Abstract
A highly effective method for derivatizing 2,1-borazaronaphthalene cores using ammonium alkylbis(catecholato)silicates via photoredox/nickel dual catalysis is reported. By forging Csp3–Csp2 bonds via this approach, alkyl fragments with various functional groups can be introduced to the azaborine core, affording previously inaccessible heterocyclic isosteres in good to excellent yields. The base-free, room temperature conditions outlined allow sensitive functional group tolerance, even permitting the cross-coupling of unprotected primary and secondary amines.
Graphical Abstract
Azaborines are boron-, nitrogen-, and carbon-containing heteroaromatic compounds that have been recognized as viable iso-steric species for aryl- and heteroaryl cores.1 The B–N bond serves as a replacement for a C=C bond in arenes, and substitution in this manner affords molecules having similar, but not identical, electronic and steric properties.2 Consequently, B–N/C=C isosterism has attracted the attention of medicinal chemists because it allows the introduction of structural and electronic variety to existing drug templates, and may enhance the potency of biologically active molecules, leading to new therapeutics.3
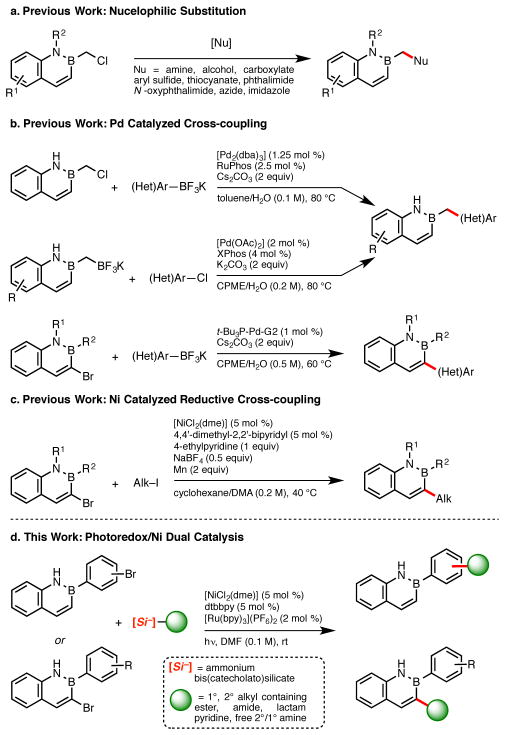
Recently, we developed a rapid synthetic route to 2,1-borazaronaphthalenes,4 in addition to methods for further elaborating the structural diversity of these cores.5 Post annulation modifications included nucleophilic substitution of 2-chloromethyl-2,1-borazaronaphthalene (Scheme 1a),6 and various Pd-catalyzed functionalization processes (Scheme 1b).7 Additionally, alkyl groups could be introduced at the 3-position of the naphthyl azaborine via Ni-catalyzed reductive coupling of 1° and 2° alkyl iodides (Scheme 1c).8 This latter protocol enabled the formation of Csp3–Csp2 bonds between the naphthalene isostere core and an alkyl chain. The introduction of functionalized alkyl moieties is attractive, particularly for medicinal chemistry applications.9 Addition of such groups could provide a means of increasing solubility of drug candidates, hence improving their ADME (Absorption, Distribution, Metabolism, and Excretion) properties.10 Unfortunately, current conditions for incorporation of alkyl chains have several drawbacks, among them the need for excess terminal reductant, intolerance of protic functional groups, and the limited accessibility/high cost of alkyl iodides.
Scheme 1.
2,1-Borazaronaphthalene Functionalization by (a) Nucleophilic Substitution; (b) Pd Catalyzed Cross-coupling; (c) Ni Catalyzed Reductive Cross-coupling; (d) Photoredox/Ni Cross-Coupling.
Very recently, our group and others reported a new synthetic paradigm to enable the formation of C–C bonds under extremely mild reaction conditions: i.e., visible light photoredox/Ni dual catalytic cross-coupling. 11,12 Utilization of this reaction manifold has allowed the formation of Csp3–Csp2 bonds between benzylic,11a,13 secondary alkyl,12a,13 primary alkyl,13 α-alkoxy,11d,12a,f and α-amino11b,12c,d,f Csp3-hybridized radical precursors with a variety of (hetero)aryl and/or alkenyl halide electrophiles. These radical precursors are oxidizable species such as organotrifluoroborates, carboxylic acids, and alkylsilicates. The latter, specifically ammonium alkylbis(catecholato)silicates,13a,b represent practical, highly versatile radical precursors because of their low oxidation potentials, the innocuous byproducts generated during SET-mediated fragmentation, and the near-neutral reaction conditions. Taken together, photoredox/Ni dual catalysis employing ammonium alkylsilicates offers a cross-coupling approach that possesses excellent functional group tolerance and ease of operation.13a,b With this newly developed paradigm in mind and the goal of accessing more highly elaborated and functionalized 2,1-borazaronaphthalene cores, we assessed whether these substrates were amenable to this means of Csp3–Csp2 bond formation.
From the outset, we chose to focus our study on azaborines having an aryl ring off the boron atom because such derivatives have been found to possess enhanced stability relative to those possessing other substituents on boron. Using the previously reported reaction conditions for the cross-coupling of aryl bromides with silicates {2 mol % [Ru(bpy)3](PF6)2, 5 mol % [NiCl2(dme)], 5 mol % dtbbpy in DMF (0.1 M)}, the reaction of 2-(4-bromophenyl)-2,1-borazaronaphthalene 1a with cyclohexylsili-cate13a affords coupled product 2a in 97% isolated yield (Scheme 2) (dtbbpy: 4,4′-di-tert-butyl-2,2′-dipyridyl; bpy: 2,2′-dipyridyl). Substrate 1a was further coupled with various silicates, affording alkylated azaborines 2b–n in excellent yield with both 2° and 1° alkylsilicates. Isobutyl, bicyclic, and 3,3,3-trifluoropropyl fragments were amenable to cross-coupling (2b–c, e) as were radicals containing various functional groups, including an ether (2d), ester (2f–g), amide (2h) and even a lactam (2i).14 Alkylsilicates containing Lewis basic residues such as pyridyl (2j), benzylalkylamino (2l) and secondary alkylamino (2m) were similarly well tolerated. In the case of piperazine (2k) and primary amine (2n) -containing silicates, lower yields were obtained (47% and 68%, respectively), with unreacted bromoazaborine remaining even after extended reaction time. The rationale for the lower yield observed using the unprotected piperazine and the previously successful 3-aminopropyl silicate remains elusive.13a Despite this, the late-stage incorporation of these basic substituents may be highly attractive to medicinal chemists seeking to improve the ADME properties of prospective azaborine targets. To assess further the robustness of the described protocol, the synthesis of the amine-containing compound 2m was scaled to one gram (3.5 mmol). Neither the yield nor the reaction time was compromised in this transformation, confirming the scalability of this reaction.
Scheme 2.
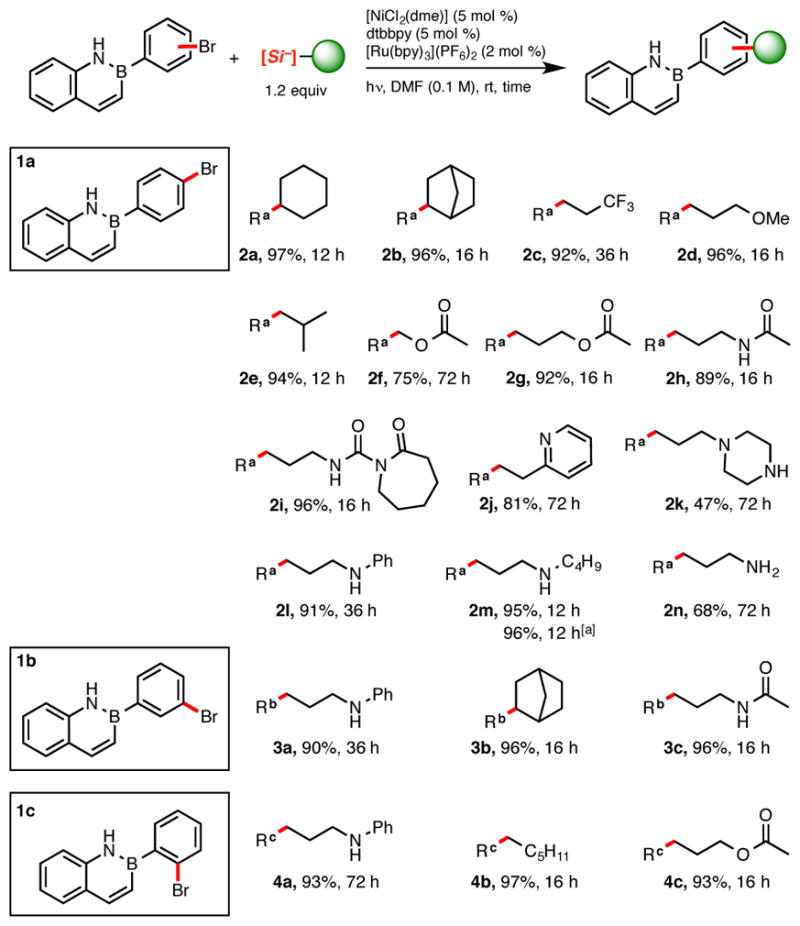
Photoredox Cross-coupling of Ammonium Alkylbis(catecholato)silicates with 2-(Bromophenyl)-2,1-borazaronaphthalenes.
aReaction carried out on 3.5 mmol using blue LEDs; all other reactions were carried out on a 0.5 mmol scale of 1.
The azaborine scope was further extended to meta and ortho substituted 2-(bromophenyl)-2,1-borazaronaphthalenes (Scheme 2, 1b and 1c, respectively). It appeared that the positioning of the bromine atom on the B-aryl group did not affect the reaction, affording coupled products 3a–c and 4a–c in excellent yields. Interestingly, ortho-substituted azaborines (1c, 4a–c) were isolated as oils, whereas nearly all other compounds (with the exception of 2k) were obtained as fluffy powders. This disparity is most likely due to a perturbation of the molecular packing caused by the steric constraints imparted by ortho substitution. Positioning of an alkyl chain at this position on the B-aryl group likely prevents coplanarity, disrupting intermolecular π–stacking.
Next we examined the photoredox/Ni cross-coupling performed directly on the azaborinyl ring itself. Therefore, a series of 3-bromo-2-phenyl-2,1-borazaronaphthalene derivatives were prepared in high yield via selective electrophilic bromination (Scheme 3).4
Scheme 3.
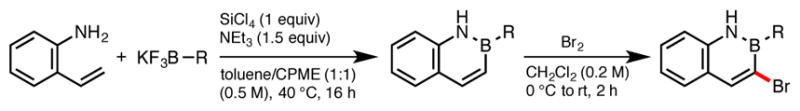
Synthesis of Brominated 2,1-Borazaronaphthalene Cores.
Brominated azaborines 1d–h were found to be excellent electrophiles in this type of cross-coupling, affording coupled product in good to excellent yields (Scheme 4). Indeed, a variety of groups could be installed at the 3-position on the azaborine core (5a–5f, 6–9). Notably, reaction times were often shorter when performing these couplings. Such acceleration may be due to improved rates of oxidative addition into the C–Br bond, illustrating the lower aromatic character of the azaborines relative to that of all-carbon arenes. Substitution on the aryl group off the boron did not seem to affect cross-coupling. B-4-Trifluoromethylphenyl (1e) and B-benzodioxanyl substituents (1f) were both well tolerated, affording coupling products 6 and 7 in good and excellent yields, respectively. An ortho-difluoro-substituted azaborine (1g) did not impede the reaction and afforded the corresponding alkylated product 8 almost quantitatively. Whereas selectivity can sometimes be problematic when using dihalogenated systems in transition metal catalyzed cross-couplings, total selectivity for the bromine over the chlorine was observed in the case of 1h, leaving the latter intact for further modification.
Scheme 4.
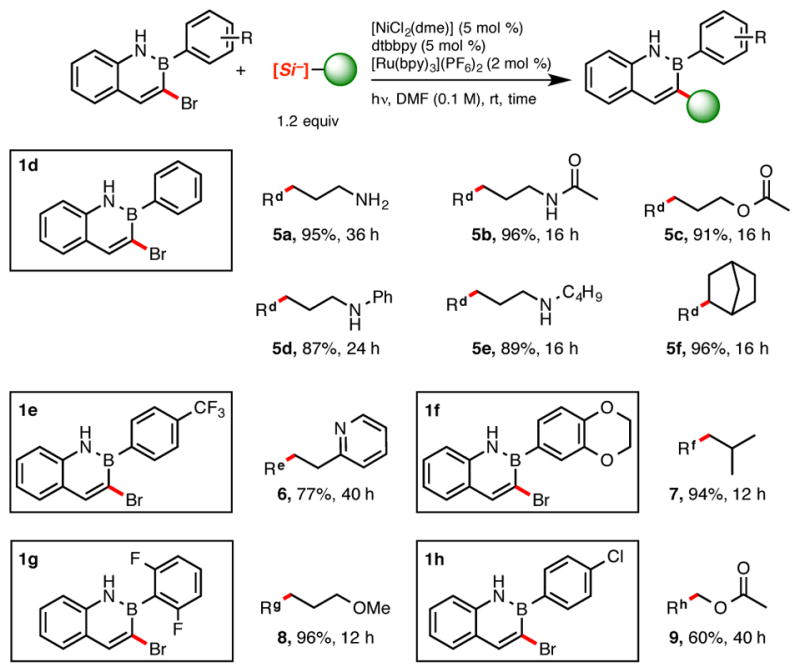
2,1-Borazaronaphthalene Scope with both Primary and Secondary Ammonium Alkylbis(catecholato)silicates.
In conclusion, several 2,1-borazaronaphthalene derivatives were efficiently alkylated using ammonium alkylsilicates via photoredox/Ni dual catalysis. This selective, high yielding, and very mild approach to azaborinyl functionalization enables the rapid construction of a library of naphthalene isosteres with numerous types of functional groups at room temperature and near-neutral conditions. In fact, the modular nature of constructing the borazaronaphthalene core, combined with the selective and regiocomplementary means of elaborating this core, provide a means to access chemical space in a manner that is challenging, if not impossible, to achieve in the parent naphthalene systems themselves. The methods outlined in this report add further to this versatility, and may allow enhancement of ADME properties of target structures, thus improving their viability as potential isosteres for drug molecules.
Supplementary Material
Acknowledgments
This research was supported by the NIGMS (R01 GM-111465 and GM-113878) and Eli Lilly. We thank Kingson Lin (University of Pennsylvania) for the preparation of alkylsilicates and Dr. Christopher B. Kelly (University of Pennsylvania) for helpful discussions. Frontier Scientific is acknowledged for their generous donation of potassium organotrifluoroborates. Evonik Industries are acknowledged for their generous donation of organotrimethoxysilanes.
Footnotes
The authors declare no competing financial interest
Experimental procedures, compound characterization data, and NMR spectra for all compounds. The Supporting Information is available free of charge on ACS Publications website at DOI: XXX
References
- 1.(a) Francotte P, Goffin E, Fraikin P, Graindorge E, Lestage P, Danober L, Challal S, Rogez N, Nosjean O, Caignard DH, Pirotte B, de Tullio P. J Med Chem. 2013;56:7838. doi: 10.1021/jm400676g. [DOI] [PubMed] [Google Scholar]; (b) Pirotte B, de Tullio P, Florence X, Goffin E, Somers F, Boverie S, Lebrun P. J Med Chem. 2013;56:3247. doi: 10.1021/jm301743b. [DOI] [PubMed] [Google Scholar]; (c) Meanwell NA. J Med Chem. 2011;54:2529. doi: 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]; (d) Patani GA, LaVoie EJ. Chem Rev. 1996;96:3147. doi: 10.1021/cr950066q. [DOI] [PubMed] [Google Scholar]
- 2.Zhou HB, Nettles KW, Bruning JB, Kim Y, Joachimiak A, Sharma S, Carlson KE, Stossi F, Katzenellenbogen BS, Greene GL, Katzenellenbogen JA. Chem Biol. 2007;14:659. doi: 10.1016/j.chembiol.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 3.(a) Liu L, Marwitz AJ, Matthews BW, Liu SY. Angew Chem, Int Ed. 2009;48:6817. doi: 10.1002/anie.200903390. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Baldock C, Rafferty JB, Sedelnikova SE, Baker PJ, Stuitje AR, Slabas AR, Hawkes TR, Rice DW. Science. 1996;274:2107. doi: 10.1126/science.274.5295.2107. [DOI] [PubMed] [Google Scholar]; (c) Davis MC, Franzblau SG, Martin AR. Bioorg Med Chem Lett. 1998;8:843. doi: 10.1016/s0960-894x(98)00126-7. [DOI] [PubMed] [Google Scholar]; (d) Grassberger MA, Turnowsky F, Hildebrand J. J Med Chem. 1984;27:947. doi: 10.1021/jm00374a003. [DOI] [PubMed] [Google Scholar]; (e) Baker SJ, Ding CZ, Akama T, Zhang YK, Hernandez V, Xia Y. Future Med Chem. 2009;1:1275. doi: 10.4155/fmc.09.71. [DOI] [PubMed] [Google Scholar]; (f) Vlasceanu A, Jessing M, Kilburn JP. Bioorg Med Chem. 2015;23:4453. doi: 10.1016/j.bmc.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Wisniewski SR, Guenther CL, Argintaru OA, Molander GA. J Org Chem. 2014;79:365. doi: 10.1021/jo402616w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For an overview of the synthetic methods for accessing azaborine derivatives, see: Bosdet MJD, Piers WE. Can J Chem. 2009;87:8.Campbell PG, Marwitz AJV, Liu SY. Angew Chem, Int Ed. 2012;51:6074. doi: 10.1002/anie.201200063.Morgan MM, Piers WE. Dalton Trans. doi: 10.1039/c5dt03991f.
- 6.Molander GA, Wisniewski SR, Amani J. Org Lett. 2014;16:5636. doi: 10.1021/ol502708z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Molander GA, Wisniewski SR. J Org Chem. 2014;79:6663. doi: 10.1021/jo5011894. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molander GA, Amani J, Wisniewski SR. Org Lett. 2014;16:6024. doi: 10.1021/ol5030508. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Amani J, Molander GA. Org Lett. 2015;17:3624. doi: 10.1021/acs.orglett.5b01750. [DOI] [PubMed] [Google Scholar]
- 8.Molander GA, Wisniewski SR, Traister KT. Org Lett. 2014;16:3692. doi: 10.1021/ol501495d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Rombouts FJR, Tovar F, Austin N, Tresadern G, Trabanco AA. J Med Chem. 2015;58:9287. doi: 10.1021/acs.jmedchem.5b01088. [DOI] [PubMed] [Google Scholar]; (b) Foye WO, Lemke TL, Williams DA. Foye’s Principles of Medicinal Chemistry. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2013. [Google Scholar]
- 10.(a) Kerns EH, Di L. Drug-like Properties: Concepts, Structure Design and Methods. 1. Elsevier; Burlington, MA: 2008. [Google Scholar]; (b) Leeson P. Nature. 2012;481:455. doi: 10.1038/481455a. [DOI] [PubMed] [Google Scholar]; (c) Manallack DT, Prankerd RJ, Yuriev E, Oprea TI, Chalmers DK. Chem Soc Rev. 2013;42:485. doi: 10.1039/c2cs35348b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seminal reports: Tellis JC, Primer DN, Molander GA. Science. 2014;345:433. doi: 10.1126/science.1253647.Zuo ZW, Ahneman DT, Chu LL, Terrett JA, Doyle AG, MacMillan DWC. Science. 2014;345:437. doi: 10.1126/science.1255525.Kalyani D, McMurtrey KB, Neufeldt SR, Sanford MS. J Am Chem Soc. 2011;133:18566. doi: 10.1021/ja208068w.Ye Y, Sanford MS. J Am Chem Soc. 2012;134:9034. doi: 10.1021/ja301553c.Sahoo B, Hopkinson MN, Glorius F. J Am Chem Soc. 2013;135:5505. doi: 10.1021/ja400311h.
- 12.Recent advances in photoredox dual catalysis: Primer DN, Karakaya I, Tellis JC, Molander GA. J Am Chem Soc. 2015;137:2195. doi: 10.1021/ja512946e.Karakaya I, Primer DN, Molander GA. Org Lett. 2015;17:3294. doi: 10.1021/acs.orglett.5b01463.El Khatib M, Serafim RAM, Molander GA. Angew Chem, Int Ed. 2016;55:254. doi: 10.1002/anie.201506147.Rueping M, Koenigs RM, Poscharny K, Fabry DC, Leonori D, Vila C. Chem-Eur J. 2012;18:5170. doi: 10.1002/chem.201200050.Shu XZ, Zhang M, He Y, Frei H, Toste FD. J Am Chem Soc. 2014;136:5844. doi: 10.1021/ja500716j.Noble A, McCarver SJ, MacMillan DWC. J Am Chem Soc. 2015;137:624. doi: 10.1021/ja511913h.Chu L, Lipshultz JM, MacMillan DWC. Angew Chem, Int Ed. 2015;54:7929. doi: 10.1002/anie.201501908.Le CC, MacMillan DWC. J Am Chem Soc. 2015;137:11938. doi: 10.1021/jacs.5b08304.
- 13.(a) Jouffroy M, Primer DN, Molander GA. J Am Chem Soc. 2016;138:475. doi: 10.1021/jacs.5b10963. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Patel NR, Kelly CB, Jouffroy M, Molander GA. Org Lett. 2016;18:764. doi: 10.1021/acs.orglett.6b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Corce V, Chamoreau LM, Derat E, Goddard JP, Ollivier C, Fensterbank L. Angew Chem, Int Ed. 2015;54:11414. doi: 10.1002/anie.201504963. [DOI] [PubMed] [Google Scholar]
- 14.Ammonium organobis(catecholato)silicates used in this study represent nearly all the substructures that are currently commercially available. Methods for accessing silicates having shorter alkyl chains (e.g., aminomethyl- and aminoethylsilicates) are under development.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.