Abstract
A number of proteomic and peptidomic analyses of urine from diabetic subjects have been published in the quest for a biomarker that predicts progression of nephropathy. Less attention has been paid to the relationships between urinary proteins and the underlying biological processes revealed by the analyses. In this review, we focus on the biological processes identified by studying urinary proteins and protein-protein interactions at each stage of diabetic nephropathy to provide an overview of the events underlying progression of kidney disease reflected in the urine. In uncomplicated diabetes, proteomic/peptidomic analyses indicate that early activation of fibrotic pathways in the kidney occurs before the onset of microalbuminuria. In incipient nephropathy, when albumin excretion rates are abnormal, proteomic/peptidomic analyses suggest that changes in glomerular permselectivity and tubular reabsorption account, at least in part, for the proteins and peptides that appear in the urine. Finally, overt nephropathy is characterized by proteins involved in wound healing, ongoing fibrosis, and inflammation. These findings suggest that there is a spectrum of biological processes in the diabetic kidney and that assessing protein networks may be more informative than individual markers with respect to the stage of disease and the risk of progression.
Keywords: bioinformatics, diabetic kidney disease, urinary proteomics
Microalbuminuria is currently the most reliable predictor of diabetic nephropathy.1–3 However, recent evidence challenges this notion. The Joslin Study of Natural History of Microalbuminuria demonstrated that the likelihood of regression from microalbuminuria to normal urinary albumin excretion outweighs the likelihood of progression to overt proteinuria.4 Other longitudinal, observational studies have replicated these results in children and adults, in type 1 and type 2 diabetes, and in individuals from North America, Asia, and Europe.5–9 Taken together, these studies provide strong evidence that microalbuminuria may not be the ideal marker of progression after all.
Advances in mass spectrometry have enhanced our ability to identify thousands of proteins and peptides in urine in a single analysis—some of which may serve as new markers. This large-scale study of proteins is termed proteomics, whereas the study of naturally occurring peptides generated by endogenous protease activity is termed peptidomics. Urinary proteomics and peptidomics add different dimensions to the investigation of underlying biology. Their application in urine has important clinical implications for diabetic kidney disease, given that urine can be collected noninvasively with relative ease and is directly produced by the kidneys.10 As such, changes in the relative abundance of urinary proteins and peptides may reflect changes in protein expression, deposition, or turnover in the diabetic kidney.
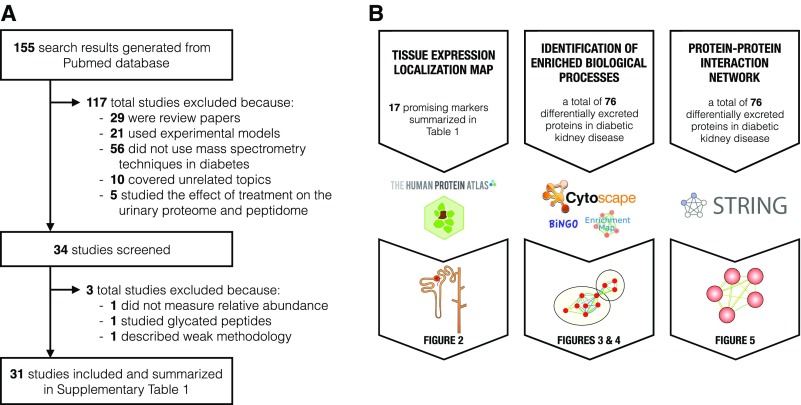
We reviewed the literature on urinary proteomics/peptidomics, biomarkers, and diabetic kidney disease in humans (Figure 1A, Supplemental Table 1). We selected the most robust candidate markers at each stage of diabetic kidney disease and then highlighted their roles in biological processes that may contribute to progression from uncomplicated diabetes to incipient diabetic nephropathy to overt diabetic nephropathy (Table 1). Although reviews on similar topics exist, none integrate findings across studies and assess their biological implications on mechanisms underlying diabetic kidney disease progression.
Figure 1.
Summary of literature search and bioinformatic analyses. (A) Out of the 155 search results, 34 studies were screened and 31 relevant urinary proteomic/peptidomics studies in diabetes were included into our review. Reasons for study exclusion are shown for the initial screening and final inclusion. (B) The protein datasets, bioinformatic tools and software, and output figures are outlined for each of the bioinformatic analyses.
Table 1.
Characteristics of promising markers for each stage of diabetic kidney disease
| Stage of Diabetic Kidney Disease | Protein | Description | Direction of Urinary Excretiona | Nephron Segment | Biological Processes | Validated |
|---|---|---|---|---|---|---|
| Uncomplicated Diabetes | α1-antitrypsin (SERPINA1) | 40-kD serine protease inhibitor | ↑ (9 of 9) | PT | Coagulation, inflammation | ELISA, SRM |
| Clusterin (CLU) | 50-kD nuclear form | ↑ (3 of 4) | PT | Complement activation, inflammation, lipid metabolism | ELISA | |
| 75-kD secretory form | ||||||
| Type I collagen (COL1A1/COL1A2) | >130-kD extracellular matrix protein | ↓ (8 of 8) | NS | Extracellular matrix organization | None | |
| Heparan sulfate proteoglycan (HSPG2) | 469-kD basement membrane protein | ↓ (2 of 2) | NS | Blood vessel development, extracellular matrix organization | None | |
| Osteopontin (SPP1) | 35-kD secreted phosphoprotein | ↑ (1 of 1) | LoH, DCT* | Adhesion, tissue development | None | |
| Uromodulin (UMOD) | 85-kD GPI-anchored glycoprotein | ↓ (11 of 11) | LoH, DCT* | Tissue development | ELISA, SRM | |
| Incipient Diabetic Nephropathy | α1-acid glycoprotein 1 (ORM1) | 24-kD positive acute-phase reactant | ↑ (5 of 5) | G | Inflammation, immune system, transport | ELISA, immuno-turbidimetry |
| Cubilin (CUBN) | 399-kD endocytic receptor | ↑ (1 of 1) | PT* | Endocytosis, lipid metabolism | None | |
| Haptoglobin (HP) | 45-kD positive acute-phase reactant | ↑ (2 of 4) | NS | Immune system response, inflammation | ELISA, SRM | |
| Megalin (LRP2) | 522-kD endocytic receptor | ↑ (1 of 1) | PT* | Endocytosis, tissue development | None | |
| Mannan-binding lectin serine protease 2 (MASP2) | 76-kD serine protease | ↑ (1 of 2) | NS | Complement activation, immune system response | SRM | |
| Transferrin (TF) | 77-kD plasma carrier of iron | ↑ (2 of 2) | G | Iron ion homeostasis, transport | SRM | |
| Overt Diabetic Nephropathy | α2-HS-glycoprotein (AHSG) | 39-kD plasma carrier | ↑ (5 of 5) | G, PT | Endocytosis, inflammation, tissue development | None |
| β2-microglobulin (B2M) | 14-kD MHC class I component | ↑ (5 of 5) | G, PT | Ion homeostasis, immune system response | None | |
| Hemopexin (HPX) | 59-kD plasma carrier of heme | ↑ (1 of 1) | G | Iron ion homeostasis, transport | None | |
| Retinol-binding protein 4 (RBP4) | 23-kD plasma carrier of retinol | ↑ (5 of 6) | PT* | Immune system response, tissue development, transport | ELISA | |
| Transthyretin (TTR) | 55-kD homotetrameric plasma carrier of RBP4 and thyroxine | ↓ (4 of 7) | PT | Extracellular matrix organization, transport | ELISA |
The asterisk (*) denotes high enrichment in and/or specificity to the kidney. ↑, increased urinary excretion in cases compared to controls; PT, proximal tubules; SRM, selected reaction monitoring; ↓, decreased urinary excretion in cases compared to controls; NS, not specific to the kidneys or a particular segment; LoH, loop of Henle; DCT, distal convoluted tubules; G, glomerulus.
Fraction of studies that reported the specific direction of urinary excretion (in cases versus controls) out of all studies that identified differential excretion of the protein (shown in brackets).
Integrating Data with Bioinformatics
Proteins and peptides are interconnected in large networks, which can be altered in disease. Expression profiles vary according to the type of cell, tissue, organ, or specimen, which can shed light on the location of injury.11,12 Furthermore, multiple datasets of potential biological markers can be more useful when considered together, as opposed to in isolation, so that the most robust and consistent network of biological functions may be identified. This “mechanistic” approach can be used to explore and address the current gaps in knowledge underlying the pathophysiology and progression of diabetic nephropathy.
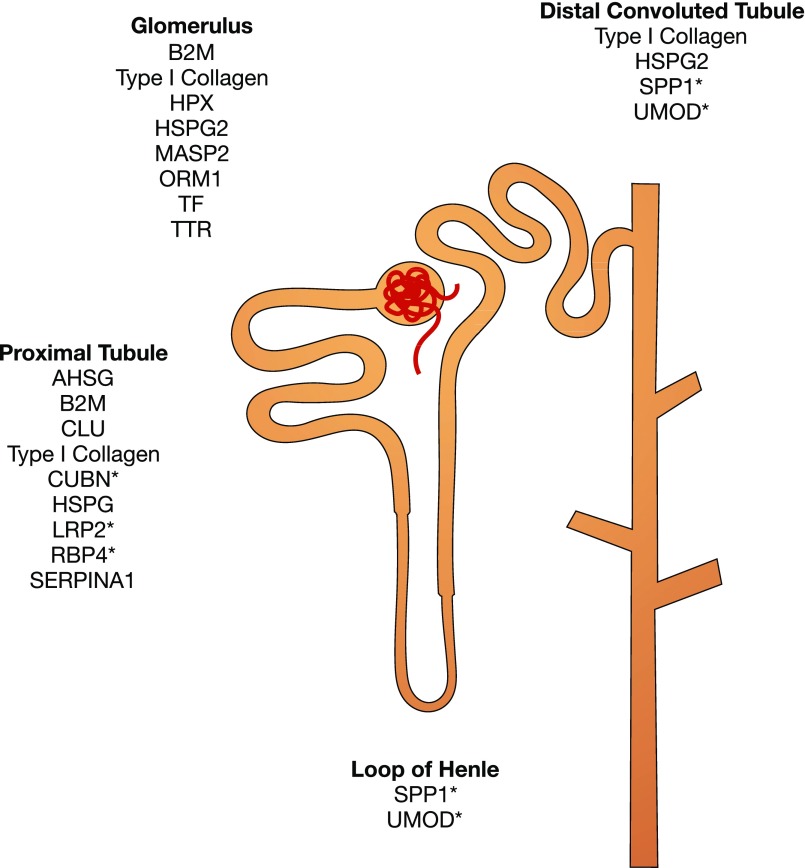
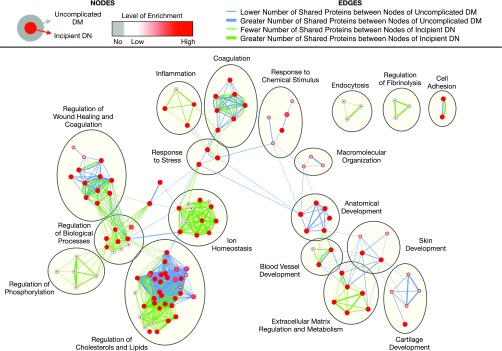
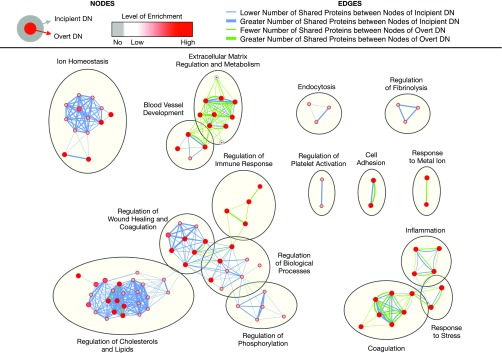
We thus investigated the biological implications of differentially excreted urinary proteins in diabetic kidney disease by taking advantage of published, relevant data (Figure 1B). Candidate markers with differential excretion between cases and controls were extracted from all 31 studies (Supplemental Table 1). All candidates were weighted equally when inputted into the network analyses. However, we selected a handful of promising candidates for each stage of diabetic kidney disease on the basis of (1) between-group differential excretion rates in at least two studies, (2) support from verification and validation, and/or (3) high enrichment and/or specificity in the kidney (Table 1). For the localization analysis, these promising candidates were mapped onto specific nephron segments on the basis of their renal expression in normal tissues using the Human Protein Atlas11 and inferred site of injury using the existing literature (Figure 2). For the functional analysis, we deduced biological processes implicated in the progression of diabetic kidney disease using the Biological Networks Gene Ontology13 and Enrichment Map14 plug-ins of Cytoscape software for the entire set of proteins associated with each stage of diabetic kidney disease (Figures 3 and 4). For the purposes of this review, we compared sequential stages of diabetic kidney disease to better understand transitions and progression, although we realize that this construct is somewhat artificial. Furthermore, we constructed protein-protein interaction networks with the Search Tool for the Retrieval of Interacting Genes/Proteins (Figure 5).15 Overall, these analyses have enabled us to comprehensively identify common and stage-specific biological processes in diabetic kidney disease by including all proteins associated with a particular stage of disease.
Figure 2.
Localization of the most promising urinary markers of diabetic kidney disease in different nephron segments. Proteins were mapped according to their normal and induced expression in renal tissues using the Human Protein Atlas12 and the existing literature. The asterisk (*) denotes high protein enrichment and/or specificity in the segment. AHSG, α2-HS-glycoprotein; B2M, β2-microglobulin; CLU, clusterin; CUBN, cubilin; HPX, hemopexin; HSPG2, heparan sulfate proteoglycan; LRP2, megalin; MASP2, mannan-binding lectin serine protease 2; ORM1, α1-acid glycoprotein 1; RBP4, retinol-binding protein 4; SERPINA1, α1-antitrypsin; SPP1, osteopontin; TF, transferrin; TTR, transthyretin; UMOD, uromodulin.
Figure 3.
Comparison of enriched biological processes in uncomplicated diabetes and incipient diabetic nephropathy. Significantly enriched biological processes were identified for each stage using Biological Networks Gene Ontology with Benjamini and Hochberg multiple testing correction (P<0.05) and then run on Enrichment Map with Jaccard coefficient of 0.5 (P value cut-off = 0.001; false discovery rate Q-value cut-off = 0.05). Each node represents an enriched biological process. Red node colors correspond to high enrichment, whereas gray node colors correspond to no enrichment. As shown in the figure legend, the outer circle color corresponds to the level of enrichment in uncomplicated diabetes, whereas the inner circle color corresponds to that in incipient diabetic nephropathy. Edge thickness denotes the amount of overlapping markers between two connected nodes within uncomplicated diabetes (blue) and within incipient diabetic nephropathy (green). DM, diabetes mellitus; DN, diabetic nephropathy.
Figure 4.
Comparison of enriched biological processes in incipient diabetic nephropathy and overt diabetic nephropathy. Significantly enriched biological processes were identified for each stage using Biological Networks Gene Ontology with Benjamini and Hochberg multiple testing correction (P<0.05) and then run on Enrichment Map with Jaccard coefficient of 0.5 (P value cut-off = 0.001; false discovery rate Q-value cut-off = 0.05). As shown in the figure legend, the outer circle color corresponds to the level of enrichment in incipient diabetic nephropathy, whereas the inner circle color corresponds to that in overt diabetic nephropathy. Edge thickness denotes the amount of overlapping markers between two connected nodes within incipient diabetic nephropathy (blue) and within overt diabetic nephropathy (green). DM, diabetes mellitus; DN, diabetic nephropathy.
Figure 5.
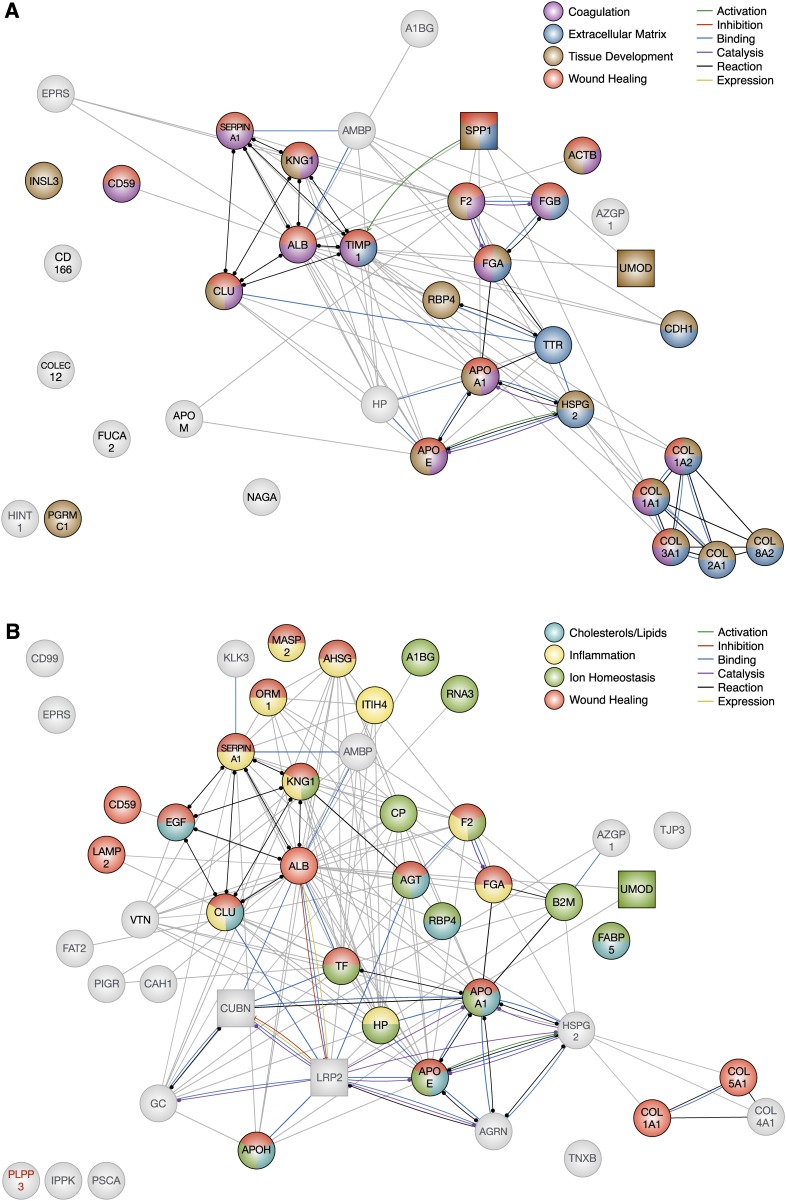
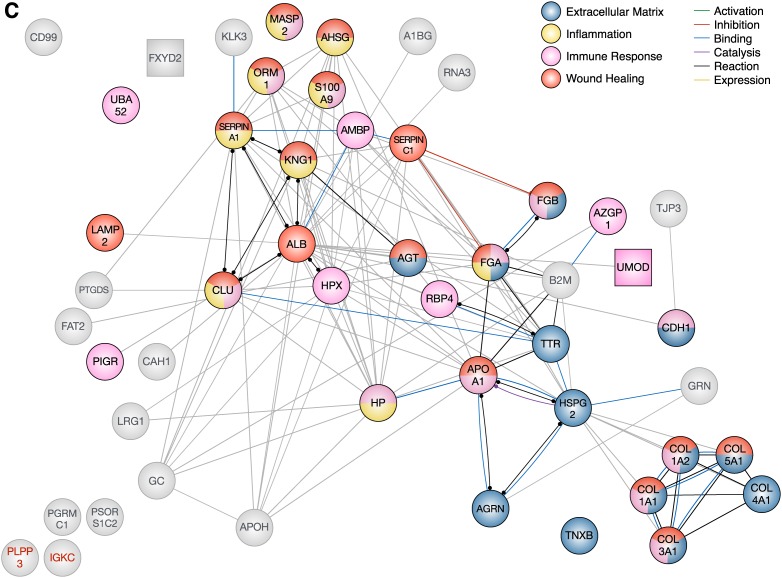
Protein-protein interaction networks for differentially excreted proteins in diabetic kidney disease using the Search Tool for the Retrieval of Interacting Genes/Proteins v10 database. Each node represents a candidate marker. Node colors illustrate a protein’s involvement in the four significantly enriched biological processes (identified by Biological Networks Gene Ontology) for each stage. Gray nodes are not associated with any of the four significantly enriched biological processes. Square nodes indicate high enrichment in and/or specificity to the kidney. Some proteins were not found in the Search Tool for the Retrieval of Interacting Genes/Proteins database and were labeled in red (e.g., PLPP3). The edges represent protein-protein interactions, and the nature of the protein-protein interactions are color-coded as indicated in the figure legend (e.g., binding interactions in blue and unspecified interactions in gray). (A) Uncomplicated diabetes was characterized by coagulation, extracellular matrix, and tissue development, which are also activated in wound healing (n=35). (B) Incipient diabetic nephropathy was characterized by regulation of cholesterols and lipids, inflammation, ion homeostasis, and wound healing (n=47). (C) Overt diabetic nephropathy was characterized by extracellular matrix, inflammation, immune system, and wound healing (n=50).
Uncomplicated Diabetes
Uncomplicated diabetes is the earliest stage of diabetic kidney disease. Some maladaptive changes such as renal hyperfiltration and hypertrophy are often present at time of diagnosis of diabetes.16 Renal hyperfiltration has been linked to the initiation and progression of diabetic nephropathy.17,18 Many patients develop lesions such as mesangial expansion, glomerular basement membrane thickening, and podocyte loss.16,19 These functional and structural abnormalities distinguish the diabetic kidney from a healthy kidney before the onset of microalbuminuria. However, current urinary markers are not sensitive enough to confidently detect these changes. Hence, there is a pressing need for accurate and reliable markers of early diabetic kidney injury.
In healthy adolescents with type 1 diabetes, Meier and colleagues identified a urinary proteome profile, which was different from that of healthy, age-matched controls.20 Without the use of tandem mass spectrometry, differences could not be assigned to specific proteins. Nevertheless, this study demonstrated that differences could be detected early in diabetic kidney disease, setting the stage for later studies. In the largest urinary peptidome study of diabetes to date, Maahs and colleagues demonstrated lower urinary levels of collagen and uromodulin in diabetic cases with normal renal function compared with controls.21 Zhang and colleagues found two urinary fragments of fibrinogen α chain and prothrombin—two important and closely related players in coagulation—which were associated with type 2 diabetes (particularly in those with high glycated hemoglobin, HbA1c).22 In total, 35 protein candidates have been identified as potential markers of early, uncomplicated diabetes.
Early Diabetes: a Tubular Pathology?
Diabetic kidney disease is often described as a glomerular pathology, wherein injury to the glomerulus not only precedes but typically outweighs progressive injury to the tubulo-interstitium.19,23,24 However, the differential excretion of tubular proteins such as uromodulin and osteopontin suggests that the tubular compartment is an important site of early injury (Figure 2). Other lines of evidence also support the notion that early changes in the tubulointerstitium contribute to the development and progression of diabetic nephropathy.25,26 In fact, regression of microalbuminuria in type 1 diabetes was significantly associated with lower urinary levels of tubular injury markers: kidney injury molecule-1 and N-acetyl-β-D-glucosaminidase.26,27 Targeted attempts to identify and treat early tubular injury may prevent or delay diabetic nephropathy.
Coagulation and Fibrosis May Be Activated in Chronic Hyperglycemia
In our functional analysis, the 35 differentially excreted proteins in uncomplicated diabetes were associated with cell adhesion, coagulation, extracellular matrix regulation and metabolism, macromolecular organization, regulation of wound healing and coagulation, regulation of cholesterols and lipids, responses to chemical stimulus and stress, and (“anatomic,” “blood vessel,” “cartilage,” and “skin”) tissue development. Processes appear as clustered nodes in the enrichment map comparing uncomplicated diabetes and incipient diabetic nephropathy (Figure 3). The majority of processes in uncomplicated diabetes were also enriched in incipient diabetic nephropathy, except for macromolecular organization. Coagulation, tissue development, and extracellular matrix regulation and metabolism were among the most significantly enriched processes. Interestingly, these biological processes play important roles within the broader biological processes of wound healing.
In fact, coagulation is one of the first processes activated in wound healing, facilitating leukocyte entry into the site of injury.28 Increased urinary excretion of thrombin and fibrinogen in uncomplicated diabetes21,22 suggests that coagulation may be activated in early diabetes (Figure 5A). Experimental studies have shown that increased activation of coagulation in diabetes impaired glomerular permselectivity and induced apoptosis of endothelial cells and podocytes.29
Tissue development, in the context of wound healing, relies on normal extracellular matrix organization. Platelets release TGFβ to stimulate the deposition and suppress the degradation of extracellular matrix,30 which replaces the fibrin clot and acts as a scaffold for healing tissues. As the wound heals, TGFβ levels subside, and the deposited matrix is degraded, restoring homeostasis.30 Chronic injury, however, overrides the tight control of TGFβ. The subsequent accumulation of extracellular matrix, as evidenced by significant reductions of urinary fibrillar collagens (e.g., type I and III) and heparan sulfate proteoglycan in complicated diabetes compared with healthy controls,21,31,32 hinders cellular migration and proper tissue development, culminating in scar tissue and fibrosis. Interestingly, urinary excretion of nonfibrillar type VIII collagen was also diminished in uncomplicated diabetes,21 possibly as a result of impaired turnover and increased accumulation in the glomerulus. Studies have shown that its upregulated expression in the kidney may be specific to diabetes and may contribute to profibrotic TGFβ-driven pathophysiologic processes such as the proliferation of renal fibroblasts and mesangial cells.33,34 Blood vessel development is also greatly impaired by fibrosis,35 contributing to the development and progression of renal lesions in diabetic animals.36,37
Although renal fibrosis is a hallmark of late diabetic nephropathy, extracellular matrix accumulation in early, uncomplicated diabetes seems plausible (Figure 5A). Moreover, mesangial matrix expansion has been well documented at this stage of diabetic kidney disease.16,38,39
Incipient Diabetic Nephropathy
Between 20% and 30% of patients with diabetes advance to incipient diabetic nephropathy,5,40,41 defined by the onset of persistent microalbuminuria. Although hyperfiltration may persist, some patients experience a progressive decline in GFR.42 Incipient diabetic nephropathy usually develops after 10–15 years of type 1 diabetes, but may already be present when type 2 diabetes is diagnosed.16
In their comparison of patients with incipient diabetic nephropathy and controls with uncomplicated diabetes, Jin and colleagues performed isobaric tag for relative and absolute quantitation to identify and multiple reaction monitoring to verify differential excretion of α1-antitrypsin, transferrin, ceruloplasmin, albumin, haptoglobin, vitamin D–binding protein, α1-acid glycoprotein 1, and prostate stem cell antigen.43 Thrailkill and colleagues posited that increased urinary excretion of megalin and cubilin contributed to the increased urinary excretion of other proteins, such as albumin, transferrin, and vitamin D–binding protein, in urine.44 Focusing on peptidomics, Merchant and colleagues performed a case-control study, comparing “decliner” patients whose GFRs progressively declined by 3.3% per year during follow-up and “nondecliner” controls whose GFRs remained relatively stable.45 Differentially excreted peptides derived from extracellular matrix elements (e.g., types IV and V collagen, tenascin-X), cell adhesion molecules (e.g., zona occludens 3, protocadherin FAT 2), and the enzyme inositol pentakisphosphate 2-kinase and were associated with progressive renal function decline. We identified a total of 47 differentially excreted proteins in incipient diabetic nephropathy, 36% of which were also identified in uncomplicated diabetes.
Urinary Markers Reflect Defects in the Glomerulus and Proximal Tubules
Localization of markers within the glomerulus and proximal tubules may reflect impaired glomerular permselectivity and proximal tubular reabsorption (Figure 2). Within the nephron segments, reduced urinary excretion of type IV collagen and heparan sulfate proteoglycan likely reflects progressive basement membrane thickening of the glomerulus and proximal tubules.16,46 Although a healthy glomerular filtration barrier with normal permselectivity limits entry of large macromolecules into Bowman’s space, some proteins are filtered, including trace amounts of albumin.47 However, nearly all of these filtered proteins are reabsorbed into the proximal tubules via megalin/cubilin-mediated endocytosis.48 Interestingly, endocytosis was among the enriched biological processes in incipient diabetic nephropathy (Figure 3). Several of the differentially excreted proteins (e.g., albumin, clusterin, apos, vitamin D–binding protein, and transferrin) are known ligands of megalin and cubilin,49 suggesting tubular dysfunction before development of overt proteinuria.
Inflammation, Cholesterol and Lipid Dysregulation, and Ion Imbalance Underlie Incipient Diabetic Nephropathy
Despite the overlap between uncomplicated diabetes and incipient diabetic nephropathy, regulation of cholesterols and lipids, inflammation, and ion homeostasis emerged as the top biological processes in incipient diabetic nephropathy (Figures 3 and 4). Endocytosis, regulation of phosphorylation, and regulation of fibrinolysis were exclusively enriched in this stage of disease. Endocytosis is the primary mechanism for reabsorption of proteins via megalin/cubilin, as previously discussed. Phosphorylation is a common post-translational modification used in several signaling cascades (e.g., of EGF and thrombin). Fibrinolysis refers to the breakdown to fibrin clots, which are formed from fibrinogen by thrombin during coagulation, which were discussed in the uncomplicated diabetes section.
Inflammation, similar to coagulation and the regulation of extracellular matrix in uncomplicated diabetes, is linked to the wound healing pathways. In wound healing, macrophages infiltrate the site of injury during inflammation to phagocytose cellular debris, which in turn facilitates the migration and proliferation of other cells.28 Eventually, inflammation is turned “off” to allow for the resolution of injury through tissue proliferation and remodeling.28 However, this inflammatory state is sustained by chronic hyperglycemia, predisposing to progressive diabetic kidney disease.50 Urinary markers of inflammation include several acute-phase reactant proteins such as α1-acid glycoprotein 1, haptoglobin, clusterin, α2-HS-glycoprotein, and mannan-binding lectin serine protease 2 (Figure 5B).
The transport and metabolism of cholesterols and lipids are regulated, in part, by the apo family.51 Several members (e.g., apo A, E, H, clusterin/J) were differentially excreted throughout the progression of diabetic kidney disease (Figure 5) and significantly contributed to the enrichment of cholesterols and lipids in diabetic nephropathy (Figure 4). Studies have shown that diabetes impairs insulin- and leptin-mediated regulation of cholesterol and lipid, which increases the risk of cardiovascular disease.52,53 Interestingly, impaired cholesterol esterification and efflux have been linked to podocyte injury.54 Cholesterols also play an important role in cell membrane integrity. In diabetes, overexpression of cholesterol in platelet membranes impaired fluidity and in turn heightened platelet sensitivity to thrombin,55 perhaps contributing to increased thrombosis and vascular injury. There could be an interesting interplay between coagulation, blood vessel development, and cholesterol and lipid regulation. However, unlike other processes, the enrichment of cholesterol and lipid regulation was largely driven by apos as well as other high-abundance plasma proteins. Although these biological processes may reflect systemic changes, these plasma proteins may be detected in the urine as a result of impaired glomerular permselectivity and tubular reabsorption.
Maintaining ion homeostasis is an important function of the kidney, especially the proximal tubule. These segments are largely responsible for the bulk reabsorption of a variety of electrolytes including sodium, chloride, bicarbonate, and phosphate. Interestingly, iron ion homeostasis emerged as one of the most enriched individual biological processes within the broader cluster of ion homeostasis, a finding that might implicate oxidative stress in incipient diabetic nephropathy.56,57 Iron catalyzes the formation of hydroxyl radical, a reactive oxygen species, via the Haber–Weiss reaction as a catalyzing agent.58,59 Iron metabolism and transport are regulated by haptoglobin and transferrin (Figure 5B), which were elevated in the urines of patients with diabetic kidney disease.
Overt Diabetic Nephropathy
The onset of proteinuria, or macroalbuminuria, marks late-stage diabetic kidney disease.16 Functionally, urinary albumin excretion continues to rise, whereas GFR declines.60,61 Structurally, there is evidence of glomerulosclerosis and tubular atrophy. Nearly 10% of patients with diabetes progress further to ESRD62 and require RRT such as dialysis and transplantation.
Dihazi and colleagues found increased urinary levels of β2-microglobulin and decreased levels of ubiquitin-60S ribosomal protein L40 from 38 patients with diabetic nephropathy, compared with 45 healthy controls.63 These proteins are ubiquitously expressed in the body and likely derived from the circulation. As such, their presence in the urine may reflect impaired glomerular permselectivity. Sharma and colleagues identified α1-antitrypsin as the primary marker between cases and controls, but were limited by a small sample size of eight patients.64 The presence of α1-antitrypsin in urine is consequently not specific to a single stage of diabetic kidney disease. Rao and colleagues focused on low-abundance proteins by immunodepleting albumin, immunoglobin G and A, α1-antitrypsin, transferrin, and haptoglobin.65 Transthyretin and α2-HS-glycoprotein among others were identified as potential markers. Otu and colleagues characterized the urinary proteome for diabetic nephropathy in Pima Indians of Southern Arizona.66 This particular demographic has been extensively studied for its predisposition to type 2 diabetes and vascular complications.67,68 Limitations of this proteomic study include the lack of tandem mass spectrometry, survivor bias, and the questionable stability of urine samples stored for >10 years. Although 50 proteins were differentially excreted in overt diabetic nephropathy, only nine were exclusive to this stage. Consequently, we hypothesize that the biological processes underlying overt diabetic nephropathy were also enriched in previous stages.
Glomerular Dysfunction Predominantly Manifests in Late Disease
Defects in permselectivity and reabsorption continue to prevail in overt diabetic nephropathy, as exemplified by the increased urinary presence of several carrier proteins (e.g., α2-HS-glycoprotein, hemopexin, transthyretin) from the plasma compartment.
Underlying Processes in Overt Diabetic Nephropathy Were Likely Active in Previous Stages
In overt diabetic nephropathy, the significantly enriched biological processes included extracellular matrix regulation and metabolism, inflammation, and regulation of the immune response (Figure 4). Interestingly, the number of proteins involved in the regulation of the immune response greatly outnumbered those involved in wound healing, although there is significant overlap between both processes (Figure 5C). The immune system plays an integral role in successful wound healing, promoting the infiltration of immune cells into the site of injury and regulating inflammation.28 For the most part, these biological processes (e.g., extracellular matrix regulation and metabolism, inflammation, regulation of the immune response, and cell adhesion) were also present in the earlier stages (Figures 3 and 4), underlining their persistent role in the progression of diabetic kidney disease.
Diabetic Kidney Disease as a Whole
Overall, the majority of enriched biological processes were identified at all stages of diabetic kidney disease. These processes include coagulation, inflammation, cell adhesion, regulation of the immune system, extracellular matrix metabolism, tissue and blood vessel development, endothelial cell proliferation, and cell adhesion (Figures 3 and 4). Interestingly, these processes all fall within the spectrum of wound healing—the umbrella term of complex biological processes involved in the body’s response to injury and subsequent repair.28 The identification of wound healing processes in early, uncomplicated diabetes indicates a degree of renal injury long before the onset of microalbuminuria. Even though urinary markers were identified in different studies, they were highly connected on the basis of our analysis of protein-protein interactions (Figure 5). For example, the presence of α1-antitrypsin alone in urine would not have made a compelling argument for coagulation in early diabetes. However, we found supporting evidence in that thrombin, antithrombin, fibrinogen, and kininogen were also identified in urine—all of which are involved in coagulation.69,70 As such, stage-specific networks of proteins may be more informative than individually quantified proteins, due to the complexity of biology.
There are several limitations to our analyses. First, we assumed that the proteins measured in the urine reflected biological processes in the diabetic kidney. Given the protein networks enriched in protein-protein interactions, it seems unlikely that the proteins were found in the urine by chance. In a study on the characterization of the normal urinary proteome, nearly 70% of urinary proteins were likely derived from the kidney,71 supporting the use of urine as a surrogate biospecimen for kidney tissue. Second, our bioinformatic analyses did not include quantitative results as these were not available for all reviewed studies. Given the differences in methodology, it would also be difficult to normalize data across studies. Third, our bioinformatic analyses was largely tailored to proteomic data. Differentially excreted peptides were incorporated into protein networks as their precursor proteins. Although the field of “mechanistic peptidomics” is relatively new, there are tools currently available for the prediction of proteases involved in the generation of naturally occurring peptides (e.g., Proteasix72) and the mapping of peptides onto their native protein sequence (e.g., Peptide Extractor73). Such analyses will enhance our understanding of the proteolytic processes that may be altered in diabetic kidney disease. Nevertheless, our findings identified major biological processes that develop early, persist throughout, and become dominant at different stages in the progression of diabetic kidney disease.
Over 75 differentially excreted urinary proteins were identified by studies using urinary proteomic and peptidomic approaches, but only a minority of candidates have been verified and validated. Our bioinformatic analysis of the biological implications of the candidate markers uncovered stage-specific and overarching mechanisms potentially acting on the diabetic kidney. Urinary markers in uncomplicated diabetes indicated early activation of renal fibrosis and the importance of tubular injury. Incipient diabetic nephropathy was predominantly characterized by impaired glomerular permselectivity and tubular reabsorption via inflammation and ion dysregulation. These processes continued to play a significant role in overt diabetic nephropathy, highlighted by wound healing, progressive fibrosis, and chronic inflammation. Overall, we demonstrate how proteomic/peptidomic datasets may be combined and integrated to highlight the most robust markers and to characterize the biological context for the proteins.
Disclosures
None.
Supplementary Material
Acknowledgments
J.A.D.V. is supported by the Banting & Best Diabetes Centre–Novo Nordisk Scholarship. A.K. is supported by the Kidney Foundation of Canada operating grant and the Kidney Research Scientist Core Education and National Training program/Canadian Institutes of Health Research (CIHR) salary and infrastructure support for new investigators. J.W.S. is supported by operating grants from the Heart and Stroke Foundation of Canada and the CIHR.
J.A.D.V. wrote the manuscript. All authors contributed equally to researching data for the article, and reviewing and editing the manuscript before submission.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016091018/-/DCSupplemental.
References
- 1.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H: Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1: 1430–1432, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Parving HH, Oxenbøll B, Svendsen PA, Christiansen JS, Andersen AR: Early detection of patients at risk of developing diabetic nephropathy. A longitudinal study of urinary albumin excretion. Acta Endocrinol (Copenh) 100: 550–555, 1982 [DOI] [PubMed] [Google Scholar]
- 3.Mogensen CE: Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med 310: 356–360, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS: Regression of microalbuminuria in type 1 diabetes. N Engl J Med 348: 2285–2293, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Schultz CJ, Konopelska-Bahu T, Dalton RN, Carroll TA, Stratton I, Gale EA, Neil A, Dunger DB; Oxford Regional Prospective Study Group : Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Diabetes Care 22: 495–502, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Giorgino F, Laviola L, Cavallo Perin P, Solnica B, Fuller J, Chaturvedi N: Factors associated with progression to macroalbuminuria in microalbuminuric Type 1 diabetic patients: The EURODIAB Prospective Complications Study. Diabetologia 47: 1020–1028, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Araki S, Haneda M, Sugimoto T, Isono M, Isshiki K, Kashiwagi A, Koya D: Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes 54: 2983–2987, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Roy MS, Affouf M, Roy A: Six-year incidence of proteinuria in type 1 diabetic African Americans. Diabetes Care 30: 1807–1812, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Son MK, Yoo HY, Kwak BO, Park HW, Kim KS, Chung S, Chae HW, Kim HS, Kim DH: Regression and progression of microalbuminuria in adolescents with childhood onset diabetes mellitus. Ann Pediatr Endocrinol Metab 20: 13–20, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konvalinka A, Scholey JW, Diamandis EP: Searching for new biomarkers of renal diseases through proteomics. Clin Chem 58: 353–365, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Pontén F, Jirström K, Uhlen M: The human protein atlas--a tool for pathology. J Pathol 216: 387–393, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Uhlen M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F: Proteomics. Tissue-based map of the human proteome. Science 347: 1260419, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Maere S, Heymans K, Kuiper M: BiNGO: A Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21: 3448–3449, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Merico D, Isserlin R, Stueker O, Emili A, Bader GD: Enrichment map: A network-based method for gene-set enrichment visualization and interpretation. PLoS One 5: e13984, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C: STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43: D447–D452, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mogensen CE, Christensen CK, Vittinghus E: The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 32[Suppl 2]: 64–78, 1983 [DOI] [PubMed] [Google Scholar]
- 17.Ruggenenti P, Porrini EL, Gaspari F, Motterlini N, Cannata A, Carrara F, Cella C, Ferrari S, Stucchi N, Parvanova A, Iliev I, Dodesini AR, Trevisan R, Bossi A, Zaletel J, Remuzzi G; GFR Study Investigators : Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 35: 2061–2068, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG: Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52: 691–697, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier M, Kaiser T, Herrmann A, Knueppel S, Hillmann M, Koester P, Danne T, Haller H, Fliser D, Mischak H: Identification of urinary protein pattern in type 1 diabetic adolescents with early diabetic nephropathy by a novel combined proteome analysis. J Diabetes Complications 19: 223–232, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Maahs DM, Siwy J, Argilés A, Cerna M, Delles C, Dominiczak AF, Gayrard N, Iphöfer A, Jänsch L, Jerums G, Medek K, Mischak H, Navis GJ, Roob JM, Rossing K, Rossing P, Rychlík I, Schiffer E, Schmieder RE, Wascher TC, Winklhofer-Roob BM, Zimmerli LU, Zürbig P, Snell-Bergeon JK: Urinary collagen fragments are significantly altered in diabetes: A link to pathophysiology. PLoS One 5: e13051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang M, Fu G, Lei T: Two urinary peptides associated closely with type 2 diabetes mellitus. PLoS One 10: e0122950, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauer SM: Structural-functional correlations of diabetic nephropathy. Kidney Int 45: 612–622, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Chavers BM, Bilous RW, Ellis EN, Steffes MW, Mauer SM: Glomerular lesions and urinary albumin excretion in type I diabetes without overt proteinuria. N Engl J Med 320: 966–970, 1989 [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Brezniceanu ML, Wei CC, Chénier I, Sachetelli S, Zhang SL, Filep JG, Ingelfinger JR, Chan JS: Overexpression of angiotensinogen increases tubular apoptosis in diabetes. J Am Soc Nephrol 19: 269–280, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonventre JV: Can we target tubular damage to prevent renal function decline in diabetes? Semin Nephrol 32: 452–462, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH, Krolewski AS, Bonventre JV: Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-β-D-glucosaminidase. Kidney Int 79: 464–470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurtner GC, Werner S, Barrandon Y, Longaker MT: Wound repair and regeneration. Nature 453: 314–321, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Gilbert RE, Marsden PA: Activated protein C and diabetic nephropathy. N Engl J Med 358: 1628–1630, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Border WA, Noble NA: Transforming growth factor beta in tissue fibrosis. N Engl J Med 331: 1286–1292, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Rossing K, Mischak H, Dakna M, Zürbig P, Novak J, Julian BA, Good DM, Coon JJ, Tarnow L, Rossing P; PREDICTIONS Network : Urinary proteomics in diabetes and CKD. J Am Soc Nephrol 19: 1283–1290, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewandowicz A, Bakun M, Kohutnicki R, Fabijańska A, Kistowski M, Imiela J, Dadlez M: Changes in urine proteome accompanying diabetic nephropathy progression. Pol Arch Med Wewn 125: 27–38, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Gerth J, Cohen CD, Hopfer U, Lindenmeyer MT, Sommer M, Gröne HJ, Wolf G: Collagen type VIII expression in human diabetic nephropathy. Eur J Clin Invest 37: 767–773, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Loeffler I, Hopfer U, Koczan D, Wolf G: Type VIII collagen modulates TGF-β1-induced proliferation of mesangial cells. J Am Soc Nephrol 22: 649–663, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA: Abnormal angiogenesis in diabetic nephropathy. Diabetes 58: 1471–1478, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto Y, Maeshima Y, Kitayama H, Kitamura S, Takazawa Y, Sugiyama H, Yamasaki Y, Makino H: Tumstatin peptide, an inhibitor of angiogenesis, prevents glomerular hypertrophy in the early stage of diabetic nephropathy. Diabetes 53: 1831–1840, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Ichinose K, Maeshima Y, Yamamoto Y, Kitayama H, Takazawa Y, Hirokoshi K, Sugiyama H, Yamasaki Y, Eguchi K, Makino H: Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes 54: 2891–2903, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC: Structural-functional relationships in diabetic nephropathy. J Clin Invest 74: 1143–1155, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steffes MW, Osterby R, Chavers B, Mauer SM: Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes 38: 1077–1081, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH: Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes 39: 1116–1124, 1990 [DOI] [PubMed] [Google Scholar]
- 41.Newman DJ, Mattock MB, Dawnay AB, Kerry S, McGuire A, Yaqoob M, Hitman GA, Hawke C: Systematic review on urine albumin testing for early detection of diabetic complications. Health Technol Assess 9: iii–vi, xiii–163, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Krolewski AS, Niewczas MA, Skupien J, Gohda T, Smiles A, Eckfeldt JH, Doria A, Warram JH: Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care 37: 226–234, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin J, Ku YH, Kim Y, Kim Y, Kim K, Lee JY, Cho YM, Lee HK, Park KS, Kim Y: Differential proteome profiling using iTRAQ in microalbuminuric and normoalbuminuric type 2 diabetic patients. Exp Diabetes Res 2012: 168602, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thrailkill KM, Nimmo T, Bunn RC, Cockrell GE, Moreau CS, Mackintosh S, Edmondson RD, Fowlkes JL: Microalbuminuria in type 1 diabetes is associated with enhanced excretion of the endocytic multiligand receptors megalin and cubilin. Diabetes Care 32: 1266–1268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merchant ML, Perkins BA, Boratyn GM, Ficociello LH, Wilkey DW, Barati MT, Bertram CC, Page GP, Rovin BH, Warram JH, Krolewski AS, Klein JB: Urinary peptidome may predict renal function decline in type 1 diabetes and microalbuminuria. J Am Soc Nephrol 20: 2065–2074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brito PL, Fioretto P, Drummond K, Kim Y, Steffes MW, Basgen JM, Sisson-Ross S, Mauer M: Proximal tubular basement membrane width in insulin-dependent diabetes mellitus. Kidney Int 53: 754–761, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Pollock CA, Poronnik P: Albumin transport and processing by the proximal tubule: Physiology and pathophysiology. Curr Opin Nephrol Hypertens 16: 359–364, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Hryciw DH, Lee EM, Pollock CA, Poronnik P: Molecular changes in proximal tubule function in diabetes mellitus. Clin Exp Pharmacol Physiol 31: 372–379, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Christensen EI, Birn H: Megalin and cubilin: Multifunctional endocytic receptors. Nat Rev Mol Cell Biol 3: 256–266, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Navarro-González JF, Mora-Fernández C: The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19: 433–442, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Phillips MC: Molecular mechanisms of cellular cholesterol efflux. J Biol Chem 289: 24020–24029, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Rourke L, Yeaman SJ, Shepherd PR: Insulin and leptin acutely regulate cholesterol ester metabolism in macrophages by novel signaling pathways. Diabetes 50: 955–961, 2001 [DOI] [PubMed] [Google Scholar]
- 53.O’Rourke L, Gronning LM, Yeaman SJ, Shepherd PR: Glucose-dependent regulation of cholesterol ester metabolism in macrophages by insulin and leptin. J Biol Chem 277: 42557–42562, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Pedigo CE, Ducasa GM, Leclercq F, Sloan A, Mitrofanova A, Hashmi T, Molina-David J, Ge M, Lassenius MI, Forsblom C, Lehto M, Groop PH, Kretzler M, Eddy S, Martini S, Reich H, Wahl P, Ghiggeri G, Faul C, Burke GW 3rd, Kretz O, Huber TB, Mendez AJ, Merscher S, Fornoni A: Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J Clin Invest 126: 3336–3350, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winocour PD, Watala C, Perry DW, Kinlough-Rathbone RL: Decreased platelet membrane fluidity due to glycation or acetylation of membrane proteins. Thromb Haemost 68: 577–582, 1992 [PubMed] [Google Scholar]
- 56.Swaminathan S, Fonseca VA, Alam MG, Shah SV: The role of iron in diabetes and its complications. Diabetes Care 30: 1926–1933, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Dixon SJ, Stockwell BR: The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10: 9–17, 2014 [DOI] [PubMed] [Google Scholar]
- 58.Haber F, Weiss J: The catalytic decomposition of hydrogen peroxide by iron salts. Proc R Soc A Math Phys Eng Sci 147: 332–351, 1934 [Google Scholar]
- 59.Kehrer JP: The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 149: 43–50, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS: Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 18: 1353–1361, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Pavkov ME, Knowler WC, Lemley KV, Mason CC, Myers BD, Nelson RG: Early renal function decline in type 2 diabetes. Clin J Am Soc Nephrol 7: 78–84, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C: Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 294: 1782–1787, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Dihazi H, Müller GA, Lindner S, Meyer M, Asif AR, Oellerich M, Strutz F: Characterization of diabetic nephropathy by urinary proteomic analysis: Identification of a processed ubiquitin form as a differentially excreted protein in diabetic nephropathy patients. Clin Chem 53: 1636–1645, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Sharma K, Lee S, Han S, Lee S, Francos B, McCue P, Wassell R, Shaw MA, RamachandraRao SP: Two-dimensional fluorescence difference gel electrophoresis analysis of the urine proteome in human diabetic nephropathy. Proteomics 5: 2648–2655, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Rao PV, Lu X, Standley M, Pattee P, Neelima G, Girisesh G, Dakshinamurthy KV, Roberts CT Jr, Nagalla SR: Proteomic identification of urinary biomarkers of diabetic nephropathy. Diabetes Care 30: 629–637, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Otu HH, Can H, Spentzos D, Nelson RG, Hanson RL, Looker HC, Knowler WC, Monroy M, Libermann TA, Karumanchi SA, Thadhani R: Prediction of diabetic nephropathy using urine proteomic profiling 10 years prior to development of nephropathy. Diabetes Care 30: 638–643, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Knowler WC, Bennett PH, Hamman RF, Miller M: Diabetes incidence and prevalence in Pima Indians: A 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol 108: 497–505, 1978 [DOI] [PubMed] [Google Scholar]
- 68.Nelson RG, Newman JM, Knowler WC, Sievers ML, Kunzelman CL, Pettitt DJ, Moffett CD, Teutsch SM, Bennett PH: Incidence of end-stage renal disease in type 2 (non-insulin-dependent) diabetes mellitus in Pima Indians. Diabetologia 31: 730–736, 1988 [DOI] [PubMed] [Google Scholar]
- 69.Furie B, Furie BC: The molecular basis of blood coagulation. Cell 53: 505–518, 1988 [DOI] [PubMed] [Google Scholar]
- 70.Long AT, Kenne E, Jung R, Fuchs TA, Renné T: Contact system revisited: An interface between inflammation, coagulation, and innate immunity. J Thromb Haemost 14: 427–437, 2016 [DOI] [PubMed] [Google Scholar]
- 71.Pieper R, Gatlin CL, McGrath AM, Makusky AJ, Mondal M, Seonarain M, Field E, Schatz CR, Estock MA, Ahmed N, Anderson NG, Steiner S: Characterization of the human urinary proteome: A method for high-resolution display of urinary proteins on two-dimensional electrophoresis gels with a yield of nearly 1400 distinct protein spots. Proteomics 4: 1159–1174, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Klein J, Eales J, Zürbig P, Vlahou A, Mischak H, Stevens R: Proteasix: A tool for automated and large-scale prediction of proteases involved in naturally occurring peptide generation. Proteomics 13: 1077–1082, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Guerrero A, Dallas DC, Contreras S, Chee S, Parker EA, Sun X, Dimapasoc L, Barile D, German JB, Lebrilla CB: Mechanistic peptidomics: Factors that dictate specificity in the formation of endogenous peptides in human milk. Mol Cell Proteomics 13: 3343–3351, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.