Abstract
IgA nephropathy frequently leads to progressive CKD. Although interest surrounds use of immunosuppressive agents added to standard therapy, several recent studies have questioned efficacy of these agents. Depleting antibody–producing B cells potentially offers a new therapy. In this open label, multicenter study conducted over 1-year follow-up, we randomized 34 adult patients with biopsy–proven IgA nephropathy and proteinuria >1 g/d, maintained on angiotensin–converting enzyme inhibitors or angiotensin receptor blockers with well controlled BP and eGFR<90 ml/min per 1.73 m2, to receive standard therapy or rituximab with standard therapy. Primary outcome measures included change in proteinuria and change in eGFR. Median baseline serum creatinine level (range) was 1.4 (0.8–2.4) mg/dl, and proteinuria was 2.1 (0.6–5.3) g/d. Treatment with rituximab depleted B cells and was well tolerated. eGFR did not change in either group. Rituximab did not alter the level of proteinuria compared with that at baseline or in the control group; three patients in each group had ≥50% reduction in level of proteinuria. Serum levels of galactose-deficient IgA1 or antibodies against galactose-deficient IgA1 did not change. In this trial, rituximab therapy did not significantly improve renal function or proteinuria assessed over 1 year. Although rituximab effectively depleted B cells, it failed to reduce serum levels of galactose-deficient IgA1 and antigalactose–deficient IgA1 antibodies. Lack of efficacy of rituximab, at least at this stage and severity of IgA nephropathy, may reflect a failure of rituximab to reduce levels of specific antibodies assigned salient pathogenetic roles in IgA nephropathy.
Keywords: IgA nephropathy, proteinuria, rituximab
IgA nephropathy (IgAN) is the most common primary glomerular disease in the world.1,2 Among patients with reduced renal function and proteinuria >1 g/24 h, outcomes remain poor. Up to 50% of such patients will progress to ESRD over 10 years.3,4 Trials have established the benefit of agents that antagonize the renin-angiotensin-aldosterone system (RAAS) in reducing or delaying progression,5,6 but even patients treated with RAAS blockade still face deterioration of their kidney function over time.
The presence of inflammation on histologic examination of the kidney in IgAN along with the presence of immune complexes in the kidney suggest that immunosuppression would be beneficial in this disease. Indeed, many small studies have suggested that corticosteroid therapy may be effective in attenuating proteinuria and retarding progression, but they are attended by risks for serious adverse events.7–9 However, a recent highly publicized study questioned the efficacy of corticosteroids and other immunosuppression in IgAN.10 In this study, compared with the control group (without immunosuppression), the rate of decrease of eGFR was not reduced in patients treated with corticosteroids alone (patients with eGFR≥60 ml/min) or those who were treated with corticosteroids and cyclophosphamide for 3 months followed by corticosteroids and azathioprine thereafter (patients with eGFR between 30 and 60 ml/min per 1.73 m2). Similarly, immunosuppressive therapy with other approaches, such as mycophenolate mofetil, has yielded conflicting findings with regards to efficacy in retarding progressive disease.11,12 Thus, there remains a strong need to find better, safer therapies for this disease.
A potentially novel therapy is suggested on the basis of the current understanding of the pathogenesis of IgAN. A key event is the development of IgG and/or IgA autoantibodies against polymeric galactose–deficient IgA1 (Gd-IgA1), the levels of which are elevated in the circulation of most patients with IgAN.13,14 Increased circulatory levels of autoantigen or autoantibody levels correlate with increased risk of progression.15,16 Because B cell–depleting therapies are now known to be effective in many renal diseases mediated by the presence of autoantibodies (for example, membranous nephropathy, lupus nephritis, and ANCA-associated vasculitis),17–19 such an approach is appealing in IgAN, because it would deplete antibody–producing B cells and presumably, the autoantibodies that drive progression of this CKD. Indeed, isolated case reports support this approach, because rituximab, which depletes CD20–bearing B cells, reportedly improved renal function and reduced proteinuria in individuals with IgAN or the associated systemic disease, Henoch–Schönlein purpura with nephritis (HSPN).20,21
We conducted an open label, multicenter, randomized, controlled study to formally assess the hypothesis that B cell depletion with rituximab, added to the standard of care, would decrease the production of IgG anti–Gd-IgA1 autoantibodies and reduce immune complex formation and glomerular inflammation, leading thereby to less proteinuria and renal dysfunction in patients with IgAN at high risk of progression.
Results
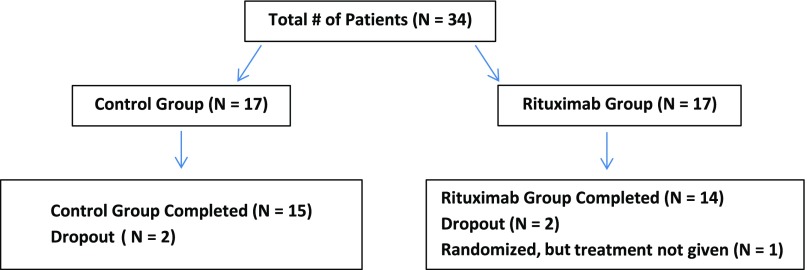
Thirty-four patients met inclusion criteria for this study and were randomized, leading to 17 subjects in the rituximab group as well as in the standard care group. Their ultimate disposition is shown in Figure 1. Two patients had HSPN with inactive systemic features and were randomly divided between the two groups. Six of the rituximab and five of the standard care patients had received prior corticosteroids. Baseline characteristics are detailed in Table 1. All subjects had biopsy-proven IgAN, with <50% glomerular sclerosis or interstitial fibrosis. All patients were maintained on RAAS inhibitors, with five patients on combined therapy with angiotensin–converting enzyme inhibitor and angiotensin receptor blocker treatment, three in the rituximab arm and two in the conservative group. BP was well controlled, averaging 122/79±10/11 mmHg. The median baseline serum creatinine concentration was 1.4 (0.8–2.4) mg/dl. Randomization resulted in a higher but not statistically significant baseline serum creatinine concentration in the rituximab group compared with the standard care group (1.7 [0.8–2.3] mg/dl versus 1.3 [0.8–2.4] mg/dl, respectively), but the resultant eGFR was significantly lower in the rituximab group (Table 1). The baseline proteinuria of all subjects was 2.1 (range =0.6–5.3) g/d, and the difference between the rituximab group and the standard care group was not statistically significant, although it trended higher in the rituximab group (2.6 versus 1.7 g/d). There was no difference in components of the Oxford classification score22 between the two groups (rituximab group versus standard care group: M1, 100% versus 94%; E1, 41% versus 24%; S1, 82% versus 88%; T1–T2, 35% versus 50%, respectively; all nonsignificant) (Table 1). The standard care group was significantly younger and had a greater percentage of men than women. Two subjects in the standard care group dropped out as did three in the rituximab group, such that 15 subjects completed the study in the standard care group and 14 completed it in the rituximab group; all 16 rituximab-treated subjects had post-treatment data for the intention-to-treat analysis (they received their first round of rituximab).
Figure 1.
Trial organization. Patients were randomly assigned, in a 1:1 ratio, to rituximab and control groups. No patient was withdrawn from the study due to an adverse event.
Table 1.
Baseline characteristics
| Baseline Characteristics | All Patients, n=34 | Control, n=17 | Rituximab, n=17 | P Value |
|---|---|---|---|---|
| Age, yr | 40 (21–63) | 33 (21–59) | 43 (29–63) | 0.04 |
| Sex, women, men | 9, 25 | 2, 15 | 7, 10 | 0.12 |
| Weight, kg | 89.7 (57–120) | 92.3 (57–119) | 85.1 (61–120) | 0.46 |
| Race | 0.07 | |||
| White | 24 | 15 | 9 | 0.07 |
| Black | 1 | 0 | 1 | 0.07 |
| Asian/Pacific Islanders | 6 | 2 | 4 | 0.07 |
| Hispanic/Latino | 3 | 0 | 3 | 0.07 |
| Systolic BP | 121 (102–147) | 118 (102–147) | 124 (107–144) | 0.17 |
| Diastolic BP | 78 (56–106) | 78 (56–106) | 81 (59–94) | 0.24 |
| Creatinine, mg/dl | 1.4 (0.8–2.4) | 1.3 (0.8–2.4) | 1.7 (0.8–2.3) | 0.07 |
| eGFR | 49 (30–122) | 61 (32–122) | 40 (30–78) | 0.02 |
| 24-h Proteinuria, g | 2.1 (0.6–5.3) | 1.7 (0.6–4.0) | 2.6 (0.9–5.3) | 0.09 |
| Oxford pathology score, % | ||||
| M0/M1 | 3/97 | 6/94 | 0/100 | 0.30 |
| E0/E1 | 68/32 | 76/24 | 59/41 | 0.30 |
| S0/S1 | 15/85 | 12/88 | 18/82 | 0.60 |
| T0/T1/T2 | 56/41/3 | 53/47/0 | 65/29/6 | 0.30 |
Data presented as median and range.
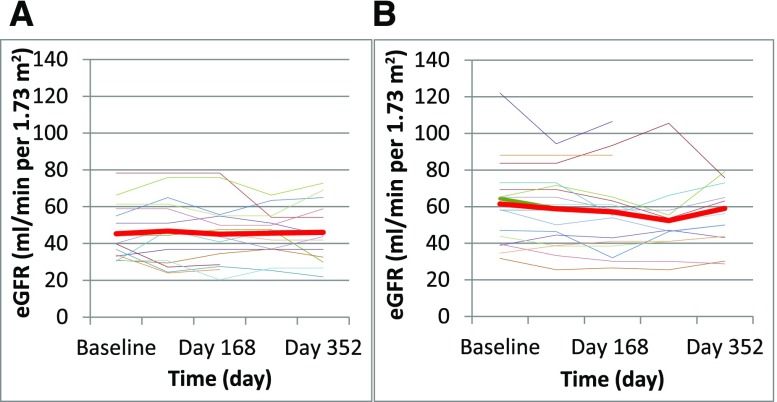
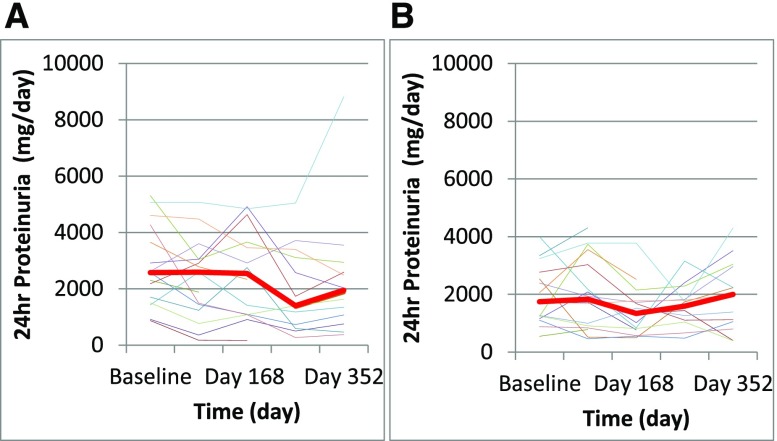
BP was stable and well controlled throughout the study, and it was consistently comparable between treatment groups. Kidney function was also stable throughout the study in both groups, whether assessed by eGFR or serum creatinine concentration (Figure 2, Table 1). The other primary outcome, proteinuria at 12 months, was not changed from baseline in either group. In the rituximab group, median proteinuria decreased from 2.6 (range =0.9–5.3) g/d to 1.9 (range =0.4–8.8) g/d, but this change was not statistically significant (P=0.30). The final proteinuria in the rituximab group was not different from the proteinuria in the standard care group, which was unchanged from baseline: 1.8 (range =0.5–4.0) g/d to 1.8 (range =0.4–4.3) g/d (Figure 3). There was no statistical difference found using absolute level of quantitative proteinuria or the change of proteinuria during the study period. Three subjects in each group experienced a >50% reduction in proteinuria from baseline. Six subjects in the rituximab group and four in the control group had a >25% reduction in proteinuria.
Figure 2.
eGFR trends in (A) rituximab versus (B) control groups. The red line represents average data.
Figure 3.
Proteinuria trends in (A) rituximab versus (B) control groups. The red line represents median data.
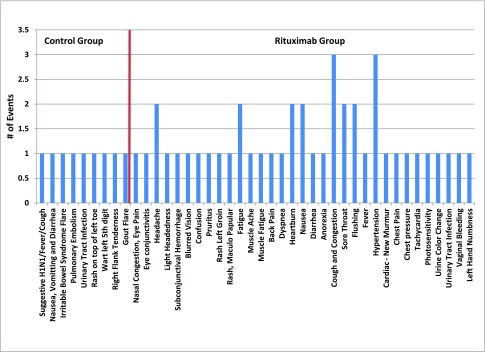
As expected, rituximab depleted B cells as measured 6 months into the study. Peripheral blood B cells (CD19+) decreased from a median of 84 (range =41–222) cells per microliter prerituximab to 0 (range =0–45) cells per microliter postrituximab (only one patient exhibited partial depletion of CD19+ B cells to 45 and 68 cells per microliter at 6 and 12 months, respectively). CD19+ B cells depletion was sustained at 12 months. There was no significant effect on serum Ig levels, including total IgA levels (Table 2). There were no differences in serum levels of Gd-IgA1 or anti–Gd-IgA1 autoantibodies in the rituximab compared with control group at baseline or during the study. The serum levels did not change during observation. There was no correlation between changes in serum levels of Gd-IgA1 or anti–Gd-IgA1 autoantibody and proteinuria during the study (data not shown). There was no serious adverse event in either group during the study, although adverse events per subject were more common in the rituximab group, including mild infections (Figure 4).
Table 2.
Disease markers and outcomes in control versus rituximab treatment groups
| Markers and Outcomes | Baseline | End of Study | ||||
|---|---|---|---|---|---|---|
| Control, n=11 | Rituximab, n=11 | P Value | Control, n=10 | Rituximab, n=10 | P Value | |
| Biochemical markersa | ||||||
| IgA, mg/ml | 4.7±2.0 | 5.0±1.8 | 0.68 | 4.6±1.9 | 4.4±1.4 | 0.77 |
| Gd-IgA1, per 100 ng IgA, U | 56.8±6.9 | 54.8±10.0 | 0.58 | 58.9±5.6 | 60.5±13.0 | 0.73 |
| IgG autoantibodies, U/ml | 858.4±647.3 | 1492.5±1672.9 | 0.25 | 1075±908.7 | 1751.3±2469.4 | 0.42 |
| Outcomes, n | ||||||
| Patients with >50% reduction in proteinuria | — | — | — | 3 | 3 | >0.99 |
| Patients with >50% increase in proteinuria | — | — | — | 2 | 1 | 0.54 |
| Patients with <500 mg/d protein | — | — | — | 2 | 2 | >0.99 |
—, not applicable.
Data presented as mean ±SD.
Figure 4.
Adverse events in the control group and the rituximab group. Type of event and number of each of these events are depicted, with the control group to the left of the red line and the rituximab group to the right of the red line.
Discussion
Our study enrolled 34 subjects with IgAN who were likely to progress (on the basis of decreased eGFR and substantial proteinuria), assigning 17 to treatment with rituximab and resulting in 16 receiving therapy and having outcome data. At baseline, there were no differences in 24-hour proteinuria or serum creatinine concentration between the active treatment group and control group treated with diet, RAAS blockade, and fish oil. Although there was a trend toward lower proteinuria after rituximab treatment, neither group experienced ;a significant reduction in proteinuria, and a significant number of individuals did not manifest a substantial, sustained reduction in proteinuria or improvement in renal function. There were equal numbers of subjects achieving a 50% or greater reduction in proteinuria (three of 16 in the rituximab arm and three of 15 in the standard therapy arm) and similar numbers of subjects achieving a 25% or greater reduction (six of 16 rituximab and four of 15 standard care). There was no significant difference in kidney function over the 1 year of follow-up. Two rituximab subjects and one standard care subject had a 25% or greater increase in eGFR, and one rituximab subject and no standard care subjects had a >25% reduction in eGFR. Thus, over the time course studied and for this stage and severity of IgAN, treatment with rituximab failed to significantly reduce proteinuria or benefit renal function.
We believe that these negative findings with regards to the efficacy of rituximab are important for several reasons. First, these data add another type of immunosuppression to the current list of such agents wherein there is no clear evidence of therapeutic efficacy. Second, our findings call for circumspection and restraint in considering the use of this agent in patients with IgAN at this stage of the disease who exhibit increasing proteinuria and/or decline in eGFR, despite conservative therapy and/or other immunosuppressive regimens. Third, because rituximab is not without significant adverse effects and risks, the current failure to observe a beneficial effect at this stage of the disease argues against its use in this setting, thereby sparing patients from unjustified risks.
In our study, we confirmed that rituximab achieved its stated objective of effectively depleting CD19+ B cells, the latter considered, in earlier studies, a primary producer of IgA and a contributor to the pathogenesis of IgAN.23 We also assessed the effect of rituximab on serum levels of Gd-IgA1 and anti–Gd-IgA1 IgG autoantibodies and found, much to our surprise, that levels of neither were reduced. Serum Gd-IgA1 levels are known to be genetically codetermined,24,25 although the levels can be further enhanced by some cytokines).26 Thus, it is not clear whether a B cell depletion protocol that would significantly reduce serum levels of Igs, including Gd-IgA1, would have a long-lasting effect on serum Gd-IgA1 levels. We regard the absence of reduction in the autoantibody production as notable for at least two reasons that center on pathogenetic and therapeutic considerations. The pathogenesis of IgAN conforms to a four-hit process: (1) circulating levels of Gd-IgA1 are increased, (2) anti–Gd-IgA1 autoantibodies are produced, (3) complexes containing the two are formed in the systemic circulation, and (4) deposition of these complexes in the glomerulus ensues, leading to glomerular inflammation and injury.13,14,27 The failure of rituximab to reduce the serum levels of the anti–Gd-IgA1 autoantibodies may thus account for the absence of a beneficial effect of rituximab on the course of IgAN at this stage. Another consideration is that increased circulating levels of anti–Gd-IgA1 IgG autoantibodies may originate from plasma cells in bone marrow. Plasma cells are derived from B cells but in the process, have lost CD19 expression. It is thus possible that our failure to observe efficacy of rituximab may reflect that, although CD19+ B cells are effectively depleted by rituximab, plasma cells are not, and it is these latter cells, rather than the CD19+ expressing B cells, that account for production of antiglycan antibodies. This consideration may also be germane to other antibody–driven nephropathies in which rituximab use is considered: if CD19+ plasma cells are the source of such antibodies, rituximab may not be effective. There is a similarity of our results with those showing an ineffectiveness of rituximab to induce remission in patients with moderately active ulcerative colitis who had not responded to oral corticosteroids.28 Both IgAN and ulcerative colitis display a prominent involvement of the mucosal immune system, and B cell depletion therapy does not seem to provide long-lasting effects. In contrast, patients with rheumatoid arthritis or primary membranous nephropathy benefit from B cell depletion therapy.18,19 These disease-specific and divergent effects thus raise the following questions. Is it the significant role of mucosal immunity in IgAN and ulcerative colitis that renders B cell depletion therapy less effective? Are the autoantibodies in patients with primary membranous nephropathy of systemic origin, and thus, are the antigen-specific cells in these subjects an easier target of rituximab? As a recent review concluded, we have much more to learn about B cell biology to be able to design optimal approaches to test for therapeutic response to B cell–targeted agents.29
This trial is limited by the relatively small numbers of patients studied. However, we wish to emphasize that this is the first randomized, controlled study that prospectively evaluated the use of B cell depletion added to standard care in patients with IgAN. Moreover, given the nature of IgAN, this study serves as a relatively large series of patients treated with biopsy-defined disease who were given full doses of rituximab, resulting in effective B cell depletion. The presence of a control group allows us to conclude that results were not different in the rituximab group than in the standard care group. It is impossible to rule out individual responses, but if present, they were the exception rather than the rule. Our experience is also limited in that our patients receiving rituximab had significant proteinuria and abnormal kidney function, perhaps making it too late in their disease to respond to any immunotherapy, including B cell depletion. Patients in the rituximab group were older and had lower eGFR at baseline (by 21 ml/min per 1.73 m2), more than expected by the age difference. This finding suggests that the disease was of longer duration in the rituximab group, which may have biased the outcomes, despite equal Oxford MEST scores at baseline. However, all patients had biopsies within 2 years before treatment, and those with advanced glomerulosclerosis or interstitial fibrosis (>50%) were excluded. Two patients with HSPN were included, but their outcomes did not influence the results. The rituximab group was more racially and ethnically diverse, but an analysis, restricted to white patients only also revealed an absence of an effect of rituximab on proteinuria and eGFR (data not shown). Thus, because reduction in proteinuria is an important determinant in outcome,30 if there were signals of benefit in this population, we should have been alerted by this trial. The implications of the numerical, not statistically significant, fall in proteinuria in the rituximab-treated group merit comment. To achieve 80% power for any significant reduction in proteinuria to be possibly observed, our results show that a randomized study comparing the change in proteinuria for rituximab with control would require 272 patients. The need to treat such a large number of patients for any statistical change in proteinuria to be evinced thus seriously questions whether any meaningful pathogenetic and/or therapeutic importance can be applied to this numerical reduction in proteinuria observed in the rituximab-treated group.
Finally, although the presence of circulatory Gd-IgA1 and IgG autoantibodies to this protein are thought to be central to the pathogenesis and progression of IgAN, our data strikingly show no favorable effect of rituximab on these Igs (either Gd-IgA1 or autoantibodies to Gd-IgA1), supporting the lack of clinical evidence of benefit observed in the study. Because the serum levels of Gd-IgA1 and anti–Gd-IgA1 autoantibodies predict disease progression15,16 and disease recurrence,31 both Gd-IgA1 and the corresponding autoantibodies may be valid candidate biomarkers for assessment of responses to treatment.27
In summary, in patients with IgAN and relatively high risk for progressive renal dysfunction, treatment with rituximab resulted in statistically insignificant reductions in proteinuria or partial remissions that could not be distinguished from the response to supportive therapy alone. These data do not support the use of rituximab for the treatment of IgAN, at least for this stage and severity of the disease. Future studies could consider examining earlier use of B cell depletion in IgAN or longer follow-up periods or focus on other approaches to reduce the progression of renal disease. Finally, if CD19+ cells of B cell lineage, such as plasma cells, are confirmed as the source of autoantigen and autoantibody production in IgAN, it is possible that rituximab may not be effective at any stage and severity for this disease.
Concise Methods
The protocol was approved by the institutional review board of each participating center. The study was registered with clinicaltrials.gov, protocol NCT00498368. Informed consent was obtained before all study procedures, and the study adhered to the Declaration of Helsinki.
Subjects
Adults, ages 18–70 years old, with biopsy-proven IgAN shown within 2 years of enrollment were included. Patients were excluded if their biopsy showed >50% glomerular sclerosis or interstitial fibrosis or >10% glomerular crescents. Baseline Oxford classification score22 was recorded in blinded fashion by an expert renal pathologist (S.S.). eGFR (by Modification of Diet in Renal Disease [MDRD]) or measured creatinine clearance had to be <90 and >30 ml/min per 1.73 m2. To establish continued risk, baseline proteinuria needed to be >1000 mg/d while on stable doses of angiotensin–converting enzyme inhibitor, angiotensin receptor blocker, or renin inhibitor therapy for at least 2 months. However, patients who were on dual therapy with agents that inhibit angiotensin II required a lower proteinuria threshold of >500 mg/d. Baseline BP was controlled to <130/80 mmHg. Patients with secondary forms of IgAN, such as cirrhosis, were excluded, although subjects with HSPN could be included. Patients were excluded if they had previously received rituximab, were receiving other immunosuppressive therapy, or had ever received >6 months of prednisone or other systemic corticosteroid therapy in the past. There was no corticosteroid exposure within 3 months of study initiation. Randomization was done centrally by a random assignment by prefilled envelopes. The study was unblinded as to treatment arm.
Treatment and Follow-Up
Fish oil supplements were required at a minimal dose of 3 g/d. Subjects were randomly assigned to receive rituximab or continue standard care. The study was an open label trial; those assigned to rituximab received a 1-g infusion of rituximab followed by an identical dose 2 weeks later. All patients were premedicated with acetaminophen (1 g) and diphenhydramine HCl (50 mg) by mouth from 30 to 60 minutes before the start of an infusion. Premedication with corticosteroids (10 mg dexamethasone intravenously) was also given 30 minutes before the first infusion of each series of rituximab. They received an identical 2-g course of rituximab 6 months later. Subjects were assessed at least every 3 months or as needed for clinical events. This assessment included physical examination, a questionnaire for adverse events, and measurement of routine hematology, serum chemistry, timed urine protein excretion, and, for those assigned to rituximab, B cell subsets. eGFR was calculated by the four–component MDRD formula using serum creatinine concentration measured in the central laboratory. Follow-up was considered complete at 12 months.
Outcomes
The primary outcome measures were the change in proteinuria and the change in eGFR from baseline to 12 months. The secondary outcome was safety related, comparing overall adverse events and monitoring for potential intervention–specific complications, such as infusion-related reactions, hypogammaglobulinemia, and infections.
Determination of Serum Total IgA and Serum Levels of Gd-IgA1
The concentration of serum total IgA was measured by ELISA.32 Briefly, 96-well plates were coated with goat F(ab′)2 anti–human IgA (Jackson ImmunoResearch Laboratories), blocked with 1% BSA in PBS plus 0.05% Tween 20 (PBS-T), washed, and incubated with serially diluted samples or standard serum with known concentration of IgA. Bound IgA was detected by biotinylated goat F(ab′)2 anti–human IgA (GenWay Biotech Inc.) followed by incubation with horseradish peroxidase–conjugated streptavidin (ExtrAvidin-Peroxidase; Sigma-Aldrich) and developed with the peroxidase chromogenic substrate o-phenylenediamine-H2O2. OD was measured at 490 nm on an EL808 Microplate Reader, and the concentration of IgA in the samples was calculated on the basis of a calibration curve of standard serum.
Gd-IgA1 was measured by lectin ELISA.26,33 Briefly, 96-well plates coated with goat F(ab′)2 anti–human IgA were blocked with 1% BSA in PBS-T, washed, and incubated with serum samples added at dilutions corresponding to 100 ng IgA per well overnight at 4°C. Captured IgA was desialylated using neuraminidase.26 Gd-IgA1 was detected using biotinylated lectin from Helix aspersa (HAA; Sigma-Aldrich) specific for terminal N-acetylgalactosamine and diluted to final concentration of 2 μg/ml in 1% BSA in PBS-T. HAA was detected by ExtrAvidin-Peroxidase and developed with the peroxidase chromogenic substrate o-phenylenediamine-H2O2. OD was measured at 490 nm using an EL808 Microplate Reader. The serum Gd-IgA1 concentration was expressed in units defined as the ratio of OD determined for individual samples and a standard Gd-IgA1 (Ale) myeloma protein; 100 U Gd-IgA1 was defined as the OD of HAA lectin binding to 50 ng standard Gd-IgA1 (Ale).
Determination of Serum Levels of IgG Autoantibody Specific for Gd-IgA1
Serum levels of IgG autoantibody specific for Gd-IgA1 were measured by ELISA34; 96-well plates were coated with 3 μg/ml Gd-IgA1 myeloma protein (Ale). Plates were washed and then blocked with 1% BSA in PBS-T. Samples of serum were diluted 500-fold in PBS. Bound IgG was detected with a biotin–labeled F(ab′)2 fragment of goat IgG anti–human IgG antibody (Invitrogen). ExtrAvidin-Peroxidase was added, and the reaction was developed with the peroxidase chromogenic substrate o-phenylenediamine-H2O2. OD was measured at 490 nm using an EL808 Microplate Reader. Serum levels of IgG autoantibody specific for Gd-IgA1 were expressed in units (1 U IgG autoantibody defined as the OD of 1.0 measured at 490 nm) using a standard recombinant IgG autoantibody specific for Gd-IgA1.34
Statistical Analyses
Data were stored centrally within a secure database. Our preliminary experience (one investigator) with rituximab in IgAN indicated that approximately 80% of patients would achieve a significant reduction in proteinuria. On the basis of the one-half width of a 95% confidence interval, the study targeted 25 patients per arm to achieve 80% power and allowed for an interim analysis after 15 per arm were recruited. Outcome variables were measured as median and range. Primary outcomes of the rituximab and standard care groups were compared using the Wilcoxon rank sum test, rejecting the null hypothesis at a P value of <0.05. Within-group comparisons were done with a Wilcoxon signed rank test, again with a P value <0.05 denoting a significant variance. Fisher exact test and chi-squared analysis were used to measure categorical variables. Between-group analyses were performed according to the principle of intention to treat using all patients who received any dose of rituximab as part of the treatment group.
Disclosures
J.N. and B.A.J. are founders of Reliant Glycosciences, LLC (Birmingham, AL). F.C.F. has received unrestricted grant support from Genentech/Roche, Inc (South San Francisco, CA).
Acknowledgments
This study was an investigator-initiated study sponsored by Genentech/Roche, Inc. and the Fulk Family Foundation.
The abstract was previously published at the American Society of Nephrology Meeting, November 5–8, 2015, San Diego, CA, abstract number 6192.
The sponsors had no role in study design, protocol development, data analysis, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Li LS, Liu ZH: Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int 66: 920–923, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Zaza G, Bernich P, Lupo A; “Triveneto” Register of Renal Biopsies (TVRRB) : Incidence of primary glomerulonephritis in a large North-Eastern Italian area: A 13-year renal biopsy study. Nephrol Dial Transplant 28: 367–372, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Barratt J, Feehally J: IgA nephropathy. J Am Soc Nephrol 16: 2088–2097, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Barbour SJ, Reich HN: Risk stratification of patients with IgA nephropathy. Am J Kidney Dis 59: 865–873, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Boyd JK, Cheung CK, Molyneux K, Feehally J, Barratt J: An update on the pathogenesis and treatment of IgA nephropathy. Kidney Int 81: 833–843, 2012 [DOI] [PubMed] [Google Scholar]
- 6.D’Amico G: Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 36: 227–237, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Chen N: Treatment of progressive IgA nephropathy: An update. Contrib Nephrol 181: 75–83, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F: Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol 15: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, Wang H: Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis 53: 26–32, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JF, Hilgers RD, Floege J; STOP-IgAN Investigators : Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373: 2225–2236, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Tang SC, Tang AW, Wong SS, Leung JC, Ho YW, Lai KN: Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int 77: 543–549, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Hogg RJ, Bay RC, Jennette JC, Sibley R, Kumar S, Fervenza FC, Appel G, Cattran D, Fischer D, Hurley RM, Cerda J, Carter B, Jung B, Hernandez G, Gipson D, Wyatt RJ: Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. Am J Kidney Dis 66: 783–791, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai KN, Tang SCW, Schena FP, Novak J, Tomino Y, Foggo AB, Glassock RJ: IgA nephropathy. Nat Rev Dis Primers 2016 Feb 11: 16001. doi: 10.1038/nrdp.2016.1 [DOI] [PubMed] [Google Scholar]
- 15.Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, Mariat C, Tomino Y, Julian BA, Novak J: Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 23: 1579–1587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao N, Hou P, Lv J, Moldoveanu Z, Li Y, Kiryluk K, Gharavi AG, Novak J, Zhang H: The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int 82: 790–796, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U; RAVE-ITN Research Group : Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221–232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T: Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 350: 2572–2581, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, Hogan MC, Dillon JJ, Hickson LJ, Li X, Cattran DC; Mayo Nephrology Collaborative Group : Rituximab therapy in idiopathic membranous nephropathy: A 2-year study. Clin J Am Soc Nephrol 5: 2188–2198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pindi Sala T, Michot JM, Snanoudj R, Dollat M, Estève E, Marie B, Taoufik Y, Delfraissy JF, Lazure T, Lambotte O: Successful outcome of a corticodependent Henoch-Schönlein purpura adult with rituximab. Case Rep Med 2014: 619218, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnithorne KJ, Atkinson TP, Hinze CH, Nogueira JB, Saeed SA, Askenazi DJ, Beukelman T, Cron RQ: Rituximab therapy for severe refractory chronic Henoch-Schönlein purpura. J Pediatr 155: 136–139, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Yuling H, Ruijing X, Xiang J, Yanping J, Lang C, Li L, Dingping Y, Xinti T, Jingyi L, Zhiqing T, Yongyi B, Bing X, Xinxing W, Youxin J, Fox DA, Lundy SK, Guohua D, Jinquan T: CD19+CD5+ B cells in primary IgA nephropathy. J Am Soc Nephrol 19: 2130–2139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lomax-Browne HJ, Visconti A, Pusey CD, Cook HT, Spector TD, Pickering MC, Falchi M: IgA1 glycosylation is heritable in healthy twins [published online ahead of print June 16, 2016]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, Lifton RP, Mestecky J, Novak J, Julian BA: Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol 19: 1008–1014, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki H, Raska M, Yamada K, Moldoveanu Z, Julian BA, Wyatt RJ, Tomino Y, Gharavi AG, Novak J: Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem 289: 5330–5339, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novak J, Rizk D, Takahashi K, Zhang X, Bian Q, Ueda H, Ueda Y, Reily C, Lai LY, Hao C, Novak L, Huang ZQ, Renfrow MB, Suzuki H, Julian BA: New insights into the pathogenesis of IgA nephropathy. Kidney Dis (Basel) 1: 8–18, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leiper K, Martin K, Ellis A, Subramanian S, Watson AJ, Christmas SE, Howarth D, Campbell F, Rhodes JM: Randomised placebo-controlled trial of rituximab (anti-CD20) in active ulcerative colitis. Gut 60: 1520–1526, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Hoffman W, Lakkis FG, Chalasani G: B cells, antibodies, and more. Clin J Am Soc Nephrol 11: 137–154, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reich HN, Troyanov S, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry : Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Berthelot L, Robert T, Vuiblet V, Tabary T, Braconnier A, Dramé M, Toupance O, Rieu P, Monteiro RC, Touré F: Recurrent IgA nephropathy is predicted by altered glycosylated IgA, autoantibodies and soluble CD89 complexes. Kidney Int 88: 815–822, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, Huang WQ, Anreddy SR, Hall S, Hastings MC, Lau KK, Cook WJ, Novak J: Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 71: 1148–1154, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, Novak L, Julian BA, Tomana M, Wyatt RJ, Edberg JC, Alarcón GS, Kimberly RP, Tomino Y, Mestecky J, Novak J: IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest 118: 629–639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J: Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]