Abstract
Objective
We examined patterns of change in adiposity across four decades starting in young adulthood and their relationships with midlife cardiometabolic outcomes.
Methods
BMI was assessed at average age 20, 40, 56 and 62 years in 977 male veterans from the Vietnam Era Twin Study of Aging. Age 62 (range 56–66) cardiometabolic outcomes included hypertension, diabetes, dyslipidemia, inflammation, and ischemic heart disease. Analyses included latent growth modeling (LGM), latent class growth modeling (LCGM), and logistic regression models.
Results
Linear BMI slope was associated with all outcomes. Accelerated (quadratic) BMI slope was significantly associated with greater risk for hypertension, diabetes, dyslipidemia, and inflammation; odds ratios ranged from 1.93 (diabetes) to 3.15 (dyslipidemia). Initial BMI did not predict later outcomes. Linear slope contributed significant unique variance for diabetes and dyslipidemia even controlling for age 62 BMI. LCGM revealed three trajectories. Men with the relatively stable, lower BMI trajectory had significantly better outcomes than those with trajectories with accelerated increases, especially those including obesity.
Conclusions
How individuals reach late midlife BMI is important. Steepness of BMI change across 40 years from young adulthood to late midlife, in addition to late midlife BMI itself, was robustly associated with greater risk for poor cardiometabolic outcomes.
Keywords: Body mass index, BMI, longitudinal, aging, inflammation, VETSA
Introduction
Obesity poses a serious public health concern.1, 2 It has been established as a prominent risk factor for cardiovascular disease (CVD), the leading cause of morbidity and mortality among adults in the United States (U.S.), and is linked to many detrimental cardiometabolic outcomes.2–6 In the Framingham Heart Study, higher adiposity at approximately age 50 (midlife) was associated with reduced survival and 52% higher lifetime risk for CVD by age 95; men with no cardiometabolic risk factors at midlife were highly unlikely to develop heart disease in late life.5
Midlife is a transitional period for men’s health with rapid increases in rates of CVD, high cholesterol, diabetes, and hypertension after age 50; moreover, over half of deaths due to CVD occur in men.7 Measured rates of body mass index (BMI), an indicator of adiposity, in adult men age 20 and over in the U.S. indicate that age-adjusted obesity (BMI ≥30 kg/m2) rose progressively from an estimated 31.6% in 1999–2002 to 35.5% in 2009–2010.8 From 2005 to 2014, age-adjusted obesity rates appeared to stabilize in adult men.9
Longitudinal patterns of change in adiposity are understudied, in particular the period from young adulthood (ages 19–29) through midlife (ages 45–64). One study identified four longitudinal BMI trajectories from age 18 to age 49 years10; self-reports of hypertension, diabetes and high cholesterol were lowest in the normal BMI trajectory and highest in the two trajectories that included obesity. Studies of adults across shorter periods of time demonstrated mixed associations between change in BMI and various CVD risk factors.11–14 In earlier studies by our group, being overweight in young adulthood significantly predicted age 48 diabetes but not hypertension.14 In addition, we found significant shared genetic and environmental influences between adiposity and all other components of the metabolic syndrome (i.e., hypertension, insulin resistance, cholesterol, triglycerides).15 Adiposity was the only component genetically associated with all other components.
Objectives of this study were a) to assess change and patterns of change in adiposity from young adulthood to late midlife (age 62 years); and b) to examine associations between change in adiposity and late midlife cardiometabolic outcomes. We hypothesized that a steeper BMI slope over time would be associated with higher rates of diabetes, dyslipidemia, heart disease, hypertension, and inflammation.
Methods
Participants were 977 men in the Vietnam Era Twin Study of Aging follow-up assessment [VETSA 2; mean age 61.6 years (range 56–66)] with complete data.16 The VETSA project is a longitudinal study of risk and protective factors for cognitive aging in a community-dwelling sample of veterans from across the U.S..
Recruitment. In the first VETSA assessment (VETSA 1: 2002–2008) the 1237 participants (mean age 56 years; range 51–60) were a simple random sample from the all-male Vietnam Era Twin Registry (VETR).17 The VETR was established as a research registry of twins who had both served in the U.S. military between 1965 and 1975. VETSA 1 eligibility included: being 51 to 59 years old when recruited and both members of a twin pair agreeing to participate.18 Recruitment rate was 44%; we view this positively since the study involved high burden – a three days/two night commitment due to travel to University of California San Diego (UCSD) or Boston University (BU) for assessment, limited enrollment windows (due to batching), and the requirement that both brothers enroll. Ethical approval of the research protocol was provided by the UCSD Human Research Protection Program (HRPP) and the BU Charles River Campus Institutional Review Board (IRB). More details about the recruitment and methods are in the supplement.
The VETSA 2 follow-up occurred approximately six years after VETSA 1 (2008–2014); 1016 men from VETSA 1 participated in the follow-up (82% retention). The most common single reason for attrition was death (N=56).
The VETSA participants comprise a relatively representative epidemiological sample of community-based men with regard to marital, work, income, and health characteristics of American men in their age range based on U.S. Census and Center for Disease Control data (CDC).19 Prevalence of self-reported chronic health problems was also generally comparable with CDC rates for men. VETSA participants constitute a sample with no major chronic childhood health problems.
Procedures
The VETR also serves as a data archive for data previously collected on the registry sample; those data are available to other researchers on request. This study incorporates data from four data collections: military induction data procured from military records by the VETR and a National Heart, Lung, and Blood Institute (NHLBI) funded study conducted in 199020 (both archived at the VETR) as well as the two waves of VETSA data collection conducted by our research group.16
Height, weight, pulse, and blood pressure (BP) were measured objectively at military induction (mean age 20 years; range 17–25). Height and weight at mean age 40 years (range 35–46) were collected with a self-report health survey mailed to all VETR twins in 1990 as part of an NHLBI funded study.20 Relevant military record and 1990 survey data were provided to VETSA researchers by the VETR.
VETSA 1 and 2 data were collected in person at either UCSD or BU. Height, weight, pulse, and blood pressure (BP) were measured objectively. Blood chemistries were only obtained in VETSA 2.15 Participants who did not fast for at least 9 hours were excluded from analyses. Following written informed consent, blood was drawn by a certified phlebotomist. Assays were conducted at the same certified laboratory to ensure comparability (Quest Diagnostics/Nichols Institute, San Juan Capistrano, CA).
From here forward we refer to data collection timepoints as age 20 (or baseline), age 40, age 56 and age 62.
Measures
BMI
Height and weight were measured at age 20; at that time the majority of men were not obese [2.3% (N=22) men with BMI ≥30 kg/m2].17 Age 40 height and weight were self-reported. Self-reported height and weight are considered valid measures, but are biased toward underreport of weight (especially in women, overweight adults, and adults over age 45) and overreport of height.21, 22 At ages 56 and 62, participants were weighed on a digital scale after removing shoes and heavy outer clothing. Height was assessed, in stocking feet, with a stadiometer. Self-reported height at age 40 correlated r=.94 with measured height at age 56; measured height at ages 56 and 62 correlated r=.96. Age 40 self-reported weight correlated r=.86 with weight at age 56; measured weight at ages 56 and 62 correlated r=.92.
After being transformed to metric scales, BMI was calculated as kg/m2. BMI ranges are: normal: 18.5 to 24.9 kg/m2; overweight: 25.0 to 29.9; level 1 obesity: 30.0 to 34.9; level 2 obesity: 35.0 to 39.99, and level 3 obesity: 40.0 and greater.23 More details about BMI measurements are available elsewhere.14, 15 BMI was our only indicator of adiposity with data from four timepoints. From this point forward we refer to BMI at age 20 and age 62 as BMI20 and BMI62.
Age 62 (VETSA 2) cardiometabolic measures.
Hypertension
Age 62 BP was based on the average of four systolic (SBP) readings and the four diastolic (DBP) readings taken during the assessment day. In the morning and afternoon, after sitting quietly for five minutes, BP was taken twice with an automated blood pressure machine with a one-minute break between readings. Individuals with either SBP > 140 or DBP > 90 mm hg 24, or who took anti-hypertensive medication, were classified as hypertensive (75.1%).
Diabetes
Fasting insulin was assayed via a sensitive electrochemiluminescent immunoassay (ECLIA); levels of insulin greater than 117 pmol/L were considered at risk. Fasting plasma glucose was assayed with spectrophotometry as part of a comprehensive metabolic panel; glucose levels greater than 5.54 mmol/L were considered at risk. Diabetes was defined as having at-risk levels of insulin or glucose, and/or taking a prescription medication for diabetes (51.7% at risk).
Dyslipidemia
Triglycerides and HDL-cholesterol were assayed as part of a lipid panel via spectrophotometry. At risk HDL-cholesterol was defined as levels < 1.03 mmol/L (28.9%); at risk triglycerides was classified as > 1.68 mmol/L (31.5%). At risk overall cholesterol was defined as being at risk for either HDL or triglycerides or taking cholesterol-lowering medication (72% at risk).
Inflammation
High-sensitivity C-reactive protein, a protein measured in blood that indicates inflammation, was assayed using nephelometry.25 At risk inflammation was defined as C-reactive protein > 28.5 nmol/L (27.5%).
Ischemic heart disease (IHD)
Presence/absence of IHD at age 62 (18%) was coded using a validated population-based index.26 Items included self-reported heart attack/myocardial infarction, presence of angina and/or heart surgery (e.g., stent placement, angioplasty, coronary artery bypass). Presence of angina was operationalized as a positive Rose Angina score and/or a prescription for nitroglycerin.27
Covariates and demographic data. Covariates included age at VETSA 2, lifetime education, ethnicity (white non-Hispanic vs. other), and tobacco smoking (never N=339, past N=443, current N=195). The low family-of-origin socioeconomic status (SES) demographic variable was operationalized as the father having less than high school education, and occupation of unskilled or semi-skilled laborer.28
Data analysis
Latent Growth Models (LGMs). LGM analysis was used to estimate the BMI growth patterns/slopes across four timepoints from early adulthood to late midlife; having four timepoints allows the quadratic term to be tested in the model.
A two-level unconditional model (a model with only time as a covariate) was analyzed in order to accommodate the repeated measures nested within individuals. The age variable was rescaled by both centering at initial age of 20 and weighting by the time intervals.
-
Level 1:
-
Level 2:
π0i=β00+ν0i
π1i=β10+ν1i
π2i=β20+ν2i
Composite:
Where BMIij and ageij represent BMI and age for individual i at jth measurement; π0i, π1i and π2i represent individual i’s initial BMI, linear growth rate and quadratic growth rate of BMI; εij is level 1 residual, represents the portion of individual i’s BMI at jth measurement not accounted for by the model; β00, β10 and β20 represent population average initial BMI, linear growth rate and quadratic growth rate of BMI; ν0i, ν1i and ν2i are level 2 residual, represent deviations from the average initial BMI, linear growth rate and quadratic growth rate of BMI.
Latent Class Growth Model (LCGM). LCGM analysis was used to identify distinct subgroups with similar BMI change patterns across the four timepoints. Each latent class consists of specific intercept and slopes allowing linear and quadratic patterns of BMI change. Latent classes were identified starting with one class, then adding another, one at a time. The Lo-Mendell-Rubin (LMR) likelihood ratio test was used to examine the better fit of (k+1)-class model versus k-class model (k≥1).29 The significance of the test suggests (k+1)-class-model fits the data better. Otherwise the k-class model fits the data better and thus the k class model is selected as best fitting.
LGM and LCGM analyses were performed using Mplus version 7.4.30 Twin clustering of the data was accounted for in all analyses. Parameters were estimated using maximum likelihood (ML) estimation based on probability density function of normal distribution of random error εij and random effect ν0i, ν1i and ν2i.
Empirical Bayes estimates of each individual's initial BMI (intercept), linear slope and quadratic slope were calculated and used as predictors in subsequent analyses; Type III results are shown in tables. All subsequent statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Analyses were conducted on dichotomous outcome measures representing risk for hypertension, diabetes, dyslipidemia, inflammation, and IHD at age 62. To examine associations between risk measures at age 62 and BMI intercept and growth rates while controlling for confounding variables, SAS PROC SURVEYREG/PROC SURVEYLOGISTIC was used to account for the twin clustering. Models adjusted for age, education, ethnicity, smoking, and twin clustering.
All p-values are 2-tailed; p-values <0.05 were considered statistically significant.
Results
Descriptive data. Participants were predominantly white non-Hispanic (90%). Approximately 25% came from low SES families. At age 20, participants were typically single (never married) with 12.3 years of education (see Table 1). By age 62, only 5% were single and 78% currently married. Lifetime education was 13.9 years. Mean BMI increased from 22.7 kg/m2 (SD 3.0) at baseline, to 25.8 (SD 3.4) at age 40, to 29.3 (SD 4.9) and 29.9 (SD 5.2) at ages 56 and 62, respectively. At baseline, 2.3% of participants were obese; by age 62, 42% were obese (28.4%/8.8%/4.9% in levels 1/2/3 obesity respectively).
Table 1.
Descriptive Data
| AGE 20 (Baseline) |
AGE 62 (VETSA 2) |
|
|---|---|---|
| Mean (SD) or % | ||
| Age (Years) | 19.8 (1.3; range 17–25) | 61.6 (2.4; range 56–66) |
| Ethnicity (% Non-Hispanic white) | 90% | |
| Marital status (% single) | 92.7% | 4.70% |
| Education (Years) | 12.3 (1.2) | 13.9 (2.1) |
| Low SES Family (%) | 24.7% | |
| Systolic BP | 124.6 (10.8) | 128.1 (16.1) |
| Diastolic BP | 74.2 (8.0) | 78.6 (9.0) |
| Height (in) | 68.9 (2.6) | 69.1 (2.6) |
| Weight (lbs) | 153.4 (22.7) | 203.3 (40.0) |
| BMI (kg/m2) | 22.7 (3.0) | 29.9 (5.2) |
| Normal BMI (%) | 741 (75.8%) | 148 (15.2%) |
| Overweight (%) | 173 (17.7%) | 416 (42.6%) |
| Obese (%) | 22 (2.3%) | 412 (42.2%) |
| Level 1 | 22 (2.3%) | 278 (28.5%) |
| Level 2 | 0 | 86 (8.8%) |
| Level 3 | 0 | 48 (4.9%) |
| Smoking (VETSA 2) | ||
| Never | 339 (34.7%) | |
| Former | 443 (45.3%) | |
| Current | 195 (20.0%) | |
Notes. Single = never married; BP= Blood Pressure; BPM=beats per minute; Ethnicity=non-Hispanic white versus Other; Low SES=Father less than high school education and unskilled/semi-skilled laborer reported at VETSA 1 (age 56); BMI=Body Mass Index. Normal BMI: ≥ 18.5 and <25.0 kg/m2; Overweight BMI ≥25 and <30; Obese BMI: BMI ≥ 30; level 1 obesity: BMI ≥30.0 and <35.0; level 2 obesity: BMI ≥35.0 and <40.0, level 3 obesity: BMI ≥40.0.
Latent growth model of BMI change. An unconditional LGM with fixed and random effects for the intercept, linear, and quadratic slopes provided the best fit to the data (Details in Supplemental Table S1). Fixed effect estimates were significant for the intercept (b=22.64; p<.0001), linear (b=3.20; p<.0001) and quadratic slopes (b=.22; p=.002), respectively, indicating an average BMI of 22.64 at age 20 years, with a positive linear increase of 3.2 BMI units every two decades at the centering age of 20 years, and accelerating BMI increase of .22 BMI units every two decades across the age range.
Individual level intercepts and slopes were used to estimate risk for cardiometabolic outcomes at age 62. In models adjusting for age, education, ethnicity, smoking, and twin clustering, BMI linear and quadratic slopes were both significantly associated with risk for hypertension, diabetes, dyslipidemia, and inflammation (Table 2). The significant positive quadratic effect indicates that an accelerated increase in BMI was associated with poorer outcomes. Where both linear and quadratic slopes were significantly associated with outcomes, odds ratios ranged from 1.25 (inflammation) to 1.34 (diabetes) for the linear slope, and from 1.93 (diabetes) to 3.15 (dyslipidemia) for the quadratic slope. Prevalence of IHD was associated with linear, but not quadratic, BMI slope. As indicated by the odds ratios for intercept, BMI20 did not predict any age 62 outcomes. Current and past smokers were at higher risk for IHD; odds ratios for current smoking and inflammation were also significant. No other covariates were significantly associated with the cardiometabolic outcomes.
Table 2.
BMI Intercept (at Age 20) and Slope Derived from Latent Growth Modeling as Predictors of Cardiometabolic Outcomes at Age 62
| Hypertension Odds Ratio (95% CI) |
Diabetes Odds Ratio (95% CI) |
Dyslipidemia Odds Ratio (95% CI) |
Inflammation Odds Ratio (95% CI) |
Ischemic Heart Disease Odds Ratio (95% CI) |
|
|---|---|---|---|---|---|
| Intercept (BMI Age 20) | 1.08 (0.97,1.20) | 1.02 (0.93,1.12) | 0.97 (0.88,1.07) | 1.00 (0.91,1.10) | 0.99 (0.88,1.10) |
| Linear slope | 1.28 (1.10,1.50) | 1.34 (1.16,1.54) | 1.33 (1.13,1.57) | 1.25 (1.11,1.41) | 1.21 (1.05,1.39) |
| Quadratic slope | 3.03 (1.75,5.26) | 1.93 (1.14,3.27) | 3.15 (1.75,5.67) | 3.10 (1.86,5.17) | 1.38 (0.79,2.41) |
| Age (VETSA 2) | 1.03 (0.96,1.10) | 1.04 (0.97,1.11) | 1.00 (0.94,1.07) | 1.04 (0.98,1.12) | 0.93 (0.86,1.01) |
| Education (Lifetime years) | 1.02 (0.94,1.11) | 1.00 (0.93,1.08) | 0.97 (0.89,1.05) | 1.04 (0.96,1.13) | 0.98 (0.89,1.07) |
| Ethnicity (Non-Hispanic White vs. other) |
1.06 (0.62,1.81) | 1.01 (0.63,1.62) | 1.36 (0.79,2.33) | 0.82 (0.49,1.36) | 1.12 (0.61,2.07) |
| Smoking (VETSA 2) | |||||
| Current smokers vs. Never smokers | 0.98 (0.61,1.57) | 0.90 (0.58,1.38) | 1.51 (0.94,2.44) | 2.96 (1.92,4.56) | 2.79 (1.57,4.94) |
| Former smokers vs. Never smokers | 1.16 (0.80,1.69) | 1.20 (0.85,1.69) | 1.17 (0.82,1.65) | 1.00 (0.69,1.46) | 2.83 (1.79,4.50) |
Notes: BMI=Body Mass Index (kg/m2); inflammation measured with high sensitivity C-Reactive Protein; Models include intercept (age 20 BMI), Age (years), education (Lifetime in years), ethnicity (0=other; 1=non-Hispanic white) and smoking (0= Never smokers; 1= Former smokers; 2= Current smokers; Never smoker as reference group) as covariates in models with family as a random effect. Bold indicates values significant with 95% CI. Age measured in years. CI=confidence interval. Age centered at 20 years. VETSA 2 measures = Age 62; BMI intercept and slope derived from empirical Bayes estimates.
Because individuals with a steeper slope would tend to have higher BMI62, it could be that it is only useful to know BMI62 and not rate of change over time. To address this question, we first examined cross-sectional associations between BMI62 and outcomes. Cross-sectional associations between BMI62 and cardiometabolic outcomes were all significant (Table 3).
Table 3.
Cross-Sectional Associations Between BMI at Age 62 and Cardiometabolic Outcomes at Age 62
| Hypertension Odds Ratio (95% CI) |
Diabetes Odds Ratio (95% CI) |
Dyslipidemia Odds Ratio (95% CI) |
Inflammation Odds Ratio (95% CI) |
Ischemic Heart Disease Odds Ratio (95% CI) |
|
|---|---|---|---|---|---|
| Intercept (BMI Age 20) | 0.99 (0.89,1.09) | 0.92 (0.83,1.01) | 0.87 (0.79,0.97) | 0.93 (0.83,1.03) | 0.93 (0.82,1.05) |
| BMI Age 62 | 1.15 (1.10,1.21) | 1.14 (1.09,1.18) | 1.17 (1.11,1.24) | 1.14 (1.10,1.19) | 1.08 (1.02,1.13) |
| Age (VETSA 2) | 1.03 (0.96,1.10) | 1.04 (0.97,1.11) | 1.00 (0.93,1.07) | 1.04 (0.97,1.11) | 0.93 (0.86,1.01) |
| Education (Lifetime years) | 1.02 (0.94,1.11) | 1.00 (0.93,1.08) | 0.97 (0.90,1.06) | 1.04 (0.97,1.13) | 0.98 (0.89,1.07) |
| Ethnicity (Non-Hispanic white vs. other) | 1.04 (0.61,1.78) | 0.98 (0.62,1.57) | 1.33 (0.78,2.26) | 0.81 (0.49,1.35) | 1.10 (0.60,2.02) |
| Smoking (Age 62) | |||||
| Current smokers vs. Never smokers | 0.99 (0.62,1.58) | 0.90 (0.59,1.39) | 1.53 (0.94,2.47) | 2.94 (1.91,4.52) | 2.81 (1.59,4.98) |
| Former smokers vs. Never smokers | 1.16 (0.80,1.69) | 1.20 (0.86,1.69) | 1.16 (0.82,1.64) | 1.00 (0.69,1.46) | 2.85 (1.79,4.52) |
Note. BMI=Body mass index (kg/m2); inflammation measured with high sensitivity C-Reactive Protein; Models include intercept (age 20 BMI), Age (years), education (years), ethnicity (0=other; 1=non-Hispanic white) and smoking (0= Never smokers; 1= Former smokers; 2= Current smokers; Never smoker = reference group) as covariates in models with family as a random effect. Bold indicates values significant with 95% CI. Age measured in years. VETSA 2 measures = Age 62.
We then examined the effect of the LGM-derived slope variables and BMI62 when both variables were in the same model. For those models, we re-centered the LGM analyses at age 62. By examining Type III effects, these models allowed us to test whether slope was uniquely associated with outcomes over and above BMI62 (Table 4). There were significant odds ratios for linear slope for diabetes (OR=1.29) and dyslipidemia (OR=1.42). Odds ratios for BMI62 were all close to 1.00 and nonsignificant. Smoking was the only other variable that was a significant unique predictor; the odds ratio was 2.96 for inflammation and 2.79 for IHD for current versus never smokers, and 2.83 for IHD for former versus never smokers. There were no significant odds ratios for either slope measure or BMI62 for hypertension, inflammation, and IHD. However, it seems likely this lack of significance is due to collinearity rather than the fact that these measures are not predictive (see Discussion).
Table 4.
BMI Intercept (at Age 62) and Slope Derived from Latent Growth Modeling as Predictors of Cardiometabolic Outcomes at Age 62
| Hypertension Odds Ratio (95% CI) |
Diabetes Odds Ratio (95% CI) |
Dyslipidemia Odds Ratio (95% CI) |
Inflammation Odds Ratio (95% CI) |
Ischemic Heart Disease Odds Ratio (95% CI) |
|
|---|---|---|---|---|---|
| Intercept (BMI Age 62) | 1.08 (0.97, 1.20) | 1.02 (0.93, 1.12) | 0.97 (0.88, 1.07) | 1.00 (0.91, 1.10) | 0.99 (0.88, 1.10) |
| Linear slope (BMI) | 1.10 (0.88, 1.38) | 1.29 (1.05, 1.60) | 1.42 (1.13, 1.80) | 1.25 (0.99, 1.58) | 1.24 (0.94, 1.63) |
| Quadratic slope (BMI) | 1.48 (0.65, 3.40) | 0.63 (0.28, 1.42) | 0.85 (0.36, 1.98) | 1.24 (0.59, 2.63) | 0.61 (0.25, 1.46) |
| Age (VETSA 2) | 1.03 (0.96, 1.10) | 1.04 (0.97, 1.11) | 1.00 (0.94, 1.07) | 1.04 (0.98, 1.12) | 0.93 (0.86, 1.01) |
| Education (Lifetime years) | 1.02 (0.94, 1.11) | 1.00 (0.93, 1.08) | 0.97 (0.89, 1.05) | 1.04 (0.96, 1.13) | 0.98 (0.89, 1.07) |
| Ethnicity (Non-Hispanic White vs. other) |
1.06 (0.62, 1.81) | 1.01 (0.63, 1.62) | 1.36 (0.79, 2.33) | 0.82 (0.49, 1.36) | 1.12 (0.61, 2.07) |
| Smoking (VETSA 2) | |||||
| Current smokers vs. Never smokers | 0.98 (0.61, 1.57) | 0.90 (0.58, 1.38) | 1.51 (0.94, 2.44) | 2.96 (1.92, 4.56) | 2.79 (1.57, 4.94) |
| Former smokers vs. Never smokers | 1.16 (0.80, 1.69) | 1.20 (0.85, 1.69) | 1.17 (0.82, 1.65) | 1.00 (0.69, 1.46) | 2.83 (1.79, 4.50) |
Notes: BMI=Body Mass Index (kg/m2); inflammation measured with high sensitivity C-Reactive Protein; Models include intercept (age 20 BMI), Age (years), education (Lifetime in years), ethnicity (0=other; 1=non-Hispanic white) and smoking (0= Never smokers; 1= Former smokers; 2= Current smokers; Never smoker as reference group) as covariates in models with family (twin clustering) as a random effect. Bold indicates values significant with 95% CI. Age measured in years. CI=confidence interval. Age centered at 62 years. VETSA 2 measures = Age 62; BMI intercept and slope derived from empirical Bayes estimates.
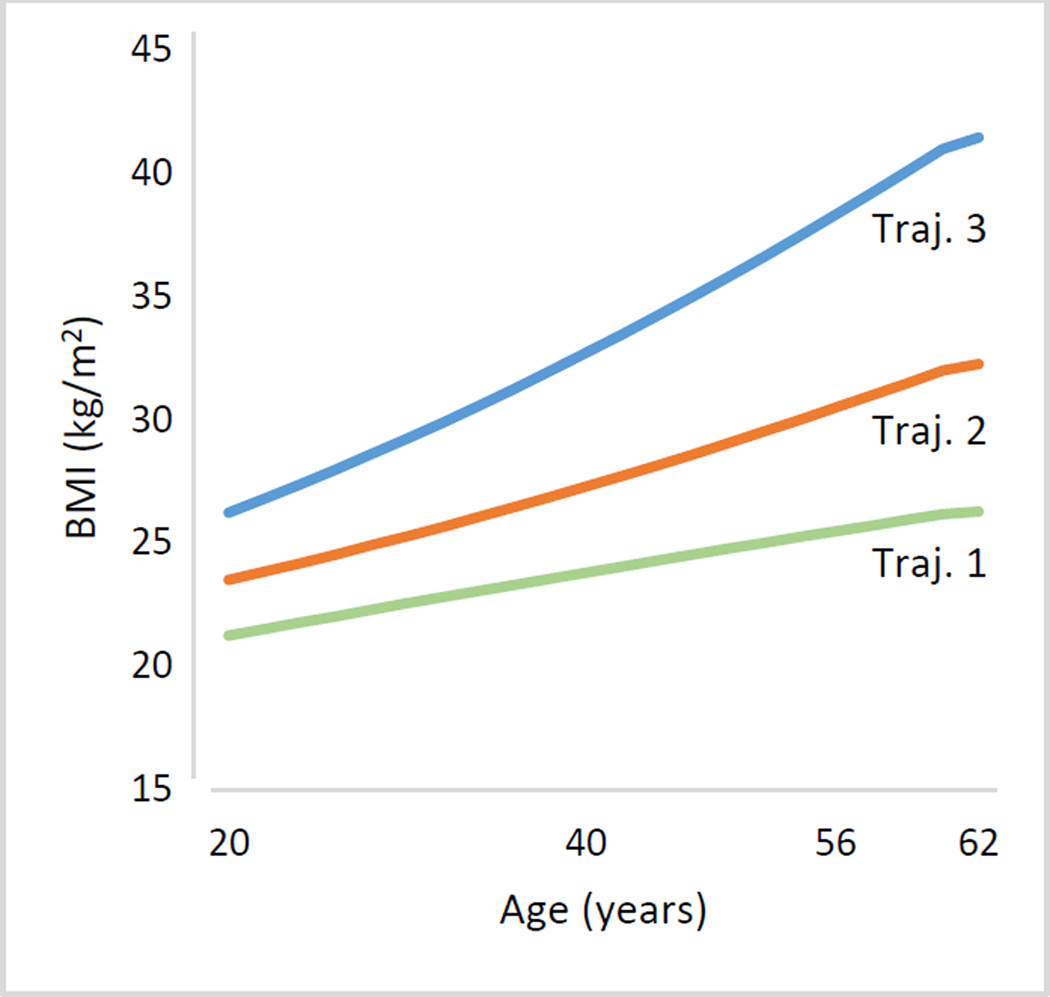
Latent class growth models and heterogeneity of change. LCGMs were tested to determine the number of distinct latent classes best representing the longitudinal BMI data. Using the LMR test as the index of model fit, the best fitting model identified three BMI trajectory classes/trajectories (Fig. 1 and Table 5). Terms for intercept, linear and quadratic slope for trajectories 2 and 3 were significant, but for trajectory 1 only the intercept and linear terms were significant.
Figure 1.
Three latent class trajectory groups representing change in body mass index from age 20 (T0) to age 40 (T1), age 56 (T2), and age 62 (T3). Trajectory is abbreviated as Traj; body mass index as BMI.
Table 5.
Latent Class Growth Modeling over Four Timepoints with Linear and Quadratic Terms
| Model | Entropy | LMR | LMR p |
|---|---|---|---|
| 1 | |||
| 2 | 0.90 | 1610.66 | 0.0052 |
| 3 | 0.86 | 821.02 | 0.0003 |
| 4 | 0.85 | 373.01 | 0.3040 |
| 3-Class Model Parameters | |||
|---|---|---|---|
| Class 1 (N=490) | Class 2 (N=400) | Class 3 (N=87) | |
| Intercept | 21.26 (20.99, 21.53)** | 23.52 (CI: 23.13, 23.92)** | 26.25 (CI: 25.17, 27.34)** |
| Linear | 2.66 (CI: 2.28, 3.05)** | 3.31 (CI: 2.84, 3.78)** | 5.65 (CI: 3.85, 7.44)** |
| Quadratic | −0.10 (CI: −0.26, 0.05) | 0.47 (CI: 0.24, 0.69)** | 0.91 (CI: 0.05, 1.78)* |
Notes: BIC = Bayesian information criterion; LMR=Lo-Mendell-Rubin likelihood ratio test; Model 3 (3-class model, bold font) has best fit. In the text, to avoid confusion, class 1 corresponds to trajectory 1; class 2 to trajectory 2; class 3 to trajectory 3.
p=.04;
p<.001
As can be seen in Fig. 1, trajectory 1 (N=490, 50%) had the lowest BMI at all time points; baseline BMI was 21.26 kg/m2 (SD 2.1), rising to 26.1 (SD 5.2) by age 62. Trajectory 2 (N=400; 41%) baseline BMI was in normal range (23.6, SD 2.7) rising to level 1 obesity (mean BMI 32.1, SD 2.5) by age 62. Trajectory 3 (N=87; 9%) was overweight at baseline (mean BMI 26.3, SD 3.5), increased to level 1 obesity by age 40, and level 3 obesity by age 62 (mean BMI 41.0, SD 4.2). Significant quadratic terms for trajectories 2 and 3 indicate accelerating BMI over time.
Odds ratios for the rates of cardiometabolic outcomes associated with the three BMI trajectories are shown in Table 6. Compared with trajectory 1, trajectory 2 had significantly greater risk for hypertension, diabetes, dyslipidemia, and high inflammation, but not IHD; odds ratios ranged from 1.81 (diabetes) to 2.31 (hypertension). Trajectory 3, compared with trajectory 1, had significantly higher risks for all outcomes; odds ratios ranged from 1.86 (IHD) to 8.08 (hypertension). Trajectory 3, compared with trajectory 2, had significantly greater risk for hypertension (OR=3.49), diabetes (OR=1.69), and inflammation (OR=2.29) but not dyslipidemia or IHD. After applying the false discovery rate (FDR) adjustment,31 all comparisons remained significant except for the odds ratio of IHD (Trajectory 1 vs 3). Descriptive data for the trajectories are in supplemental Tables S2 and S3.
Table 6.
Comparisons Between Trajectory Groups for Cardiometabolic Outcomes at Age 62
| BMI Trajectory Group |
Hypertension Odds Ratio (95% CI) |
Diabetes Odds Ratio (95% CI) |
Dyslipidemia Odds Ratio (95% CI) |
High Inflammation Odds Ratio (95% CI) |
Ischemic Heart Disease Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| 2 vs 1 | 2.31 (1.62,3.30) | 1.81 (1.34,2.45) | 1.92 (1.39,2.65) | 2.08 (1.49, 2.91) | 1.41 (0.95,2.09) |
| 3 vs 1 | 8.08 (2.70,24.00) | 3.06 (1.80,5.22) | 3.44 (1.58,7.49) | 4.77 (2.85,7.99) | 1.86 (1.02,3.39) |
| 3 vs 2 | 3.49 (1.16,10.53) | 1.69 (1.01,2.85) | 1.80 (0.82,3.95) | 2.29 (1.40,3.76) | 1.32 (0.75,2.31) |
Notes: BMI=Body mass index (kg/m2). CI= confidence intervals; Trajectory 1 (N=490); Trajectory 2 (N=400); Trajectory 3 (N=87); Bold indicates values significant with 95% CI. Models adjusted for age, education, ethnicity, smoking, and twin clustering. IHD no longer significant after adjusting for false discovery rate (p=.13)
Discussion
Across four decades, men with accelerated increases in BMI were at significantly higher risk for hypertension, diabetes, dyslipidemia, and inflammation at age 62, regardless of young adult BMI. Latent class growth curve analyses identified three latent classes representing BMI trajectories. Comparisons of the three trajectories revealed health benefits for participants who maintained BMI at lower and relatively stable levels. Trajectory 1, with an average change of 5 kg/m2 across four decades, had the lowest levels of cardiometabolic risks. Trajectory 3, with three out of four timepoints at obese levels and an average change of 15 kg/m2 had significantly higher risk for all outcomes except IHD compared with other trajectories. However even moderate increases in BMI as shown by trajectory 2 (average change of 9 kg/m2) put participants at higher risk. In the Framingham Heart Study, men with no cardiometabolic risk factors at midlife were highly unlikely to develop heart disease and had better survival in late life5. Maintaining BMI close to normal range may be one path contributing to lower cardiometabolic risks. Higher BMI, but also more rapidly increasing BMI, appear to reflect increased cardiometabolic risks.
In models that simultaneously included slopes and BMI62 (Table 4), linear slope had unique predictive value for diabetes and dyslipidemia after accounting for BMI62, indicating that rate of change (i.e., how one got to BMI62) and not just where one ended up makes a difference with respect to these outcomes. Slope measures had significant odds ratios for all outcomes in LGMs with slope and BMI20 (Table 2); there were significant odds ratios for BMI62 for all cardiometabolic outcomes in cross-sectional analyses (Table 3), and trajectory comparisons showed significant odds ratios for cardiometabolic outcomes (Table 6). As such, we think the most parsimonious explanation is that results including BMI62 as an intercept are due to collinearity rather than the fact that the slope measures or BMI62 are not predictive of hypertension, inflammation or IHD.
These results have potential implications for later life cognitive and brain health. Obesity and metabolic dysregulation appear to be related to Alzheimer’s disease pathophysiology; being overweight or obese in midlife appears to be detrimental for late-life cognition, brain structure, and brain function.32 In the Whitehall II study of midlife adults through average age 61, higher BMI was associated with poorer cognition; age 61 cognitive effects were stronger for those who were obese at two of three timepoints.33 Shared genetic factors are also implicated in the association between BMI and cognitive function.34, 35 For instance, in a genome-wide association study meta-analysis, the most strongly enriched gene sets for BMI and obesity involved critical brain pathways regulating appetite, insulin synthesis and processing, and energy metabolism in the hypothalamus and pituitary as well as synaptic plasticity and cellular mechanisms mediating learning and memory in the hippocampus.35 Finally, adults ages 50–75 self-reporting larger weight gain in adulthood had significantly shorter telomeres, a potential biomarker for aging.36
A limitation of our study is that height and weight were self-reported at age 40 which may influence growth curves.21, 22 On the other hand, having objectively measured BMI at three of the four timepoints is a strength. Waist circumference is sometimes considered a better indicator of adiposity than BMI; however, we had only two timepoints with waist circumference measures, thus not allowing for growth curve analyses.37 Moreover, BMI and waist circumference were correlated .90 and cross-sectional results at age 62 (not shown) were virtually the same. The sample is primarily non-Hispanic white male veterans, so generalizability to women and other groups is unclear; recent longitudinal studies find some sex differences in associations with BMI.38 It is a strength that VETSA comprises a national sample of male veterans, but their military service meant that they were healthy at baseline. Despite initial selection for good health, sample demographics and health statistics paralleled CDC and census statistics for U.S. men. In addition, veterans are an often overlooked cohort of adults.39 Given that this is a twin sample, there may be greater homogeneity than would be expected in a nontwin study. Finally, we do not address genetic and environmental influences on BMI change, which may contribute to differential vulnerability; BMI is highly heritable and different genes may affect BMI at different ages.14, 40
Despite these limitations, this study—to our knowledge—is unique in the richness of its longitudinal BMI data starting in young adulthood, and objectively measured metabolic outcomes at late midlife. This large age-homogeneous sample allowed for in-depth examination of BMI change and heterogeneity across an important transitional age period when cardiometabolic dysregulation and cardiovascular disease become more prevalent.35 Studies focused on only elderly adults may already have high levels of selective attrition due to high morbidity and mortality associated with obesity—especially among men. We provided evidence from multiple analytic approaches that increases in BMI—in particular, accelerated change—from young adulthood to midlife are significantly associated with risk for poorer cardiometabolic outcomes.
Supplementary Material
What is already known about this subject?
Previous research found that cardiometabolic risks at midlife predicted cardiovascular outcomes in late life.
Few studies examine associations between changes in body mass index (BMI) from young adulthood to late midlife and cardiometabolic risks.
What does this study add?
Across four decades, from approximately age 20 to age 62, accelerated BMI gain was significantly associated with greater risk for hypertension, diabetes, dyslipidemia, and inflammation.
Men with lower and relatively stable BMI had significantly better midlife outcomes than men with BMI trajectories in the obese range.
Acknowledgments
Content of this manuscript is the responsibility of the authors and does not represent official views of NIH, or the Veterans’ Administration. U.S. Department of Veterans Affairs, Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University provided invaluable assistance in the conduct of the VET Registry. The authors gratefully acknowledge the continued cooperation of the twins and the efforts of many staff members.
Funding:
The study was supported by awards from the National Institutes of Health/National Institute on Aging [R01s AG 050595, AG018386, AG022381, AG022982 to W.S.K.; R01 AG018384 to M.J.L.; R03 AG 046413 to C.E.F, and K08 AG047903 to M.S.P].
Footnotes
Disclosure: The authors declare no conflict of interest
References
- 1.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the united states in the 21st century. N Engl J Med. 2005;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: Findings from the national health and nutrition examination survey, 1999 to 2004. J Am Coll Surg. 2008;207(6):928–934. doi: 10.1016/j.jamcollsurg.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89(6):2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 4.Reis JP, Allen N, Gunderson EP, et al. Excess body mass index-and waist circumference-years and incident cardiovascular disease: The cardia study. Obesity. 2015;23(4):879–885. doi: 10.1002/oby.21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 6.Twig G, Yaniv G, Levine H, et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med. 2016 doi: 10.1056/NEJMoa1503840. [DOI] [PubMed] [Google Scholar]
- 7.Murray CJ, Atkinson C, Bhalla K, et al. The state of us health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among us adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the united states, 2005 to 2014. JAMA. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Østbye T, Malhotra R, Landerman LR. Body mass trajectories through adulthood: Results from the national longitudinal survey of youth 1979 cohort (1981–2006) Int J Epidemiol. 2011;40(1):240–250. doi: 10.1093/ije/dyq142. [DOI] [PubMed] [Google Scholar]
- 11.Norman J, Bild D, Lewis C, Liu K, West DS. The impact of weight change on cardiovascular disease risk factors in young black and white adults: The cardia study. Int J Obes. 2003;27(3):369–376. doi: 10.1038/sj.ijo.0802243. [DOI] [PubMed] [Google Scholar]
- 12.Dowd JB, Zajacova A. Long-term obesity and cardiovascular, inflammatory, and metabolic risk in us adults. Am J Prev Med. 2014;46(6):578–584. doi: 10.1016/j.amepre.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Tirosh A, Shai I, Afek A, et al. Adolescent bmi trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364(14):1315–1325. doi: 10.1056/NEJMoa1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franz CE, Grant MD, Jacobson KC, et al. Genetics of body mass stability and risk for chronic disease: A 28-year longitudinal study. Twin Res Hum Genet. 2007;10(4):537–545. doi: 10.1375/twin.10.4.537. [DOI] [PubMed] [Google Scholar]
- 15.Panizzon MS, Hauger RL, Sailors M, et al. A new look at the genetic and environmental coherence of metabolic syndrome components. Obesity. 2015;23(12):2499–2507. doi: 10.1002/oby.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kremen WS, Franz CE, Lyons MJ. Vetsa: The vietnam era twin study of aging. Twin Res Hum Genet. 2013;16(01):399–402. doi: 10.1017/thg.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisen SA, True WR, Goldberg J, Henderson W, Robinette CD. The vietnam era twin (vet) registry: Method of construction. Acta Genet Med Gemellol. 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- 18.Kremen WS, Thompson-Brenner H, Leung YJ, et al. Genes, environment, and time: The vietnam era twin study of aging (vetsa) Twin Res Hum Genet. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- 19.Schoenborn CA, Heyman KM. U.S. Department of Health and Human Services Centers for Disease Control and Prevention. Hyattsville, MD: 2009. Health characteristics of adults aged 55 years and over: United states, 2004–2007. editor. [Google Scholar]
- 20.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The vietnam era twin registry. Twin Res. 2002;5(5):476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 21.Connor-Gorber S, Tremblay A, Moher D, Gorber B. A comparison of direct vs. Self-report measures for assessing height, weight and body mass index: A systematic review. Obes Rev. 2007;8:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 22.Villanueva EV. The validity of self-reported weight in us adults: A population based cross-sectional study. BMC Public Health. 2001;1(1):11. doi: 10.1186/1471-2458-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flegal KM, Kit BK, Graubard BI. Body mass index categories in observational studies of weight and risk of death. Am J Epidemiol. 2014;180(3):288–296. doi: 10.1093/aje/kwu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aronow WS, Fleg JL, Pepine CJ, et al. Accf/aha 2011 expert consensus document on hypertension in the elderly: A report of the american college of cardiology foundation task force on clinical expert consensus documents. Circulation. 2011;123(21):2434–2506. doi: 10.1161/CIR.0b013e31821daaf6. [DOI] [PubMed] [Google Scholar]
- 25.Mora S, Musunuru K, Blumenthal RS. The clinical utility of high-sensitivity c-reactive protein in cardiovascular disease and the potential implication of jupiter on current practice guidelines. Clin Chem. 2009;55(2):219–228. doi: 10.1373/clinchem.2008.109728. [DOI] [PubMed] [Google Scholar]
- 26.Xian H, Scherrer JF, Franz CE, et al. Genetic vulnerability and phenotypic expression of depression and risk for ischemic heart disease in the vietnam era twin study of aging. Psychosom Med. 2010;72(4):370–375. doi: 10.1097/PSY.0b013e3181d28125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lampe FC, Walker M, Lennon LT, Whincup PH, Ebrahim S. Validity of a self-reported history of doctor-diagnosed angina. J Clin Epidemiol. 1999;52(1):73–81. doi: 10.1016/s0895-4356(98)00146-2. [DOI] [PubMed] [Google Scholar]
- 28.Hollingshead AB, Redlich FC. Social class and mental illness: A community study. 1958. Am J Public Health. 2007;97(10):1756–1757. doi: 10.2105/ajph.97.10.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo Y, Mendell N, Rubin D. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- 30.Muthen LK, Muthen BO. Mplus user's guide. 7. Los Angeles, CA: Muthén & Muthén; 2015. [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 32.Bischof GN, Park DC. Obesity and aging: Consequences for cognition, brain structure, and brain function. Psychosom Med. 2015;77(6):697–709. doi: 10.1097/PSY.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh-Manoux A, Czernichow S, Elbaz A, et al. Obesity phenotypes in midlife and cognition in early old age: The whitehall ii cohort study. Neurology. 2012;79(8):755–762. doi: 10.1212/WNL.0b013e3182661f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marioni RE, Yang J, Dykiert D, et al. Assessing the genetic overlap between bmi and cognitive function. Mol Psychiatry. 2016 doi: 10.1038/mp.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muezzinler A, Mons U, Dieffenbach AK, et al. Body mass index and leukocyte telomere length dynamics among older adults: Results from the esther cohort. Exp Gerontol. 2015;74:1–8. doi: 10.1016/j.exger.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89(2):500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Bonsdorff MB, Tormakangas T, Rantanen T, et al. Early life body mass trajectories and mortality in older age: Findings from the helsinki birth cohort study. Ann Med. 2015;47(1):34–39. doi: 10.3109/07853890.2014.963664. [DOI] [PubMed] [Google Scholar]
- 39.Department of Veterans Affairs Office of the Actuary. The veteran population projection model 2011 (vetpop2011) 2013 [Google Scholar]
- 40.Jelenkovic A, Yokoyama Y, Sund R, et al. Zygosity differences in height and body mass index of twins from infancy to old age: A study of the codatwins project. Twin Res Hum Genet. 2015;18(5):557–570. doi: 10.1017/thg.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.