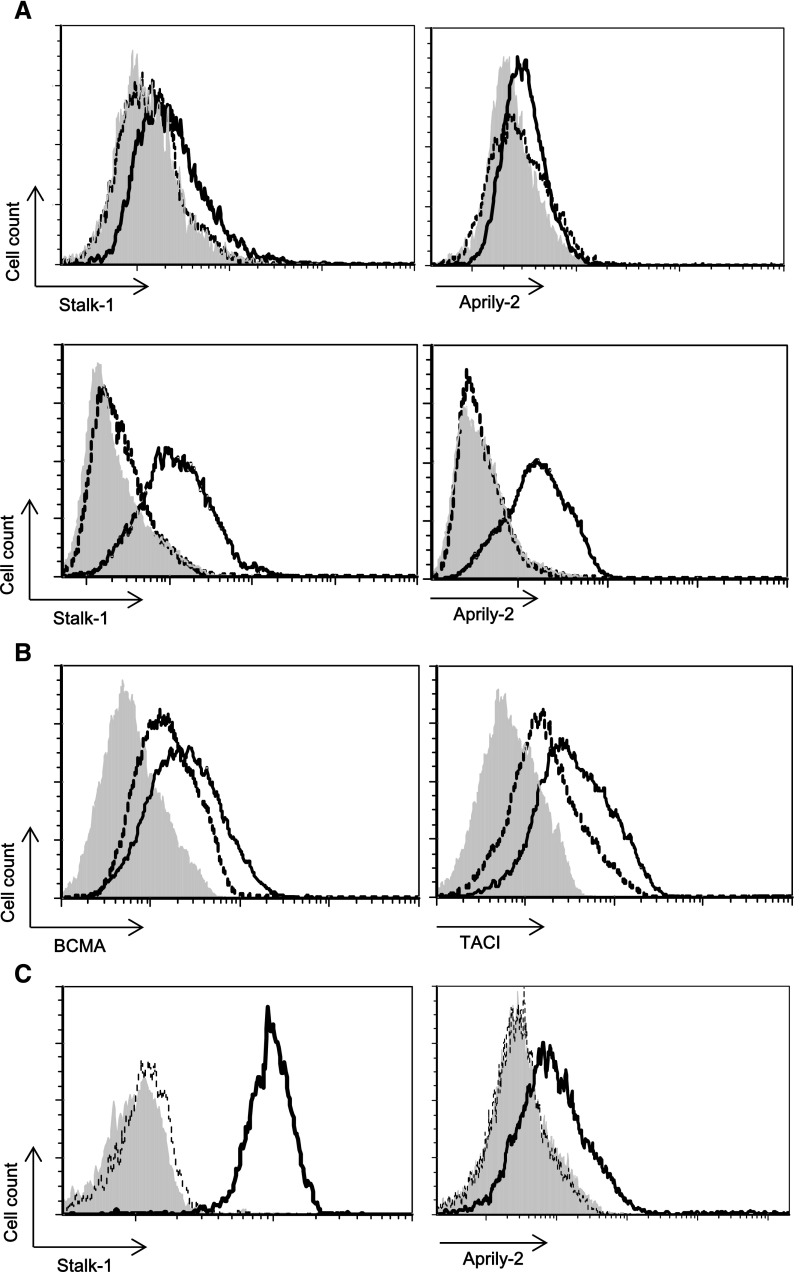
The Stalk-1 staining pattern obtained in GC B cells from patients with IgAN was clearly different from the one in PMN cells described previously11 (Figure 3A). We additionally performed immunohistochemical analysis with Aprily-2 antibody, which recognizes the secreted part of APRIL.29 We observed that the Aprily-2 staining colocalized with Stalk-1 in tonsillar GCs from patients with IgAN (Figure 3B). Such colocalization of Stalk-1 and Aprily-2 has never been observed in healthy and pathologic tissues.30 A recent study reported a possible expression of APRIL-δ and -ζ, APRIL isoforms lacking the consensus motif for the furin convertase, in B cell precursor acute lymphoblastic leukemia,31 and such isoforms could be stained with both Stalk-1 and Aprily-2. RT-PCR analyses revealed that tonsillar B cells from patients with IgAN and patients with CT indeed expressed APRIL-δ and -ζ in addition to the common furin-cleavable APRIL-α (Figure 3C). Real-time qPCR further showed that the abundances of APRIL-α and APRIL-δ/ζ mRNA in tonsillar B cells of patients with IgAN were significantly higher than those in patients with CT (Figure 3D).
Figure 3.
Tonsillar GC B cells of IgAN express cleavable and uncleavable APRIL. (A) IgAN tonsils were stained for Stalk-1. A representative GC B cell is shown. The picture shown is representative of 56 patients with IgAN. (B) IgAN tonsils were costained for Stalk-1 (green) and Aprily-2 (red). A representative GC is shown. Scale bars, 20 μm. (C) Predicted amino acid sequences of different isoforms of APRIL. The GenBank accession numbers for APRIL-α, -δ, and -ζ are NM_003808, NM_001198622, and NM_001198623.1, respectively. The furin cleavable site lacking in APRIL-δ and -ζ is highlighted in gray. Identities are indicated by dashes, and deletions are indicated by dots. Numbers indicate amino acid positions. (D) Correlation between APRIL-α and -δ/ζ mRNA expression in purified tonsillar B cells from patients with IgAN (n=20) and patients with CT (n=6). Both APRIL-α and -δ/ζ mRNA expressions in tonsillar B cells were significantly higher in patients with IgAN. Bars represent the mean±SEM. **P<0.05.
TLR9 Stimulation Induces APRIL Expression in GC B Cells
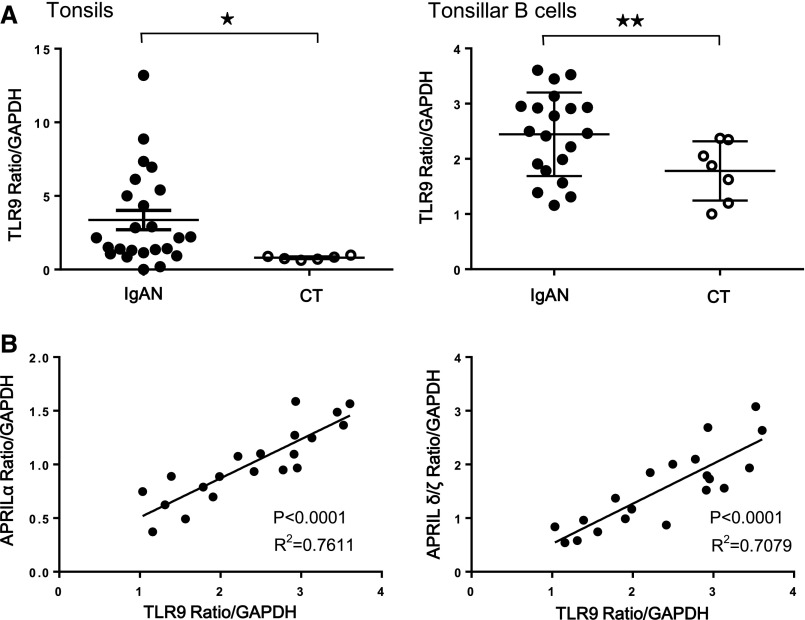
Levels of TLR9 mRNA in whole tonsils and tonsillar B cells of patients with IgAN were significantly higher than those of patients with CT (Figure 4A), and an increase of TLR9 mRNA well correlated with an increase of APRIL-α and APRIL-δ/ζ mRNA in tonsillar B cells of patients with IgAN (Figure 4B).
Figure 4.
Correlation between TLR9 and APRIL mRNA expressions in patients with IgAN. (A) TLR9 mRNA expressions in whole tonsils (left panel) and purified tonsillar B cells (right panel) were significantly higher in IgAN. Bars represent the mean±SEM. *P<0.01; **P<0.05. (B) TLR9 and APRIL-α (left panel) or -δ/ζ (right panel) mRNA expressions in tonsillar B cells were well correlated in patients with IgAN.
We next stimulated whole tonsillar cells from patients with CT with the TLR9 ligand CpG-oligodeoxynucleotide (CpG-ODN) and analyzed APRIL expression on CD19+ B cells. A daily stimulation induced a reactivity of CD19+ cells with Stalk-1 and Aprily-2 antibodies starting at day 3, with a maximum seen at day 7, in CD19+ cells (Figure 5A). The APRIL reactivity was observed intracellularly with a limited signal at the cells surface. The weak surface APRIL expression on CpG–stimulated B cells was consistent with the absence of surface staining observed ex vivo. Repeated TLR9 stimulation weakly but clearly upregulated expression of the APRIL receptors TACI and BCMA as well (Figure 5B). Consistent with analyses in Figure 5A, the same APRIL reactivities were observed in studies with purified tonsillar B cells at day 7 (Figure 5C). Taken together, these indicate a putative autocrine APRIL signaling in CpG–stimulated B cells.
Figure 5.
TLR9 activation induces APRIL expression in tonsillar B cells. (A) Tonsillar B cells isolated from patients with CT were stimulated daily with 10 μg/ml CpG. APRIL expression is shown on viable (upper panel) and permeabilized (lower panel) gated CD19+ B cells. (B) Surface expressions of TACI and BCMA are also shown. (C) CD19+ B cells from patients with CT were purified on an FACS ARIA (BD Pharmingen) by positive selection. Purified CD19+ B cells were stimulated daily with 10 μg/ml CpG. APRIL expression is shown on permeabilized cells. Shaded histograms represent control isotype–matched reactivity. Dotted and straight lines represent indicated antibody reactivities on control and CpG-ODN–stimulated cells, respectively, at day 7. Histogram plots are representative of at least three experiments performed with tonsils from independent patients.
APRIL Expression in Tonsillar GC Is Associated with the Severity of IgAN and Treatment Responses to Tonsillectomy
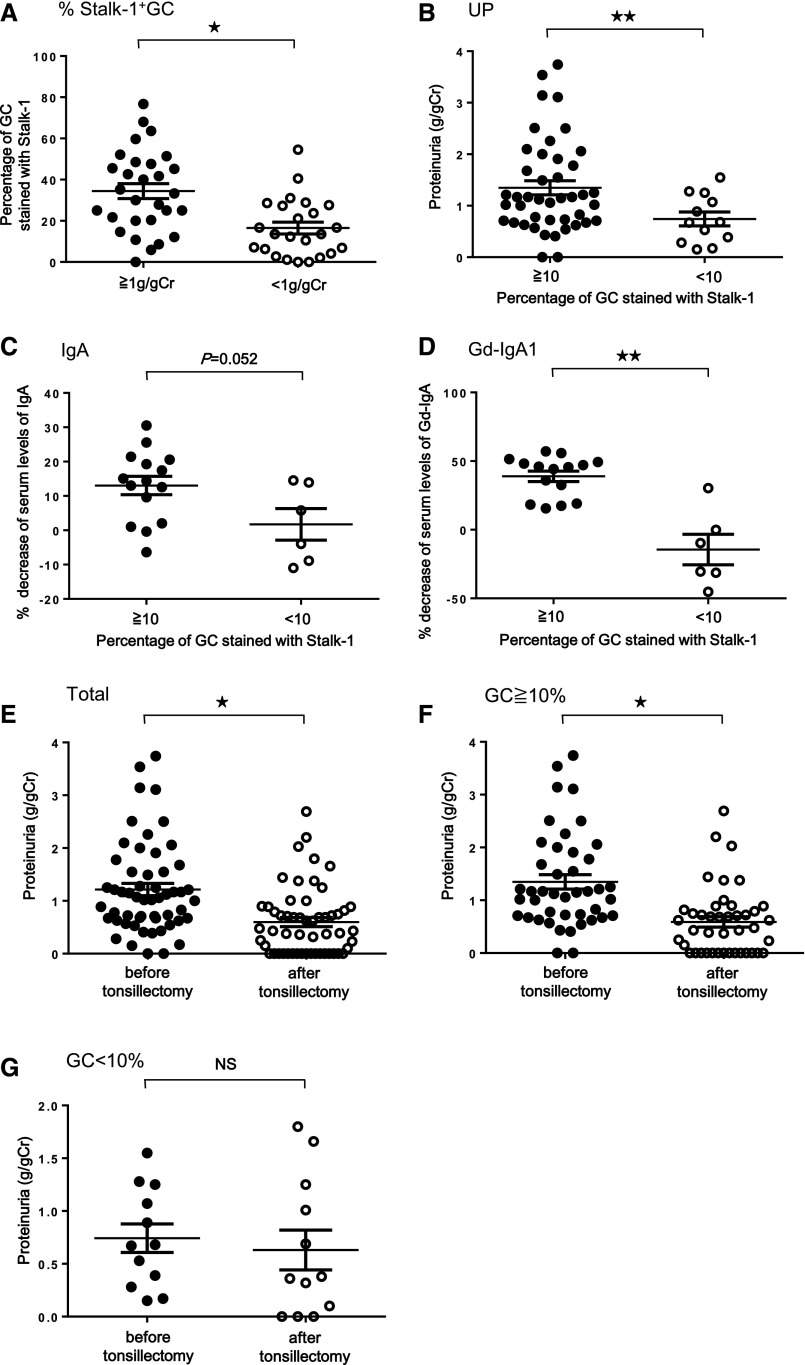
We assessed whether the extent of Stalk-1+GC in tonsils may affect the severity of IgAN and therapeutic responses to tonsillectomy. The percentage of Stalk-1+GC was significantly higher in patients with IgAN with an elevated proteinuria (Figure 6A) (P<0.01). Figure 1D shows that even patients with CT showed Stalk-1+GC but at <10%, suggesting that Stalk-1+GC≥10% in tonsils may be more characteristic of IgAN. Therefore, we next focused on patients with IgAN with Stalk-1+GC>10%. Urinary protein levels in patients with IgAN with Stalk-1+GC≥10% were significantly higher than those in patients with IgAN with Stalk-1+GC<10% (Figure 6B) (P<0.05). Percentage of patients with IgAN whose proteinuria decreased >50% after the tonsillectomy alone was significantly higher in patients with Stalk-1+GC≥10% (62.8%) than those with Stalk-1+GC<10% (25.0%; P=0.02) (Table 2). Notably, the efficacy of tonsillectomy was similarly observed in patients with severe cases of IgAN who showed proteinuria >0.5 g/g creatinine (P=0.05) independent of basal levels of proteinuria before the treatment (Supplemental Table 1). Change of serum levels of whole IgA and Gd-IgA1 before and after tonsillectomy was then evaluated. Significantly higher decrease of serum Gd-IgA1 (P<0.05) but not whole IgA was observed in patients with IgAN with Stalk-1+GC≥10% (Figure 6, C and D). We also compared proteinuria levels before and after tonsillectomy in patients with IgAN (Figure 6E), those with Stalk-1+GC≥10% (Figure 6F), and those with Stalk-1+GC<10% (Figure 6G). There were significant differences before and after tonsillectomy in the preceding two groups (P<0.01), despite no significance in patients with IgAN with Stalk-1+GC<10%.
Figure 6.
Correlation between APRIL expression in tonsillar GC and disease activity in patients with IgAN. (A) Percentage of Stalk-1+GC in tonsils of patients with IgAN and their proteinuria level. The percentage of Stalk-1+GC in patients with IgAN with proteinuria >1 g/g creatinine (Cr) was significantly higher than that in those with proteinuria <1 g/g Cr. *P<0.01. (B) Comparison of proteinuria level, (C) percentage decrease of the serum IgA, and (D) the serum Gd-IgA1 levels between patients with IgAN with the percentage of Stalk-1+GC≥10% and those with the percentage of Stalk-1+GC<10%. Patients with IgAN with Stalk-1+GC≥10% showed significantly higher proteinuria before tonsillectomy and larger decrease of serum levels of Gd-IgA1 after tonsillectomy than those with Stalk-1+GC<10%. **P<0.05. (E–G) Comparison of proteinuria levels before and after tonsillectomy in (E) patients with IgAN (*P<0.01), (F) those with Stalk-1+GC≥10% (*P<0.01), and (G) those with Stalk-1+GC<10%. There were significant differences before and after tonsillectomy in the preceding two groups, despite no significance in patients with IgAN with Stalk-1+GC<10%. The average duration from tonsillectomy to quantification of these clinical parameters was 69.2±47.2 days.
Table 2.
The correlation between Stalk-1+GC and the severity or the efficacy of tonsillectomy in patients with IgAN
| Stalk-1+GC<10%, n=12 | Stalk-1+GC≥10%, n=43 | P Value | |
|---|---|---|---|
| Proteinuria (g/g creatinine) before treatment | 0.69±0.48 | 1.35±0.9 | 0.03 |
| Patients whose proteinuria decreased >50% after tonsillectomy, % | 25.0 (3 of 12) | 62.8 (27 of 43) | 0.02 |
Discussion
Mucosal immune dysregulation has already been reported in the pathogenesis of IgAN. However, the underlying mechanism remains unclear. APRIL and B cell–activating factor belonging to the TNF family derived from myeloid cells, such as neutrophils, monocytes, and dendritic cells, are TNF superfamily members best known for their roles in the survival and maturation of B cells. Recent studies revealed that mature B cell neoplasms, including chronic lymphocytic leukemia,32 follicular lymphoma, and diffuse large B cell lymphoma, may start to produce APRIL33,34 by themselves. This aberrant production of APRIL was also observed in B cells from patients suffering from autoimmune diseases, such as SLE.35 In fact, repeated stimulation of B cells induced their expression of APRIL.36 This study shows, for the first time, an aberrant upregulation of APRIL in tonsillar GC B cells from patients with IgAN.
In GC B cells from IgAN, we observed that the reactivity of the two APRIL-specific antibodies, Stalk-1 and Aprily-2, was clearly different from the one obtained in other organs. Indeed, we observed an uncommon costaining of Stalk-1 and Aprily-2, revealing the presence of a full-length form of APRIL. The detection of the two furin uncleavable isoforms of APRIL, APRIL-δ and -ζ mRNA, is consistent with this observation. This uncleavable full–length APRIL was detected intracellularly and most likely stored in vesicles, warranting further investigations (Figure 3A).
Exacerbation of IgAN on upper respiratory infections allows speculation on the participation of exogenous antigens in disease progression. The palatine tonsils have a unique cellular composition in the reticulated subepithelium, which is ideal for productive antigen sampling for rapid and broad defense against microorganisms at the gate of the respiratory and digestive tracts. Transient mucosal activation of a pattern recognition receptor, such as TLR, by pathogen–associated molecular patterns in IgAN-prone mice is sufficient to exacerbate this disease, with rapid serum elevation of IgA and ICs.23 We recently showed that tonsillar levels of TLR9 expression but not those of other TLRs were associated with the disease activity of IgAN and clinical outcome of tonsillectomy.24–28 Furthermore, the TLR9 genotype was strongly associated with histologic severity of IgAN.23 Genome-wide scan identifies a copy number variable region at 3p21.1 that influences the TLR9 expression levels in patients with IgAN.37 Accordingly, these findings suggest that tonsillar TLR9 signaling pathways may be involved in the pathogenesis of human IgAN. TLR9 ligand CpG-ODN increased the expressions of the APRIL receptors BCMA and TACI on B cells and enhanced B cell activation and Ig secretion.38,39 We are reporting here that chronic CpG-ODN stimulation induced APRIL production by tonsillar B cells. The findings of this study provide a rationale for tonsillectomy and indicate that the TLR9-APRIL axis is a promising specific target for future treatment apart from nonspecific immunosuppressants or tonsillectomy. Also, although the possible contribution of specific exogenous antigens to the pathogenesis of IgAN, including Haemophilus parainfluenzae, has been discussed,40 there are no consistent antigens yet. Our previous and recent studies regarding the involvement of TLR9 in the pathogenesis of human and murine IgAN indicate that specific antigens are not required for the development of IgAN. However, it seems that there is a biased bacterial flora in tonsil of human IgAN,41 suggesting that actual exogenous antigens in the pathogenesis may be limited.
We observed that tonsillar levels of APRIL correlated with disease activity and treatment responses to tonsillectomy, indicating that tonsillar GC B cells may be involved in the pathogenesis via their production of APRIL. It is now widely accepted that Gd-IgA1 and related ICs are essential effector molecules to induce glomerular damages in IgAN.7,42 Serum levels of these molecules, indeed, have clinical diagnostic potential for the assessment of prognosis and disease activity for IgAN, independent of information from renal biopsy.42–45 Reports showed abnormal glycosylation of tonsillar IgA46,47 and aberrant cytokine profiles in tonsillar B cells, leading to the underglycosylation of IgA1 in patients with IgAN.48–51 Because total IgA is decreased by approximately 10% on average after tonsillectomy alone in patients with IgAN27 and because patients who showed a large decrease of serum IgA after the tonsillectomy had better clinical outcome, the palatine tonsil was hypothesized to be a major delivery source of nephritogenic IgA.27 Indeed, we recently showed that Gd-IgA1 was significantly decreased after tonsillectomy in patients with IgAN, who also showed a significant improvement in urinalysis just after tonsillectomy.26 This study further showed that patients with IgAN with abundant expression of Stalk-1 in tonsillar GC showed more proteinuria and better clinical outcomes after the tonsillectomy, including improvement of proteinuria and decrease of serum Gd-IgA1 levels. These findings suggest that palatine tonsils with overexpression of APRIL may be one of the major delivery source of nephritogenic IgA. However, some patients notably showed improved hematuria and serum Gd-IgA1 levels after steroid pulse therapy after the tonsillectomy compared with just after tonsillectomy, suggesting that Gd-IgA1–producing cells may also be localized outside the tonsils.26 Recent data have revealed that some of the NALT–derived and –activated B cells and even tonsillar B cells can migrate from inductive mucosal sites to systemic effector sites, including bone marrow, through guiding adhesion molecules and chemokine/chemokine receptors.52,53 In addition, our recent study revealed that IgA1 secreted by Epstein–Barr virus–immortalized B cells from the peripheral blood of patients with IgAN was mostly polymeric with Gd sialylated O-glycans.54 These findings support the hypothesis that Gd-IgA1–producing B cells may travel between the tonsils and systemic lymphoid organs and produce the nephritogenic IgA outside of mucosal sites. Moreover, bone marrow transplantation with an IgAN donor reconstitutes IgAN in humans and mice,55,56 suggesting that Gd-IgA1–producing B cells could localize in the bone marrow. Indeed, it has been reported that IgA plasma cells containing subclass IgA1 were increased in patients with IgAN compared with controls.57,58 It has also been shown that there was an increase in the proportion of IgA+ cells that express J-chain mRNA, which is essential for the production of dimeric IgA, in the bone marrow of patients with IgAN.59 These lines of evidence emphasize the possibility that the bone marrow could be the production site of the IgA1 found in the circulation and mesangial deposits in patients with IgAN. Thus, we can speculate that mucosally primed/activated Gd–IgA1–producing B cells are disseminated to systemic organs, such as lymph nodes and tonsils, or the bone marrow.
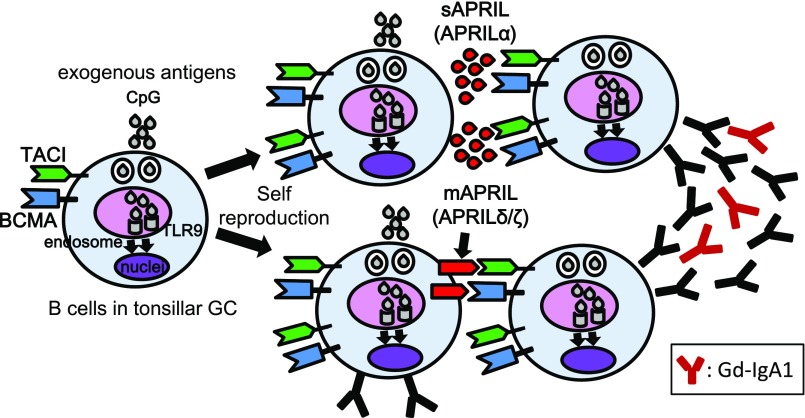
In conclusion, APRIL+GC B cells in tonsils may determine the disease activity of patients with IgAN, presumably via production of Gd-IgA1 before IC formation. Aberrant TLR9 activation in tonsillar B cells may be involved in the underlying mechanisms of the tonsillar overexpression of APRIL, partly with APRIL variants, in IgAN (Figure 7).
Figure 7.
Crosstalk between APRIL and TLR9 on B cells in tonsillar GCs of patients with IgAN. This study revealed aberrant APRIL expression in tonsillar GC B cells from patients with IgAN. On the basis of our findings, we hypothesize that activation of intracellular TLR9 through exogenous antigens may be involved in this overexpression consisting of not only APRIL-α but also, uncleaved APRIL, such as APRIL-δ/ζ in tonsillar GC B cells of patients with IgAN. This TLR9 activation also upregulates expression of TACI and BCMA and increases both BCR signaling and APRIL sensitivity. This aberrant APRIL expression may induce long-term survival of GC B cells responsible for the production of aberrant antibodies, including Gd-IgA1, and thereby, contribute to subsequent progression of IgAN.
Concise Methods
Patients and Treatment Protocol
Fifty-five patients with biopsy-proven IgAN (26 men) and 12 patients with CT (six men) who had undergone tonsillectomy at the Department of Otorhinolaryngology of Juntendo University Hospital, Narita Memorial Hospital, or Tokyo Metropolitan Health and Medical Treatment Corporation, Okubo Hospital were included in this study. Patient demographics and clinical characteristics are summarized in Table 1 and Supplemental Table 2. Both before and after tonsillectomy, patients with IgAN were evaluated for the following clinical outcomes: proteinuria (grams per gram creatinine) and serum levels of IgA and Gd-IgA1. The average duration from tonsillectomy to quantification of these clinical parameters was 69.2±47.2 days. This study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the institutional review board of each hospital. Informed consents were obtained from all patients before inclusion in the study.
Table 1.
Profiles of patients with IgAN and CT just before tonsillectomy
| Patients profile | IgAN | CT |
|---|---|---|
| n | 55 | 12 |
| Age, yr | 35.1 | 31.4 |
| Men, % | 47.3 (26:29) | 50 (6:6) |
| Duration from onset to tonsillectomy, yr | 8.5±8.8 | — |
| sCr, mg/dl | 0.86±0.3 | 0.6±0.14 |
| BUN, mg/dl | 13.1±3.2 | 11.5±3.6 |
| eGFR, ml/min per 1.73 m2 | 79.5±28.1 | 116.4±26.7 |
| Proteinuria-to-urine Cr ratio, g/g Cr | 1.22±0.86 | — |
| Hematuria (RBCs per HPF) | ||
| 1–4 | 8 | — |
| 5–9 | 9 | — |
| 10–15 | 3 | — |
| 16–20 | 11 | — |
| 21–25 | 5 | — |
| 26–30 | 3 | — |
| >30 | 16 | — |
Values are means±SD. Hematuria was assessed by assigning scores according to the number of red blood cells (RBCs) per high-power field (HPF). —, not done; sCr, serum creatinine; Cr, creatinine.
Real–Time Quantitative RT-PCR and Sequencing
qPCR on RNA isolated from tonsillar tissues and cells was performed as described previously.27 A Homo sapiens–specific Taqman gene expression assay (Life Technologies, Carlsbad, CA) was purchased for the TNF (ligand) superfamily, member 13 (APRIL; Hs00601664_g1) and TLR9 (Hs00370913_s1) as well as for an endogenous control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Hs99999905_m1).
A SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and a 7500 Real-Time PCR System (Applied Biosystems) were also used for qPCR for APRIL-α and -δ/ζ expression. Transcript levels were normalized by GAPDH. The following primers synthesized by Life Technologies were used: APRIL-α, 5′-AGGAGAGCAGTGCTCACCC-3′ and 5′-CTCCAGCATCCTGGATTCGG-3′; APRIL-δ, 5′-AGAGTCTCCCGGAGCAGCAG-3′ and 5′-CTCCAGCATCCTGGATTCGG-3′; and GAPDH, 5′-TGACTCCGACCTTCACCTTC-3′ and 5′-CTCTGCTCCTCCTGTTCGAC-3′.
For sequencing purposes, total RNA from tonsillar B cells was extracted using the QIA Shredder (Qiagen, Valencia, CA) and the RNeasy Mini Kit (Qiagen). The PCR was performed with Takara Ex Taq DNA Polymerase (Takara, Shiga, Japan). PCR fragments were isolated from 2% agarose gels using a gel extraction kit (Qiagen). PCR products were ligated into the pMD20 vector (Takara). All standard cloning and plasmid propagation were performed in Escherichia coli strain INVαF′ (Invitrogen, Basel, Switzerland). Clones were first screened by restriction enzyme digestion (EcoRI and SphI; Takara), and subsequent RT-PCRs were subjected to sequence analysis with primers Seq Forward M13 Primer RV (5′-CAGGAAACAGCTATGAC-3′) and Seq Reverse M13 Primer M4 (5′-GTTTTCCCAGTCACGAC-3′). The REH cell line was obtained from American Type Culture Collection (Manassas, VA).
Immunohistochemical Analyses
For immunohistochemistry, the paraformaldehyde–fixed, paraffin–embedded tonsillar samples from patients with IgAN and patients with CT were cut at a thickness of 3 μm. All sections were deparaffinized in xylene followed by 100% ethanol and then, placed in a freshly prepared methanol/0.3% H2O2 solution for 10 minutes. Microwave antigen retrieval was performed with a hot 0.01 mol/L citrate buffer for 20 minutes. The sections were cooled to room temperature and then, blocked with a blocking solution (DS Pharma Biomedical, Osaka, Japan). The primary antibody Stalk-1 was used at 5 μg/ml. The polyclonal rabbit antiserum Stalk-1 was raised against a peptide in the membrane-proximal part of the APRIL extracellular domain remaining associated with the cell membrane after furin cleavage.29 The secondary antibody was a horseradish peroxidase–labeled anti–rabbit antibody (1:50; Dako, Tokyo, Japan). Sections were washed with (PBS; pH 7.4) three times after each incubation. An individual GC was considered a Stalk-1+GC when 25% of its area was covered by Stalk-1+ cells. Image acquisition was performed with a ×40 objective. Cell numeration was performed in a tissue area of 30 mm2. Staining was evaluated by two nephrologists who were blinded to patients’ clinical data (Table 2).
For immunofluorescence staining, tonsillar tissues were mounted in optimal cutting temperature compound (Sakura Finetek, Inc., Tokyo, Japan), immersed in liquid nitrogen, and stored at −80°C. These frozen specimens were cut into 3-mm sections and fixed with 4% paraformaldehyde at −20°C for 10 minutes. Paraformaldehyde–fixed frozen tonsillar tissue sections were then stained with Stalk-1, Aprily-2 (mouse IgG; 2 μg/ml), antielastase (NP57; mouse IgG; 1:200; Dako), anti-CD19 (LE-CD19; mouse IgG; 1:100; Dako), anti-human IgG (3E8; mouse IgG; 0.5 μg/ml; Santa Cruz Biotechnology, Dallas, TX), anti-human IgA (47C12; mouse IgG; 1:200; Santa Cruz), and anti-human IgM (R1/69; mouse IgG; 1 μg/ml; Santa Cruz Biotechnology). The mAb Aprily-2 was raised against the C–terminal TNF homology domain of APRIL secreted on furin cleavage.29 Slides were incubated with the following secondary reagents: Alexa 488–conjugated anti–rabbit Ig (Invitrogen) and Alexa 555–conjugated anti–mouse Ig (Invitrogen). Nuclei were visualized with 4′,6-diamidine-2′phenylindole dihydrochloride (Boehringer Mannheim, Indianapolis, IN). Images were acquired with a confocal laser scanning microscope (Fluoview FV1000; Olympus, Tokyo, Japan). The number of Stalk-1+ elastase+ cells was quantified with the KS400 Image Analysis System (Carl Zeiss GmbH, Oberkochen, Germany) by two nephrologists.
ELISA for Gd-IgA1
The serum level of Gd-IgA1 was measured by lectin ELISA using GalNAc-specific lectin from Helix aspersa (HAA; Sigma-Aldrich, St. Louis, MO) as previously reported.45,54,60,61 Diluted sera were added at 100 ng per well of serum IgA. The captured IgA was treated with 10 mU/ml neuraminidase (Roche Diagnostics, Indianapolis, IN) to remove terminal sialic acid residues.54,60 The desialylated IgA1 was then reacted with biotin-labeled HAA, and subsequently developed absorbance was measured at 490 nm. The HAA reactivity of IgA1 in each sample was then calculated as OD units per 100 ng serum IgA. Naturally Gd-IgA1 (Ale) myeloma protein60 was treated with neuraminidase and used as the standard. Serum level of total Gd-IgA1 was expressed in relative units calculated by multiplying the normalized HAA reactivity by the amount of IgA in the serum sample (milligrams per milliliter).
Tonsillar Cell Preparation
After surgery, tonsil samples were dissected into small pieces in 2 mg/ml collagenase intravenously (Worthington Biochemical Corporation, Lakewood, NJ) and filtered on a 100-μm cell strainer. Tonsil cell stimulation was performed daily with 10 μg/ml CpG-ODN 1826 and control ODN 1982 (Microsynth, Balgach, Switzerland). Tonsillar B cells were purified using a Dynabeads Untouched Human B Cells Kit (Invitrogen) according to the manufacturer’s instructions.
Flow Cytometric Analyses
In total, 1×106 tonsillar cells were stained for flow cytometry. The cells were preincubated with Fc receptor blocking reagent (MBL, Aichi, Japan) and incubated for 30 minutes at 4°C with FITC–conjugated mouse anti–human CD19 antibody (BioLegend, San Diego, CA), APC mouse anti–human CD38 (BD Pharmingen, San Jose, CA), and PE mouse anti–human IgD (BD Pharmingen). Biotinylated anti-TACI and BCMA have been previously described.62 PE-conjugated streptavidin was from BD Pharmingen. Total staining was performed after permeabilization with a Cytofix/Cytoperm Solution (BD Pharmingen) for 30 minutes at 4°C. After washing twice in Perm/Wash solution, cells were incubated for 30 minutes at 4°C with Stalk-1 (5 μg/ml) and Aprily-2 (5 μg/ml). Brilliant Violet 421 donkey anti–rabbit or mouse IgG (BioLegend) was used as secondary antibody. After washing the cells twice in BD Perm/Wash solution, labeled cells were analyzed by flow cytometry using a FACSVerse Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ) and the FlowJo program (TreeStar, Inc., Ashland, OR).
Statistical Analyses
Statistical analyses were performed using GraphPad PRISM software, version 6.0 (GraphPad Software, La Jolla, CA). Comparisons between groups were analyzed by the Mann–Whitney U test. Spearman regression analysis was used to analyze the correlation between two variables. Differences at P<0.05 were considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Drs. Takaaki Oobayashi, Kanae Yoshikawa, and Hiroshi Kitagawa for preparation of tonsillar samples. Technical assistance was provided by Ms. Terumi Shibata, Dr. Masao Kihara, Dr. Akemi Kawasaki, Dr. Takako Ikegami, and Dr. Tomomi Ikeda (Division of Molecular and Biochemical Research, Juntendo University Graduate School of Medicine).
This study was supported by grants-in-aid from the Ministry of Health, Labor and Welfare of Japan (to M. Muto, H.S., M. Maiguma, and Y.S.) and grants from the Japanese Swiss Science and Technology Cooperation Program, the Strategic International Research Cooperative Program, the Japan Science and Technology Agency (to Y.T., B.H., and Y.S.), the Foundation Finovi (to B.H.), Institut National de la Santé et de la Recherche Médicale (to B.H.), and Joseph Fourier University (to B.H.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016050496/-/DCSupplemental.
References
- 1.Barratt J, Feehally J: IgA nephropathy. J Am Soc Nephrol 16: 2088–2097, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Appel GB, Waldman M: The IgA nephropathy treatment dilemma. Kidney Int 69: 1939–1944, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Suzuki Y, Suzuki H, Sato D, Kajiyama T, Okazaki K, Hashimoto A, Kihara M, Yamaji K, Satake K, Nakata J, Aizawa M, Novak J, Tomino Y: Reevaluation of the mucosa-bone marrow axis in IgA nephropathy with animal models. Adv Otorhinolaryngol 72: 64–67, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Suzuki Y, Tomino Y: Potential immunopathogenic role of the mucosa-bone marrow axis in IgA nephropathy: Insights from animal models. Semin Nephrol 28: 66–77, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Kokubo T, Hiki Y, Iwase H, Horii A, Tanaka A, Nishikido J, Hotta K, Kobayashi Y: Evidence for involvement of IgA1 hinge glycopeptide in the IgA1-IgA1 interaction in IgA nephropathy. J Am Soc Nephrol 8: 915–919, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J: Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int 52: 509–516, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahne M, Kataoka T, Schröter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE, Sordat B, Rimoldi D, Tschopp J: APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med 188: 1185–1190, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Fraga M, Fernández R, Albar JP, Hahne M: Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep 2: 945–951, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A: Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26: 812–826, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, Donze O, Frossard C, Chizzolini C, Favre C, Zubler R, Guyot JP, Schneider P, Roosnek E: APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest 118: 2887–2895, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthes T, Dunand-Sauthier I, Santiago-Raber ML, Krause KH, Donze O, Passweg J, McKee T, Huard B: Production of the plasma-cell survival factor a proliferation-inducing ligand (APRIL) peaks in myeloid precursor cells from human bone marrow. Blood 118: 1838–1844, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Yu G, Boone T, Delaney J, Hawkins N, Kelley M, Ramakrishnan M, McCabe S, Qiu WR, Kornuc M, Xia XZ, Guo J, Stolina M, Boyle WJ, Sarosi I, Hsu H, Senaldi G, Theill LE: APRIL and TALL-I and receptors BCMA and TACI: System for regulating humoral immunity. Nat Immunol 1: 252–256, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, Mizoguchi E, Geha RS: Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci USA 101: 3903–3908, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, Schneider P, Huard B, Lambert PH, Siegrist CA: APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood 111: 2755–2764, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Chu VT, Beller A, Rausch S, Strandmark J, Zänker M, Arbach O, Kruglov A, Berek C: Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 40: 582–593, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Chu VT, Fröhlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, Lee JJ, Löhning M, Berek C: Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol 12: 151–159, 2011 [DOI] [PubMed] [Google Scholar]
- 18.McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA, Ward L, Lawson MA, Macpherson AJ, McCoy KD, Pei Y, Novak L, Lee JY, Julian BA, Novak J, Ranger A, Gommerman JL, Browning JL: Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 121: 3991–4002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han SS, Yang SH, Choi M, Kim HR, Kim K, Lee S, Moon KC, Kim JY, Lee H, Lee JP, Jung JY, Kim S, Joo KW, Lim CS, Kang SW, Kim YS, Kim DK: The role of TNF superfamily member 13 in the progression of IgA nephropathy [published online ahead of print April 11, 2016]. J Am Soc Nephrol doi:10.1681/ASN.2015060677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu XQ, Li M, Zhang H, Low HQ, Wei X, Wang JQ, Sun LD, Sim KS, Li Y, Foo JN, Wang W, Li ZJ, Yin XY, Tang XQ, Fan L, Chen J, Li RS, Wan JX, Liu ZS, Lou TQ, Zhu L, Huang XJ, Zhang XJ, Liu ZH, Liu JJ: A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat Genet 44: 178–182, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Krieg AM: Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov 5: 471–484, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Goodnow CC: Immunology. Discriminating microbe from self suffers a double toll. Science 312: 1606–1608, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Suzuki Y, Narita I, Aizawa M, Kihara M, Yamanaka T, Kanou T, Tsukaguchi H, Novak J, Horikoshi S, Tomino Y: Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol 19: 2384–2395, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kajiyama T, Suzuki Y, Kihara M, Suzuki H, Horikoshi S, Tomino Y: Different pathological roles of toll-like receptor 9 on mucosal B cells and dendritic cells in murine IgA nephropathy. Clin Dev Immunol 2011: 819646, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiguma M, Suzuki Y, Suzuki H, Okazaki K, Aizawa M, Muto M, Tomino Y: Dietary zinc is a key environmental modifier in the progression of IgA nephropathy. PLoS One 9: e90558, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakata J, Suzuki Y, Suzuki H, Sato D, Kano T, Yanagawa H, Matsuzaki K, Horikoshi S, Novak J, Tomino Y: Changes in nephritogenic serum galactose-deficient IgA1 in IgA nephropathy following tonsillectomy and steroid therapy. PLoS One 9: e89707, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato D, Suzuki Y, Kano T, Suzuki H, Matsuoka J, Yokoi H, Horikoshi S, Ikeda K, Tomino Y: Tonsillar TLR9 expression and efficacy of tonsillectomy with steroid pulse therapy in IgA nephropathy patients. Nephrol Dial Transplant 27: 1090–1097, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T, Kanamaru Y, Liu C, Suzuki Y, Tada N, Okumura K, Horikoshi S, Tomino Y: Negative regulation of inflammatory responses by immunoglobulin A receptor (FcαRI) inhibits the development of Toll-like receptor-9 signalling-accelerated glomerulonephritis. Clin Exp Immunol 166: 235–250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwaller J, Schneider P, Mhawech-Fauceglia P, McKee T, Myit S, Matthes T, Tschopp J, Donze O, Le Gal FA, Huard B: Neutrophil-derived APRIL concentrated in tumor lesions by proteoglycans correlates with human B-cell lymphoma aggressiveness. Blood 109: 331–338, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Burjanadze M, Matthes T, McKee T, Passweg J, Huard B: In situ detection of APRIL-rich niches for plasma-cell survival and their contribution to B-cell lymphoma development. Histol Histopathol 24: 1061–1066, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Maia S, Pelletier M, Ding J, Hsu YM, Sallan SE, Rao SP, Nadler LM, Cardoso AA: Aberrant expression of functional BAFF-system receptors by malignant B-cell precursors impacts leukemia cell survival. PLoS One 6: e20787, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kern C, Cornuel JF, Billard C, Tang R, Rouillard D, Stenou V, Defrance T, Ajchenbaum-Cymbalista F, Simonin PY, Feldblum S, Kolb JP: Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood 103: 679–688, 2004 [DOI] [PubMed] [Google Scholar]
- 33.He B, Chadburn A, Jou E, Schattner EJ, Knowles DM, Cerutti A: Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol 172: 3268–3279, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Gupta M, Dillon SR, Ziesmer SC, Feldman AL, Witzig TE, Ansell SM, Cerhan JR, Novak AJ: A proliferation-inducing ligand mediates follicular lymphoma B-cell proliferation and cyclin D1 expression through phosphatidylinositol 3-kinase-regulated mammalian target of rapamycin activation. Blood 113: 5206–5216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu VT, Enghard P, Schürer S, Steinhauser G, Rudolph B, Riemekasten G, Berek C: Systemic activation of the immune system induces aberrant BAFF and APRIL expression in B cells in patients with systemic lupus erythematosus. Arthritis Rheum 60: 2083–2093, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Chu VT, Enghard P, Riemekasten G, Berek C: In vitro and in vivo activation induces BAFF and APRIL expression in B cells. J Immunol 179: 5947–5957, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Sallustio F, Cox SN, Serino G, Curci C, Pesce F, De Palma G, Papagianni A, Kirmizis D, Falchi M, Schena FP; European IgAN Consortium : Genome-wide scan identifies a copy number variable region at 3p21.1 that influences the TLR9 expression levels in IgA nephropathy patients. Eur J Hum Genet 23: 940–948, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J, Gross JA, Dillon SR, Min JK, Elkon KB: Increased BCMA expression in lupus marks activated B cells, and BCMA receptor engagement enhances the response to TLR9 stimulation. Autoimmunity 44: 69–81, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Katsenelson N, Kanswal S, Puig M, Mostowski H, Verthelyi D, Akkoyunlu M: Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur J Immunol 37: 1785–1795, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Suzuki S, Nakatomi Y, Sato H, Tsukada H, Arakawa M: Haemophilus parainfluenzae antigen and antibody in renal biopsy samples and serum of patients with IgA nephropathy. Lancet 343: 12–16, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Nagasawa Y, Iio K, Fukuda S, Date Y, Iwatani H, Yamamoto R, Horii A, Inohara H, Imai E, Nakanishi T, Ohno H, Rakugi H, Isaka Y: Periodontal disease bacteria specific to tonsil in IgA nephropathy patients predicts the remission by the treatment. PLoS One 9: e81636, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki Y, Suzuki H, Yasutake J, Tomino Y: Paradigm shift in activity assessment of IgA nephropathy - optimizing the next generation of diagnostic and therapeutic maneuvers via glycan targeting. Expert Opin Biol Ther 15: 583–593, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, Mariat C, Tomino Y, Julian BA, Novak J: Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 23: 1579–1587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki Y, Matsuzaki K, Suzuki H, Okazaki K, Yanagawa H, Ieiri N, Sato M, Sato T, Taguma Y, Matsuoka J, Horikoshi S, Novak J, Hotta O, Tomino Y: Serum levels of galactose-deficient immunoglobulin (Ig) A1 and related immune complex are associated with disease activity of IgA nephropathy. Clin Exp Nephrol 18: 770–777, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasutake J, Suzuki Y, Suzuki H, Hiura N, Yanagawa H, Makita Y, Kaneko E, Tomino Y: Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol Dial Transplant 30: 1315–1321, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horie A, Hiki Y, Odani H, Yasuda Y, Takahashi M, Kato M, Iwase H, Kobayashi Y, Nakashima I, Maeda K: IgA1 molecules produced by tonsillar lymphocytes are under-O-glycosylated in IgA nephropathy. Am J Kidney Dis 42: 486–496, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Inoue T, Sugiyama H, Hiki Y, Takiue K, Morinaga H, Kitagawa M, Maeshima Y, Fukushima K, Nishizaki K, Akagi H, Narimatsu Y, Narimatsu H, Makino H: Differential expression of glycogenes in tonsillar B lymphocytes in association with proteinuria and renal dysfunction in IgA nephropathy. Clin Immunol 136: 447–455, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Liu H, Peng Y, He L, Zhang Y, Xie Y, Peng X, Liu C, Liu F: Expression and correlation analysis of IL-4, IFN-γ and FcαRI in tonsillar mononuclear cells in patients with IgA nephropathy. Cell Immunol 289: 70–75, 2014 [DOI] [PubMed] [Google Scholar]
- 49.He L, Peng Y, Liu H, Yin W, Chen X, Peng X, Shao J, Liu Y, Liu F: Activation of the interleukin-4/signal transducer and activator of transcription 6 signaling pathway and homeodomain-interacting protein kinase 2 production by tonsillar mononuclear cells in IgA nephropathy. Am J Nephrol 38: 321–332, 2013 [DOI] [PubMed] [Google Scholar]
- 50.He L, Peng Y, Liu H, Yin W, Chen X, Peng X, Shao J, Liu Y, Liu F: Th1/Th2 polarization in tonsillar lymphocyte form patients with IgA nephropathy. Ren Fail 36: 407–412, 2014 [DOI] [PubMed] [Google Scholar]
- 51.Suzuki H, Raska M, Yamada K, Moldoveanu Z, Julian BA, Wyatt RJ, Tomino Y, Gharavi AG, Novak J: Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem 289: 5330–5339, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brandtzaeg P, Johansen FE: Mucosal B cells: Phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev 206: 32–63, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P: The immune geography of IgA induction and function. Mucosal Immunol 1: 11–22, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, Novak L, Julian BA, Tomana M, Wyatt RJ, Edberg JC, Alarcón GS, Kimberly RP, Tomino Y, Mestecky J, Novak J: IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest 118: 629–639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imasawa T, Nagasawa R, Utsunomiya Y, Kawamura T, Zhong Y, Makita N, Muso E, Miyawaki S, Maruyama N, Hosoya T, Sakai O, Ohno T: Bone marrow transplantation attenuates murine IgA nephropathy: Role of a stem cell disorder. Kidney Int 56: 1809–1817, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Suzuki H, Suzuki Y, Aizawa M, Yamanaka T, Kihara M, Pang H, Horikoshi S, Tomino Y: Th1 polarization in murine IgA nephropathy directed by bone marrow-derived cells. Kidney Int 72: 319–327, 2007 [DOI] [PubMed] [Google Scholar]
- 57.van den Wall Bake AW, Daha MR, Radl J, Haaijman JJ, Van der Ark A, Valentijn RM, Van Es LA: The bone marrow as production site of the IgA deposited in the kidneys of patients with IgA nephropathy. Clin Exp Immunol 72: 321–325, 1988 [PMC free article] [PubMed] [Google Scholar]
- 58.van den Wall Bake AW, Daha MR, Evers-Schouten J, van Es LA: Serum IgA and the production of IgA by peripheral blood and bone marrow lymphocytes in patients with primary IgA nephropathy: Evidence for the bone marrow as the source of mesangial IgA. Am J Kidney Dis 12: 410–414, 1988 [DOI] [PubMed] [Google Scholar]
- 59.Harper SJ, Allen AC, Pringle JH, Feehally J: Increased dimeric IgA producing B cells in the bone marrow in IgA nephropathy determined by in situ hybridisation for J chain mRNA. J Clin Pathol 49: 38–42, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, Huang WQ, Anreddy SR, Hall S, Hastings MC, Lau KK, Cook WJ, Novak J: Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 71: 1148–1154, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Moore JS, Kulhavy R, Tomana M, Moldoveanu Z, Suzuki H, Brown R, Hall S, Kilian M, Poulsen K, Mestecky J, Julian BA, Novak J: Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol 44: 2598–2604, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matthes T, McKee T, Dunand-Sauthier I, Manfroi B, Park S, Passweg J, Huard B: Myelopoiesis dysregulation associated to sustained APRIL production in multiple myeloma-infiltrated bone marrow. Leukemia 29: 1901–1908, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.