Abstract
Palau has a rich heritage of conservation that has evolved from the traditional moratoria on fishing, or “bul”, to more western Marine Protected Areas (MPAs), while still retaining elements of customary management and tenure. In 2003, the Palau Protected Areas Network (PAN) was created to conserve Palau’s unique biodiversity and culture, and is the country’s mechanism for achieving the goals of the Micronesia Challenge (MC), an initiative to conserve ≥30% of near-shore marine resources within the region by 2020. The PAN comprises a network of numerous MPAs within Palau that vary in age, size, level of management, and habitat, which provide an excellent opportunity to test hypotheses concerning MPA design and function using multiple discreet sampling units. Our sampling design provided a robust space for time comparison to evaluate the relative influence of potential drivers of MPA efficacy. Our results showed that no-take MPAs had, on average, nearly twice the biomass of resource fishes (i.e. those important commercially, culturally, or for subsistence) compared to nearby unprotected areas. Biomass of non-resource fishes showed no differences between no-take areas and areas open to fishing. The most striking difference between no-take MPAs and unprotected areas was the more than 5-fold greater biomass of piscivorous fishes in the MPAs compared to fished areas. The most important determinates of no-take MPA success in conserving resource fish biomass were MPA size and years of protection. Habitat and distance from shore had little effect on resource fish biomass. The extensive network of MPAs in Palau likely provides important conservation and tourism benefits to the Republic, and may also provide fisheries benefits by protecting spawning aggregation sites, and potentially through adult spillover.
Introduction
Palau has a rich tradition of fisheries management and stewardship of its waters [1–4]. Traditionally, Palau had strong community control that closed areas to fishing through implementation of traditional moratoria on fishing, or “bul”, prohibiting all use for a restricted period, but usually not indefinitely [5–7]. This localized adaptive management was based on customary knowledge and practices, and was responsive to changes in resource abundance [1].
Conservation in Palau has evolved from the traditional “bul” to more western Marine Protected Areas (MPAs). The government of Palau was instrumental in establishing the Micronesia Challenge–a conservation initiative to protect >30% of the marine ecosystems of the region by 2020 through the establishment of local Protected Areas Network (PAN) [8–10]. The PAN was established by national law in 2003, and created a framework for a national system of marine and terrestrial protected areas. Currently, there are 35 MPAs throughout Palau, encompassing all major habitat types, ranging from nearshore mangroves and seagrass beds to offshore coral reefs, with > 45% of the country's nearshore waters under some form of protection [11–13]. These MPAs range in management from complete no-take to subsistence fishing only, and not all are included in the PAN.
The people of Palau and other tropical island nations rely heavily on coral reefs for the ecosystem services they provide, such as protection from storms, food provisioning, perpetuation of cultural practices, and revenue from tourism [4, 14–16]. Palau is one of the world’s top dive destinations, with tourists coming to experience its high biodiversity and unique marine ecosystems [17–19]. In recent years, tourism has contributed roughly three quarters of GDP growth, more than 80% of exports of goods and services, 15% of total tax revenue, and 40% of total employment [20].
Due to local and global threats, coral reefs are becoming increasingly degraded worldwide, necessitating better conservation and management measures [21–23]. MPAs have proven to be an effective ecosystem-based management tool to conserve biodiversity and manage fisheries [24–26]. By protecting populations, habitats, and ecosystems within their borders, no-take MPAs provide a spatial refuge for the entire ecological system they contain and provide a powerful buffer against anthropogenic effects and natural variability [27–30]. In addition to resource management, MPAs also contribute to the long-term livelihoods of island people though the strong cultural and economic connections between islanders and the sea, as well as their interdependence on a healthy marine environment for survival and prosperity [31].
The effectiveness of MPAs can be influenced by their size, shape, age, level of protection, and the movement patterns of individual species [32–36]. Fully protected areas have been shown to have much greater conservation benefits compared with areas under lesser levels of protection [37]. It is assumed that larger MPAs are more effective because they protect a greater amount and diversity of habitats, and encompass and protect critical habitats or processes that maintain populations and ecosystem stability, which provides protection for a wider range of species and buffers against losses associated with environmental fluctuations and large-scale disturbances [38–41]. Large MPAs are more likely to contain fully functional ecosystems and suffer less from outside effects since they have a smaller perimeter-to-area ratio [42–43].
While several meta-analyses of MPAs have not shown an effect of reserve size [44–45], these studies contained relatively few large no-take areas, and the wide range of locations and biogeographic affinities examined may mask the effects of MPA size [45]. A meta-analysis of 19 European no-take MPAs found that for every 1-fold increase in no-take MPA size, there was a 35% increase in the density of commercial fishes [35]. Edgar and Barrett [46] compared four no-take MPAs in Tasmania with unprotected reference regions and found that the largest MPA had higher fish species richness, higher density of large fish, and larger-sized exploitable fishes when compared with fished reference sites.
Decadal-scale observations of no-take MPAs have shown direct effects on target species typically occurring within 5 years, with most target species showing initial direct effects, but their trajectories were highly variable based on the life history characteristics of the species examined [36, 47–48]. The average time for indirect effects that occur through cascading trophic interactions took 13 years or more to develop [36], and many non-fishery species did not show any response to protection at all [48]. A study of MPAs in eastern Australia showed that many of the targeted taxa examined were more abundant in large no-take MPAs within a few years of the establishment compared with the small no-take MPAs and the fished sites [49]. Collectively, these studies show that MPA effects can be slow, complex, and species-specific.
The objectives of this study were to examine the effectiveness of Palau’s MPAs relative to comparable fished areas, and to determine which factors lead to better success among these MPAs. A subset of Palau’s MPAs have been monitored for a number of years, but prior to our study no comprehensive evaluation of the efficacy of these MPAs has been conducted. We used integrated survey methods, across multiple taxonomic groups, conducted at the same time to compare these MPAs to one another and to comparable adjacent habitats. This approach provided a robust comparison among these MPAs and between these MPAs and reference areas, and while it represents a snapshot in time, this work complements the information currently being collected over a longer time period.
Methods
Ethics statement
Data were collected by all authors in a collaborative effort. Non-invasive research was conducted, which included photographs and visual estimates described in the methods. The Republic of Palau granted all necessary permission to conduct this research. No vertebrate sampling was conducted and therefore no approval was required by the University of Hawaii Institutional Animal Care and Use Committee. Our data are available at Data Dryad: doi:10.5061/dryad.tp3j5.
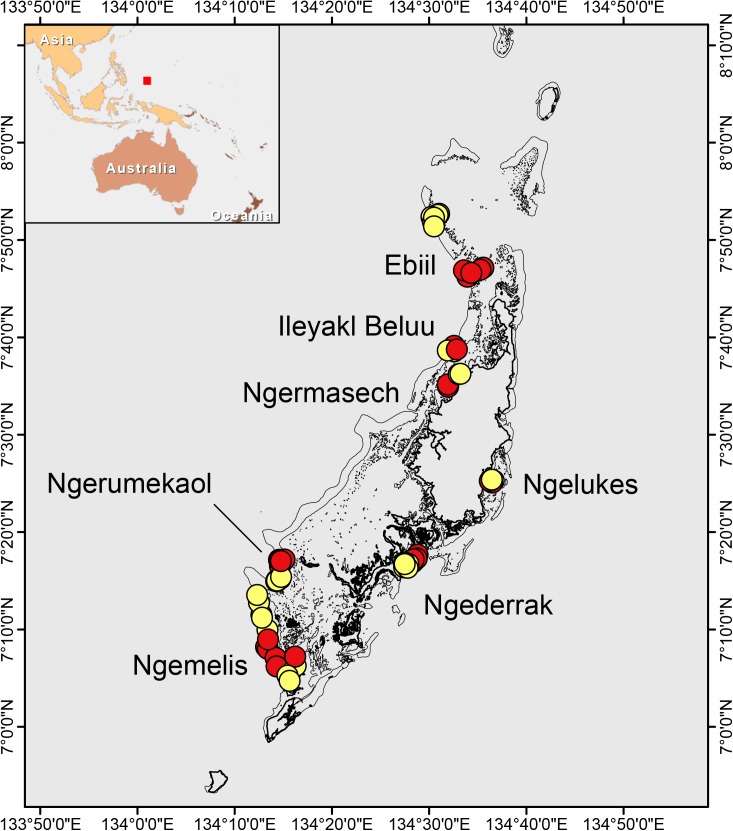
Of the 35 MPAs within the PAN, many protect nearshore mangrove, estuary, or seagrass habitats, while others are species-specific (e.g., clams, crabs) management areas, or remote atolls. We examined a subset of MPAs within the Palau PAN that were completely no-take areas, except for Ngemelis, which prohibits fishing within dive and snorkel sites and was considered as no-take for this study. We compared ecosystem characteristics within these areas to similar adjacent unprotected habitats (Fig 1). In the case of Ebiil, the control site was ~ 10 km to the north to incorporate comparable channel habitats. Previously created digital benthic habitat maps for all MPAs and adjacent habitats [50] were used to create a spatially-explicit stratified, random sampling design. Habitat features were mapped by visually interpreting multispectral satellite imagery and random sampling points were assigned within the major hard bottom geomorphic strata (e.g., forereef, patch reefs, channels) common to the MPA and their adjacent area. All adjacent area samples were > 500 m from the nearest MPA boundary. The MPAs ranged in age from 17 to 38 years of protection and from 0.4 km2 to 40 km2 in size (Table 1). The size range of these MPAs was representative of most of the MPAs within the PAN (range: 0.04–98.00 km2, median = 0.90 km2). All surveys were conducted in September 2014.
Fig 1. Locations of the Marine Protected Areas (red) and adjacent open sites (yellow) in Palau.
Table 1. Characteristics of Marine Protected Areas in Palau surveyed during the 2014 expedition.
N is the number of transects at each MPA, divided equally between the two depth strata (10 and 20 m). An equal number of samples were conducted at adjacent areas open to fishing.
| Name | State | Year est. | Size (km2) | N benthos | N fishes | Habitat tabitatypes | Restrictions |
|---|---|---|---|---|---|---|---|
| Ebiil | Ngerchelong | 1999 | 37.9 | 12 | 36 | Reef, channel | No fishing |
| Ngermasech | Ngardmau | 1998 | 3.3 | 4 | 12 | Mangrove, seagrass, coral reef | No entry, no fishing |
| Ngederrak | Koror | 2001 | 5.9 | 12 | 36 | Seagrass & reef flat | No entry, no fishing |
| Ngerumekaol | Koror | 1976 | 3.5 | 12 | 36 | Reef | No fishing |
| Ngemelis | Koror | 1995 | 40.3 | 16 | 48 | Islands & reefs | No fishing w/in dive & snorkel sites |
| Ngelukes | Ngchesar | 2002 | 1.0 | 4 | 12 | Patch reef | No entry, no fishing |
| Ileyakl Beluu | Ngardmau | 2005 | 0.4 | 4 | 12 | Reef | No entry, no fishing |
Benthos
Characterization of the benthos was conducted along 50 m-long transects oriented parallel to the shoreline at two depth strata (20 and 10 m). For algae, corals, and other sessile invertebrates, we used a line-point intercept methodology along each transect, recording the species or taxa found every 20 cm on the measuring tape. Benthic organisms were identified to the lowest possible taxonomic level, with overall benthic cover classified into major functional groupings (hard coral, soft coral, bare substrate, turf algae, macroalgae, blue-green algae, crustose coralline algae [CCA], soft sediment, seagrass, and sponge) for analyses.
Fishes
At each of two depth strata within a site (20 and 10 m), divers counted and estimated lengths for select fishes (see below for details) encountered within fixed-length (25-m) belt transects whose widths differed depending on direction of swim. All fish ≥ 20 cm total length (TL) were tallied within a 4-m wide strip surveyed on an initial “swim-out” as the transect line was laid (transect area = 100 m2). All fishes < 20 cm TL were tallied within a 2-m wide strip surveyed on the return swim back along the laid transect line (transect area = 50 m2). The fish survey was limited to species from 17 families, which comprised most of the fish biomass on the reef and were important fisheries or ecological species (Acanthuridae, Caesionidae, Carangidae, Carcharhinidae, Haemulidae, Kyphosidae, Labridae, Lethrinidae, Lutjanidae, Mullidae, Muraenidae, Scaridae, Scombridae, Serranidae, Siganidae, Sphyraenidae, Zanclidae) (S1 Table). This dataset resulted in density and length estimates for 165 species and of these, 139 (from 15 families) were considered primary targeted resource species. These were species important for commercial, cultural, or subsistence fishing in Palau based on discussions with local fishers, scientists, and resource managers.
The survey methodology was designed to minimize bias associated with in situ underwater visual censuses [51]. Constraints on the focal window size and survey duration for the swim-out limited problems of over-counting large-bodied, vagile species. Use of 2 transect areas (4-m vs. 2-m lanes) compensated for some of the size-specific differences in density, namely that larger-bodied fish are typically less abundant than their smaller-bodied counterparts, addressing some concerns of differing patterns of variance across size classes [52].
The biomass of individual fishes was estimated using the allometric length-weight conversion: W = aTLb, where parameters a and b are species-specific constants, TL is total length in cm, and W is weight in grams. Length-weight fitting parameters were obtained from FishBase [53]. The sum of all individual weights and numerical densities was used to estimate biomass density by species. Fishes were categorized into four trophic groups (piscivore, herbivore, secondary consumer, and planktivore) based on published literature.
Statistical analyses
Benthic community composition among MPAs and adjacent open areas was compared using permutation-based multivariate analysis of variance (PERMANOVA, PRIMER v6, [54]). A Bray–Curtis similarity matrix was created from percent cover of major benthic components and arcsine square root transformed prior to conducting the PERMANOVA. Management (MPA vs. open) was treated as a fixed factor and location was nested within management and treated as a random factor. Similarity of Percentages (SIMPER) was used to determine the benthic functional groups most responsible for the percentage dissimilarities between management regimes (MPA vs. open) using Bray-Curtis similarity analysis of hierarchical agglomerative group average clustering [55].
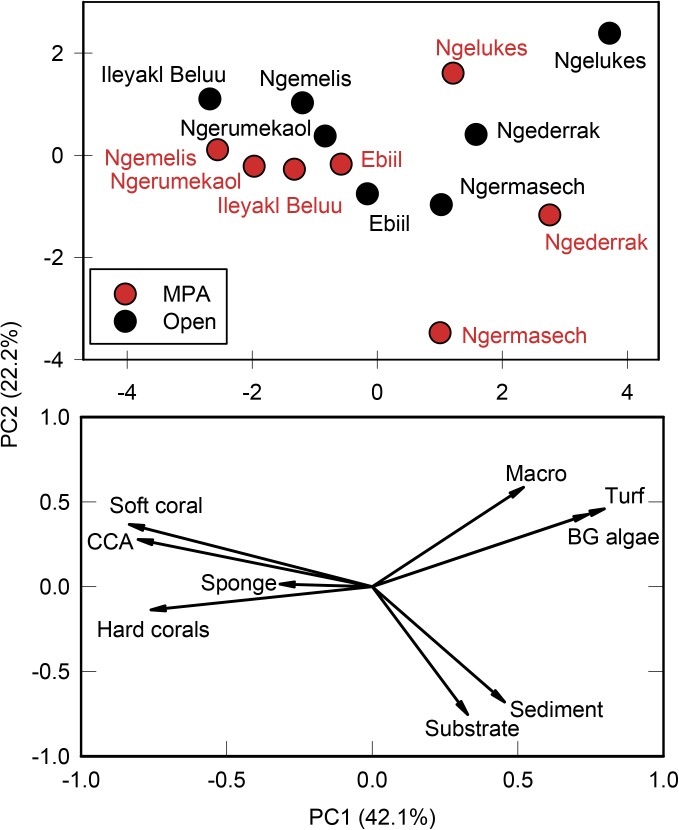
To explore the gradients in benthic community structure among sites, we performed a principal components analysis (PCA) on the percent cover of major benthic functional groups. Data were arcsine square root transformed to conform to the assumptions of the PCA. Non-metric multi-dimensional scaling (nMDS) analysis was conducted using PRIMER v6 [54] to examine differences in resource fish biomass among locations and between management regimes. A Bray–Curtis similarity matrix was constructed based on resource fish biomass, which was square root transformed prior to analysis.
Percent live coral cover was compared among locations and between management regimes using a generalized linear model (GLM) with a normal distribution and identity link function. Management (MPA vs. open) was treated as a fixed factor and locations were nested within management. Data were arcsine square root transformed prior to analysis. Percent live coral cover between MPA and open pairs of sites were tested using contrasts of the least squares means. Resource and non-resource fish biomass was compared using a GLM with a Poisson distribution and log link function, with contrasts between inside and outside MPAs performed as described above. Fish trophic biomass among locations was compared in a similar manner. All GLM analyses were performed using JMP Pro 12.2 [56].
To describe the pattern of fish trophic structure within MPAs and their relationship to MPA characteristics, we performed direct gradient analysis (redundancy analysis: RDA) using the ordination program CANOCO version 5.0 [57]. The RDA introduces a series of explanatory (environmental) variables and resembles the model of multivariate multiple regression, allowing us to determine what linear combinations of these explanatory variables determine the gradients. Data were centered, standardized, and log transformed fish trophic biomass by MPA. Explanatory variables consisted of MPA age, MPA size, distance from closest land, live coral cover, and benthic habitat characteristics [PC1, PC2]). PC1 and PC2 from the benthic PCA were used as variables to describe the benthic community among MPAs. To rank explanatory MPA variables in their importance for being associated with the structure of the fish assemblages, we used a forward selection where the statistical significance of each variable was judged by a Monte-Carlo unrestricted permutation test with 499 permutations [58].
Results
Benthic communities
Benthic community composition was not significantly different between MPAs and adjacent open areas (PERMANOVA pseudo-F1,127 = 0.44, p = 0.81, Table 2). Hard coral accounted for 50.6% (± 21.7 sd) of the overall benthic cover, followed by bare substrate (15.3% ± 15.1), CCA (9.1% ± 9.6), blue-green algae (6.2% ± 13.4), and macroalgae (6.0% ± 9.6). Based on SIMPER analysis, the average dissimilarity of benthic community composition between MPAs and open areas was only 33.4%. Although percent cover of hard coral was similar between MPAs and open areas (51.0 and 50.3%, respectively), it comprised 18.1% of the dissimilarity between management regimes. Bare substrate accounted for an additional 14.0% of the dissimilarity between management regimes, followed by blue-green algae (13.5), and CCA (11.9%).
Table 2. Comparison of benthic community composition among MPAs and adjacent open areas based on permutation-based multivariate analysis of variance (PERMANOVA).
| Source | df | MS | Pseudo-F | P(perm) |
|---|---|---|---|---|
| Management | 1 | 1092 | 0.44 | 0.813 |
| Location(Management) | 12 | 3155 | 8.37 | 0.001 |
| Residuals | 114 | 377 | ||
| Total | 127 |
The first two principal component axes (PC1 and PC2) described over 64% of the variation in benthic cover data (Fig 2). Forereef MPAs (Ebiil, Ileyakl Beluu, Ngemelis, and Ngerumekaol) and their adjacent open sites clustered together in ordination space, while inshore areas (e.g., Ngelukes, Ngermasech, and Ngederrak) were distinct from the forereef areas and there was less concordance between paired protected and open sites within these inshore areas. PC1 described the gradient from offshore to inshore sites, with the major loadings being soft coral, CCA, and coral in the offshore direction and turf algae, blue-green algae, and macroalgae loading towards the inshore areas. PC2 was weakly associated with management, with the major loadings being bare substrate and sediment towards the bottom of the biplot (MPAs), and algae (macroalgae, turf, blue-green) towards the top (open areas). Macroalgae was, on average, 46% higher in open areas compared to MPAs, although overall macroalgae cover was extremely low (~6%). Bare substrate was 33% higher in MPAs compared with open areas, and sediment was 89% greater inside MPAs although again, the overall cover of sediment was low (1.3% inside MPAs and 2.5% outside).
Fig 2. Principal component analysis (PCA) of major benthic groups from all sites.
Percent cover data were arcsine square root transformed prior to analysis. Top figure shows site separation while lower figure shows drivers that explain the most variance in the principal components.
Coral cover was not significantly different between MPAs and adjacent unprotected sites (χ2 1, 128 = 0.46, p = 0.50), except for the Ngederrak MPA, which had coral cover nearly two times lower than the adjacent open area (χ2 1, 24 = 9.54, p = 0.002). We found the highest coral cover in the Ngerumekaol MPA (68.2%), Ngerumekaol open area (62.9%), Ngemelis MPA (55.5%), and Ileyakl Beluu MPA (55.3%). The lowest coral cover was in the Ngederrak MPA (21.5%), which was affected more severely by the typhoon in 2013 than the adjacent open area [59].
Fishes
Fish biomass
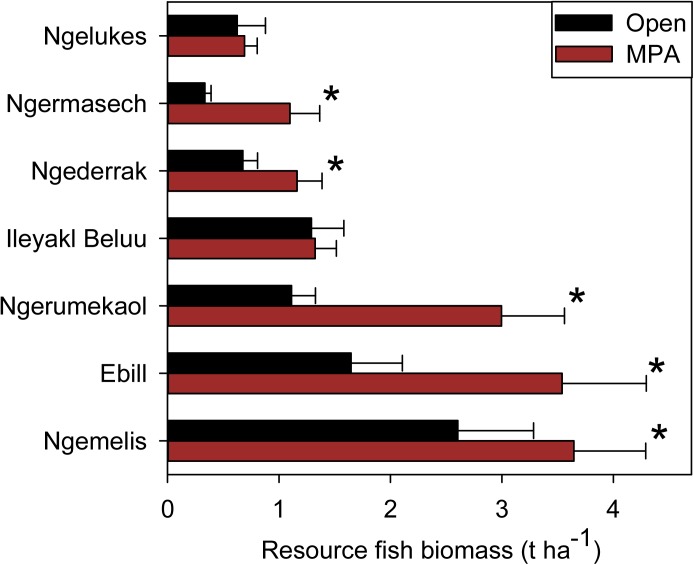
There were no significant differences in resource and non-resource fish biomass between depth strata (GLM, p > 0.05 for both), and samples were subsequently pooled. There was a highly significant difference in overall resource fish biomass between MPAs and open areas (χ2 1, 384 = 19.4, p < 0.001), but no significant difference in non-resource fish biomass (χ2 1, 384 = 0.20, p = 0.67). Resource fish biomass was significantly higher in five (Ebiil, Ngerumekaol, Ngederrak, Ngemelis, and Ngermasech) of the seven MPAs compared to their adjacent open areas (Fig 3). The most pronounced differences were found in the Ngermasech and Ngerumekaol MPAs, which had resource fish biomass 3.3 and 2.7 times higher, respectively, compared to their adjacent open areas. Variations in resource biomass within locations were relatively low, ranging from a CV of 11.6% at Ileyakl Beluu to 34.9% at Ngemelis
Fig 3. Comparison of resource fish biomass (t ha-1, mean ± standard error) inside and outside MPAs.
Asterisks denote MPA/open pairs that are significantly different.
Locations were well separated in ordination space based on fish species biomass (Fig 4). The first nMDS axis showed a strong gradient from nearshore to offshore locations moving from left to right along this axis. The second nMDS axis showed a gradient from MPAs to open areas moving from the bottom up along this axis, with the exception of the Ngederrak MPA, which was at the top of this axis.
Fig 4. Nonmetric multidimensional scaling plot of mean fish biomass for each MPA and adjacent open areas.
Arrows denote the direction and magnitude from open area to MPA in ordination space. Stress = 0.11.
Fish size and trophic structure
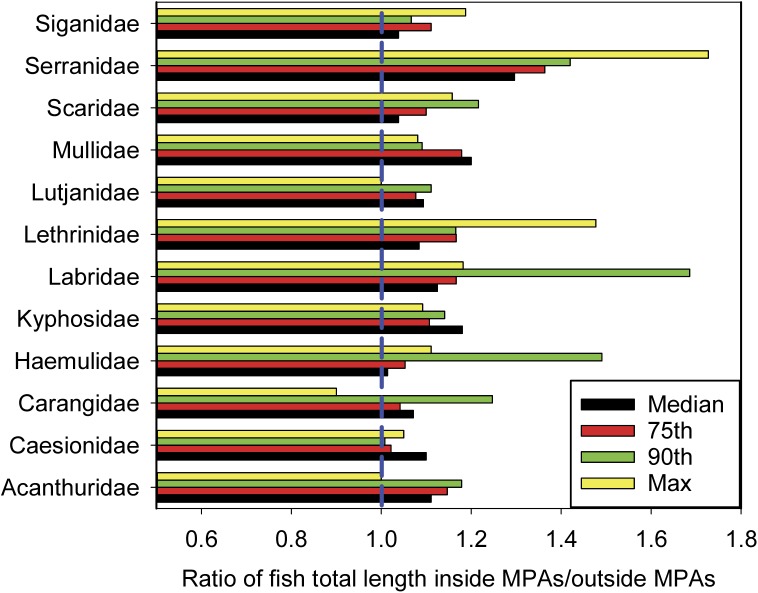
Examination of fish sizes inside vs. outside MPAs showed larger lengths inside MPAs for median, 75th and 90th percentiles, and maximum size for nearly all major families of fishes surveyed (Fig 5). Wrasses (Labridae), groupers (Serranidae), emperors (Lethrinidae), and grunts (Haemulidae) showed the largest differences.
Fig 5. Ratio of fish lengths (TL) by family inside versus outside MPAs based on median, 75th and 90th percentiles, and maximum size.
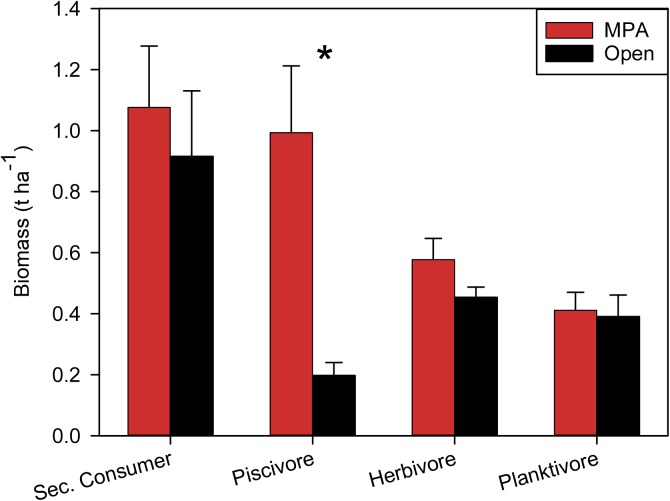
The interaction between management and biomass by trophic group was significant (χ2 1, 1536 = 70.2, p < 0.001). Contrasts in biomass between MPAs and open areas within trophic groups showed highly significant differences for top predators (χ2 1, 384 = 78.9, p < 0.001), but not for any other trophic group (all p > 0.05) (Fig 6). Top predators accounted for 32.5% of the biomass in MPAs, but only 10% in adjacent open areas. Secondary consumers comprised 35% of the biomass inside MPAs and 47% in open areas. Herbivores accounted for 18.9% of the biomass inside MPAs and 23.2% in open areas. Planktivores comprised 13% of the biomass within MPAs and nearly 20% in open areas.
Fig 6. Biomass (t ha-1, mean ± standard error) by fish trophic groups and management (open to fishing and MPA).
The asterisk identifies significant differences between MPA and adjacent open area.
Comparison of MPAs
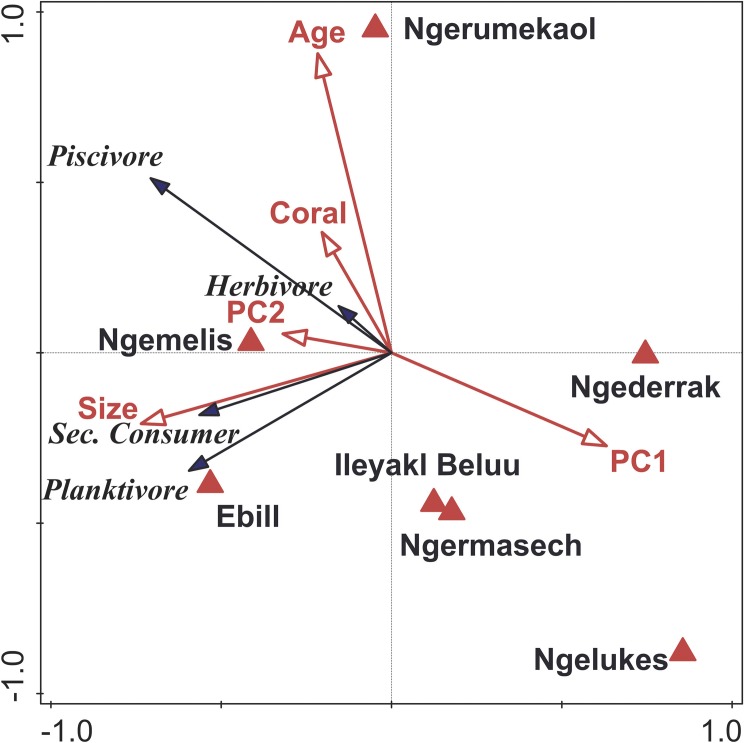
Our data show strong separation among MPAs based on fish trophic biomass (Fig 7, Table 3). The first two axes of the RDA biplot explained 53.5% of the trophic group variance and 96% of the trophic groups and MPA variables relationship (Table 3). In terms of trophic biomass structure, piscivores explained 50.0% of the cumulative fraction of variation explained by Axis 1, followed by planktivores, which explained an additional 26.2% of the cumulative variation. The only significant explanatory MPA variables involved in this ordination were MPA size and age, which were orthogonal to one another in ordination space. MPA size explained 52.2% of the variability in the fish trophic structure and MPA variable matrix, and separated MPAs along Axis 1. Years of protection (MPA age) explained 39.7% of the variability in this matrix and separated MPAs along Axis 2.
Fig 7. Biplot of results of redundancy analysis on fish biomass of trophic groups with MPA variables (MPA age, MPA size, distance from land, live coral cover, and benthic habitat [PC1, PC2]).
Data were centered, standardized, and log transformed fish biomass for trophic groups by MPA. MPA characteristics were centered and standardized prior to analysis.
Table 3. A. Results of redundancy analysis (RDA) on log-transformed, fish biomass data for trophic groups with MPA variables (size, age, distance from land, coral cover, PC1, and PC2).
B. Conditional effects of Monte-Carlo permutation results on the redundancy analysis (RDA).
| A. Axes | Axis 1 | Axis 2 | Axis 3 |
| Eigenvalues | 0.38 | 0.15 | 0.01 |
| Explained variation (cumulative) | 38.38 | 53.53 | 54.78 |
| Pseudo-canonical correlation | 0.81 | 0.71 | 0.43 |
| Explained fitted variation (cumulative) | 80.83 | 92.22 | 97.11 |
| B. Variable | Pseudo-F | P | % variance explained |
| MPA size | 8.2 | 0.002 | 52.2 |
| MPA age | 7.6 | 0.002 | 39.7 |
Discussion
The majority of the no-take MPAs in Palau surveyed during our expedition are effective in conserving resource fish biomass relative to adjacent fished sites. Resource fish biomass in Ngemelis and Ebiil (> 3 t ha-1) are comparable to that of pristine sites elsewhere in the Pacific [60–61]. The most striking difference in trophic structure between MPAs and fished areas was in the biomass of top predators (sharks, jacks, and groupers), which was 5 times larger in the MPAs compared to open areas. MPA size, and to a slightly lesser extent, age explained most of the variation in fish assemblage structure, particularly for piscivores, which are a major target of the local fisheries. Larger MPAs contain a greater amount and diversity of habitats, and have been shown to possess more and larger resource fishes compared with smaller MPAs [35, 45, 48]. The life history characteristics of coral reef fishes, especially for many large-bodied predators, are such that long-term (> 10 years) protection is necessary for fully recovery of populations [36, 46–48]. Several of the MPAs assessed in this study were specifically designed to protect these predator species, especially grouper spawning aggregations, which are particularly susceptible to overfishing [12, 62].
Palau possesses some of the best preserved and managed coral reefs remaining in the western Pacific [63–64], where much of the world’s marine biodiversity lies [65]. The level of enforcement of these MPAs is high, by most standards, due to strong local community support and patrolling [13]. Conservation rangers were present at every MPA we surveyed and there is general support for the PAN in Palau [13].
The use of traditional ecological knowledge in the establishment of Palau’s PAN has provided a customary framework to support western management, thereby creating greater acceptance by the local communities who manage these MPAs. The PAN consists of a wide variety of habitats and management regimes, ranging from complete no-take to subsistence fishing only. While our results only pertain to fully protected areas in the PAN, they may also have implications for other protected areas in the network.
There were no differences in coral cover and benthic community structure between MPAs and adjacent unprotected areas, therefore the greater abundance of resource fish inside MPAs is likely due to protection and not to differences in the state of the benthic communities. We did not detect differences in non-resource fish biomass, providing further evidence for the positive effects of protection from fishing. This highlights the fact that fishing, rather than other anthropogenic influences (e.g., pollution, habitat degradation) or intrinsic differences in local productivity or habitat quality, is likely primarily responsible for the observed differences in fish biomass between MPAs and adjacent areas open to fishing.
The habitat at forereef sites, for both MPAs and open areas, was dominated by CCA and hard coral, while turf algae, blue-green algae, and macroalgae characterized the inshore areas. Although macroalgae cover was low overall, it was nearly twice as high in open areas and may partially be in response to the higher herbivorous fish biomass in the MPAs compared with the open areas. Inshore areas, particularly around the large island of Babeldaob, suffer from the effects of sedimentation and pollution [4, 7, 66]. Both MPAs and areas open to fishing in these inshore areas had lower coral cover and high cover of macroalgae compared with more offshore reefs. Despite the poor habitat quality, inshore MPAs performed better than inshore areas open to fishing in terms of accumulating resource fish biomass. Reducing the effects of sedimentation and pollution in these inshore areas will likely improve fish biomass within these MPAs, as well as the areas open to fishing [10].
While our results are only a snapshot in time, they indicate that the no-take MPAs in Palau that we surveyed are meeting the goal of conservation of resource fishes. MPAs benefit adjacent fisheries by protecting large spawning individuals and through the spillover of adults into fished areas [67–70]. Networks of MPAs provide an option for increasing the ecological and economic benefits often provided by single MPAs [71]. The effectiveness of Palau’s extensive network of MPAs may likely benefit the nearshore fisheries of the entire country and improve the resilience of coral reefs by reducing their vulnerability to global climate change, and promote rapid recovery from natural impacts such as typhoons [58].
A comprehensive study by Houk et al. [10] used a robust and consistent methodology to examine the coral reef ecosystem condition in six jurisdictions across Micronesia: (i) the Marshall Islands, the states of (ii) Kosrae, (iii) Pohnpei, (iv) Chuuk, and (v) Yap, which comprise the Federated States of Micronesia, and (vi) Commonwealth of the Northern Mariana Islands. Using a number of biological metrics of fish and benthic assemblage structure, they found that only 42% of the major reef habitats examined exceeded the ecosystem-condition threshold of 70% established by the Micronesia Challenge [10]. MPAs in these jurisdictions showed little influence when grouped together across the region, emphasizing the limited amount of area currently located within MPAs in these other locations and the need for increased protection and better management, similar to those adopted by Palau.
Palau generates substantial income from tourism. A recent economic study in Palau showed that divers would be willing to pay more for diving in no-take MPAs because of more and larger fishes [72]. The economic benefits of more protection of just two charismatic species (Napoleon wrasse [maml] and bumphead parrotfish [kemedukl], currently protected in Palau) would be 100 to 1,000 times greater than the market value if those species were fished [72]. In addition, the value of live sharks in the water brings in $1.9 million to Palau’s economy through dive tourism, compared to $10,800 if these sharks were killed for sale [15]. These results suggest that greater levels of protection may bring greater economic revenue to Palau and could provide a model for other Pacific islands.
MPA effectiveness in Palau has been enhanced through the use of traditional knowledge combined with expert science and the development of MPA networks. Ownership, legacy, stewardship, and responsibility are essential elements of Palau’s approach to resource management and conservation [4]. Traditional approaches were, and still are, effective in managing human impacts on coral reefs and related resources in Palau [1, 73], and model legislation (Palau’s Marine Protection Act of 1994) was based on this traditional knowledge for protecting specific spawning sites and establishing fisheries closures.
While Palau’s MPAs are doing well relative to nearby areas open to fishing, previous work on spawning aggregation closures [12] and communications with fishermen indicate that fish abundance in Palau was much greater in the past. While in the ecosystem health of Palau’s MPAs are likely below historical baselines, they represent a step in the right direction towards recovery of the marine ecosystem, which is so critical to Palau and its people. The recent creation of the Palau National Marine Sanctuary protects ~500,000 km2 of its offshore waters, representing 80% of the country’s EEZ [74]. The protection provided by this new, large MPA around Palau could support increased diving tourism revenues, improve local fisheries, and ensure the long-term sustainability of marine resources.
Supporting information
Primary resource species denoted as yes. Main diet categories: herbivore, planktivore, secondary consumer, and piscivore.
(DOCX)
Acknowledgments
We would like to thank Koror State, Ngerchelong State, Ngchesar State, Ngarmdau State and Peleliu State for giving us permission to enter conduct surveys in their no entry MPAs. The National Marine Research Permit was granted by Ministry of Natural Resources, Environment and Tourism. We should like to acknowledge the Minister of Natural Resources, Environment and Tourism, Honorable F. Umiich Sengebau for his help and support. We would also like to acknowledge the fantastic logistical support provided by the Palau International Coral Reef Center. Thank you to Fish’n Fins, Tova and Navot Bornovski, and the captain and crew of the Ocean Hunter III for their help and support during the expedition.
Data Availability
Our data are available at Data Dryad: doi:10.5061/dryad.tp3j5.
Funding Statement
This work was supported by the National Geographic Society, www.nationalgeographic.com, ES; Blancpain, http://www.blancpain.com/, ES; Davidoff Cool Water, http://www.zinodavidoff.com/, ES; Jynwel Foundation, jynwelfoundation.org, ES; The Leona M. and Harry B. Helmsley Charitable Trust, www.helmsleytrust.org, ES; Lindblad Expeditions, www.expeditions.com, ES. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Johannes RE. Words of the lagoon: fishing and marine lore in the Palau district of Micronesia. Berkeley: University of California Press; 1981. [Google Scholar]
- 2.Johannes RE. The case for data-less marine resource management: examples from tropical nearshore finfisheries. Trends Ecol Evol. 1998;13: 243–246. [DOI] [PubMed] [Google Scholar]
- 3.Takeda J, Mad PK. Traditional Palauan lunar calendar and the fishing-gleaning activities of reef flats and/or in lagoons in the western Caroline Islands, Micronesia. Kagoshima Univ. Res. Center S. Pac. 1996;15: 91–106. [Google Scholar]
- 4.Richmond RH, Rongo T, Golbuu Y, Victor S, Idechong N, Davis G, et al. Watersheds and coral reefs: conservation science, policy, and implementation. BioScience. 2007;57: 598–607. [Google Scholar]
- 5.Johannes RE. Traditional marine conservation methods in Oceania and their demise. Annu Rev Ecol Evol Syst. 1978;9: 349–364. [Google Scholar]
- 6.Hughes JD. Palau: A parable for the twenty-first century. Capitalism Nature Socialism. 2005;16: 85–88. [Google Scholar]
- 7.Golbuu Y, Wolanski E, Harrison P, Richmond RH, Victor S, Fabricius KE. Effects of land-use change on characteristics and dynamics of watershed discharges in Babeldaob, Palau, Micronesia. J Mar Biol. 2010;7: 2011. [Google Scholar]
- 8.Baker N, Beger M, McClennen C, Ishoda A, Edwards F. Reimaanlok: a national framework for conservation area planning in the Marshall Islands. J Mar Biol. 2010;29: 2011. [Google Scholar]
- 9.Jupiter S, Mangubhai S, Kingsford RT. Conservation of biodiversity in the Pacific Islands of Oceania: challenges and opportunities. Pac Conserv Biol. 2014;20: 206–220. [Google Scholar]
- 10.Houk P, Camacho R, Johnson S, McLean M, Maxin S, Anson J, et al. The Micronesia Challenge: assessing the relative contribution of stressors on coral reefs to facilitate science-to-management feedback. PloS ONE. 2015;18: e0130823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marino S, Bauman A, Miles J, Kitalong A, Bukurou A, Mersai C, et al. The state of coral reef ecosystems of Palau. In: Wadell JE, Clarke AM, editors. The state of coral reef ecosystems of the United States and Pacific Freely Associated States. NOAA Technical Memorandum NOS NCCOS 73. 2008. pp. 511–539.
- 12.Golbuu Y, Friedlander AM. Spatial and temporal characteristics of grouper spawning aggregations in marine protected areas in Palau, western Micronesia. Estuar Coast Shelf Sci. 2011;92: 223–231. [Google Scholar]
- 13.Gruby RL, Basurto X. Multi-level governance for large marine commons: politics and polycentricity in Palau's protected area network. Environ Sci Policy. 2013;33: 260–272. [Google Scholar]
- 14.Fitzpatrick SM, Kataoka O. Prehistoric fishing in Palau, Micronesia: evidence from the northern Rock Islands. Archaeol Oceania. 2005;40: 1–3. [Google Scholar]
- 15.Vianna GM, Meekan MG, Pannell DJ, Marsh SP, Meeuwig JJ. Socio-economic value and community benefits from shark-diving tourism in Palau: a sustainable use of reef shark populations. Biol Cons. 2012;145: 267–277. [Google Scholar]
- 16.Koshiba S, Besebes M, Soaladaob K, Ngiraingas M, Isechal AL, Victor S, et al. 2000 years of sustainable use of watersheds and coral reefs in Pacific Islands: A review for Palau. Estuar Coast Shelf Sci. 2014;144: 19–26. [Google Scholar]
- 17.Dawson MN, Martin LE, Penland LK. Jellyfish swarms, tourists, and the Christ-child. Hydrobiologia. 2001;451: 131–144. [Google Scholar]
- 18.Yamashita S. The Japanese encounter with the South: Japanese tourists in Palau. Contemp Pac. 2000;12: 437–463. [Google Scholar]
- 19.Poonian C, Davis PZ, McNaughton CK. Impacts of Recreational Divers on Palauan Coral Reefs and Options for Management. Pac Sci. 2010;64: 557–565. [Google Scholar]
- 20.International Monetary Fund (IMF). Republic of Palau. IMF Country Report No. 14/110. 2014; https://www.imf.org/external/pubs/ft/scr/2014/cr14110.pdf
- 21.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413: 591–596. 10.1038/35098000 [DOI] [PubMed] [Google Scholar]
- 22.Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318: 1737–1742. 10.1126/science.1152509 [DOI] [PubMed] [Google Scholar]
- 23.Hughes TP, Graham NA, Jackson JB, Mumby PJ, Steneck RS. Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol. 2010;25: 633–642. 10.1016/j.tree.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 24.Browman HI, Cury PM, Hilborn R, Jennings S, Lotze HK, Mace PM, et al. Ecosystem-based management. Mar Ecol Prog Ser. 2004;274: 269–303. [Google Scholar]
- 25.Palumbi SR, Sandifer PA, Allan JD, Beck MW, Fautin DG, Fogarty MJ, et al. Managing for ocean biodiversity to sustain marine ecosystem services. Front Ecol Environ. 2009;7: 204–211. [Google Scholar]
- 26.Ruckelshaus M, Klinger T, Knowlton N, DeMaster DP. Marine ecosystem-based management in practice: scientific and governance challenges. BioScience. 2008;58: 53–63. [Google Scholar]
- 27.Sumaila UR, Guénette S, Alder J, Chuenpagdee R. Addressing ecosystem effects of fishing using marine protected areas. ICES J Mar Sci. 2000;57: 752–760. [Google Scholar]
- 28.Roberts CM, Hawkins JP, Gell FR. The role of marine reserves in achieving sustainable fisheries. Philos Trans R Soc Lond B Biol Sci. 2005;360: 123–32. 10.1098/rstb.2004.1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellwood DR, Hughes TP, Folke C, Nyström M. Confronting the coral reef crisis. Nature. 2004;429: 827–833. 10.1038/nature02691 [DOI] [PubMed] [Google Scholar]
- 30.Halpern BS, Regan HM, Possingham HP, McCarthy MA. Accounting for uncertainty in marine reserve design. Ecol Lett. 2006;9: 2–11. 10.1111/j.1461-0248.2005.00827.x [DOI] [PubMed] [Google Scholar]
- 31.Friedlander AM. Marine protected areas In: Gillespie R, Clague DA, editors. Encyclopedia of Islands. Berkeley: University of California Press; 2009. pp. 607–610. [Google Scholar]
- 32.Attwood CG, Bennett BA. Modelling the effect of marine reserves on the recreational shore-fishery of the south-western Cape, South Africa. S Afr J Mar Sci. 1995;16: 227–40. [Google Scholar]
- 33.Russ GR, Alcala AC. Do marine reserves export adult fish biomass? Evidence from Apo Island, central Philippines. Mar Ecol Prog Ser. 1996;132: 1–9. [Google Scholar]
- 34.Friedlander AM, Brown EK, Jokiel PL, Smith WR, Rodgers KS. Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian archipelago. Coral Reefs. 2003;22: 291–305. [Google Scholar]
- 35.Claudet J, Osenberg CW, Benedetti‐Cecchi L, Domenici P, García‐Charton JA, Pérez‐Ruzafa Á, et al. Marine reserves: size and age do matter. Ecol Lett. 2008;11: 481–489. 10.1111/j.1461-0248.2008.01166.x [DOI] [PubMed] [Google Scholar]
- 36.Babcock RC, Shears NT, Alcala AC, Barrett NS, Edgar GJ, Lafferty KD, et al. Decadal trends in marine reserves reveal differential rates of change in direct and indirect effects. P Natl Acad Sci USA. 2010;107: 18256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.e Costa BH, Claudet J, Franco G, Erzini K, Caro A, Gonçalves EJ. A regulation-based classification system for Marine Protected Areas (MPAs). Mar Policy. 2016;72: 192–198. [Google Scholar]
- 38.Dayton PK, Sala E, Tegner MJ, Thrush S. Marine reserves: parks, baselines, and fishery enhancement. Bull Mar Sci. 2000;66: 617–34. [Google Scholar]
- 39.Allison GW, Gaines SD, Lubchenco J, Possingham HP. Ensuring persistence of marine reserves: catastrophes require adopting an insurance factor. Ecol Appl. 2003;13: S8–24. [Google Scholar]
- 40.Airamé S, Dugan JE, Lafferty KD, Leslie H, McArdle DA, Warner RR. Applying ecological criteria to marine reserve design: a case study from the California Channel Islands. Ecol Appl. 2003;13: S170–184. [Google Scholar]
- 41.Toonen RJ, Wilhelm TA, Maxwell SM, Wagner D, Bowen BW, Sheppard CR, et al. One size does not fit all: the emerging frontier in large-scale marine conservation. Mar Poll Bull. 2013;15: 7–10. [DOI] [PubMed] [Google Scholar]
- 42.Bartholomew A, Bohnsack JA, Smith SG, Ault JS, Harper DE, McClellan DB. Influence of marine reserve size and boundary length on the initial response of exploited reef fishes in the Florida Keys National Marine Sanctuary, USA. Landscape Ecol. 2008;23: 55–65. [Google Scholar]
- 43.McLeod E, Salm R, Green A, Almany J. Designing marine protected area networks to address the impacts of climate change. Front Ecol Environ. 2009;7: 362–70. [Google Scholar]
- 44.Halpern BS. The impact of marine reserves: do reserves work and does reserve size matter?. Ecol Appl. 2003;13: S117–137. [Google Scholar]
- 45.Lester SE, Halpern BS, Grorud-Colvert K, Lubchenco J, Ruttenberg BI, Gaines SD, et al. Biological effects within no-take marine reserves: a global synthesis. Mar Ecol Prog Ser. 2009;384: 33–46. [Google Scholar]
- 46.Edgar GJ, Barrett NS. Effects of the declaration of marine reserves on Tasmanian reef fishes, invertebrates and plants. Exp Mar Biol Ecol. 1999;242: 107–44. [Google Scholar]
- 47.Russ GR, Alcala AC. Marine reserves: long-term protection is required for full recovery of predatory fish populations. Oecologia. 2004;138: 622–627. 10.1007/s00442-003-1456-4 [DOI] [PubMed] [Google Scholar]
- 48.Barrett NS, Edgar GJ, Buxton CD, Haddon M. Changes in fish assemblages following 10 years of protection in Tasmanian marine protected areas. J Exp Mar Biol Ecol. 2007;345: 141–157. [Google Scholar]
- 49.Malcolm HA, Jordan A, Creese RG, Knott NA. Size and age are important factors for marine sanctuaries: evidence from a decade of systematic sampling in a subtropical marine park. Aquat Conserv. 2016;26: 1090–1106. [Google Scholar]
- 50.Battista TA, Costa BM, Anderson SM. Shallow-Water Benthic Habitats of the Republic of Palau. 2007; NOAA Technical Memorandum. NOS NCCOS 59, Biogeography Branch. Silver Spring, MD.
- 51.Mapstone BD, Ayling AM. An investigation of the optimum methods and unit sizes or the visual estimation of abundance of some coral reef organisms. 1998; Great Barrier Reef Mar Park Auth Res Publ No. 47, Townsville, QLD.
- 52.Friedlander AM, Sandin SA, DeMartini EE, Sala E. Spatial patterns of the structure of reef fish assemblages at a pristine atoll in the central Pacific. Mar Ecol Prog Ser. 2010;410: 219–231. [Google Scholar]
- 53.Froese R, Pauly D. FishBase; 2011. World Wide Web electronic publication. www.fishbase.org. Accessed 11 June 2016.
- 54.Anderson MJ, Gorley RN, Clarke KR. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. Plymouth, UK. PRIMER-E, 2008.
- 55.Clarke KR. Non-parametric multivariate analyses of changes in community structure. Aust J Ecol. 1993;18: 117–143. [Google Scholar]
- 56.JMP Pro 12.2, SAS Institute Inc., Cary, NC, 1989–2007.
- 57.ter Braak CJF, Šmilauer P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0. Ithaca, NY, USA. Microcomputer Power: 2012.
- 58.ter Braak CJ, Verdonschot PF. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci. 1995;57: 255–289. [Google Scholar]
- 59.Gouezo M, Golbuu Y, van Woesik R, Rehm L, Koshiba S, Doropoulos C. Impact of two sequential super typhoons on coral reef communities in Palau. Mar Ecol Prog Ser. 2015;540: 73–85. [Google Scholar]
- 60.Knowlton N, Jackson JB. Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol. 2008;6: e54 10.1371/journal.pbio.0060054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandin SA, Smith JE, DeMartini EE, Dinsdale EA, Donner SD, et al. Degradation of coral reef communities across a gradient of human disturbance. PLoS ONE 2008;3(2): e1548 10.1371/journal.pone.0001548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Mitcheson, Cornish A, Domeier M, Colin PL, Russell M, Lindeman KC. A global baseline for spawning aggregations of reef fishes. Conserv Biol. 2008;22: 1233–44. 10.1111/j.1523-1739.2008.01020.x [DOI] [PubMed] [Google Scholar]
- 63.Golbuu Y, Bauman A, Kuartei J, Victor S. The state of coral reef ecosystem of Palau. In: Wadell J, editor. The state of coral reef ecosystems of the United States and Pacific freely associated states: 2005. Silver Spring, MD. NOAA Technical Memorandum NOS NCCOS 11, pp 488–507.
- 64.Golbuu Y, Victor S, Penland L, Idip D Jr, Emaurois C, Okaji K, et al. Palau’s coral reefs show differential habitat recovery following the 1998-bleaching event. Coral Reefs. 2007;26: 319–332. [Google Scholar]
- 65.Tittensor DP, Mora C, Jetz W, Lotze HK, Ricard D, Berghe EV, et al. Global patterns and predictors of marine biodiversity across taxa. Nature. 2010;466: 1098–1101. 10.1038/nature09329 [DOI] [PubMed] [Google Scholar]
- 66.Golbuu Y, Van Woesik R, Richmond RH, Harrison P, Fabricius KE. River discharge reduces reef coral diversity in Palau. Marine Poll Bull. 2011;62: 824–831. [DOI] [PubMed] [Google Scholar]
- 67.Russ GR, Alcala AC, Maypa AP. Spillover from marine reserves: the case of Naso vlamingii at Apo Island, the Philippines. Mar Ecol Prog Ser. 2003;264: 15–20. [Google Scholar]
- 68.Russ GR, Alcala AC, Maypa AP, Calumpong HP, White AT. Marine reserve benefits local fisheries. Ecol Appl. 2004;14: 597–606. [Google Scholar]
- 69.Tupper MH. Spillover of commercially valuable reef fishes from marine protected areas in Guam, Micronesia. Fish Bull. 2007;105: 527–537. [Google Scholar]
- 70.Harmelin-Vivien M, Le Diréach L, Bayle-Sempere J, Charbonnel E, García-Charton JA, Ody D, et al. Gradients of abundance and biomass across reserve boundaries in six Mediterranean marine protected areas: Evidence of fish spillover?. Biol Conserv. 2008;141: 1829–1839. [Google Scholar]
- 71.Grorud-Colver K, Claudet J, Tissot BN, Caselle JE, Carr MH, Day JC, et al. Marine protected area networks: assessing whether the whole is greater than the sum of its parts. PloS ONE, 2014;9(8): e102298 10.1371/journal.pone.0102298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koike H, Friedlander A, Oleson K, Koshiba S, Polloi K. Final Report on Diver’s Perception Survey for Palau’s Kemedukl and Maml: Stock Assessment for Humphead Wrasse and Bumphead Parrotfish. Koror, Palau.PICRC Technical Report 14–02. Palau International Coral Reef Center. http://picrc.org/picrcpage/wp-content/uploads/2016/01/WTP_Survey_For_Maml_Kemedukl_FINAL.pdf
- 73.Johannes RE. Traditional coral-reef fisheries management In: Birkeland C, editor. Life and death of coral reefs. New York: Chapman and Hall; 1997. pp. 380–385. [Google Scholar]
- 74.Lubchenco J, Grorud-Colvert K. Making waves: The science and politics of ocean protection. Science., 2015;340: 382–383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary resource species denoted as yes. Main diet categories: herbivore, planktivore, secondary consumer, and piscivore.
(DOCX)
Data Availability Statement
Our data are available at Data Dryad: doi:10.5061/dryad.tp3j5.