Abstract
Tight control of extracellular and intracellular inorganic phosphate (Pi) levels is critical to most biochemical and physiologic processes. Urinary Pi is freely filtered at the kidney glomerulus and is reabsorbed in the renal tubule by the action of the apical sodium-dependent phosphate transporters, NaPi-IIa/NaPi-IIc/Pit2. However, the molecular identity of the protein(s) participating in the basolateral Pi efflux remains unknown. Evidence has suggested that xenotropic and polytropic retroviral receptor 1 (XPR1) might be involved in this process. Here, we show that conditional inactivation of Xpr1 in the renal tubule in mice resulted in impaired renal Pi reabsorption. Analysis of Pi transport in primary cultures of proximal tubular cells or in freshly isolated renal tubules revealed that this Xpr1 deficiency significantly affected Pi efflux. Further, mice with conditional inactivation of Xpr1 in the renal tubule exhibited generalized proximal tubular dysfunction indicative of Fanconi syndrome, characterized by glycosuria, aminoaciduria, calciuria, and albuminuria. Dramatic alterations in the renal transcriptome, including a significant reduction in NaPi-IIa/NaPi-IIc expression, accompanied these functional changes. Additionally, Xpr1-deficient mice developed hypophosphatemic rickets secondary to renal dysfunction. These results identify XPR1 as a major regulator of Pi homeostasis and as a potential therapeutic target in bone and kidney disorders.
Keywords: phosphate homeostasis, kidney, retroviral receptor XPR1, Fanconi syndrome, hypophosphatemic rickets
The xenotropic and polytropic retrovirus receptor 1 (XPR1) has long been considered as a candidate component of the inorganic phosphate (Pi) efflux mechanism because of its high degree of homology with PHO1 protein in plants, which has been shown to mediate Pi transport from roots to shoots.1,2 However, evidence has only recently emerged supporting a role of XPR1 in Pi transport. Battini and colleagues have shown in vitro that XPR1 depletion or inhibition results in a marked decrease in Pi efflux.3 They also demonstrated that XBRD, a XPR1 ligand derived from the X-MLV envelope glycoprotein, could efficiently inhibit Pi efflux, thereby providing evidence on the direct role of XPR1 in Pi transport. Wege and Poirier have demonstrated that ectopically expressed mouse XPR1 mediates Pi efflux in tobacco leaves.4 Most recently, Legati et al. have shown an association between genetic polymorphisms in Xpr1 and primary familial brain calcification disorder.5 However, the role of XPR1 in the maintenance of Pi homeostasis remains unknown. Here, we addressed this issue in mice deficient for Xpr1 in the nephron.
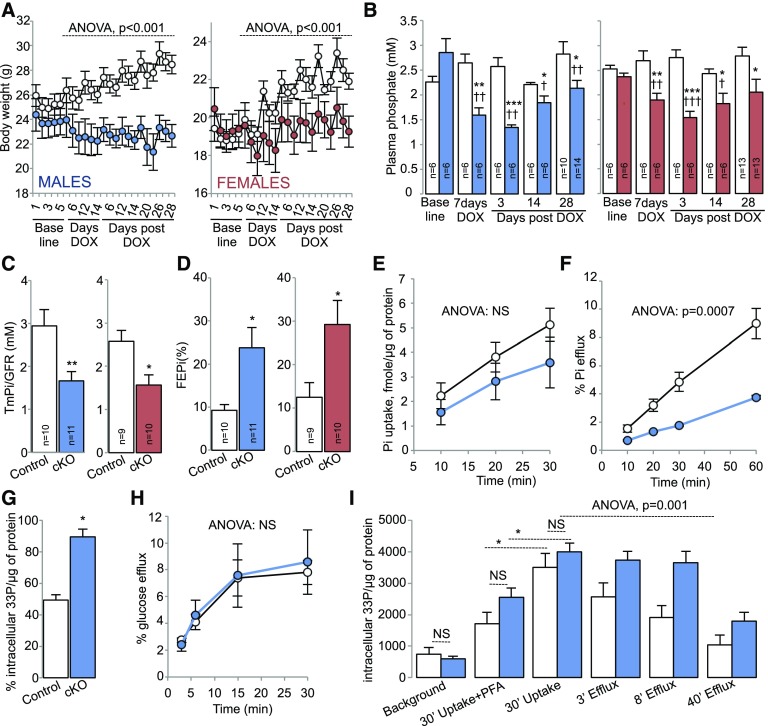
Because Xpr1-null mice exhibit embryonic lethality (viable pups: wild type, 254; heterozygous, 384; null, 0), we generated mice with a doxycycline (DOX)-inducible, Pax8-rtTA–driven,6 conditional deletion of Xpr1 in the renal tubule (Xpr1lox/lox/Pax8-rtTA/LC1 mice, hereafter referred to as conditional knockout [cKO] mice). Littermate Xpr1lox/lox mice treated with DOX were used as controls. Males and females were investigated separately to assess possible sex differences. As shown in Supplemental Figure 1, DOX treatment resulted in a significant reduction in Xpr1 mRNA and protein levels in whole kidneys and in microdissected proximal tubules of cKO mice. The decrease in renal XPR1 expression was accompanied by a progressively increasing difference in body weight between control and cKO mice that reached −20.4% (cKO males) and −12.1% (cKO females) 28 days after the end of DOX treatment (Figure 1A). Assessment of renal Pi handling revealed that cKO mice exhibited hypophosphatemia (Figure 1B), phosphaturia (transient in males, Supplemental Figure 2), inappropriately low maximal tubular reabsorption of Pi per volume of glomerular filtrate (TmPi/GFR) (Figure 1C), and significantly increased fractional excretion of Pi (Figure 1D). Furthermore, we assessed the role of XPR1 in Pi efflux in primary cultures of proximal tubular cells isolated from kidneys of DOX-untreated control and cKO mice. Xpr1 deficiency was induced ex vivo by 24 hours of DOX exposure. Twenty four hours after the end of DOX treatment, the Xpr1 mRNA expression was significantly decreased in the proximal tubular cells isolated from cKO mice, as assessed by quantitative PCR (Xpr1 mRNA expression in cKO versus control cells: 18.9±7.3%; n=3; P=0.01, t test). As shown in Figure 1E, proximal tubular cells from cKO mice had a nonsignificant trend toward lower [33Pi]phosphate uptake. In contrast, [33P]phosphate efflux was strongly affected by XPR1 deficiency (Figure 1F). The latter correlated with higher percentage of [33P]phosphate remaining in the proximal tubular cells from cKO mice after 60 minutes of efflux (Figure 1G). Importantly, efflux of [14C]glucose was not different between proximal tubular cells isolated from kidneys of control or cKO mice (Figure 1H), indicating that the short-term ex-vivo Xpr1 deficiency did not result in the overall depression of efflux transport activity. The Pi efflux was also evaluated in renal tubules freshly isolated from kidneys of control or cKO mice treated with DOX for 5 days. As shown in Figure 1I, the 30-minute [33P]phosphate uptake was similar in both genotypes, and was significantly reduced in the presence of phosphonoformic acid (PFA), a low potency competitive inhibitor of apical Na+/Pi cotransporters. The persistent Pi uptake in the presence of PFA likely results from partial inhibition of apical Pi transport, but importantly, the fraction of PFA-sensitive Pi uptake was not different between genotypes. At the end of the 30-minute uptake period, the [33P]phosphate was removed from the bath and Pi efflux was measured. In tubules isolated from kidneys of cKO mice, the Pi efflux was significantly slower compared with control mice, providing further evidence for an XPR1-mediated Pi efflux. Pi efflux is generally considered to occur through the basolateral membrane; indeed, an apical Pi efflux is very unlikely because intracellular Pi concentration remains far below the thermodynamic equilibrium for Na+-dependent Pi transporters.7 Collectively, these experiments demonstrate a critical role of XPR1 in Pi efflux from renal tubular cells, and suggest Xpr1 deficiency as the primary cause of phosphaturia in cKO mice.
Figure 1.
Altered renal handling of Pi in cKO mice. White circles/bars indicate control mice. Blue and red circles/bars indicate male or female cKO mice, respectively. (A) Body weight in control and cKO male (left panel) or female (right panel) mice. The body weight was measured during 5 days preceding DOX treatment (baseline), during the 2-week period of DOX treatment (days DOX), and during 28 days after DOX withdrawal (days post DOX). n=6 in each group; ANOVA. (B) Plasma Pi levels in control and cKO male (left panel) or female (right panel) mice. Plasma Pi concentration was measured on the day preceding the 2-week period of DOX treatment (baseline), on day 7 of DOX treatment (7 days DOX), and on days 3, 14, 21, and 28 after DOX withdrawal (days post DOX). *P<0.05; **P<0.01; ***P<0.001; t test, statistical significance between control and cKO mice. †P<0.05; ††P<0.01; †††P<0.001; t test, statistical significance between plasma Pi levels measured at baseline and plasma Pi levels measured on day 7 of DOX treatment or after DOX withdrawal (days 3, 14, and 28). (C) TmPi/GFR in control and cKO male (left panel) or female (right panel) mice. The TmPi/GFR was determined on day 28 after DOX withdrawal. *P<0.05; **P<0.01; t test. (D) FEPi in control and cKO male (left panel) or female (right panel) mice. The FEPi was determined on day 28 after DOX withdrawal. *P<0.05; t test. (E) [33Pi]phosphate uptake in primary cultures of proximal tubule cells isolated from DOX-untreated control or male cKO mice. Cells were exposed to DOX for 24 hours and the [33Pi]phosphate uptake was measured 24 hours after the end of DOX treatment (see Supplemental Material for details). n=4 in each group; ANOVA. (F) [33P]phosphate efflux from primary cultures of control or cKO proximal tubule cells. n=4 in each group; ANOVA. For (E), (F), and (H), white and blue circles indicate primary cultures of control or cKO proximal tubule cells, respectively. (G) [33P]phosphate remaining in primary cultures of control or cKO proximal tubule cells at the end of the efflux experiment (60 minutes of efflux); n=4 in each group. *P<0.05; t test. (H) [14C]glucose efflux from primary cultures of control or cKO proximal tubule cells. n=4 in each group; ANOVA. (I) [33P]phosphate uptake (30 minutes) and efflux (3 minutes, 8 minutes, and 40 minutes) from renal tubules freshly isolated from kidneys of control or cKO mice induced with DOX for 5 days (for efflux experiments, the 30-minute [33P]phosphate uptake was set as the zero time point). Pi uptake was determined in the presence or absence of 5 mmol PFA. Pi efflux was measured in the presence of 5 mmol PFA (see Supplemental Material for details). Background represents nonspecific binding of [33P]phosphate to the renal tubules. n=4 in each group. The difference in the efflux kinetics was evaluated by ANOVA (genotype–time interaction). The differences in background, 30’ uptake + PFA, and 30’ uptake conditions was evaluated by t test. Numbers inside of bars represent the number of animals. Data are mean±SEM. *P<0.05. FEPi, fractional excretion of Pi.
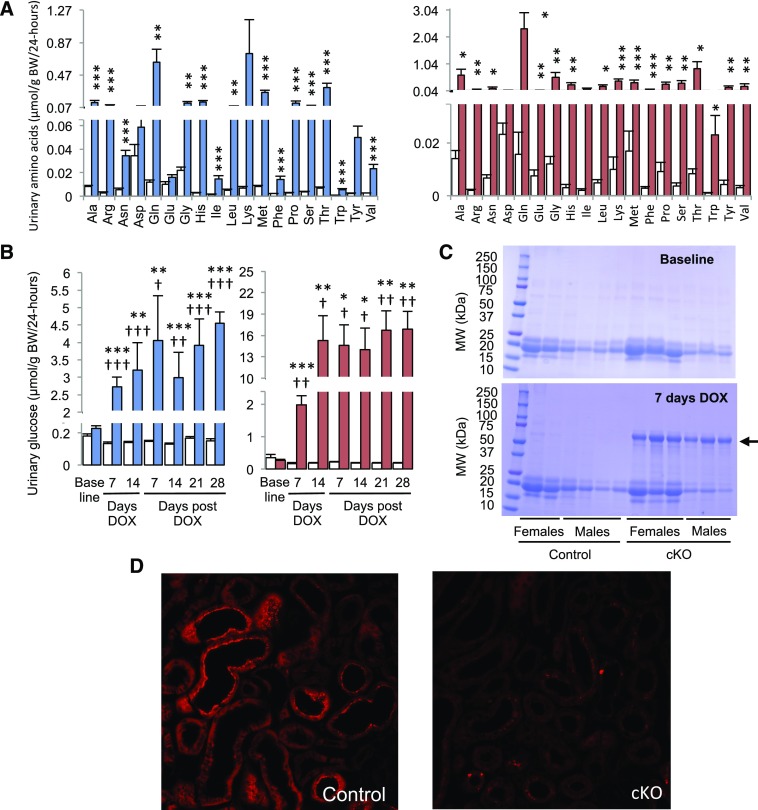
Analysis of urine samples revealed that 1 week after beginning DOX treatment, cKO mice developed generalized proximal tubule dysfunction, or renal Fanconi syndrome, characterized by aminoaciduria (Figure 2A), glycosuria (Figure 2B), albuminuria (Figure 2C), magnesuria (Supplemental Figure 3A), calciuria (Supplemental Figure 3B), lower urinary pH (Supplemental Figure 4A), polyuria (Supplemental Figure 4B), and decreased urine osmolality (Supplemental Figure 4C). Transcriptome analysis (GSE87450) of kidneys from control and cKO mice (males) revealed dramatic changes in expression levels of RNAs encoding proteins involved in apical Pi reabsorption (NaPi-IIa [Slc34a1]: −7.19-fold; NaPi-IIc [Slc34a3]: −25.37-fold), glucose reabsorption (SGLT2 [Slc5a2]: −2.88-fold; GLUT2 [Slc2a2]: −2.87-fold), amino acid transport (LAT2 [Slc7a8]: −4.59-fold; BAT1 [Slc7a9]: −4.24-fold; LAT1 [Slc7a7]: −3.36-fold; 4F2hc [Slc3a2]: −1.83-fold), and in endocytic receptors required for reuptake of filtered albumin in the proximal tubule (megalin [Lrp2]: −3.52-fold; cubilin [Cubn]: −3.40-fold) (Supplemental Table 1). The impairment in tubular albumin reabsorption was assessed functionally by confocal microscopy analysis of kidney slices prepared from kidneys of mice intravenously injected with fluorescent albumin (Texas Red albumin). As shown in Figure 2D, Texas Red albumin was abundantly present in the subapical region of the proximal tubular cells in kidneys of control mice, whereas the fluorescence intensity was significantly lower in kidneys of cKO mice.
Figure 2.
XPR1 deficiency in the nephron is associated with aminoaciduria, glucosuria, albuminuria, and impaired albumin reabsorption in the proximal tubule. (A) Aminoaciduria in cKO mice. The urinary excretion rate of 19 of 20 proteinogenic amino acids (at the exception of cysteine) was measured by mass spectrometry on urine collected on day 28 after DOX withdrawal. White bars indicate the urinary excretion rates of amino acids in control mice. Blue and red bars indicate the urinary excretion rates of amino acids in male or female cKO mice, respectively; n=6 in each group. *P<0.05; **P<0.01; ***P<0.001; t test. (B) Glucosuria in cKO mice. The urinary excretion rate of glucose was measured on urine collected on the day preceding the 2-week period of DOX treatment (baseline), on days 7 and 14 of DOX treatment (days DOX), and on days 7, 14, 21, and 28 after DOX withdrawal (days post DOX). White bars indicate the urinary excretion rates of glucose in control mice (n=6 for males and n=4 for females). Blue and red bars indicate the urinary excretion rates of glucose in male or female cKO mice, respectively (n=6 for males and n=6 for females). *P<0.05; **P<0.01; ***P<0.001; t test, statistical significance between control and cKO mice. †P<0.05; ††P<0.01; †††P<0.001; t test, statistical significance between the urinary excretion rates of glucose measured at baseline and the urinary excretion rates of glucose measured during the period of DOX treatment or after DOX withdrawal. (C) Albuminuria associated with XPR1 deficiency. Urine (5 μl) was run on SDS-PAGE and stained with Coomassie blue. Urine was collected from the same mice on the day preceding the 2-week period of DOX treatment (baseline) or on day 7 of DOX treatment (7 days DOX). The albumin band (67 kDa) is indicated by an arrow. (D) Decreased tubular reabsorption of Texas Red (TR)-albumin in kidneys of cKO mice. Confocal microscopy analysis of kidney slices prepared from perfusion-fixed kidneys of TR-albumin–injected control (left panel) or cKO (right panel) mice. Mice were euthanized 5 minutes after intravenous injection of TR-albumin. Data are mean±SEM. Original magnification, ×40 in D.
The kidneys of cKO mice exhibited reduced expression of genes encoding mitochondrially located proteins (Supplemental Figure 5, A and B) despite normal mitochondrial biogenesis (Supplemental Figure 5C) and apparently normal mitochondria, as examined by electron microscopy (Supplemental Figure 5E). The NAD+/NADH ratio was significantly reduced in kidneys of cKO mice, suggesting a shift in the metabolic status resulting from the XPR1 deficiency (Supplemental Figure 5D).
The GFR was significantly decreased in male cKO mice, along with an increase in plasma creatinine levels in cKO mice of both sexes (Table 1). The cKO mice exhibited slightly higher calcemia, whereas plasma levels of glucose, sodium, and potassium, and plasma osmolality were not different from controls (Table 1). Plasma aldosterone levels were significantly increased, suggesting extracellular volume contraction in cKO mice (Table 1).
Table 1.
Plasma chemistry and GFR in control and cKO mice euthanized on day 28 after DOX withdrawal
| Plasma | Males | Females | ||||
|---|---|---|---|---|---|---|
| Control | cKO | P Value | Control | cKO | P Value | |
| Osmolality, mosm/kg H2O | 325.6±1.1 (6) | 324.6±5.6 (6) | 0.86 | 324.4±2.2 (4) | 323.8±2.1 (6) | 0.86 |
| Ca2+, mM | 2.12±0.01 (4) | 2.18±0.02 (5) | 0.03 | 2.11±0.02 (5) | 2.41±0.06 (4) | 0.001 |
| Na+, mM | 156.5±2.2 (6) | 159.9±4.2 (6) | 0.44 | 158.6±1.2 (5) | 160.9±2.89 (4) | 0.58 |
| K+, mM | 4.04±0.16 (6) | 3.64±0.20 (3) | 0.16 | 3.74±0.29 (5) | 3.19±0.09 (4) | 0.09 |
| Creatinine, μM | 11.03±1.03 (16) | 21.24±1.68 (15) | 1.26×10−5 | 14.03±1.69 (16) | 20.16±1.37 (16) | 0.01 |
| GFR (inulin), μl/min | 235±13 (5) | 147±24 (5) | 0.01 | |||
| Aldosterone, pg/ml | 202.46±65.27 (8) | 408.25±56.76 (8) | 0.03 | 222.02±54.06 (9) | 506.85±78.97 (8) | 0.01 |
| FGF23, pg/ml | 122.95±15.57 (5) | <30 (4) | 0.001 | 142.61±20.37 (4) | <30 (5) | 9.3×10−5 |
| CTX1, pg/ml | 5.95±0.87 (4) | 17.71±2.34 (4) | 0.003 | 6.56±0.30 (4) | 30.31±12.85 (4) | 0.11 |
| 1.25(OH)2-D3 nmol/ml | 88.97±13.41 (5) | 66.04±5.97 (5) | 0.16 | 56.53±4.57 (5) | 55.86±6.13 (5) | 0.93 |
| PTH, pg/ml | 20.29±7.30 (6) | 34.05±11.34 (6) | 0.33 | 39.25±12.75 (6) | 15.11±6.83 (6) | 0.12 |
| ALP activity, U/L | 3.87±1.81 (4) | 3.77±0.75 (4) | 0.96 | 9.06±4.43 (4) | 7.48±3.45 (4) | 0.79 |
| TRAP, ng/ml | 124.13±13.02 (4) | 92.54±12.13 (4) | 0.13 | 98.07±11.79 (4) | 62.12±6.89 (4) | 0.03 |
| Osteocalcin, ng/ml | 66.13±18.61 (6) | 210.79±59.18 (4) | 0.03 | 104.59±23.77 (4) | 145.92±14.79 (3) | 0.23 |
Data are means±SEM (n). P values calculated using unpaired t test. CTX1, C-terminal telopeptides of type I collagen; PTH, parathyroid hormone; ALP, alkaline phosphatase; TRAP, tartrate-resistant acid phosphatase.
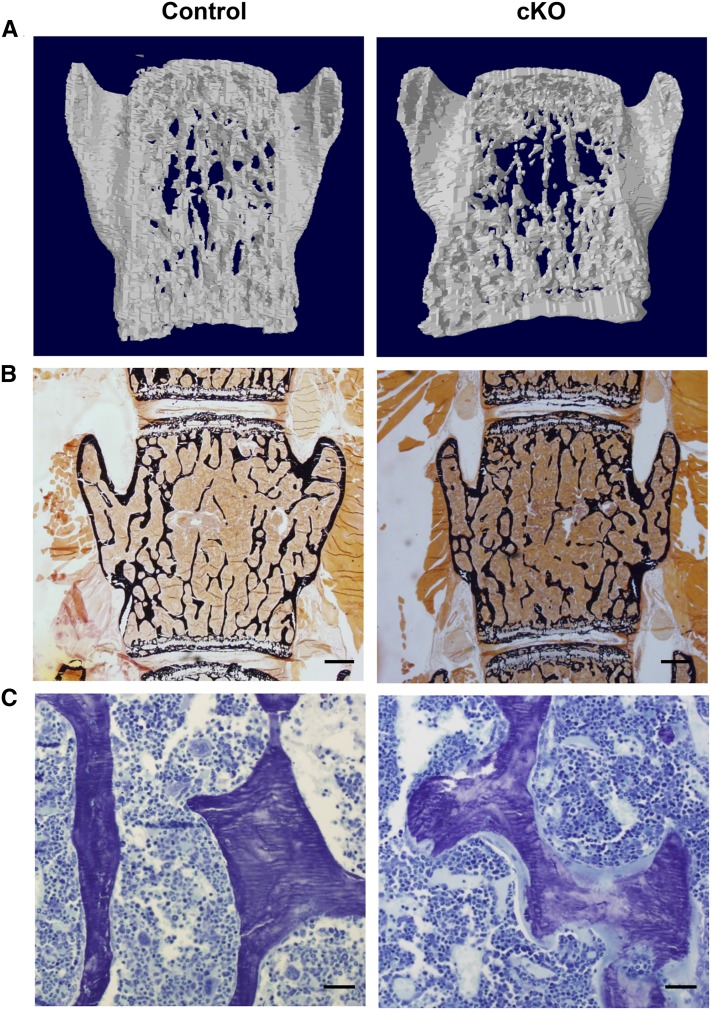
Hypophosphatemia and decreased TmPi/GFR prompted us to study the bone phenotype in cKO mice. Analysis of vertebrae by microcomputed tomography revealed severely decreased bone mineral density, bone volume per total volume, trabecular thickness, and trabecular number in male cKO mice, and similar but milder features in female cKO mice (Figure 3A, Supplemental Table 2). The microcomputed tomography analysis of femora showed significantly decreased thickness and tissue mineral density in the distal diaphyseal and metaphyseal cortical bone in male cKO mice, with similar but nonsignificant changes in female cKO mice, and largely unaffected trabecular bone of the distal metaphysis (Supplemental Table 3).
Figure 3.
XPR1 deficiency in the nephron causes vertebral osteomalacia in male mice. (A) Three-dimensional reconstructions of 400-μm thick coronal sections of L5 vertebral bodies scanned by microcomputed tomography revealed an impaired trabecular network in male cKO mice (images representative of two control and three cKO mice). (B) Calcium staining of vertebral sections by von Kossa revealed no significant change in trabecular bone of cKO animals. (C) Toluidine Blue staining of vertebral bone revealed a prominent osteoidosis (osteoid seam in light blue) in cKO mice. (B, C) Representative images of five control and five cKO mice; 4-μm thick sections of nondecalcified bone viewed under 2× (B) or 20× (C) magnification; scale bars, 500 μm (B) and 50 μm (C). Mice were euthanized on day 28 after DOX withdrawal.
Vertebral specimens of male control and cKO mice were further analyzed by nondecalcified bone histomorphometry. Although not clearly visible at low magnification (Figure 3B), high magnification analysis showed a striking increase in all unmineralized osteoid parameters in cKO mice (Supplemental Table 4). The excessive osteoid in vertebrae of cKO mice is distinctly visible on a representative image of Toluidine Blue staining (Figure 3C). Cellular osteoblast parameters (the number of osteoblasts and the osteoblast surface) were unchanged in cKO mice, whereas the number of osteoclasts was increased (Supplemental Table 4). Collectively, these data reveal a highly excessive fraction of unmineralized bone in cKO mice, consistent with rickets.
To gain further insight into the molecular mechanisms underlying the defective bone mineralization in cKO mice, we measured plasma levels of hormones involved in calcium/phosphate homeostasis and bone turnover biomarkers (Table 1). Most strikingly, fibroblast growth factor 23 (FGF23) levels were undetectable in cKO mice of both sexes, whereas 1,25-dihydroxyvitamin D3 [1,25(OH)2-D3, or calcitriol] and parathyroid hormone levels were unchanged. Collagen degradation product CTX1 was significantly increased in male cKO mice, and a nonsignificant trend in the same direction was found in female cKO mice, suggesting an increase in bone resorption consistent with the increased osteoclast numbers observed. However, alkaline phosphatase activity was unchanged. The levels of the osteoblast-produced hormone osteocalcin were increased in male cKO mice. To summarize, distinct signs of overall altered bone turnover were present in cKO mice.
To conclude, mice deficient for Xpr1 in the renal tubule develop complete Fanconi syndrome and hypophosphatemic rickets. The severity of renal dysfunction was similar in cKO mice of both sexes, whereas the bone phenotype was more prominent in males compared with females, an observation that has been made in human patients.8 Hypophosphatemic rickets represents a heterogeneous entity that can be further divided into conditions associated with high FGF23 levels and suppressed 1,25(OH)2-D3, such as X-linked hypophosphatemic rickets and autosomal recessive hypophosphatemic rickets, or with low FGF23 and high 1,25(OH)2-D3 levels, found when defects of renal phosphate transport are present. Indeed, mutations of NaPi-IIa and NaPi-IIc, the two sodium-phosphate cotransporters present in the brush border of the proximal tubule, lead to hereditary hypophosphatemic rickets with hypercalciuria.9,10 Here, we provide evidence for involvement of XPR1 in hypophosphatemic rickets associated with low FGF23 levels and normal 1,25(OH)2-D3 levels, reminiscent of hereditary hypophosphatemic rickets with hypercalciuria. Furthermore, we show that renal XPR1 is essential for phosphate homeostasis and bone physiology, and open new avenues for treatment options.
Concise Methods
Detailed methods are described in the Supplemental Material.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Drs. Jean-Luc Battini and Yves Poirier for helpful discussions, Dr. Florence Morgenthaler (Cellular Imaging Facility, University of Lausanne, Lausanne, Switzerland) for help with microcomputed tomography analysis, and the Lausanne Genomic Technologies Facility for transcriptome analysis.
This work was supported by the Swiss National Science Foundation Research grants 31003A-149440 (to D.F.) and 310030-163340 (to O.B.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016070726/-/DCSupplemental.
References
- 1.Hamburger D, Rezzonico E, MacDonald-Comber Petétot J, Somerville C, Poirier Y: Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14: 889–902, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefanovic A, Arpat AB, Bligny R, Gout E, Vidoudez C, Bensimon M, Poirier Y: Over-expression of PHO1 in Arabidopsis leaves reveals its role in mediating phosphate efflux. Plant J 66: 689–699, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Giovannini D, Touhami J, Charnet P, Sitbon M, Battini JL: Inorganic phosphate export by the retrovirus receptor XPR1 in metazoans. Cell Reports 3: 1866–1873, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Wege S, Poirier Y: Expression of the mammalian Xenotropic Polytropic Virus Receptor 1 (XPR1) in tobacco leaves leads to phosphate export. FEBS Lett 588: 482–489, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Legati A, Giovannini D, Nicolas G, López-Sánchez U, Quintáns B, Oliveira JR, Sears RL, Ramos EM, Spiteri E, Sobrido MJ, Carracedo Á, Castro-Fernández C, Cubizolle S, Fogel BL, Goizet C, Jen JC, Kirdlarp S, Lang AE, Miedzybrodzka Z, Mitarnun W, Paucar M, Paulson H, Pariente J, Richard AC, Salins NS, Simpson SA, Striano P, Svenningsson P, Tison F, Unni VK, Vanakker O, Wessels MW, Wetchaphanphesat S, Yang M, Boller F, Campion D, Hannequin D, Sitbon M, Geschwind DH, Battini JL, Coppola G: Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat Genet 47: 579–581, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, Horst J, von Knebel Doeberitz M, Niggli FK, Kriz W, Gröne HJ, Koesters R: An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14: 979–984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman D, Bartlett S, Radda G, Ross B: Energetics of sodium transport in the kidney. Saturation transfer 31P-NMR. Biochim Biophys Acta 762: 325–336, 1983 [DOI] [PubMed] [Google Scholar]
- 8.Beck-Nielsen SS, Brusgaard K, Rasmussen LM, Brixen K, Brock-Jacobsen B, Poulsen MR, Vestergaard P, Ralston SH, Albagha OM, Poulsen S, Haubek D, Gjørup H, Hintze H, Andersen MG, Heickendorff L, Hjelmborg J, Gram J: Phenotype presentation of hypophosphatemic rickets in adults. Calcif Tissue Int 87: 108–119, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM: Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet 78: 193–201, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H: SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78: 179–192, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.