Abstract
An absolute, supraphysiologic elevation in GFR is observed early in the natural history in 10%–67% and 6%–73% of patients with type 1 and type 2 diabetes, respectively. Moreover, at the single-nephron level, diabetes-related renal hemodynamic alterations—as an adaptation to reduction in functional nephron mass and/or in response to prevailing metabolic and (neuro)hormonal stimuli—increase glomerular hydraulic pressure and transcapillary convective flux of ultrafiltrate and macromolecules. This phenomenon, known as glomerular hyperfiltration, classically has been hypothesized to predispose to irreversible nephron damage, thereby contributing to initiation and progression of kidney disease in diabetes. However, dedicated studies with appropriate diagnostic measures and clinically relevant end points are warranted to confirm this assumption. In this review, we summarize the hitherto proposed mechanisms involved in diabetic hyperfiltration, focusing on ultrastructural, vascular, and tubular factors. Furthermore, we review available evidence on the clinical significance of hyperfiltration in diabetes and discuss currently available and emerging interventions that may attenuate this renal hemodynamic abnormality. The revived interest in glomerular hyperfiltration as a prognostic and pathophysiologic factor in diabetes may lead to improved and timely detection of (progressive) kidney disease, and could provide new therapeutic opportunities in alleviating the renal burden in this population.
Keywords: diabetes, diabetic nephropathy, glomerular hyperfiltration, glomerular filtration rate, albuminuria
Driven by the ever-increasing prevalence of diabetes, diabetic kidney disease (DKD) has become the most common cause of CKD, leading to ESRD, cardiovascular events, and premature death in developed and developing countries.1 In order to reduce the onset and progression of DKD, current management focuses on prevention, early identification, and treatment. Diabetes and nephrology guidelines advocate strict glycemic and BP targets, the latter for which renin-angiotensin system (RAS) inhibitors are recommended in diabetes patients with2 and without3 albuminuria. Despite increased efforts that stabilized incidence rates for ESRD attributable to DKD in the United States over the last 5 years, the number of patients with renal impairment due to diabetes is still increasing.4 Therefore, improved and timely strategies are needed.
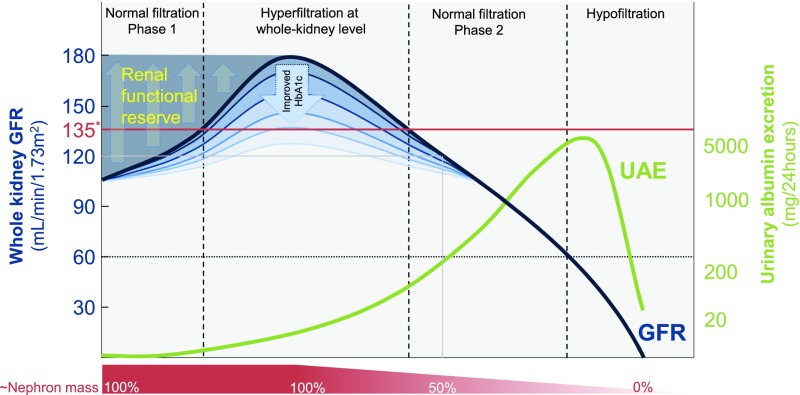
In addition to albuminuria, reduced GFR is a pivotal marker in predicting the risk for ESRD and renal death in diabetes, whereas the role of increased GFR is uncertain. In the classic, five-stage, proteinuric pathway of DKD, the initial phase is characterized by an absolute, supraphysiologic increase in whole-kidney GFR (i.e., the sum of filtration in all functioning nephrons) (Figure 1). This early clinical entity, known as glomerular hyperfiltration, is the resultant of obesity and diabetes-induced changes in structural and dynamic factors that determine GFR.5 Reported prevalences of hyperfiltration at the whole-kidney level vary greatly: between 10% and 67% in type 1 diabetes mellitus (T1DM) (with GFR values up to 162 ml/min per 1.73 m2), and 6%–73% in patients with type 2 diabetes (T2DM) (up to 166 ml/min per 1.73 m2, Table 1). In general, GFR increases by about 27% and 16% in recently diagnosed patients with T1DM6 and T2DM,7 respectively. The prevailing hypothesis is that hyperfiltration in diabetes precedes the onset of albuminuria and/or decline in renal function, and predisposes to progressive nephron damage by increasing glomerular hydraulic pressure (PGLO) and transcapillary convective flux of ultrafiltrate and, although modestly, macromolecules (including albumin). Furthermore, increased GFR in single remnant nephrons—to compensate for reduced nephron numbers8,9 and/or caused by stimuli of the diabetes phenotype—is proposed to accelerate renal function decline in longer-standing diabetes.
Figure 1.
Classic course of whole-kidney GFR and UAE according to the natural (proteinuric) pathway of DKD. Peak GFR may be seen in prediabetes or shortly after diabetes diagnosis, and can reach up to 180 ml/min in the case of two fully intact kidneys. Strict control of HbA1c and initiation of other treatments (such as RAS inhibition) mitigate this initial response. Two normal filtration phases can be encountered, in which GFR may be for instance 120 ml/min (indicated with the gray line): one at 100% of nephron mass and one at approximately 50% of nephron mass. Thus, whole-kidney GFR may remain normal even in the presence of considerable loss of nephron mass, as evidenced by a recent autopsy study.121 Assessing renal functional reserve and/or UAE may help identify the extent of subclinically inflicted loss of functional nephron mass. *Whole-kidney hyperfiltration is generally defined as a GFR that exceeds approximately 135 ml/min, and is indicated with the red line. Heterogeneity of single-nephron filtration rate and nonproteinuric pathway122 of DKD are not illustrated.
Table 1.
Prevalence studies of hyperfiltration in diabetes
| Study Author(s) and Year | N | Diabetes Duration | Baseline HbA1c, % | GFR Method | GFR, ml/min per 1.73 m2 | HF Threshold, ml/min per 1.73 m2 | Prevalence of HF, % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | HF | NH | All | HF | NH | All | HF | NH | |||||
| T1DM | |||||||||||||
| Kalk et al. (1990)131 | 127 | 8 | 6 | 10.8 | 51Cr-EDTA | 129 | 162 | 107 | 135 | 34 | |||
| Azevedo and Gross (1991)132 | 21 | 5 | 7 | 10.1 | 10.7 | 51Cr-EDTA | 156 | 107 | 134 | 48 | |||
| Marre et al. (1992)133 | 50 | 12 | 11 | 9.1 | 8.2 | 51Cr-EDTA | 148 | 111 | 125 | 42 | |||
| Cotroneo et al. (1998)134 | 177 | 51Cr-EDTA | 135 | 56 | |||||||||
| Caramori et al. (1999)135 | 33 | 7 | 51Cr-EDTA | 155 | 108 | 134 | 63 | ||||||
| Dahlquist et al. (2001)136 | 60 | 29 | Inulin | 125 | 50 | ||||||||
| Amin et al. (2005)137 | 308 | 5 | 10.4 | Inulinb | 125 | 67 | |||||||
| Vervoort et al. (2005)138 | 54 | 5 | 8 | 9 | 8.4 | 8.3 | Inulin | 121 | 143 | 114 | 130 | 24 | |
| Steinke et al. (2005)139 | 107 | 8 | 8.6 | Inulin | 142 | 130 | 63 | ||||||
| Ficociello et al. (2009)67 | 426 | 14 | 12 | 14 | 8.6 | 8.1 | eGFR | 155 | 122 | 134 (M)/149 (F)a | 24 | ||
| Thomas et al. (2012)68 | 2318 | 18 | 11 | 19 | 8.8 | 8.2 | eGFR | 125 | 10 | ||||
| Bulum et al. (2013)140 | 313 | eGFR | 125 | 12 | |||||||||
| T2DM | |||||||||||||
| Palmisano and Lebovitz (1989)141 | 72 | 125I-iothalamate | 140 | 25 | |||||||||
| Lebovitz and Palmisano (1990)142 | 71 | 125I-iothalamate | 140 | 35 | |||||||||
| Marre et al. (1992)133 | 19 | 13 | 6 | 6.8 | 7.6 | 51Cr-EDTA | 134 | 108 | 125 | 32 | |||
| Norwack et al. (1992)143 | 16 | 0.5 | 6.5 | Inulin | 133 | 141 | 44 | ||||||
| Vora et al. (1992)144 | 110 | 51Cr-EDTA | 140 | 16 | |||||||||
| Gragnoli et al. (1993)145 | 163 | 99mTc-DTPA | 139 | 6 | |||||||||
| Silveiro et al. (1993)146 | 71 | 7 | 6 | 10.4 | 9.4 | 51Cr-EDTA | 147 | 110 | 137.1 | 21 | |||
| Bruce et al. (1994)147 | 15 | 51Cr-EDTA | 166 | 140 | 73 | ||||||||
| Lee et al. (1995)148 | 284 | 51Cr-EDTA | 140 | 23 | |||||||||
| Silveiro et al. (1996)63 | 32 | 51Cr-EDTA | 137 | 40 | |||||||||
| Keller et al. (1996)149 | 85 | 1 | 9.1 | Inulin | 136 | 131 | 58 | ||||||
| Chaiken et al. (1998)150 | 194 | 125I-iothalamate | 140 | 17 | |||||||||
| Guizar et al. (2001)151 | 28 | 0.3 | 6.2 | 99mTc-DTPA | 140 | 140b | 72 | ||||||
| Premaratne et al. (2005)152 | 662 | 99mTc-DTPA | 130 | 7/17d | |||||||||
| Jin et al. (2006)153 | 93 | 11 | 7 | 8.1 | 7.0 | Iohexol | 141 | 99 | Age-adjustedc | 17 | |||
| Ruggenenti et al. (2012)62 | 600 | 7 | 6 | 7 | 6.2 | 6.7 | 6.1 | Iohexol | 101 | 132 | 96 | 120 | 15 |
| Guo et al. (2016)154 | 3301 | eGFR | 138 | 12 | |||||||||
| T1DM and T2DM | |||||||||||||
| Zhao et al. (2015)155 | 3492 | 8 | 8 | 9.7 | 9.0 | 99mTc-DTPA | 140 | 88 | 129 | 10 | |||
HF, hyperfiltration; NH, nonhyperfiltration; M, males; F, females; 51Cr-EDTA, chromium 51–labeled EDTA; 99mTc-DTPA, 99mTc-labeled diethylenetriaminepenta-acetic acid.
HF definition was sex-specific.
HF was additionally defined as <10% increase in GFR after an acute protein load.
HF was defined as GFR greater than the mean GFR + 1.96 SD of control subjects, after adjustment for age.
Correction for age-related GFR decline increased HF prevalence from 7% to 17%.
This review summarizes proposed factors that underlie hyperfiltration in diabetes, and addresses evidence of this phenomenon as predictor and pathophysiologic factor in DKD. Furthermore, we discuss lifestyle and (emerging) pharmacologic interventions that may attenuate hyperfiltration.
Definition and Measurement
“Whole-Kidney” Hyperfiltration
Although a generally accepted definition is lacking, reported thresholds to define hyperfiltration vary between 130 and 140 ml/min per 1.73 m2 in subjects with two functioning kidneys,10 which corresponds to a renal function that exceeds two SD above mean GFR in healthy individuals.11 Notably, use of any set GFR cutoff does not consider differences between sexes and distinct ethnic populations,10 nephron endowment at birth,12 and age-related GFR decline.10,13 Identification of hyperfiltration in clinical practice and systematic studies is complicated by intra- and interday GFR fluctuations,14,15 and the inaccuracy of available serum creatinine–based GFR estimates.16 As such, the Cockroft–Gault, Modification of Diet in Renal Disease, and Chronic Kidney Disease Epidemiology Collaboration 2009 equations systematically underestimate GFR in diabetes, and progressively more so with increasing GFR.16 This seems due to changes in tubular creatinine secretion in the setting of obesity, hyperglycemia, and hyperfiltration, although high glucose concentrations also lead to overestimation of serum creatinine when the Jaffe reaction is used.16 eGFR on the basis of serum cystatin C is suggested to more accurately reflect renal function in patients with diabetes and normal or elevated GFR.17,18 Nevertheless, renal clearance techniques using inulin, or its more widely used alternative sinistrin, are required for gold standard measurement of GFR.19 However, because inulin and sinistrin require labor-intensive analysis, alternative well recognized, although less accurate, exogenous filtration markers across GFR values are widely used in clinical practice and research, such as (125I-labeled) iothalamate, iohexol, 51Cr-labeled ethylenediaminetetra-acetic acid, and 99mTc-labeled diethylenetriaminepenta-acetic acid.19,20
“Single-Nephron” Hyperfiltration
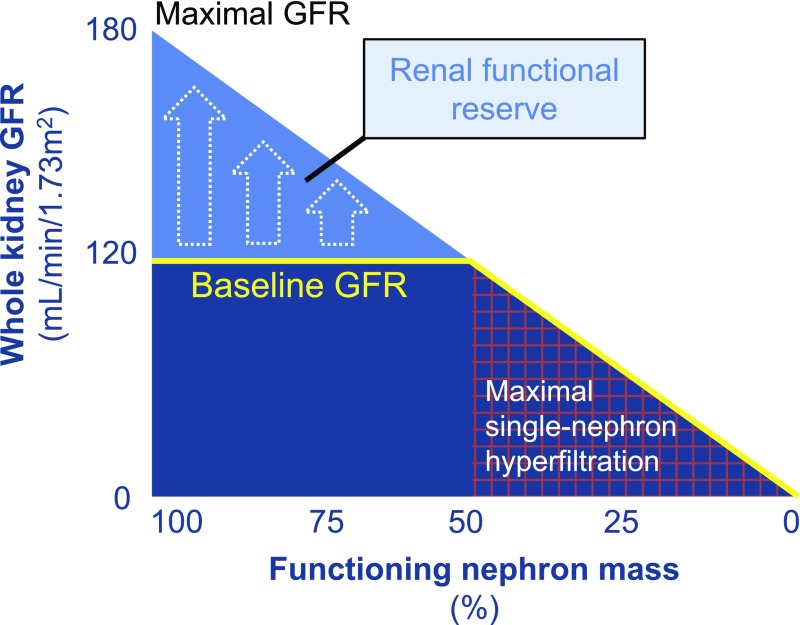
The definition of hyperfiltration at the whole-kidney level disregards conditions in single nephrons, for which two distinct (frequently co-occurring) elements seem to be involved. First, in the natural history of DKD, with irreversible damage to progressively more glomeruli, remnant nephrons undergo functional and structural hypertrophy (glomeruli and associated tubules), thereby striving to maintain whole-kidney filtration and reabsorption within the normal range.21 Second, and regardless of renal mass, metabolic and (neuro)hormonal stimuli that prevail in diabetes and/or obesity (as discussed below) enhance filtration in single nephrons, even when whole-kidney GFR does not exceed 130–140 ml/min per 1.73 m2 (Figure 1). Given these considerations, hyperfiltration has also been defined as a filtration fraction11,22 (FF; the ratio between GFR and effective renal plasma flow [ERPF]) above 17.7%±2.8%, i.e., the mean±SD in healthy 22–25–year-old humans.23 In support of such a definition, a mean FF of 24% is observed in adolescents with uncomplicated T1DM and a GFR of 178 ml/min per 1.73 m2, whereas FF is 17% in those with a GFR of 111 ml/min per 1.73 m2.24 ERPF is measured using para-aminohippuric acid, radioiodine-labeled hippuran, or 99mTc-labeled mercaptoacetyltriglycine, which are removed from the circulation during a single pass through the kidney by approximately 90%,25 75%,25 or 55%,26 respectively. Whether FF is a valid approximation of PGLO is subject to debate, as the latter can only be directly measured by micropuncture. However, in humans there is no alternative,27 other than estimation with Gomez equations (using measured GFR and ERPF, and total protein).28,29 Some authors propose that a stress test, which is capable of exploiting the entire filtration capacity of the kidneys (known as the renal functional reserve; i.e., by means of a high-protein load, or infusion of amino acids or dopamine), could be a significant tool to identify a hyperfiltering state in patients with whole-kidney GFR within normal range, assuming that a preexisting elevation of PGLO and ERPF will prevent a rise in GFR (Figure 2).30,31 However, utility of such a diagnostic measure remains uncertain, as variability of renal functional reserve testing makes an impaired GFR response to a stimulus difficult to identify and hard to interpret.
Figure 2.
Schematic representation of renal functional reserve. Renal functional reserve is defined as the capacity of the kidney to compensate or increase its function in states of demand (e.g., high protein or fluid intake, pregnancy) or disease (e.g., diabetes, CKD).31 In early diabetes, when nephron mass is still >50%, renal functional reserve may be reduced due to prevailing metabolic and (neuro)hormonal factors that increase baseline GFR. In later stages, additional renal hemodynamic adaptations occur in response to reduced renal mass, leading to continuous maximal use of glomerular filtration capacity.
Pathogenesis of Hyperfiltration in Diabetes
Pathogenesis of hyperfiltration in diabetes is complex, comprising numerous mechanisms and mediators, with a prominent role for hyperglycemia and distorted insulin levels,32 especially in early diabetes33 and prediabetes.34 As such, prevalence of diabetes-related hyperfiltration may have been dropped due to earlier diagnosis and modern day stricter control of hyperglycemia and other factors (e.g., angiotensin II by means of RAS blockade). For example, reducing glycated hemoglobin A1c (HbA1c) from 10% to 7%, which could be considered adequate glycemic control,35 normalized measured GFR from 149 to 129 ml/min per 1.73 m2 (16% reduction) in patients with T1DM on insulin pump therapy, whereas no effect on GFR was observed in the control group that continued conventional insulin treatment without changes in HbA1c.36 Notably, independent of diabetes and glucose levels,37 body weight also augments GFR (by about 15% in obese37 to about 56% in severely obese nondiabetic subjects38,39). Thus, especially in T2DM, hyperfiltration likely develops after and on top of body weight–induced increases in GFR, although such longitudinal data are not available. The mechanisms of hyperfiltration, which may overlap and act in concert, are briefly discussed at ultrastructural, vascular, and tubular level.
Ultrastructural Changes
From the onset of diabetes, the kidneys grow large due to expanded nephron size (particularly hypertrophy of the proximal tubule).32,40 This phenomenon is most likely caused by various cytokines and growth factors in response to hyperglycemia,41 although obesity may also independently contribute to nephromegaly.11,42 Although increased kidney size36,43 and filtration surface area per glomerulus44 have been linked to hyperfiltration, it has been proven difficult to separate cause from effect.40 Some have suggested that (compensatory) hypertrophy occurs as a result of hyperfiltration.45 However, in animal studies, hypertrophy precedes hyperfiltration.41 Inhibition of the rate-limiting enzyme ornithine decarboxylase to reduce early diabetic tubular hypertrophy and—likely subsequent—proximal hyper-reabsorption of sodium (see below) diminishes hyperfiltration in direct proportion to the effect on kidney size in diabetic rats.46 Because tubular growth reverses slowly, and normalization of kidney size may not be achieved in patients with diabetes even after strict glycemic control, hyperfiltration could endure due to persistent tubular enlargement and changes in tubular functions.
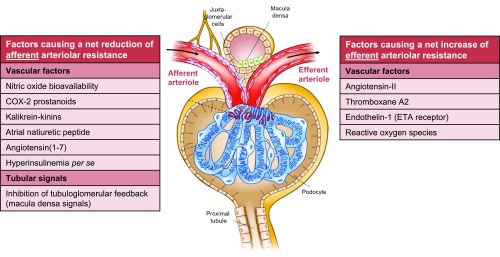
Vascular Theory
According to the “vascular theory,” hyperfiltration results from imbalance of vasoactive humoral factors that control pre-and postglomerular arteriolar tone leading to hyperfiltration, as depicted in Figure 3.8,32 Preferential sites of action of these factors are derived from infusion or blockade studies in preclinical models and humans, in which reduced FF is frequently related to a vasodilatory effect on the efferent arteriole or vasoconstrictive effect on the afferent arteriole. However, FF reduces also with proportional decreases in efferent and afferent arteriolar resistance (as the former decreases FF more than the latter increases FF), which denotes that changes in FF are not necessarily indicative for selective alteration in segmental vascular resistance (Supplemental Figure 1).47 As various vasoactive mediators are released or activated after a meal, they may be effectors in postprandial hyperfiltration (Figure 3).48 In addition, amino acids from digested proteins may directly49,50 and indirectly48 increase tubular reabsorption of sodium and subsequently inactivate tubuloglomerular feedback (TGF; see below).
Figure 3.
Schematic (net) effect of factors implicated in the pathogenesis of glomerular hyperfiltration in diabetes. Several vascular and tubular factors32,48,123–126 are suggested to result in a net reduction in afferent arteriolar resistance, thereby increasing (single-nephron) GFR. Effects of insulin per se seem to depend on insulin sensitivity.96,97 A net increase in efferent arteriolar resistance—leading to increased GFR—is proposed for other vascular factors.32,42,71,124,127 Growth hormone128 and insulin-like growth factor-1129 likely increase filtration by augmenting total renal blood flow, without specific arteriolar preference. Glucagon and vasopressin seem to (principally) act through TGF.48 Intrinsic defects of electromechanical coupling or alterations in signal transduction in afferent arterioles may impair vasoactive responses to renal hemodynamic (auto)regulation.32 Augmented filtration by increases in the ultrafiltration coefficient, and net filtration pressure via reduction in intratubular volume and subsequent hydraulic pressure in Bowman’s space are not depicted. Several vascular factors may be released or activated after a (high-protein) meal (e.g., nitric oxide, cyclooxygenase-2 prostanoids, angiotensin II),48,50,130 whereas TGF becomes (further) inhibited, through increased amino acid- (and glucose) coupled sodium reabsorption in the proximal tubule49,50 and/or increased glucagon/vasopressin-dependent sodium reabsorption in the thick ascending limb.48 These changes may collectively play a part in postprandial hyperfiltration. COX-2, cyclooxygenase-2; ETA, endothelin A receptor.
Tubular Theory
The “tubular theory” of hyperfiltration describes diabetes-related abnormalities in the close interaction between the glomerulus and tubule. It proposes that enhanced proximal tubular sodium (and glucose) reabsorption, paralleled by tubular growth32 and upregulation of sodium-glucose cotransporters (SGLTs) and sodium-hydrogen exchanger (NHE)3, leads to a reduction in afferent arteriolar resistance and increase in single-nephron GFR through inhibition of TGF (Figure 3).32,42,51 The raised intrarenal pressure in obese patients—due to increased intra-abdominal pressure and accumulation of peri-renal fat—compresses the thin loops of Henle, which may add to enhanced tubular sodium reabsorption.52–54 Finally, diabetes-associated tubular hyperplasia and hypertrophy32 and proximal tubular hyper-reabsorption reduce intratubular pressure and hydraulic pressure in Bowman’s space, which further perpetuates hyperfiltration by increasing the net hydraulic pressure gradient.55,56
Clinical Significance of Hyperfiltration in Diabetes
Elucidating the significance of hyperfiltration as an independent renal risk factor in diabetes is complicated by the complex multifactorial etiology of DKD, and the lack of dedicated studies that assess the influence of sustained or altered whole-kidney hyperfiltration and FF on long-term renal outcome. Hyperfiltration per se does not seem to fully explain adverse renal outcome, as the risk for ESRD in transplant donors (in which single-nephron GFR is typically increased by about 60%–70%)57 is very low.58 However, it may be suggested that the stimulus and/or prevailing diabetes play a part in the pathogenesis of hyperfiltration-induced renal damage. As such, an evaluation of 52,998 living kidney donors revealed that non-insulin-dependent diabetes was among the strongest predictors of developing ESRD after 15-years of follow up (hazard ratio, 3.01; 95% confidence interval, 1.91 to 4.74).59 To date, studies that report on the effects of whole-kidney level hyperfiltration in diabetes are observational in nature, whereas the clinical significance of single-nephron hyperfiltration in all phases of DKD is best deduced from RAS blockade trials. Finally, a potential pathophysiologic role of postprandial hyperfiltration in DKD is suggested in small-sized studies. We will discuss the significance of diabetic hyperfiltration using this somewhat artificial distinction.
Whole-Kidney Hyperfiltration and Renal End Points: Observational Studies
Several epidemiologic studies in diabetes report associations between supraphysiologic GFR in diabetes and all-cause mortality.60,61 Furthermore, longitudinal cohort studies of 3–18 years’ duration show that GFR declines more rapidly in patients with T1DM and T2DM with whole-kidney hyperfiltration compared with those with normal GFR at baseline.34,62–64 However, as GFR remained in the normal range at end of follow-up (i.e., ≥100 ml/min per 1.73 m2), it is unclear whether these observations indicate (pharmacologic) resolution of hyperfiltration (i.e., restoration of renal functional reserve), or loss of nephron mass. The latter is suggested in a recent 6-year observational cohort study, in which rapid eGFR decline was associated with baseline hyperfiltration and renal impairment in 509 patients with T1DM.65
Additionally, numerous studies reported on the association of whole-kidney hyperfiltration with onset and progression of the surrogate renal end point albuminuria (Table 2). In a systematic review and meta-analysis of ten cohort studies involving 780 patients with T1DM, followed for a mean of 11.2 years,66 the pooled odds for developing albuminuria in patients with measured whole-kidney hyperfiltration at baseline was 2.71 (95% confidence interval, 1.20 to 6.11). In contrast, other large-sized studies that estimated GFR did not detect such an association.67,68 Moreover, several studies suggest that the absence of whole-kidney hyperfiltration in T1DM has a negative predictive value of approximately 95% for albuminuria development.69,70 In a post hoc analysis of 600 patients with T2DM, patients with persistent measured hyperfiltration, compared with those with normofiltration at inclusion or in whom hyperfiltration was ameliorated by metabolic and BP control at 6 months, were more likely to develop microalbuminuria or macroalbuminuria over a follow-up of 4 years (hazard ratio, 2.23; 95% confidence interval, 1.1 to 4.3).62 These observations were maintained even after adjustment for various risk factors, including HbA1c, BP, and duration of diabetes. However, other reported series in T2DM, which were either smaller-sized or used eGFR, are not in line with these results (Table 2).
Table 2.
Observational studies on the association of hyperfiltration and albuminuria progression or nonprogression in diabetes
| Study Author(s) and Year | Baseline MA Status | N | Follow-Up, yr | Baseline HbA1c, % | GFR Method | Baseline GFR, ml/min per 1.73 m2 | HF Threshold, ml/min per 1.73 m2a | Prevalence of HF, % | Risk Estimate | Summarized albuminuria risk | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | P | NP | All | P | NP | All | P | NP | P | NP | |||||||
| T1DM | |||||||||||||||||
| Mogensen (1986)156a | N | 12 | 166 | 138 | ↑ | ||||||||||||
| Lervang et al. (1988)157 | N | 29 | 8 | 21 | 18# | 9.3* | 7.2* | Inulin | 142# | 147# | OR, 0.67* | = | |||||
| Azevedo and Gross (1991)132 | N | 21 | 0 | 21 | 3.4 | 10.4 | 51Cr-EDTA | 134 | = | ||||||||
| Lervang et al. (1992)158 | N | 34 | 17 | 17 | 12# | 10.8* | 9* | 51Cr-EDTA | ∼136# | 134# | 137# | OR, 0.45* | = | ||||
| Rudberg et al. (1992)70 | N | 53 | 18 | 35 | 8 | 11.8 | Inulin | 135 | ∼150 | ∼130 | 119 | ↑ | |||||
| Bognetti et al. (1993)159 | N | 38 | 7 | 31 | 2.5 | 8.8 | 51Cr-EDTA | 135 | 43 | 52 | OR, 0.89 | = | |||||
| Chiarelli et al. (1995)69 | N | 46 | 8 | 38 | 10 | 9.7 | 12.2 | 9.5 | 51Cr-EDTA | ∼142 | ∼169 | 140 | 87 | 42 | OR, 9.97* | ↑ | |
| Yip et al. (1996)160 | N | 50 | 7 | 43 | 9.6 | ∼9.9 | 51Cr-EDTA | ∼135 | 135 | 57 | 49 | OR, 1.00* | = | ||||
| Caramori et al. (1999)135 | N | 33 | 3 | 30 | 8.4 | 9.9 | 11.4* | 9.9* | 51Cr-EDTA | 134 | 100 | 60 | OR, 4.95* | ↑ | |||
| Dahlquist et al. (2001)136 | N | 60 | 19 | 41 | 8 | 11.9 | 12.2 | 11.8 | Inulin | ∼135 | ∼139 | 129 | 125 | 84 | OR, 3.81 | ↑ | |
| Amin et al. (2005)137 | N | 273 | 30 | 243 | 10.9 | ∼9.9# | 11.4 | 9.7 | Inulinb | ∼142 | 167 | 139 | 125 | 97 | 64 | OR, 16.44* | ↑ |
| Steinke et al. (2005)139 | N | 107c | 8 | 99 | 5 | ∼8.5 | 9.2 | 8.4 | Inulin | ∼144 | 163 | 143 | 130 | 88 | 61 | OR, 4.48* | ↑ |
| Zerbini et al. (2006)161 | N | 146 | 27 | 119 | 9.5 | ∼9.2 | 9.8 | 9 | 51Cr-EDTA | ∼120 | 122 | 118 | OR, 2.01* | = | |||
| Ficociello et al. (2009)67 | N | 426 | 94 | 332 | 15 | ∼8.2 | eGFR | ∼130 | 134 (M)/149 (F)d | 21 | 25 | HR, 0.8 | = | ||||
| Thomas et al. (2012)68 | N | 2318 | 162 | 2156 | 5.2# | ∼8.3 | 9.2 | 8.2 | eGFR | e | e | = | |||||
| Mogensen and Christensen (1984)162 | N/MA | 43 | 16 | 27 | 10.4 | 6.9* | 7.4* | 125I-iothalamate | 158 | 134 | OR, 33.12* | ↑ | |||||
| Mogensen and Christensen (1985)163 | N/MA | 31 | 9 | 22 | 11.7 | 125I-iothalamate | 140 | R 0.78f | ↑ | ||||||||
| Jones et al. (1991)164 | N/MA | 50 | 6 | 44 | 4.7 | ∼9.9 | 51Cr-EDTA | 135 | = | ||||||||
| Bangstad et al. (2002)165 | N/MA | 18 | 3 | 15 | 8 | 10.1 | Inulin | 143 | 150 | 143 | ↑/= | ||||||
| Mathiesen et al. (1997)166 | MA | 40 | 14 | 26 | 5 | ∼8.7 | 9.2 | 8.4 | 51Cr-EDTA | ∼120 | 122 | 115 | = | ||||
| Couper et al. (1997)167 | MA | 59 | 15 | 44 | 2.3# | ∼9.9 | 10.8 | 9.7 | 99mTc-DTPA | “no difference” | = | ||||||
| Amin et al. (2005)137 | MA | 35 | 9 | 26 | 10.9 | 10.8# | 12.1 | 10.3 | Inulinb | 134 | 132 | 135 | 125 | 57 | 72 | OR, 0.79 | = |
| T2DM | |||||||||||||||||
| Silveiro et al. (1996)63 | N | 32 | 9 | 23 | 5 | 51Cr-EDTA | ∼128 | 123 | 129 | 137 | 43 | 40 | OR, 1.13 | = | |||
| Nelson et al. (1996)7 | N | 24 | 4 | Iothalamate | = | ||||||||||||
| Murussi et al. (2006)168 | N | 50 | 14 | 36 | 9.3 | ∼6.9 | 7.5 | 6.7 | 51Cr-EDTA | 121 | 128 | 118 | 137 | 38 | 22 | OR, 1.94 | = |
| Murussi et al. (2007)169 | N | 158 | 41 | 117 | 8 | 6.9 | 7.3 | 6.8 | eGFR | ∼103 | 93 | 107 | ↓ | ||||
| Viswanathan et al. (2012)170 | N | 152 | 67 | 85 | 11# | ∼9.9 | 10.4 | 9.5 | eGFR | ∼101 | 93 | 108 | ↓ | ||||
| Ruggenenti et al. (2012)62 | N/MA | 600 | 62 | 538 | 4# | 6.2 | Iohexol | 101 | 120 | 17 | 7 | HR, 2.26 | ↑ | ||||
| Yokoyama et al. (2011)171 | Any | 1002 | 77 | 925 | 3.8# | ∼6.7 | ∼6.9 | ∼6.7 | eGFR | ∼79 | ∼77 | ∼79 | = | ||||
Progression (P) or nonprogression (NP) to microalbuminuria or macroalbuminuria. HF, hyperfiltration; N, normoalbuminuria; ↑, increased albuminuria risk; *, adapted from Magee and colleagues;66 #, median; OR, odds rate; =, no effect on albuminuria risk; 51Cr-EDTA, 51Cr-labeled ethylenediaminetetra-acetic acid; ∼, calculated mean; M, males; F, females; MA, microalbuminuria; R, standardized beta; 99mTc-DTPA, 99mTc-labeled diethylenetriaminepenta-acetic acid; ↓, decreased albuminuria risk; HR, hazard ratio.
Retrospective cohort study.
GFR was measured 5 years after cohort entry, which was set as baseline value.
Of the 170 patients in the full cohort 63 were excluded, primarily due to the lack of persistent MA.
HF definition was sex specific.
GFR was estimated using Modification of Diet in Renal Disease, Chronic Kidney Disease Epidemiology Collaboration 2009, Cockcroft–Gault, and cystatin C–based formulae. Multiple definitions were used to define HF.
Correlation between baseline GFR and UAE at follow-up.
Despite suggestive evidence that whole-kidney hyperfiltration could contribute to DKD development and progression in T1DM and perhaps T2DM, interpretation of the data is hampered by variations in metabolic control, BP, diabetes duration, and other confounding factors, as well as potential publication bias. To date, no prospective studies with adequate measured and hard end points have investigated the renoprotective potential of controlling early hyperfiltration.
Single-Nephron Hyperfiltration and Renal End Points: RAS Blockade Trials
As angiotensin II induces a net increase in postglomerular resistance,71 reducing its action with an angiotensin converting enzyme inhibitor or angiotensin receptor blocker (ARB) lowers FF and PGLO.72 Consequently, RAS blockers are known to variably increase serum creatinine, which may raise up to 30% in patients with CKD in the first month after treatment initiation, and is generally reversible after drug discontinuation.73 Furthermore, 3-week enalapril treatment reduced GFR and FF in 11 adolescents with uncomplicated T1DM and whole-kidney hyperfiltration.24
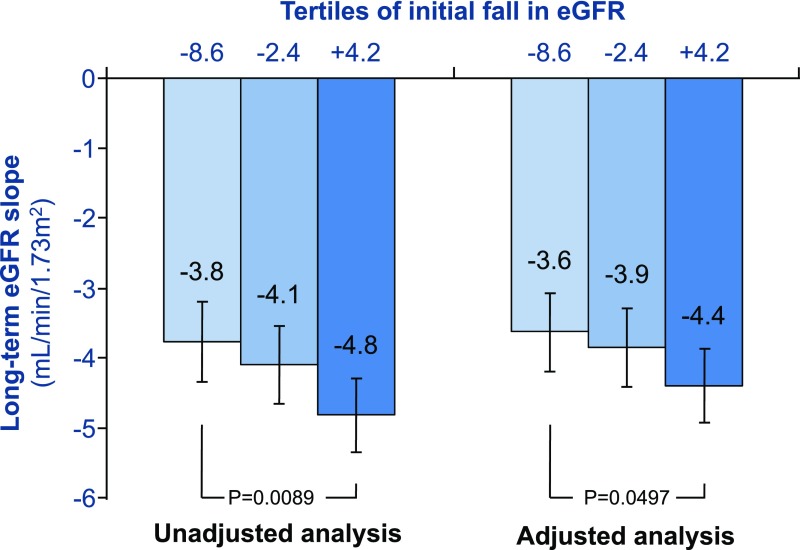
Pivotal trials in patients with T1DM and T2DM, which indicated that RAS blockade reduces the rate of developing albuminuria and hard renal end points, independent from BP lowering, have placed these drugs at the cornerstone of renoprotective management.74 Notably, a greater initial fall in eGFR portends a slower subsequent decline in renal function in patients with T2DM assigned to the ARB losartan (Figure 4), which supports the notion that reducing single-nephron hyperfiltration ameliorates DKD risk.75 However, as there is a close relationship between PGLO and urinary albumin excretion (UAE),76 and RAS blockade benefits both renal risk factors, the independent contribution of each to long-term renal preservation remains unknown.
Figure 4.
An acute fall in eGFR in losartan-assigned T2DM patients with DKD is inversely correlated with the long-term eGFR slope, after correction for sex, baseline eGFR, diastolic BP, hemoglobin, and urinary albumin-to-creatinine ratio. Data adapted from Holtkamp and colleagues.75
Postprandial Hyperfiltration and Renal End Points: Speculative Studies
The pathophysiologic role of meal-induced increases in (single-nephron) GFR, known as postprandial hyperfiltration, in the onset or progression of CKD is a re-emerging field of study, especially in the context of high-protein diets that aim to induce weight-loss in obesity and T2DM. As such, in a 7-day crossover study in healthy young men, high-protein intake (2.4 g/kg per day) compared with normal protein intake (1.2 g/kg per day) increased measured GFR, FF, and 24-hour UAE.77 As humans largely reside in the postprandial state, the excessive and prolonged metabolic and hormonal disturbances occurring after meal ingestion in diabetes could, in theory, unfavorably influence kidney function, and predispose to renal damage. Interestingly, a blunted rise in GFR after amino acid infusion or protein loading in the presence of a RAS inhibitor has been widely described, suggesting an added renoprotective benefit of these drugs.73,78,79 Yet, the long-term effect of diet-induced renal hemodynamic alterations (and its amelioration), independent of e.g., an increased renal acid load, on renal outcome in diabetes remains unclear.
Current and Emerging Treatment Options
Although glucose-lowering per se ameliorates diabetic hyperfiltration, especially in early-onset diabetes,80 some antihyperglycemic drugs exhibit glucose-independent properties that may directly and/or indirectly benefit this renal risk factor. Here, we briefly discuss a selection of currently available or promising emerging antihyperglycemic (Table 3) and other (nonantihyperglycemic) (Table 4) interventions that may favorably affect renal hemodynamics in human diabetes.
Table 3.
Current and emerging antihyperglycemic treatment options with the potential to reduce hyperfiltration in diabetes
| Treatment | FDA-Approved Compounds | Route of Administration | Mode of Action | (Potential) Adverse Eventsa | Potential Hyperfiltration-Reducing Mechanismb |
|---|---|---|---|---|---|
| SGLT2 inhibitor | Canagliflozin | Oral | ↑ Urinary glucose excretion | Genital mycotic infections, urinary tract infections, ketoacidosisc, breast/bladder cancerc, bone fracturesc, lower limb amputationsc | Weight loss, BP ↓ |
| Dapagliflozin | TGF activation, PBOW ↑ | ||||
| Empagliflozin | |||||
| Dual SGLT1/SGLT2 inhibitor | Phase-3 development | Oral | ↑ Urinary glucose excretion | Largely uncertain. Genital mycotic infections, urinary tract infections, GI side effects (nausea, diarrhea), ketoacidosisc | Weight loss, BP ↓ |
| ↓ GI glucose uptake | GI absorption rate ↓ | ||||
| ANP ↓, GLP-1 ↑ | |||||
| TGF activation, PBOW ↑ | |||||
| GLP-1 receptor agonist | Albiglutide (QW) | Injectable | ↑ Insulin secretion (glucose-dependent) | GI side effects (nausea, vomiting, diarrhea), acute gallstone disease, pancreatitisc, pancreatic cancerc | Weight loss, BP ↓ |
| Dulaglutide (QW) | ↓ Glucagon secretion (glucose-dependent) | Gastric emptying rate ↓d | |||
| Exenatide (QW, BID) | ↓ Gastric emptyingd | Glucagon ↓, RAS ↓172 | |||
| Liraglutide (QD) | ↑ Satiety | TGF activation, PBOW ↑ | |||
| Lixisenatide (QD) | |||||
| Semaglutide (QD) | |||||
| DPP-4 inhibitor | Alogliptin | Oral | ↑ Insulin secretion (glucose-dependent) | Nasopharyngitis, heart failurec, | Weight loss, BP ↓ |
| Linagliptin | ↓ Glucagon secretion (glucose-dependent) | pancreatitisc, pancreatic cancerc | Ultrafiltration coefficient ↓173 | ||
| Saxagliptin | Glucagon ↓, RAS ↓172 | ||||
| Sitagliptin | TGF activation, PBOW ↑ | ||||
| Thiazolidinedione | Pioglitazone | Oral | ↑ Insulin sensitivity | Edema and heart failure, weight gain, bone fractures, bladder cancerc, CV eventsc | NO-bioavailability efferent arteriole ↑ |
| Rosiglitazone | ↓ Hepatic glucose production | TGF signaling ↑ | |||
| Insulin | Insulin lispro | Injectable | Hypoglycemia, weight gain | Postprandial IGF-1–dependent renal vasodilation ↓ | |
| ↑ Glucose disposal | |||||
| ↓ Hepatic glucose production | |||||
| Glucagon receptor antagonist | Phase-2 development | Oral/injectable | ↓ Glucagon action | Uncertain | TGF activation |
FDA, Food and Drug Administration; ↑, increase; PBOW, hydraulic pressure in Bowman’s space; ↓, decrease; GI, gastro-intestinal; ANP, atrial natriuretic peptide; QW, once weekly; BID, twice daily; QD, once daily; CV, cardiovascular; NO, nitric oxide; IGF, insulin-like growth factor.
The list of adverse events does not aim to be exhaustive.
Potential mechanisms beyond glucose reduction are listed.
Uncertain safety issues.
Effect on gastric emptying is only sustained with short-action GLP-1 receptor agonists.
Table 4.
Current and emerging nonantihyperglycemic treatment options with hyperfiltration-reducing potential in diabetes
| Treatment | Intervention/Primary Indication | (Potential) Adverse Eventsa | Potential Hyperfiltration-Reducing Mechanism |
|---|---|---|---|
| Nonpharmacologic interventions | |||
| Nutritional “therapy” | ↓ (High)-protein intake | Decreased muscle mass, physical weakness, compromised immune response, decreased bone mineral density | TGF activation, PBOW ↑ |
| ↓ Salt restriction in diabetes | Reduced antihypertensive efficacy | TGF activation, PBOW ↑ | |
| Continuous positive airway pressure | ↓ Obstructive sleep apnea | Irritation at mask contact points, dryness/irritation of nasal and pharyngeal membranes, eye irritation, nasal congestion and rhinorrhea, claustrophobia, headache, gastric and bowel distention, pneumothorax, recurrent ear and sinus infections | SNS-induced efferent arteriolar resistance ↓174 |
| ANP ↓174 | |||
| Bariatric surgery | ↓ Body weight | Peri- and postoperative complications, reoperation, GI side effects (nausea, vomiting, diarrhea, dumping syndrome), hypoglycemia, nutritional deficiencies, gallstone disease | (Pre-)diabetes ↓, BP ↓ |
| Ultrafiltration coefficient ↓, renal plasma flow ↓ | |||
| GLP-1 ↑175 | |||
| TGF activation | |||
| Renal sympathetic denervation | ↓ BP | Procedure-related events (renal artery dissection and stenosis, brachycardia, and vascular access complications), postprocedural hypotension | Glomerular size ↓176 |
| Norepinephrine-induced efferent vasoconstriction ↓176 | |||
| Dopamine-induced vasodilation ↓176 | |||
| Pharmacologic | |||
| Carbonic anhydrase inhibitor | ↓ Na+/Cl− and bicarbonate reabsorption in proximal tubule | Metabolic acidosis, polyuria, paresthesia, tinnitus, dysgeusia, loss of appetite, GI side effects (nausea, vomiting, diarrhea) | TGF activation, PBOW ↑ |
| Mineralocorticoid receptor antagonist | ↑ Natriuresis (potassium-sparing) | Hyperkalemia, renal dysfunction, leg cramps, GI side effects (bleeding/ulceration, nausea, vomiting, gastritis, diarrhea), leukopenia/thrombocytopenia | TGF sensitivity ↑ |
| ↓ BP | Spironolactone: gynecomastia, erectile dysfunction, menstrual irregularities | ||
| Endothelin A receptor antagonist | ↓ Albuminuria | Fluid retention–related events (peripheral, pulmonary, and facial edema; anemia), congestive heart failure, weight increase | Net efferent arteriolar resistance ↓ |
| COX-2 inhibitor | ↓ Inflammation | CV events, peripheral edema, hypertension, renal injury, GI side effects (bleeding/ulceration, dyspepsia, abdominal pain, diarrhea), upper respiratory tract infections | COX-2 prostanoids ↓177 |
| ↓ Pain | RAS ↓177 | ||
| Thromboxane A2 ↓178 | |||
| PKC-β inhibitor | Diabetic retinopathy | Dyspepsia, first-degree atrioventricular block, superficial thrombosis, increased blood creatinine phosphokinase, micturition urgency, skin discoloration | Angiotensin II–induced vasoconstriction ↓179,180 |
| C-peptide | Improved functional and structural organ-system abnormalities in diabetes181 | Experimental phase | Afferent arteriolar resistance ↑182 |
| Efferent arteriolar resistance ↓182 | |||
↓, decrease; Pbow, hydraulic pressure in Bowman's; ↑, increase; SNS, sympathetic nervous system; ANP, atrial natriuretic peptide; Na+/Cl−, sodium chloride; GI, gastrointestinal; COX, cyclooxygenase; CV, cardiovascular; PKC, protein kinase C.
The list of adverse events does not aim to be exhaustive.
Antihyperglycemic Drugs
SGLT2 Inhibitors
By concomitantly blocking glucose and sodium reabsorption in the proximal tubule, SGLT2 inhibitors not only improve glycemic control by inducing glycosuria in diabetes, but also increase urinary sodium excretion. Their proximal natriuretic effect may be enhanced by accompanied functional blockade of NHE3.81 Thus, SGLT2 inhibition could reduce (single-nephron) hyperfiltration in diabetes by (1) restoring sodium-chloride concentration at the macula densa and subsequent TGF-mediated afferent arteriolar vasoconstriction,82,83 and (2) increasing intraluminal volume causing a retrograde increase in hydraulic pressure in Bowman’s space, which constrains filtration pressure.56 Furthermore, SGLT2 inhibitors consistently reduce bodyweight and BP, and may influence several vascular mediators of renal hemodynamics in both the fasting and postprandial state (e.g., a decrease in atrial natriuretic peptide and insulin, and an increase in glucagon, RAS components, and glucagon-like peptide 1 [GLP–1]).
In an 8-week add-on to insulin study, empagliflozin in uncomplicated T1DM patients with whole-kidney hyperfiltration (mean GFR 172±23 ml/min per 1.73 m2) demonstrated a glucose-independent 19% decrease in GFR, which was paralleled by a decline in ERPF and estimated PGLO and increase in afferent arteriolar resistance, as assessed by the Gomez equations.82,83 Finally, as the rise in circulating RAS components may have blunted the renal hemodynamic effect of empagliflozin in these RAS blockade naïve T1DM patients, it is tempting to speculate that combined use of SGLT2 inhibitors and angiotensin converting enzyme inhibitors/ARBs may lead to synergistic renoprotective effects through combined blockade of neurohormonal and tubular factors.84 Surprisingly, FF increased during euglycemic-clamp conditions in the hyperfiltering patients, underlining the difficulty to unambiguously assess intrarenal hemodynamic changes. In longer-term trials in patients with T2DM, SGLT2 inhibitors initially reduce eGFR over a wide range of baseline values, which appears to be hemodynamically regulated as the reduction reverses after a washout period.85 In EMPA-REG OUTCOME, 48 months of empagliflozin versus placebo treatment in 7020 high-risk patients with T2DM induced an eGFR trajectory reminiscent of RAS blockade (Figure 5), and resulted in a 46% reduction in the composite of serum creatinine doubling (accompanied by eGFR of ≤45 ml/min per 1.73 m2), ESRD, or renal death.86 Notably, over the 34 days after empagliflozin discontinuation, a weekly increase in eGFR of approximately 0.5 ml/min per 1.73 m2 was observed, as compared with a small decrease in the placebo group. Other long-term SGLT2 inhibition studies in T2DM patients with primary or secondary renal outcomes are underway.76 Finally, the gastrointestinal effects of novel dual SGLT2/SGLT1 inhibitors (e.g., reduced gastric emptying rate and intestinal glucose uptake) could theoretically also contribute to PGLO reduction after meal ingestion.
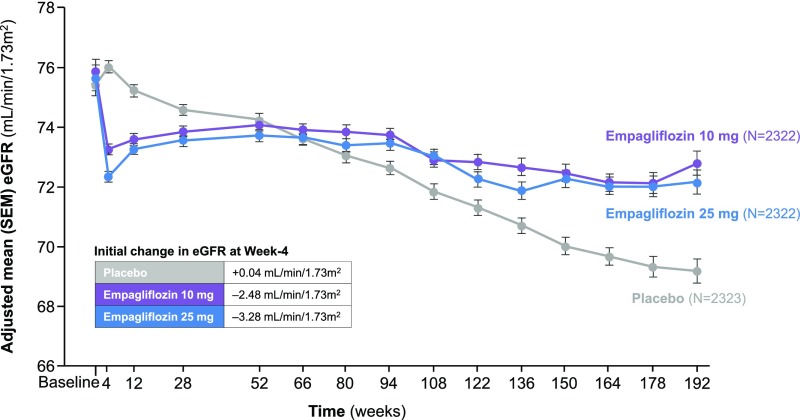
Figure 5.
Renal function trajectory in the EMPA-REG OUTCOME trial. In this study, 7020 patients with T2DM at high cardiovascular risk were randomly assigned to receive the SGLT2 inhibitor empagliflozin (10 or 25 mg once daily) or placebo. After an initial drop in eGFR documented at week 4, renal function stabilized in empagliflozin-treated patients over the ensuing follow-up period, whereas among those patients receiving placebo, a steady decline of 1.67 ml/min per 1.73 m2 per year in eGFR was observed. After 34 days of cessation of the study drug, the initial decrease in eGFR in all empagliflozin-treated patients was completely reversed with an adjusted mean difference from placebo in the change from baseline eGFR of 4.7 ml/min per 1.73 m2 (not depicted). Adapted from Wanner and colleagues.86
GLP-1–Based Therapies
GLP-1 receptor agonists (GLP-1RA) and dipeptidyl peptidase (DPP)–4 inhibitors are associated with renal hemodynamic effects, potentially beyond glycemic control. As such, native GLP-1 infusion reduced creatinine clearance–measured GFR in obese, insulin resistant, hyperfiltering males, 25% of whom were diagnosed with T2DM.87 The long-acting GLP-1RA liraglutide reversibly reduced measured GFR and UAE in an uncontrolled open-label study involving 31 patients with T2DM.88 These observations have been attributed to a GLP-1–mediated inhibition of NHE3 (which assembles with DPP-4 in the proximal tubular brush border), thereby reducing proximal sodium reabsorption and GFR through activation of TGF.51 However, acute administration of GLP-1RA left GFR unaffected in patients with T2DM with normal renal function.89,90 Moreover, treatment with liraglutide or the DPP-4 inhibitor sitagliptin compared with placebo in normoalbuminuric patients with T2DM (mean GFR 83 ml/min per 1.73 m2 and FF 23.7%) did not affect eGFR after 2 weeks, nor were there changes in inulin and para-aminohippuric acid–measured renal hemodynamics after 12 weeks.91 However, although 12-weeks’ liraglutide treatment nonsignificantly reduced mean GFR of 75 by 5 ml/min per 1.73 m2 in 27 albuminuric patients with T2DM with albuminuria, in a placebo-controlled crossover study, GFR decreased by >30% in the two patients with whole-kidney hyperfiltration.92 Of future interest are postprandial renal hemodynamic actions of short-acting GLP-1RA (which have sustained inhibitory effects on gastric emptying rate and glucagon levels) or DPP-4 inhibitors.
Thiazolidinediones
Twelve-weeks’ treatment with the thiazolidinedione rosiglitazone in patients with T2DM with and without albuminuria reduced GFR and FF.93 These observations were explained by vasodilator actions at the efferent arteriole through increased nitric oxide bioavailability.93,94 Studies in diabetic rats suggest that restoration of TGF signaling may also play a role.95
Insulin
In the fasting state, insulin has been reported to either increase GFR and ERPF, or to have neutral effects, which seems to be dependent on insulin sensitivity.96,97 Interestingly, in T2DM with macroalbuminuria, the fast-acting insulin lispro blunted postprandial increase in GFR and RPF versus regular insulin, possibly due to inhibition of insulin-like growth factor-1–dependent renal vasodilation.98
Glucagon Receptor Antagonists
Hyperglucagonemia in the fasting and postprandial state contributes to elevated blood glucose and hyperfiltration in diabetes.48,99 Interestingly, glucagon levels increase in the course of DKD.100 Selective blockade of the glucagon receptor as a novel glucose-lowering target in diabetes could favorably influence renal hemodynamics.48
Nonantihyperglycemic Interventions
Nutritional “Therapy”
Improving the diet in diabetes may ameliorate DKD risk, but defining an optimal regime is heavily debated. Importantly, examining its independent influence on (postprandial) hyperfiltration and subsequent renal outcome is virtually impossible, as confounding factors are legion. Nevertheless, extremes of macronutrient intake, especially that of protein, should generally be avoided to reduce hyperfiltration and renal risk.101 As such, in (pre)hypertensive patients of the OmniHeart study, a high-protein diet (+10% of energy from protein) increased fasting eGFR by approximately 4 ml/min per 1.73 m2 compared with diets replacing protein with either carbohydrate or fat.102 Furthermore, guidelines direct to reduce sodium intake to <2000 mg/d in order to prevent renal disease in diabetes.76 However, clinicians may be reluctant to advocate sodium restriction in diabetes. This is fueled on the one hand by the hypothesis of a “salt-paradox” in diabetes (i.e., a rise in single nephron GFR in response to salt restriction, due to enhanced sensitivity of proximal tubular sodium reabsorption and subsequent inhibition of TGF),103 and on the other by concerns about sympathetic nervous system and RAS activation with a low-salt diet.104
Weight Loss
Although overweight and obesity are independently associated with increases in GFR, ERPF, and FF,38,105 hyperfiltration is absent in obese nondiabetic patients when GFR and RPF are indexed for individuals’ body surface area (BSA) in many,11 but not all, studies.105 The rationale for BSA adjustments comes from observations in mammals that GFR and ERPF are proportional to kidney size, which in turn is typically proportional to body size. Also, dependency of kidney and body size is assumed, as the main function of the kidneys is to regulate total body volume and waste.106 However, BSA normalizations may not be appropriate given that individuals are endowed with a set number of nephrons, which do not change with weight gain.106 In addition, formulas like the Du Bois and Du Bois may not be accurate in severely obese (T2DM) subjects.106 Gastroplasty-induced weight loss from 145 to 97 kg reduced (nonindexed) GFR, ERPF, FF, and albuminuria in nondiabetic subjects.39 Notably, bariatric surgery in severely obese subjects, of whom 38% had diabetes, has recently been shown to reduce the 4.4-year risk for an eGFR decline of ≥30% and doubling of serum creatinine or ESRD by 58% and 57%, respectively, compared with a matched nonoperated cohort.77
Diuretics
The carbonic anhydrase inhibitor acetazolamide decreases sodium, chloride, and bicarbonate reabsorption at the level of the proximal tubule. Although acetazolamide is rarely used as a diuretic because its long-term natriuretic effect is modest,107 several studies have shown that this drug markedly reduces GFR in T1DM with whole-kidney hyperfiltration108,109 and DKD,110 likely by TGF activation and independent from sodium balance.107 Loop diuretics may not affect TGF, because inhibition of the Na-K-2Cl–cotransporter also blocks solute transport into macula densa cells,107 although discussion is ongoing.111 Thiazide diuretics and epithelial sodium channel blockers act distally of the macula densa and do not influence TGF signals. However, (novel selective nonsteroidal) mineralocorticoid receptor antagonists (e.g., spironolactone, eplerenone, finerenone) do induce an initial acute fall in eGFR in T2DM,112–114 possibly by increasing TGF sensitivity,115 which predicts a later favorable influence on the course of renal function.114
Endothelin-A Receptor Antagonists
Increased endothelin-1 concentrations contribute to DKD development by increasing PGLO, podocyte damage, and permeability to albumin. Conversely, selective endothelin-A receptor antagonists (e.g., avosentan and atrasentan), which alleviate vasoconstriction of the efferent renal arteriole, were shown to increase renal blood flow and reduce renal vascular resistance and FF in hypertensive CKD patients.116 In line with these hemodynamic observations, long-term treatment with endothelin-A receptor antagonists reduced residual albuminuria by 35%–50% and seemingly preserved renal function in patients with T2DM that were optimally treated for their DKD.117,118 As the antiproteinuric effect of this drug class is already evident after 1 week of treatment, and in concert with eGFR returns to pretreatment levels after cessation of therapy, a hemodynamic nature of response is suggested.117,119
Concluding Remarks
CKD due to diabetes continues to rise, indicating that current strategies in managing DKD do not suffice to halt renal risk in this population. Accumulating evidence suggests a prognostic and pathogenic role of glomerular hyperfiltration in the initiation and progression of DKD. However, especially as hyperfiltration and albuminuria are renal hemodynamically linked,76 dedicated prospective studies are needed to confirm whether targeting hyperfiltration improves clinically relevant end points (i.e., 30% or 40% eGFR decline,120 ESRD, and/or renal death).76 Several antihyperglycemic and nonhyperglycemic interventions are associated with ameliorated hyperfiltration. Whether these treatments add benefit in the ongoing search for renal risk reduction in diabetes is worth investigating in specifically designed (renoprotection) trials using active comparators, especially in patients with hyperfiltration at baseline.
Disclosures
H.J.L.H. has consulted for AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Janssen, and ZS-Pharma (all honoraria paid to employer).
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016060666/-/DCSupplemental.
References
- 1.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME: Diabetic kidney disease: A report from an ADA Consensus Conference. Diabetes Care 37: 2864–2883, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Kidney Foundation : KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 60: 850–886, 2012 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association : Cardiovascular disease and risk management, section 8. In standards of medical care in diabetes-2016. Diabetes Care 39(Suppl 1): S60–S71, 2016 [DOI] [PubMed] [Google Scholar]
- 4.United Stated Renal Data System (USRDS). 2016 Annual Data Report, Vol 1, CKD, Chapter 1. Available at: http://www.usrds.org. Accessed January 3, 2017
- 5.Pollak MR, Quaggin SE, Hoenig MP, Dworkin LD: The glomerulus: The sphere of influence. Clin J Am Soc Nephrol 9: 1461–1469, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christiansen JS, Gammelgaard J, Frandsen M, Parving HH: Increased kidney size, glomerular filtration rate and renal plasma flow in short-term insulin-dependent diabetics. Diabetologia 20: 451–456, 1981 [DOI] [PubMed] [Google Scholar]
- 7.Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, Hirschman GH, Myers BD; Diabetic Renal Disease Study Group : Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N Engl J Med 335: 1636–1642, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Brenner BM, Lawler EV, Mackenzie HS: The hyperfiltration theory: A paradigm shift in nephrology. Kidney Int 49: 1774–1777, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM: Hyperfiltration in remnant nephrons: A potentially adverse response to renal ablation. Am J Physiol 241: F85–F93, 1981 [DOI] [PubMed] [Google Scholar]
- 10.Cachat F, Combescure C, Cauderay M, Girardin E, Chehade H: A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. Clin J Am Soc Nephrol 10: 382–389, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW: Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat Rev Nephrol 8: 293–300, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Rossing P, Tarnow L, Nielsen FS, Hansen BV, Brenner BM, Parving HH: Low birth weight. A risk factor for development of diabetic nephropathy? Diabetes 44: 1405–1407, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Rius F, Pizaro E, Salinas I, Lucas A, Sanmarti A, Romero R: Age as a determinant of glomerular filtration rate in non-insulin-dependent diabetes mellitus. Nephrol Dial Transplant 10: 1644–1647, 1995 [PubMed] [Google Scholar]
- 14.Hansen HP, Hovind P, Jensen BR, Parving HH: Diurnal variations of glomerular filtration rate and albuminuria in diabetic nephropathy. Kidney Int 61: 163–168, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Kwong YT, Stevens LA, Selvin E, Zhang YL, Greene T, Van Lente F, Levey AS, Coresh J: Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis 56: 39–49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaspari F, Ruggenenti P, Porrini E, Motterlini N, Cannata A, Carrara F, Jiménez Sosa A, Cella C, Ferrari S, Stucchi N, Parvanova A, Iliev I, Trevisan R, Bossi A, Zaletel J, Remuzzi G; GFR Study Investigators : The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney Int 84: 164–173, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Cherney DZ, Sochett EB, Dekker MG, Perkins BA: Ability of cystatin C to detect acute changes in glomerular filtration rate provoked by hyperglycaemia in uncomplicated Type 1 diabetes. Diabet Med 27: 1358–1365, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH: Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: Results of a 4-year follow-up study. J Am Soc Nephrol 16: 1404–1412, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens LA, Levey AS: Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 20: 2305–2313, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Soveri I, Berg UB, Björk J, Elinder CG, Grubb A, Mejare I, Sterner G, Bäck SE; SBU GFR Review Group : Measuring GFR: A systematic review. Am J Kidney Dis 64: 411–424, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Brenner BM: Hemodynamically mediated glomerular injury and the progressive nature of kidney disease. Kidney Int 23: 647–655, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Huang SH, Sharma AP, Yasin A, Lindsay RM, Clark WF, Filler G: Hyperfiltration affects accuracy of creatinine eGFR measurement. Clin J Am Soc Nephrol 6: 274–280, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabelink TJ, Koomans HA, Boer WH, van RJ, Dorhout Mees EJ: Lithium clearance in water immersion-induced natriuresis in humans. J Appl Physiol (1985) 66: 1744–1748, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA: Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol 17: 1703–1709, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Hutchings M, Hesse B, Grønvall J, Olsen NV: Renal 131I-hippuran extraction in man: Effects of dopamine. Br J Clin Pharmacol 54: 675–677, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bubeck B, Brandau W, Weber E, Kälble T, Parekh N, Georgi P: Pharmacokinetics of technetium-99m-MAG3 in humans. J Nucl Med 31: 1285–1293, 1990 [PubMed] [Google Scholar]
- 27.Griffin KA, Kramer H, Bidani AK: Adverse renal consequences of obesity. Am J Physiol Renal Physiol 294: F685–F696, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Bjornstad P, Skrtic M, Lytvyn Y, Maahs DM, Johnson RJ, Cherney DZ: The Gomez' equations and renal hemodynamic function in kidney disease research [published online ahead of print September 7, 2016]. Am J Physiol Renal Physiol 10.1152/ajprenal.00415.2016 [DOI] [PMC free article] [PubMed]
- 29.Gomez DM: Evaluation of renal resistances, with special reference to changes in essential hypertension. J Clin Invest 30: 1143–1155, 1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chawla LS, Ronco C: Renal stress testing in the assessment of kidney disease. Kidney International Reports 1: 57–63, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma A, Mucino MJ, Ronco C: Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract 127: 94–100, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Vallon V, Komers R: Pathophysiology of the diabetic kidney. Compr Physiol 1: 1175–1232, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jerums G, Premaratne E, Panagiotopoulos S, MacIsaac RJ: The clinical significance of hyperfiltration in diabetes. Diabetologia 53: 2093–2104, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Melsom T, Schei J, Stefansson VT, Solbu MD, Jenssen TG, Mathisen UD, Wilsgaard T, Eriksen BO: Prediabetes and risk of glomerular hyperfiltration and albuminuria in the general nondiabetic population: A prospective cohort study. Am J Kidney Dis 67: 841–850, 2016 [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association : 5. Glycemic targets. Diabetes Care 39[Suppl 1]: S39–S46, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Wiseman MJ, Saunders AJ, Keen H, Viberti G: Effect of blood glucose control on increased glomerular filtration rate and kidney size in insulin-dependent diabetes. N Engl J Med 312: 617–621, 1985 [DOI] [PubMed] [Google Scholar]
- 37.Ribstein J, du Cailar G, Mimran A: Combined renal effects of overweight and hypertension. Hypertension 26: 610–615, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U: Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol 278: F817–F822, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y: The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 14: 1480–1486, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Hostetter TH: Hypertrophy and hyperfunction of the diabetic kidney. J Clin Invest 107: 161–162, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bak M, Thomsen K, Christiansen T, Flyvbjerg A: Renal enlargement precedes renal hyperfiltration in early experimental diabetes in rats. J Am Soc Nephrol 11: 1287–1292, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Chagnac A, Herman M, Zingerman B, Erman A, Rozen-Zvi B, Hirsh J, Gafter U: Obesity-induced glomerular hyperfiltration: Its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant 23: 3946–3952, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Mogensen CE, Andersen MJ: Increased kidney size and glomerular filtration rate in early juvenile diabetes. Diabetes 22: 706–712, 1973 [DOI] [PubMed] [Google Scholar]
- 44.Hirose K, Tsuchida H, Osterby R, Gundersen HJ: A strong correlation between glomerular filtration rate and filtration surface in diabetic kidney hyperfunction. Lab Invest 43: 434–437, 1980 [PubMed] [Google Scholar]
- 45.Fine L: The biology of renal hypertrophy. Kidney Int 29: 619–634, 1986 [DOI] [PubMed] [Google Scholar]
- 46.Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V: Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest 107: 217–224, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carmines PK, Perry MD, Hazelrig JB, Navar LG: Effects of preglomerular and postglomerular vascular resistance alterations on filtration fraction. Kidney Int Suppl 20: S229–S232, 1987 [PubMed] [Google Scholar]
- 48.Bankir L, Roussel R, Bouby N: Protein- and diabetes-induced glomerular hyperfiltration: Role of glucagon, vasopressin, and urea. Am J Physiol Renal Physiol 309: F2–F23, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Gonska T, Hirsch JR, Schlatter E: Amino acid transport in the renal proximal tubule. Amino Acids 19: 395–407, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Premen AJ: Potential mechanisms mediating postprandial renal hyperemia and hyperfiltration. FASEB J 2: 131–137, 1988 [DOI] [PubMed] [Google Scholar]
- 51.Muskiet MH, Smits MM, Morsink LM, Diamant M: The gut-renal axis: Do incretin-based agents confer renoprotection in diabetes? Nat Rev Nephrol 10: 88–103, 2014 [DOI] [PubMed] [Google Scholar]
- 52.Alonso-Galicia M, Dwyer TM, Herrera GA, Hall JE: Increased hyaluronic acid in the inner renal medulla of obese dogs. Hypertension 25: 888–892, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE: Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 7: 75–88, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE: Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 12: 1211–1217, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Persson P, Hansell P, Palm F: Tubular reabsorption and diabetes-induced glomerular hyperfiltration. Acta Physiol (Oxf) 200: 3–10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H: Glomerular hyperfiltration in experimental diabetes mellitus: Potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Mueller TF, Luyckx VA: The natural history of residual renal function in transplant donors. J Am Soc Nephrol 23: 1462–1466, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Steiner RW: The risks of living kidney donation. N Engl J Med 374: 479–480, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Grams ME, Sang Y, Levey AS, Matsushita K, Ballew S, Chang AR, Chow EK, Kasiske BL, Kovesdy CP, Nadkarni GN, Shalev V, Segev DL, Coresh J, Lentine KL, Garg AX; Chronic Kidney Disease Prognosis Consortium: Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med 374: 411–421, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG; Chronic Kidney Disease Prognosis Consortium : Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 380: 1662–1673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groop PH, Thomas MC, Moran JL, Wadèn J, Thorn LM, Mäkinen VP, Rosengård-Bärlund M, Saraheimo M, Hietala K, Heikkilä O, Forsblom C; FinnDiane Study Group : The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58: 1651–1658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruggenenti P, Porrini EL, Gaspari F, Motterlini N, Cannata A, Carrara F, Cella C, Ferrari S, Stucchi N, Parvanova A, Iliev I, Dodesini AR, Trevisan R, Bossi A, Zaletel J, Remuzzi G; GFR Study Investigators : Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 35: 2061–2068, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silveiro SP, Friedman R, de Azevedo MJ, Canani LH, Gross JL: Five-year prospective study of glomerular filtration rate and albumin excretion rate in normofiltering and hyperfiltering normoalbuminuric NIDDM patients. Diabetes Care 19: 171–174, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Thomson HJ, Ekinci EI, Radcliffe NJ, Seah JM, MacIsaac RJ, Jerums G, Premaratne E: Elevated baseline glomerular filtration rate (GFR) is independently associated with a more rapid decline in renal function of patients with type 1 diabetes. J Diabetes Complications 30: 256–261, 2016 [DOI] [PubMed] [Google Scholar]
- 65.Bjornstad P, Cherney DZ, Snell-Bergeon JK, Pyle L, Rewers M, Johnson RJ, Maahs DM: Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with Type 1 diabetes. Nephrol Dial Transplant 30: 1706–1711, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG: Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52: 691–697, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Ficociello LH, Perkins BA, Roshan B, Weinberg JM, Aschengrau A, Warram JH, Krolewski AS: Renal hyperfiltration and the development of microalbuminuria in type 1 diabetes. Diabetes Care 32: 889–893, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomas MC, Moran JL, Harjutsalo V, Thorn L, Wadén J, Saraheimo M, Tolonen N, Leiviskä J, Jula A, Forsblom C, Groop PH; FinnDiane Study Group : Hyperfiltration in type 1 diabetes: Does it exist and does it matter for nephropathy? Diabetologia 55: 1505–1513, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Chiarelli F, Verrotti A, Morgese G: Glomerular hyperfiltration increases the risk of developing microalbuminuria in diabetic children. Pediatr Nephrol 9: 154–158, 1995 [DOI] [PubMed] [Google Scholar]
- 70.Rudberg S, Persson B, Dahlquist G: Increased glomerular filtration rate as a predictor of diabetic nephropathy--an 8-year prospective study. Kidney Int 41: 822–828, 1992 [DOI] [PubMed] [Google Scholar]
- 71.Denton KM, Fennessy PA, Alcorn D, Anderson WP: Morphometric analysis of the actions of angiotensin II on renal arterioles and glomeruli. Am J Physiol 262: F367–F372, 1992 [DOI] [PubMed] [Google Scholar]
- 72.Anderson S, Rennke HG, Brenner BM: Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest 77: 1993–2000, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bakris GL, Weir MR: Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: Is this a cause for concern? Arch Intern Med 160: 685–693, 2000 [DOI] [PubMed] [Google Scholar]
- 74.Lambers Heerspink HJ, de Borst MH, Bakker SJ, Navis GJ: Improving the efficacy of RAAS blockade in patients with chronic kidney disease. Nat Rev Nephrol 9: 112–121, 2013 [DOI] [PubMed] [Google Scholar]
- 75.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, Parving HH, Brenner BM, Shahinfar S, Lambers Heerspink HJ: An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80: 282–287, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Muskiet MH, Tonneijck L, Smits MM, Kramer MH, Heerspink HJ, van Raalte DH: Pleiotropic effects of type 2 diabetes management strategies on renal risk factors. Lancet Diabetes Endocrinol 3: 367–381, 2015 [DOI] [PubMed] [Google Scholar]
- 77.Chang AR, Chen Y, Still C, Wood GC, Kirchner HL, Lewis M, Kramer H, Hartle JE, Carey D, Appel LJ, Grams ME: Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int 90: 164–171, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Böhler J, Woitas R, Keller E, Reetze-Bonorden P, Schollmeyer PJ: Effect of nifedipine and captopril on glomerular hyperfiltration in normotensive man. Am J Kidney Dis 20: 132–139, 1992 [DOI] [PubMed] [Google Scholar]
- 79.Tietze IN, Sørensen SS, Ivarsen PR, Nielsen CB, Pedersen EB: Impaired renal haemodynamic response to amino acid infusion in essential hypertension during angiotensin converting enzyme inhibitor treatment. J Hypertens 15: 551–560, 1997 [DOI] [PubMed] [Google Scholar]
- 80.Mogensen CE, Andersen MJ: Increased kidney size and glomerular filtration rate in untreated juvenile diabetes: Normalization by insulin-treatment. Diabetologia 11: 221–224, 1975 [DOI] [PubMed] [Google Scholar]
- 81.Pessoa TD, Campos LC, Carraro-Lacroix L, Girardi AC, Malnic G: Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol 25: 2028–2039, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M: Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Skrtić M, Yang GK, Perkins BA, Soleymanlou N, Lytvyn Y, von Eynatten M, Woerle HJ, Johansen OE, Broedl UC, Hach T, Silverman M, Cherney DZ: Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia 57: 2599–2602, 2014 [DOI] [PubMed] [Google Scholar]
- 84.Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ: Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther 345: 464–472, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilbert RE: Sodium-glucose linked transporter-2 inhibitors: Potential for renoprotection beyond blood glucose lowering? Kidney Int 86: 693–700, 2014 [DOI] [PubMed] [Google Scholar]
- 86.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von EM, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators : Empagliflozin and progression of kidney disease in Type 2 diabetes. N Engl J Med 375: 323–334, 2016 [DOI] [PubMed] [Google Scholar]
- 87.Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C: Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 89: 3055–3061, 2004 [DOI] [PubMed] [Google Scholar]
- 88.von Scholten BJ, Hansen TW, Goetze JP, Persson F, Rossing P: Glucagon-like peptide 1 receptor agonist (GLP-1 RA): Long-term effect on kidney function in patients with type 2 diabetes. J Diabetes Complications 29: 670–674, 2015 [DOI] [PubMed] [Google Scholar]
- 89.Skov J, Pedersen M, Holst JJ, Madsen B, Goetze JP, Rittig S, Jonassen T, Frøkiaer J, Dejgaard A, Christiansen JS: Short-term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes: A randomized clinical trial. Diabetes Obes Metab 18: 581–589, 2016 [DOI] [PubMed] [Google Scholar]
- 90.Tonneijck L, Smits MM, Muskiet MH, Hoekstra T, Kramer MH, Danser AH, Diamant M, Joles JA, van Raalte DH: Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: A randomised, double-blind, placebo-controlled trial. Diabetologia 59: 1412–1421, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tonneijck L, Smits MM, Muskiet MH, Hoekstra T, Kramer MH, Danser AH, Ter Wee PM, Diamant M, Joles JA, van Raalte DH: Renal effects of DPP-4 inhibitor sitagliptin or GLP-1 receptor agonist liraglutide in overweight patients with Type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 39: 2042–2050, 2016 [DOI] [PubMed] [Google Scholar]
- 92.von Scholten BJ, Persson F, Rosenlund S, Hovind P, Faber J, Hansen TW, Rossing P: The effect of liraglutide on renal function: A randomized clinical trial [published online ahead of print October 17, 2016]. Diabetes Obes Metab 10.1111/dom.12808 [DOI] [PubMed] [Google Scholar]
- 93.Pistrosch F, Herbrig K, Kindel B, Passauer J, Fischer S, Gross P: Rosiglitazone improves glomerular hyperfiltration, renal endothelial dysfunction, and microalbuminuria of incipient diabetic nephropathy in patients. Diabetes 54: 2206–2211, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Arima S, Kohagura K, Takeuchi K, Taniyama Y, Sugawara A, Ikeda Y, Abe M, Omata K, Ito S: Biphasic vasodilator action of troglitazone on the renal microcirculation. J Am Soc Nephrol 13: 342–349, 2002 [DOI] [PubMed] [Google Scholar]
- 95.Asakura J, Hasegawa H, Takayanagi K, Shimazu T, Suge R, Shimizu T, Iwashita T, Tayama Y, Matsuda A, Kanozawa K, Araki N, Mitarai T: Renoprotective effect of pioglitazone by the prevention of glomerular hyperfiltration through the possible restoration of altered macula densa signaling in rats with type 2 diabetic nephropathy. Nephron, Exp Nephrol 122: 83–94, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Schmidt A, Pleiner J, Schaller G, Roden M, Dallinger S, Mayer G, Schmetterer L, Wolzt M: Renal hemodynamic effects of somatostatin are not related to inhibition of endogenous insulin release. Kidney Int 61: 1788–1793, 2002 [DOI] [PubMed] [Google Scholar]
- 97.Ter Maaten JC, Bakker SJ, Serné EH, Moshage HJ, Donker AJ, Gans RO: Insulin-mediated increases in renal plasma flow are impaired in insulin-resistant normal subjects. Eur J Clin Invest 30: 1090–1098, 2000 [DOI] [PubMed] [Google Scholar]
- 98.Ruggenenti P, Flores C, Aros C, Ene-Iordache B, Trevisan R, Ottomano C, Remuzzi G: Renal and metabolic effects of insulin lispro in type 2 diabetic subjects with overt nephropathy. Diabetes Care 26: 502–509, 2003 [DOI] [PubMed] [Google Scholar]
- 99.Lefèbvre PJ, Paquot N, Scheen AJ: Inhibiting or antagonizing glucagon: Making progress in diabetes care. Diabetes Obes Metab 17: 720–725, 2015 [DOI] [PubMed] [Google Scholar]
- 100.Wang X, Yang J, Chang B, Shan C, Xu Y, Zheng M, Wang Y, Ren H, Chen L: Glucagon secretion is increased in patients with Type 2 diabetic nephropathy. J Diabetes Complications 30: 488–493, 2016 [DOI] [PubMed] [Google Scholar]
- 101.Jain N, Reilly RF: Effects of dietary interventions on incidence and progression of CKD. Nat Rev Nephrol 10: 712–724, 2014 [DOI] [PubMed] [Google Scholar]
- 102.Juraschek SP, Appel LJ, Anderson CA, Miller ER 3rd: Effect of a high-protein diet on kidney function in healthy adults: Results from the OmniHeart trial. Am J Kidney Dis 61: 547–554, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vallon V, Blantz RC, Thomson S: Glomerular hyperfiltration and the salt paradox in early [corrected] type 1 diabetes mellitus: A tubulo-centric view. J Am Soc Nephrol 14: 530–537, 2003 [DOI] [PubMed] [Google Scholar]
- 104.Lambers Heerspink HJ, Navis G, Ritz E: Salt intake in kidney disease--a missed therapeutic opportunity? Nephrol Dial Transplant 27: 3435–3442, 2012 [DOI] [PubMed] [Google Scholar]
- 105.Wuerzner G, Pruijm M, Maillard M, Bovet P, Renaud C, Burnier M, Bochud M: Marked association between obesity and glomerular hyperfiltration: A cross-sectional study in an African population. Am J Kidney Dis 56: 303–312, 2010 [DOI] [PubMed] [Google Scholar]
- 106.Levey AS, Kramer H: Obesity, glomerular hyperfiltration, and the surface area correction. Am J Kidney Dis 56: 255–258, 2010 [DOI] [PubMed] [Google Scholar]
- 107.Zingerman B, Herman-Edelstein M, Erman A, Bar Sheshet Itach S, Ori Y, Rozen-Zvi B, Gafter U, Chagnac A: Effect of acetazolamide on obesity-induced glomerular hyperfiltration: A randomized controlled trial. PLoS One 10: e0137163, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hannedouche T, Lazaro M, Delgado AG, Boitard C, Lacour B, Grünfeld JP: Feedback-mediated reduction in glomerular filtration during acetazolamide infusion in insulin-dependent diabetic patients. Clin Sci (Lond) 81: 457–464, 1991 [DOI] [PubMed] [Google Scholar]
- 109.Slomowitz LA, Bergamo R, Hirschberg R, Grosvenor M, Kopple JD: Enalapril attenuates the renal hemodynamic effect of acetazolamide in patients with diabetes mellitus: Possible implications for tubuloglomerular feedback. Am J Nephrol 16: 315–319, 1996 [DOI] [PubMed] [Google Scholar]
- 110.Skøtt P, Hommel E, Bruun NE, Arnold-Larsen S, Parving HH: Effects of acetazolamide on kidney function in type 1 (insulin-dependent) diabetic patients with diabetic nephropathy. Diabetologia 31: 806–810, 1988 [DOI] [PubMed] [Google Scholar]
- 111.Huang X, Dorhout Mees E, Vos P, Hamza S, Braam B: Everything we always wanted to know about furosemide but were afraid to ask. Am J Physiol Renal Physiol 310: F958–F971, 2016 [DOI] [PubMed] [Google Scholar]
- 112.Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM; Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group : Effect of finerenone on albuminuria in patients with diabetic nephropathy: A randomized clinical trial. JAMA 314: 884–894, 2015 [DOI] [PubMed] [Google Scholar]
- 113.Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Patni R, Beckerman B: Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 1: 940–951, 2006 [DOI] [PubMed] [Google Scholar]
- 114.Morales E, Millet VG, Rojas-Rivera J, Huerta A, Gutiérrez E, Gutiérrez-Solís E, Egido J, Praga M: Renoprotective effects of mineralocorticoid receptor blockers in patients with proteinuric kidney diseases. Nephrol Dial Transplant 28: 405–412, 2013 [DOI] [PubMed] [Google Scholar]
- 115.de Paula RB, da Silva AA, Hall JE: Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension 43: 41–47, 2004 [DOI] [PubMed] [Google Scholar]
- 116.Goddard J, Johnston NR, Hand MF, Cumming AD, Rabelink TJ, Rankin AJ, Webb DJ: Endothelin-A receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure: A comparison of selective and combined endothelin receptor blockade. Circulation 109: 1186–1193, 2004 [DOI] [PubMed] [Google Scholar]
- 117.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, Correa-Rotter R, Kohan D, Lambers Heerspink HJ, Makino H, Perkovic V, Pritchett Y, Remuzzi G, Tobe SW, Toto R, Viberti G, Parving HH: The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 25: 1083–1093, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G; ASCEND Study Group : Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 21: 527–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kohan DE, Pritchett Y, Molitch M, Wen S, Garimella T, Audhya P, Andress DL: Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol 22: 763–772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, Cheung AK, Coresh J: GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 64: 821–835, 2014 [DOI] [PubMed] [Google Scholar]
- 121.Klessens CQ, Woutman TD, Veraar KA, Zandbergen M, Valk EJ, Rotmans JI, Wolterbeek R, Bruijn JA, Bajema IM: An autopsy study suggests that diabetic nephropathy is underdiagnosed. Kidney Int 90: 149–156, 2016 [DOI] [PubMed] [Google Scholar]
- 122.Porrini E, Ruggenenti P, Mogensen CE, Barlovic DP, Praga M, Cruzado JM, Hojs R, Abbate M, de Vries AP; ERA-EDTA diabesity working group : Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol 3: 382–391, 2015 [DOI] [PubMed] [Google Scholar]
- 123.Jaffa AA, Rust PF, Mayfield RK: Kinin, a mediator of diabetes-induced glomerular hyperfiltration. Diabetes 44: 156–160, 1995 [DOI] [PubMed] [Google Scholar]
- 124.Sasson AN, Cherney DZ: Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes 3: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tikellis C, Brown R, Head GA, Cooper ME, Thomas MC: Angiotensin-converting enzyme 2 mediates hyperfiltration associated with diabetes. Am J Physiol Renal Physiol 306: F773–F780, 2014 [DOI] [PubMed] [Google Scholar]
- 126.Zhang PL, Mackenzie HS, Troy JL, Brenner BM: Effects of an atrial natriuretic peptide receptor antagonist on glomerular hyperfiltration in diabetic rats. J Am Soc Nephrol 4: 1564–1570, 1994 [DOI] [PubMed] [Google Scholar]
- 127.Serri O, Beauregard H, Brazeau P, Abribat T, Lambert J, Harris A, Vachon L: Somatostatin analogue, octreotide, reduces increased glomerular filtration rate and kidney size in insulin-dependent diabetes. JAMA 265: 888–892, 1991 [PubMed] [Google Scholar]
- 128.Christiansen JS, Gammelgaard J, Frandsen M, Orskov H, Parving HH: Kidney function and size in type 1 (insulin-dependent) diabetic patients before and during growth hormone administration for one week. Diabetologia 22: 333–337, 1982 [DOI] [PubMed] [Google Scholar]
- 129.Hirschberg R, Brunori G, Kopple JD, Guler HP: Effects of insulin-like growth factor I on renal function in normalmen. Kidney Int 43: 387–397, 1993 [DOI] [PubMed] [Google Scholar]
- 130.Michell AR, Debnam ES, Unwin RJ: Regulation of renal function by the gastrointestinal tract: Potential role of gut-derived peptides and hormones. Annu Rev Physiol 70: 379–403, 2008 [DOI] [PubMed] [Google Scholar]
- 131.Kalk WJ, Osler C, Taylor D, Panz VR, Esse JD, Reinach SG: The prevalence of micro-albuminuria and glomerular hyperfiltration in young patients with IDDM. Diabetes Res Clin Pract 8: 145–153, 1990 [DOI] [PubMed] [Google Scholar]
- 132.Azevedo MJ, Gross JL: Follow-up of glomerular hyperfiltration in normoalbuminuric type 1 (insulin-dependent) diabetic patients. Diabetologia 34: 611, 1991 [DOI] [PubMed] [Google Scholar]
- 133.Marre M, Hallab M, Roy J, Lejeune JJ, Jallet P, Fressinaud P: Glomerular hyperfiltration in type I, type II, and secondary diabetes. J Diabetes Complications 6: 19–24, 1992 [DOI] [PubMed] [Google Scholar]
- 134.Cotroneo P, Manto A, Todaro L, Manto A Jr, Pitocco D, Saponara C, Vellante C, Maussier ML, D’Errico G, Magnani P, Ghirlanda G: Hyperfiltration in patients with type I diabetes mellitus: A prevalence study. Clin Nephrol 50: 214–217, 1998 [PubMed] [Google Scholar]
- 135.Caramori ML, Gross JL, Pecis M, de Azevedo MJ: Glomerular filtration rate, urinary albumin excretion rate, and blood pressure changes in normoalbuminuric normotensive type 1 diabetic patients: An 8-year follow-up study. Diabetes Care 22: 1512–1516, 1999 [DOI] [PubMed] [Google Scholar]
- 136.Dahlquist G, Stattin EL, Rudberg S: Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrol Dial Transplant 16: 1382–1386, 2001 [DOI] [PubMed] [Google Scholar]
- 137.Amin R, Turner C, van Aken S, Bahu TK, Watts A, Lindsell DR, Dalton RN, Dunger DB: The relationship between microalbuminuria and glomerular filtration rate in young type 1 diabetic subjects: The Oxford Regional prospective Study. Kidney Int 68: 1740–1749, 2005 [DOI] [PubMed] [Google Scholar]
- 138.Vervoort G, Veldman B, Berden JH, Smits P, Wetzels JF: Glomerular hyperfiltration in type 1 diabetes mellitus results from primary changes in proximal tubular sodium handling without changes in volume expansion. Eur J Clin Invest 35: 330–336, 2005 [DOI] [PubMed] [Google Scholar]
- 139.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M; International Diabetic Nephopathy Study Group : The early natural history of nephropathy in Type 1 Diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 54: 2164–2171, 2005 [DOI] [PubMed] [Google Scholar]
- 140.Bulum T, Kolarić B, Prkacin I, Duvnjak L: Hyperfiltration in normoalbuminuric type 1 diabetic patients: Relationship with urinary albumin excretion rate. Coll Antropol 37: 471–476, 2013 [PubMed] [Google Scholar]
- 141.Palmisano JJ, Lebovitz HE: Renal function in black Americans with type II diabetes. J Diabet Complications 3: 40–44, 1989 [DOI] [PubMed] [Google Scholar]
- 142.Lebovitz HE, Palmisano J: Cross-sectional analysis of renal function in black Americans with NIDDM. Diabetes Care 13: 1186–1190, 1990 [DOI] [PubMed] [Google Scholar]
- 143.Nowack R, Raum E, Blum W, Ritz E: Renal hemodynamics in recent-onset type II diabetes. Am J Kidney Dis 20: 342–347, 1992 [DOI] [PubMed] [Google Scholar]
- 144.Vora JP, Dolben J, Dean JD, Thomas D, Williams JD, Owens DR, Peters JR: Renal hemodynamics in newly presenting non-insulin dependent diabetes mellitus. Kidney Int 41: 829–835, 1992 [DOI] [PubMed] [Google Scholar]
- 145.Gragnoli G, Signorini AM, Tanganelli I, Fondelli C, Borgogni P, Borgogni L, Vattimo A, Ferrari F, Guercia M: Prevalence of glomerular hyperfiltration and nephromegaly in normo- and microalbuminuric type 2 diabetic patients. Nephron 65: 206–211, 1993 [DOI] [PubMed] [Google Scholar]
- 146.Silveiro SP, Friedman R, Gross JL: Glomerular hyperfiltration in NIDDM patients without overt proteinuria. Diabetes Care 16: 115–119, 1993 [DOI] [PubMed] [Google Scholar]
- 147.Bruce R, Rutland M, Cundy T: Glomerular hyperfiltration in young Polynesians with type 2 diabetes. Diabetes Res Clin Pract 25: 155–160, 1994 [DOI] [PubMed] [Google Scholar]
- 148.Lee KU, Park JY, Hwang IR, Hong SK, Kim GS, Moon DH, Kim SB, Park JS: Glomerular hyperfiltration in Koreans with non-insulin-dependent diabetes mellitus. Am J Kidney Dis 26: 722–726, 1995 [DOI] [PubMed] [Google Scholar]
- 149.Keller CK, Bergis KH, Fliser D, Ritz E: Renal findings in patients with short-term type 2 diabetes. J Am Soc Nephrol 7: 2627–2635, 1996 [DOI] [PubMed] [Google Scholar]
- 150.Chaiken RL, Eckert-Norton M, Bard M, Banerji MA, Palmisano J, Sachimechi I, Lebovitz HE: Hyperfiltration in African-American patients with type 2 diabetes. Cross-sectional and longitudinal data. Diabetes Care 21: 2129–2134, 1998 [DOI] [PubMed] [Google Scholar]
- 151.Guízar JM, Kornhauser C, Malacara JM, Amador N, Barrera JA, Esparza R: Renal functional reserve in patients with recently diagnosed Type 2 diabetes mellitus with and without microalbuminuria. Nephron 87: 223–230, 2001 [DOI] [PubMed] [Google Scholar]
- 152.Premaratne E, Macisaac RJ, Tsalamandris C, Panagiotopoulos S, Smith T, Jerums G: Renal hyperfiltration in type 2 diabetes: Effect of age-related decline in glomerular filtration rate. Diabetologia 48: 2486–2493, 2005 [DOI] [PubMed] [Google Scholar]
- 153.Jin Y, Moriya T, Tanaka K, Matsubara M, Fujita Y: Glomerular hyperfiltration in non-proteinuric and non-hypertensive Japanese type 2 diabetic patients. Diabetes Res Clin Pract 71: 264–271, 2006 [DOI] [PubMed] [Google Scholar]
- 154.Guo K, Zhang L, Zhao F, Lu J, Pan P, Yu H, Bao Y, Chen H, Jia W: Prevalence of chronic kidney disease and associated factors in Chinese individuals with type 2 diabetes: Cross-sectional study. J Diabetes Complications 30: 803–810, 2016 [DOI] [PubMed] [Google Scholar]
- 155.Zhao F, Zhang L, Lu J, Guo K, Wu M, Yu H, Zhang M, Bao Y, Chen H, Jia W: The chronic kidney disease epidemiology collaboration equation improves the detection of hyperfiltration in Chinese diabetic patients. Int J Clin Exp Med 8: 22084–22097, 2015 [PMC free article] [PubMed] [Google Scholar]
- 156.Mogensen CE: Early glomerular hyperfiltration in insulin-dependent diabetics and late nephropathy. Scand J Clin Lab Invest 46: 201–206, 1986 [DOI] [PubMed] [Google Scholar]
- 157.Lervang HH, Jensen S, Brøchner-Mortensen J, Ditzel J: Early glomerular hyperfiltration and the development of late nephropathy in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 31: 723–729, 1988 [DOI] [PubMed] [Google Scholar]
- 158.Lervang HH, Jensen S, Brøchner-Mortensen J, Ditzel J: Does increased glomerular filtration rate or disturbed tubular function early in the course of childhood type 1 diabetes predict the development of nephropathy? Diabet Med 9: 635–640, 1992 [DOI] [PubMed] [Google Scholar]
- 159.Bognetti E, Meschi F, Bonfanti R, Gianolli L, Chiumello G: Decrease of glomerular hyperfiltration in short-term diabetic adolescents without microalbuminuria. Diabetes Care 16: 120–124, 1993 [DOI] [PubMed] [Google Scholar]
- 160.Yip JW, Jones SL, Wiseman MJ, Hill C, Viberti G: Glomerular hyperfiltration in the prediction of nephropathy in IDDM: A 10-year follow-up study. Diabetes 45: 1729–1733, 1996 [DOI] [PubMed] [Google Scholar]
- 161.Zerbini G, Bonfanti R, Meschi F, Bognetti E, Paesano PL, Gianolli L, Querques M, Maestroni A, Calori G, Del Maschio A, Fazio F, Luzi L, Chiumello G: Persistent renal hypertrophy and faster decline of glomerular filtration rate precede the development of microalbuminuria in type 1 diabetes. Diabetes 55: 2620–2625, 2006 [DOI] [PubMed] [Google Scholar]
- 162.Mogensen CE, Christensen CK: Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 311: 89–93, 1984 [DOI] [PubMed] [Google Scholar]
- 163.Mogensen CE, Christensen CK: Blood pressure changes and renal function in incipient and overt diabetic nephropathy. Hypertension 7: II64–II73, 1985 [DOI] [PubMed] [Google Scholar]
- 164.Jones SL, Wiseman MJ, Viberti GC: Glomerular hyperfiltration as a risk factor for diabetic nephropathy: Five-year report of a prospective study. Diabetologia 34: 59–60, 1991 [DOI] [PubMed] [Google Scholar]
- 165.Bangstad HJ, Østerby R, Rudberg S, Hartmann A, Brabrand K, Hanssen KF: Kidney function and glomerulopathy over 8 years in young patients with Type I (insulin-dependent) diabetes mellitus and microalbuminuria. Diabetologia 45: 253–261, 2002 [DOI] [PubMed] [Google Scholar]
- 166.Mathiesen ER, Feldt-Rasmussen B, Hommel E, Deckert T, Parving HH: Stable glomerular filtration rate in normotensive IDDM patients with stable microalbuminuria. A 5-year prospective study. Diabetes Care 20: 286–289, 1997 [DOI] [PubMed] [Google Scholar]
- 167.Couper JJ, Clarke CF, Byrne GC, Jones TW, Donaghue KC, Nairn J, Boyce D, Russell M, Stephens M, Raymond J, Bates DJ, McCaul K: Progression of borderline increases in albuminuria in adolescents with insulin-dependent diabetes mellitus. Diabet Med 14: 766–771, 1997 [DOI] [PubMed] [Google Scholar]
- 168.Murussi M, Gross JL, Silveiro SP: Glomerular filtration rate changes in normoalbuminuric and microalbuminuric Type 2 diabetic patients and normal individuals A 10-year follow-up. J Diabetes Complications 20: 210–215, 2006 [DOI] [PubMed] [Google Scholar]
- 169.Murussi M, Campagnolo N, Beck MO, Gross JL, Silveiro SP: High-normal levels of albuminuria predict the development of micro- and macroalbuminuria and increased mortality in Brazilian Type 2 diabetic patients: An 8-year follow-up study. Diabet Med 24: 1136–1142, 2007 [DOI] [PubMed] [Google Scholar]
- 170.Viswanathan V, Tilak P, Kumpatla S: Risk factors associated with the development of overt nephropathy in type 2 diabetes patients: A 12 years observational study. Indian J Med Res 136: 46–53, 2012 [PMC free article] [PubMed] [Google Scholar]
- 171.Yokoyama H, Kanno S, Takahashi S, Yamada D, Honjo J, Saito K, Sone H, Haneda M: Risks for glomerular filtration rate decline in association with progression of albuminuria in type 2 diabetes. Nephrol Dial Transplant 26: 2924–2930, 2011 [DOI] [PubMed] [Google Scholar]
- 172.Tonneijck L, Muskiet MH, Smits MM, van Raalte DH, Diamant M: Combining incretin-based drugs and RAAS inhibitors: More cons than pros? Lancet Diabetes Endocrinol 2: 684–685, 2014 [DOI] [PubMed] [Google Scholar]
- 173.Tanaka T, Higashijima Y, Wada T, Nangaku M: The potential for renoprotection with incretin-based drugs. Kidney Int 86: 701–711, 2014 [DOI] [PubMed] [Google Scholar]
- 174.Kinebuchi S, Kazama JJ, Satoh M, Sakai K, Nakayama H, Yoshizawa H, Narita I, Suzuki E, Gejyo F: Short-term use of continuous positive airway pressure ameliorates glomerular hyperfiltration in patients with obstructive sleep apnoea syndrome. Clin Sci (Lond) 107: 317–322, 2004 [DOI] [PubMed] [Google Scholar]
- 175.Holst JJ, Madsbad S: Mechanisms of surgical control of type 2 diabetes: GLP-1 is key factor. Surg Obes Relat Dis 12: 1236–1242, 2016 [DOI] [PubMed] [Google Scholar]
- 176.Luippold G, Beilharz M, Mühlbauer B: Chronic renal denervation prevents glomerular hyperfiltration in diabetic rats. Nephrol Dial Transplant 19: 342–347, 2004 [DOI] [PubMed] [Google Scholar]
- 177.Cherney DZ, Miller JA, Scholey JW, Bradley TJ, Slorach C, Curtis JR, Dekker MG, Nasrallah R, Hébert RL, Sochett EB: The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes 57: 688–695, 2008 [DOI] [PubMed] [Google Scholar]
- 178.McAdam BF, Byrne D, Morrow JD, Oates JA: Contribution of cyclooxygenase-2 to elevated biosynthesis of thromboxane A2 and prostacyclin in cigarette smokers. Circulation 112: 1024–1029, 2005 [DOI] [PubMed] [Google Scholar]
- 179.Cherney DZ, Konvalinka A, Zinman B, Diamandis EP, Soosaipillai A, Reich H, Lorraine J, Lai V, Scholey JW, Miller JA: Effect of protein kinase Cbeta inhibition on renal hemodynamic function and urinary biomarkers in humans with type 1 diabetes: A pilot study. Diabetes Care 32: 91–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nagahama T, Hayashi K, Ozawa Y, Takenaka T, Saruta T: Role of protein kinase C in angiotensin II-induced constriction of renal microvessels. Kidney Int 57: 215–223, 2000 [DOI] [PubMed] [Google Scholar]
- 181.Wahren J, Kallas A, Sima AA: The clinical potential of C-peptide replacement in type 1 diabetes. Diabetes 61: 761–772, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nordquist L, Wahren J: C-Peptide: The missing link in diabetic nephropathy? Rev Diabet Stud 6: 203–210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.