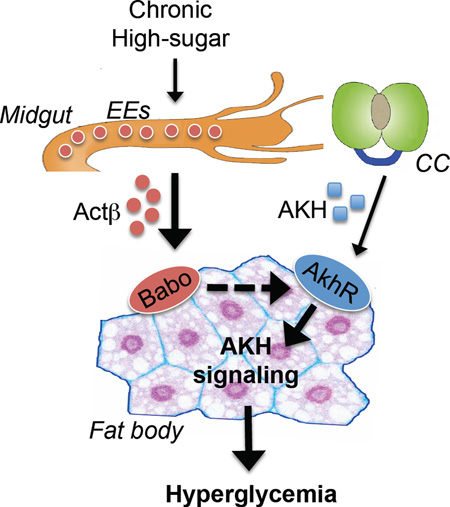
Abstract
While high-caloric diet impairs insulin response to cause hyperglycemia, whether and how counter-regulatory hormones are modulated by high-caloric diet is largely unknown. We find that enhanced response of Drosophila adipokinetic hormone (AKH, the glucagon homolog) in the fat body is essential for hyperglycemia associated with a chronic high-sugar diet. We show that the activin type I receptor Baboon (Babo) autonomously increases AKH signaling without affecting insulin signaling in the fat body via, at least, increase of Akh receptor (AkhR) expression. Further, we demonstrate that Activin-β (Actβ), an activin ligand predominantly produced in the enteroendocrine cells (EEs) of the midgut, is up-regulated by chronic high-sugar diet and signals through Babo to promote AKH action in the fat body, leading to hyperglycemia. Importantly, activin signaling in mouse primary hepatocytes also increases glucagon response and glucagon-induced glucose production, indicating a conserved role for activin in enhancing AKH/glucagon signaling and glycemic control.
Graphical Abstract
Introduction
In both vertebrates and invertebrates, Insulin and counter-regulatory hormones have evolved to maintain stable circulating carbohydrate levels in response to nutritional and environmental cues (Haselton and Fridell, 2010; Unger, 1971). Insulin promotes circulating carbohydrate clearance, while counter-regulatory hormones increase carbohydrate release into circulation. However, high-caloric diets can cause an imbalance between insulin and counter-regulatory hormone regulations resulting in elevated glycemic levels, a condition known as hyperglycemia (Buettner et al., 2007; Musselman et al., 2011). Much has been learned about how impaired insulin action or insulin resistance contributes to the carbohydrate metabolic dysregulation associated with hyperglycemia (Frojdo et al., 2009; McNelis and Olefsky, 2014). By contrast, very little is known about the role of counter-regulatory hormone regulation of glycemic control in caloric overload conditions.
Glucagon, an important insulin counter-regulatory hormone, is secreted from pancreatic α-cells during fasting or exercise and promotes glucose production in the liver via glucagon receptor signaling (i.e. GCGR/cAMP/PKA signaling) (Jiang and Zhang, 2003; Unger and Cherrington, 2012). Glucagon action in hepatocytes is abnormally increased in response to a high-caloric diet in obese or diabetic subjects, and contributes to glucose intolerance and hyperglycemia (Matsuda et al., 2002; Sheng et al., 2012). Importantly, inhibition of hepatic glucagon signaling using antisense oligonucleotides has been shown to improve glucose tolerance and to restore normal glycemic levels in diet-induced obese or diabetic mice (Liang et al., 2004). A similar effect of glucagon enhancement has also been reported in diabetic patients (Ali and Drucker, 2009). Previous studies have indicated that the inflammatory factor NIK and the ER stress sensor IRE1α are involved in hepatic glucagon signal transduction in obese mice under high-fat diet feeding (Mao et al., 2011; Sheng et al., 2012).
As a homolog of mammalian glucagon, Drosophila adipokinetic hormone (AKH) is a well-established insulin counter-regulatory hormone involved in carbohydrate metabolic homeostasis (Galikova et al., 2015; Kim and Rulifson, 2004; Lee and Park, 2004). Under starvation conditions, AKH is secreted from corpora cardiaca (CC) endocrine cells and promotes nutrient utilization in the fat body, leading to increased glycemic levels in circulation. The prohormone processing and secretion regulations of AKH and glucagon are similar. AKH also activates a G protein-coupled receptor, AkhR, and triggers cAMP/PKA signaling to regulate carbohydrate metabolism in the fat body, which is reminiscent of glucagon signaling (Bharucha et al., 2008; Gronke et al., 2007; Kim and Rulifson, 2004; Lee and Park, 2004; Rhea et al., 2010). Further, similar to the effects of high-fat diet in mammals, subjecting Drosophila to a chronic high-sugar diet has been shown to increase adiposity, impair insulin signaling in the fat body, and cause hyperglycemia in larvae (Musselman et al., 2011). Thus, we reasoned that investigating how AKH response is regulated by high-sugar diet would provide novel insights into how carbohydrate imbalance and hyperglycemia develop in response to excess caloric intake.
In this study, we uncover that fat body AKH action is abnormally enhanced in the context of a high-sugar diet, causing hyperglycemia. We further demonstrate that, in response to high-sugar feeding, midgut produces Actβ to increase AKH response in the fat body via activation of Babo/dSmad2 signaling and elevation of AkhR expression, thus leading to a hyperglycemic state.
Results
Regulation of carbohydrate metabolism by AKH
To characterize the role of AKH at the organismal level, we used RNAi to knockdown the Akh receptor (AkhR) in various metabolic tissues and measured circulating carbohydrate levels. Knockdown of AkhR in the larval fat body, but not in neurons, the midgut, or muscle, led to a significant reduction in circulating levels of both glucose and trehalose, a disaccharide form of glucose that is the major carbohydrate in the fly hemolymph (Fig. 1A–B and Fig. S1A–B, S1D–E). Knockdown of AkhR in the adult fat body also led to a significant reduction in glycemic levels, i.e. circulating glucose and trehalose (Fig. S3H). AkhR knockdown in the larval fat body dramatically decreased glucose/trehalose storage but increased glycogen storage (Fig. S1C), suggesting that AKH signaling in the fly fat body regulates circulating carbohydrate levels via both glycogen breakdown and glucose/trehalose production.
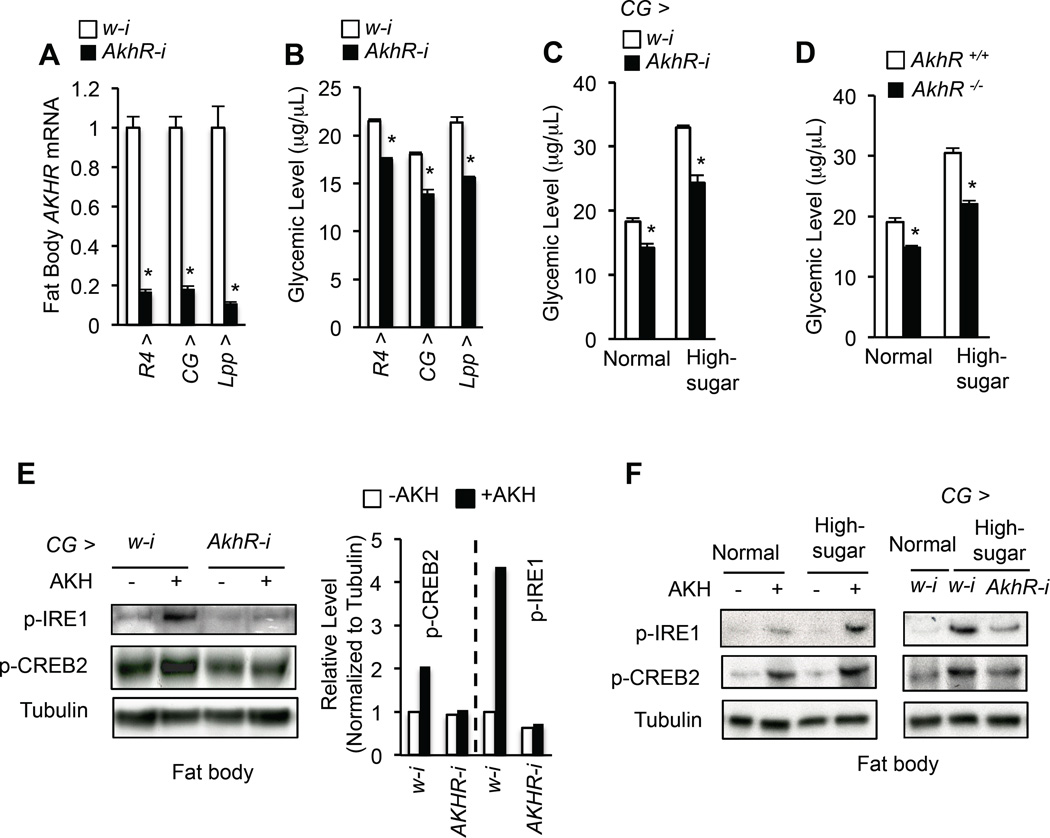
Figure 1. Enhanced AKH signaling contributes to hyperglycemia in response to a high-sugar diet.
(A, B) Fat body AkhR mRNA levels (A, n=4, 40 larval fat bodies) and glycemic levels (B, circulating glucose + trehalose, n=4, 40 larvae) in 3rd instar larvae expressing RNAi against w (white, control) or AkhR with different fat body Gal4 drivers. (C, D) Glycemic levels in CG > AkhR-i (CG-Gal4/+; UAS-AkhR-RNAi/+) (C) or AkhR −/− (D) 3rd instar larvae were fed with normal or high-sugar food (n=4, 40 larvae). (E) Immunoblots (left) and quantification (right) of p-IRE1 and p-CREB2 in freshly isolated 3rd instar larval fat bodies that were treated with or without 1 µM synthetic AKH peptide for 30 min. (F) Immunoblots of p-IRE1 and p-CREB2 in fresh isolated wild type 3rd instar larval fat bodies treated with or without 1 µM synthetic AKH peptide for 30 min (left) and in fresh isolated fat bodies of 3rd instar larvae fed with or without high-sugar diet (right). Data are presented as means ± SEM. * p < 0.05.
Mammalian studies have shown that glucagon activates PKA, leading to increased phosphorylation of IRE1α and CREB, CRTC2 nuclear translocation, and CREB transcriptional activity (Mao et al., 2011; Wang et al., 2009). To address whether the AKH signal is transduced in a similar manner in Drosophila, we generated a Drosophila S2R+ cultured cell line stably expressing AkhR. The addition of synthetic AKH to the culture media led to increased phosphorylation, not nuclease activity, of IRE1, the fly ortholog of IRE1α, in a dose- and PKA-dependent manner, and promoted nuclear translocation of Crtc, the fly ortholog of CRTC2 (Fig.S1F–G and S1J). Consistently, treatment with synthetic AKH of wild type larval fat bodies also led to an increase in phosphorylation of IRE1 and CREB2, the fly ortholog of CREB. Moreover, knockdown of AkhR potently blocked this AKH-induced increase in p-IRE1 and p-CREB2 levels (Fig. 1E, antibody that recognizes p-CREB2 in Fig. S1H). We also found that AKH signaling led to an increase in CREB2 transcriptional activity in the fat body (Fig. S1I). Altogether, our results indicate that AKH signaling in the fly fat body is analogous to glucagon signaling in the mammalian liver.
A chronic high-sugar diet promotes glycemic level by enhancing AKH action
An increase in AkhR expression in the larval fat body was previously shown to be associated with chronic high-sugar feeding (Musselman et al., 2013); however, whether AKH directly contributes to high-calorie diet-induced hyperglycemia is not known. To address this question, we fed larvae a chronic high-sugar diet to induce hyperglycemia and monitored AKH signaling. Compared to the larvae fed normal food, larvae fed a high-sugar diet showed an increase in both their response to synthetic AKH stimulation and homeostatic AKH signaling output in the fat body, as evidenced by increased p-IRE1 and p-CREB2 levels (Fig. 1F), indicating that a chronic high-sugar diet enhances larval fat body AKH response. Abolishing AKH signaling via disruption of AkhR, as achieved using either a null mutation of AkhR or fat body-specific RNAi, significantly alleviated both increased AKH signaling and hyperglycemia associated with high-sugar diet (Fig. 1C–D, 1F). These data indicate that high-sugar diet-induced hyperglycemia is attributable to increased AKH signaling in the fat body.
Impaired insulin signaling is known to elevate glycemic levels and contribute to high-sugar diet-induced hyperglycemia (Musselman et al., 2011). AkhR knockdown in the fat body is sufficient to restore normal glycemic levels in the context of InR deficiency, which dramatically impairs insulin signaling and leads to elevated glycemic levels (Fig. S1K–M). Thus, we next asked whether AKH signaling suppresses hyperglycemia by promotion of insulin signaling under a normal diet. However, knockdown of AkhR failed to affect Akt phosphorylation or FoxO target gene expression (4E-BP and InR) (Fig. S3E–F), two major readouts of insulin signaling (Owusu-Ansah et al., 2013; Song et al., 2010). Thus, fat body AKH signaling does not impinge on insulin signaling to impact glycemic control.
An AKH-induced hyperglycemia model in Drosophila
Ectopic Akh overexpression in the fat body using R4-Gal4 (R4 > Akh) results in hyperglycemia (Fig. 2A) (Lee and Park, 2004), suggesting that ectopic Akh expression activates AKH signaling in the fat body in an autocrine manner. Consistently, Akh overexpression leads to a dramatic increase in fat body AKH signaling (Fig. 2B). Further, fat body-specific knockdown of AKH/glucagon signaling components significantly affected AKH-induced hyperglycemia (Fig. 2C).
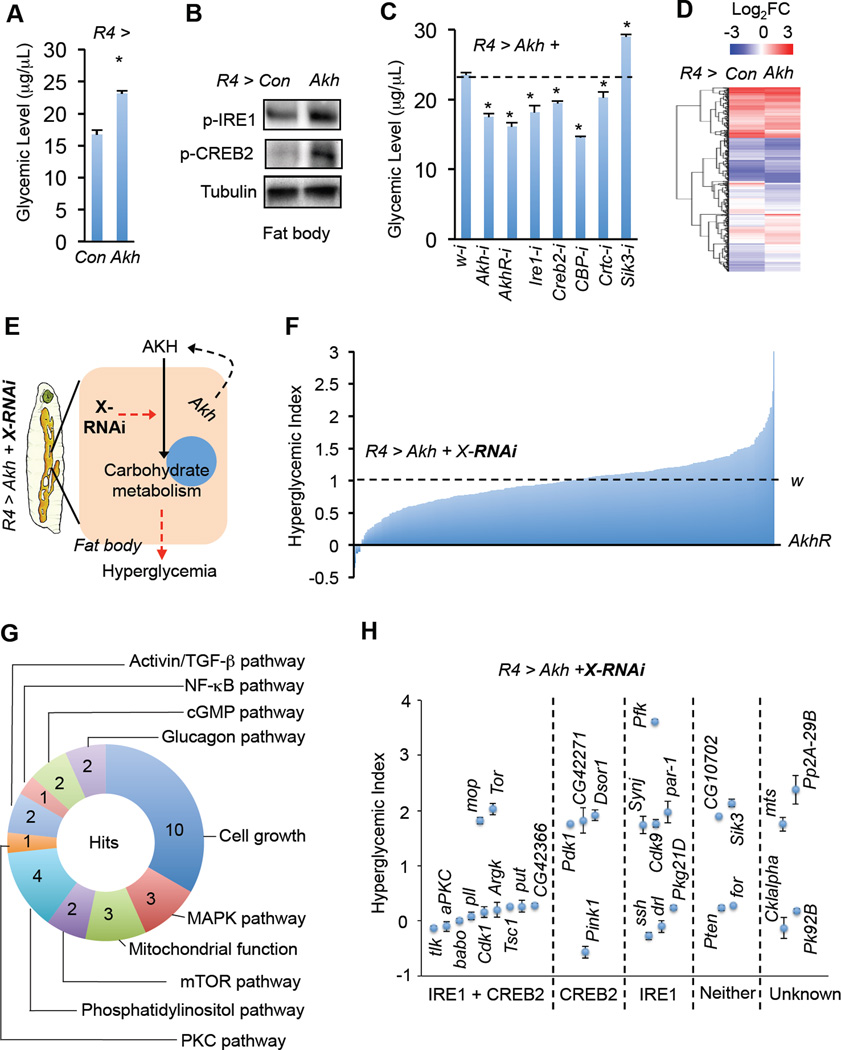
Figure 2. An AKH-induced hyperglycemia model and in vivo RNAi screen.
(A–D) Glycemic levels (glucose + trehalose) (A, n=4, 40 larvae), immunoblots (B), and gene expression (D) in fat bodies from R4 > AKH (UAS-Akh/+; R4-Gal4/+) 3rd instar larvae. (C) Glycemic levels of R4 > AKH larvae with knockdown of AKH/glucagon signaling components (n=4, 40 larvae). Schematic (E) and results (F) of the in vivo RNAi screen of kinases and phosphatases in AKH-induced hyperglycemic flies. Glycemic levels in R4 > Akh + w-i (UAS-Akh/+; R4-Gal4/UAS-w-RNAi) and R4 > Akh + AkhR-i (UAS-Akh/+; R4-Gal4/UAS-AkhR-RNAi) were normalized to 1 (100%) and 0, respectively (n=3, 30 larvae). (G) Annotation of novel positive gene ‘hits’ identified in the in vivo RNAi screen (> 1.7 or < 0.3 as cutoffs) for signaling pathways or biological processes. (H) 30 hits modulate AKH-induced hyperglycemia via regulation of p-IRE1 or p-CREB2. Data are presented as means ± SEM. * p < 0.05.
Little is known about the molecular mechanism by which AKH regulates carbohydrate metabolism. We thus analyzed the fat body transcriptome via RNA-seq and found that ectopic AKH expression led to significant changes in the expression of 702 genes in the fat body, with 364 down-regulated and 338 up-regulated (Fig. 2D and Table S1). Gene Ontology (GO) enrichment analysis of the differentially-expressed genes revealed a significant enrichment of genes involved in carbohydrate metabolism, including glycogen and sucrose metabolism, glycolysis and/or gluconeogenesis, and the TCA cycle (Fig. S2A). Genes involved in AKH-associated carbohydrate metabolism were further validated by qPCR and genetic analysis (Fig. S2C–D). Consistent with activation of CREB2, GO analysis revealed that CREB2 target genes were significantly enriched (Fig. S2B). Interestingly, the targets of other transcriptional factors than CREB2 were also enriched (Fig. S2B), suggesting potential cross-talk between AKH signaling and other pathways. Note that FoxO target genes were not significantly enriched, consistent with our observation that knockdown of AkhR failed to affect insulin/FoxO signaling (Fig. S3E–F).
In vivo RNAi screen for regulators of AKH signaling
The AKH-induced hyperglycemia model described above allowed us to perform an in vivo RNAi screen to discover physiological regulators of AKH signaling (Fig. 2E). Specifically, transgenic RNAi fly stocks targeting 305 kinases and phosphatases, many of which were previously validated (Sopko et al., 2014), were crossed to R4 > Akh flies and glycemic levels (glucose + trehalose) of their offspring were measured. We used glycemic levels as the major readout in this screen as we found it to be more reliable and inclusive than monitoring signaling (i.e. p-IRE1, p-CREB2, or CREB transcriptional activity; data not shown). The screen assay is robust, as knockdown of glucagon/AKH signaling components consistently affected glycemic levels (Fig. 2C). We normalized glycemic changes to both negative (w-i, 1, 100% hyperglycemia) and positive (AkhR-i, 0, 0% hyperglycemia) controls using the formula NG RNAi = (RG RNA-i – RG AkhR-i) / (RG w-i – RG AkhR-i) (NG, normalized glycemic levels; RG, raw glycemic levels) (Fig. 2F). A total of 30 candidate genes were identified that significantly affected AKH-induced hyperglycemia (>1.7 or <0.3, hyperglycemic index) (Table S2). These genes have been previously implicated in various biological processes. (Fig. 2G and Table S2). Among the candidates, the mammalian homologs of Pfk and Sik3 have previously been shown to be involved in glucagon signaling (Pilkis et al., 1982; Wang et al., 2011). Consistently, knockdown of Pfk and Sik3 in the Drosophila fat body significantly enhanced AKH-induced hyperglycemia (hyperglycemic index values of 3.6 and 2.1, respectively) (Table S2).
In addition to changes in glycemic levels, knockdown of a few genes also resulted in changes in larval fat body morphology, developmental delay, and/or pupae lethality. However, we did not observe a strong correlation between these effects and glycemic changes (Fig. S2E and Table S2). To further assess how these 30 genes regulate AKH action, AKH signaling outputs were measured and potential mechanisms annotated based on their differential effects on p-IRE1 or p-CREB2 levels (Fig. 2H and Fig. S2F–G).
We also performed a small secondary RNAi screen in wild type larvae and found that knockdown of these candidates consistently affect glycemic levels in the absence of AKH overexpression (Fig. S3A).
Babo regulates AKH response and carbohydrate metabolism
RNAi stocks targeting Babo and Punt (Put), which encode a type I and II TGF-β receptors, respectively, were among the reagents exerting the greatest suppression of AKH-induced hyperglycemia in the primary RNAi screen (Table S2 and Fig. 2H). In Drosophila there are three different type I TGF-β receptors (Thick-Vein (Tkv), Saxophone (Sax) and Babo) and two shared type II receptors (Put and Wishful-thinking (Wit)), which respond to different ligands and activate distinct downstream transcription factors (Brummel et al., 1999; Gesualdi and Haerry, 2007). Unlike our observation with knockdown of Babo, knockdown of the other two type I receptors, Tkv or Sax, failed to affect AKH-induced hyperglycemia (Fig. 3A). Consistently, only knockdown of dSmad2 (the transcription factor downstream of Babo), but not Mad (the transcription factor downstream of Tkv/Sax), significantly suppressed AKH-induced hyperglycemia (Fig. 3A).
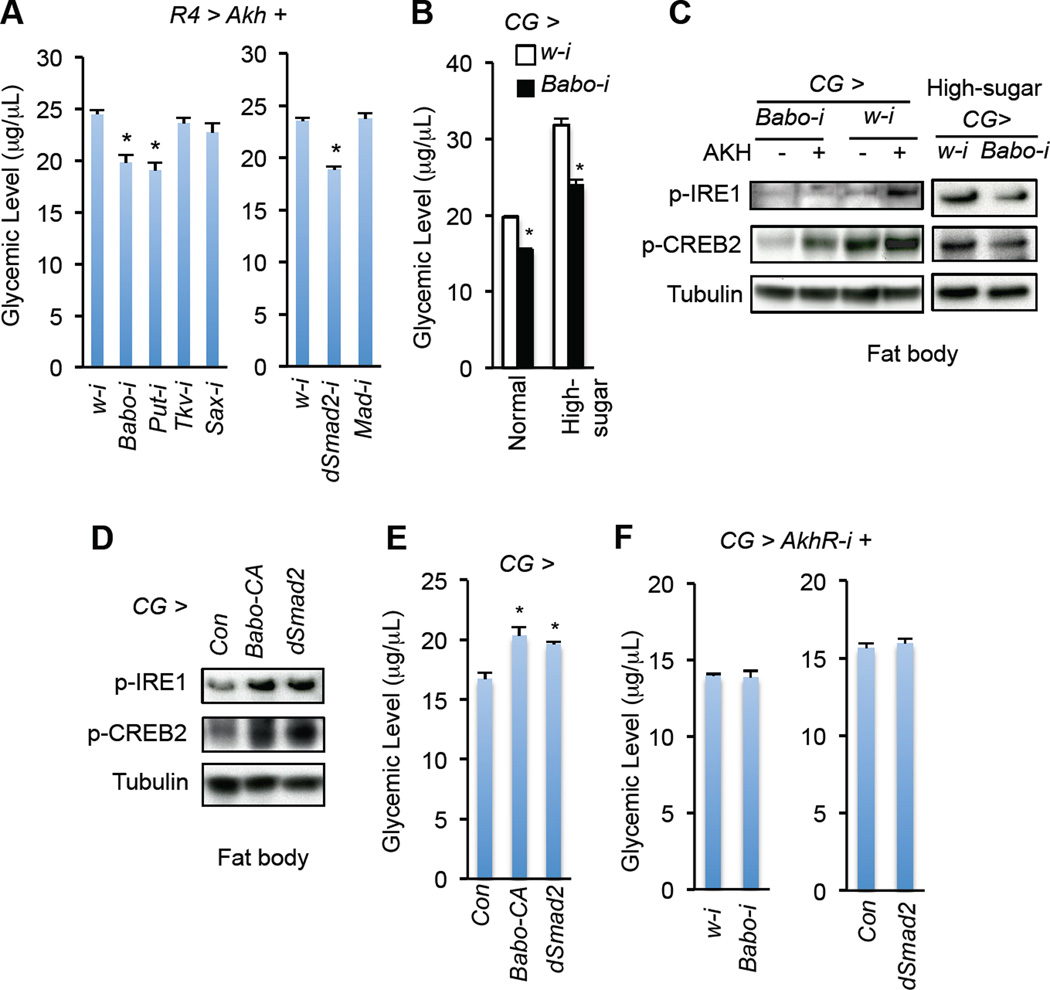
Figure 3. Babo signaling regulates AKH action and glycemic control.
(A) Effects of knockdown of individual TGF-β-like receptors (left) or downstream transcription factors (right) in the fat body on glycemic levels (glucose + trehalose) in R4 > Akh 3rd instar larvae (UAS-Akh/+; R4-Gal4/UAS-RNAi) (n=4, 40 larvae). (B) Glycemic levels in CG > Babo-i larvae (CG-Gal4/+; UAS-babo-RNAi/+) fed with normal or high-sugar food (n=4, 40 larvae). (C–D) Immunoblots of protein from freshly isolated larval fat bodies that were treated with or without 1 µM synthetic AKH peptide for 30 min (C, left) or freshly isolated fat bodies of 3rd instar larvae fed a high-sugar diet (C, right) or a normal diet (D). (E) Glycemic levels in Babo or dSmad2 overexpressing 3rd instar larvae (n=4, 40 larvae). (F) Glycemic levels in 3rd instar larvae bearing Babo knockdown (left, CG-Gal4/+; UAS-AkhR-RNAi/UAS-Babo-RNAi) or dSmad2 overexpression (right, CG-Gal4/+; UAS-AkhR-RNAi/UAS-dSmad2) in the context of AkhR deficiency in fat body (n=4, 40 larvae). Data are presented as means ± SEM. * p < 0.05.
To examine whether Babo signaling directly regulates AKH signaling, we treated larval fat bodies bearing Babo RNAi with synthetic AKH peptides. Interestingly, Babo RNAi potently blocked the AKH-induced increase in p-CREB2 and p-IRE1 (Fig. 3C). Suppression of Babo signaling using RNAi in the wild-type fat bodies significantly decreased glycemic levels under normal conditions (Fig. S3B). Babo RNAi further alleviated hyperglycemia and enhancement of AKH signaling in the fat body associated with high-sugar diet (Fig. 3B–C). Moreover, activation of Babo signaling via overexpression of either active Babo or dSmad2 in the fat body was sufficient to increase AKH signaling and glycemic levels in wild type larvae (Fig. 3D–E), indicating that Babo signaling directly enhances the fat body responses to AKH.
To determine whether the impact of Babo signaling on carbohydrate metabolism is dependent on AKH response, we manipulated Babo signaling in AkhR-deficient larval fat bodies. Interestingly, neither Babo knockdown nor dSmad2 overexpression affected glycemic levels when AkhR was knocked down in the larval fat body (Fig. 3F). Similar results were observed in adults (Fig. S3H). Taken together, our results suggest that Babo signaling regulates carbohydrate metabolism and glycemic levels via modulation of the fat body response to AKH.
Importantly, knockdown of Babo in the fat body failed to affect insulin signaling, as indicated by p-Akt level and expression of FoxO targets (4E-BP and InR), or dILP2-induced hypoglycemia (Fig. S3E–G). These results indicate that the effects of Babo signaling on glycemic control are independent of insulin/Akt/FoxO signaling in larval fat bodies.
Babo signaling regulates AkhR mRNA expression
AkhR is essential for AKH signaling and carbohydrate metabolic regulation, and AkhR mRNA levels correlate with chronic high-sugar diet-induced hyperglycemia (Musselman et al., 2013) (Fig. S3D). Therefore, we hypothesized that Babo affects AKH signaling and high-sugar-induced hyperglycemia via, at least, control of AkhR mRNA levels. Consistent with our hypothesis, reducing Babo signaling led to a dramatic reduction in fat body AkhR mRNA levels under both normal and high-sugar diet conditions (Fig. S3C–D). We further found that overexpression of dSmad2 in the fat body led to a significant increase in AkhR mRNA levels (Fig. S3D). As Babo/dSmad2 signaling regulates sugar metabolism in an AkhR-dependent manner (Fig. 3F), our results collectively indicate that Babo/dSmad2 signaling mediates fat body AKH response via AkhR transcriptional regulation.
Actβ/Babo signaling modulates AKH response in the fat body
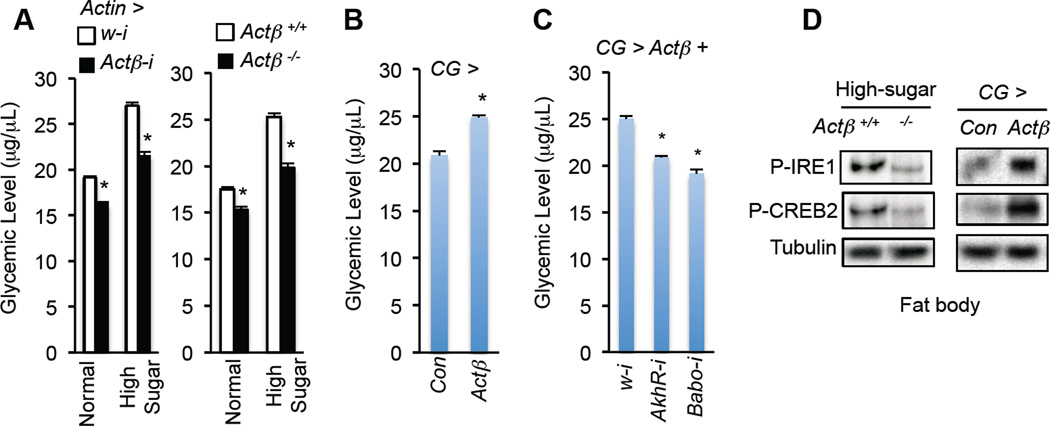
Babo expression is not elevated in the fat body in response to high-sugar diet (Fig. S4A). Thus, we examined the role of ligands that activate Babo in modulating AKH action. Two activin ligands, Actβ and Daw, regulate Babo/dSMAD2 signaling in Drosophila (Gesualdi and Haerry, 2007). To address whether one or both is involved in Babo-mediated AKH signaling, we first assessed the impact of Actβ on carbohydrate metabolism via a loss-of-function mutation (Actβ ed80) (Zhu et al., 2008) or ubiquitous knockdown (Actin > Actβ-i). In both conditions, we observed a decrease in glycemic levels (Fig. 4A and Fig. S4B–D). Reduction of Actβ expression led to a decrease in the fat body AKH response and hyperglycemia under high-sugar diet conditions (Fig. 4A, 4D). Conversely, ectopic expression of Actβ in the larval fat body was sufficient to increase fat body AKH signaling and glycemic levels (Fig. 4B, 4D and Fig. S4E–F). The increase in glycemic levels was potently diminished by knockdown of either Babo or AkhR expression in the fat body (Fig. 4C and Fig. S4G). These data indicate that Actβ is involved in Babo-mediated AKH response and carbohydrate metabolism in the fat body.
Figure 4. Actβ signals through Babo to affect AKH signaling in the fat body.
(A) Glycemic levels (glucose + trehalose) in 3rd instar larvae with systemic Actβ knockdown (left, Actin-Gal4/UAS-Actβ-RNAi) or knockout (right, actβ ed80 or Actβ −/−) fed a standard or high-sugar diet. (B, C) Glycemic levels in CG > Actβ (CG-Gal4/+; UAS-Actβ/+) larvae (B) or CG > Actβ larvae with gene knockdown (CG-Gal4, tub-Gal80ts/+; UAS-Actβ/UAS-RNAi) (C) (n=4, 40 larvae). (D) Immunoblots in freshly isolated fat bodies of Actβ−/− larvae fed a high-sugar diet (left) or CG > Actβ larvae fed normal food (right). Data are presented as means ± SEM. * p < 0.05.
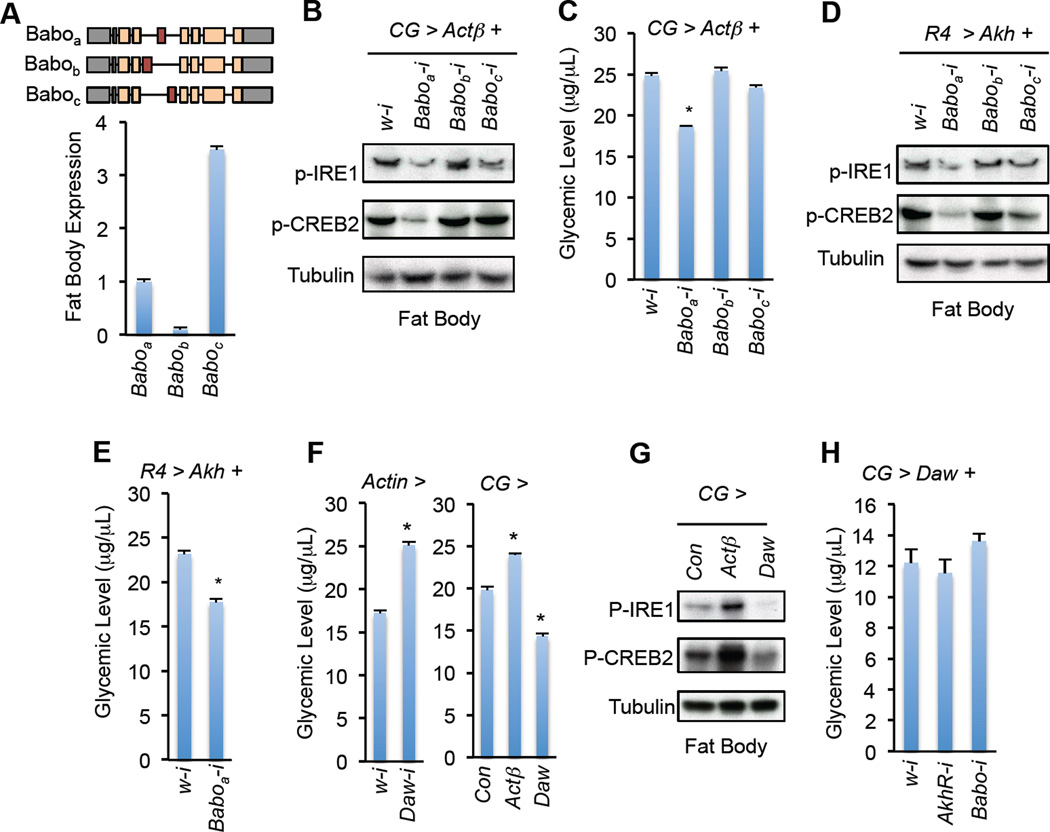
Different Babo isoforms respond to individual ligands and result in diverse physiological outputs (Gesualdi and Haerry, 2007; Jensen et al., 2009). Baboa and Baboc are highly expressed in the larval fat body as revealed by RNA-seq analysis (Fig. 5A). We determined which Babo isoform(s) is required for Actβ regulation of AKH action using RNAi targeting distinct isoforms (Awasaki et al., 2011). Strikingly, only knockdown of Baboa, but not Babob or Baboc, in the larval fat body significantly decreased Actβ-induced AKH signaling and hyperglycemia (Fig. 5B–C). Based on the similarity between Actβ and Baboa in neuronal development and growth regulation (Gesualdi and Haerry, 2007; Zhu et al., 2008), our results suggest that Actβ regulate carbohydrate metabolism in the fat body via Baboa. Moreover, knockdown of Baboa also significantly alleviated AKH signaling and hyperglycemia in the AKH-overexpression flies (Fig. 5D–E). Our results thus demonstrate that Actβ/Baboa signaling modulates AKH activity and glycemic control in Drosophila.
Figure 5. Baboa responds to Actβ regarding modulation of AKH action and glycemia.
(A) Schematic and relative FPKM value of different Babo isoforms indicated by RNA-seq of 3rd instar larval fat body (n=3, 30 larval fat bodies). (B, C) Fat body immunoblots (B) and glycemic levels (C) (n=4, 40 larvae) of CG > Actβ larvae with knockdown of Babo isoforms. Genotype is CG-Gal4, tub-Gal80ts /+; UAS-Actβ/UAS-RNAi. (D, E) Fat body immunoblots (D) and glycemic levels (E) (n=4, 40 larvae) of R4 > Akh larvae with knockdown of Babo isoforms. Genotype is UAS-Akh/+; R4-Gal4/UAS-RNAi. (F) Glycemic levels in Actin > Daw-i larvae (left, n=4, 40 larvae) and in larvae with fat body overexpression of Actβ or Daw (right, n=4, 40 larvae). (G) Immunoblots of fat body in larvae with fat body overexpression of Actβ or Daw. (H) Glycemic levels in 3rd instar larvae with fat body overexpression of Daw plus knockdown of AkhR or Babo (n=4, 40 larvae). The genotype is CG-Gal4, tub-Gal80ts/+; UAS-Daw/UAS-RNAi. Data are presented as means ± SEM. * p < 0.05.
Daw does not enhance AKH signaling in the fat body
The activin ligand Daw was previously shown to activate Baboc signaling and control glycemic levels (Ghosh and O’Connor, 2014; Jensen et al., 2009). Consistently, we observed that systemic knockdown of Daw decreased, whereas overexpression of Daw in the fat body increased, glycemic levels (Fig. 5F). However, unlike Actβ, Daw overexpression failed to enhance AKH signaling and AkhR- or Babo-loss in the fat body had little effect on Daw-associated glycemic changes (Fig. 5G–H). Thus, Daw is not likely to regulate glycemic levels by increasing AKH action in the fat body.
We further tested the role of Myo, another activin ligand, which is produced by muscles and functions via Babo/MAPK, but not Babo/dSmad2, signaling in the fat body (Demontis et al., 2014). Ectopic expression of Myo in the larval muscle or fat body failed to enhance AKH signaling (data not shown).
Activin signaling enhances glucagon action in mouse hepatocytes
As activin signaling pathways are highly conserved between Drosophila and mammals, we hypothesized that, as in Drosophila, mammalian activin signaling might also regulate liver glucose metabolism via modulation of glucagon response. We test this using primary cultured mouse hepatocytes. Similar to the increase in AkhR mRNA levels by Actβ in Drosophila, stimulation by Activin A led to a significant increase in expression of the glucagon receptor (GCGR) in primary hepatocytes (Fig. S5A). Activin A further significantly enhanced glucagon signaling, as indicated by increased p-CREB and p-IRE1α levels, as well as glucagon-induced glucose production in primary hepatocytes (Fig. S5B–C). Treatment with the glucagon receptor antagonist, Cpd1 (Qureshi et al., 2004), potently blocked the effects of Activin A on glucagon-induced glucose production (Fig. S5C), demonstrating a glucagon-dependent mechanism. We also noticed an increase in glucose production under Activin A treatment alone (Fig. S5C), suggesting potential mechanism(s) independent of glucagon as well.
Next, we examined whether inhibition of activin signaling attenuates hepatic glucagon response by treating with the inhibitor SB 431542 (SB) (Inman et al., 2002). SB led to a significant suppression of activin signaling, as indicated by p-SMAD3, and alleviated activin A-enhanced glucagon signaling and hepatic glucose production (Fig. S5B–C). Interestingly, similar results were observed for TGF-β1. Specifically, we observed that TGF-β1, but not Myostatin (data not shown), significantly increased GCGR expression, glucagon signaling, and glucagon-induced glucose production in primary mouse hepatocytes (Fig. S5D–F). Taken together, our results indicate that, in mammals, activin signaling regulates hepatic carbohydrate metabolism, at least, via modulation of the glucagon response (Fig. S5G).
Midgut-derived Actβ regulates fat body AKH action and glycemic levels
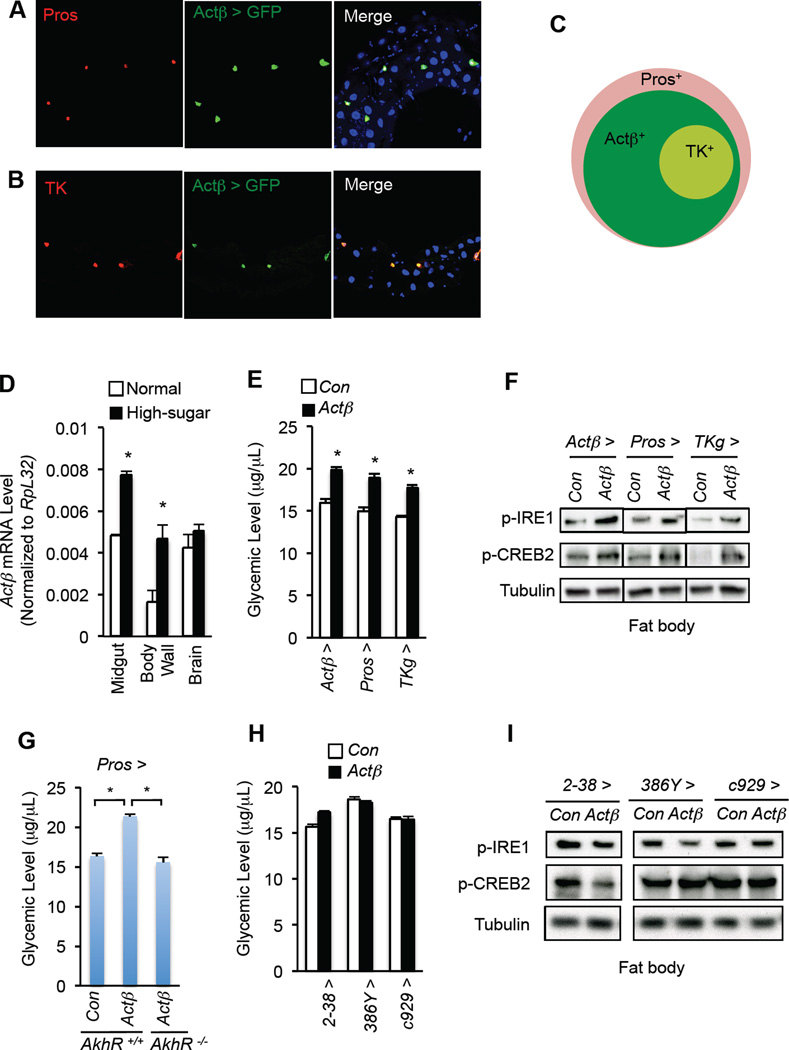
We next examined the source(s) of Actβ that regulates AKH action and carbohydrate metabolism in the fat body. Consistent with previous results (Gibbens et al., 2011; Kim and O’Connor, 2014; Ting et al., 2007), we observed Actβ expression in the larval central neuron system (CNS), neuroendocrine cells, and motor neurons using Actβ-Gal4-driving GFP expression (Fig. S6A, C). Strikingly, we also observed Actβ expression in most enteroendocrine cells (EEs), as indicated with the EE marker Prospero (Pros), in the larval midgut (Fig. 6A and Fig. S6B). Actβ-expressing EEs also produce Tachykinin (TK), an abundant gut hormone (Amcheslavsky et al., 2014; Song et al., 2014), in the posterior midgut (Fig. 6B–C). Actβ expression in the midgut was confirmed by qPCR and further was found to be upregulated in response to a chronic high-sugar diet (Fig. 6D). High-sugar diet also increased Actβ induction in the motor neurons in the body wall, but not in the brain (Fig. 6D). Importantly, overexpression of Actβ using Actβ-Gal4 potently enhanced AKH signaling in the fat body and led to hyperglycemia (Fig. 6E–F), mimicking the impact of a high-sugar diet on Actβ elevation and carbohydrate metabolic regulation.
Figure 6. Enteroendocrine cell-derived Actβ modulates AKH action in the fat body.
(A, B) Expression of Actβ > GFP (green, UAS-srcGFP/+; Actβ-Gal4/+) in EEs, indicated by Pros (A, red) or TK (B, red), in 3rd larval posterior midgut. (C) Schematic Actβ > GFP expression pattern in larval EEs. (D) Actβ mRNA levels in different larval tissues under high sugar diet. (E–I) Glycemic levels (glucose + trehalose) (E, H) (n=4, 40 larvae) and fat body immunoblots (F, I) in Actβ-overexpressing larvae. (G) Glycemic levels in AkhR +/+; Pros > Con (AkhR +/AkhR +; Pros-Gal4/+), AkhR +/+; Pros > Actβ (AkhR +/AkhR +; Pros-Gal4/UAS-Actβ), and AkhR −/−; Pros > Actβ (AkhR /AkhR; Pros-Gal4/UAS- Actβ) larvae (n=4, 40 larvae per group). Data are presented as means ± SEM. * p < 0.05.
To determine which cell types or tissues produce Actβ to enhance AKH response in the fat body, we used different Gal4 drivers to specifically overexpress Actβ in EEs (i.e. using Pros-Gal4 or TKg-Gal4) or in motor neurons (2–38-Gal4) (Fig. S6C). Surprisingly, Actβ overexpression in all EEs (Pros-Gal4) or only in TK-expressing EEs (TKg-Gal4) was sufficient to increase AKH signaling in the fat body, as well as glycemic levels (Fig. 6E–F). Notably, the elevation in glycemia associated with Actβ overexpression in EEs was significantly diminished by AkhR deficiency (Fig. 6G), indicating that the hyperglycemia associated with EE-derived Actβ is dependent on AKH signaling. By contrast, Actβ overexpression in motor neurons (2–38-Gal4) failed to enhance fat body AKH signaling activity (Fig. 6H–I). We also excluded the effects of neuroendocrine cell-derived Actβ, as neither fat body AKH signaling nor glycemic levels were affected following Actβ overexpression in neuroendocrine cells (386Y–Gal4 and c929-Gal4) (Fig. 6H–I and Fig. S6C). Therefore, our results indicate that Actβ production in EEs remotely regulates fat body AKH response and glycemic homeostasis.
A high-sugar diet leads to an increase in the number of Actβ-producing EEs
The Drosophila midgut is an important endocrine organ that senses environmental stresses and nutrient availability and produces multiple peptides or hormones that regulate systemic lipid and carbohydrate metabolism (Chng et al., 2014; Li et al., 2016; Song et al., 2014). We next investigated how chronic high-sugar feeding regulates Actβ expression in the EEs. To determine whether the effects of Actβ reflect an acute or a chronic condition, 3rd instar larvae were fed a high-sugar diet for 6h, a time sufficient to evoke an acute response. In contrast to chronic feeding, acute feeding of a high-sugar diet failed to elevate midgut Actβ expression, AKH signaling or glycemic levels (Fig. S6D–F). We next asked whether hemocyte infiltration-associated immune and/or inflammatory responses, as suggested by induction of Jak/Stat or NF-κB signaling (Woodcock et al., 2015), contribute to high-caloric diet-induced Actβ induction in EEs. However, we failed to observe any changes in hemocyte infiltration (Hml > GFP) in the midgut, and did not observe changes in Jak/Stat signaling (Stat-GFP reporter) or NF-κB signaling (Dpt-GFP reporter) in EEs (Fig. S7A–C), suggesting that immune and/or inflammatory responses do not directly regulate Actβ expression in EEs.
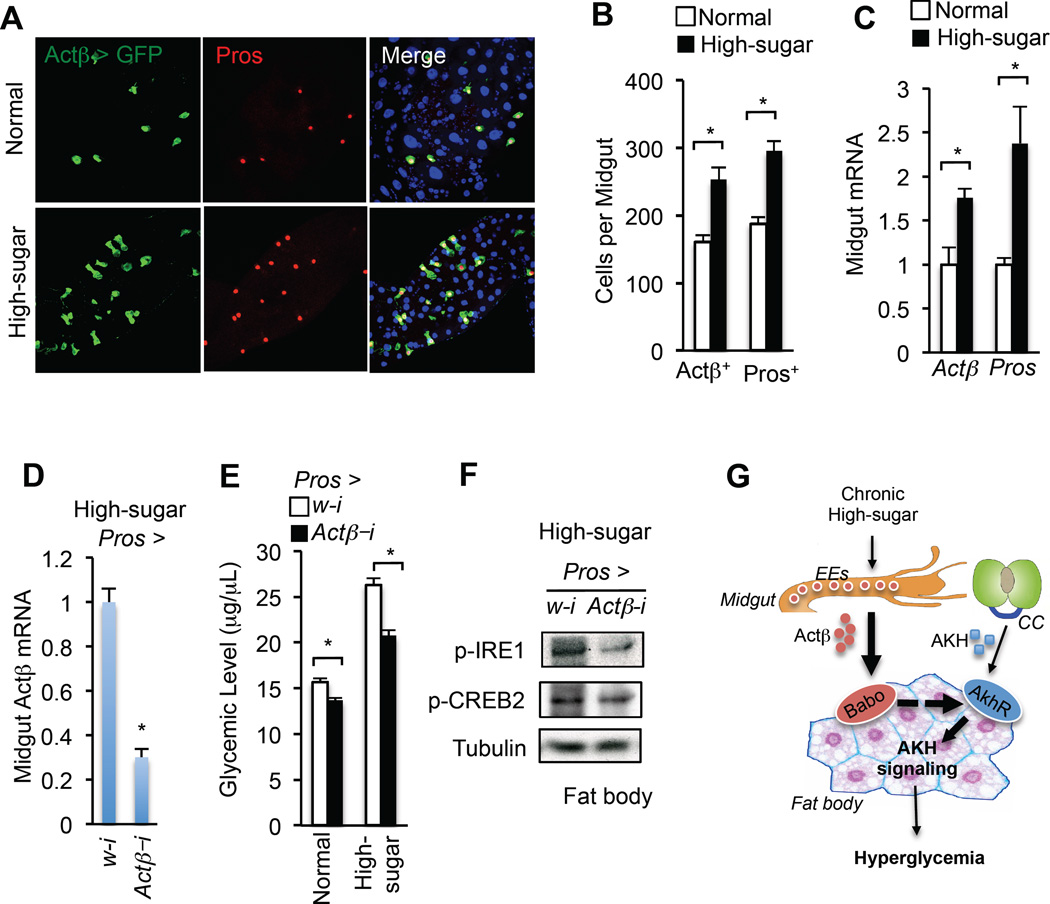
During this analysis we did note a striking observation that the number of EEs and the expression of EE marker Pros in the midgut were potently increased by chronic high-sugar feeding (Fig. 7A–C and Fig. S7A–C). These results indicate that chronic high-sugar feeding perturbs midgut homeostasis and leads to an increase in EE production. Consistently, the number of Actβ-producing EEs was also significantly increased under chronic high-sugar feeding (Fig. 7A–B). In contrast, the overall midgut size, as well as the mass of intestinal stem cells (ISCs) and enterocytes (ECs), were significantly decreased (Fig. S7D–E). The qPCR results also revealed that mRNA levels of the ISC marker esg and EC marker Pdm1 were significantly decreased (Fig. S7F).
Figure 7. Chronic high-sugar diet increases EE number and Actβ production in the larval midgut.
(A–C) Confocal image (A), number of Actβ > GFP or Pros+ cells (B) (n=15, 15 larval guts), and gene expression (C) (n=4, 40 larval guts), in the larval midgut under high-sugar diet. (D–F) Midgut Actβ mRNA (D) (n=4, 40 larval guts), glycemic levels (glucose + trehalose) (E) (n=4, 40 larvae), and (F) immunoblots of fat body from Pros > Actβ-i larvae (Pros-Gal4/UAS-Actβ-RNAi) fed a chronic high-sugar diet. (G) The midgut-to-fat-body axis in glycemic control. Chronic high-sugar diet leads to an increase in Actβ production in the EEs of larval midgut and enhances the fat body AKH signaling, resulting in hyperglycemia, via Actβ/Babo signaling. Data are presented as means ± SEM. * p < 0.05.
As starvation causes effects that are similar to what we observe with chronic high-sugar feeding, including suppressed insulin signaling and increased AKH signaling (Fig. S7G, I), we further examined whether starvation affects EE number and Actβ expression in the midgut. 3rd instar larvae were cultured for 6h on food containing only agar. However, we failed to observe an induction in the number of EEs, Actβ expression in the midgut, or AkhR expression in the fat body (Fig. S7G–H). These results indicate that Actβ levels are not induced by acute nutrient deprivation.
Finally, we examined whether the increase in EE-derived Actβ is essential for high-sugar diet-induced hyperglycemic levels by knocking down Actβ specifically in EEs. Knockdown of Actβ using the Pros-Gal4 driver caused a dramatic decrease in Actβ mRNA levels in the midgut under chronic high-sugar diet (Fig. 7D). Importantly, Actβ knockdown in EEs significantly lowered the increases in both AKH signaling in the fat body and glycemic levels associated with chronic high-sugar feeding (Fig. 7E–F). Taken together, our results indicate that chronic high-sugar feeding perturbs homeostasis of EEs and increases Actβ production in the midgut, leading to a non-cell autonomous enhancement of AKH signaling in the fat body and hyperglycemia (Fig. 7G).
Discussion
Chronic high-caloric diets have been reported to impair insulin action and elevate glycemic levels in both vertebrates and invertebrates (Musselman et al., 2011; Pasco and Leopold, 2012; Susini et al., 1979). Using Drosophila as a conserved model of metabolic regulation, we demonstrate that enhanced AKH action, which mobilizes energy storage and increases circulating carbohydrate levels as an insulin counter-regulatory hormone, is also essential for high-sugar diet-induced hyperglycemia. We show that chronic high-sugar feeding abnormally augments AKH sensitivity in the fat body, consistent with previous observation of increased AkhR expression in this tissue (Musselman et al., 2013). Importantly, impaired AKH signaling in the fat body due to AkhR deficiency significantly alleviates the hyperglycemia induced by chronic high-sugar feeding or insulin resistance. However, acute high-sugar feeding fails to increase AKH action or glycemic level, indicating a pathogenic, but not physiological, enhancement of AKH response in the fat body under chronic high-sugar diet. Altogether, our study identifies an insulin-independent mechanism for diet-associated hyperglycemia.
Identification of regulators of AKH signaling
To study how intracellular AKH signaling is enhanced, we performed an in vivo RNAi screen targeting kinases and phosphatases and identified 30 potential novel candidates that modulate AKH signaling and glycemic control. Future studies that aim to elucidate how these candidates regulate AKH action in the fat body in response to a chronic high-sugar diet will offer a more comprehensive understanding of diet-associated hyperglycemic pathogenesis beyond insulin regulation.
In addition to AKH signaling in the fat body as presented here, AKH production or secretion from CC cells may also contribute to diet-associated hyperglycemia. Interestingly, several metabolic regulators, including AMPK and water sensor ppk28, have previously been shown to affect AKH production and/or secretion (Braco et al., 2012; Kim and Neufeld, 2015; Kim and Rulifson, 2004; Waterson et al., 2014). Thus, regulation of AKH production and/or secretion in the context of high-caloric feeding will be another interesting question to address.
Actβ/Baboa regulate AKH response in the fat body
Our results demonstrate that Actβ-induced Babo action in the fat body potentiates AKH signaling. Specifically, we found that Babo signaling triggers an increase in dSmad2 transcriptional activity and AkhR expression. Interestingly, in addition to Actβ, Babo also responds to other activin ligands, including Daw and Myo. Among these activin ligands, only Actβ and Daw signal through dSmad2 (Gesualdi and Haerry, 2007). However, several lines of evidence suggest that Actβ and Daw exert different effects on Babo signaling and trigger distinct downstream outputs. First, Daw deficiency results in reduced insulin signaling and hyperglycemia, but fat body overexpression of an active form of Babo fails to restore normal glycemic levels in Daw mutants (Ghosh and O’Connor, 2014). Consistently, our results revealed that Babo deficiency in the fat body fails to affect Daw-induced hypoglycemia (Fig. 5H), indicating that Daw regulation of carbohydrate metabolism is independent of fat body Babo signaling. A plausible explanation is that Daw controls dILP2 secretion to modulate systemic insulin effects in a non-cell autonomous manner (Bai et al., 2013; Ghosh and O’Connor, 2014). In contrast, Actβ promotion of AKH signaling is dependent on Babo function in the fat body. Second, Daw preferentially targets Baboc (Jensen et al., 2009), but the Babo isoform used for Actβ signaling has not previously been reported. In this study we demonstrate that Actβ modulates AKH signaling and hyperglycemia via Baboa in the fat body, but not via Babob or Baboc. Collectively, we conclude that Actβ/Baboa regulates AKH signaling in the fat body, whereas Daw/Baboc regulates dILP2 secretion in CNS, to differentially control carbohydrate homeostasis. Note that we exclude the possibility that Mav, another activin ligand, is relevant to AKH signaling as it has only a slight effect on Babo signaling in vivo (M. O’Connor, unpublished data). We also exclude another activin ligand, Myo, which signals through Babo/MAPK in the fat body (Demontis et al., 2014), as Myo shows no effects on AKH signaling (unpublished data).
Actβ produced in midgut EEs affects fat body AKH signaling
One of our striking findings is that Actβ produced by midgut EEs affects the fat body AKH signaling, implicating a midgut-to-fat-body axis in glycemic control. Actβ has been previously characterized as a neuronal factor and is abundantly expressed in the central and peripheral nervous systems (PNS) to modulate neuronal development and activity (Kim and O’Connor, 2014; Zhu et al., 2008). We demonstrate for the first time that Actβ is abundantly expressed in EEs of the larval midgut. Midgut EEs are key endocrine cells that sense environmental cues, including stresses and dietary nutrients, and secrete gut hormones that modulate the functions of other tissues (Chng et al., 2014; Li et al., 2016; Song et al., 2014). Strikingly, Actβ overexpression in EEs (Pros-Gal4) leads to a dramatic increase in hyperglycemia in a manner dependent on Akh response in the fat body, suggesting inter-organ communication between the midgut and the fat body. Further, using a midgut-specific TKg-Gal4 that targets TK EEs (i.e. about ~23% of Actβ-positive EEs), we show that overexpression of Actβ only in a few EEs is sufficient to lead to a significant increase in fat body AKH signaling activity and glycemic levels. These results are consistent with a model in which Actβ production in midgut EEs affects fat body physiology. Finally, knockdown of Actβ in the midgut using RNAi potently alleviates the increased fat body AKH action and hyperglycemia associated with a chronic high-sugar diet, indicating an essential role for midgut Actβ in diet-associated fat body AKH action and carbohydrate metabolism.
We also noticed that Actβ overexpression in neuroendocrine cells (386Y–or c929-Gal4) or in motor neurons (2–38-Gal4) failed to enhance AKH response in the fat body. Possibly, Actβ may function as a regional hormone, as the larval midgut is surrounded by the fat body, which is relatively far from neuroendocrine cells in the brain and motor neurons in the body wall. Alternatively, only mature Actβ hormone that is produced from the midgut through specific post-translational modifications is able to enhance AKH response in the fat body. In support of this model, different processing regulations of the Ast B prohormone in EEs and neurons have been observed in Drosophila (Baggerman et al., 2002; Reiher et al., 2011). The release of active mammalian TGF-β is also context-dependent of distinct activation/localization of TGF-β complexes (Annes et al., 2003).
Intriguingly, we found that a chronic high-sugar diet is associated with an increase in EE number. Several signaling pathways have been implicated in ISC proliferation and EE differentiation in the adult midgut (Zeng et al., 2015), in particular JAK/STAT signaling (Lin et al., 2010). Interestingly, we have observed an increase in JAK/STAT signaling in ISCs under chronic high-sugar feeding (data not shown). Further, Upd3, a Jak/Stat ligand, is elevated in both hemocytes (Woodcock et al., 2015) and enterocytes (data not shown) under high-caloric diet. Further studies that elucidate in detail the signaling crosstalk that controls EE generation in the late larval midgut will be required to understand how a chronic high-sugar diet increases EE number and Actβ production.
Relevance to Mammalian Physiology
Maintenance of stable circulating carbohydrate levels via a balance between insulin and counter-regulator hormones, particularly glucagon, is conserved in mammals and Drosophila. Whereas insulin regulation is well studied, less is known about AKH/glucagon. Here, we demonstrate that AKH action in the Drosophila fat body is highly similar to glucagon action in the mammalian liver with regards to diet-induced hyperglycemia. Interestingly, and reminiscent of AKH regulation in Drosophila, liver glucagon action is aberrantly enhanced in high-fat diet conditions and a reduction of glucagon signaling specifically in the liver improves glucose tolerance in type II diabetic or insulin resistant subjects (Ali and Drucker, 2009; Liang et al., 2004; Matsuda et al., 2002). Moreover, newly-characterized physiological regulators of mouse glucagon signaling, including the unfolded protein response (UPR) sensor IRE1α and the inflammatory NF-κB signaling (Mao et al., 2011; Sheng et al., 2012), have been shown to play similar roles in AKH regulation in Drosophila (Fig. 2). Thus, our study provides a conserved model of glucagon regulation and will help to comprehensively characterize regulatory networks involved in liver glucagon signaling.
Like the response of Drosophila Babo to Actβ, the mammalian receptors ALK4 and ALK5 respond to the ligands activin A, TGF-β1, and Myostatin to activate Smad2/3 transcriptional activity (Pauklin and Vallier, 2015; Rebbapragada et al., 2003). We found that long-term treatment with activin A or TGF-β1, but not Myostatin, potently enhances glucagon signaling to increase glucagon-induced glucose production in mouse primary hepatocytes. Activin A and TGF-β1 are produced in multiple organs in mammals, including EEs, and regulate liver growth and inflammation (Bogunovic et al., 2007; Kreidl et al., 2009; La Rosa et al., 2004). Enhanced glucagon signaling and increased activin A/TGF-β1 levels have been coincidently observed in obese or diabetic animals (Mao et al., 2011; Sheng et al., 2012; Yadav et al., 2011; Zaragosi et al., 2010). Importantly, mice with a mutation in Inhba, which encodes the inhibin βA subunit of Activin A, are lean and show improved glucose tolerance and hyperglycemia (Li et al., 2009). TGF-β1 or Smad3 deficiency also protects mice from diet-induced diabetes (Yadav et al., 2011). These observations are consistent with our proposal that activin A/TGF-β1 regulates hepatic glucagon action and glucose production to impact glycemic homeostasis. However, consistent with an in vitro study indicating that acute treatment of activin A alone promotes liver glycogen breakdown and glucose production (Kojima et al., 1995), we also observed a basal induction of glucose production by activin A alone, suggesting the presence of a glucagon-independent mechanism. Thus, it will be of interest to characterize the nature and role of glucagon-dependent and -independent mechanisms in regulating liver glucose production under different conditions, such as long- or short-term activin ligand treatment.
Methods
Drosophila studies
Strains and culture methods used in this study are listed in the Sup Methods.
High-sugar diet and carbohydrate measurement
25% extra sucrose was added into the standard diet to generate the high-sugar diet food. Determination of carbohydrate levels has previously been described (Kwon et al., 2015; Song et al., 2010). Trehalase (E-TREH) and glucose reagent (K-GLUC) were obtained from Megazyme.
RNA-seq transcriptome analysis
10 fat bodies from late 3rd instar larvae of each genotype were collected for RNA-seq. Details on samples preparation and data analyses are in the Sup. Methods. RNA-seq data were deposited in the Gene Expression Omnibus (Accession number GSE92350).
Generation of stable AkhR-expressing S2R+ cells
A full-length AkhR cDNA was generated by RT-PCR using primers 5’-CCGGAATTCGAGGCAAATCCTTGATGCAG and 5’-AGAATGCGGCCGCACTTCTGGCGGATCGGGGAT, verified by DNA sequencing, and inserted into vector pAC5-Stable2-Puro (Gonzalez et al., 2011). Stable cell selection is described in the Sup. Methods.
Primary hepatocyte culture, HGP assays, and immunoblots
Mouse primary hepatocytes preparation and HGP assays have been previously described (Hong et al., 2015; Sheng et al., 2012). Immunoblots were performed as previously described (Song et al., 2010).
qPCR
10 fat bodies, 10 midguts, 10 body walls, or 10 brains from late 3rd instar larvae of each genotype were collected for RNA extraction and qPCR analysis as previously described (Song et al., 2014). Primers are listed in the Sup. Methods.
Immunostaining
Immunostainings of S2R+ cells and larval midgut and brain have been previously described (Song et al., 2010; Song et al., 2014).
Statistical Analyses
Data are presented as the mean ± SEM. Student’s t tests were used to compare two groups. p < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank the Transgenic RNAi Project (TRiP) and Bloomington Drosophila Stock Center (BDSC) for providing RNAi stocks; Ronald Kühnlein for AkhR stocks; Richard Binari, Young Kwon, Stephanie Mohr, Richelle Sopko, Ilia Droujinine, Charles Xu, and Xiaochun Ni for comments. This work was supported in part by the American Diabetes Association and the Joslin Pilot and Feasibility Program. Work in the O’Connor lab is supported by NIH grant R01 GM095746. Work in the Pissios lab is supported by NIH grant R01 DK083694. Work in the Perrimon lab is supported by NIH and HHMI. N.P. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
W. S. conceived the study and designed and performed experiments. D. C. and Y. H. performed RNA-seq and bioinformatics analysis. D. C., B. S. and N. W. performed RNAi screen. S. H. and P. P. performed primary hepatocyte experiments. C. Z. and M. B. O. generated transgenic flies. W. S. and N. P. discussed results and wrote manuscript.
References
- Ali S, Drucker DJ. Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. Am J Physiol Endocrinol Metab. 2009;296:E415–E421. doi: 10.1152/ajpendo.90887.2008. [DOI] [PubMed] [Google Scholar]
- Amcheslavsky A, Song W, Li Q, Nie Y, Bragatto I, Ferrandon D, Perrimon N, Ip YT. Enteroendocrine cells support intestinal stem-cell-mediated homeostasis in Drosophila. Cell Rep. 2014;9:32–39. doi: 10.1016/j.celrep.2014.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Huang Y, O’Connor MB, Lee T. Glia instruct developmental neuronal remodeling through TGF-beta signaling. Nat Neurosci. 2011;14:821–823. doi: 10.1038/nn.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggerman G, Cerstiaens A, De Loof A, Schoofs L. Peptidomics of the larval Drosophila melanogaster central nervous system. J Biol Chem. 2002;277:40368–40374. doi: 10.1074/jbc.M206257200. [DOI] [PubMed] [Google Scholar]
- Bai H, Kang P, Hernandez AM, Tatar M. Activin signaling targeted by insulin/dFOXO regulates aging and muscle proteostasis in Drosophila. PLoS Genet. 2013;9:e1003941. doi: 10.1371/journal.pgen.1003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha KN, Tarr P, Zipursky SL. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J Exp Biol. 2008;211:3103–3110. doi: 10.1242/jeb.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Dave SH, Tilstra JS, Chang DT, Harpaz N, Xiong H, Mayer LF, Plevy SE. Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1770–G1783. doi: 10.1152/ajpgi.00249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braco JT, Gillespie EL, Alberto GE, Brenman JE, Johnson EC. Energy-dependent modulation of glucagon-like signaling in Drosophila via the AMP-activated protein kinase. Genetics. 2012;192:457–466. doi: 10.1534/genetics.112.143610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummel T, Abdollah S, Haerry TE, Shimell MJ, Merriam J, Raftery L, Wrana JL, O’Connor MB. The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development. Genes Dev. 1999;13:98–111. doi: 10.1101/gad.13.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- Chng WB, Bou Sleiman MS, Schupfer F, Lemaitre B. Transforming growth factor beta/activin signaling functions as a sugar-sensing feedback loop to regulate digestive enzyme expression. Cell Rep. 2014;9:336–348. doi: 10.1016/j.celrep.2014.08.064. [DOI] [PubMed] [Google Scholar]
- Demontis F, Patel VK, Swindell WR, Perrimon N. Intertissue control of the nucleolus via a myokine-dependent longevity pathway. Cell Rep. 2014;7:1481–1494. doi: 10.1016/j.celrep.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frojdo S, Vidal H, Pirola L. Alterations of insulin signaling in type 2 diabetes: a review of the current evidence from humans. Biochim Biophys Acta. 2009;1792:83–92. doi: 10.1016/j.bbadis.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Galikova M, Diesner M, Klepsatel P, Hehlert P, Xu Y, Bickmeyer I, Predel R, Kuhnlein RP. Energy Homeostasis Control in Drosophila Adipokinetic Hormone Mutants. Genetics. 2015;201:665–683. doi: 10.1534/genetics.115.178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesualdi SC, Haerry TE. Distinct signaling of Drosophila Activin/TGF-beta family members. Fly (Austin) 2007;1:212–221. doi: 10.4161/fly.5116. [DOI] [PubMed] [Google Scholar]
- Ghosh AC, O’Connor MB. Systemic Activin signaling independently regulates sugar homeostasis, cellular metabolism, and pH balance in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2014;111:5729–5734. doi: 10.1073/pnas.1319116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbens YY, Warren JT, Gilbert LI, O’Connor MB. Neuroendocrine regulation of Drosophila metamorphosis requires TGFbeta/Activin signaling. Development. 2011;138:2693–2703. doi: 10.1242/dev.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, Martin-Ruiz I, Jimenez S, Pirone L, Barrio R, Sutherland JD. Generation of stable Drosophila cell lines using multicistronic vectors. Sci Rep. 2011;1:75. doi: 10.1038/srep00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronke S, Muller G, Hirsch J, Fellert S, Andreou A, Haase T, Jackle H, Kuhnlein RP. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol. 2007;5:e137. doi: 10.1371/journal.pbio.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselton AT, Fridell YW. Adult Drosophila melanogaster as a model for the study of glucose homeostasis. Aging (Albany NY) 2010;2:523–526. doi: 10.18632/aging.100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Moreno-Navarrete JM, Wei X, Kikukawa Y, Tzameli I, Prasad D, Lee Y, Asara JM, Fernandez-Real JM, Maratos-Flier E, et al. Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nat Med. 2015;21:887–894. doi: 10.1038/nm.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Jensen PA, Zheng X, Lee T, O’Connor MB. The Drosophila Activin-like ligand Dawdle signals preferentially through one isoform of the Type-I receptor Baboon. Mech Dev. 2009;126:950–957. doi: 10.1016/j.mod.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284:E671–E678. doi: 10.1152/ajpendo.00492.2002. [DOI] [PubMed] [Google Scholar]
- Kim J, Neufeld TP. Dietary sugar promotes systemic TOR activation in Drosophila through AKH-dependent selective secretion of Dilp3. Nat Commun. 2015;6:6846. doi: 10.1038/ncomms7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, O’Connor MB. Anterograde Activin signaling regulates postsynaptic membrane potential and GluRIIA/B abundance at the Drosophila neuromuscular junction. PLoS One. 2014;9:e107443. doi: 10.1371/journal.pone.0107443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Kojima I, Nobusawa R, Zhang YQ, Sekine N, Mine T, Shibata H. Attenuation of glycogenolytic action of activin A in intact rat liver. Am J Physiol. 1995;269:E846–E851. doi: 10.1152/ajpendo.1995.269.5.E846. [DOI] [PubMed] [Google Scholar]
- Kreidl E, Ozturk D, Metzner T, Berger W, Grusch M. Activins and follistatins: Emerging roles in liver physiology and cancer. World J Hepatol. 2009;1:17–27. doi: 10.4254/wjh.v1.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, Perrimon N. Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev Cell. 2015;33:36–46. doi: 10.1016/j.devcel.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa S, Uccella S, Marchet S, Capella C, Lloyd RV. Localization of inhibins and activins in normal endocrine cells and endocrine tumors of the gut and pancreas: an immunohistochemical and in situ hybridization study. J Histochem Cytochem. 2004;52:217–225. doi: 10.1177/002215540405200210. [DOI] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Song J, Zaytseva YY, Liu Y, Rychahou P, Jiang K, Starr ME, Kim JT, Harris JW, Yiannikouris FB, et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature. 2016;533:411–415. doi: 10.1038/nature17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shen JJ, Bournat JC, Huang L, Chattopadhyay A, Li Z, Shaw C, Graham BH, Brown CW. Activin signaling: effects on body composition and mitochondrial energy metabolism. Endocrinology. 2009;150:3521–3529. doi: 10.1210/en.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, She P, Jetton TL, Demarest KT. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes. 2004;53:410–417. doi: 10.2337/diabetes.53.2.410. [DOI] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. J Mol Cell Biol. 2010;2:37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- Mao T, Shao M, Qiu Y, Huang J, Zhang Y, Song B, Wang Q, Jiang L, Liu Y, Han JD, et al. PKA phosphorylation couples hepatic inositol-requiring enzyme 1alpha to glucagon signaling in glucose metabolism. Proc Natl Acad Sci U S A. 2011;108:15852–15857. doi: 10.1073/pnas.1107394108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Defronzo RA, Glass L, Consoli A, Giordano M, Bressler P, Delprato S. Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism. 2002;51:1111–1119. doi: 10.1053/meta.2002.34700. [DOI] [PubMed] [Google Scholar]
- McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, Cagan RL, Baranski TJ. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech. 2011;4:842–849. doi: 10.1242/dmm.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman LP, Fink JL, Ramachandran PV, Patterson BW, Okunade AL, Maier E, Brent MR, Turk J, Baranski TJ. Role of fat body lipogenesis in protection against the effects of caloric overload in Drosophila. J Biol Chem. 2013;288:8028–8042. doi: 10.1074/jbc.M112.371047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Song W, Perrimon N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell. 2013;155:699–712. doi: 10.1016/j.cell.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco MY, Leopold P. High sugar-induced insulin resistance in Drosophila relies on the lipocalin Neural Lazarillo. PLoS One. 2012;7:e36583. doi: 10.1371/journal.pone.0036583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauklin S, Vallier L. Activin/Nodal signalling in stem cells. Development. 2015;142:607–619. doi: 10.1242/dev.091769. [DOI] [PubMed] [Google Scholar]
- Pilkis SJ, El-Maghrabi MR, McGrane M, Pilkis J, Claus TH. Regulation by glucagon of hepatic pyruvate kinase, 6-phosphofructo 1-kinase, and fructose-1,6-bisphosphatase. Fed Proc. 1982;41:2623–2628. [PubMed] [Google Scholar]
- Qureshi SA, Rios Candelore M, Xie D, Yang X, Tota LM, Ding VD, Li Z, Bansal A, Miller C, Cohen SM, et al. A novel glucagon receptor antagonist inhibits glucagon-mediated biological effects. Diabetes. 2004;53:3267–3273. doi: 10.2337/diabetes.53.12.3267. [DOI] [PubMed] [Google Scholar]
- Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230–7242. doi: 10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiher W, Shirras C, Kahnt J, Baumeister S, Isaac RE, Wegener C. Peptidomics and peptide hormone processing in the Drosophila midgut. J Proteome Res. 2011;10:1881–1892. doi: 10.1021/pr101116g. [DOI] [PubMed] [Google Scholar]
- Rhea JM, Wegener C, Bender M. The proprotein convertase encoded by amontillado (amon) is required in Drosophila corpora cardiaca endocrine cells producing the glucose regulatory hormone AKH. PLoS Genet. 2010;6:e1000967. doi: 10.1371/journal.pgen.1000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng L, Zhou Y, Chen Z, Ren D, Cho KW, Jiang L, Shen H, Sasaki Y, Rui L. NF-kappaB-inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat Med. 2012;18:943–949. doi: 10.1038/nm.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Ren D, Li W, Jiang L, Cho KW, Huang P, Fan C, Song Y, Liu Y, Rui L. SH2B regulation of growth, metabolism, and longevity in both insects and mammals. Cell Metab. 2010;11:427–437. doi: 10.1016/j.cmet.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Veenstra JA, Perrimon N. Control of lipid metabolism by tachykinin in Drosophila. Cell Rep. 2014;9:40–47. doi: 10.1016/j.celrep.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopko R, Foos M, Vinayagam A, Zhai B, Binari R, Hu Y, Randklev S, Perkins LA, Gygi SP, Perrimon N. Combining genetic perturbations and proteomics to examine kinase-phosphatase networks in Drosophila embryos. Dev Cell. 2014;31:114–127. doi: 10.1016/j.devcel.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susini C, Lavau M, Herzog J. Adrenaline responsiveness of glucose metabolism in insulin-resistant adipose tissue of rats fed a high-fat diet. Biochem J. 1979;180:431–433. doi: 10.1042/bj1800431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting CY, Herman T, Yonekura S, Gao S, Wang J, Serpe M, O’Connor MB, Zipursky SL, Lee CH. Tiling of r7 axons in the Drosophila visual system is mediated both by transduction of an activin signal to the nucleus and by mutual repulsion. Neuron. 2007;56:793–806. doi: 10.1016/j.neuron.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH. Glucagon and the insulin: glucagon ratio in diabetes and other catabolic illnesses. Diabetes. 1971;20:834–838. doi: 10.2337/diab.20.12.834. [DOI] [PubMed] [Google Scholar]
- Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Moya N, Niessen S, Hoover H, Mihaylova MM, Shaw RJ, Yates JR, 3rd, Fischer WH, Thomas JB, Montminy M. A hormone-dependent module regulating energy balance. Cell. 2011;145:596–606. doi: 10.1016/j.cell.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460:534–537. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterson MJ, Chung BY, Harvanek ZM, Ostojic I, Alcedo J, Pletcher SD. Water sensor ppk28 modulates Drosophila lifespan and physiology through AKH signaling. Proc Natl Acad Sci U S A. 2014;111:8137–8142. doi: 10.1073/pnas.1315461111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock KJ, Kierdorf K, Pouchelon CA, Vivancos V, Dionne MS, Geissmann F. Macrophage-derived upd3 cytokine causes impaired glucose homeostasis and reduced lifespan in Drosophila fed a lipid-rich diet. Immunity. 2015;42:133–144. doi: 10.1016/j.immuni.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragosi LE, Wdziekonski B, Villageois P, Keophiphath M, Maumus M, Tchkonia T, Bourlier V, Mohsen-Kanson T, Ladoux A, Elabd C, et al. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes. 2010;59:2513–2521. doi: 10.2337/db10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Han L, Singh SR, Liu H, Neumuller RA, Yan D, Hu Y, Liu Y, Liu W, Lin X, et al. Genome-wide RNAi screen identifies networks involved in intestinal stem cell regulation in Drosophila. Cell Rep. 2015;10:1226–1238. doi: 10.1016/j.celrep.2015.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CC, Boone JQ, Jensen PA, Hanna S, Podemski L, Locke J, Doe CQ, O’Connor MB. Drosophila Activin- and the Activin-like product Dawdle function redundantly to regulate proliferation in the larval brain. Development. 2008;135:513–521. doi: 10.1242/dev.010876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.