Abstract
Soil and rhizosphere bacteria produce an array of secondary metabolites including a wide range of volatile organic compounds (VOCs). These compounds play an important role in the long-distance interactions and communication between (micro)organisms. Furthermore, bacterial VOCs are involved in plant pathogens inhibition and induction of soil fungistasis and suppressivenes. In the present study, we analysed the volatile blend emitted by the rhizospheric isolate Pseudomonas donghuensis P482 and evaluated the volatile effect on the plant pathogenic fungi and bacteria as well as one oomycete. Moreover, we investigated the role of the GacS/GacA system on VOCs production in P. donghuensis P482. The results obtained demonstrated that VOCs emitted by P. donghuensis P482 have strong antifungal and antioomycete, but not antibacterial activity. The production of certain volatiles such as dimethyl sulfide, S-methyl thioacetate, methyl thiocyanate, dimethyl trisulfide, 1-undecan and HCN is depended on the GacS/GacA two-component regulatory system. Apparently, these compounds play an important role in the pathogens suppression as the gacA mutant entirely lost the ability to inhibit via volatiles the growth of tested plant pathogens.
Introduction
Volatile organic compounds (VOCs) are commonly produced by all organisms including bacteria, fungi and plants. They are low molecular mass molecules (<300 Da) able to penetrate soil and rhizosphere. VOCs synthesized by soil and plant-associated microorganisms have been shown to suppress the growth of plant pathogenic microorganisms indicating that these compounds could be one of the important mechanisms for biological control of plant diseases [1,2].
Recent studies, demonstrated that VOCs can also mediate a variety of interactions between microorganisms and their environment [3]. Indeed, in nature, volatiles are considered to mediate or participate in different intra- and interspecies communication [4,5]. Microbial volatiles can induce systemic resistance of plants or modulate plant growth [6–8]. In bacterial-bacterial interactions they can affect quorum sensing, biofilm formation, secondary metabolites production, antibiotic resistance and virulence [3,5,9,10].
Many volatiles emitted by soil bacteria have antifungal activities and reduce hyphal extension as well as hyphal biomass [4,9]. Hence, bacterial volatiles are considered as a major contributor to soil fungistasis [11,12].
Pseudomonas species, which are frequently isolated from soil and rhizosphere are well known as plant growth-promoting rhizobacteria (PGPR) that positively affect plant growth and health by inhibiting the growth of plant pathogens and/or by inducing systemic resistance (ISR) to plant [13]. These bacteria produced numerous secondary metabolites, which have been studied for their antimicrobial activity towards fungi and oomycetes and, to a lesser extent, towards bacteria [13–18]. Among these antimicrobials, Pseudomonas strains produce an array of volatile bioactive metabolites, including hydrogen cyanide (HCN), which may participate in the inhibition of many metalloenzymes [19–22].
The production of secondary metabolites in many bacteria, as well as in Pseudomonas, is often regulated by GacS/GacA two-component regulatory system [23]. This system consists of a membrane-bound sensory kinase GacS (which senses yet unknown biotic and abiotic signals) and a transcriptional response regulator GacA [24]. Genetically obtained mutants in the gene encoding one of these proteins result in the loss of the antimicrobials production including HCN [25,26]. However, it was also discovered that in P. fluorescens SBW25 GacS mutants show enhanced antimicrobial activity [27].
Pseudomonas donghuensis P482, used in this study, is a tomato rhizosphere isolate belonging to the recently established new species of Pseudomonas genus [28]. P. donghuensis P482 is capable of inhibiting the growth of several plant pathogens, including the stone fruit pathogen P. syringae [29] and the various strains of soft rot Enterobacteriaceae from the genus of Dickeya and Pectobacterium (formerly Erwinia) [29]. The genetic background of the observed antibacterial activity against Dickeya and Pectobacterium has been recently unveiled [18]. P. donghuensis P482 also inhibits the growth of the fungal plant pathogen Rhizoctonia solani [30], yet, little is known about antifungal properties of this strain, as well as the inhibitory capacity of the VOCs blend produced by P. donghuensis P482 on the fungal and bacterial pathogens.
In this study, the VOCs blend emitted by P. donghuensis P482 strain was analysed and evaluated for its effect on the plant pathogenic fungi and oomycete as well as on phylogenetically different bacteria. Furthermore, we investigated the role of the GacS/GacA system in regulation of P. donghuensis P482 VOCs production.
The results obtained demonstrated that VOCs emitted by P. donghuensis P482 have strong antifungal and antioomycete, but not antibacterial activity. Furthermore, our results revealed that the production of certain antifungal volatiles is depended the GacS/GacA two-component regulatory system.
Material and methods
Strains and growth conditions
Bacterial strains used in this study are listed in Table 1. All strains were maintained on 1/10 TSB agar plates (5.0 g L-1 NaCl, 1.0 g L-1 KH2PO4; 3 g L-1 oxoid tryptic soy broth (TSBA); 20 g L-1 Merck Agar, pH 6.5; Garbeva and de Boer, 2009) or LB (Novagen, Germany) at 25°C, when necessary supplemented with kanamycin (30 μg ml-1 Sigma-Aldrich, USA). For the growth of the mating strain E. coli ST18 the medium was supplemented with 5-aminolevulonic acid (ALA) 30 μg ml-1 (Sigma-Aldrich, USA) and the bacteria were grown at 37°C. All fungi and oomycetes cultures were pre-grown on 1/2 potato dextrose agar (PDA; 29 g L−1 Oxoid CM139; [31]) for one-week prior to use them in the experiments.
Table 1. The list of the bacterial, fungal and oomycetes strains used in this study.
| Strain | Origin/ features | reference |
|---|---|---|
| Pseudomonas donghuensis P482 | Tomato rhizosphere isolate—a wild type strain | [29] |
| KN3318 | P. donghuensis P482 mutant carrying an inbuilt pKNOCK-Km suicide vector in gacA gene (locus BV82_3318); KmR BV82_3318::pKnock-Km | This study |
| Escherichia coli ST18 | Donor strain for diparental mating; pro thi hsdR+ TpR SmR; Chromosome::RP4-2 Tc::Mu-Kan::Tn7/λpir ΔhemA | [32] |
| Escherichia coli DH5α | Cloning strain; fhuA2 lac(del)U169 phoA glnV44 Φ80' lacZ(del)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17,high efficiency transformation strain | [33] |
| Agrobacterium sp. AD140 | NIOO-KNAW collection | [9] |
| Pseudomonas fluorescens AD21 | NIOO-KNAW collection | [9] |
| Rhizoctionia solani AG2.2IIIB | Plant pathogen NIOO-KNAW collection | [9] |
| Fusarium culmorum PV | Plant pathogen NIOO-KNAW collection | [9] |
| Verticulum dahliae JR | Plant pathogen NIOO-KNAW collection | [9] |
| Pythium ultimum P17 | Plant pathogen NIOO-KNAW collection | [9] |
Construction of the gacA mutant
A locus of gacA gene was identified and annotated in P. donghuensis P482 genome (JHTS00000000.1; [30]) based on ten sequences of the homologous genes present in pseudomonas.com database [34]. The mutation was introduced using pKnock-Km system [35] as previously described by Krzyzanowska et al. [18]. Briefly, the fragment of interest (413 bp of gacA gene) was amplified with Hot Start II Phusion DNA polymerase (Thermo Scientific, USA) and the primer pair gaca_insF/gaca_insR (S1 Fig) in conditions recommended by the supplier. A PCR product obtained was cloned between the KpnI/SalI restriction sites of the pKNOCK-Km suicide vector [35]. The resulting construct pKN3318 was introduced into E. coli ST18 [31] via electroporation (Gene Pulser Xcell™, Bio-Rad). Subsequently the positive E. coli transformants were selected on LB medium with ALA and kanamycin. The generated plasmid was transferred to P. donghuensis P482 by biparental mating. Donor carrying pKN3318 plasmid and recipient strains were grown overnight. From each culture 2 ml was spin down and rinsed twice with 0,9% NaCl (Avantor, Poland). Pellets were suspended in 25 μl LB, pooled together and transferred as a droplet onto LB agar plate with ALA then incubated 20h at 37°C. The macro-colony established on the plate was collected and re-suspended in 1 ml LB medium. One hundred microliter aliquots of the suspension and serial dilutions (10−1, 10−2, 10−3) were plated on LB agar supplemented with 30μg·ml−1 kanamycin but lacking ALA, thus preventing the growth of the E.coli ST18. Nine P. donghuensis P482 transconjugants obtained were screened for the presence of the pKNOCK-Km insertion with the primer pair gaca_outF/gaca_outR (S1 Table). To confirm that the suicide vector had incorporated into the target loci, genomic DNA of each mutant was used as template in a sequencing reaction with primer F_outof_pKNOCK. The results obtained enabled mapping of the pKNOCK-Km insertion site to the genome of the P. donghuensis P482 strain. Two of obtained mutants (KN3318-1 and KN3318-2) were sequenced by Oligo.pl (Warsaw, Poland) that finally confirmed presence of pKnock-Km insert in gacA gene (locus BV82_3318). One of the sequenced strains (KN3318-1) was chosen and used for further experiments as KN3318.
Effect of volatiles produced by P. donghuensis P482 on fungi and oomycete growth
To investigate the VOCs effect on the growth of the tested fungi and oomycete bottom-top approach was applied as described previously by Garbeva et al., [9]. Bacteria suspensions washed with 10 mM phosphate buffer were spread at the bottom part of the plates on 20 ml 1/10 TSB agar. For a control, a sterile phosphate buffer was plated. The top part (the lid) of Petri dish contained 12 ml water agar (WA) and the 6 mm-in-diameter PDA disc with fungi/oomycete was placed in the center; the plates were sealed with the parafilm, and incubated at 25°C.
When the effect of the pure compounds: S-methyl thioacetate (MTA), dimethyl disulfide (DMDS) or dimethyl trisulfide (DMTS) (Sigma-Aldrich, Germany), was tested, the bottom part of the plate contained a paper disc soaked with one or two (mixed) pure compounds. In every treatment with single compound 1 μl was added (MTA- 11.4 μM, DMDS– 11.1 μM, DMTS– 9.5 μM), for the mix 1 μl of each compound was used. The plates were immediately sealed with the parafilm and incubated at 25°C.
Effect of volatiles produced by P. donghuensis P482 on bacteria growth
Two compartment Petri dishes with 10 ml of 1/10 TSB per each part were used to evaluate the effect of VOCs produced by P. donghuensis P482 on two bacterial species: Agrobacterium sp. AD140 and P. fluorescens AD21. One side of the plate was inoculated with 50 μl of 105 cfu ml-1 P. donghuensis P482 and incubated for 2 days. Afterwards overnight liquid cultures of the model strains AD140 and AD21 were added at the other compartment of the plate at different bacteria densities (10μl drops). Enumeration of AD140 and AD21 was performed under the binocular after overnight incubation in 25°C, all the experimental setups were prepared in triplicates.
Hydrogen cyanide production
For hydrogen cyanide (HCN) detection in the headspace of P. donghuensis P482, a method adapted from Castric and Castric [36] was used. Sterile Whatmann paper (3 mm thick and size 128 x 86 mm) was soaked with suspension containing 5 mg ml-1 of copper(II) ethyl acetoacetate (Sigma-Aldrich, USA) and 4,4'-methylenebis-(N,N-dimethylaniline) (Sigma-Aldrich, USA) in chloroform and dried in sterile conditions. One hundred μl of 1/10 TSB medium was poured into the wells of the 96-well plate. Three different treatments were prepared i) 1/10 TSB without any supplementation, ii) 1/10 TSB supplemented with 50μM FeCl3 (Avantor, Poland), and iii) 1/10 TSB supplemented with 20 μM glycine (Sigma-Aldrich, USA). All the treatments were prepared in duplicates. Wells were inoculated with 1 μl overnight culture of P. donghuensis P482, KN3318 or the HCN non-producer E. coli DH5α. All bacterial cultures were diluted to 108 cfu ml-1. Plate was covered with freshly prepared and dried Whatmann paper and the plastic cover. The plate was pressed using six large binder clips and incubated 24 h at 25°C. After this time the development of the blue color, indicating HCN production was examined.
Volatile trapping and GC-MS analysis
P. donghuensis P482 and KN3318 mutant were grown in triplicates on 1/10 TSB agar medium, non-inoculated medium was used as a control. Emitted volatile compounds were collected in the steel traps with 150 mg Tenax TA and 150 mg Carbopack B (Markes International Ltd, Llantrisant, UK) applied to the specially adapted glass petri dishes [9]. Traps were removed, capped and stored at 4°C until GC-Q-TOF analysis. Volatiles were desorbed from the traps using an automated thermodesorption unit (model UnityTD-100, Markes International Ltd., Llantrisant, UK) at 210°C for 12 min (Helium flow 50 mL/min) and trapped on cold trap at -10°C. The trapped volatiles were introduced into the GC-QTOF (model Agilent 7890B GC and the Agilent 7200A QTOF, Santa Clara, USA) by heating the cold trap for 3 min to 280°C with split ratio set to 1:20. The column used was a 30 × 0.25 mm ID RXI-5MS, film thickness 0.25 μm (Restek 13424–6850, Bellefonte, PA, USA). Temperature program used was as follows: 39°C for 2 min, from 39°C to 95°C at 3.5°C/min, then to 165°C at 6°C/min, to 250°C at 15°C/min and finally to 300°C at 40°C/min, hold 20 min. The volatiles were detected by the MS operating at 70 eV in EI mode. Mass spectra were acquired in full scan mode (30–400 amu, 4 scans/s). Identification of metabolites was performed using NIST-MS Search and accurate mass and spectra match factor using NIST 2014 V2.20 (National Institute of Standards and Technology, USA, http://www.nist.gov) and Wiley 9th edition spectral libraries and by their linear retention indexes (lri). The lri values were compared with those found in the NIST and in the in-house NIOO lri database. The LRI values were calculated using an alkane calibration mix before the measurements in combination with AMDIS 2.72 (National Institute of Standards and Technology, USA).
Statistical analysis
For the metabolomics analysis the acquired raw MS data was extracted to m/z format with MassHunter Qualitative Analysis Software V B.07.00 (Agilent Technologies, Santa Clara, CA, USA) and processed with MZMine V 2.21 (Copyright © 2005–2012 MZmine Development Team, [37]) to create m/z and peak intensity table that could be used as input file to MetaboAnalyst 3.0 software (http://www.metaboanalyst.ca/MetaboAnalyst [38]). Before the statistical analysis, data was filtered using Interquantile range (IQR) and normalized by the log transformation with automatic scaling. On the pretreated dataset following analyses were performed: clustering using partial least squares—discriminant analysis (PLS-DA), One-way Analysis of Variance (ANOVA) and Hierarchical Clustering. The statistical analyses of fungal antagonistic tests and bacterial enumeration were carried out with Excel using ANOVA and Student’s t-Test. Data were considered to be statistically different at p≤ 0.05.
Results
Effect of volatiles emitted by P. donghuensis P482 on the fungal, oomycete and bacterial growth
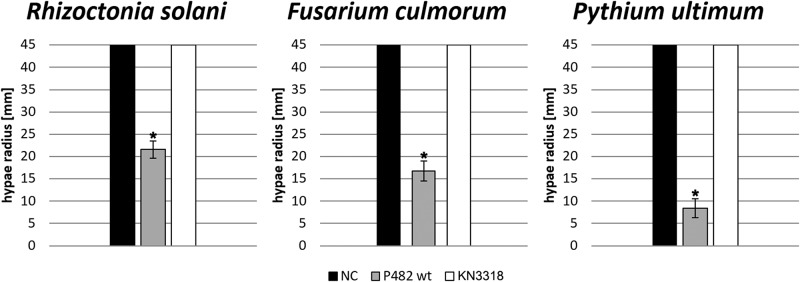
The growth of the 2 fungal (R. solani AG2.2IIIB and F. culmorum PV) and one oomcycete (P. ultimum P17) pathogens was significantly inhibited when exposed to the volatiles emitted by P. donghuensis P482 wt (Fig 1). The hyphal inhibition varied from about 50 to 80% reduction of fungal and oomycete growth as compared to the control. The strongest inhibition (>80%) was observed on the oomycete pathogen P. ultimum P17. The lowest, yet still significant (about 50%) growth reduction was observed on the R. solani strain.
Fig 1. Average hyphae radius after 4 days (Rhizoctonia solani), 7 days (Fusarium cilmorum) and 5 days (Pythium ultimum) of incubation under influence of volatiles emitted by P. donghuensis P482 wt, KN3318 mutant and non-treated control (NC).
Significant difference between sample and control are indicated by asterisk (one-way ANOVA), error bars represents standard deviation of the mean.
The growth of V. dahliae in the given experimental condition was very slow and after 14 days of incubation the hypha expanded only about 5–6 mm in case of the non-treated control. However, when exposed to volatiles emitted by P. donghuensis P482 the growth of V. dahliae was completely suppressed (S2 Fig).
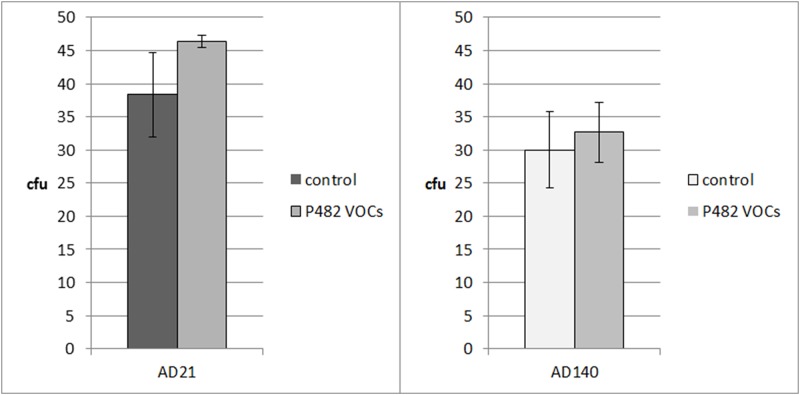
The exposure of bacterial strains Agrobacterium sp. AD 140 and Pseudomonas fluorescens AD21 to the volatiles emitted by P. donghuensis P482 did not cause any significant difference in their growth (Fig 2).
Fig 2. Number of colony forming units (cfu) of Pseudomonas fluorescens AD21 and Agrobacterium sp. AD140 after overnight exposure to volatiles emitted by P. donghuensis P482 and control without bacterial volatiles.
Error bars represents standard deviation of the mean.
GacA deficiency abolished P. donghuensis P482 volatiles-mediated inhibition of the tested fungal and oomycete pathogens
The results obtained with the GacA-deficient mutant KN3318 revealed that the mutant lost entirely its volatile-mediated inhibition resulting in hypha extension of the tested fungal and oomycete pathogens similar to that of the controls (Fig 1). In case of the tested strain of V. dahliae the growth of hypha was as slow as observed for the untreated control (S2 Fig). However, when exposed to volatiles emitted by KN3318, the change in a phenotype of V. dahliae mycelium was observed in comparison to the non-treated control. The untreated mycelium was black in the center but in the case of exposure to KN3318- emitted volatiles it remains white (S2 Fig). Microscopic observation revealed that this difference in coloration is due to the formation of microsclerotia by the fungus (pigmented aggregates). This phenomenon was not observed under the exposure to P. donghuensis P482 wt.
GC-MS and metabolomics analysis of volatiles emitted by P. donghuensis P482
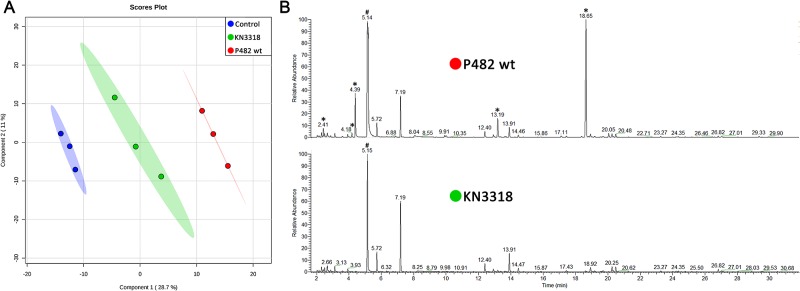
The volatolomic analysis of P. donghuensis P482 wt and its GacA-deficient mutant KN33318 revealed that their volatile profiles differ. Clear separations between control, wild type and mutant were obtained in PSL-DA score plot (Fig 3A). Five major peaks with RT 2.41, 4.18, 4.39, 13.19 and 18.65 were not detected in the headspace of KN3318 mutant and the control consisting only of media without bacteria (Fig 3B and 3C). The volatiles emitted by P. donghuensis P482 wt strain but not present in the KN3318 mutant headspace were identified as dimethyl sulfide, S-methyl thioacetate, methyl thiocyanate, dimethyl trisulfide and 1-undecan (Table 2). Two compounds namely dimethyl trisulfide and S-methyl thioacetate were confirmed with commercially available authentic standards. In both P. donghuensis P482 wt and the GacA KN33318 mutant the highly abundant peak with RT 5.14 was identified as dimethyl disulfide.
Fig 3. Data obtained from MetaboAnalyst 3.0 and GC-MS results.
(A) PLS-DA plot of VOC’s produced by P. donghuensis P482 and KN3318 mutant with media as a control (triplicates); (B) TIC chromatographs from GC-MS analysis showing different peaks between P. donghuensis P482 and KN3318 mutant. Significant peaks that differ between wild type and mutant are indicated by asterisk. Dimethyl disulfide (DMDS) is indicated by “#”.
Table 2. Volatile compounds identified in the headspace P. donghuensis P482 wt not present in the KN3318 gacA mutant culture.
| RT | Compound name | ERI | Formula |
|---|---|---|---|
| 2.41 | dimethyl sulfide | 540 | C2H6S |
| 4.18 | S-methyl thioacetate | 701 | C3H6OS |
| 4.39 | methyl thiocyanate | 709 | C2H3NS |
| 13.19 | dimethyl trisulfide | 966 | C2H3S3 |
| 18.65 | 1-undecan | 1091 | C11H22 |
| N/A* | hydrogen cyanide | N/A | HCN |
RT- retention time in min and ERI- experimental retention index;
* Hydrogen cyanide could not be detected/measured using GC-QTOF and was measured as described by Castric and Castric [36]
Effect of S-methyl thioacetate, dimethyl disulfide and dimethyl trisulfide on fungal and oomycete growth
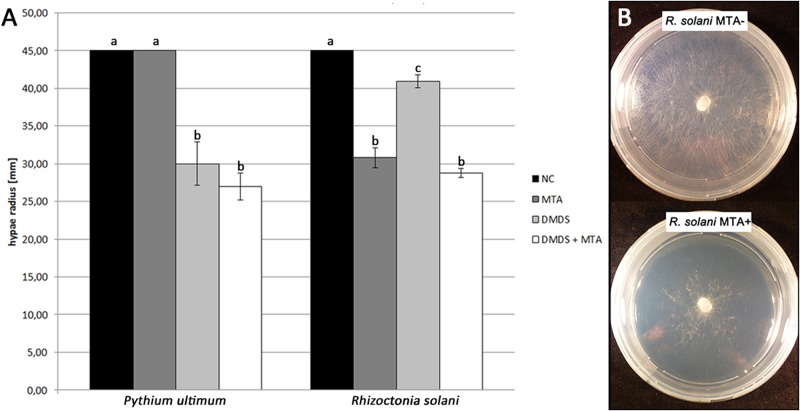
Pure S-methyl thioacetate (MTA), found to be produced by P. donghuensis P482 wt but not by the GacA-deficient KN33318 mutant, was tested on two selected plant pathogens: R. solani and P. ultimum using the bottom-top approach. For oomycete P. ultimum there was no significant difference between pathogen treated with MTA and non-treated control but in case of R. solani the growth inhibition was significant and comparable to the effect of volatile blend produced by P. donghuensis P482 wt (Fig 4) indicating that this particular compound is important for the growth inhibition of R. solani. The pure compound dimethyl disulfide caused significant inhibition on both R. solani and P. ultimum. Slightly stronger inhibition (but not significant) was observed when MTA was applied in combination with dimethyl disulfide (Fig 4). Complete growth inhibition of both R. solani and P. ultimum was observed when dimethyl trisulfide was applied (data not shown).
Fig 4.
(A) Average hyphae radius of P. ultimum and R. solani after 4 days of incubation exposed and not exposed (NC) to pure volatile organic compounds: S-methyl thioacetate (MTA), dimethyl disulfide (DMDS) and combination of MTA and DMDS. Significant difference between samples are indicated by different letters (one-way ANOVA, post hoc t-test p < 0.05), error bars represents standard deviation of the mean. (B) Photos of R. solani cultures exposed (+) and not exposed (-) to MTA.
Hydrogen cyanide production
Production of the hydrogen cyanide (HCN) was detected in all media variants only in Pseudomonas donghuensis P482 but not in GacA-deffecient KN3318 mutant or E. coli (S3 Fig).
Supplementation of the growing medium (1/10 TSB) with 50μM glycine resulted in the darker blue color in comparison to P. donghuensis P482 wt grown only in basal medium or medium supplemented with iron.
Discussion
Soil and rhizosphere bacteria produce a large amount of secondary metabolites, which have many different physiochemical and biological properties. Beside well documented, soluble antimicrobial compounds bacteria emit a wide range of VOCs that hold a strong inhibitory capacity [5,9,20,39]. In this study, we attempted to determine the VOCs emitted by the rhizospheric isolate P. donghuensis P482, their antimicrobial activity and the role of the two-component GacS/GacA regulatory system in VOCs production.
Our results revealed that P. donghuensis P482 strain is emitting VOCs with strong antifungal and antioomycete properties and significantly inhibited the plant pathogens R. solani, F. culmorum, V. dahliae and P. ultimum. Interestingly GacA-deficient mutant lost entirely the ability to inhibit via volatiles the tested plant pathogens. The metabolomics analysis revealed clear differences in volatile profiles between the P. donghuensis P482 and the GacA mutant KN3318. Five compounds detected only in the headspace of P. donghuensis P482 but not in the headspace of GacA-deficient mutant were identified as dimethyl sulfide, S-methyl thioacetate, methyl thiocyanate, dimethyl trisulfide and 1-undecan.
The 1-undecan together with dimethyl disulfide were the most abundant volatiles detected in the headspace of P. donghuensis P482 which is in line with other studies on VOCs produced by plant-associated Pseudomonas [20,21]. In the recent study of Hunziker et al [21] the 1-undecene, emitted by plant-associated Pseudomonas, was indicated as the main and active compound with antioomycete properties and with strong inhibitory power to hinder Phytophtora infestans growth and development [21].
Interestingly, our results revealed that the production of dimethyl disulfide in contrast to the other sulfur containing volatiles namely dimethyl sulfide, S-methyl thioacetate, methyl thiocyanate, and dimethyl trisulfide, was not regulated by the two-component GacS/GacA system, as it was detected both in the headspace of P. donghuensis P482 wt and GacA deficient mutant (Fig 3B).
The sulfur containing volatile S-methyl thioacetate was tested individually or in combination with dimethyl disulfide for the ability to inhibit the growth of the model plant pathogens R. solani and P. ultimum. Individually applied, the S-methyl thioacetate revealed only anti-fungal activity but did not inhibit the growth of the oomycete P. ultimum. This apparent difference in sensitivity of the oomycete to volatiles may be related to the cell wall composition and structure, which is different from that of fungi.
Pinpointing of the observed effects to a single volatile compound can be complicated, because fungi and oomycetes might react to a blend of volatiles, rather than to single compounds. For example, when S-methyl thioacetate was applied in combination with other sulfur containing volatiles such as dimethyl disulfide or dimethyl triulfide stronger inhibition of both R. solani and P. ultimum was observed. Although numerous studies including this one are trying to pinpoint a single volatile compound responsible for antifungal and antioomycete properties most probably the synergistic or additive effect of several compounds are responsible for the strong antimicrobial volatile activity [12,21].
Beside volatile organic compounds many bacteria are producing large amount of inorganic compounds such as hydrogen cyanide or ammonia [8,21,40] already demonstrated to possess strong antimicrobial properties [21,41,42]. Our results revealed that the production of the volatile hydrogen cyanide in P. donghuensis P482 is also regulated by the two-component GacS/GacA system as reported for other Pseudomonas and Chromobacterium species [43,44].
While many study tested the effect of bacterial VOCs on various fungi and oomycete relatively few studies have reported on VOCs with antibacterial activities [5,45]. We revealed that the VOCs produced by P. donghuensis P482 did not influence the growth of the tested rhizosphere isolates P. fluorescens AD21 and Agrobacterium sp. AD140 (nor Dickeya solani IFB 102 strain (S. Jafra personal communication)). This is in line with the recent study of Garbeva et al., [46] where P. fluorescens Pf0-1 was exposed to VOCs produced by 4 phylogenetically different bacterial isolates (Collimonas pratensis, Serratia plymuthica, Paenibacillus sp., and Pedobacter sp.) growing in sand containing artificial root exudates. Their results revealed that the bacterial VOCs rather stimulated than inhibited the growth of P. fluorescens Pf0-1. However, bacterial growth suppression was reported for dimethyl trisulfide emitted by Pseudomonas strains against the Agrobacterium sp. causing crown-gall diseases [19,47]. Recently, Tyc et al. [45] tested the two commonly produced bacterial VOCs namely dimethyl di- and trisulfide for their effect on bacteria. The experiments revealed strong inhibition on the tested bacterial model organisms, only when applied in the highest tested concentration of 50 μM [45]. However, it is unclear at what concentration these VOCs are produced in the natural environment.
It has been speculated that variations in sensitivity of bacteria to volatiles may possibly be mediated by an ATP-dependent efflux mechanism, which has been investigated for several terpene compounds against Pseudomonas aeruginosa [48] as well as the ability of the VOCs to disintegrate the outer membrane [49]. Hence, the outcome of bacterial volatile-mediated interactions can strongly vary depending on the interacting partners.
In conclusion, our work revealed that the rhizospheric isolate P. donghuensis P482 emits VOCs with strong antifungal and antioomycete activity and it is depended on the GacS/GacA two-component regulatory system. Further studies are needed to explore the antimicrobial volatile power of this isolate under natural conditions e.g. in greenhouse and field experiments.
Supporting information
Locus BV82_3318 was identified as gacA gene. Place of the homologous recombination is indicated by the “X” sign between the plasmid and the sequence, black arrows shows the places of primers hybridization (primers sequences are listed in S1 Table).
(DOCX)
(DOCX)
Change of color from white to blue indicates the production of the hydrogen cyanide.
(DOCX)
(DOCX)
Acknowledgments
This study was funded by the Polish National Science Centre research grant no. DEC-2012/07/B/NZ9/01623 and the Netherlands Organization for Scientific Research (NWO), VIDI personal grant (864.11.015). We thank Saskia Gerards and Hans Zweers (Netherlands Institute of Ecology) for technical assistance. This is publication 6259 of the NIOO-KNAW.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Support was provided by Polish National Science Centre research grant no. DEC-2012/07/B/NZ9/01623 and the Netherlands Organization for Scientific Research (NWO), (864.11.015).
References
- 1.Kai M, Haustein M, Molina F, Petri A, Scholz B, Piechulla B. Bacterial volatiles and their action potential. Appl Microbiol Biotechnol. 2009;81: 1001–1012. 10.1007/s00253-008-1760-3 [DOI] [PubMed] [Google Scholar]
- 2.Garbeva P, de Boer W. Inter-specific interactions between carbon-limited soil bacteria affect behavior and gene expression. Microb Ecol. 2009;58: 36–46. 10.1007/s00248-009-9502-3 [DOI] [PubMed] [Google Scholar]
- 3.Audrain B, Létoffé S, Ghigo J-M. Airborne bacterial interactions: functions out of thin air? Front Microbiol. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Effmert U, Kalderás J, Warnke R, Piechulla B. Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol. 2012;38: 665–703. 10.1007/s10886-012-0135-5 [DOI] [PubMed] [Google Scholar]
- 5.Schmidt R, Cordovez V, de Boer W, Raaijmakers J, Garbeva P. Volatile affairs in microbial interactions. ISME J. 2015;9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryu C-M, Farag MA, Hu C-H, Reddy MS, Kloepper JW, Paré PW. Bacterial volatiles induce systemic resistance in Arabidopsis. PLANT Physiol. 2004;134: 1017–1026. 10.1104/pp.103.026583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farag MA, Zhang H, Ryu C-M. Dynamic chemical communication between plants and bacteria through airborne signals: induced resistance by bacterial volatiles. J Chem Ecol. 2013;39: 1007–1018. 10.1007/s10886-013-0317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blom D, Fabbri C, Eberl L, Weisskopf L. Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl Environ Microbiol. 2011;77: 1000–1008. 10.1128/AEM.01968-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garbeva P, Hordijk C, Gerards S, de Boer W. Volatiles produced by the mycophagous soil bacterium Collimonas. FEMS Microbiol Ecol. 2014;87: 639–649. 10.1111/1574-6941.12252 [DOI] [PubMed] [Google Scholar]
- 10.Kim K, Lee S, Ryu C-M. Interspecific bacterial sensing through airborne signals modulates locomotion and drug resistance. Nat Commun. 2013;4: 1809 10.1038/ncomms2789 [DOI] [PubMed] [Google Scholar]
- 11.Zou C-S, Mo M-H, Gu Y-Q, Zhou J-P, Zhang K-Q. Possible contributions of volatile-producing bacteria to soil fungistasis. Soil Biol Biochem. 2007;39: 2371–2379. [Google Scholar]
- 12.Garbeva P, Hol WHG, Termorshuizen AJ, Kowalchuk GA, de Boer W. Fungistasis and general soil biostasis—A new synthesis. Soil Biol Biochem. 2011;43: 469–477. [Google Scholar]
- 13.Haas D, Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol. 2005;3: 307–319. 10.1038/nrmicro1129 [DOI] [PubMed] [Google Scholar]
- 14.Weller DM. Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology. 2007;97: 250–256. 10.1094/PHYTO-97-2-0250 [DOI] [PubMed] [Google Scholar]
- 15.Gross H, Loper JE. Genomics of secondary metabolite production by Pseudomonas spp. Nat Prod Rep. 2009;26: 1408–1446. 10.1039/b817075b [DOI] [PubMed] [Google Scholar]
- 16.Pierson LS, Pierson EA, Pierson EA. Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes. Appl Microbiol Biotechnol. 2010;86: 1659–1670. 10.1007/s00253-010-2509-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M. Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev. 2010; 34: 1037–1062 10.1111/j.1574-6976.2010.00221.x [DOI] [PubMed] [Google Scholar]
- 18.Krzyżanowska DM, Ossowicki A, Rajewska M, Maciąg T, Jabłońska M, Obuchowski M, et al. When genome-based approach meets the “old but good”: revealing genes involved in the antibacterial activity of Pseudomonas sp. P482 against soft rot pathogens. Front Microbiol. 2016;7: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dandurishvili N, Toklikishvili N, Ovadis M, Eliashvili P, Giorgobiani N, Keshelava R, et al. Broad-range antagonistic rhizobacteria Pseudomonas fluorescens and Serratia plymuthica suppress Agrobacterium crown gall tumours on tomato plants. J Appl Microbiol. 2011;110: 341–352. 10.1111/j.1365-2672.2010.04891.x [DOI] [PubMed] [Google Scholar]
- 20.De Vrieze M, Pandey P, Bucheli TD, Varadarajan AR, Ahrens CH, Weisskopf L, et al. Volatile organic compounds from native potato-associated Pseudomonas as potential anti-oomycete agents. Front Microbiol. 2015;6: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunziker L, Bönisch D, Groenhagen U, Bailly A, Schulz S, Weisskopf L. Pseudomonas strains naturally associated with potato plants produce volatiles with high potential for inhibition of Phytophthora infestans. Appl Environ Microbiol. 2015;81: 821–830. 10.1128/AEM.02999-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumer C, Haas D. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch Microbiol. 2000;173: 170–177. [DOI] [PubMed] [Google Scholar]
- 23.Sonnleitner E, Haas D. Small RNAs as regulators of primary and secondary metabolism in Pseudomonas species. Applied Microbiology and Biotechnology. 2011. pp. 63–79. [DOI] [PubMed] [Google Scholar]
- 24.Heeb S, Haas D. Regulatory roles of the GacS / GacA two-component system in plant-associated and other Gram-negative bacteria. Mol Plant-Microbe Interact. 2001;14: 1351–1363. 10.1094/MPMI.2001.14.12.1351 [DOI] [PubMed] [Google Scholar]
- 25.Laville J, Voisard C, Keel C, Maurhofer M, Défago G, Haas D. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc Natl Acad Sci U S A. 1992;89: 1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, et al. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol. 1997;24: 309–319. [DOI] [PubMed] [Google Scholar]
- 27.Cheng X, de Bruijn I, van der Voort M, Loper JE, Raaijmakers JM. The Gac regulon of Pseudomonas fluorescens SBW25. Environ Microbiol Rep. 2013;5: 608–619. 10.1111/1758-2229.12061 [DOI] [PubMed] [Google Scholar]
- 28.Gao J., Xie G., Peng F., Xie Z, Pseudomonas donghuensis sp. nov., exhibiting high-yields of siderophore. Antonie Van Leeuwenhoek. 2015, 7: 83–94. [DOI] [PubMed] [Google Scholar]
- 29.Krzyzanowska DM, Potrykus M, Golanowska M, Polonis K, Gwizdek-Wisniewska A, Lojkowska E, et al. Rhizosphere bacteria as potential biocontrol agents against soft rot caused by various Pectobacterium and Dickeya spp. strains. J Plant Pathol. 2012;94: 367–378. [Google Scholar]
- 30.Krzyzanowska DM, Ossowicki A, Jafra S. Genome Sequence of Pseudomonas sp. Strain P482, a tomato rhizosphere isolate with broad-spectrum antimicrobial activity. Genome Announc. 2014;2: 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiddaman PJ, Rossall S. The production of antifungal volatiles by Bacillus subtilis. J Appl Bacteriol. Blackwell Publishing Ltd; 1993;74: 119–126. [DOI] [PubMed] [Google Scholar]
- 32.Thoma S, Schobert M. An improved Escherichia coli donor strain for diparental mating. FEMS Microbiol Lett. 2009;294: 127–132. [DOI] [PubMed] [Google Scholar]
- 33.Techniques for transformation of E. coli. in DNA cloning: a practical approach. Edited by: Glover DM and Hames BD. IRL Press, Oxford, United Kingdom; 1985. [Google Scholar]
- 34.Winsor GL, Griffiths EJ, Lo R, Dhillon BK, Shay JA, Brinkman FSL. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 2016;44: D646–653. 10.1093/nar/gkv1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexeyev MF. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. Biotechniques. 1999;26: 824–6, 828 Available: http://www.ncbi.nlm.nih.gov/pubmed/10337469 [DOI] [PubMed] [Google Scholar]
- 36.Castric KF, Castric P a. Method for rapid detection of cyanogenic bacteria. Appl Environ Microbiol. 1983;45: 701–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katajamaa M, Miettinen J, Oresic M. MZmine: toolbox for processing and visualization of mass spectrometry based molecular profile data. Bioinformatics. 2006;22: 634–636. 10.1093/bioinformatics/btk039 [DOI] [PubMed] [Google Scholar]
- 38.Xia J, Sinelnikov I V., Han B, Wishart DS. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 2015;43: W251–W257. 10.1093/nar/gkv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kai M, Vespermann A, Piechulla B. The growth of fungi and Arabidopsis thaliana is influenced by bacterial volatiles. Plant Signal Behav. 2008;3: 482–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weise T, Kai M, Piechulla B. Bacterial ammonia causes significant plant growth inhibition. PLoS One. Public Library of Science; 2013;8: e63538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramette A, Moe Y. Prevalence of Fuorescent pseudomonads producing antifungal phloroglucinols and/or hydrogen cyanide in soils naturally suppressive or conducive to tobacco black root rot’. FEMS Microbiol Ecol. 2003;44: 35–43. 10.1111/j.1574-6941.2003.tb01088.x [DOI] [PubMed] [Google Scholar]
- 42.Lanteigne C, Gadkar VJ, Novinscak A, Filion M. Production of DAPG and HCN by Pseudomonas sp. LBUM300 Contributes to the Biological Control of Bacterial Canker of Tomato. Phytopathol. 2012;102: 967–973. [DOI] [PubMed] [Google Scholar]
- 43.Pessi G, Haas D. Transcriptional control of the hydrogen cyanide biosynthetic genes hcnABC by the anaerobic regulator ANR and the quorum-sensing regulators LasR and RhlR in Pseudomonas aeruginosa. J Bacteriol. 2000;182: 6940–6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blom D, Fabbri C, Connor EC, Schiestl FP, Klauser DR, Boller T, et al. Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ Microbiol. 2011; 13: 3047–3058. 10.1111/j.1462-2920.2011.02582.x [DOI] [PubMed] [Google Scholar]
- 45.Tyc O, Zweers H, de Boer W, Garbeva P. Volatiles in inter-specific bacterial interactions. Front Microbiol. 2015;6: 1412 10.3389/fmicb.2015.01412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garbeva P, Hordijk C, Gerards S, de Boer W. Volatile-mediated interactions between phylogenetically different soil bacteria. Front Microbiol. Frontiers; 2014;5: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Popova AA, Koksharova OA, Lipasova VA, Zaitseva J V, Katkova-Zhukotskaya OA, Eremina SI, et al. Inhibitory and toxic effects of volatiles emitted by strains of Pseudomonas and Serratia on growth and survival of selected microorganisms, Caenorhabditis elegans, and Drosophila melanogaster. Biomed Res Int. 2014;2014: 125704 10.1155/2014/125704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox SD, Markham JL. Susceptibility and intrinsic tolerance of Pseudomonas aeruginosa to selected plant volatile compounds. J Appl Microbiol. 2007;103: 930–936. 10.1111/j.1365-2672.2007.03353.x [DOI] [PubMed] [Google Scholar]
- 49.Longbottom CJ, Carson CF, Hammer KA, Mee BJ, Riley T V. Tolerance of Pseudomonas aeruginosa to Melaleuca alternifolia (tea tree) oil is associated with the outer membrane and energy-dependent cellular processes. J Antimicrob Chemother. Oxford University Press; 2004;54: 386–392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Locus BV82_3318 was identified as gacA gene. Place of the homologous recombination is indicated by the “X” sign between the plasmid and the sequence, black arrows shows the places of primers hybridization (primers sequences are listed in S1 Table).
(DOCX)
(DOCX)
Change of color from white to blue indicates the production of the hydrogen cyanide.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.