Abstract
The renin-angiotensin system (RAS) has a pivotal role in the maintenance of extracellular volume homeostasis and blood pressure through complex mechanisms. Apart from the well known systemic RAS, occurrence of a local RAS has been documented in multiple tissues, including the kidney. A large body of recent evidence from pharmacologic and genetic studies, particularly those using various transgenic approaches to manipulate intrarenal levels of RAS components, has established the important role of intrarenal RAS in hypertension. Recent studies have also begun to unravel the molecular mechanisms that govern intrarenal RAS activity. This local system is under the control of complex regulatory networks consisting of positive regulators of (pro)renin receptor, Wnt/β-catenin signaling, and PGE2/PGE2 receptor EP4 subtype, and negative regulators of Klotho, vitamin D receptor, and liver X receptors. This review highlights recent advances in defining the regulation and function of intrarenal RAS as a unique entity separate from systemic angiotensin II generation.
Keywords: intrarenal renin-angiotensin system, (pro)renin receptor, prostaglandin E4 receptors, Klotho, β-catenin
The renin-angiotensin system (RAS) has been known for over a century, since the first discovery of renin by Tigerstedt and Bergman in 1898.1 The systemic RAS requires interaction of multiple organs involving liver production of angiotensinogen (AGT) which is converted to angiotensin I (Ang I) by renin, a protease produced by juxtaglomerular apparatus (JGA), followed by a second cleavage to angiotensin II (Ang II) by angiotensin converting enzyme (ACE) located on the surface of lung endothelium. Apart from the well known systemic RAS, production of multiple RAS components has been found in a variety of tissues including the kidney.2,3 Inappropriate activation of intrarenal RAS has been recognized as an important mechanism for hypertension and renal disease. To date, the anti-RAS regimen represents the cornerstone therapy for both diseases. No matter hypertension or renal disease, there is no clear evidence for increased plasma renin activity (PRA), renin, or Ang II. Furthermore, the RAS interventions are capable of lowering blood pressure (BP) in the presence of suppressed or elevated PRA4 despite a wide range of data variability.5,6 These findings have led to the hypothesis that these inhibitors may exert a large part of their effect at a local level.
The general knowledge of intrarenal RAS has already been covered by a number of comprehensive reviews.7–9 The major objective of this article is to review recent advances in defining the intrarenal RAS, including its function, and positive and negative regulators in the settings of hypertension and renal injury.
Existence of Intrarenal RAS
As opposed to the systemic RAS, the circulation-borne endocrine system, the intrarenal RAS refers to a local autocrine/paracrine system in the kidney which involves both angiotensin-dependent and independent actions. A hallmark of intrarenal RAS is the high level of intratubular Ang II that exceeds the plasma concentration.10,11 Strong evidence suggests that intrarenal RAS contains all elements necessary to generate Ang II. AGT is synthesized in the proximal tubule (PT) and can be secreted to the tubular lumen12,13 or act within the PT.7 During systemic Ang II infusion, AGT expression in the renal cortex and urinary AGT excretion are elevated in an Ang II type 1 receptor–dependent (AT1R-dependent) manner.14,15 Consistent with this finding, overexpression of an intracellular cyan fluorescent Ang II in the PT via AT1R induces renal cortical mRNA and protein expression of AGT, without affecting circulating levels of AGT or renin activity.16 Immunoreactive renin is found in principal cells of the connecting segment and collecting duct (CD) of both murine and human kidneys secreted to tubular lumen in response to salt depletion.17 Subsequently, CD renin is shown to be upregulated under pathologic conditions such as Ang II–induced hypertension18 and diabetes.19 Both ACE and AT1R are found abundantly throughout the apical nephron surface. Direct evidence for local synthesis of Ang II is demonstrated by using radiolabeled Ang II.20 Although circulating 125I-Ang II is accumulated in the renal tissue sites, the endogenous renal Ang II levels are up to 100 times higher than the plasma levels of endogenous Ang II.20 This result was further validated by the substitution of isoleucine (Ile[5] Ang II) at position five with valine (Val[5]-Ang II).21
The complexity of intrarenal RAS stems from the evidence supporting the circulating source of intrarenal RAS components. Nearly all major components of the RAS including AGT, prorenin, renin, Ang I, and Ang II will be filtered by the glomerulus and taken up by the PT. The uptake of circulating Ang II by the PT is mediated by AT1R22–27 as well as the multiligand endocytic receptor megalin.28,29 Besides Ang II, other RAS components including AGT, prorenin, Ang I, and Ang II are also taken up by the PT through the same megalin-dependent mechanism.28,29 The seminal work by Matsusaka et al. demonstrates hepatic origin of intrarenal AGT5,30; renal AGT protein and Ang II levels are unaffected by renal-specific AGT deletion using the KAP-Cre but are significantly reduced by liver-specific AGT deletion. A caveat is that urinary AGT is reduced (by approximately 50%) in renal-specific AGT knockout (KO) mice not in liver-specific AGT KO mice. Furthermore, the valuable models are only analyzed under basal condition and after podocyte injury.5,30 These results can’t rule out the possible role of intrarenal AGT under other physiologic or pathologic conditions. Recently, another strain of renal-specific AGT KO mice generated by using an inducible Pax8-rtTA system shows a remarkable reduction of urinary AGT associated with hypotension.31 However, this study is limited in that the Pax8-rtTA system also causes partial deletion of AGT in the liver as reflected by reduced circulating AGT level. Therefore, more vigorous studies are needed to determine the contribution of intrarenal AGT versus liver AGT to the overall control of BP as well as kidney injury.
Role of Intrarenal RAS in Hypertension
Several lines of pharmacologic and genetic evidence demonstrate an essential role of intrarenal RAS in the pathogenesis of experimental hypertension. When ACE inhibitor lisinopril was administered systemically, Ang II–infused mice became normotensive with attenuation of the upregulation of components of intrarenal RAS, particularly CD renin, ACE, and AT1R.32,33 Despite the limitations of the systemic approach, this study provides the first functional evidence that the pressor response of the end product Ang II relies on the upstream enzyme ACE which drives in situ Ang II synthesis. Subsequent studies using genetic approaches to manipulate a key component of the RAS at the level of the whole nephron or a specific nephron segment greatly facilitate understanding of the role of intrarenal RAS in hypertension. Bernstein’s group generated mice lacking renal ACE but having sufficient ACE in other tissues (termed ACE 3/3 and ACE 10/10) that were able to maintain normal serum levels of Ang II and normal kidney structure and BP under basal condition.34 The genetic ablation of renal ACE remarkably attenuated the pressor response to Ang II infusion at 400 ng/kg per minute, accompanied with reduced renal Ang II content and suppressed expression of renal Na+ transporters.35 Additionally, ACE 10/10 mice were also protected against L-NAME–induced hypertension.36 Together, these results suggest that ACE-dependent activation of intrarenal RAS may represent a common pathway leading to pressor responses to different hypertensive stimuli. Of note, these models were generated by using a promoter-swamping strategy so that the control of ACE expression is switched from the endogenous ACE promoter to liver-specific albumin promoter (3/3) or macrophage-specific c-fms promoter (10/10). Therefore, it remains uncertain whether the BP phenotype is directly related to the lack of ACE expression in the kidney versus the blood vessels or other tissues. To address this issue, the use of a nephron-specific deletion approach will be needed.
Studies using renal crosstransplantation demonstrated a significant role of renal AT1R in BP regulation independent of aldosterone.37 Furthermore, conditional deletion of AT1R in the PT reduced baseline BP and attenuated Ang II–induced hypertension.38 In the PT, AT1R activation appears to primarily target Na+/H+ exchanger 3 to induce Na+ retention and hypertension.7
The functional role of CD renin has been examined by Ramkumar et al., who generated mice with CD-specific overexpression or deletion of renin.39,40 Overexpression of renin in the CD causes spontaneous hypertension.40,41 Although deletion of renin in the CD didn’t produce major disturbances in Na+ and water balance and BP, the null mice were protected against Ang II–induced hypertension.39 These results support an important role of CD renin in BP regulation.
As discussed above, the functional contribution of intrarenal RAS to hypertension is tested mostly by using the Ang II–infusion model which is of limited relevance due to the nonphysiologic doses of Ang II. In fact, alteration of intrarenal RAS has been documented in several other models of experimental hypertension, such as Dahl salt-sensitive rats;42 two-kidney, one clip Goldblatt hypertension;43 and spontaneously hypertensive rats.44 These models have a better relevance to human hypertension. Defining the functional role of intrarenal RAS in each of these models is expected to offer new perspectives on the pathophysiology of intrarenal RAS during hypertension.
Role of Intrarenal RAS in Renal Disease
An inappropriate activation of intrarenal RAS has been implicated in a variety of animal models of renal disease, such as 5/6 nephropathy, adriamycin nephropathy, unilateral ureteral obstruction,45 and polycystic kidney disease,46 as an initial response to hypoperfusion and an important driver of the disease progression.47,48 The upregulation of multiple RAS components was usually detected in the absence of increased PRA or plasma Ang II.47 Urinary AGT has been shown to be a strong predictor of intrarenal RAS activity and renal injury in both animal and clinical studies.49–51
To date, the use of RAS inhibitors such as ACEi or AT1 blockers remains the cornerstone therapy for amelioration of albuminuria and renal disease progression. Yet, there is no clear evidence for enhancement of systemic RAS in patients with renal disease. Along this line, although ACEi treatment may acutely lower circulating Ang II, its long-term therapy in a subset of patients raises Ang II or aldosterone concentrations back to the baseline level.52 Overall, the concept of intrarenal RAS in renal disease has been well established. However, a number of issues still need to be resolved. For example, the precise intrarenal sites of RAS activation during renal injury and the detailed regulatory mechanisms still largely remain elusive. Furthermore, most studies in this area are limited in their descriptive or correlative nature. The precise contribution of intrarenal RAS to renal disease as compared with that of systemic RAS needs to be determined by functional studies, particularly those using mice with genetic manipulations of RAS components in a tissue-specific manner without perturbing systemic RAS.
Potential Regulators of Intrarenal RAS
(Pro)Renin Receptor as a Positive Regulator of Intrarenal RAS
In 2002, Nguyen et al. cloned a specific receptor for prorenin and renin, termed (pro)renin receptor (PRR).53 PRR is a unique 350–amino acid transmembrane protein consisting of a large N-terminal extracellular domain, a single transmembrane protein, and a short cytoplasmic domain.54 The extracellular domain is cleaved to generate a soluble form of PRR (sPRR) via furin or ADAM19.55 This cleavage results in three isoforms: the full length PRR, sPRR, and the intracellular domain M8.9. It is increasingly evident that PRR serves a multitude of functions in regulating embryogenesis, balancing sodium and water, modulating acid secretion, etc.56–60 Of note, complete PRR deletion in vertebrates leads to developmental alterations and early embryonic lethality probably as a result of PRR’s role in regulation of vacuolar H+-ATPase and Wnt/β-catenin signaling.61 Moreover, nephron-specific deletion of PRR causes severe autophagic defects in renal medullary tubules and acidosis.62
The association between PRR and RAS has been extensively investigated but highly debated. Since its first identification from human mesangial cells, PRR was thought to be a component of the RAS on the basis of in vitro evidence.53 However, subsequent animal studies were unable to prove the renin-regulatory role of PRR.63 In particular, overexpression of human PRR failed to affect tissue Ang II concentrations.64 The definitive evidence for PRR as a component in Wnt/β-catenin signaling during embryogenesis56,65 and as an accessory protein of vacuolar ATPase66 further questioned its role in RAS regulation. Unraveling this issue has been difficult due to the controversial PRR inhibitor HRP67 and also the lack of viable PRR knockout mice.
Within the kidney, PRR is predominantly expressed in the intercalated cells of the CD.68 The expression of PRR in the CD is stimulated by chronic Ang II infusion69 or sodium depletion.59,60 In cultured CD cells, PRR expression was stimulated by low salt or Ang II and the stimulation was potentiated by the combined treatments.70 In light of the colocalization of PRR with renin in the CD, it is conceivable that PRR may regulate renin activity in the distal nephron, particularly during Ang II–induced hypertension.
Functional studies showed that a newly developed PRR decoy inhibitor PRO2071 administered via intramedullary infusion technique remarkably suppressed the increases in urinary and renal medullary renin activity during Ang II–induced hypertension.72 In vitro evidence further demonstrated that the action of PRO20 in inhibiting renin activity was direct.72 In agreement with these results, CD-specific deletion of PRR reduced the basal urinary renin activity by approximately 40% and almost completely abolished its response to Ang II at 300 ng/kg per minute, in parallel with the suppressed hypertensive response.73 This BP phenotype was similar to that of nephron PRR KO mice generated by Ramkumar et al. when Ang II was infused at 600 ng/kg per minute.74 However, another strain of nephron PRR KO model generated by Trepiccione et al.62 exhibited normal BP response to Ang II at 1 µg/kg per minute; renal Ang II level in these null mice was also unaltered. The exact reason for this discrepancy is unclear but could be related to differences in experimental protocols such as the doses of Ang II.
Despite the emphasis on the potential renin-regulatory role of PRR as discussed above, it is increasingly evident that PRR can also act in an RAS-independent manner. In cultured mpkCCD cells, activation of PRR by prorenin in the nanomolar range induced epithelial sodium channel activity, an effect that was unaffected by AT1 blockade.75 This result was recapitulated by using freshly isolated cortical CD with single-channel patch-clamp recording.74 In contrast, renin was largely ineffective in activating the Na+ channel. The in vitro data favors prorenin, but not renin, as a candidate physiologic ligand of PRR. However, it remains elusive whether the prorenin/PRR interaction truly occurs in vivo. PRR’s nanomolar affinity for prorenin/renin is many orders of magnitude above their levels in blood.76 Indeed, deletion of PRR in the CD or the nephron produces a urine concentrating defect that is not seen in the CD renin KO model.39 Future studies are needed to determine whether prorenin or renin is the true physiologic ligand of PRR.
PGE2/EP4 Pathway as a Positive Regulator of Intrarenal RAS
E series prostaglandins (PGs) have long been recognized as important regulators of renin secretion from the JGA.77–79 In early studies in the isolated rabbit JGA, renin secretion in response to low NaCl was virtually abolished by nonspecific cyclooxygenase (COX) inhibition with flufenamic acid or flurbiprofen.80 Subsequently, a large body of experimental evidence demonstrated that PGE2 derived from COX-2 serves as a dominant mechanism in mediating renin secretion from the JGA via EP2 and EP4 receptors which signal through the cAMP pathway.81,82
Besides the JGA, the CD is another major site of both production and action of PGE2. Among the microdissected nephron segments, the highest amount of PGE2 was detected in the CD.83 At the distal nephron, PGE2 exerts complex roles in regulation of Na+ and water transport depending on a specific EP subtype.84 The PGE2/EP4 pathway is well recognized as an antidiuretic mechanism85,86 that complements vasopressin (AVP) action in the CD. Recent pharmacologic87 and conditional EP4 knockout studies86 provide compelling evidence to support antidiuretic action of EP4 receptors in the CD which is mediated by upregulation of AQP2 expression. Although most of the previous studies focused on the direct action of PGE2 in regulation of tubular transport, recent studies suggested that the PGE2/EP4 pathway may modulate CD function via a previously undescribed mechanism involving concomitant activation of PRR and local renin response in the distal nephron.72,87 The capability of EP4 to independently stimulate PRR and renin make it an effective regulator of intrarenal RAS leading to increased fluid reabsorption in the distal nephron. This mechanism contributes to physiologic maintenance of fluid balance during water deprivation.87 The discovery of the PGE2/EP4/PRR pathway in the distal nephron is also of importance in BP regulation. Our studies suggest that inappropriate activation of this pathway contributes to Ang II–induced hypertension.72 EP4 receptors signaling works through a number of pathways involving cAMP/PKA, phosphatidylinositol 3-kinase, and AKT.88,89 Among these candidate signaling mechanisms downstream of EP4 receptors, cAMP/PKA, but not AKT or phosphatidylinositol 3-kinase, is shown to mediate the upregulation of PRR in the CD cells.87
The fluid-retaining and prohypertensive action of EP4 receptors in the distal nephron is opposite to the well recognized vasodilatory property of this EP subtype. In most vascular beds, PGE2 functions as a vasodilator to buffer the action of vasoconstrictive stimuli such as Ang II. Central to the buffering actions of PGE2 are the vasodilatory EP4 receptors found in both vascular smooth muscle cells and endothelial cells. Endothelial EP4 receptors contribute to the acute vasorelexation of aortic rings induced by PGE2 through cGMP-dependent dephosphorylation of eNOS at Thr495. It was recently shown that inducible vascular smooth muscle cell EP4 deletion impairs PGE2-induced mesentery artery relaxation but fails to affect Ang II–induced hypertension.90 This result suggests that vascular EP4 receptors may be less important for BP regulation despite their role in regulation of vascular tone.
Wnt/β-Catenin Signaling as a Positive Regulator of Intrarenal RAS
Wnt/β-catenin signaling is an evolutionarily conserved signaling cascade that plays a pivotal role in regulating embryogenesis and tissue hemostasis.91,92 Emerging evidence demonstrates that activation of Wnt/β-catenin signaling underlies pathogenesis of CKD, including diabetic nephropathy, polycystic kidney disease, chronic allograft nephropathy, etc.93–95 A link between Wnt/β-catenin signaling and intrarenal RAS is suggested by Zhou et al. who used a bioinformatics approach to demonstrate that β-catenin targeted putative T cell factor/lymphoid enhancer–binding factor binding sites found in promoter regions of multiple RAS genes including AGT, renin, ACE, AT1R, and AT2R.96 It is interesting to note that despite their opposite roles in renal physiology and pathophysiology, AT1R and AT2R are both targeted by Wnt/β-catenin signaling. This raises a question as to whether Wnt/β-catenin signaling affects other components of the protective RAS axis such as the ACE2/Ang1-7/MasR axis. Additionally, the PRR gene also contains multiple T cell factor/lymphoid enhancer–binding factor binding sites in its promoter region, raising a possibility that PRR may also be a target gene of Wnt/β-catenin signaling. On the other hand, Ang II stimulates β-catenin signaling in cultured M-1 cortical CD cells leading to enhancement of fibronectin and collagen I as well as cyclin D1 and c-myc.97 Therefore, there appears to be a mutually stimulatory relationship between Ang II and Wnt/β-catenin signaling during renal fibrosis.
The complex relationship between Wnt/β-catenin signaling and intrarenal RAS is also reflected by PRR as an upstream component of the Wnt/β-catenin pathway.56,65 We recently reported that sPRR is produced from intercalated cells of the CD and acts in a paracrine manner to interact with principal cell frzzled-8 (FZD8), leading to activation of β-catenin pathway and thus increasing AQP2 transcription and urine concentration.98 On the basis of these observations, we propose the following hypothetic model: Wnt/β-catenin signaling pathway and intrarenal RAS may interact with each other to form a positive feedback loop where PRR upregulates the β-catenin pathway that in turn stimulates expression of multiple RAS genes. Activation of this positive feedback loop may underlie pathophysiology of hypertension and renal injury.
Klotho as a Negative Regulator of Intrarenal RAS
Klotho is a well known antiaging gene as highlighted by the prominent ageing phenotype of Klotho mutant mice, including shortened lifespan and cardiovascular disease.99 Within the kidney, Klotho is selectively expressed in the distal convoluted tubule and the PT, serving as an obligatory coreceptor for fibroblast growth factor 23100 to control phosphate reabsorption. Apart from the involvement in phosphate metabolism, Klotho exerts a multitude of beneficial activities against hypertension and renal disease.45,99,101–103
Increasing evidence demonstrates that the renoprotective action of Klotho is conferred through inhibition of intrarenal RAS. In various rodent models of renal disease including 5/6 nephropathy, adriamycin nephropathy, and unilateral ureteral obstruction, renal Klotho expression is suppressed but RAS components are upregulated; administration of exogenous Klotho through hydrodynamic-based gel ameliorates renal pathologies associated with abolishment of the induction of RAS components.45 It has further been shown that Klotho may inhibit intrarenal RAS by targeting Wnt/β-catenin signaling as suggested by the observation that Klotho directly binds multiple Wnts, including Wnt1, Wnt4, and Wnt7a, to block Wnt-triggered nuclear translocation of β-catenin.104 These observations have been extended by the study of Zhou et al., who showed that Klotho exerted a direct inhibitory effect on aldosterone synthesis in adrenal glands.105 It is likely that Klotho may exert a multitude of actions to mitigate the activation of intrarenal RAS as well as systemic aldosterone production. Overall, strong evidence demonstrates the suppression of renal Klotho expression in renal disease and more vigorous functional studies are needed to define the renoprotective action of this antiaging protein as well as its relationship with intrarenal RAS.
Nuclear Receptors as Regulators of Intrarenal RAS
The nuclear receptor family of transcription factors acts primarily via interacting with consensus elements in the promoter regions of the target genes and plays diverse and important roles in development and the regulation of normal physiologic functions, particularly energy metabolism.106 A number of nuclear receptors such as peroxisome proliferator–activated receptors,107,108 liver X receptor (LXR),98 and vitamin D receptor (VDR)109 have been implicated in the regulation of plasma volume and electrolyte homeostasis, a primary function of the RAS. It is conceivable that a crosstalk between nuclear receptors and the RAS may exist.
VDR is a well established negative regulator of the RAS.110,111 Multiple clinical studies revealed an inverse relationship between plasma 1,25 (OH) 2D3 concentrations and the BP and/or plasma renin activity in hypertensive patients as well as in normal subjects.112–114 More definitive evidence linking VDR and the RAS came from the cardiovascular phenotype of VDR null mice that displayed hypertension and cardiac hypertrophy associated with increases in renin and Ang II levels in the plasma as well as in renin, AGT, and AT1R, and PRR in inflammatory cells.109,110 Although this model doesn’t allow differentiating the involvement of systemic versus local RAS, the renin response to low salt and volume stimuli, a measurement of systemic RAS, remains intact. At cellular level, VDR directly suppresses renin gene transcription by interfering with cAMP responsive elements in the renin gene promoter.115 In rats with 5/6 nephrectomy, VDR activation by paricalcitol decreases expression of multiple RAS genes including renin, AGT, AT1R, and PRR in the remnant kidney and improves hypertension and kidney injury.116 These results seem to suggest that VDR may primarily target the local RAS to confer cardiovascular and renal protection. Indeed, multiple small clinical studies showed an inverse association between serum vitamin D levels and hypertension.117–119 However, recent intervention trials reveal no significant effect of vitamin D supplementation on BP in hypertensive patients.120–123 As noted by Beveridge et al.,124 these trials have a number of limitations. For example, the largest trials to date enrolled only a few hundred patients. Other limitations of these trails include limited representation by black individuals and short duration of the studies. It is known that blacks in the United States have higher rates of hypertension and cardiovascular disease associated with lower circulating levels of 25-hydroxyvitamin D as compared with whites.125 Therefore, larger trials with improved representations by blacks for a longer duration of treatment will be needed. Another consideration is that the inconsistent results may be related to varied vitamin D dosages. Lastly, although BP is the primary outcome of these trials, the effect of vitamin D supplementation on cardiovascular events and renal disease remains unclear.
LXRs heterodimerize with the retinoid X receptor to regulate transcription of target genes involved in cholesterol, fatty acid, and glucose metabolism.126–128 LXRs have an established role in reverse cholesterol transport which leads to cholesterol efflux from peripheral tissues to the liver.129 We recently discovered that administration of an LXR against TO901317 in mice induces polyuria, polydipsia, hypo-osmotic urine, and downregulation of renal AQP2 expression, all indicative of nephrogenic diabetes insipidus.98 This observation reveals a novel diuretic role of renal LXRs. This study further provides a mechanism by which LXRs control urine concentrating capability via suppressing renal PRR/sPRR and intrarenal RAS.72,130 This concept aligns well with the observation that chronic TO901317 treatment suppresses the induction of renin, AT1R, and ACE in the heart and kidney induced by a nonpressor dose of isoproterenol in wild-type but not LXRα KO mice.131 The RAS-inhibitory and diuretic activities of TO901317 are likely ascribed to LXRα. However, during an acute setting, LXR activation stimulates renin expression at the JGA.132 The underlying mechanism and physiologic significance of the distinct effects of LXR activation on RAS under the two different experimental settings certainly warrant further investigation.
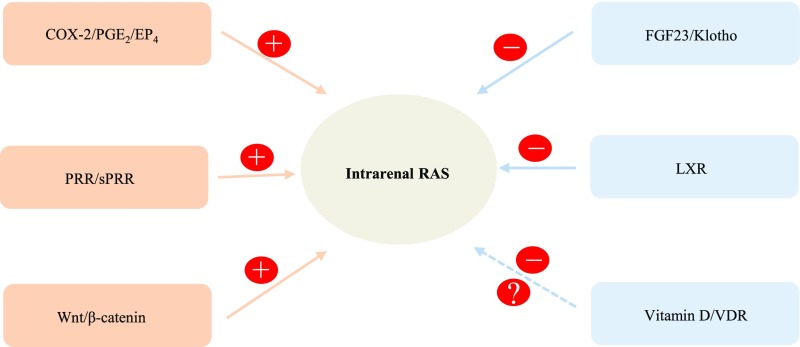
In conclusion, a large body of experimental evidence has firmly established the concept of intrarenal RAS as a unique entity despite the circulating source of some intrarenal RAS components such as AGT. In general, intrarenal RAS is featured as a local feedforward system for augmentation of intrarenal generation or actions of RAS components, which is counterintuitive to the normal feedback regulation. This local system plays an important role in pathogenesis of hypertension and renal disease as well as in physiologic regulation of fluid homeostasis. Recent studies have identified several important regulatory pathways that can affect intrarenal RAS (Figure 1), including PRR, Wnt/β-catenin signaling, and the PGE2/EP4 pathway in the positive arm, and Klotho, VDR, and LXR in the negative arm. The balance between the positive and negative regulatory pathways may be an important determinant of intrarenal RAS activity. A better understanding of the molecular basis for integrative control of intrarenal RAS may offer new perspectives on both pathophysiology and therapy for hypertension and renal disease. Optimism has been generated from the therapeutic potential of new inhibitors of PRR (PRO20)72 and Wnt/β-catenin signaling (ICG-001),133 and activators of LXR (TO901317)134 and Klotho (soluble Klotho)135 in cardiovascular and/or renal diseases. In addition, the clinical implication is also suggested by circulating levels of sPRR101 and soluble Klotho136,137 as a predictor of a decline of renal function in patients with CKD.
Figure 1.
Schematic illustration of regulatory networks that control intrarenal RAS activity. This local system is subjected to tight control by complex regulatory networks consisting of both positive regulators of (pro)renin receptor, Wnt/β-catenin signaling, and the PGE2/EP4 pathway, and negative regulators of fibroblast growth factor 23/Klotho, vitamin D/VDR, and LXRs. Imbalance of the two opposing regulatory networks may be an important determinant of intrarenal RAS activity. FGF23, Fibroblast Growth Factor 23; LXR, liver X receptor; VDR, vitamin D receptor.
Disclosures
None.
Acknowledgments
This work was supported by National Institutes of Health grants DK104072 and DK094956, Veterans Affairs Merit Review from the Department of Veterans Affairs, and National Natural Science Foundation of China grants 91439205 and 31330037. T.Y. is Research Career Scientist in the Department of Veterans Affairs.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Tigerstedt R, Bergman PG: Niere und kreislauf. Skand Arch Physiol 8: 223–271, 1898 [Google Scholar]
- 2.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA: Intratubular renin-angiotensin system in hypertension. Hypertension 57: 355–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzaki Y, Prieto-Carrasquero MC, Kobori H: Intratubular renin-angiotensin system in hypertension. Curr Hypertens Rev 2: 151–157, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dzau VJ: Evolving concepts of the renin-angiotensin system. Focus on renal and vascular mechanisms. Am J Hypertens 1: 334S–337S, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Matsusaka T, Niimura F, Pastan I, Shintani A, Nishiyama A, Ichikawa I: Podocyte injury enhances filtration of liver-derived angiotensinogen and renal angiotensin II generation. Kidney Int 85: 1068–1077, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones MR, Sealey JE, Laragh JH: Effects of angiotensin receptor blockers on ambulatory plasma renin activity in healthy, normal subjects during unrestricted sodium intake. Am J Hypertens 20: 907–916, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Zhuo JL, Ferrao FM, Zheng Y, Li XC: New frontiers in the intrarenal renin-angiotensin system: A critical review of classical and new paradigms. Front Endocrinol (Lausanne) 4: 166, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobori H, Nangaku M, Navar LG, Nishiyama A: The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Navar LG, Prieto MC, Satou R, Kobori H: Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol 11: 180–186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navar LG, Imig JD, Zou L, Wang CT: Intrarenal production of angiotensin II. Semin Nephrol 17: 412–422, 1997 [PubMed] [Google Scholar]
- 11.Navar LG, Nishiyama A: Why are angiotensin concentrations so high in the kidney? Curr Opin Nephrol Hypertens 13: 107–115, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Niimura F, Okubo S, Fogo A, Ichikawa I: Temporal and spatial expression pattern of the angiotensinogen gene in mice and rats. Am J Physiol 272: R142–R147, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ: In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest 85: 417–423, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobori H, Harrison-Bernard LM, Navar LG: Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol 12: 431–439, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lara LS, McCormack M, Semprum-Prieto LC, Shenouda S, Majid DS, Kobori H, Navar LG, Prieto MC: AT1 receptor-mediated augmentation of angiotensinogen, oxidative stress, and inflammation in ANG II-salt hypertension. Am J Physiol Renal Physiol 302: F85–F94, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhuo JL, Kobori H, Li XC, Satou R, Katsurada A, Navar LG: Augmentation of angiotensinogen expression in the proximal tubule by intracellular angiotensin II via AT1a/MAPK/NF-кB signaling pathways. Am J Physiol Renal Physiol 310: F1103–F1112, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM: Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension 34: 1265–1274, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC: Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension 57: 594–599, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J: The collecting duct is the major source of prorenin in diabetes. Hypertension 51: 1597–1604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Kats JP, Schalekamp MA, Verdouw PD, Duncker DJ, Danser AH: Intrarenal angiotensin II: Interstitial and cellular levels and site of production. Kidney Int 60: 2311–2317, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Shao W, Seth DM, Navar LG: Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol 296: F1067–F1071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li XC, Hopfer U, Zhuo JL: AT1 receptor-mediated uptake of angiotensin II and NHE-3 expression in proximal tubule cells through a microtubule-dependent endocytic pathway. Am J Physiol Renal Physiol 297: F1342–F1352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XC, Carretero OA, Navar LG, Zhuo JL: AT1 receptor-mediated accumulation of extracellular angiotensin II in proximal tubule cells: Role of cytoskeleton microtubules and tyrosine phosphatases. Am J Physiol Renal Physiol 291: F375–F383, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XC, Zhuo JL: In vivo regulation of AT1a receptor-mediated intracellular uptake of [125I]Val5-ANG II in the kidneys and adrenals of AT1a receptor-deficient mice. Am J Physiol Renal Physiol 294: F293–F302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li XC, Navar LG, Shao Y, Zhuo JL: Genetic deletion of AT1a receptors attenuates intracellular accumulation of ANG II in the kidney of AT1a receptor-deficient mice. Am J Physiol Renal Physiol 293: F586–F593, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG: Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: Role of AT(1) receptor. Hypertension 39: 116–121, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Zou LX, Imig JD, Hymel A, Navar LG: Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens 11: 570–578, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F: Intrarenal renin angiotensin system revisited: Role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem 285: 41935–41946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roksnoer LC, Heijnen BF, Nakano D, Peti-Peterdi J, Walsh SB, Garrelds IM, van Gool JM, Zietse R, Struijker-Boudier HA, Hoorn EJ, Danser AH: On the origin of urinary renin: A translational approach. Hypertension 67: 927–933, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I: Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol 23: 1181–1189, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramkumar N, Stuart D, Calquin M, Wang S, Niimura F, Matsusaka T, Kohan DE: Possible role for nephron-derived angiotensinogen in angiotensin-II dependent hypertension. Physiol Rep 4: e12675, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG: Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol 298: F150–F157, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Villalobos RA, Satou R, Seth DM, Semprun-Prieto LC, Katsurada A, Kobori H, Navar LG: Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension 53: 351–355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole J, Quach DL, Sundaram K, Corvol P, Capecchi MR, Bernstein KE: Mice lacking endothelial angiotensin-converting enzyme have a normal blood pressure. Circ Res 90: 87–92, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA: The absence of intrarenal ACE protects against hypertension. J Clin Invest 123: 2011–2023, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giani JF, Shah KH, Khan Z, Bernstein EA, Shen XZ, McDonough AA, Gonzalez-Villalobos RA, Bernstein KE: The intrarenal generation of angiotensin II is required for experimental hypertension. Curr Opin Pharmacol 21: 73–81, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM: Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM: AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab 13: 469–475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramkumar N, Stuart D, Rees S, Hoek AV, Sigmund CD, Kohan DE: Collecting duct-specific knockout of renin attenuates angiotensin II-induced hypertension. Am J Physiol Renal Physiol 307: F931–F938, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramkumar N, Ying J, Stuart D, Kohan DE: Overexpression of renin in the collecting duct causes elevated blood pressure. Am J Hypertens 26: 965–972, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Ramkumar N, Kohan DE: Role of collecting duct renin in blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 305: R92–R94, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Kobori H, Nishiyama A, Abe Y, Navar LG: Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 41: 592–597, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YG, Lee SH, Kim SY, Lee A, Moon JY, Jeong KH, Lee TW, Lim SJ, Sohn IS, Ihm CG: Sequential activation of the intrarenal renin-angiotensin system in the progression of hypertensive nephropathy in Goldblatt rats. Am J Physiol Renal Physiol 311: F195–F206, 2016 [DOI] [PubMed] [Google Scholar]
- 44.Takenaka T, Suzuki H, Furukawa T, Ogata Y, Saruta T: Role of intrarenal renin-angiotensin system on pressure-natriuresis in spontaneously hypertensive rats. Clin Exp Hypertens A 12: 1377–1394, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Zhou L, Mo H, Miao J, Zhou D, Tan RJ, Hou FF, Liu Y: Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am J Pathol 185: 3211–3223, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saigusa T, Dang Y, Bunni MA, Amria MY, Steele SL, Fitzgibbon WR, Bell PD: Activation of the intrarenal renin-angiotensin-system in murine polycystic kidney disease. Physiol Rep 3: 12405, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graciano ML, Cavaglieri RC, Dellê H, Dominguez WV, Casarini DE, Malheiros DM, Noronha IL: Intrarenal renin-angiotensin system is upregulated in experimental model of progressive renal disease induced by chronic inhibition of nitric oxide synthesis. J Am Soc Nephrol 15: 1805–1815, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Rafiq K, Noma T, Fujisawa Y, Ishihara Y, Arai Y, Nabi AH, Suzuki F, Nagai Y, Nakano D, Hitomi H, Kitada K, Urushihara M, Kobori H, Kohno M, Nishiyama A: Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation 125: 1402–1413, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Chen C, Tian J, Zha Y, Xiong Y, Sun Z, Chen P, Li J, Yang T, Ma C, Liu H, Wang X, Hou FF: Urinary angiotensinogen level predicts AKI in acute decompensated heart failure: A prospective, two-stage study. J Am Soc Nephrol 26: 2032–2041, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobori H, Harrison-Bernard LM, Navar LG: Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int 61: 579–585, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A: Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 18: 1558–1565, 2007 [DOI] [PubMed] [Google Scholar]
- 52.MacFadyen RJ, Lee AF, Morton JJ, Pringle SD, Struthers AD: How often are angiotensin II and aldosterone concentrations raised during chronic ACE inhibitor treatment in cardiac failure? Heart 82: 57–61, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD: Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burcklé C, Bader M: Prorenin and its ancient receptor. Hypertension 48: 549–551, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G: Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 53: 1077–1082, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C: Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327: 459–463, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Lu X, Wang F, Liu M, Yang KT, Nau A, Kohan DE, Reese VR, Richardson RS, Yang T: Activation of ENaC in collecting duct cells by prorenin and its receptor PRR: Involvement of Nox4-derived hydrogen peroxide. Am J Physiol Renal Physiol 310: F1243–F1250, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang T: Crosstalk between (pro)renin receptor and COX-2 in the renal medulla during angiotensin II-induced hypertension. Curr Opin Pharmacol 21: 89–94, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang J, Siragy HM: Sodium depletion enhances renal expression of (pro)renin receptor via cyclic GMP-protein kinase G signaling pathway. Hypertension 59: 317–323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matavelli LC, Huang J, Siragy HM: In vivo regulation of renal expression of (pro)renin receptor by a low-sodium diet. Am J Physiol Renal Physiol 303: F1652–F1657, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sihn G, Rousselle A, Vilianovitch L, Burckle C, Bader M: Physiology of the (pro)renin receptor: Wnt of change? Kidney Int 78: 246–256, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Trepiccione F, Gerber SD, Grahammer F, López-Cayuqueo KI, Baudrie V, Păunescu TG, Capen DE, Picard N, Alexander RT, Huber TB, Chambrey R, Brown D, Houillier P, Eladari D, Simons M: Renal Atp6ap2/(pro)renin receptor is required for normal vacuolar H+-ATPase function but not for the renin-angiotensin system. J Am Soc Nephrol 27: 3320–3330, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krop M, Lu X, Danser AH, Meima ME: The (pro)renin receptor. A decade of research: What have we learned? Pflugers Arch 465: 87–97, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H: Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol 18: 1789–1795, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Nguyen G: Renin, (pro)renin and receptor: An update. Clin Sci (Lond) 120: 169–178, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Rousselle A, Sihn G, Rotteveel M, Bader M: (Pro)renin receptor and V-ATPase: From Drosophila to humans. Clin Sci (Lond) 126: 529–536, 2014 [DOI] [PubMed] [Google Scholar]
- 67.Feldt S, Maschke U, Dechend R, Luft FC, Muller DN: The putative (pro)renin receptor blocker HRP fails to prevent (pro)renin signaling. J Am Soc Nephrol 19: 743–748, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE: The (pro)renin receptor: Site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54: 261–269, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC: Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 57: 859–864, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gonzalez AA, Womack JP, Liu L, Seth DM, Prieto MC: Angiotensin II increases the expression of (pro)renin receptor during low-salt conditions. Am J Med Sci 348: 416–422, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li W, Sullivan MN, Zhang S, Worker CJ, Xiong Z, Speth RC, Feng Y: Intracerebroventricular infusion of the (pro)renin receptor antagonist PRO20 attenuates deoxycorticosterone acetate-salt-induced hypertension. Hypertension 65: 352–361, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang F, Lu X, Liu M, Feng Y, Zhou SF, Yang T: Renal medullary (pro)renin receptor contributes to angiotensin II-induced hypertension in rats via activation of the local renin-angiotensin system. BMC Med 13: 278, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peng K, Lu X, Wang F, Nau A, Chen R, Zhou SF, Yang T: Collecting duct (pro)renin receptor targets ENaC to mediate angiotensin II-induced hypertension [published online ahead of print April 27, 2010]. Am J Physiol Renal Physiol doi:10.1152/ajprenal.00178.2016 [DOI] [PMC free article] [PubMed]
- 74.Ramkumar N, Stuart D, Mironova E, Bugay V, Wang S, Abraham N, Ichihara A, Stockand JD, Kohan DE: Renal tubular epithelial cell prorenin receptor regulates blood pressure and sodium transport. Am J Physiol Renal Physiol 311: F186–F194, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu X, Wang F, Liu M, Yang KT, Nau A, Kohan DE, Reese V, Richardson RS, Yang T: Activation of ENaC in collecting duct cells by prorenin and its receptor PRR: Involvement of Nox4-derived hydrogen peroxide. Am J Physiol Renal Physiol 310: F1243–F1250, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Danser AH: The role of the (pro)renin receptor in hypertensive disease. Am J Hypertens 28: 1187–1196, 2015 [DOI] [PubMed] [Google Scholar]
- 77.Schnermann J, Briggs JP: Synthesis and secretion of renin in mice with induced genetic mutations. Kidney Int 81: 529–538, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schnermann J, Briggs JP: Tubular control of renin synthesis and secretion. Pflugers Arch 465: 39–51, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mann B, Hartner A, Jensen BL, Kammerl M, Krämer BK, Kurtz A: Furosemide stimulates macula densa cyclooxygenase-2 expression in rats. Kidney Int 59: 62–68, 2001 [DOI] [PubMed] [Google Scholar]
- 80.Greenberg SG, Lorenz JN, He XR, Schnermann JB, Briggs JP: Effect of prostaglandin synthesis inhibition on macula densa-stimulated renin secretion. Am J Physiol 265: F578–F583, 1993 [DOI] [PubMed] [Google Scholar]
- 81.Schweda F, Klar J, Narumiya S, Nüsing RM, Kurtz A: Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol 287: F427–F433, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Pöschke A, Kern N, Maruyama T, Pavenstädt H, Narumiya S, Jensen BL, Nüsing RM: The PGE(2)-EP4 receptor is necessary for stimulation of the renin-angiotensin-aldosterone system in response to low dietary salt intake in vivo. Am J Physiol Renal Physiol 303: F1435–F1442, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Farman N, Pradelles P, Bonvalet JP: Determination of prostaglandin E2 synthesis along rabbit nephron by enzyme immunoassay. Am J Physiol 251: F238–F244, 1986 [DOI] [PubMed] [Google Scholar]
- 84.Breyer MD, Jacobson HR, Breyer RM: Functional and molecular aspects of renal prostaglandin receptors. J Am Soc Nephrol 7: 8–17, 1996 [DOI] [PubMed] [Google Scholar]
- 85.Olesen ET, Rützler MR, Moeller HB, Praetorius HA, Fenton RA: Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc Natl Acad Sci USA 108: 12949–12954, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao M, Cao R, Du S, Jia X, Zheng S, Huang S, Han Q, Liu J, Zhang X, Miao Y, Kang J, Gustafsson JA, Guan Y: Disruption of prostaglandin E2 receptor EP4 impairs urinary concentration via decreasing aquaporin 2 in renal collecting ducts. Proc Natl Acad Sci USA 112: 8397–8402, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang F, Lu X, Peng K, Fang H, Zhou L, Su J, Nau A, Yang KT, Ichihara A, Lu A, Zhou SF, Yang T: Antidiuretic action of collecting duct (pro)Renin receptor downstream of vasopressin and PGE2 receptor EP4. J Am Soc Nephrol 27: 3022–3034, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Breyer MD, Breyer RM: Prostaglandin receptors: Their role in regulating renal function. Curr Opin Nephrol Hypertens 9: 23–29, 2000 [DOI] [PubMed] [Google Scholar]
- 89.Vo BT, Morton D Jr, Komaragiri S, Millena AC, Leath C, Khan SA: TGF-β effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology 154: 1768–1779, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thibodeau JF, Holterman CE, He Y, Carter A, Cron GO, Boisvert NC, Abd-Elrahman KS, Hsu KJ, Ferguson SS, Kennedy CR: Vascular smooth muscle-specific EP4 receptor deletion in mice exacerbates angiotensin II-induced renal injury. Antioxid Redox Signal 25: 642–656, 2016 [DOI] [PubMed] [Google Scholar]
- 91.Angers S, Moon RT: Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10: 468–477, 2009 [DOI] [PubMed] [Google Scholar]
- 92.Schmidt-Ott KM, Barasch J: WNT/beta-catenin signaling in nephron progenitors and their epithelial progeny. Kidney Int 74: 1004–1008, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He W, Kang YS, Dai C, Liu Y: Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou T, He X, Cheng R, Zhang B, Zhang RR, Chen Y, Takahashi Y, Murray AR, Lee K, Gao G, Ma JX: Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia 55: 255–266, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, Hou FF, Kahn M, Liu Y: Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol 26: 107–120, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cuevas CA, Gonzalez AA, Inestrosa NC, Vio CP, Prieto MC: Angiotensin II increases fibronectin and collagen I through the β-catenin-dependent signaling in mouse collecting duct cells. Am J Physiol Renal Physiol 308: F358–F365, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu X, Wang F, Xu C, Soodvilai S, Peng K, Su J, Zhao L, Yang KT, Feng Y, Zhou S-F, Gustafsson J-A, Yang T: Soluble (pro)renin receptor via β-catenin enhances urine concentration capability: A target of liver X receptor. Proc Natl Acad Sci USA 113: E1898–E1906, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 100.Hu MC, Kuro-o M, Moe OW: Klotho and chronic kidney disease. Contrib Nephrol 180: 47–63, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hamada K, Taniguchi Y, Shimamura Y, Inoue K, Ogata K, Ishihara M, Horino T, Fujimoto S, Ohguro T, Yoshimoto Y, Ikebe M, Yuasa K, Hoshino E, Iiyama T, Ichihara A, Terada Y: Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol 17: 848–856, 2013 [DOI] [PubMed] [Google Scholar]
- 102.Yang HC, Deleuze S, Zuo Y, Potthoff SA, Ma LJ, Fogo AB: The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol 20: 2380–2388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R: In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 39: 838–843, 2002 [DOI] [PubMed] [Google Scholar]
- 104.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y: Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 24: 771–785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou X, Chen K, Wang Y, Schuman M, Lei H, Sun Z: Antiaging gene Klotho regulates adrenal CYP11B2 expression and aldosterone synthesis. J Am Soc Nephrol 27: 1765–1776, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gustafsson JA: Historical overview of nuclear receptors. J Steroid Biochem Mol Biol 157: 3–6, 2016 [DOI] [PubMed] [Google Scholar]
- 107.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T: Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci USA 102: 9406–9411, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD: Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med 11: 861–866, 2005 [DOI] [PubMed] [Google Scholar]
- 109.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP: 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li YC: Discovery of vitamin D hormone as a negative regulator of the renin-angiotensin system. Clin Chem 60: 561–562, 2014 [DOI] [PubMed] [Google Scholar]
- 111.Li YC: Vitamin D receptor signaling in renal and cardiovascular protection. Semin Nephrol 33: 433–447, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kristal-Boneh E, Froom P, Harari G, Ribak J: Association of calcitriol and blood pressure in normotensive men. Hypertension 30: 1289–1294, 1997 [DOI] [PubMed] [Google Scholar]
- 113.Lind L, Hänni A, Lithell H, Hvarfner A, Sörensen OH, Ljunghall S: Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens 8: 894–901, 1995 [DOI] [PubMed] [Google Scholar]
- 114.Resnick LM, Müller FB, Laragh JH: Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med 105: 649–654, 1986 [DOI] [PubMed] [Google Scholar]
- 115.Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC: 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem 282: 29821–29830, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Freundlich M, Quiroz Y, Zhang Z, Zhang Y, Bravo Y, Weisinger JR, Li YC, Rodriguez-Iturbe B: Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int 74: 1394–1402, 2008 [DOI] [PubMed] [Google Scholar]
- 117.Scragg R, Sowers M, Bell C: Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens 20: 713–719, 2007 [DOI] [PubMed] [Google Scholar]
- 118.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM: Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med 152: 307–314, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Witham MD, Nadir MA, Struthers AD: Effect of vitamin D on blood pressure: A systematic review and meta-analysis. J Hypertens 27: 1948–1954, 2009 [DOI] [PubMed] [Google Scholar]
- 120.Margolis KL, Ray RM, Van Horn L, Manson JE, Allison MA, Black HR, Beresford SA, Connelly SA, Curb JD, Grimm RH Jr, Kotchen TA, Kuller LH, Wassertheil-Smoller S, Thomson CA, Torner JC; Women’s Health Initiative Investigators : Effect of calcium and vitamin D supplementation on blood pressure: The women’s health initiative randomized trial. Hypertension 52: 847–855, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, Alvarez JA, Boxer RS, Dalbeni A, Gepner AD, Isbel NM, Larsen T, Nagpal J, Petchey WG, Stricker H, Strobel F, Tangpricha V, Toxqui L, Vaquero MP, Wamberg L, Zittermann A, Witham MD; D-PRESSURE Collaboration : Effect of vitamin D supplementation on blood pressure: A systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med 175: 745–754, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pilz S, Gaksch M, Kienreich K, Grübler M, Verheyen N, Fahrleitner-Pammer A, Treiber G, Drechsler C, Ó Hartaigh B, Obermayer-Pietsch B, Schwetz V, Aberer F, Mader J, Scharnagl H, Meinitzer A, Lerchbaum E, Dekker JM, Zittermann A, März W, Tomaschitz A: Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 65: 1195–1201, 2015 [DOI] [PubMed] [Google Scholar]
- 123.Wu SH, Ho SC, Zhong L: Effects of vitamin D supplementation on blood pressure. South Med J 103: 729–737, 2010 [DOI] [PubMed] [Google Scholar]
- 124.Beveridge LA, Witham MD: Controversy in the link between vitamin D supplementation and hypertension. Expert Rev Cardiovasc Ther 13: 971–973, 2015 [DOI] [PubMed] [Google Scholar]
- 125.Artaza JN, Contreras S, Garcia LA, Mehrotra R, Gibbons G, Shohet R, Martins D, Norris KC: Vitamin D and cardiovascular disease: Potential role in health disparities. J Health Care Poor Underserved 22[Suppl]: 23–38, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berkenstam A, Gustafsson JA: Nuclear receptors and their relevance to diseases related to lipid metabolism. Curr Opin Pharmacol 5: 171–176, 2005 [DOI] [PubMed] [Google Scholar]
- 127.Alberti S, Steffensen KR, Gustafsson JA: Structural characterisation of the mouse nuclear oxysterol receptor genes LXRalpha and LXRbeta. Gene 243: 93–103, 2000 [DOI] [PubMed] [Google Scholar]
- 128.Zelcer N, Hong C, Boyadjian R, Tontonoz P: LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 325: 100–104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ: Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289: 1524–1529, 2000 [DOI] [PubMed] [Google Scholar]
- 130.Wang F, Lu X, Peng K, Du Y, Zhou SF, Zhang A, Yang T: Prostaglandin E-prostanoid4 receptor mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Hypertension 64: 369–377, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kuipers I, van der Harst P, Kuipers F, van Genne L, Goris M, Lehtonen JY, van Veldhuisen DJ, van Gilst WH, de Boer RA: Activation of liver X receptor-alpha reduces activation of the renal and cardiac renin-angiotensin-aldosterone system. Lab Invest 90: 630–636, 2010 [DOI] [PubMed] [Google Scholar]
- 132.Morello F, de Boer RA, Steffensen KR, Gnecchi M, Chisholm JW, Boomsma F, Anderson LM, Lawn RM, Gustafsson JA, Lopez-Ilasaca M, Pratt RE, Dzau VJ: Liver X receptors alpha and beta regulate renin expression in vivo. J Clin Invest 115: 1913–1922, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y: Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol 22: 1642–1653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang M, Wang R, Sun J, Yu K, Chen B, Xu L, Zhao B, Wang H: The liver X receptor agonist TO901317 protects mice against cisplatin-induced kidney injury. Exp Biol Med (Maywood) 240: 1717–1727, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hum JM, O’Bryan LM, Tatiparthi AK, Cass TA, Clinkenbeard EL, Cramer MS, Bhaskaran M, Johnson RL, Wilson JM, Smith RC, White KE: Chronic hyperphosphatemia and vascular calcification are reduced by stable delivery of soluble Klotho [published online ahead of print November 11, 2016]. J Am Soc Nephrol doi 10.1681/ASN.2015111266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto S, Ikebe M, Yuasa K, Yamanaka S, Sugiura T, Terada Y: Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 16: 722–729, 2012 [DOI] [PubMed] [Google Scholar]
- 137.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, Yamanari T, Kikumoto Y, Uchida HA, Kitamura S, Maeshima Y, Nakamura K, Ito H, Makino H: A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One 8: e56695, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]