Abstract
A key component of both innate and adaptive immunity, new understandings of the complement system are expanding its roles beyond that traditionally appreciated. Evidence is accumulating that complement has an intracellular arsenal of components that provide not only immune defense, but also assist in key interactions for host cell functions. Although early work has primarily centered on T cells, the intracellular complement system likely functions in many if not most cells of the body. Some of these functions may trace their origins to the primitive complement system that began as a primeval form of C3 likely tasked for protection from intracellular pathogen invasion. This later expanded to include extracellular defense as C3 became a secreted protein to patrol the vasculature. Other components were added to the growing system including regulators to protect host cells from the indiscriminate effects of this potent system. Contemporary cells may retain some of these vestigial remnants. We now know that a) C3 serves as a damage-associated molecular pattern (in particular by coating pathogens that translocate into cells), b) most cells store C3 and recycle C3(H2O) for immediate use, and c) C3 assists in cellular survival and metabolic reprogramming. Other components also are part of this hidden arsenal including C5, properdin, factors H and B, and complement receptors. Importantly, better definition of the intracellular complement system may translate into new target discovery to assist in creating the next generation of complement therapeutics.
Keywords: complement, T cells, intracellular complement system, CD46, C3, C3(H2O), factor H, properdin, inflammasome
1.0 Introduction
The complement system traces its origins to more than a billion years ago when primitive proteins evolved to protect the cell from pathogens and to engage in intracellular metabolic processes. The complement system’s sophistication has grown enormously over the millennia yet has retained some of these ancient functions. An emerging concept is that the location of complement expression, in part, dictates its function [reviewed in (Kolev, Friec, et al., 2014) and (Hess and Kemper, 2016)]. Thus, the role of complement’s intracellular arsenal may be just as important as its better known actions in the circulation.
This review focuses on expanding our view of complement pointing to major new intracellular roles. In this inner universe of interactions, C3 (among other components) has several newly recognized roles. These include being a damage-associated molecular pattern (DAMP) that can enhance intracellular innate immunity (Tam, Bidgood, et al., 2014), a controller of cell survival (Liszewski, Kolev, et al., 2013) and a component of an extracellular/intracellular recycling pathway (Elvington, 2016). Further, autocrine activation of complement regulator CD46 (also known as membrane cofactor protein) via T-cell derived C3b plays a key role in nutrient uptake and enhances cellular metabolism essential for Th1 responses (Kolev, Dimeloe, et al., 2015). Additionally, C3 and factor H (FH) act as chaperones for the processing of apoptotic cargo (Baudino, Sardini, et al., 2014; Martin, Leffler, et al., 2016), while release of intracellular properdin and C3 stores in neutrophils may quickly instigate and enhance local complement activation against pathogens. More recently, intracellular C5 activation has been shown to be essential for NLRP3 inflammasome assembly in CD4+ T cells (Arbore, West, et al., 2016), suggesting a critical link between these two components of innate immunity that determines effector responses. Thus, the full importance and mechanisms of complement’s hidden intracellular arsenal continue to be elucidated. Other intracellular players including the C3a receptor (C3aR), the C5a receptor (C5aR), and factor B (FB) also are currently being evaluated (Hess and Kemper, 2016).
2.0 The Complement Pathways
Our traditional understanding of complement traces its origins to about 125 years ago when the system was identified as a relatively unstable and heat-labile lytic substance in blood (plasma and serum) that “complemented” antibodies in lysing bacteria.
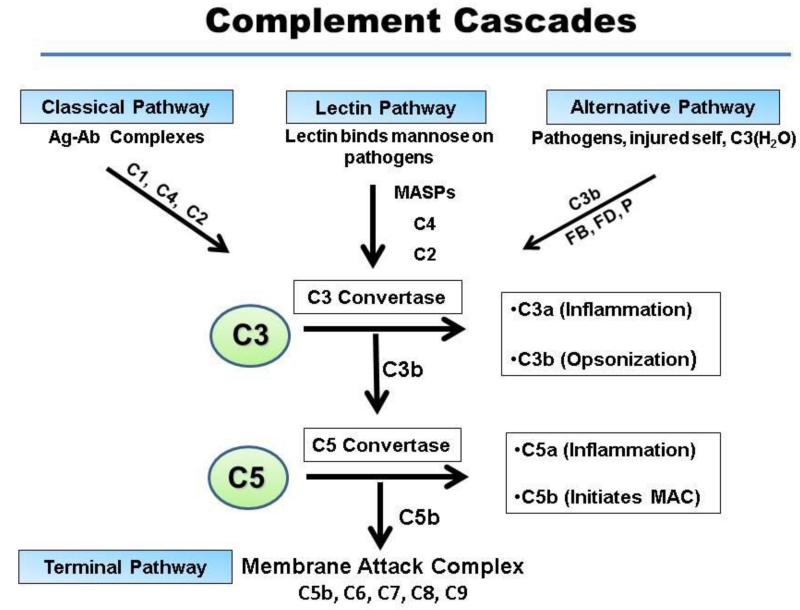
Since then, our knowledge of complement has grown immensely. We now know that the system consists of three major pathways of activation that ultimately result in the generation of a common lytic complex. Two target-specific initiating arms are the classical pathway (CP) and lectin pathway (LP) (Figure 1). A third arm, the alternative pathway (AP), is the most ancient and likely represents the original complement system. It engages robotically due to the continuous low-level turnover of one of its main components, C3. The AP has the potential to rapidly amplify in the presence of FB, factor D (FD) and properdin. Thus, C3 is the central component as all three activation pathways merge at the step of the generation of a C3 convertase (Ricklin, Hajishengallis, et al., 2010). Further, the enzymatic generation of C3b by each cascade commonly leads to the formation of a C5 convertase as well that generates C5b to initiate the terminal pathway of complement, appropriately named the membrane attack complex (MAC). The latter disrupts the integrity of the pathogen’s cell membrane, often resulting in lysis (Bubeck, 2014).
Figure 1.
Traditional extracellular roles of the complement cascades. The three pathways of complement activation are shown. Although each is triggered independently, yet they all merge at the step of C3 activation. The classical pathway (CP) is initiated by the binding of antibody to antigen and the lectin pathway (LP) by the binding of lectin to an oligosaccaride. The alternative pathway (AP) turns over continuously, generating small amounts of C3(H20) and C3. The AP rapidly amplifies in the presence of pathogens or injured tissue. C3(H20) behaves like C3b in that it can bind FB to initiate the AP. Activation of the complement system leads to inflammation (release of anaphylatoxins C3a and C5a), opsonization (the coating of targets with C3b and/or C4b), and membrane perturbation (formation of the membrane attack complex, MAC, C5b-C9).
The convertases generate (i) C3b that deposits on pathogens serves both as a ligand for receptors in addition to being a central player in the AP’s feedback loop and (ii) C3a and C5a that act as anaphylatoxins by engaging cells bearing their distinct receptors and (iii) C5b-C9, the MAC .
Strict control of this potent system is exerted in the fluid phase (plasma) and on healthy self-tissue at each of the major steps in the pathway: initiation or recognition, amplification leading to C3 and then C5 cleavage and formation of C5b-9. This is accomplished by regulatory proteins in plasma and on cells. About one-third of complement’s ~ 60 proteins function in its regulation (Zipfel and Skerka, 2009).
Pertinent to this review are the Regulators of Complement Activation (RCA) gene family whose proteins interact with and control C3 and C4 activation fragments [reviewed in (Kim and Song, 2006; Rodriguez de Cordoba and Goicoechea de Jorge, 2008; Zipfel and Skerka, 2009)]. This genetically-, structurally-, and functionally- related group is tightly clustered at 1q3.2 and includes the genes (in order) for membrane cofactor protein (MCP; CD46), complement receptors one (CR1; CD35) and two (CR2; CD21), decay accelerating factor (DAF; CD55), C4b-binding protein (C4BP), and FH and its family of like and related proteins.
As will be discussed, new insights reveal that some regulatory membrane-anchored proteins also are capable of intracellular signaling events that drive critical cellular functions. Further, recent studies have identified unexpected and novel roles for locally synthesized plasma proteins such as FH and properdin.
3.0 Evolution-driven multi-tasking of complement
Evolutionary theory suggests that nature “preserves” its most vital components by multi-tasking them. This certainly applies to the complement system. C3- and FB-like proteins evolved at least a billion years ago to comprise the earliest complement system (Nonaka and Kimura, 2006; Pinto, Melillo, et al., 2007). While the evolutionary evidence for such complement gene function is fragmentary, a primeval form of C3 has been identified in the phylum Porifera (sponges) (Al-Sharif, Sunyer, et al., 1998). Sponges are arguably the “sister group” of all extant animals and are representative of a small group of living fossils that are relatively simple multicellular organisms lacking true tissues or organs (Pisani, Pett, et al., 2015).
Thus, in our view, a primitive version of C3 likely comprised the first element of the original complement system and functioned intracellularly. We speculate that this intracellular C3 also engaged in key metabolic processes, some of which are retained in modern times.
With the advent of multi-cellularity and then a pumped circulatory system, C3 subsequently evolved its well-recognized role as a secreted protein, with liver cells serving as the specialized provider. C3 now became the “guardian of intravascular space” serving to enhance its powerful and protective role in plasma. While the focus of C3 for many years has been on this latter function, recent studies highlight that contemporary C3 has at least partially retained some of its more ancient roles inside the cell, on membranes and in the interstitial space.
C3 belongs to a gene family that also includes C4 and C5, which bear 26-30% identical amino acid sequence to C3 (Gros, Milder, et al., 2008). Gene duplication of C3 likely occurred at the time of jawed invertebrates (Nonaka and Kimura, 2006). Another ancient family linkage is that of the RCA members that underwent a rapid evolution in both the primary structure and the number of CCP domains, although orthologous relationships have been difficult to determine (Nonaka and Kimura, 2006).Thus, as the complement system evolved from its simple intracellular underpinnings, it became more complex and intertwined with innate and adaptive immune sytems. Deciphering and dissecting the evolution of this complex mix of ancient and modern functioning proteins may be problematic. However, further understanding of the roles of these intracellular complement players may provide an entirely new appreciation for their therapeutic targeting.
4.0 New Insights on the Roles of Intracellular C3
Recent studies have expanded our knowledge of C3 beyond that of the central component of the complement pathways that functions in the plasma and on host membranes. A new appreciation is emerging for other roles of C3, especially from the vantage point of inside the cell. For example, while systemic and local complement activation were thought to occur primarily extracellularly, new studies demonstrate that protease-driven activation of C3 inside CD4+ T cells results in CD46 and C3a-mediated signaling that regulates the induction of Th1 and Th17 responses (Hess and Kemper, 2016; Kolev, Dimeloe, et al., 2015; Liszewski, Kolev, et al., 2013). In another example, intracellular C3 proteins and CD46 crosstalk mediate nutrient uptake and sensing in human T cells in order to alter glycolysis and respiration, i.e., processes required for Th1 responses (Hess and Kemper, 2016; Kolev, Dimeloe, et al., 2015). Additionally, C3 plays a role in detecting and disabling intracellular pathogens (Tam, Bidgood, et al., 2014) and activated C3 serves as a chaperone for the intracellular processing of apoptotic cargo (Baudino, Sardini, et al., 2014). Finally, a new recycling pathway has been identified for intracellular uptake of C3 [in the form of C3(H2O)] and its partial return to the extracellular milieu (Elvington, 2016). Thus, these new and exciting findings provide a fresh appreciation of the many important roles for this versatile molecule.
4.1 C3-Mediated “Tagging” of Pathogens
Pathogens such as viruses and bacteria can evade the host’s immunological response as they invade the body’s extracellular fluids, and, in some cases, target host cells for intracellular replication. As an example, Listeria monocytogenes temporarily overcomes the host response by producing numerous virulence factors allowing it to evade the phagosome, replicate, hijack host actin filaments and spread between cells through protrusions of the host cell membrane (Calame, Mueller-Ortiz, et al., 2016). Among viruses, adenovirus and rhinovirus can lyse the endosome, while poliovirus and coxsackievirus form pores in the endosomal membrane (Tam, Bidgood, et al., 2014) to evade the host response.
Because of its sentry-like function, complement is always “on guard” in the intravascular and extracellular spaces. This is accomplished via low-grade, continuous engagement of the AP that quickly fires via its feedback loop in the setting of ‘danger’ to irreversibly tag infectious microbes with C3 fragments. While complement-mediated host defense begins in vertebrates by immune attack in body fluids, new studies have identified a nontraditional role in that complement continues defensive strategies inside the cell.
A recent study demonstrated that, despite attachment of complement fragments on pathogens, when opportunistic microbes evade host defenses and succeed in entering a cell, complement has another trick up its sleeve; that is, intracellular defenses aimed at sensing of complement-coated viruses and bacteria become activated and lead to amplification of intracellular immune responses (Tam, Bidgood, et al., 2014) (Figure 2). In an informative series of experiments, Tam et al. initially infected human embryonic kidney (HEK) 293T cells [carrying a nuclear factor-κB- (NF-κB) driven luciferase reporter] with adenovirus type 5 vector (AdV) previously treated with serum. AdV coated with C3 fragments (C3b and iC3b) on the viral membrane then entered these cells, which immediately engaged intracellular defense mechanisms via potent signaling pathways. Key observations included:
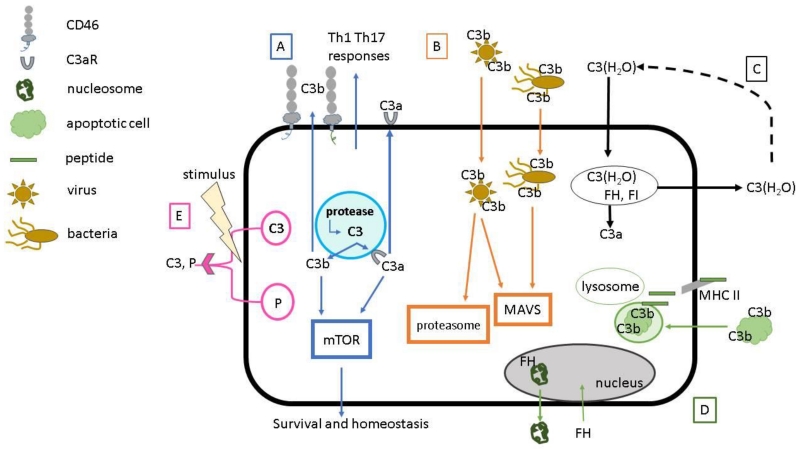
Figure 2.
Emerging roles of an intracellular complement system. This system has multiple mechanisms for cell defense, survival and homeostasis, most of which have been demonstrated to operate in many cell types. (A) Intracellular cleavage of C3 stores by a protease generates C3a and C3b (Kolev, Dimeloe, et al., 2015; Liszewski, Kolev, et al., 2013). In CD4+ T cells, this process is essential for induction of T cell effector function. Upon activation, the generated C3b regulates the necessary increase in metabolic processes through mTOR required for a Th1 response, while the C3a, together with the C3aR, are translocated to the cell surface. Additionally, tonic C3a generation is necessary for homeostatic T cell survival. (B) Extracellular opsonization of viruses and bacteria allows for, upon uptake by Ig and complement receptors, intracellular sensing and subsequent activation of mitochondrial antiviral signaling (MAVS) and proteasome mediated viral degradation (Tam, Bidgood, et al., 2014). (C) C3(H2O) is continuously taken up from blood and a majority returned to the cell exterior under steady-state conditions (Elvington, 2016). The C3(H2O) stores retained are a continuous source of intracellular C3a and other fragments. (D) Opsonization of apoptotic cells mediates their intracellular trafficking, delays fusion with the lysosome and regulates presentation of apoptotic cell-associated antigens on MHC II (Baudino, Sardini, et al., 2014). FH also associates with nucleosomes in apoptotic cells, resulting in an anti-inflammatory response (Martin, Leffler, et al., 2016). Not shown is that FH internalized by apoptotic cell facilitates enhanced cleavage of intracellular C3 and thereby apoptotic cell opsonization. (E) Release of intracellular stores of C3 and properdin (P) may be a mechanism to rapidly initiate local complement activation at the site of infection or injury (cell membrane or interstitial space) (Kouser, Abdul-Aziz, et al., 2013; Spitzer, Mitchell, et al., 2007). These five pathways likely interact. C3a and C3b, generated by each, may engage one or more signaling pathways involving Th1/Th17, mTOR, MAVS and nuclear machinery. P, properdin; FH, factor H; FI, factor I; MAVS, mitochondrial antiviral signaling.
Infected cells detected a variety of C3 fragment-tagged pathogens (including RNA and DNA non-enveloped viruses and cytosolic bacteria).
Cytoplasmic sensing of C3 activated the mitochondrial antiviral signaling (MAVS) pathways that lead to potent responses by NF- κB, activating protein 1 (AP-1) and interferon regulatory factors-3, -5, and -7 transcription pathways. As a result, their induction enhanced proinflammatory cytokine production such as interleukin-6, tumor necrosis factor, CCL4, IL-1β and interferon-β. These data suggested that intracellular C3 fragments serve as a DAMP to enhance intracellular innate immunity.
Tagging of the virus with C3 enabled a direct intracellular effector response to restrict viral infection via degradation (mediated by the AAA-adenosine triphosphatase valosin-containing protein and by the proteasome).
Viruses differed in their ability to defend against this innate sensing of C3 fragments and to evade intracellular complement immunity, either by inactivating complement components (e.g., C1) or by expressing different proteases that cleaved C3, thus antagonizing C3-mediated signaling.
Attesting to its evolutionary importance, intracellular immunity directed by C3 was shown to be conserved among mammals by using both serum as well as cell lines from different species such as humans, mice and cats.
In summary, this study showed that cells possess an evolutionarily conserved intracellular early warning system activated by complement-coated pathogens to provide protection against a diverse array of intracellular pathogens. This mechanism utilizes the activation of C3 as a DAMP to activate innate immunity.
4.2 Intracellular activation of C3
Although hepatocytes are the main source for the constitutive secretion of C3, other cells also secrete smaller amounts of C3 or can upregulate C3 secretion as part of the acute phase response (Sacks and Zhou, 2012). The local production of complement components, in particular C3, may play important roles for immune function including protecting the cell membrane and interstitial space (an alarm system to a pathogen’s presence) (Freeley, Kemper, et al., 2016).
An emerging paradigm suggests that immune cell-derived and intrinsically-acting complement activation fragments are important drivers, especially for modulating adaptive T cell immunity (Heeger and Kemper, 2012; Kolev, Le Friec, et al., 2013). Additionally, local production by immunocompetent cells helps shape adaptive immune responses (Carroll, 2004; Kerekes, Cooper, et al., 2001; Kerekes, Prechl, et al., 1998; Price, Schaumburg, et al., 2005). Further, C3b, produced early after activation of human CD4+ T cells by anti-CD3, rapidly increases after co-stimulation (Cardone, LeFriec, et al., 2010). These findings prompted a series of investigations demonstrating that activated CD4+ T cells generated C3a and C3b more rapidly than could be accounted for by de novo expression and indicated the presence of intracellular stores of C3 (Liszewski, Kolev, et al., 2013) (Figure 2). In these studies, cathepsin L partially colocalized with C3 and was the enzyme responsible for cleavage of C3 into C3a and C3b. Once generated intracellularly, the C3a engaged its cognate receptor (C3aR) and moved to the cell surface. C3b also was transported to the cell surface where it engaged CD46, suggesting an autocrine mechanism that polarizes naïve CD4+ T cells towards a Th1 phenotype upon activation.
In addition to its role for cleaving C3 intracellularly, CTSL also migrated to the cell surface where it mediated extracellular activation of C3. However, if incubated with increasing amounts of cell-permeable CTSL inhibitor, CD4+ T cells became apoptotic within 8-12 hours. Since mTOR (mammalian target of rapamycin) is a kinase that senses and integrates environmental signals to regulate metabolic activity in cells and plays a key role for cell survival, mTOR phosphorylation (Laplante and Sabatini, 2012) was also investigated. Surprisingly, inhibition of CTSL also reduced mTOR phosphorylation in resting as well as in activated CD4+ T cells. Additionally, knock down of C3aR expression with siRNAs in resting CD4+ T cells induced a comparable decrease in cell viability and mTOR activity. The addition of extracellular C3a did not rescue either the reduced mTOR phosphorylation or apoptosis. Together, these results indicate that intracellular C3a generation and C3aR activation create a C3a-C3aR axis that contributes to homeostatic control of mTOR activity and CD4+ T cell survival.
A question raised by these studies was why CD4+ T cells from C3-deficient patients (that do not produce IFN-γ) survive and proliferate normally (Ghannam, Pernollet, et al., 2008; Le Friec, Sheppard, et al., 2012). RT-PCR and other analyses of C3-deficient patient samples demonstrated the presence of C3 mRNA and normal amounts of intracellular C3a protein, respectively. The authors speculated that cells from serum C3-deficient patients do not secrete C3 or C3 fragments but by some mechanism can still generate sufficient intracellular C3a for cell survival (Liszewski, Kolev, et al., 2013).
On the opposite end of the spectrum, patients with juvenile idiopathic arthritis (JIA) have autoreactive effector T cells producing increased amounts of IFN-γ in their inflamed synovial tissue. They also have protein kinase B (PKB) hyperactivation, rendering the cells resistant to suppression by regulatory T cells (Wehrens, Mijnheer, et al., 2011). PKB activation is required for mTOR function (Verbist, Wang, et al., 2012). CD4+ T cells derived from the synovial fluid of a JIA patient demonstrated increased intracellular C3a, heightened mTOR activity and enhanced proinflammatory cytokine production (Liszewski, Kolev, et al., 2013). Further, ex-vivo CTSL inhibition in synovial fluid-derived CD4+ cells from patients with inflammatory arthritis (either JIA or rheumatoid arthritis) ‘normalized’ the pro-inflammatory pattern, suggesting the value of novel therapeutic approaches for modulating intracellular C3 activation (Liszewski, Kolev, et al., 2013).
While most of the studies in this report were directed to CD4+ T cells, intracellular C3 stores and “tonic” generation of intracellular C3a were identified in freshly isolated monocytes, neutrophils, CD8+ T cells, B cells, cultured epithelial cells, endothelial cells and fibroblasts (Liszewski, Kolev, et al., 2013). The apparent universal nature of such an intracellular C3-activating mechanism also suggests that it provides broad homeostatic functions for both immune and non-immune cells. In the healthy host, this mechanism likely contributes to intracellular host immunity and cell survival. However, dysregulation of this system could have important ramifications relating to other diseases ranging from autoimmunity to cancer.
Important questions and issues remaining include defining the cell-specific C3-activating proteases in different cell populations, characterizing how the system is regulated and dysregulated, identifying which C3 fragments (e.g., iC3b, C3dg, or novel fragments) may be involved in the system, and extending this knowledge to a new dimension of therapeutics relative to intracellular complement activation.
4.3 Complement and intracellular metabolism
Emerging evidence has linked crosstalk between innate and adaptive immunity with homeostasis of cellular metabolism and, when altered, to metabolic disorders such as obesity, diabetes mellitus and fatty liver disease [reviewed in (Phieler, Garcia-Martin, et al., 2013)]. In these disorders, metabolic products such as insulin, free fatty acids and excess glucose promote complement activation, resulting in increased proinflammatory derivatives (C3a, C3a-desArg and C5a), which can upregulate triglyceride synthesis and free fatty acid uptake, and polarize macrophages to a proinflammatory phenotype. Additionally, adipocytes themselves secrete proinflammatory factors such as Factor D (Choy, Rosen, et al., 1992), setting up a vicious cycle that suggests the complement system plays an important role in influencing the physiology and pathology of metabolically-active organs such as the pancreas, adipose tissue, and the liver (Phieler, Garcia-Martin, et al., 2013).
A recent study demonstrated the complement system also plays a vital role as an intracellular metabolic rheostat in human CD4+ T cells (Kolev, Dimeloe, et al., 2015); i.e., activation of T cells resulted in increased amino acid uptake, glycolytic flux and oxidative phosphorylation. Given that mTOR had previously been shown as an important link between intracellular C3a generation and CD4+ T cell survival, mTOR signaling was also examined (Figure 2). mTOR signals via two distinct multi-molecular complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (Laplante and Sabatini, 2012). It is well known that mTORC1 activity induces enzymes needed for glycolysis and for CD4+ T cells and is required for normal Th1 and Th17 cell induction (Pollizzi and Powell, 2014). Kolev et al demonstrated that, in activated CD4+ T cells, CD46 was necessary for mTORC1 activity. Additionally, they showed that its engagement by intracellular generated C3b stimulated and sustained mTORC1 activation and thus regulated cellular metabolic processes such as glycolysis and oxidative phosphorylation (Kolev, Dimeloe, et al., 2015). Along with their previous findings, these data suggested that, in addition to a C3a-C3aR axis that contributes to the homeostatic control of mTOR activity, an autocrine “C3b-CD46 axis” also acts as a link between complement and metabolic checkpoint indicators that modulate nutrient influx and metabolic reprogramming in human CD4+ T cells.
The story becomes even more interesting because human CD46 consists of not one, but rather a family of four primary isoforms that co-exist in distinct ratios on most cells. While most cell types in an individual tend to express the same ratio, cell-specific isoform expression patterns have been identified (Johnstone, Russell, et al., 1993; Liszewski, Kemper, et al., 2005; Post, Liszewski, et al., 1991). Each isoform shares the identical amino-terminal portion consisting of four CCPs that house the sites for ligand (C3b/C4b) interaction. This is followed by an alternatively spliced region for O-glycosylation, a juxtamembraneous 12 amino acid domain of unknown significance followed by a hydrophobic transmembrane region and a charged cytoplasmic anchor. The carboxyl-terminal tail is also alternatively spliced. It contains nonhomologous cytoplasmic tails of either 16 or 23 amino acids (CYT-1 and CYT-2, respectively) (Figure 2). Both CYT-1 and CYT-2 of CD46 bear signaling motifs that can be activated and CYT-2 has a putative nuclear localization signal (Freeley, Kemper, et al., 2016; Liszewski, Kemper, et al., 2005; Ni Choileain and Astier, 2012). However, the cellular roles played by each are still being elucidated.
Building on their findings showing that CD46 was necessary for sustaining mTORC1 activation, Kolev et al. elucidated the role of CD46 cytoplasmic tails in the so-called “C3b-CD46 metabolism axis” and provided key evidence for the important role played by CD46 cytoplasmic tail ‘switching’ to support CD4+ T cell responses (Kolev, Dimeloe, et al., 2015). Following TCR engagement, C3b increased on the surface of stimulated CD4+ T cells by one hour and served as a ligand for CD46, consistent with earlier studies (Cardone, LeFriec, et al., 2010; Liszewski, Kolev, et al., 2013). CD46 costimulation resulted in nuclear translocation of its cytoplasmic tail. Further, TCR activation increased expression of CD46 isoforms bearing CYT-1 that mediated upregulation of glucose transporter 1 (GLUT1; SLC2A1) and the L-type amino-acid transporter, LAT1. GLUT1 is important for cell growth, expansion and functionality, and the induction of LAT1 enhances leucine uptake. The latter stimulates mTORC1 activity via a sensor complex at the lysosomal surface (Boothby, Raybuck, et al., 2015). Subsequently, amino acid sensing via mTORC1 provides for CD4+ T cell maturation and IFN-γ production (i.e., a Th1 response). These phenotypic changes suggest that intracellularly generated complement proteins engage in crosstalk that may underlie an “intracellular stress detection system” and may reprogram intracellular metabolic pathways for a desired effector response (Kolev, Friec, et al., 2014). Specifically, these key interactions help explain how activated CD4+ T cells, which can exhibit a remarkable enhancement of metabolism, provide for their continuous supply of nutrients that are needed for effector responses. Aberrant activation of these intracellular metabolic pathways may potentiate tumor proliferation in non-immune cells, for example, via increased glycolysis (Warburg effect) and/or the persistent activation of the PI3K-Akt-mTOR pathway, among other mechanisms that need to be explored (Hess and Kemper, 2016).
Kolev et al also examined CD4+ T cells from CD46-deficient patients (Kolev, Dimeloe, et al., 2015). These CD4+ T cells cannot establish Th1 induction, suggesting that the C3b-CD46 crosstalk was necessary for Th1 induction [reviewed in (Yamamoto, Fara, et al., 2013)]. Predictably, their activated CD4+ T cells also failed to upregulate the molecular components of the metabolic program associated with Th1 induction, including enhanced glycolysis and oxidative phosphorylation. However, IFN-γ production could be re-established by retrovirus-mediated CD46 CYT-1 expression, confirming the role of the isoform bearing the CYT-1in Th1-specific responses.
Further, having examined the induction and effector phases following CYT-1 T cell activation, the Th1 cell contraction and induction of an IL-10 response phase was assessed (Kolev, Dimeloe, et al., 2015). Consistent with the other findings, CD46 isoform expression correlated with the Th1 life cycle changes during contraction. That is, as CD4+ T cells switched from a IFN-γ producing Th1 phenotype towards an IL-10 producing T-regulatory cell phenotype, CD46 reverted to CYT-2 dominance producing a reduction of GLUT1 and LAT1 as well as downregulation of oxidative phosphorylation and glycolysis. These studies indicate that CD46 is a key sensor in a metabolic network that responds to variable nutrient environments, affecting human CD4+ T cell phenotype and function. A role for these mechanisms in other cell types requires further study.
To understand the mechanism by which CD46 activation affects these metabolic pathways, King et al. activated human CD4+ T cells using antibodies to CD3 and CD46. Employing microarray analysis of activated human CD4+ T cells, they demonstrated that miR-150 was preferentially downregulated after CD3/CD46 costimulation, especially compared to CD3/CD28 costimulation. They found that this downregulation was critical for enhanced upregulation of GLUT1 expression and the relative proportion of cytokine (IFN- γ and IL-10) secretion from human CD4+ T cells (King, Esguerra, et al., 2016). The relative expression of miR-150 was significantly higher in IL-10 secreting human CD4+ T cells compared to IFN- γ secreting human CD4+ T cells. Thus, it appears that CD46 controls both Th1 activation and regulation via a miR-150-dependent mechanism. These important findings have broken new ground to further elaborate the emerging role of the intracellular complement system and its members not only in immune defense but also in homeostasis and metabolic reprogramming.
4.4 C3 and factor H as chaperones of intracellular apoptotic cargo
Similar to the findings of Tam et al. where C3 fragment ‘tagging’ of pathogens activated a MAVS response leading to restriction of viral replication and induction of proinflammatory cytokines (Tam, Bidgood, et al., 2014), another report demonstrated that C3 activation fragments deposited on apoptotic cells to: a) elicit engulfment by antigen presenting cells, b) mediate lysosomal fusion and processing of cell debris and c) regulate antigen presentation (Baudino, Sardini, et al., 2014) (Figure 2).
Although similar phagocytic mechanisms mediate uptake of a pathogen, subsequent cellular responses may differ dramatically for phagocytosis of apoptotic cells. For example, Baudino et al. explored the role of C3 in the handling of dying cells (Baudino, Sardini, et al., 2014). In particular, they investigated intracellular mechanisms that become engaged following extracellular C3 opsonization of apoptotic cells. Their hypothesis was that, since C3 deficiency does not commonly predispose to autoimmunity unlike deficiency of other early complement components such as C1q and C4, C3 likely has a modifying role in disease pathogenesis.
Using a murine model, they investigated the role of C3 in homologous T-cell responses to apoptotic cell-associated antigens (Baudino, Sardini, et al., 2014). They compared phagosome maturation and MHC class II presentation of peptides in either C3-deficient or C3-sufficient cells. Consistent with the hypothesis that C3 deficiency leads to impaired antigen-specific T cell proliferation, they determined that lack of C3 opsonization in C3-deficient mice accelerated phagosome maturation by enhancing the fusion of the apoptotic cargo with lysosomes in bone marrow-derived dendritic cells. This was also associated with a reduction in MHC Class II presentation of apoptotic-cell associated antigens, which resulted in impaired antigen-specific T-cell proliferation in vitro and in vivo. Also, pre-opsonizing the apoptotic cells with C3 activation fragments restored the trafficking and reversed the T-cell stimulation defects. These findings indicate that opsonization with C3 activation fragments can ‘chaperone’ the processing of an apoptotic cargo to delay phagosome maturation and prolong intracellular compartment retention. This process facilitates peptide loading on MHC class II molecules and thereby serves to modulate T cell responses to self-antigens derived from apoptotic cells.
Another complement player involved in intracellular activation during apoptosis is the regulator FH (Martin, Leffler, et al., 2016) (Figure 2). Apoptotic cells bind FH while simultaneously initiating complement activation via an interaction with a C1 complex to ensure efficient opsonization and disposal of coated debris, processes crucial for preventing excessive complement activation, autoimmunity and inflammation (Botto and Walport, 2002; Trouw, Bengtsson, et al., 2007). Dysregulation of this protective mechanism may contribute to a variety of diseases. Martin et al sought to examine FH-apoptotic cell interactions in a model of systemic lupus erythematosus (SLE) and age-related macular degeneration (Martin, Leffler, et al., 2016). They discovered that FH was actively and rapidly internalized by apoptotic cells, including retinal pigmented epithelial cells. Following internalization, FH enhanced CTSL-mediated cleavage of endogenous C3. They also determined that endogenous C3 was cleaved into the opsonin iC3b that became exposed on the cell surface upon apoptosis induction.
Further, the authors showed that the internalized FH coated intracellular nucleosomes during apoptosis. Nucleosomes are major autoantigens (especially for SLE patients) that are released into the cytosol during apoptosis and positively correlate with disease activity (Williams, Malone, et al., 2001). Unexpectedly, this coating enhanced the release of nucleosomes from apoptotic cells. However, it also facilitated uptake of nucleosomes by monocytes and induced an anti-inflammatory biased cytokine profile. These findings were supported by in vivo studies from CFH−/−MRL-lpr mice, which showed higher levels of circulating nucleosomes and necrotic cells as compared to their CFH+/+ littermates. The authors also demonstrated that FH facilitated phagocytosis of nucleosomes and regulated in vivo nucleosome and apoptotic cell clearance in the SLE MRL-lpr murine model.
4.5 Uptake of C3(H2O) into cells
Although the presence of intracellular C3 stores has been identified (Liszewski, Kolev, et al., 2013), its source and composition are being further evaluated (Elvington, 2016). It has been shown that many types of cells can ‘load’ C3 from the external environment (Elvington, 2016). Specifically, only the inactivated form of C3 [i.e., C3(H2O)] was internalized, not native C3. The loading was rapid, saturable, inhibited at 4°C, and sensitive to competition with unlabeled C3(H2O). Under steady state conditions, a substantial amount of the loaded C3(H2O) was released into the surrounding milieu. Further, a fraction of the C3(H2O) stores were cleaved to generate C3a. The mechanisms driving these events are under investigation. Thus, this study suggests that in addition to local synthesis and storage, another means for acquiring intracellular C3 stores is by uptake from the blood (Elvington, 2016). In both cases, the C3 may assist in the rapid response of the host to danger as well as for homeostatic responses (Kolev, Dimeloe, et al., 2015; Liszewski, Kolev, et al., 2013). Teasing out the specific roles and interactions of the endogenous and exogenously loaded C3 stores will be an important area of investigation. Under appropriate conditions, the C3 within cells can readily generate C3a and other fragments to provide a potent form of innate defense.
5.0 Other intracellular components
The full scope of complement’s hidden intracellular arsenal continues to be discovered and defined. For example, polymorphonuclear neutrophils (PMN) contain intracellular stores of properdin (P) as well as C3 as a mechanism for their quick release into the local environment (Kouser, Abdul-Aziz, et al., 2013).
More recently, intracellular C5 activation has been shown to be necessary for NLRP3 inflammasome assembly in human CD4+ T cells, and this assembly is modulated by the differential activation of C5aR1 versus the surface-expressed C5aR2 (Arbore, West, et al., 2016). Thus, more components of complement’s intracellular arsenal are being increasingly recognized both for an immediate as well as a sustained response. The intracellular role of other players such as the C3a receptor (C3aR), C4 and factor B is only now being evaluated (Hess and Kemper, 2016).
Using properdin as an example of pre-existing intracellular complement stores made locally available upon neutrophil activation, we envision the utility of its quick release especially as it relates to pathogen invasion. In this case, properdin, a positive regulator of complement activation, is needed to enhance AP response. The strongest evidence for properdin being required for AP activation in host defense is that deficiency of properdin is highly associated with Neisseria infections. Patients have not been shown to be susceptible to any other conditions. Additionally, the deposition of complement C3b is not sufficient to protect against this pathogen. The MAC must be generated. Presumably, local turnover of C3 to C3a and C3b (via the AP) and subsequent generation of C5 convertases (subsequently resulting in the generation of MAC) early on requires release of properdin by PMNs. These data suggest that at least for humans, the MAC and properdin (via the AP) are solely needed for the host to defend itself against this lethal pathogen of the bloodstream and meninges. The likely explanation is that to efficiently generate MAC on Neisseria (as the organism is dividing when its membrane is not wholly protected from a lytic agent), properdin is necessary (Agarwal, Ferreira, et al., 2010; Spitzer, Mitchell, et al., 2007).
A likely host defense strategy, then, is that PMNs quickly migrate (i.e., chemotax) to the site of infection or injury and immediately release C3 and properdin locally. Thereby, they could rapidly facilitate/trigger AP activation and inhibit an organism from entering the blood stream. Thus, intracellular stores may be key to preventing infections with Neisseria. Further, local synthesis and storage in a vesicle for release at a site of infection by a single cell type may be critical early on for initiating complement activation. This points to PMN and their ‘complement cargo,’ working together, to begin AP activation. We envision this as one mechanism whereby the earliest responders to a pathogen or a non-infectious, yet injurious, stimulus results in downstream pro-inflammatory sequelae.
Another scenario is that complement stores are released intracellularly to mediate critical processes involved in host defense. Two activities that one could envisage are release of C3a from stores derived either from native C3 or by the uptake of C3(H2O) [Figure 2]. A variety of proteases are capable of generating this anaphylatoxin. Further, the C3(H2O) can engage FB to form C3bB. FD or related protease would then be required to cleave FB into Bb and Ba. Properdin could also be released to stabilize the enzyme complex. This form of intracellular complement activation is largely theoretical, but at least C3a release from native C3 or C3(H2O) has been demonstrated in several cell types (Liszewski, Kolev, et al., 2013; Peake, O’Grady, et al., 1999). Additionally, the requirement of intracellular C5 activation for the assembly of the NLRP3 inflammasome in human CD4+ T cells further suggests that, in addition to directing immune cell phenotypes (such as Th1 polarization of CD4+ T cells by C3a-C3aR and CD46-C3b axes), intracellular complement activation may be playing a critical role in generating a sustained response that is altered in both infectious and non-infectious inflammatory diseases (Arbore, West, et al., 2016). That these proinflammatory pathways can be differentially modulated by receptors intrinsic to the complement pathway - specifically C5aR1 and C5aR2 in case of NLRP3 assembly - suggests that this area is ripe for investigation to identify novel therapeutic avenues for targeting inflammation (Morgan and Harris, 2015).
6 Conclusions
While we are only beginning to appreciate the exciting discovery of a seemingly ubiquitous and potent intracellular complement system arsenal, there are more questions than answers at present. In which cells does this intracellular system operate? What other pathways are engaged and for what reasons? What cell-specific mechanisms drive these events? How can this information be leveraged to create the next generation of complement therapeutics?
It is clear that new roles for intracellular complement will continue to expand beyond that of a simple mechanism for innate defense and an adjunct to immunity. In the future, we will better understand the other key roles for this ancient system including being a player in metabolic reprogramming and regulator of fundamental cellular processes. As each role is defined, new opportunities for therapeutic intervention will arise from the “inside out.
New studies have demonstrated major new intracellular roles for complement:
C3 is a damage-associated molecular pattern that can enhance intracellular innate immunity
C3 helps control cell survival
C3(H2O) is component of an extracellular/intracellular recycling pathway
Autocrine activation of complement regulator CD46 via T-cell derived C3b plays a key role in nutrient uptake and enhances cellular metabolism essential for Th1 responses
C3 and factor H act as chaperones for the processing of apoptotic cargo
Release of intracellular properdin and C3 stores in neutrophils may quickly instigate and enhance local complement activation against pathogens
Intracellular C5 activation has been shown to be essential for NLRP3 inflammasome assembly in CD4+ T cells suggesting a critical link between these two components of innate immunity that determines effector responses.
The full importance and mechanisms of complement’s hidden intracellular arsenal continue to be elucidated. Other intracellular players including the C3a receptor (C3aR), the C5a receptor (C5aR), and factor B (FB) also are currently being evaluated. These new roles also suggest new therapeutic approaches.
Acknowledgements
Support was provided by the National Institutes of Health (R01 GM0099111 and R01 AI041592 to J.P.A.), the National Institutes of Health Training in the Immunobiology of Rheumatic Disease (2T32 AR007279, to M.E.) and the National Institutes of Health Training Grant in the Principles of Pulmonary Research (5T32 HL007317, to H.S.K.). Research reported in this publication is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number P30AR048335 and the National Institutes of Health Grant for the Washington University Institute of Clinical and Translational Sciences (3UL1 TR000448).
Abbreviations
- CP
classical pathway
- LP
lectin pathway
- AP
alternative pathway
- MAC
membrane attack complex
- FB
factor B
- FH
factor H
- DAMP
damage-associated molecular pattern
- mTOR
mammalian target of rapamycin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have declared no conflicting financial interests exist.
References
- Agarwal S, Ferreira VP, Cortes C, Pangburn MK, Rice PA, Ram S. An evaluation of the role of properdin in alternative pathway activation on Neisseria meningitidis and Neisseria gonorrhoeae. Journal of immunology. 2010;185:507–516. doi: 10.4049/jimmunol.0903598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sharif WZ, Sunyer JO, Lambris JD, Smith LC. Sea urchin coelomocytes specifically express a homologue of the complement component C3. J.Immunol. 1998;160:2983–2997. [PubMed] [Google Scholar]
- Arbore G, West EE, Spolski R, Robertson AA, Klos A, Rheinheimer C, et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4(+) T cells. Science. 2016;352:aad1210. doi: 10.1126/science.aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudino L, Sardini A, Ruseva MM, Fossati-Jimack L, Cook HT, Scott D, et al. C3 opsonization regulates endocytic handling of apoptotic cells resulting in enhanced T-cell responses to cargo-derived antigens. Proc Natl Acad Sci U S A. 2014;111:1503–1508. doi: 10.1073/pnas.1316877111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby MR, Raybuck AL, Cho SH. Complementing T Cells’ Functions: Bringing in Metabolism Matters. Immunity. 2015;42:977–979. doi: 10.1016/j.immuni.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Botto M, Walport MJ. C1q, autoimmunity and apoptosis. Immunobiology. 2002;205:395–406. doi: 10.1078/0171-2985-00141. [DOI] [PubMed] [Google Scholar]
- Bubeck D. The making of a macromolecular machine: assembly of the membrane attack complex. Biochemistry. 2014;53:1908–1915. doi: 10.1021/bi500157z. [DOI] [PubMed] [Google Scholar]
- Calame DG, Mueller-Ortiz SL, Wetsel RA. Innate and adaptive immunologic functions of complement in the host response to Listeria monocytogenes infection. Immunobiology. 2016;221:1407–1417. doi: 10.1016/j.imbio.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone J, LeFriec G, Vantourout P, Roberts A, Fuchs A, Jackson I, et al. Complement regulator CD46 promotes immunoregulation by distinct effects on conventional and unconventional human T cells. Nature immunology. 2010;11:862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MC. The complement system in regulation of adaptive immunity. Nature immunology. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- Choy LN, Rosen BS, Spiegelman BM. Adipsin and an endogenous pathway of complement from adipose cells. J Biol Chem. 1992;267:12736–12741. [PubMed] [Google Scholar]
- Elvington M, Liszewski MK, Kulkarni HS, Atkinson JP. A C3(H2O) recycling and degradation pathway of the intracellular complement system. Immunobiology. 2016;221 [Google Scholar]
- Freeley S, Kemper C, Le Friec G. The “ins and outs” of complement-driven immune responses. Immunol Rev. 2016;274:16–32. doi: 10.1111/imr.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannam A, Pernollet M, Fauquert JL, Monnier N, Ponard D, Villiers MB, et al. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. Journal of immunology. 2008;181:5158–5166. doi: 10.4049/jimmunol.181.7.5158. [DOI] [PubMed] [Google Scholar]
- Gros P, Milder FJ, Janssen BJ. Complement driven by conformational changes. Nat Rev Immunol. 2008;8:48–58. doi: 10.1038/nri2231. [DOI] [PubMed] [Google Scholar]
- Heeger PS, Kemper C. Novel roles of complement in T effector cell regulation. Immunobiology. 2012;217:216–224. doi: 10.1016/j.imbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Hess C, Kemper C. Complement-Mediated Regulation of Metabolism and Basic Cellular Processes. Immunity. 2016;45:240–254. doi: 10.1016/j.immuni.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RW, Russell SM, Loveland BE, McKenzie IF. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Molecular immunology. 1993;30:1231–1241. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- Kerekes K, Cooper PD, Prechl J, Jozsi M, Bajtay Z, Erdei A. Adjuvant effect of gamma-inulin is mediated by C3 fragments deposited on antigen-presenting cells. J Leukoc Biol. 2001;69:69–74. [PubMed] [Google Scholar]
- Kerekes K, Prechl J, Bajtay Z, Jozsi M, Erdei A. A further link between innate and adaptive immunity: C3 deposition on antigen-presenting cells enhances the proliferation of antigen-specific T cells. Int Immunol. 1998;10:1923–1930. doi: 10.1093/intimm/10.12.1923. [DOI] [PubMed] [Google Scholar]
- Kim DD, Song WC. Membrane complement regulatory proteins. Clin Immunol. 2006;118:127–136. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- King BC, Esguerra JL, Golec E, Eliasson L, Kemper C, Blom AM. CD46 Activation Regulates miR-150-Mediated Control of GLUT1 Expression and Cytokine Secretion in Human CD4+ T Cells. J Immunol. 2016;196:1636–1645. doi: 10.4049/jimmunol.1500516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev M, Dimeloe S, Le Friec G, Navarini A, Arbore G, Povoleri GA, et al. Complement regulates nutrient influx and metabolic reprogramming during Th1 cell responses. Immunity. 2015;42:1033–1047. doi: 10.1016/j.immuni.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev M, Friec GL, Kemper C. Complement - tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–820. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- Kolev M, Le Friec G, Kemper C. The role of complement in CD4(+) T cell homeostasis and effector functions. Semin Immunol. 2013;25:12–19. doi: 10.1016/j.smim.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Kouser L, Abdul-Aziz M, Nayak A, Stover CM, Sim RB, Kishore U. Properdin and factor h: opposing players on the alternative complement pathway “see-saw”. Frontiers in immunology. 2013;4:93. doi: 10.3389/fimmu.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Friec G, Sheppard D, Whiteman P, Karsten CM, Shamoun SA, Laing A, et al. The CD46-Jagged1 interaction is critical for human TH1 immunity. Nat Immunol. 2012;13:1213–1221. doi: 10.1038/ni.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszewski MK, Kemper C, Price JD, Atkinson JP. Emerging roles and new functions of CD46. Springer Semin Immunopathol. 2005;27:345–358. doi: 10.1007/s00281-005-0002-3. [DOI] [PubMed] [Google Scholar]
- Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39:1143–1157. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Leffler J, Smolag KI, Mytych J, Bjork A, Chaves LD, et al. Factor H uptake regulates intracellular C3 activation during apoptosis and decreases the inflammatory potential of nucleosomes. Cell Death Differ. 2016;23:903–911. doi: 10.1038/cdd.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BP, Harris CL. Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discov. 2015;14:857–877. doi: 10.1038/nrd4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Choileain S, Astier AL. CD46 processing: a means of expression. Immunobiology. 2012;217:169–175. doi: 10.1016/j.imbio.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka M, Kimura A. Genomic view of the evolution of the complement system. Immunogenetics. 2006;58:701–713. doi: 10.1007/s00251-006-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake PW, O’Grady S, Pussell BA, Charlesworth JA. C3a is made by proximal tubular HK-2 cells and activates them via the C3a receptor. Kidney Int. 1999;56:1729–1736. doi: 10.1046/j.1523-1755.1999.00722.x. [DOI] [PubMed] [Google Scholar]
- Phieler J, Garcia-Martin R, Lambris JD, Chavakis T. The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol. 2013;25:47–53. doi: 10.1016/j.smim.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto MR, Melillo D, Giacomelli S, Sfyroera G, Lambris JD. Ancient origin of the complement system: emerging invertebrate models. Adv Exp Med Biol. 2007;598:372–388. doi: 10.1007/978-0-387-71767-8_26. [DOI] [PubMed] [Google Scholar]
- Pisani D, Pett W, Dohrmann M, Feuda R, Rota-Stabelli O, Philippe H, et al. Genomic data do not support comb jellies as the sister group to all other animals. Proc Natl Acad Sci U S A. 2015;112:15402–15407. doi: 10.1073/pnas.1518127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14:435–446. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post TW, Liszewski MK, Adams EM, Tedja I, Miller EA, Atkinson JP. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. Journal of Experimental Medicine. 1991;174:93–102. doi: 10.1084/jem.174.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JD, Schaumburg J, Sandin C, Atkinson JP, Lindahl G, Kemper C. Induction of a regulatory phenotype in human CD4+ T cells by streptococcal M protein. Journal of immunology. 2005;175:677–684. doi: 10.4049/jimmunol.175.2.677. [DOI] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature immunology. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Cordoba S, Goicoechea de Jorge E. Translational mini-review series on complement factor H: genetics and disease associations in human complement factor H. Clinical and experimental immunology. 2008;151:1–13. doi: 10.1111/j.1365-2249.2007.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nat Rev Immunol. 2012;12:431–442. doi: 10.1038/nri3225. [DOI] [PubMed] [Google Scholar]
- Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for De Novo convertase assembly. Journal of immunology. 2007;179:2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- Tam JC, Bidgood SR, McEwan WA, James LC. Intracellular sensing of complement C3 activates cell autonomous immunity. Science. 2014;345:1134. doi: 10.1126/science.1256070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouw LA, Bengtsson AA, Gelderman KA, Dahlback B, Sturfelt G, Blom AM. C4b-binding protein and factor H compensate for the loss of membrane-bound complement inhibitors to protect apoptotic cells against excessive complement attack. The Journal of biological chemistry. 2007;282:28540–28548. doi: 10.1074/jbc.M704354200. [DOI] [PubMed] [Google Scholar]
- Verbist KC, Wang R, Green DR. T cell metabolism and the immune response. Semin Immunol. 2012;24:399–404. doi: 10.1016/j.smim.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Wehrens EJ, Mijnheer G, Duurland CL, Klein M, Meerding J, van Loosdregt J, et al. Functional human regulatory T cells fail to control autoimmune inflammation due to PKB/c-akt hyperactivation in effector cells. Blood. 2011;118:3538–3548. doi: 10.1182/blood-2010-12-328187. [DOI] [PubMed] [Google Scholar]
- Williams RC, Jr., Malone CC, Meyers C, Decker P, Muller S. Detection of nucleosome particles in serum and plasma from patients with systemic lupus erythematosus using monoclonal antibody 4H7. J Rheumatol. 2001;28:81–94. [PubMed] [Google Scholar]
- Yamamoto H, Fara AF, Dasgupta P, Kemper C. CD46: the ‘multitasker’ of complement proteins. Int J Biochem Cell Biol. 2013;45:2808–2820. doi: 10.1016/j.biocel.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nature Reviews: Immunology. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]