Abstract
Objectives
To investigate the association of sleep characteristics with prevalent hypertension, diabetes, and obesity in a multiethnic cohort.
Design
This study used a population based cross-sectional study design.
Setting
Participants were recruited between 2009 and 2011 from Chicago, Illinois and the surrounding suburbs.
Participants
Participants were 492 adults ages 35–64 years old who self-reported as white, black, Hispanic or Asian and who had a low likelihood of sleep apnea based on the apnea screening questionnaires and 1 night of apnea screening using an in-home device (apnea hypopnea index [AHI] <15 or oxygen desaturation index [ODI]<10).
Measurements
Participants wore a wrist actigraphy monitor (Actiwatch™) for 7 days. During a clinical examination, participants completed questionnaires about sleep, other health behaviors and medical history and had their blood pressure, anthropometric measures and fasting blood glucose measured; metabolic risk factors were determined based on standard clinical guidelines.
Results
The prevalence of hypertension, obesity and diabetes was 17.1%, 5.5% and 35.4%, respectively. Sleep duration was not associated with any cardiovascular risk factor. There was a significantly increased odds for hypertension (OR: 1.05 95%CI: 1.01, 1.08) and obesity (OR: 1.03 95%CI: 1.00, 1.05) associated with higher sleep fragmentation (per 1%). There was also a significantly increased odds for hypertension associated with poorer self-reported sleep quality (OR 1.14 95%CI: 1.05, 1.24 per 1 unit higher Pittsburgh Sleep Quality Index global score).
Conclusion
Objective and self-reported sleep quality may be more important than duration in relation to prevalent hypertension.
Keywords: Sleep Duration, Sleep Fragmentation, Sleep Quality, Hypertension, Diabetes, Obesity, Epidemiology
INTRODUCTION
Prior population studies describe an association between sleep duration and cardiovascular and metabolic disorders, hereafter cardiometabolic disorders, including diabetes, hypertension, and obesity.1–3 However, most of these studies have relied on self-report, which can lead to imprecise and inconsistent results, since adults may misreport their sleep.4 Much of the prior literature has typically examined the association between sleep duration and risk for cardiometabolic disease. However, this approach is limited in that sleep duration does not reflect of sleep quality. For example, although an individual’s sleep duration may be within recommended 7 or more hours, as suggested by the American Academy of Sleep Medicine and Sleep Research Society5, it is possible for this individual to have poor sleep quality, such as fragmented sleep. It is plausible that self-reported (e.g., daytime sleepiness) or objectively-determined (e.g., sleep efficiency) sleep quality is also relevant to cardiometabolic health.6 Consequently, sleep quality may provide additional insights beyond sleep duration. To our knowledge, no prior studies have investigated a combination of objectively-determined sleep characteristics and self-reported quality measures.
Prior studies by our research group7,8 and others4,9–13 describe differences in sleep characteristics by race/ethnicity. These differences may be particularly relevant given the higher prevalence of cardiovascular (e.g., hypertension) and metabolic (e.g., obesity, diabetes) risk factors in blacks, Hispanics and some Asians (particularly for diabetes).14–17 Recent reviews highlight the contribution of sleep to cardiovascular disease risk factors and highlight the potential for differences in sleep to contribute to disparities.18 Consequently, more research is needed in multi-racial/ethnic samples using both self-reported and objective assessment of sleep alongside measurements (vs. self-report) of cardiovascular disease risk factors.
Prior literature has not screened for sleep apnea or taken into risk for sleep apnea using objective screening tools. Sleep apnea is estimated to affect 10–20% of the population and rates have gone up over time.19 Sleep apnea is inversely associated with sleep duration and quality. Hypoxia is independently associated with endothelial dysfunction, which underlies cardiometabolic disorders.20,21 Because sleep interruption occurs during the sleep period, self-reported sleep is plagued by misreporting in persons with apnea. While the use of objective measures of sleep can overcome this limitation, characterization of breathing, chest movements and oxygen saturation overnight is required, at a minimum, to capture apnea risk. No prior population-based studies have captured this information alongside objectively measured sleep even though apnea could confound the association of sleep with cardiometabolic disorders. Characterization of apnea risk is required in order to test the association of short sleep independent of apnea on cardiometabolic risk.
Our first objective was to expand beyond the current literature to determine whether objectively-determined sleep duration and sleep quality are associated with cardiometabolic disorders (i.e., diabetes, hypertension and obesity) in adults who have a low likelihood of having sleep apnea. Because prior reports have suggested there is a u-shaped association with too much or too little sleep resulting in an increased odds of negative health outcomes, we hypothesized that sleep duration would display a u-shaped association with cardiometabolic risk factors and that sleep quality would be inversely associated with prevalence. We evaluated whether any observed associations remained following adjustment for demographic characteristics, health behaviors and other clinical characteristics. Our second objective was to utilize this multi-racial/ethnic sample that includes white, black, Hispanic and Asian adults in roughly equal numbers to explore whether the relationship between sleep and cardiometabolic risk factors is moderated by race (i.e., effect measure modification). Further, given differences in sleep by gender22, we also tested effect modification by gender.
METHODS
Participants
The Chicago Area Sleep Study was a cross-sectional study conducted between 2009 and 2011.23 Approximately 650 participants aged 35–64 years from Chicago, Illinois and the surrounding suburbs were recruited from neighborhoods with heavy concentrations of adults from the racial and ethnic groups of interest (i.e., white, black, Hispanic and Asian) using commercial telephone lists from Info USA. Hispanics were defined as any individual claiming Hispanic ethnicity; they were not included in the White, Black, or Asian category to ensure the groups were mutually exclusive. We attempted to restrict our recruitment of Asians to those of East Asian descent based on self-report (e.g., Chinese, Korean, Japanese and Vietnamese), given the variation in cardiometabolic risk profiles among individuals from across the Asian continent.24 For example, rates of diabetes are lower among East Asians than Southeast Asians (e.g., Filipinos).16 Further details on the methods of recruitment are published elsewhere.23
Study Design
Individuals who met the inclusion criteria of having a body mass index (BMI) <35 kg/m2, and a low likelihood of sleep apnea as determined by the Berlin questionnaire and the Snoring, Tiredness, Observed apnea, blood Pressure, Body mass index, Age, Neck circumference and Gender (STOP-BANG) questionnaire were invited to attend a clinical examination.25,26 At the initial examination, participants were consented and given all questionnaires and sleep equipment. They were instructed to wear the sleep watch for 7 days and the apnea screening device for 1 night and to return to the clinic for a second examination within 8 to 14 days when all cardiovascular risk factors were measured. The study protocol was approved by the Institutional Review Board at Northwestern University. Informed consent was obtained for all participants involved.
Sleep Characteristics
Participants wore an actigraphy device (the Actiwatch™ 2, Phillips Respironics, Bend, Oregon) on their wrist for one week. The actigraphy device contains a piezoelectric accelerometer that captures movement in orthogonal dimensions. These movements were collected in 30 second epochs and plotted as a histogram and the device software used an algorithm to determine sleep duration based on the absence of movement during time in bed. The device also contains a marker which participants were asked to press any time they went to bed or woke up. Additionally, participants recorded bed times, wake times, and any nap times in a Karolinska sleep dairy. A member of the research team identified sleep intervals using the sleep logs and event markers. To ensure quality control, a 10% random sample of sleep records was re-read by a second member of the research team. Sleep duration was based on the total amount of sleep obtained during their primary sleep period (which was nocturnal for most participants). The bed time and wake time for this primary sleep period was identified based on the sleep logs and event markers. We then averaged across all available days of rerecording, which was 7 days for most subjects and therefore no weighting by day of the week was required. Sleep fragmentation is a measure of restlessness during sleep that sums the percentage of sleep spent moving (more than two activity counts in a one minute period) and the percentage of consecutive phases of no movement that are less than one minute in duration determined using the actigraph. Higher values indicate sleep that is more fragmented. The total scores for the Pittsburgh Sleep Quality Index (PSQI; score range 5 to 18) and Epworth Sleepiness Scale (ESS; score range 6 to 22) were used to measure perceived global sleep quality and daytime sleepiness, respectively, and both have been validated for that purpose.27,28 There were more final scores missing for the PSQI survey than for the other measures (n = 153) because it is required that the first nine questions be answered – a single missing question in this section prevents the whole survey from being scored.28
Cardiometabolic Factors
The cardiometabolic risk factors of interest were hypertension, obesity, and diabetes. Participants were asked to fast for a minimum of 12 hours before their examination and to bring all of their prescription and over the counter medications with them to the examination. Measurements were collected between 7:30am and 11:00am. Blood pressure was measured at the clinical examination with an automated cuff after five minutes of rest; three measurements were collected and the final two were averaged. Hypertension was determined if any of the following criteria were met: systolic blood pressure ≥ 140 mmHg, a diastolic blood pressure ≥ 90 mmHg, or a self-reported use of anti-hypertensive medications. Height and weight were measured using a stadiometer and balance scale, respectively; BMI was determined as weight (kg)/height (meters)2. Obesity was defined as a BMI ≥ 30 kg/m2 for non-Asians and a BMI ≥ 27.5 kg/m2 for Asians.29 Blood was drawn into citrate vacutainer tubes while participants were seated. The tubes were centrifuged at 3,000 rpm at 4°C for 20 minutes then stored at −70°C. Fasting glucose levels were determined by plasma spectrophotometry and hemoglobin A1c levels were determined from whole blood immunoturbidimetric assay. Diabetes was determined if participants had any of the following: fasting glucose ≥ 126 mg/dL, a hemoglobin A1c ≥ 6.5%, or a self-reported use of diabetes control medications.30 All three factors were treated as dichotomous variables.
Other Covariates
Self-reported questionnaires were used to measure demographic and lifestyle behaviors. Demographic characteristics assessed were age, race, gender, socioeconomic status (as determined by education in years), and work status (unemployed/retired, day-shift, or night-shift). The lifestyle covariates were smoking status and alcohol consumption status. Smoking and alcohol consumption were categorized into either current, former, or never. Waist circumference was measured in duplicate from participants standing up right at the visual midway point between xiphoid process of the sternum and umbilicus. Measurements were captured to the nearest 0.5 cm and averaged. Depressive symptoms were assessed using the Centers for Epidemiologic Studies-Depression (CES-D) scale; a score greater than or equal to 16 is associated with major depressive disorder 31,32.
To restrict the sample to participants with a low likelihood of sleep apnea, at the first visit, participants were asked to wear the ApneaLinkPlus® apnea-screening device, using a combination of devices including the nasal cannula, a chest belt to detect respiratory effort, and a pulse oximeter to measure oxygen saturation, for one night. Post hoc exclusions for analysis were made for the following reasons: participants who did not wear the device for at least 4 hours; participants with AHI ≥15; participants with an oxygen desaturation index (ODI) ≥10.
Statistical Methods
SAS software version 9.4, copyright © 2012 SAS Institute Inc., Cary, NC, USA, was used to complete all statistical analyses. ANOVA tests for means and chi-squared tests for proportions were used to compare the distributions of the covariates by sleep duration category. Our primary hypothesis testing was conducted using binomial logistic regression analysis; odds ratios and 95% confidence intervals are presented. The covariates included in the adjusted model were: age, gender, race/ethnicity, education, work status, smoking status, alcohol status, and waist circumference.
Preliminary analyses were used to compare numerous strategies to determine the best method to model potential non-linearity for objective sleep duration. We assessed the crude scatterplots, categorized duration into common sleep categories (less than six hours, six to seven hours, seven to eight hours, and greater than eight hours), categorized duration into quartiles, and utilized a quadratic model. Ultimately, sleep duration was centered at 7.0 hours (the approximate mean for the sample) and the absolute value of the difference from 7.0 was used as a linear exposure. This method provided more stable and interpretable odds ratio estimates.
Individuals missing a PSQI score were excluded from the analyses where PSQI is the primary exposure. The 153 participants with missing PSQI scores were not different from those who did not have a missing PSQI score (data not shown) and no imputation methods were used to predict the scores since scoring protocols indicate it is not appropriate to do so based on the other completed PSQI questions.28 For all exposures, a p-value less than 0.05 was considered statistically significant. Effect modification was determined if the chi-square values corresponding to the multiplicative interaction terms between the main exposure and the covariate of interest (i.e., race, gender) were statistically significant at P < 0.05.
RESULTS
Participants
Of the 599 participants who completed the clinical examination, 50 participants were excluded because they had a high likelihood of sleep apnea based on the ApneaLink screening, 19 participants were excluded because apnea status could not be determined (i.e., <4 hours or technical failure), and 20 participants were using medications that would interfere with sleep habits (i.e., medications to treat sleep problems, hypnotic antidepressants, sedating antidepressants or stimulants). This left 510 participants who met the study inclusion criteria. Of these 510, 4 were missing more than one exposure variable, 13 were missing a demographic covariate, and 1 was missing a lifestyle behavior covariate. After excluding these participants 492 individuals remained.
Of the 492 participants included in these analyses, 211 (42.9%) had objective sleep duration between 7 and 8 hours of sleep; 214 (43.5%) had fewer than 7 hours and 67 (13.6%) had more than 8 hours. The characteristics of the 492 participants are displayed in Table 1 stratified by these commonly used categories of sleep.33 Participants who slept less than 7 hours were significantly younger, less likely to be female, less likely to be white, and less likely to be retired or unemployed than those who slept 7 or more hours. When sleep duration differs from the recommended amount, waist circumference is higher. There was no statistical difference in BMI. There were no differences by education, lifestyle factors, or depressive symptoms. Sample characteristics presented by race/ethnicity are previously reported.7
Table 1.
Characteristics of the 492 Participants in the Chicago Area Sleep Study Population by Categories of Sleep Duration, Chicago, Illinois 2009–2011
| Overall | Less Than 7 Hours | 7–8 Hours (recommended) | Greater Than 8 Hours | P value | |
|---|---|---|---|---|---|
| N | 492 | 214 (43.5%) | 211 (42.9%) | 67 (13.6%) | |
|
| |||||
| Demographics | |||||
| Age (years) | 47.8 (8.2) | 46.7 (7.7) | 48.3 (8.4) | 49.3 (9.1) | 0.033 |
| Female* | 296 (60.2%) | 113 (52.8%) | 136 (64.5%) | 47 (70.1%) | 0.009 |
| Race* | |||||
| Asian | 107 (21.7%) | 55 (25.7%) | 45 (21.3%) | 7 (10.4%) | < 0.001 |
| Black | 154 (31.3%) | 81 (37.9%) | 56 (26.5%) | 17 (25.4%) | |
| Hispanic | 103 (20.9%) | 43 (20.1%) | 44 (20.9%) | 16 (23.9%) | |
| White | 128 (26.0%) | 35 (16.4%) | 66 (31.3%) | 27 (40.3%) | |
| Education* | |||||
| Less Than High School (0–11) | 288 (58.5%) | 19 (8.9%) | 24 (11.4%) | 4 (6.0%) | 0.388 |
| High School (12–15) | 157 (31.9%) | 69 (32.2%) | 61 (28.9%) | 27 (40.3%) | |
| College (16+) | 47 (9.6%) | 126 (58.9%) | 126 (59.7%) | 36 (53.7%) | |
| Work* | |||||
| Retired/Unemployed | 151 (30.7%) | 53 (24.8%) | 69 (32.7%) | 29 (43.3%) | 0.048 |
| Employed Day Shift | 249 (50.6%) | 115 (53.7%) | 107 (50.7%) | 27 (40.3%) | |
| Employed Other Shift | 92 (18.7%) | 46 (21.5%) | 35 (16.6%) | 11 (16.4%) | |
| Lifestyle | |||||
| Smoking* | |||||
| Current | 93 (18.9%) | 43 (20.1%) | 38 (18.0%) | 12 (17.9%) | 0.737 |
| Former | 100 (20.3%) | 44 (20.6%) | 39 (18.5%) | 17 (25.4%) | |
| Never | 299 (60.8%) | 127 (59.3%) | 134 (63.5%) | 38 (56.7%) | |
| Alcohol* | |||||
| Current | 304 (61.8%) | 127 (59.3%) | 133 (63.0%) | 44 (65.7%) | 0.702 |
| Former | 103 (20.9%) | 46 (21.5%) | 46 (21.8%) | 11 (16.4%) | |
| Never | 85 (17.3%) | 41 (19.2%) | 32 (15.2%) | 12 (17.9%) | |
| Physical | |||||
| BMI (kg/m2) | 26.3 (4.5) | 26.8 (4.4) | 25.9 (4.5) | 26.2 (4.9) | 0.137 |
| Waist Circumference (cm) | 87.2 (12.4) | 88.9 (11.9) | 85.6 (12.5) | 86.6 (13.4) | 0.023 |
| Psychological Health | |||||
| Depressive Symptoms (CES-D Score ≥ 16)* | 132 (26.8%) | 60 (28.0%) | 50 (23.7%) | 22 (32.8%) | 0.295 |
Results are reported as n (%) or mean (standard deviation) with the P value for a chi-squared test of proportions (if categorical *) or F test from an ANOVA test (if continuous).
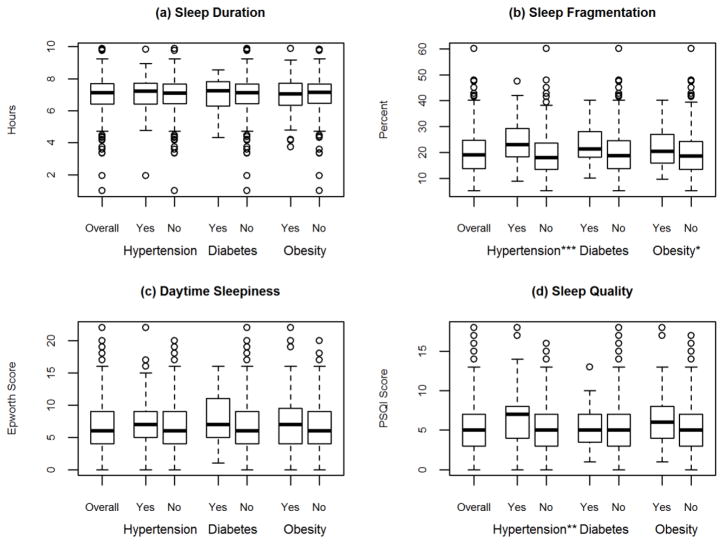
In the overall population the mean duration of sleep was 7.0 (1.1) hours, the mean fragmentation of sleep was 20.2 (8.0) percent, the mean score on the ESS was 6.9 (4.1) points, and the mean score on the PSQI was 5.8 (3.2) points. The number of patients with each cardiometabolic risk factor: 84 (17.1%) had hypertension, 27 (5.5%) had diabetes, and 120 (24.4%) were obese (Table 2). Figure 1 shows the distribution of the objective and self-reported sleep measures by cardiometabolic risk factor status.
Table 2.
The Odds Ratios for Sleep Characteristics in relation to Cardiometabolic Risk Factorsa in the Chicago Area Sleep Study (N=492), Chicago, Illinois 2009–2011.
| Unadjusted | Adjustedb | ||||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
|
Hypertension 84 (17.1%) |
Durationc | 1.13 | 0.82 , 1.55 | 1.01 | 0.69 , 1.49 |
| Fragmentationd | 1.07 | 1.04 , 1.10 | 1.05 | 1.01 , 1.08 | |
| Daytime Sleepinesse | 1.02 | 0.97 , 1.08 | 1.04 | 0.97 , 1.11 | |
| Qualityf | 1.14 | 1.05 , 1.24 | 1.07 | 0.97 , 1.18 | |
|
| |||||
|
Diabetes 27 (5.5%) |
Durationc | 1.12 | 0.67 , 1.87 | 1.19 | 0.58 , 2.46 |
| Fragmentationd | 1.04 | 1.00 , 1.09 | 1.02 | 0.95 , 1.09 | |
| Daytime Sleepinesse | 1.05 | 0.96 , 1.14 | 1.10 | 0.98 , 1.23 | |
| Qualityf | 0.97 | 0.83 , 1.12 | 0.94 | 0.78 , 1.13 | |
|
| |||||
|
Obesity 120 (35.4%) |
Durationc | 1.13 | 0.85 , 1.49 | 0.73 | 0.42 , 1.28 |
| Fragmentationd | 1.03 | 1.00 , 1.05 | 1.01 | 0.97 , 1.06 | |
| Daytime Sleepinesse | 1.01 | 0.96 , 1.06 | 1.00 | 0.92 , 1.09 | |
| Qualityf | 1.03 | 0.95 , 1.10 | 1.02 | 0.89 , 1.16 | |
Obesity is defined as a BMI greater than or equal to 30.0 (27.5 for Asians). Hypertension is defined as a seated SBP greater than or equal to 140 mmHg, a seated DBP greater than or equal to 90 mmHg, or use of medication for hypertension or high blood pressure. Diabetes is defined as a fasting blood glucose greater than or equal to 126 mg/dl or use of medication for diabetes or high blood sugar
The adjusted model includes as covariates age, gender, race/ethnicity, education, work status, smoking status, alcohol status, and waist circumference.
Unit of duration is standard deviation.
Unit of fragmentation is 1 percentage point.
Unit of Daytime Sleepiness is 1 point on Epworth.
Unit of Quality is 1 point on PSQI, limited analyses to 339 participants who had PSQI score
Figure 1.
Distribution of Objective and Self-Reported Sleep for the 492 Participants in the Chicago Area Sleep Study Population by Outcome, Chicago, Illinois 2009–2011
* p<0.05, ** p<0.005, *** p<0.0001.
There was no association between objective sleep duration or self-reported daytime sleepiness and odds of hypertension, diabetes, or obesity.
Fragmented sleep was significantly associated with odds of hypertension and obesity. For hypertension, the association was apparent in both unadjusted and adjusted models. The odds ratios in the adjusted model was 1.05 with a 95% CI: 1.01, 1.08 (shown in Table 2) suggesting that there is approximately a 5% increased odds of hypertension for each additional percentage of fragmented sleep. Fragmented sleep was only significantly associated with odds of obesity in the unadjusted model. The odds ratio was 1.03 with a 95% CI: 1.00, 1.05 (shown in Table 2) suggesting that there is approximately a 3% increased odds of obesity for each additional percentage of fragmented sleep. Fragmented sleep was not associated with odds of diabetes in either unadjusted or adjusted models.
There was a significant association between self-reported PSQI global sleep quality and odds of hypertension in the unadjusted model (odds ratio 1.14 95% CI: 1.05, 1.24) that suggested a 14% increased odds of hypertension for each additional point in the PSQI score; however, the statistical significance of this association was attenuated after adjustment for covariates. There was no statistically significant association observed between self-reported PSQI global sleep quality and odds of diabetes or obesity. These results did not change after adjustment for AHI as a continuous variable.
Neither race nor gender was an effect modifier in any association between the sleep characteristics and the cardiometabolic risk factors of interest. There was no evidence of effect modification by race/ethnicity as none of the 3 degree of freedom chi-square values exceeded 7.815, which would achieve statistical significance at P<0.05. Similarly, the multiplicative interaction terms corresponding to the sleep by gender interactions (1 degree of freedom) did not exceed 3.841 and were not significant at P<0.05.
DISCUSSION
In our population-based study of adults who had a low likelihood of obstructive sleep apnea, we observed associations of objectively-determined sleep fragmentation with hypertension and obesity and an association between perceived PSQI global sleep quality and hypertension. Despite prior research describing an association of short sleep and poor quality sleep with the prevalence of metabolic risk factors, we did not observe such an association using objectively determined sleep measures in our participants who had a low likelihood of having sleep apnea. These patterns were consistent across race/ethnic groups and among men and women.
Despite observations in other studies, objectively determined sleep duration was not associated with cardiometabolic risk factors in our study. By contrast, the Sleep Heart Health Study observed an odds ratio for hypertension of 1.66 95% CI: 1.35, 2.04 comparing those with fewer than 6 hours of sleep to those with six to nine hours of sleep.2 The Insulin Resistant Atherosclerosis Study observed an odds ratio for type 2 diabetes of 2.36 95% CI: 1.21, 3.79 when comparing those with less than 7 hours of sleep per night to those who slept at least 8 hours per night.1 And the Wisconsin Sleep Cohort Study observed a u-shaped association in which individuals who sleep above and below 7.5 hours were at a proportionally increased likelihood of obesity.3
The absence of an association with objective sleep duration in this sample may be because our sample did not include many short sleepers (i.e., <6 hours per night) when 7 days of sleep were averaged. Prior studies may have used a shorter observation period for objective measures, thus making it unlikely that they could capture “rebound sleep” that would follow very short sleep periods. Our weeklong measure may have captured this phenomenon and led to a longer “average” for the week. Similarly, short sleep may be a seminal event and when asked to self-report sleep, participants who occasionally or even regularly have nights with very short sleep may recall those nights rather than the weekly average of their sleep. However, it is not known which has a greater influence on cardiometabolic outcomes—very short sleep over a few nights or average sleep over a longer period of time.
Sleep fragmentation, an objective assessment of quality, was most strongly associated with the cardiometabolic risk factors in our study. Although we did not find a significant association between sleep fragmentation and diabetes or obesity outside of the unadjusted models, there is evidence in the literature to suggest such an association exits. The Rotterdam study found that the association between sleep duration and obesity was no longer statistically significant after adjustment for sleep fragmentation.34 Further, they found that for each additional standard deviation of sleep fragmentation there was a 0.59 kg/m2 increase in BMI.34 Their study was conducted in a population over the age of 55 years with a low likelihood of sleep apnea (defined by self-report); however, similar methods were used to obtain objective sleep measures via actigraphy. A study of weight loss in women aged 20–65 years also found that women with higher levels of sleep fragmentation experienced a lower degree of weight loss.35 It is possible that the small number of diabetes cases in our study (n=27) limited our statistical power—particularly following statistical adjustment to capture these associations.
We did find an association between sleep fragmentation and hypertension. Our observation is notable because there is evidence that sleep fragmentation is significantly associated with hypertension independent of airway resistance. A study in middle aged non-apneic snorers found that individuals with sleep fragmentation experienced a higher likelihood of hypertension than those without sleep fragmentation.36 The authors suggest that the sympathetic nervous system response to sleep fragmentation may have an effect on blood pressure and increase the odds of hypertension.36
The association in our study between the results of the PSQI and the health outcomes was limited; only the unadjusted model with hypertension was statistically significant. The Cardiovascular Health Epidemiology study similarly found that sleep duration and quality as measured by the PSQI was only moderately associated with obesity.37 Further, they found that stress was a significant modifier of the interaction and suggested the two must be measured in conjunction.37 Our study did not take into account perceived stress and this could have limited the comparability of the PSQI scores between participants.
One strength of our study is the use of wrist actigraphy to objectively measure sleep duration and quality which reduces the measurement error associated with self-reported exposures. Another strength is that we identified and excluded individuals with a high risk of apnea using screening questionnaires and one night of home sleep testing. While some participants with mild symptoms (AHI 5–15) remained in our sample, adjustment for continuous AHI score did not change the adjusted model results. Additionally, our participant sample was selected at random, not based on volunteers, and so should not be subject to the bias that could result if adults who were most interested in participating in a study about sleep were those who had sleep related problems or concerns. Finally, we enrolled approximately equal numbers of whites, blacks, Hispanics, and Asians. Social and cultural factors may also influence perceptions of the amount of sleep that is normal and can influence self-reported sleep. Individuals may misrepresent their actual sleep duration to align themselves with the amount of sleep they perceive to be desirable.38 Prior studies that include objective assessment of sleep typically only compare two race/ethnic groups (e.g., white vs. black), whereas we also captured information from Asian and Hispanic adults. By using objective measures of sleep, we overcome some of these limitations.
A limitation common to all sleep studies is that the amount of sleep that results in the best health outcomes can vary widely by individual. While population recommendations reflect the ranges that are commonly associated with the best health outcomes, there are some individuals who require more or less sleep to achieve the best health outcomes. However, this limitation does not affect the calculation of sleep fragmentation, the exposure that was most significantly associated with hypertension and obesity. That fragmentation was significant, and not duration, may be further evidence that the quality of sleep is just as, if not more, important as the amount of sleep. Our conclusions about the association of sleep with obesity are limited to those with class I obesity since the goal of our study was to identify a sample of adults who had a low likelihood of apnea. Given the positive association of obesity with apnea, we did not recruit adults who had a self-reported body mass index of 35 or greater. The use of years of education as a proxy for socioeconomic status is a potential limitation as well. Though education is an indicator likely to capture aspects of lifestyle and behavior, it can have different effects by gender, race and ethnicity and may not fully capture all aspects of socioeconomic status.39 This study is cross sectional and therefore, given the limited ability to determine temporality, there is the potential for bidirectionality of the associations identified.
As the prevalence of obesity, hypertension, and diabetes continue to increase in the population, identifying factors that could contribute to the burden of disease in the population and that can be easily measured is important. Further, our research highlights one health behavior, sleep, that is known to differ by race/ethnicity and that could be the focus of interventions which could have the significant secondary benefit of reducing disparities in cardiometabolic risk factors. Because there is no single recommended length of sleep time for optimal health given individual biological variability in sleep needs, sleep fragmentation and other measures of sleep quality warrant attention. Additional research should identify the causal pathways and further observe how their effects differ by race, ethnicity and gender.
Acknowledgments
Financial Support:
The study was funded by the National Heart, Lung and Blood Institute/National Institutes of Health grant R01HL092140
The study was funded by the National Heart, Lung, and Blood Institute/National Institutes of Health grant R01HL092140. The authors wish to thank Norrina Allen, PhD, Lihui Zhao, PhD, and the rest of the MSEB Examination Committee for reading this and contributing thoughtful comments.
Footnotes
Conflicts of Interest:
None Declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Annals of epidemiology. 2009;19(5):351–357. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. SLEEP-NEW YORK THEN WESTCHESTER- 2006;29(8):1009. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 3.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS medicine. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology (Cambridge, Mass) 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–844. doi: 10.5665/sleep.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altman NG, Izci-Balserak B, Schopfer E, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep medicine. 2012;13(10):1261–1270. doi: 10.1016/j.sleep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carnethon MR, De Chavez PJ, Zee PC, et al. Disparities in sleep characteristics by race/ethnicity in a population-based sample: Chicago Area Sleep Study. Sleep Med. 2016;18:50–55. doi: 10.1016/j.sleep.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen-Torvik LJ, De Chavez PJ, Kershaw KN, et al. The Mediation of Racial Differences in Hypertension by Sleep Characteristics: Chicago Area Sleep Study. Am J Hypertens. 2016 doi: 10.1093/ajh/hpw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep medicine reviews. 2008;12(4):289–298. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16(3):643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Practice & Research Clinical Endocrinology & Metabolism. 2010;24(5):731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Social science & medicine. 2010;71(5):1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 13.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Annals of the New York Academy of Sciences. 2008;1129(1):287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogden CL, Carroll MD, Kit BK, Flegal KM. PRevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menke A, Casagrande S, Geiss L, Cowie CC. PRevalence of and trends in diabetes among adults in the united states, 1988–2012. JAMA. 2015;314(10):1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 16.Wang EJ, Wong EC, Dixit AA, Fortmann SP, Linde RB, Palaniappan LP. Type 2 diabetes: identifying high risk Asian American subgroups in a clinical population. Diabetes Res Clin Pract. 2011;93(2):248–254. doi: 10.1016/j.diabres.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillespie CD, Hurvitz KA Centers for Disease C, Prevention. Prevalence of hypertension and controlled hypertension - United States, 2007–2010. MMWR Suppl. 2013;62(3):144–148. [PubMed] [Google Scholar]
- 18.St-Onge MP, Grandner MA, Brown D, et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation. 2016 doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopps E, Caimi G. Obstructive Sleep Apnea Syndrome: Links Betwen Pathophysiology and Cardiovascular Complications. Clin Invest Med. 2015;38(6):E362–370. doi: 10.25011/cim.v38i6.26199. [DOI] [PubMed] [Google Scholar]
- 21.Polovina MM, Potpara TS. Endothelial dysfunction in metabolic and vascular disorders. Postgrad Med. 2014;126(2):38–53. doi: 10.3810/pgm.2014.03.2739. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan V, Collop NA. Gender differences in sleep disorders. Current opinion in pulmonary medicine. 2006;12(6):383–389. doi: 10.1097/01.mcp.0000245705.69440.6a. [DOI] [PubMed] [Google Scholar]
- 23.Tosur Z, Green D, De Chavez PJ, et al. The Association between Sleep Characteristics and Prothrombotic Markers in a Population Based Sample: Chicago Area Sleep Study. Sleep Medicine. 2014 doi: 10.1016/j.sleep.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palaniappan LP, Araneta MRG, Assimes TL, et al. Call to Action: Cardiovascular Disease in Asian Americans A Science Advisory From the American Heart Association. Circulation. 2010;122(12):1242–1252. doi: 10.1161/CIR.0b013e3181f22af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108(5):822–830. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 26.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Tan K. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. The Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 30.Association AD. Position statement: Standards of Medical Care in Diabetes—2010. Diabetes Care. 2010;33:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg EL, Comstock GW, Hornstra RK. Depressed mood and subsequent physical illness. The American journal of psychiatry. 1979 [PubMed] [Google Scholar]
- 32.Comstock GW, Helsing KJ. Symptoms of depression in two communities. Psychological medicine. 1977;6(04):551–563. doi: 10.1017/s0033291700018171. [DOI] [PubMed] [Google Scholar]
- 33.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van den Berg J, Neven AK, Tulen J, et al. Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. International Journal of Obesity. 2008;32(7):1083–1090. doi: 10.1038/ijo.2008.57. [DOI] [PubMed] [Google Scholar]
- 35.Sawamoto R, Nozaki T, Furukawa T, et al. Higher sleep fragmentation predicts a lower magnitude of weight loss in overweight and obese women participating in a weight–loss intervention. Nutrition & diabetes. 2014;4(10):e144. doi: 10.1038/nutd.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Committee PAGA. Physical activity guidelines advisory committee report, 2008. Vol. 2008. Washington, DC: US Department of Health and Human Services; 2008. pp. A1–H14. [Google Scholar]
- 37.Bidulescu A, Din-Dzietham R, Coverson DL, et al. Interaction of sleep quality and psychosocial stress on obesity in African Americans: the Cardiovascular Health Epidemiology Study (CHES) BMC Public Health. 2010;10(1):581. doi: 10.1186/1471-2458-10-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basner M, Fomberstein KM, Razavi FM, et al. American time use survey: sleep time and its relationship to waking activities. Sleep. 2007;30(9):1085. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shavers VL. Measurement of socioeconomic status in health disparities research. Journal of the national medical association. 2007;99(9):1013. [PMC free article] [PubMed] [Google Scholar]