Abstract
People of African ancestry carrying certain APOL1 mutant alleles are at elevated risk of developing renal diseases. However, the mechanisms underlying APOL1-associated renal diseases are unknown. Because the APOL1 gene is unique to humans and some primates, new animal models are needed to understand the function of APOL1 in vivo. We generated transgenic Drosophila fly lines expressing the human APOL1 wild type allele (G0) or the predominant APOL1 risk allele (G1) in different tissues. Ubiquitous expression of APOL1 G0 or G1 in Drosophila induced lethal phenotypes, and G1 was more toxic than was G0. Selective expression of the APOL1 G0 or G1 transgene in nephrocytes, fly cells homologous to mammalian podocytes, induced increased endocytic activity and accumulation of hemolymph proteins, dextran particles, and silver nitrate. As transgenic flies with either allele aged, nephrocyte function declined, cell size increased, and nephrocytes died prematurely. Compared with G0-expressing cells, however, G1-expressing cells showed more dramatic phenotypes, resembling those observed in cultured mammalian podocytes overexpressing APOL1-G1. Expressing the G0 or G1 APOL1 transgene in nephrocytes also impaired the acidification of organelles. We conclude that expression of an APOL1 transgene initially enhances nephrocyte function, causing hypertrophy and subsequent cell death. This new Drosophila model uncovers a novel mechanism by which upregulated expression of APOL1-G1 could precipitate renal disease in humans. Furthermore, this model may facilitate the identification of APOL1–interacting molecules that could serve as new drug targets to treat APOL1-associated renal diseases.
Keywords: chronic kidney disease, genetic renal disease, cell biology and structure, cell survival
Studies strongly support an association between mutations in the gene encoding APOL1 (APOL1) and development of renal disease.1–6 APOL1 is the serum trypanosome lytic factor.7 A high frequency of two APOL1 risk alleles associated with renal diseases in persons of African ancestry8–10 is attributed to the evolution of trypanosome resistance to an ancestral G0 allele, whereas G1 and G2 variant risk-associated alleles confer pathogen resistance.5,6,8,9,11,12 G1 and G2 alleles are associated with diverse kidney diseases.8,13–18 APOL1 is produced by renal tubular epithelial cells and podocytes.19,20 In kidney transplantation, donor expression of APOL1 risk variants confers risk of graft failure.21–24 Overexpression of APOL1 in cultured human kidney epithelial cells induces cytotoxic effects.25–27 APOL1 is restricted to humans and some nonhuman primate species. Transgenic mice expressing APOL1-G2 developed preeclampsia and reduced podocyte cell density, but not renal disease.28 Thus, understanding APOL1 contributions to kidney disease will benefit from additional animal models.
Drosophila studies can yield insights into podocyte and proximal tubule (PT) cell biology and mechanisms underlying genetic renal disease.29–33 This model exploits structural and functional similarities of Drosophila pericardial nephrocytes (hereafter, nephrocytes) with podocytes and PT cells.34–36 Nephrocytes are renal cells performing analogous filtration and protein reabsorption functions of podocytes and PT cells, respectively.32–34,36–38 Nephrocytes form two rows of cells on either side of the fly’s tubular heart, and regulate hemolymph (insect blood) composition through filtration and protein reabsorption. The nephrocyte plasma membrane is circumferentially in-folded to form lacunar channels, spanned by extracellular domains of membrane proteins that form nephrocyte slit diaphragm filtration units.34 The nephrocyte slit diaphragm is a structural and functional homolog of the podocyte slit diaphragm. Drosophila homologs of cubilin and amnionless, protein reabsorption receptors found in PT cells, are required for nephrocyte endocytosis.33 Podocytes are difficult to study in vivo and do not maintain differentiated features in culture. Nephrocytes allow sophisticated high resolution in vivo experimental studies, and will permit genetic approaches to identify APOL1–interacting factors that contribute to kidney pathology.
We generated transgenic Drosophila fly lines expressing APOL1-G0 and APOL1-G1. We observed that ubiquitous expression of APOL1 in all tissues induced developmental lethality. Tissue-specific expression allowed development of adult flies with diverse phenotypes. In nephrocytes, APOL1 expression was first associated with increased functional activity. With age, APOL1 led to reduced function, increased cell size, abnormal vesicle acidification, and accelerated cell death. The APOL1-G1 risk allele always induced greater phenotypic severity than G0.
Results
Ubiquitous APOL1 Transgene Expression Caused Developmental Lethality
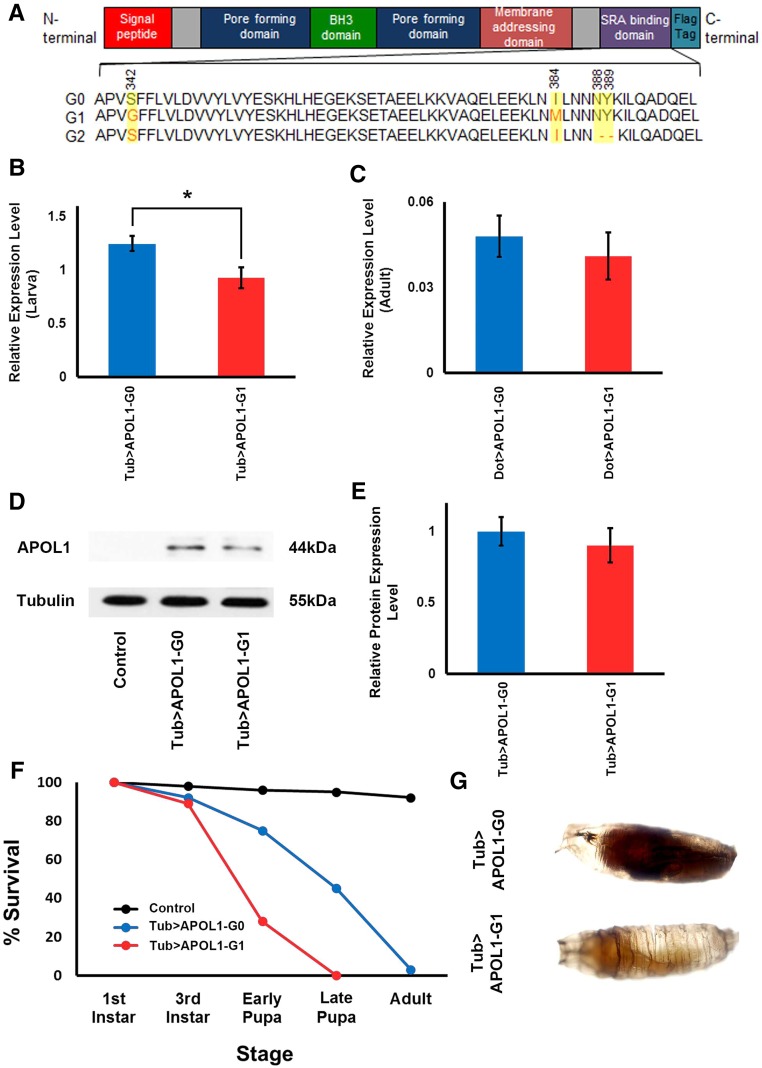
We produced transgenic39 Drosophila lines carrying human APOL1 G0 (wild type allele) or an APOL1-G1 risk allele derived from a patient with HIV affiliated nephropathy.40 Figure 1A shows the domain structure of human APOL1 protein and amino acid changes distinguishing APOL1 encoded by the G0, G1, and G2 alleles. To specify tissue and/or stage specificity of transgene expression we employed the Drosophila UAS-Gal4 system.41 Transgenes cloned downstream of a UAS promoter are expressed only in cells in which a “driver” Gal4 transcription factor is expressed under control of a tissue and/or stage-specific gene enhancer element. Thus, UAS-APOL1-G0 and UAS-APOL1-G1 transgenic fly lines were generated. The G0 and G1 expression constructs incorporated a FLAG epitope tag at the APOL1 protein C-terminus. We produced flies in which expression of these transgenes was directed by either a tubulin (Tub) gene enhancer driving expression at high levels ubiquitously (i.e., in all cells at all developmental stages), or by a Dorothy (Dot) gene enhancer driving expression in nephrocytes. RT-PCR analysis indicated that ubiquitous Tub-directed RNA expression levels of APOL1-G0 were higher than APOL1-G1 in larvae (Figure 1B), whereas Dot-directed nephrocyte RNA levels were not significantly different (Figure 1C). We confirmed ubiquitous expression of APOL1 protein in transgenic pupae by western blot analysis (Figure 1D) and quantitative analysis indicated equivalent levels of G0 and G1 forms (Figure 1E). Ubiquitous APOL1 G0 or G1 protein expression induced complete developmental lethality (no adult flies produced). G0 expression was associated with later pupal mortality, whereas G1 expression caused mortality in earlier pupae (Figure 1, F and G). These observations suggest that ubiquitous expression of APOL1 is deleterious, with G1 more toxic than G0.
Figure 1.
Ubiquitous expression of APOL1 in Drosophila is lethal. (A) APOL1 protein domain structure and amino acid changes in the SRA–binding domain distinguishing the G0 wild type and G1 and G2 risk-associated forms. The APOL1 G0 and G1 expression constructs used in this study included a C-terminal FLAG tag. (B) Transgenic APOL1 G0 and G1 ubiquitous Tub enhancer–driven RNA expression levels in larvae determined using RT-PCR. APOL1 G0 RNA levels were significantly higher than APOL1 G1 RNA levels. Experiments were performed in triplicate. *P<0.05. (C) Transgenic APOL1 G0 and G1 DoT–enhancer driven RNA expression levels in adult flies determined using RT-PCR. No significant difference was seen between APOL1 G0 and G1 RNA levels. Experiments were performed in triplicate. *P<0.05. (D) Western blot analysis using anti-FLAG antibody of total protein extracts from pupae ubiquitously expressing APOL1-G0 and APOL1-G1 constructs, and control pupae carrying only the UAS-APOL1-G0 transgene in the absence of the Tub-Gal4 driver. Tubulin was used as a constitutive control. (E) Relative levels of APOL1 on the basis of western blot analysis. No significant difference was seen between APOL1 G0 and G1 protein levels. Experiments were performed in triplicate. *P<0.05. (F) Survival curves during larva–pupa–adult stages showing control (UAS-APOL1-G0 transgene without driver) and animals ubiquitously expressing Tub enhancer–driven APOL1-G0 or APOL1-G1 transgenes. Approximately 95% of control animals emerged as adult flies. Both APOL1-G0 and APOL1-G1 expression prevented development beyond the pupa stage. The APOL1-G1 risk variant induced earlier developmental lethality than the G0 wild type. (G) Developmental lethality during late (upper, melanized) and early (lower, lightly pigmented) pupa stages induced by ubiquitous expression of APOL1 -G0 and APOL1 -G1, respectively, under the control of a Tub driver.
Tissue-Specific APOL1 Expression Caused Abnormalities in Adult Flies
Tissue-specific expression of APOL1 G0 or G1 proteins was not associated with developmental lethality. APOL1 expression in developing wing discs, somatic muscle, and nephrocytes (see below) allowed development of adult flies. This permitted evaluation of G0- and G1-associated tissue-specific phenotypes. The cells of the fly wing are produced during development of the wing imaginal disc. The wing blade is composed of a biplanar folded sheet of cells, and the morphology of the adult wing enables readout of phenotypes affecting cell viability during development. Both ms1096-GAL4 and vg-GAL4 drivers induced expression of APOL1 specifically in the wing disc, ms1096-GAL4 throughout the wing, and vg-GAL4 in the wing margin.42 The resulting phenotypes revealed increased severity of the G1 variant relative to G0 (Supplemental Figure 1). vg-GAL4 directed APOL1-G0 expression to the wings without effect on morphology. By contrast, APOL1-G1 expression led to readily-detectable abnormalities in the developing wing veins (Supplemental Figure 1A). APOL1-G0 expression driven by ms1096-GAL4 was associated with only very subtle defects in vein morphology. By contrast, ms1096-GAL4 driven APOL1-G1 expression caused pronounced defects in vein development and wing morphology (Supplemental Figure 1B). We used a mef2 gene enhancer to express APOL1 in somatic muscle cells. We observed that adult flies displayed an “up-held wings” phenotype (Supplemental Figure 1C), with APOL1-G1 expression associated with a significantly more penetrant phenotype than APOL1-G0 (Supplemental Figure 1D). We hypothesized that the “up-held wing” phenotype reflected disruption of muscle tissue. Examination of adult somatic musculature by phalloidin staining (Supplemental Figure 1E) indeed revealed that mef2-directed expression of APOL1-G0 and APOL1-G1 proteins led to disorganized and reduced muscle fibers. Again, we observed a more severe phenotype associated with G1 compared with G0 expression. We also generated adult flies expressing APOL1-G0 or G1 proteins in neurons, cardiomyocytes, hindgut cells, and hemocytes. In each case, viable adult flies were able to develop. The phenotypes are currently being analyzed.
APOL1 Affected Nephrocyte Function
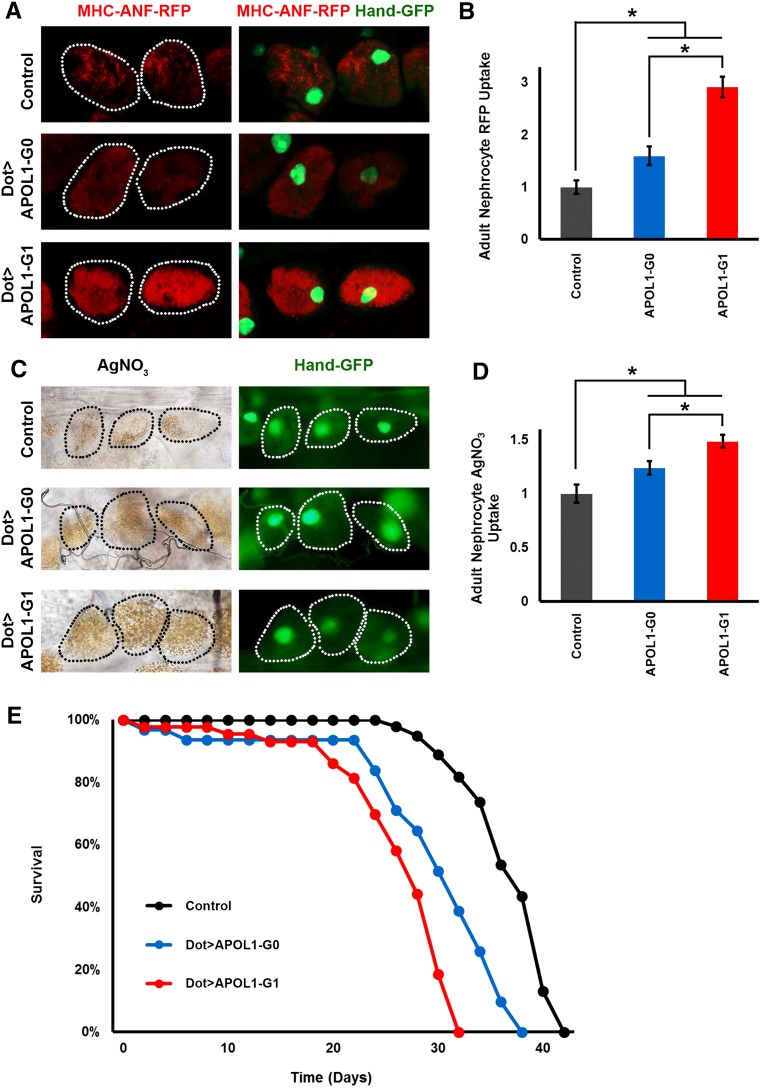
Because the Drosophila nephrocyte bears striking structural and functional homology with mammalian podocytes, we were particularly interested in analyzing the effects of nephrocyte-specific expression of APOL1. In order to measure functional changes induced by nephrocyte-specific APOL1 expression, we employed an assay to measure nephrocyte uptake of hemolymph proteins. A myosin heavy chain (MHC) promoter–driven atrial natriuretic peptide-red fluorescent protein (MHC-ANF-RFP) transgene32 was introduced into the Dot-Gal4; UAS-APOL1-G0 and Dot-Gal4; and UAS-APOL1-G1 fly lines. The MHC promoter directs muscle cell expression of a fusion protein of rat atrial natriuretic factor43—red fluorescent protein (ANF-RFP) that is secreted into the hemolymph. ANF-RFP is endocytosed by, and visualized in, nephrocytes expressing APOL1 under the control of the nephrocyte-specific Dot-Gal4 driver.32 Figure 2, A and B, shows ANF-RFP fluorescence in nephrocytes of 1-day-old (1 day postemergence) adult flies, comparing control fly nephrocytes to nephrocytes of flies expressing APOL1-G0 and APOL1-G1. APOL1-G0 protein expression led to a moderate increase in Red Fluorescence Protein (RFP) levels compared with control, with RFP distributed homogeneously throughout the nephrocyte cytoplasm. APOL1-G1 protein induced approximately three-fold greater RFP accumulation when compared with control flies, again with RFP distributed in a more uniform pattern throughout the cell cytoplasm. To further assess the effects of APOL1 on nephrocyte function, we exploited the ability of nephrocytes to take up and sequester toxins.38 Ingested silver nitrate (AgNO3) showed normal levels of sequestration in nephrocytes of 1-day-old adult control flies (Figure 2, C and D). The amount of AgNO3 sequestered in nephrocytes of APOL1-G0 and G1-expressing flies was increased by approximately 25% and 50%, respectively (Figure 2D). We hypothesized that perturbation of nephrocyte function as a result of APOL1 expression in these cells could have effects on adult fly longevity. We monitored fly survival rates under normal maintenance conditions (in this case at 29°C to optimize activity of the yeast-derived Gal4 driver protein). Under these conditions, the majority of control flies survive 30–40 days. APOL1-G1 expression in nephrocytes reduced lifespan such that most flies survived 20–30 days, and APOL1-G0 expression was associated with a less severe lifespan phenotype that was nevertheless reduced compared with controls (Figure 2E).
Figure 2.
Expression of APOL1 G0 and G1 increases the function of nephrocytes in young adult transgenic flies. (A) ANF-RFP red fluorescent fusion protein in nephrocytes of 1-day-old (1 day postemergence) adult flies. Left panels (MHC-ANF-RFP) show RFP with dotted lines indicating nephrocyte cell outlines. Right panels (MHC-ANF-RFP Hand-GFP) show merged RFP fluorescence and GFP reporter fluorescence (green, mostly nuclear). Control flies carried MHC-ANF-RFP, Hand-GFP, and Dot-GAL4 driver (without UAS-APOL1 transgene). Dot>APOL1-G0 and Dot>APOL1-G1 flies carried MHC-ANF-RFP, Hand-GFP, and Dot-GAL4 driver–directing nephrocyte-specific expression of the indicated APOL1 transgene. (B) Quantification of nephrocyte RFP fluorescence, relative to control value. For quantification, ≥20 nephrocytes were analyzed from each of three female flies per genotype. The results are presented as mean±SD. Statistical significance (*) was defined as P<0.05. (C) AgNO3 sequestration by nephrocytes of 1-day-old adult flies. Left panels show bright field views of AgNO3 taken up by nephrocytes with dotted lines indicating cell outlines. Right panels show Hand-GFP reporter fluorescence in nephrocytes (green, mostly nuclear), with dotted lines indicating cell outlines. (D) Quantification of nephrocyte AgNO3 levels, relative to control value. For quantification, ≥20 nephrocytes were analyzed from each of three female flies per genotype. The results are presented as mean±SD. Statistical significance (*) was defined as P<0.05. (E) Adult survival curves for control (without UAS-APOL1 transgene) and Dot enhancer–directed APOL1 transgene expressing flies. Nephrocyte expression of both G0 and G1 forms of APOL1 caused early mortality, although G1 expression was more toxic.
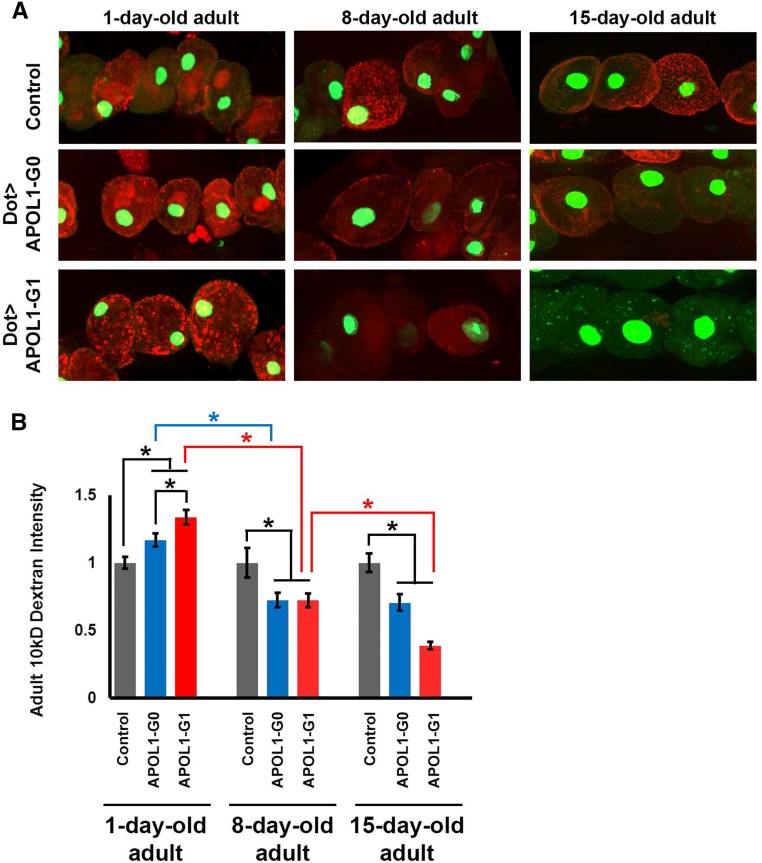
Because the effects of APOL1 nephrocyte-specific expression on survival were not evident in young flies, we tested nephrocyte function at later time points as flies aged. Because the MHC-ANF-RFP transgene is not active in older adult skeletal muscle cells, we used a previously established ex vivo functional assay that measures the capacity of dissected nephrocytes to filter and endocytose 10 kD fluorescent dextran particles.32 As shown in Figure 3, nephrocytes expressing APOL1-G0 or G1 exhibited biphasic changes in uptake of 10 kD dextran particles. At day 1, nephrocytes expressing APOL1-G0 showed significantly more fluorescent dextran accumulation relative to controls. This phenotype was significantly more pronounced in 1-day-old nephrocytes expressing APOL1-G1. In these nephrocytes, moreover, the fluorescence was qualitatively distinct from controls, appearing more punctate and more widely distributed. The APOL1-associated increase in dextran uptake was transient: 8-day-old nephrocytes expressing APOL1-G0 or G1 both showed significantly reduced fluorescent dextran relative to control nephrocytes (which did not change throughout the time course of the experiment). APOL1-G0–expressing nephrocytes were not significantly further affected by day 15. APOL1-G1 expression, by contrast, was associated with a further significant reduction in dextran uptake (Figure 3, A and B).
Figure 3.
APOL1 G0 and G1 induced biphasic age-related changes in nephrocyte uptake of fluorescent dextran particles. (A) Nephrocytes dissected from young (day 1 postemergence) APOL1-G0 and G1 transgenic flies showed increased ability to internalize 10 kD fluorescent dextran particles54 compared with control nephrocytes (carrying Dot-GAL4 driver without UAS-APOL1 transgene). By day 8, APOL1 expression was associated with dextran internalization reduced to levels significantly less than control nephrocytes. The reduced dextran internalization phenotype was relatively more severe in 15-day-old fly nephrocytes. Nephrocytes express a nuclear-localized GFP marker.55 (B) Quantification of adult nephrocyte dextran internalization. Both G0 and G1 forms of APOL1 led to increased dextran internalization at day 1, but G1 induced significantly higher levels than G0. By day 8, APOL1 was associated with significantly reduced dextran internalization relative to control nephrocytes, and levels on day 1. In 15-day-old nephrocytes expressing APOL1-G1 the dextran internalization phenotype is significantly more severe than at day 8. G0 expression, by contrast, was not associated with increased reduction in internalization capability. Control nephrocyte dextran internalization capacity remained unchanged throughout the time course of the experiment. For quantification, ≥20 nephrocytes were analyzed from each of three female flies per genotype. The results are presented as mean±SD. Statistical significance (*) was defined as P<0.05.
APOL1-Induced Nephrocyte Hypertrophy and Cell Death
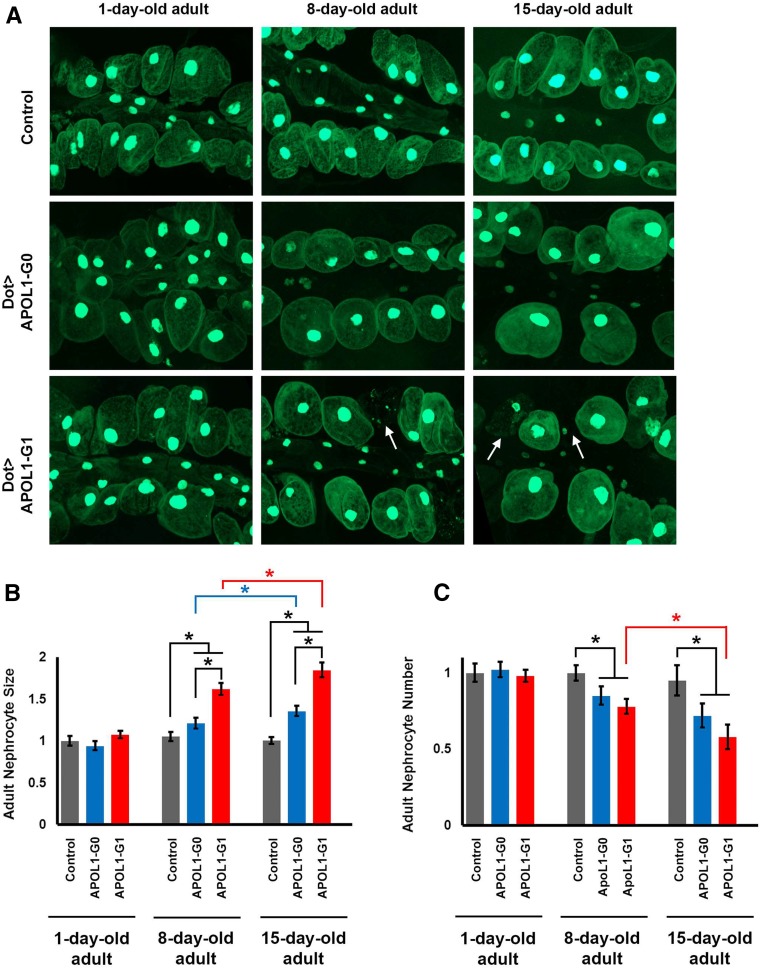
As flies aged, expression of APOL1 led to increased nephrocyte size (Figure 4, A and B). At day 1, nephrocytes expressing either APOL1-G0 or APOL1-G1 were of similar size compared with control flies. By day 8 postemergence, nephrocytes expressing G0 or G1 were significantly larger than controls (which remained unchanged throughout the experiment), and G1 expression induced significantly greater cell enlargement than G0. By day 15 both APOL1 G0 and G1 had induced significantly more hypertrophy relative to day 8, with G1 hypertrophy significantly greater than G0. APOL1-G0 or G1 expression was also associated with age-related reductions in nephrocyte cell numbers (Figure 4C) and, in flies expressing APOL1-G1, accumulation of cell debris (arrows in Figure 4A). Nephrocyte numbers were not significantly different at day 1 among control and APOL1-expressing flies. By day 8, APOL1-G0 and G1 expression both led to equivalent, significant reductions in nephrocyte numbers. By day 15 APOL1-G0 expression was not associated with further significant reductions, but G1 induced significant further nephrocyte losses.
Figure 4.
APOL1 G0 and G1 induced age-related changes in nephrocyte cell size and cell number. (A) Adult fly nephrocytes (and smaller cardiomyocytes) expressing GFP (green, the majority concentrated in nucleus) at days 1, 8, and 15 postemergence. Nephrocytes of control flies (carrying Dot-GAL4 driver without UAS-APOL1 transgene) were unchanged with respect to cell size and cell number from day 1 through day 15. Nephrocyte-specific expression of APOL1-G0 and G1 proteins, by contrast, was associated as flies aged with progressively increased cell size and decreased cell numbers. Arrows indicate cell debris among intact nephrocytes expressing APOL1 G1 at days 8 and 15. (B) Quantification of adult nephrocyte cell size at days 1, 8, and 15 postemergence. Size is indicated relative to day 1 control nephrocytes. G0 and G1 forms of APOL1 were both associated with progressively increased cell size, with G1 inducing a greater degree of cell enlargement. The size of control nephrocytes did not change significantly over the course of the experiment. (C) Quantification of adult nephrocyte cell numbers at days 1, 8, and 15. Cell numbers are indicated relative to control nephrocytes. G0 and G1 forms of APOL1 were both associated with progressively decreased cell numbers, but with G1 inducing greater reduction. The number of control nephrocytes did not change significantly over the course of the experiment. For quantification, ≥20 nephrocytes were analyzed from each of three female flies per genotype. The results are presented as mean±SD. Statistical significance (*) was defined as P<0.05.
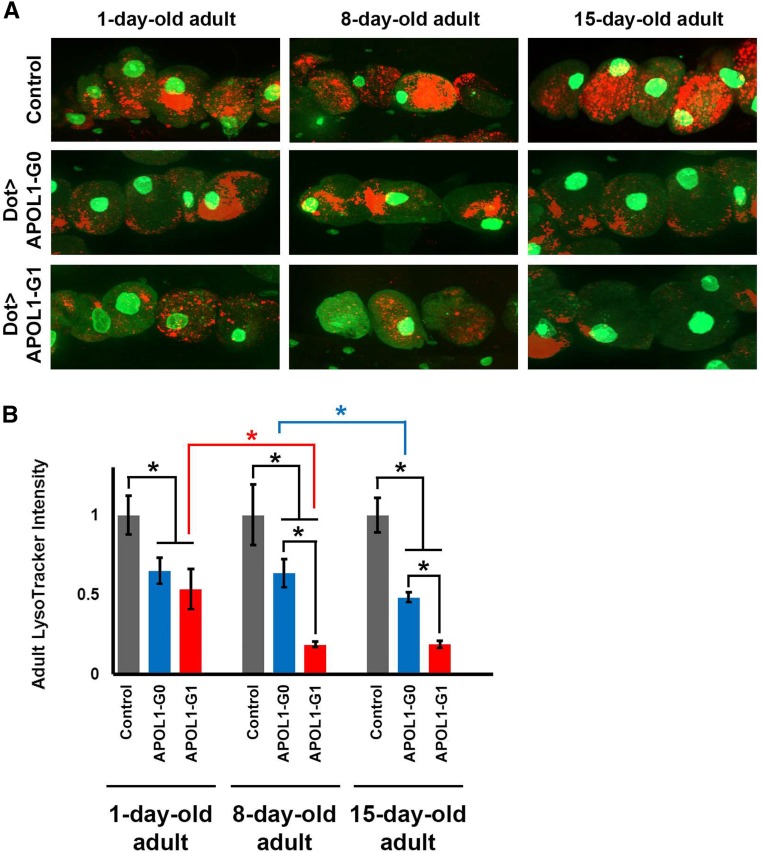
APOL1 Associated with Reduced Intracellular Acidification
We used fluorescent LysoTracker dye to examine the status of acidic vacuoles in adult fly nephrocytes from day 1 through day 15 postemergence. As shown in Figure 5, control nephrocytes exhibited the same levels of LysoTracker fluorescence throughout the 15-day study period. Expression of either APOL1-G0 or G1 was associated with equivalent and significant reductions in LysoTracker fluorescence at day 1 relative to control nephrocytes. At day 8 postemergence, LysoTracker fluorescence in G0-expressing nephrocytes was not significantly changed, but G1 expression was associated with further significant reductions in LysoTracker fluorescence relative to control and G0-expressing nephrocytes. By day 15, LysoTracker fluorescence in G0-expressing nephrocytes had undergone significant further reductions, whereas G1-expressing nephrocytes did not show further significant decrease relative to day 8 levels (Figure 5B). Thus, both APOL1-G0 and G1 expression altered levels of intracellular acidification in nephrocytes, but deacidification occurred more rapidly and more dramatically in nephrocytes expressing APOL1-G1.
Figure 5.
Expression of APOL1 G0 and G1 in nephrocytes impairs the acidification of organelles. (A) Adult fly nephrocytes expressing GFP (green, largely concentrated in the nucleus) at days 1, 8, and 15 postemergence. Nephrocytes of control flies (carrying Dot-GAL4 driver without UAS-APOL1 transgene) exhibited LysoTracker dye fluorescence54 at normal levels throughout the course of the experiment. LysoTracker fluorescence was lower in nephrocytes expressing APOL1 G0 and G1 at day 1. At day 8, APOL1 G1 led to a significantly more dramatic phenotype and remained at a low level through day 15. Further reduction in LysoTracker fluorescence was apparent by day 15 in APOL1 G0–expressing nephrocytes. (B) Quantification of adult nephrocyte LysoTracker dye fluorescence, relative to control nephrocytes, which exhibited stable levels of LysoTracker fluorescence through day 15. At day 1 postemergence, APOL1 G0– and G1-expressing nephrocytes exhibited equivalently reduced LysoTracker fluorescence compared with control cells. By day 8, APOL1 G1–associated fluorescence was reduced to its lowest level, whereas G0-expressing nephrocyte fluorescence was yet unchanged from day 1. By day 15, APOL1 G0–expressing nephrocyte fluorescence had also undergone significant reduction, although to a lesser extent than was exhibited by cells expressing G1 at day 8. For quantification, ≥20 nephrocytes were analyzed from each of three female flies per genotype. The results are presented as mean±SD. Statistical significance (*) was defined as P<0.05.
Discussion
We have developed several transgenic Drosophila lines expressing either the human APOL1 wild type G0 or G1 risk allele in different tissues, including nephrocytes. In this new animal model, we found that APOL1-G1 cloned from podocytes cultured from a child with HIV-associated nephropathy40 induced greater cytotoxicity in several tissues, including nephrocytes, when compared with the nonrisk APOL1-G0 allele. Although ubiquitous expression of either APOL1 allele throughout development led to pupa stage lethality, G1 expression caused earlier lethality. However, tissue-specific expression of APOL1 did not prevent the emergence of viable adult flies, thus permitting the analysis of different tissue-specific phenotypes. In a remarkable manner, we found that when APOL1-G0 or G1 was expressed specifically in nephrocytes, these cells showed first an upregulated endocytic activity, leading to the accumulation of hemolymph proteins. As these flies aged, the function of nephrocytes declined and the nephrocytes showed progressive enlargement and cell death. These findings suggest that nephrocytes overexpressing APOL1 failed to adapt to an increased workload, and developed chronic cytotoxic changes as the cells aged.
The expression of APOL1-G0 in Drosophila cells was associated with developmental, morphologic, and physiologic abnormalities, the underlying mechanisms of which will require extensive future studies. However, a consistent finding of our study was greater severity of observed phenotypes in flies expressing the APOL1-G1 risk allele. For example, APOL1-G1 induced earlier developmental lethality when overexpressed ubiquitously under a strong tubulin-GAL4 driver, and a more severe wing phenotype when expressed under two different wing drivers, vg-GAL4 or ms1096-GAL4. We interpret the abnormal adult wing phenotype as reflecting cell death due to APOL1-induced cytotoxicity during preadult stage development of the wing imaginal disc. Interestingly, these cytotoxic effects are remarkably similar to those previously reported in cultured renal epithelial cells overexpressing APOL1 risk variants.25–27 Taken together, these data suggest that overexpression of G1 in Drosophila cells causes greater cytotoxicity than G0. It is important to note that Drosophila lacks not only endogenous APOL1 but likely also some (or perhaps all) genes encoding factors that in humans associate with APOL1 during transport, podocyte binding, endocytosis, intracellular trafficking, and metabolic processing. Nevertheless, we show here that Drosophila is a model system in which expression of a transgenic APOL1-G1 risk allele induced phenotypes of greater severity than a wild type G0 allele.
In this study, we focused our analysis on the differential changes induced by APOL1-G0 and G1 expression in nephrocytes. As reported in previous studies, these cells share striking similarities in structure and function when compared with mammalian podocytes.32,34 The main function of nephrocytes is to filter hemolymph proteins and hence to endocytose lower mol wt proteins and toxic substances for recycling or sequestration, respectively. We observed that in young adult flies (i.e., 1 day postemergence), nephrocytes expressing APOL1-G1 accumulated more ANF-RFP (a marker of nephrocyte function), AgNO3 (a toxin that is cleared by nephrocytes), and fluorescent dextran (a marker of endocytosis), when compared with flies of similar age that express APOL1-G0, or control flies that did not carry a transgene. As these flies aged, nephrocytes expressing APOL1-G1 became much larger, reaching almost twice the size of the control nephrocytes after 15 days. During this time, however, the number of APOL1-G1–expressing nephrocytes progressively decreased, with concomitant accumulation of cell debris and dead cells. Interestingly, these findings mimic the reduction in podocyte density seen in adult transgenic mice overexpressing APOL1-G2.28 We also found that aging was associated with a progressive reduction in the function of nephrocytes that express APOL1-G1 or G0 alleles, relative to control nephrocytes. For example, by day 8 postemergence, the uptake of fluorescent dextran particles was decreased by approximately 75% relative to controls. Intriguingly, young nephrocytes expressing either APOL1-G1 or G0 alleles exhibited a highly significant reduction of acidic organelles, as determined by LysoTracker dye fluorescence, despite the significant accumulation of ANF-RFP, AgNO3, and fluorescent dextran. Furthermore, the reduced acidification phenotype became progressively more severe in aging flies that carried the G1 risk allele.
We propose that expression of APOL1-G1 in young nephrocytes increased endocytic activity, at the same time impairing organelle acidification. APOL1-G0 induced less dramatic changes. APOL1-G1 expression always led to phenotypes consistent with a gain-of-function mutation. We propose that the increased accumulation of ANF-RFP and AgNO3 in young APOL1-expressing nephrocytes reflects both increased endocytosis and accumulation of hemolymph proteins and toxins, and decreased degradation of proteins due to impaired organelle acidification. Increased endocytosis was demonstrated by increased dextran fluorescence, because short incubation times precluded measuring effects from degradation. Moreover, AgNO3 is sequestered rather than degraded so its accumulation was due to increased endocytosis. We propose that APOL1-induced impairment of organelle acidification subsequently affected degradation, because lysosomal enzymes require low pH. Nephrocyte hypertrophy represented synergy between increased endocytosis and defective acidification. As nephrocytes aged, however, they displayed abnormal enlargement associated with decreased function and further impairment of acidification leading eventually to premature cell death, as reported for cultured human podocytes and renal epithelial cells.27,44 Studies on cultured human embryonic kidney cells raise the possibility that nephrotoxic effects of APOL1 risk variants may be mediated by APOL1-induced net loss of intracellular K+ and activation of stress-activated protein kinase pathways.26 However, overexpression of APOL1 in cultured epithelial cells induced very rapid cell death. Culture conditions may not be sensitive enough to detect the early changes induced by APOL1 expression in vivo. More studies will be needed to confirm this, and to determine how APOL1-G1 increases endocytic activity and impairs acidification.
Endocytosis by podocytes maintains the glomerular filtration barrier.45,46 Human podocytes take up albumin, although the mechanisms involved are not clearly understood.47–49 Studies in rats showed that angiotensin II infusion increased albumin concentration in the subpodocyte space, possibly promoting endocytosis.50 Moreover, excessive endocytosis of albumin by podocytes appears to be a factor underlying progressive renal injury.47 It is thus tempting to speculate that APOL1 is involved in this process, and that the G1 risk variant may somehow impair adaptive processes by enhancing endocytic activity of podocytes under stress, precipitating cellular injury.
The Drosophila model provides a unique system with which to elucidate molecular mechanisms underlying APOL1-induced cytotoxicity in vivo. Overexpression of APOL1 G0 or G1 in nephrocytes first increased cell function, induced hypertrophy, and subsequently impaired acidification of organelles and accelerated cell death. These findings may yield insights into APOL1-associated renal pathogenesis. Moreover, we identified Drosophila phenotypes to exploit in designing genetic screens to identify APOL1-interacting proteins, themselves representing potential therapeutic targets.
Concise Methods
Fly Strains
Flies were reared on standard food at room temperature or 29°C for UAS-GAL4 strains. The following strains were used in this study: Hand-GFP,51 Dot-Gal4,32 MHC-ANF-RFP,32 Hand-Gal4,52 and Dmef2-Gal4;53 and the following lines are from the Bloomington Stock Center: tubulin-Gal4, ms1096-Gal4, vg-Gal4, elav-Gal4, hindgut specific-Gal4, and hml-Gal4.
DNA Cloning and Generation of Transgenic Fly Strains
The wild type APOL1-G0 cDNA was obtained from OriGene, which encodes the common 398 a.a. isoform with GenBank ID AAI43040. The APOL1-G1 cDNA was obtained from a cDNA library generated from podocytes cultured from a child with HIV-associated nephropathy.40 This APOL1-G1 differs from the wild type APOL1-G0 (AAI43040) only at S342 and I384. To generate UAS-APOL1-G0 and UAS-APOL1-G1 constructs, the above cDNAs of APOL1-G0 and G1 alleles were cloned into the pUAST vector and introduced into the germ cells of flies by standard P element–mediated germ line transformation.
qRT-PCR Analysis
RNA was isolated using Trizol Reagent (Invitrogen, Carlsbad, CA) from pupae or adult flies of the relevant genotype. RNA purity and concentration were determined using a Nanodrop-1000 (Thermo Scientific, Wilmington, DE). Total RNA (1 μg) was reverse transcribed using Superscript IV (Invitrogen). SYBR Green–based real-time qPCR (Power Cyber Mastermix; Applied Biosystems, Carlsbad, CA) was performed using a StepOne Plus (Applied Biosystems). Primer pairs specific to APOL1 were used. Quantitative values were determined using the 2−ΔΔCT method,39 normalizing to GAPDH. Values are mean±SD of three separate samples. Results were analyzed by paired t test. Statistical significance was defined as P<0.05.
Western Blotting
For each sample, ten pupae were finely ground by mortar and pestle in 100 μl H2O plus 200 μl SDS-PAGE loading buffer. The ground tissues were then sonicated, followed by centrifugation. The resulting supernatants were subjected to SDS-PAGE, transferred to nitrocellulose filters, and incubated with anti-FLAG antibody according to standard protocols. The experiment was performed in triplicate. The results are presented as mean±SD. Paired t test was used to analyze the data. Statistical significance was defined as P<0.05.
Adult Survival Assay
Within 24 hours of egg laying Drosophila larvae were transferred from 25°C to 29°C to boost UAS-transgene expression. Adult male flies were maintained in vials at 29°C in groups of 15 or fewer. Fifty flies were assayed per genotype.
Developmental Lethal, Wing, and Muscle Phenotype Assessments
To test for developmental lethality, flies of the appropriate genotype were allowed to lay eggs on standard apple juice plates at 25°C for 24 hours. Freshly emerged first instar larvae (n=110–125 per genotype) were transferred to 29°C and allowed to develop. Numbers of surviving third instar larvae, early and late pupae, and emerging adults were recorded. Wing posture phenotypes (mef2-Gal4 driver) and abnormal wing vein phenotypes (ms1096-Gal4 and vg-Gal4 drivers) were assessed by visual inspection and removal of wings for microscopic examination using a 5× objective lens. Adult muscle tissue was visualized by phalloidin (Invitrogen) staining.
RFP Uptake Assay
Flies from the MHC-ANF-RFP, Hand-GFP, and Dot-Gal4 transgenic lines were crossed to flies from the UAS-APOL1-G0 or UAS-APOL1-G1 transgenic lines at 25°C.32 One day after egg laying embryos were shifted to 29°C. RFP uptake by pericardial nephrocytes was assessed in adult flies 1 day postemergence by dissecting heart tissues into Drosophila Schneider Medium (Thermo Fisher Scientific) and examining cells by fluorescence microscopy. For quantification, ≥20 nephrocytes were analyzed from each of three female flies per genotype. The results are presented as mean±SD. Paired t test was used to analyze the data. Statistical significance was defined as P<0.05.
AgNO3 Uptake Assay
Flies of the appropriate genotype were allowed to lay eggs on standard apple juice plates for 24 hours. Freshly emerged first instar larvae were transferred to agar-only plates supplemented with regular yeast paste containing AgNO3 (2.0 g yeast in 3.5 ml 0.0005% AgNO3 solution) and allowed to develop at 29°C until adulthood.32 AgNO3 uptake by pericardial nephrocytes was assessed in adult flies 1 day postemergence by dissecting heart tissues into Drosophila Schneider Medium and examining cells by phase-contrast microscopy. For quantification, ≥20 nephrocytes were analyzed from each of three female flies per genotype. The results are presented as mean±SD. Paired t test was used to analyze the data. Statistical significance was defined as P<0.05.
Dextran Uptake Assay
Flies from the Hand-GFP and Dot-Gal4 transgenic lines were crossed to flies from the UAS-APOL1-G0 or UAS-APOL1-G1 transgenic lines at 25°C. One day after egg laying embryos were shifted to 29°C. Dextran uptake by pericardial nephrocytes was assessed ex vivo in adult flies 1 day postemergence by dissecting heart tissues into Drosophila Schneider Medium and examining cells by fluorescence microscopy after 20 minutes incubation with AlexaFluor 568-dextran (10 kD, 0.05 mg/ml). For quantification, ≥20 nephrocytes were analyzed from each of three female flies per genotype. The results are presented as mean±SD. Paired t test was used to analyze the data. Statistical significance was defined as P<0.05.
LysoTracker and Confocal Imaging
Larvae and adult flies were dissected and fixed for 10 minutes in 4% paraformaldehyde in PBS. LysoTracker (Red DND-99 from Thermo Fisher Scientific) was used according to manufacturer instructions. For quantification, ≥20 nephrocytes were analyzed from each of three female flies per genotype. The results are presented as mean±SD. Paired t test was used to analyze the data. Statistical significance was defined as P<0.05. Confocal imaging was performed with a Zeiss ApoTome.2 microscope using a ×20 Plan-Apochromat 0.8 N.A. air objective. For quantitative comparisons of intensities, common settings were chosen to avoid oversaturation. ImageJ Software Version 1.49 was used for image processing.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the Bloomington Stock Center for providing the fly stocks. We thank the Developmental Studies Hybridoma Bank at the University of Iowa for antibodies.
Z.H. is supported by the National Institutes of Health (NIH) R01 grant DK098410. P.E.R. is supported by NIH R01 grants DK49419, DK103564, and DK108368.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Identifying the Intracellular Function of APOL1,” on pages 1008–1011.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016050550/-/DCSupplemental.
References
- 1.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman DJ, Pollak MR: Genetics of kidney failure and the evolving story of APOL1. J Clin Invest 121: 3367–3374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman DJ, Pollak MR: Apolipoprotein L1 and kidney disease in African Americans. Trends Endocrinol Metab 27: 204–215, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limou S, Nelson GW, Kopp JB, Winkler CA: APOL1 kidney risk alleles: Population genetics and disease associations. Adv Chronic Kidney Dis 21: 426–433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen TK, Estrella MM, Parekh RS: The evolving science of apolipoprotein-L1 and kidney disease. Curr Opin Nephrol Hypertens 25: 217–225, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, Nolan DP, Lins L, Van Den Abbeele J, Pays A, Tebabi P, Van Xong H, Jacquet A, Moguilevsky N, Dieu M, Kane JP, De Baetselier P, Brasseur R, Pays E: Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 422: 83–87, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzur S, Rosset S, Skorecki K, Wasser WG: APOL1 allelic variants are associated with lower age of dialysis initiation and thereby increased dialysis vintage in African and Hispanic Americans with non-diabetic end-stage kidney disease. Nephrol Dial Transplant 27: 1498–1505, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Ulasi II, Tzur S, Wasser WG, Shemer R, Kruzel E, Feigin E, Ijoma CK, Onodugo OD, Okoye JU, Arodiwe EB, Ifebunandu NA, Chukwuka CJ, Onyedum CC, Ijoma UN, Nna E, Onuigbo M, Rosset S, Skorecki K: High population frequencies of APOL1 risk variants are associated with increased prevalence of non-diabetic chronic kidney disease in the Igbo people from south-eastern Nigeria. Nephron Clin Pract 123: 123–128, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Thomson R, Genovese G, Canon C, Kovacsics D, Higgins MK, Carrington M, Winkler CA, Kopp J, Rotimi C, Adeyemo A, Doumatey A, Ayodo G, Alper SL, Pollak MR, Friedman DJ, Raper J: Evolution of the primate trypanolytic factor APOL1. Proc Natl Acad Sci USA 111: E2130–E2139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, Hurles ME, McVean GA; 1000 Genomes Project Consortium : A map of human genome variation from population-scale sequencing. Nature 467: 1061–1073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen CP, Beggs ML, Saeed M, Walker PD: Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 24: 722–725, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, Birmingham DJ, Hebert LA, Hicks PJ, Segal MS, Edberg JC, Brown EE, Alarcón GS, Costenbader KH, Comeau ME, Criswell LA, Harley JB, James JA, Kamen DL, Lim SS, Merrill JT, Sivils KL, Niewold TB, Patel NM, Petri M, Ramsey-Goldman R, Reveille JD, Salmon JE, Tsao BP, Gibson KL, Byers JR, Vinnikova AK, Lea JP, Julian BA, Kimberly RP; Lupus Nephritis–End‐Stage Renal Disease Consortium : End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol 66: 390–396, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashley-Koch AE, Okocha EC, Garrett ME, Soldano K, De Castro LM, Jonassaint JC, Orringer EP, Eckman JR, Telen MJ: MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br J Haematol 155: 386–394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB; SK Investigators : Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, Winkler CA, Bowden DW, Pollak MR: The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 21: 1422–1426, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bostrom MA, Kao WH, Li M, Abboud HE, Adler SG, Iyengar SK, Kimmel PL, Hanson RL, Nicholas SB, Rasooly RS, Sedor JR, Coresh J, Kohn OF, Leehey DJ, Thornley-Brown D, Bottinger EP, Lipkowitz MS, Meoni LA, Klag MJ, Lu L, Hicks PJ, Langefeld CD, Parekh RS, Bowden DW, Freedman BI; Family Investigation of Nephropathy and Diabetes (FIND) Research Group : Genetic association and gene-gene interaction analyses in African American dialysis patients with nondiabetic nephropathy. Am J Kidney Dis 59: 210–221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR: APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol 22: 2119–2128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma L, Shelness GS, Snipes JA, Murea M, Antinozzi PA, Cheng D, Saleem MA, Satchell SC, Banas B, Mathieson PW, Kretzler M, Hemal AK, Rudel LL, Petrovic S, Weckerle A, Pollak MR, Ross MD, Parks JS, Freedman BI: Localization of APOL1 protein and mRNA in the human kidney: Nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol 26: 339–348, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee BT, Kumar V, Williams TA, Abdi R, Bernhardy A, Dyer C, Conte S, Genovese G, Ross MD, Friedman DJ, Gaston R, Milford E, Pollak MR, Chandraker A: The APOL1 genotype of African American kidney transplant recipients does not impact 5-year allograft survival. Am. J. Transplant. 12: 1924–1928, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman BI, Julian BA, Pastan SO, Israni AK, Schladt D, Gautreaux MD, Hauptfeld V, Bray RA, Gebel HM, Kirk AD, Gaston RS, Rogers J, Farney AC, Orlando G, Stratta RJ, Mohan S, Ma L, Langefeld CD, Hicks PJ, Palmer ND, Adams PL, Palanisamy A, Reeves-Daniel AM, Divers J: Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. Am. J. Transplant. 15: 1615–1622, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, Langefeld CD, Bowden DW, Hicks PJ, Stratta RJ, Lin JJ, Kiger DF, Gautreaux MD, Divers J, Freedman BI: The APOL1 gene and allograft survival after kidney transplantation. Am. J. Transplant. 11: 1025–1030, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedman BI, Pastan SO, Israni AK, Schladt D, Julian BA, Gautreaux MD, Hauptfeld V, Bray RA, Gebel HM, Kirk AD, Gaston RS, Rogers J, Farney AC, Orlando G, Stratta RJ, Mohan S, Ma L, Langefeld CD, Bowden DW, Hicks PJ, Palmer ND, Palanisamy A, Reeves-Daniel AM, Brown WM, Divers J: APOL1 genotype and kidney transplantation outcomes from deceased African American donors. Transplantation 100: 194–202, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khatua AK, Cheatham AM, Kruzel ED, Singhal PC, Skorecki K, Popik W: Exon 4-encoded sequence is a major determinant of cytotoxicity of apolipoprotein L1. Am J Physiol Cell Physiol 309: C22–C37, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olabisi OA, Zhang JY, VerPlank L, Zahler N, DiBartolo S 3rd , Heneghan JF, Schlöndorff JS, Suh JH, Yan P, Alper SL, Friedman DJ, Pollak MR: APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci USA 113: 830–837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lan X, Jhaveri A, Cheng K, Wen H, Saleem MA, Mathieson PW, Mikulak J, Aviram S, Malhotra A, Skorecki K, Singhal PC: APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. Am J Physiol Renal Physiol 307: F326–F336, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruggeman LA, Wu Z, Luo L, Madhavan SM, Konieczkowski M, Drawz PE, Thomas DB, Barisoni L, Sedor JR, O’Toole JF: APOL1-G0 or APOL1-G2 transgenic models develop preeclampsia but not kidney disease [published online ahead of print March 29, 2016]. J Am Soc Nephrol doi: 10.1681/ASN.2015111220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashraf S, Gee HY, Woerner S, Xie LX, Vega-Warner V, Lovric S, Fang H, Song X, Cattran DC, Avila-Casado C, Paterson AD, Nitschké P, Bole-Feysot C, Cochat P, Esteve-Rudd J, Haberberger B, Allen SJ, Zhou W, Airik R, Otto EA, Barua M, Al-Hamed MH, Kari JA, Evans J, Bierzynska A, Saleem MA, Böckenhauer D, Kleta R, El Desoky S, Hacihamdioglu DO, Gok F, Washburn J, Wiggins RC, Choi M, Lifton RP, Levy S, Han Z, Salviati L, Prokisch H, Williams DS, Pollak M, Clarke CF, Pei Y, Antignac C, Hildebrandt F: ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J Clin Invest 123: 5179–5189, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gee HY, Saisawat P, Ashraf S, Hurd TW, Vega-Warner V, Fang H, Beck BB, Gribouval O, Zhou W, Diaz KA, Natarajan S, Wiggins RC, Lovric S, Chernin G, Schoeb DS, Ovunc B, Frishberg Y, Soliman NA, Fathy HM, Goebel H, Hoefele J, Weber LT, Innis JW, Faul C, Han Z, Washburn J, Antignac C, Levy S, Otto EA, Hildebrandt F: ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest 123: 3243–3253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gee HY, Zhang F, Ashraf S, Kohl S, Sadowski CE, Vega-Warner V, Zhou W, Lovric S, Fang H, Nettleton M, Zhu JY, Hoefele J, Weber LT, Podracka L, Boor A, Fehrenbach H, Innis JW, Washburn J, Levy S, Lifton RP, Otto EA, Han Z, Hildebrandt F: KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J Clin Invest 125: 2375–2384, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang F, Zhao Y, Han Z: An in vivo functional analysis system for renal gene discovery in Drosophila pericardial nephrocytes. J Am Soc Nephrol 24: 191–197, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang F, Zhao Y, Chao Y, Muir K, Han Z: Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J Am Soc Nephrol 24: 209–216, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, Ruiz-Gómez M, Skaer H, Denholm B: The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simons M, Huber TB: Flying podocytes. Kidney Int 75: 455–457, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Na J, Cagan R: The Drosophila nephrocyte: Back on stage. J Am Soc Nephrol 24: 161–163, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Zhuang S, Shao H, Guo F, Trimble R, Pearce E, Abmayr SM: Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 136: 2335–2344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cagan RL: The Drosophila nephrocyte. Curr Opin Nephrol Hypertens 20: 409–415, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Xie X, Colberg-Poley AM, Das JR, Li J, Zhang A, Tang P, Jerebtsova M, Gutkind JS, Ray PE: The basic domain of HIV-tat transactivating protein is essential for its targeting to lipid rafts and regulating fibroblast growth factor-2 signaling in podocytes isolated from children with HIV-1-associated nephropathy. J Am Soc Nephrol 25: 1800–1813, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brand AH, Perrimon N: Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Gullaud M, Delanoue R, Silber J: A Drosophila model to study the functions of TWIST orthologs in apoptosis and proliferation. Cell Death Differ 10: 641–651, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Esposito T, Lea RA, Maher BH, Moses D, Cox HC, Magliocca S, Angius A, Nyholt DR, Titus T, Kay T, Gray NA, Rastaldi MP, Parnham A, Gianfrancesco F, Griffiths LR: Unique X-linked familial FSGS with co-segregating heart block disorder is associated with a mutation in the NXF5 gene. Hum Mol Genet 22: 3654–3666, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Lan X, Wen H, Lederman R, Malhotra A, Mikulak J, Popik W, Skorecki K, Singhal PC: Protein domains of APOL1 and its risk variants. Exp Mol Pathol 99: 139–144, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS: Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci USA 105: 967–972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soda K, Ishibe S: The function of endocytosis in podocytes. Curr Opin Nephrol Hypertens 22: 432–438, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schieppati A, Remuzzi G: The June 2003 Barry M. Brenner Comgan lecture. The future of renoprotection: Frustration and promises. Kidney Int 64: 1947–1955, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Okamura K, Dummer P, Kopp J, Qiu L, Levi M, Faubel S, Blaine J: Endocytosis of albumin by podocytes elicits an inflammatory response and induces apoptotic cell death. PLoS One 8: e54817, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mallipattu SK, He JC: A new mechanism for albuminuria-induced podocyte injury. J Am Soc Nephrol 24: 1709–1711, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schießl IM, Hammer A, Kattler V, Gess B, Theilig F, Witzgall R, Castrop H: Intravital imaging reveals angiotensin II-induced transcytosis of albumin by podocytes. J Am Soc Nephrol 27: 731–744, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han Z, Olson EN: Hand is a direct target of Tinman and GATA factors during Drosophila cardiogenesis and hematopoiesis. Development 132: 3525–3536, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Han Z, Yi P, Li X, Olson EN: Hand, an evolutionarily conserved bHLH transcription factor required for Drosophila cardiogenesis and hematopoiesis. Development 133: 1175–1182, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Yi P, Han Z, Li X, Olson EN: The mevalonate pathway controls heart formation in Drosophila by isoprenylation of Ggamma1. Science 313: 1301–1303, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Schell C, Baumhakl L, Salou S, Conzelmann AC, Meyer C, Helmstädter M, Wrede C, Grahammer F, Eimer S, Kerjaschki D, Walz G, Snapper S, Huber TB: N-wasp is required for stabilization of podocyte foot processes. J Am Soc Nephrol 24: 713–721, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS: CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300: 1298–1300, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.