Abstract
Fatigue that persists for 6 months or more is termed chronic fatigue. Chronic fatigue (CF) in combination with a minimum of 4 of 8 symptoms and the absence of diseases that could explain these symptoms, constitute the case definition for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). Inflammation, immune system activation, autonomic dysfunction, impaired functioning in the hypothalamic-pituitary-adrenal axis, and neuroendocrine dysregulation have all been suggested as root causes of fatigue. The identification of objective markers consistently associated with CFS/ME is an important goal in relation to diagnosis and treatment, as the current case definitions are based entirely on physical signs and symptoms. This review is focused on the recent literature related to biomarkers for fatigue associated with CFS/ME and, for comparison, those associated with other diseases. These markers are distributed across several of the body’s core regulatory systems. A complex construct of symptoms emerges from alterations and/or dysfunctions in the nervous, endocrine and immune systems. We propose that new insight will depend on our ability to develop and deploy an integrative profiling of CFS/ME pathogenesis at the molecular level. Until such a molecular signature is obtained efforts to develop effective treatments will continue to be severely limited.
Background
Fatigue is a common symptom that includes both physical and mental components. It can be an acute response to physical, mental or infectious triggers and usually decreases as the trigger recedes. It can occur in healthy people as a result of physical and/or mental exertion. Symptoms of fatigue persisting for 6 months or more define chronic fatigue (CF). A survey of 2323 residents of Norway by Loge, et al. (1998) reported CF to be endorsed by 11% of the general population. Of those respondents reporting no known disease or current heath problem, 7% had CF. In contrast, in Japan, more than half of the general adult population complained of fatigue, and more than one third of the population endorsed CF (Watanabe, et al, 2008)
Disease associated fatigue may be directly related to the disease mechanisms (primary fatigue) or may be secondary to non-disease-specific factors. Measurement of fatigue can be from either the subjective or the objective standpoint and includes physical fatigue, reduced motivation, reduced activity, and mental fatigue. The magnitude and scope of debilitating fatigue is a central component in health care where chronic illness is a growing concern. Current acute illness research models are inadequate for resolving chronic disorders affecting multiple regulatory systems and presenting with complex constellations of symptoms. Fatigue is a fundamental component in a diverse array of illnesses that affect a broad patient demographic.
There is a growing body of research directed toward understanding the biology of fatigue; this research leads us to regard fatigue as a complex construct of symptoms that emerges from alteration and/or dysfunction in the nervous, endocrine and immune systems. Identifying biomarkers of CF is an important part of this effort. The official NIH definition of a biomarker is: "a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention." (Biomarkers Definitions Working Group, 2001). In this review of recent work, we selected examples of research on CF occurring in disease with an emphasis on chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). The biomarker research examined included several levels of biology ranging from the intracellular (transcript) to the inter-cellular (cytokine and hormone) and behavioral levels (clinical and psychometric measures). Our aim in the selection of studies was not to be inclusive, but rather to include a representative sample of recent CF studies. Most commonly, CFS/ME biology and that of other illnesses has focused on the detailed characterization of individual components taken in isolation. It is now clear that further understanding of disease mechanisms will require more than a list of the abundance of gene products, proteins or cells. These various cellular and molecular components are highly inter-related. Depending on the context various biological networks will become active to ensure appropriate regulatory feedback for maintaining homeostasis. In a review of CFS/ME, published in Lancet, Prins, et al. (2006) stated: “Techniques such as bioimaging and proteomic strategies, and perhaps a systems biology approach, should be applied to try to elucidate such complicated interactions”. Advances in multiplex laboratory tests and bioinformatics allow fatigue research to advance beyond individual biomarkers to modular patterns of co-expression.
Biomarkers associated with fatigue in patients with identified diseases or syndromes other than CFS/ME (Table 1)
Table 1.
Biomarkers associated with fatigue in patients with identified diseases or syndromes (other than CFS/ME) and selected from recent studies
| Disease associated with fatigue | Biomarker associated with fatigue | Reference |
|---|---|---|
| Sjögren's syndrome | >IL-1Ra in CSF | Harboe et al, 2009 |
| Rheumatoid arthritis | >Stimulated production of IL-6 | Davis et al, 2008 |
| Depression & anxiety | Stebbings, et al, 2010 | |
| Absence of RF; >DAS-28; >pain | van Hoogmoed, et al, 2010 | |
| Systemic lupus erythematosus | >Serum IFNα | Lee et al, 2010 |
| >CRP | Rezaieyazdi, et al, 2011 | |
| Inflammatory bowel disease | >CRP | Graff et al, 2010 |
| Hemoglobin; GI symptoms; altered sleep | Jesness-Jorgensen, 2011 | |
| Multiple sclerosis | >Frequency of TNFα | Gold, et al, 2011 |
| >TNFα mRNA in T cells | Flachenecker et al, 2006 | |
| Post exercise >β-1 & β-2 adrenergic & < TLR4 by gene expression | White, et al, 2012 | |
| Obstructive sleep apnea | > Plasma sTNFRI | Al-shair, et al, 2011 |
| Hypoxia | Sickness behavior | Sherry, et al, 2009 |
| >Plasma L-1β & IL-1R1 | Basu, et al, 2005 | |
| Chronic Obstructive Pulmonary Disease | >Plasma TNFα | Mills, et al, 2008 |
| Breast cancer before treatment | >CMV antibody titers | Fagundes, et al, 2012 |
| Breast cancer soon after treatment | >sTNFRll | Bower et al, 2011b |
| Breast & prostate cancer with radiation | >Plasma CRP & IL-1Ra | Bower, et al, 2009 |
| Advanced cancer | >Plasma IL-6, TNFα | Kwak et al, 2011 |
| >Plasma cortisol, ACTH, epinephrine & norepinephrine. | Thornton, et al, 2010 | |
| Non-small cell lung cancer with concurrent chemoradiation | >Plasma IL-6, IL-10 & sTNF-R1 | Wang, et al, 2010 |
| Testicular cancer, long term survivors | >Plasma IL-1Ra & CRP | Orre, et al, 2009 |
| Breast cancer survivors | >Transcripts for NF-κB & < transcripts glucocorticoids | Bower et al, 2011a |
| >CRP | Orre, et al, 2011 | |
| >HPA and SNS dysfunction | Fernandez-de-las-Penas, 2012 | |
| Cancer | >Plasma IL-6, IL-1Rα, neopterin | Schubert, et al, 2006 review |
In a recent review, Graff, el al, (2011) noted reports of moderate to severe fatigue in 50 to 70% of the cases in immune-mediated inflammatory diseases. As reported by the New York Times (Crouse, 2011) following her withdrawal August 31, 2011 from the US Open tennis tournament, Venus Williams, winner of seven Grand Slam singles titles, said, “The fatigue is hard to explain unless you have it. Some mornings I feel really sick, like when you don’t get enough sleep or you have flu or a cold. I always have some level of tiredness. And the more I tried to push through it, the tougher it got.” Williams received the diagnosis of the autoimmune disorder, Sjögren's syndrome, a condition correlated with CF, earlier in the month. This CF is similar to “sickness behavior” in animals which is mediated by pro-inflammatory cytokines, in particular interleukin (IL)-1, acting on neuronal brain cells (Dantzer, 2001). Increased levels of IL-1 receptor antagonist (IL-1Ra) in the cerebrospinal fluid were associated with increasing fatigue in primary Sjögren's syndrome patients, supporting the suggestion that the IL-1 system is a possible biomarker of fatigue (Harboe et al., 2009).
Davis et al, 2008 reported elevation of the pro-inflammatory cytokine, IL-6, in stimulated mononuclear cells from fatigued patients with rheumatoid arthritis (RA). The strongest correlates of fatigue in the RA cohort studied by Stebbings, et al. (2010) were depression (P < 0.001) and anxiety (P < 0.001). Comparing a severely fatigued group of RA patients with cases less fatigued, van Hoogmoed, et al, (2010) reported that the proportion of patients negative for rheumatoid factor (RF) was larger in the more fatigued group. However, the DAS-28 (a measure of disease severity), the number of tender joints, swollen joints and the general health rating were significantly worse in the severely fatigued RA patients as were worse pain scores. In contrast, erythrocyte sedimentation rate, hemoglobin level and acute phase reactant C-reactive protein (CRP) did not differ between the two groups, nor did they correlate with fatigue severity. A marker of inflammation, CRP is produced mainly by hepatocytes, and its production is regulated by IL-6 (Boncler & Watała, 2009). In systemic lupus erythematous (SLE), serum values of CRP were significantly higher (p < 0.001) in patients compared with healthy controls. However, CRP did not distinguish disease severity (Rezaieyazdi, et al, 2011). According toLee et al. (2010) patients with SLE often present with flu-like symptoms including fatigue and with high serum interferon (IFN) γ levels.
In 318 individuals with inflammatory bowel disease (IBD), elevated CRP occurred in both Crohn’s disease (CD) and ulcerative colitis (UC). However extreme levels of CRP (>20mg/L) was observed most frequently in CD. High levels of fatigue occurred in 78% of patients with CD and in 67% of those diagnosed with UC (Graff, et al., 2010). In another report, CF was found in 29% (14/48) of CD and 22% (20/92) of UC compared to 11% (260/2287) of healthy controls (P < 0.001 for both diagnoses). Linear regression analysis confirmed hemoglobin values, present gastrointestinal symptoms, and altered sleep to be the most important predictors of CF (Jelsness-Jørgensen, et al., 2011). Fatigue can also be the unintended consequence of therapeutic approaches. A published example is the use of azathioprine or 6-mercaptopurine for treatment of IBD (Lee, et al., 2009).
Neurological disorders such as multiple sclerosis (MS) are associated with fatigue. MS patients with comorbid major depressive disorder (MDD) showed normal morning but elevated evening salivary cortisol levels, resulting in a flattened slope. While a higher frequency of tumor necrosis factor (TNF)α and IFNγ production by stimulated CD8+ T cells was also seen in MS patients with MDD, these markers were more closely associated with fatigue than depression (Gold, et al., 2011). Cytokine mRNA expression for TNFα was measured by real time polymerase chain reaction (RT PCR) and found to be positively correlated with fatigue in MS cases (Flachenecker, et al. 2004). A useful paradigm in biomarker research in fatiguing illnesses involves an exercise challenge model with sampling during and after the challenge. White, et al, (2012) used this approach to study patients with MS. Compared to controls, MS cases had greater post-exercise increases than controls in gene expression of β-1 and β-2 adrenergic receptors (1.4 ± 0.27- and 1.3 ± 0.06-fold increases, respectively, p = .02 and p < .001) and greater decrease in gene expression for toll-like receptor 4 (TLR4) (p = .02). Post-exercise, IL-10 and TLR4 decreases correlated with higher fatigue scores.
Hypoxia associated with stroke, heart attacks, lung disease and altitude sickness is often accompanied with sickness behavior, including fatigue, malaise and lethargy (Sherry, et al., 2009). Basu, et al.,(2005) hypothesized that these symptoms are due to the neuroinflammation subsequent to elevated cytokines and cytokine receptors, particularly elevated IL-1β and IL-1R1. Fatigued subjects with obstructive sleep apnea had elevated plasma soluble TNF receptor 1 (sTNFRI) (Al-shair, et al., 2011). Those with chronic obstructive pulmonary disease had elevated plasma TNFα (Mills, et al., 2008).
Reported by as many as 40% of cancer patients at diagnosis, cancer-related fatigue is a frequent early symptom of malignant disease that often becomes chronic. In breast cancer significant fatigue persisted in over 30% of women 10 years following chemotherapy (Bower, 2007). This is a significant patient community with breast cancer accounting for 25% of the $157 billion cost of malignant disease in the US (Radice and Redaelli, 2003). A recent study of breast cancer survivors found increased expression of genes coding for transcripts for NF-κB and decreased expression for glucocorticoids in subjects with fatigue as compared to those without (Bower et al., 2011a). Fernandez-de-las-Penas, et al (2012) reported that breast cancer survivors with the Met/Met genotype had greater hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS) dysfunction that was correlated with severe fatigue. The Met/Met genotype results from substitution of valine with methionine at codon 158 on chromosome 22q11, resulting in lower catechol-O-methyltransferase activity, which may affect estrogen metabolism.
Patients with lung cancer during concurrent chemoradiation therapy developed significant symptom burden including fatigue and elevated plasma IL-6 and sTNFRI (Wang, et al., 2010). Liu, et al, 2012 studied fatigue associated with chemotherapy in breast cancer patients and the inflammatory markers, IL-6, IL-1 receptor antagonist (IL-1Ra) before and during cycle 1 and cycle 4 of chemotherapy. Fatigue increased, levels of IL-6 increased and IL-1Ra decreased during chemotherapy. Orre, et al, (2011) reported that fatigue levels in breast cancer survivors were significantly and positively associated with CRP (p<.001) and leukocyte count (p=.018), but not with levels of IL-1Ra, IL-6, sTNF-R1 or neopterin in unadjusted analyses. Only CRP remained significantly associated with fatigue levels in the fully adjusted models (p=.020). Bower, et al, (2011b) examined the relationships of markers of inflammation, IL-1, IL-1Ra, sTNFRII and CRP, to fatigue in women with breast cancer early after primary treatment. Significant correlation was observed only with elevations in sTNFR11. Thornton, et al., (2010) looked at co-occurrence of pain, depression, and fatigue in advanced cancer. They reported that these 3 symptoms were associated with biological mediators including elevated plasma levels of cortisol and adrenocorticotropic hormone indicating HPA activation and elevated plasma epinephrine and norepinephrine indicating sympathetic nervous system activation.
Fagundes, et al, (2012) sought to determine biomarkers of fatigue that exist before cancer treatment. Relationships between the expression of latent Epstein–Barr virus (EBV) and cytomegalovirus (CMV) and fatigue were examined in 158 women newly diagnosed with breast cancer or awaiting a positive diagnostic result. Higher CMV antibody titers, but not EBV antibody titers, were associated with a greater likelihood of being fatigued. Associations between fatigue and higher CMV antibody titers remained after controlling for alcohol use, smoking, comorbidities, depressive symptoms, age, BMI, cancer stage, and sleep problems. More sleep problems and higher levels of depressive symptoms were also associated with a greater likelihood of being fatigued.
Cytokines or their receptors and antagonists were measured in 56% of the papers on disease-associated fatigue reviewed. However, the cytokines, the methods of analyses and source of samples, plasma, CSF, intracellular in lymphocytes and stimulated cell culture supernatants, varied widely among the studies. Elevated IL-6 was noted in each of the 5 papers were it was measured, as was TNFα. In only 2 of the 5 were results for both proinflammatory cytokines included. The general marker of inflammation, CRP, was elevated in those studies in which it was measured. Dysregulation of the HPA axis and SNS were, as expected, associated with CF. Analytic methods focused on the expression of individual molecules are unlikely to be fruitful in the discovery of clinically useful biomarkers. Incorporation of these markers into integrative network-based analyses will allow identifying associations at the pathway level.
Biomarkers of chronic fatigue syndrome/myalgic encephalomyelitis
We agree with the suggestion of Silverman, et al (2010) that CFS/ME is an excellent model for addressing the biology of CF. All cases of CFS/ME have CF, which is required by the case definitions (Fukuda et al., 1994; Carruthers, et al, 2011). Some relevant literature on a related syndrome, gulf war illness (GWI), also associated with CF, is included in this review. Population-based studies estimate the prevalence of CFS/ME at 0.23% to 0.41% (Reyes et al, 2003; Jason et al, 1999). Hypothetical initiating events for CFS/ME include infections, stress and exposure to toxins (Evengård & Klimas, 2002; Glaser, et al, 2005; Maes, et al, 2007). The possibility of infectious disease and the level of immune impairment have convinced several research groups involved in pathogen discovery to regard CFS/ME with interest. Duration of illness typically exceeds 10 years. Persistence may involve complex interactions of immune, autonomic and neuroendocrine regulation and remains poorly understood (Bou-Holaigah, 1995; Fuite, 2008; Hurwitz, 2009). Current CFS/ME treatments are directed at reducing symptom severity but no cure exists for this condition. It follows that this cause of chronic disability has far-reaching consequences and constitutes a significant public health concern and economic burden to society as a whole.
A sample of recent work on molecular diagnosis and associated sub-typing is shown in Table 2. In 27% of the reports cited, cytokines were studied. Fletcher, et al., (2009), in a baseline study using a multiplex chemoluminescent method (Quansys, Logan, Utah) of 16 plasma cytokines in 40 CFS/ME patients compared to 59 controls, reported higher levels of IL-4, IL-5, IL-12, lymphotoxin α (LTα), IL-1α, IL-1β, IL-6, lower amounts of IL-8, IL-13, IL-15 and not different IL-2, IFNγ, IL-10, IL-17, IL-23 and TNFα. Brenu et al., (2011) examined supernatants from PHA stimulated peripheral blood cells and found elevated levels of IL-10, IFNγ and TNFα in 95 CFS/ME patients compared to 50 controls, with both groups at rest. Natural killer (NK) cell function (NKCC) was examined by these two research groups. Brenu, et al (2011) and Fletcher, et al (2010) found NKCC to be low, confirming many past reports. Mayer, et al (2005) reported that the number of molecules of intracellular perforin per NK cells, as determined by quantitative intracellular flow cytometry, was related to NK cell function and was low in CFS/ME cases, compared to controls and also in CD3+CD8+ T cells. In contrast, Brenu, et al (2011) measured NK cell perforin by gene expression and reported it elevated in CFS/ME. The discrepancy between these two studies is unlikely to be explained by methodology because both studies reported that perforin levels were low in CD8+ T cells.
Table 2.
Biomarkers associated with fatigue in patients with identified diseases or syndromes
| Cause of Fatigue | Biomarker | Reference |
|---|---|---|
| Sjögren's syndrome | >IL-1Ra in CSF | Harboe et al, 2009 |
| Rheumatoid arthritis | >Stimulated production of IL-6 | Davis et al, 2008 |
| >IFNα | Lee, et al, 2010 | |
| Depression & anxiety | Stebbings, et al, 2010 | |
| No RF; >DAS-28; >pain | van Hoogmoed, et al, 2010 | |
| Systemic lupus erythematosus | >Serum IFNα | Lee et al, 2010 |
| Inflammatory bowel disease | >CRP | Graff et al, 2010 |
| Hemoglobin; GI symptoms; altered sleep | Jesness-Jorgensen, 2011 | |
| Multiple sclerosis | >Frequency & IFNγ of producing CD8+ T cells | Gold, et al, 2011 |
| >TNFα mRNA in T cells | Flachenecker et al, 2006 | |
| Post exercise >β-1 & β-2 adrenergic receptors & < TLR4 by gene expression | White, et al, 2012 | |
| Obstructive sleep apnea | > Plasma sTNFRI | Mills, et al, 2008 |
| Hypoxia | Sickness behavior | Sherry, et al, 2009 |
| >IPlasma L-1β & IL-1R1 | Basu, et al, 2005 | |
| Chronic Obstructive Pulmonary Disease | >Plasma TNFα | Al-shair, et al, 2011 |
| Breast cancer before treatment | >CMV antibody titers | Fagundes, et al, 2012 |
| Breast & prostate cancer with radiation | >Plasma CRP & IL-1Ra | Bower, et al, 2009 |
| Advanced cancer | >Plasma IL-6, TNFα | Kwak et al, 2011 |
| Advanced cancer | >Plasma cortisol, ACTH, epinephrine & norepinephrine | Thornton, et al, 2010 |
| Non-small cell lung cancer with concurrent chemoradiation | >Plasma IL-6, IL-10 & sTNF-R1 | Wang, et al, 2010 |
| Testicular cancer, long term survivors | >Plasma IL-1Ra & CRP | Orre, et al, 2009 |
| Breast cancer survivors | >Transcripts for NF-κB & glucocorticoids | Bower et al, 2011 |
| >CRP | Orre, et al, 2011 | |
| Cancer | >Plasma IL-6, IL-1Rα, neopterin | Schubert, et al, 2006 review |
| Major depressive disorder | >Plasma TNFα & -6 | Dowlati, et al, 2010, review |
White, et al., (2010) measured cytokines using a multiplex bead-based system (Luminex Corp, Austin, TX) in serum from 19 CFS/ME and 17 controls collected at several time points pre/post a moderate exercise challenge. In contrast to the results of Fletcher, et al, no differences were found at baseline. However, 48 hours after the challenge, 11 patients showed symptom flair. In that group, IL-1β, IL-12, IL-6, IL-8, IL-10, and IL-13 were elevated at 8 hours post exercise. Jammes, et al., (2009) and Robinson, et al. (2010) also reported a differential effect of aerobic exercise on plasma cytokines. Both studies found that the exercise elevated plasma IL-6 in controls but had no effect in CFS/ME. As shown in Table 3, an aerobic exercise challenge increased differences between CFS/ME cases and healthy controls on 8 of 9 biomarkers, plasma NPY, IL-5, IL-6, IL-10, IL-12 and TNFα. Samples were drawn at baseline, V02 max and 4 hours. It should be noted that methodology and sensitivity for cytokine measurement varied in these studies.
Table 3.
Biomarkers of chronic fatigue syndrome/myalgic encephalomyelitis
| Biomarkers Studied | Reference |
|---|---|
| >IL-10, IFNγ, TNFα by PHA stimulated lymphocytes; >CD4+CD25+ T cells expressing FoxP3 and VPACR2; <cytotoxic activity of NK and CD8+T cells; <granzyme and >perforin by gene expression. | Brenu, et al., 2011 |
| >IL-4, IL-5, IL-12, LTα, IL-1α, IL-1β, IL-6; <IL-8, IL-13, IL-15; unchanged IL-2, IFNγ, IL-17, IL-23, TNFα in plasma IL Subjects at rest. | Fletcher, et al., 2009 |
| Cytokine co-expression networks distinct in CFS/ME compared to HC and suggested persistent inflammation and humoral immune activation. | Broderick, et al., 2010 |
| <Perforin in NK cells and CD8+T cells by quantitative flow cytometry. Subjects at rest. | Maher, et al., 2005 |
| <Perforin by gene expression in GWI compared to HC at VO2Max in exercise challenge | Whistler, et al, 2009 |
| <Natural killer cell cytotoxicity; < plasma dipeptidyl peptidase IV;>T-cell activation. Subjects at rest. | Fletcher, et al., 2010 |
| Effect of exercise challenge in CFS/ME compared to HC: absence of significant increase in IL-6, IL-10, IL-12, LTα in CFS/ME | Harvey, et al., 2011 |
| In CFS/ME compared to HC: absence of significant increase in IL-6 & TNFα following exercise challenge | Jammes, et al., 2009 |
| IL-1β, IL-12, IL-6, IL-8, IL-10, and IL-13 were elevated at 8 hours post exercise in subjects showing symptom flair at 48 hours. | White, et al, 2010 |
| >NPY in CFS/ME subjects at rest by RIA; no exercise related NPY change in CFS/ME but > in HC | Fletcher, et al., 2010; Harvey, et al., 2011 |
| <Plasma CoQ10 in CFS/ME compared to HC | Maes et al., 2009 |
| <Serum vitamin E, a marker for oxidative stress | Miwa and Fujita, 2010 |
| Exercise related <plasma F(2)-isoprostanes (marker of oxidative stress); No effect of exercise on plasma IL-6 or sIL-6R in CFS/ME or HC | Robinson, et al., 2010 |
| In 71% of CFS/ME, exercise increased transcription for most sensory and adrenergic receptors and one cytokine. These correlated with fatigue and pain. No exercise related changes in HC. | Light, et al, 2012 |
| Metabolic syndrome predictors elevated in CFS/ME | Maloney, et al, 2010 |
| Increased lactate levels in ventricular cerebrospinal fluid of CFS patients. | Murrough et al. 2010 |
| Significant deficiencies in mitochondrial function in CFS/ME compared to HC | Myhill et al., 2009 |
| Quantitative proteomics using high resolution mass spectrometry of CSF | Schutzer, et al., 2011 |
| Unique CFS/ME spinal fluid proteome of 60 proteins when compared to HC and GWI. The CFS/ME and GWI patients shared 20 unique proteins | Baraniuk et al 2005 |
| > CRP in CFS/ME; >8-iso-prostaglandin F(2 alpha) isoprostanes | Spence, et al, 2008 |
| > CRP in CF cases not meeting the CFS/ME definition; no difference between CFS/ME and HC | Raison et al, 2009 |
| <basal salivary cortisol levels and illness symptoms | Torres-Harding, et al 2009 |
| <cortisol levels and flattened diurnal release of cortisol) associated with a poorer response to CBT. | Roberts, et al., 2010 |
| variations in the 5' region of NR3C1 (glucocorticoid receptor gene) | Rajeevan et al., 2007 |
| HPA axis dysfunction | Papadopoulos & Cleare, 2011 |
| HPA axis dysfunction | Ben-Zvi, et al., 2009 |
| No evidence of a biomarker using gene expression in a twin study | Byrnes et al., 2009 |
| Significant evidence for a heritable contribution to predisposition to CFS/ME | Albright, et al, 2011 |
| Gene expression revealed ‘CFS signature genes’ | Kerr, et al., 2008 |
| Reassessment CFS signature genes’ failed to confirm predictive ablity | Frampton, et al., 2011 |
Gulf War Illness (GWI) patients demonstrated impaired immune function as demonstrated by decreased NKCC and altered gene expression associated with NK cell function, including perforin. Pro-inflammatory cytokines, T-cell ratios, and salivary cortisol) were also altered in GWI cases compared to control subjects. A three point exercise challenge augmented these differences, with the most significant effects observed immediately after V02Max, possibly implicating some block in the NK and CD8 T-cells ability to respond to "stress-mediated activation" (Broderick et al., 2011a). This result has positive implications for the development of laboratory diagnostic tests for GWI and provides a paradigm for exploration of the immuno-physiological mechanisms that are operating in GWI, and similar complex syndromes (Whistler et al., 2009).
Light, et al, (2009) demonstrated, with real-time, quantitative PCR, that 19 CFS/ME patients had lower expression of beta-2 adrenergic receptors but otherwise did not differ from 16 control subjects before exercise. After a sustained moderate exercise test, CFS/ME patients showed greater increases than control subjects in gene expression for metabolite detecting receptors ASIC3, P2X4, and P2X5, for SNS receptors alpha-2A, beta-1, beta-2, and COMT and IS genes for IL10 and TLR4 lasting from 0.5 to 48 hours (P < .05). In a more recent study of moderate exercise effects on gene expression, Light, et al (2012) used a 25 minute exercise challenge with blood sampling at 0.5, 8, 24 and 48 h post exercise. The relative mRNA values from the four time-points were summed into a single measure labeled area under the curve (AUC) and then log transformed. No gene expression changes occurred following exercise in controls. In 71% of patients with CFS/ME, moderate exercise increased most sensory and adrenergic receptor's and one cytokine gene's transcription for 48 h. These post-exercise increases correlated with behavioral measures of fatigue and pain. In contrast, for the other 29% of patients with CFS/ME, adrenergic α-2A receptor's transcription was decreased at all time-points after exercise; other genes were not altered. A history of orthostatic intolerance was significantly more common in the α-2A decrease subgroup.
Spence, et al, (2008) reported increased plasma concentrations of CRP in patients with CFS/ME. In their study of 41 patients with CFS/ME and in 30 healthy subjects, plasma CRP in CFS/ME was 2.58+/−2.91 compared with 1.07+/−2.16 mg/L in HC; P<0.01). They also found elevation of a marker for oxidative stress, 8-iso-prostaglandin F(2 alpha) isoprostanes (470.7+/−250.9 compared with 331.1+/−97.6 pg/ml respectively; P<0.005). In a population-based sample in metropolitan, urban and rural areas of Georgia, CDC researchers screened 10,837 households with 21,165 residents (Raison, et al., 2008). When examined as a categorical variable (based on a cut-off of >3 mg/L), CRP was significantly higher in subjects with CFS/ME (34.38%) and in fatigued individuals who did not fulfill the diagnostic case definition (ISF) (38.05%) than in healthy controls (20.72%) (CFS/ME: OR = 2.00, 95% CI = 1.08–3.74; ISF: OR = 2.35, 95% CI = 1.38–4.00). Other variables associated with CRP >3 mg/L included sex, race, PCS score, BMI, and SDS depression score. After adjustment for age, sex, race, location of residence, BMI, depressive status and use of immune modulating medications, subjects classified as ISF continued to demonstrate increased CRP (adjusted OR = 2.34, 95% CI = 1.29–4.27, p = 0.0120). After adjustment, the association between CRP >3 mg/L and CFS/ME did not remain significant (adjusted OR = 1.62, 95% CI = 0.75–3.53, p = 0.8569). In the Copenhagen General Population Study of approximately 63,500 individuals, the distribution of circulating levels of CRP was markedly skewed to the right with 97% of the participants having CRP levels of <10 mg/L. The median plasma CRP concentration was 1.53 mg/L (IQR, 1.14–2.51) and 34% of the participants had circulating CRP levels of ≥2 mg/L (Allin and Nordestgaard, 2011).
Papadopoulos and Cleare (2011) concluded in a recent review that the clinically relevant HPA axis dysfunction occurring in CFS/ME patients includes mild hypocortisolism with attenuated diurnal variation of cortisol, enhanced negative feedback to the HPA axis and blunted HPA axis responsiveness. Dysregulation of the HPA axis was associated with a number of neuroimmune disorders including CFS/ME, which was characterized by a hypoactive rather than a hyperactive HPA axis (Roberts, et al., 2010). A theoretical analysis of HPA axis control suggested that such states might be quite stable and perpetuate as a result of physiological feedback mechanisms (Ben-Zvi et al., 2009). Lattie, et al, (2012) reported that CFS/ME cases with greater perceived stress management skills had less fatigue (p=.019) and emotional distress (p<.001), greater diurnal cortisol slope (p=.023) and lower IL-2 levels (p=.043). The influence of stress management skills on distress and fatigue appeared greatest among patients who had elevated IL-6 levels. Gaab, et al, 2005 used the Trier Social Stress Test (TSST) to determine that while cortisol responses to stress were normal, LPS induced pro-inflammatory cytokine levels were significantly attenuated in CFS/MS patients. CFS/MS patients showed an inverse response pattern in comparison to healthy controls, i.e. stimulated cytokine production decreased shortly after stress in CFS/ME patients, while it increased in controls. Torres-Harding, et al (2009) reported on the relationships between salivary cortisol levels and illness symptomatology in a group of 108 individuals with CFS/ME. Results indicated that fatigue and pain were associated with salivary cortisol levels. In particular, variance from the expected pattern of cortisol over 24 hours was associated with increased levels of fatigue. Glucocorticoid receptor gene NR3C1 was implicated as a potential mediator of CFS/ME, and suggested variations in the 5' region of NR3C1 as a possible mechanism through which the alterations in HPA axis regulation and behavioral characteristics of CFS/ME may manifest (Rajeevan et al., 2007).
A marker for chronic immune activation, dipeptyl peptidase IV/ CD26 was shown to be dysregulated in CFS/ME (Fletcher et al, 2010a). A substrate for this dipeptidase is the stress hormone, neuropeptide Y (NPY), which was elevated in CFS/ME cases (Fletcher, et al, 2010b) and correlated with symptom severity including fatigue.
Schutzer, et al., (2011) used high-resolution mass spectrometry based label-free quantitative (MS) proteomics approach to examine cerebral spinal fluid (CSF) samples from patients with CFS/ME, Lyme disease and healthy controls. These were analyzed directly employing a MS-proteomics approach. Both patient groups could be distinguished from each other and from controls based on their unique CSF proteins (p<0.01). CFS/ME (n=43) had 2,783 non-redundant proteins, Lyme disease (n=25) 2,768 proteins, and healthy normal controls, 2,630 proteins. Four components (C1S, C4B, C1QB, C1QC) that are seen with activation of the complement cascade were differentially increased in abundance consistently across the Lyme patients compared to CFS/ME. An earlier pilot study published by James Baraniuk’s group (2005) compared pooled spinal fluids from 10 CFS/ME patients, 10 GWI patients and 10 controls. They identified a unique CFS/ME spinal fluid proteome of 60 proteins when compared to healthy controls and GWI, though the CFS/ME and GWI patients shared 20 unique proteins (Baraniuk et al 2005). Among persons with CFS/ME, the number of metabolic syndrome factors was significantly correlated with worse fatigue on a standardized summary measure of fatigue (r = 0.20, P = .04) (Maloney et al, 2010). Proteins contribute to the regulation of cell metabolism both indirectly, as agents of intracellular signal transduction, and directly as enzymatic facilitators of metabolic reactions. With this evidence of an illness-specific proteome in the CNS of CFS/ME patients it may not be all that surprising to also find increased lactate levels in ventricular cerebrospinal fluid of these patients upon neuroimaging (Murrough et al. 2010). Similarly, using a targeted assay of adenosine triphosphate (ATP) availability in circulating blood neurophils, Myhill et al., (2009) pointed to significant deficiencies in mitochondrial function in CFS/ME. Though narrowly focused these studies emphasize the importance of metabolite profiling as a direct means of assessing fundamental inefficiencies in cell metabolic function. Serum vitamin E (alpha-tocopherol) concentrations were significantly lower in CFS/ME patients as compared with the control subjects, suggesting increased oxidative stress in the former (Miwa and Fujita, 2010).
Interacting with and orchestrating cell signal transduction and metabolism is the gene regulatory machinery. Broderick et al., (2006) analyzed the correlation patterns in 117 clinical variables measured in 111 female subjects and used these to isolate gene co-expression patterns characteristic of CFS/ME. These described associations between 17 transcripts related to basic cellular processes involved in cell signalign, ion transport and immune system function. The single most influential of these was sestrin 1 (SESN1), supporting the involvement of oxidative stress in CFS/ME. Kerr, et al (2008) reported on ‘CFS signature genes’ with predictive power. This set had only 5 genes out of 44 related to immune regulation. Recently, they have reassessed the CFS/ME disease predictive genes in the original study data and assessed the ability of the proposed 44-gene classifier set to discriminate between CFS/ME patients and healthy control individuals. This classifier was able to discriminate correctly between CFS/ME and healthy control samples in 95% of the training samples. However, when assessed on a new, blinded 128-sample test set only 58% of samples were predicted correctly. Using a variety of methods, it was demonstrated that the 44-gene classifier set did not robustly identify patients with CFS/ME disease (Frampton, 2011).Byrnes et al. (2009) were unable to identify a candidate biomarker for CFS/ME when comparing identical twin sets discordant for illness.
Utah researchers with access to computerized genealogical resource linking multiple generations of genealogy data with medical diagnosis data reported significant evidence for a heritable contribution to predisposition to CFS/ME. Significant excess relatedness was observed for both close (p <0.001) and distant relationships (p = 0.010). Significant excess relative risk for CFS/ME was found between first (2.70, 95% CI: 1.56–4.66), second (2.34, 95% CI: 1.31–4.19), and third degree relatives (1.93, 95% CI: 1.21–3.07) (Albright, et al, 2011).
Post-exercise mRNA increases for metabolite-detecting receptors were unique to patients with CFS/ME, whereas both patients with MS and patients with CFS/MS showed abnormal increases in adrenergic receptors. Among patients with MS, greater fatigue was correlated with blunted immune marker expression (White, et al, 2011). These post-exercise increases correlated with behavioral measures of fatigue and pain.
In current studies by the Miami/Alberta group, GWI and CFS/MS patients showed distinct differences from each other and HC that became dramatically apparent under physiological challenge. While no genes were expressed at rest with a 2-fold difference (false discovery rate FDR< 0.05) in CFS/ME over control subjects we found 18 such genes differentially expressed at rest in GWI. However, CFS/ME subjects were easily distinguished from healthy control subjects under effort due to a dramatic unresponsiveness to exercise. Indeed in moving to peak effort from rest 166 genes became differentially expressed from rest in healthy controls. In contrast 50 genes responded to challenge at peak effort in GWI but only 1 was expressed in CFS/ME at peak effort versus rest. This lack of early response in the transition from rest to peak effort explains in large part why 466 genes were expressed with a 2-fold difference (FDR<0.05) in CFS/ME versus control subjects at peak effort as opposed to 28 genes in GWI. Therefore while GWI showed a partial early response to maximal exercise challenge CFS/ME subjects were largely unresponsive in that time frame. Results such as these emphasize how the use of an exercise challenge to probe the dynamics of response offers a more sensitive measure of the differences separating these patient populations (Broderick, et al 2011b). Using a methodology originally developed by Efroni et al., (2007), Broderick, et al (2011b) went on to show that these differences in gene expression implicated 90 documented pathways with the majority being linked to immune metabolism. Examining these pathways as part of an integrated biological system the latter demonstrated that significant differences exist between CFS/ME, GWI and healthy control subjects in terms of the architecture of their active pathway networks. Indeed these significant differences in the recruitment, shedding and re-assignment of regulatory interactions would not be seen using conventional analytic methods focused on the expression of individual markers.
CONCLUSIONS
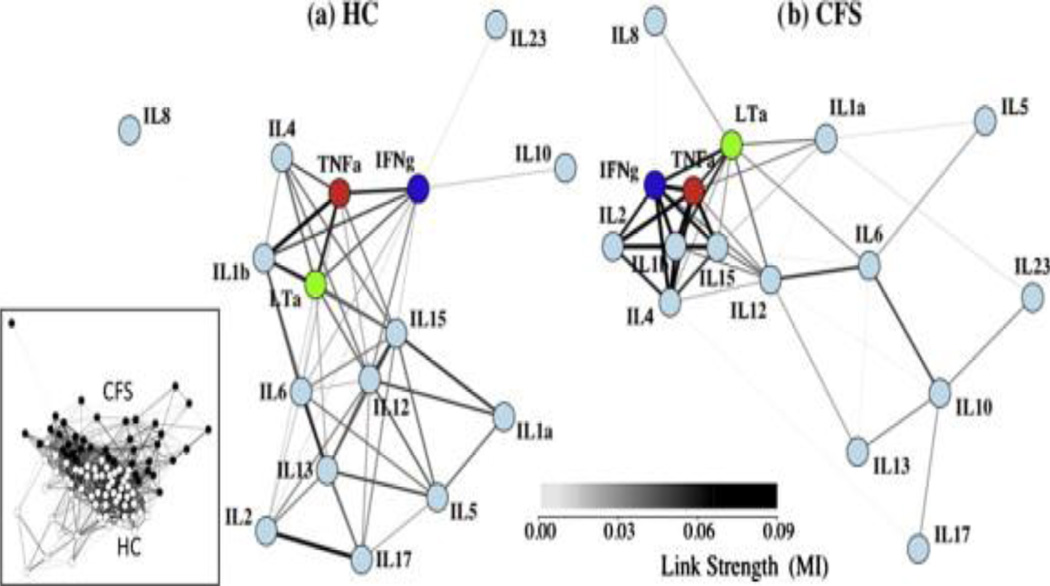
Fatigue research faces a number of methodological issues that continue to challenge the biological relevance and clinical impact of the search for useful biomarkers. In particular is the need to describe and understand the structure of complex biological networks. This will require applying novel measures to identify network functional modules within and across levels of biology, and identifying associations at the pathway level to promote increased biological relevance. Because the body operates as an autonomous, fully integrated and self-regulating system it is should not be surprising that even localized muscle fatigue will present systemic biomarkers. One can view the observed myriad of individual biomarkers as being partially overlapping manifestations of a much more fundamental and unified set of biological processes. Closely associated with the single biomarker paradigm is the conventional model of dysfunction originating from the outright failure of a single physiological component. A highly complementary paradigm and one that embraces the overarching regulatory architecture of human physiology is the concept of loss of regulatory performance. This is akin to a controller becoming de-tuned over time and under sustained adaptive load. This manifests as a change in the dynamics of response. An appropriate example would be observations of a fight-or-flight axis response to a stressor that is delayed, develops more slowly and is blunted in amplitude. The recent paper by Broderick, et al. (2010) approached the question of cytokine involvement in CFS/ME pathogenesis. Multiplex cytokine data (Fletcher, et al, 2009) was used to construct the cytokine co-expression networks, which clearly distinguished cases from controls reinforcing the importance of immune context and relative expression (Figure 1).
Figure 1. Networks of cytokine association show visibly different topologies.
A weighted spring-electrical embedding structurally reveals the subject–subject (inset) and cytokine–cytokine associations based on measurements in 59 healthy control subjects (a) and 40 CFS/ME patients (b). All edge weights are significant at p ⩽ 0.01. Separation of subjects was consistent with their assignment to diagnostic groups supporting the use of within-group variation in the estimation of mutual information for cytokine–cytokine associations.
We propose that persistent disorders such as CFS/ME and CRF likely correspond to alternative homeostatic states enabled by the body as an adaptive strategy. These alternate modes of homeostatic control result in a loss of function that is persistent because these configurations are energetically stable (Figure 2) (Ben-Zvi et al., 2009). At and around these new homeostatic states we expect a characteristic change in how the body maintains homeostasis and that this new regulatory program will support different patterns of biomarker association. For example the immune system in these individuals might now use an alternate signalign network, one that may be much less efficient. Empirically mapping networks of endocrine and immune signalign in CFS/ME and CRF will allow us to identify key changes in their basic architecture and in their capacity to support the flow of regulatory information. Indeed we have shown that such networks differ significantly in structure in GWI and CFS/ME (Fuite et al., 2008; Broderick et al., 2010; Broderick et al., 2011a) and that in many cases these structural changes occur around markers that do not change in expression across groups. This suggests that a network-based approach will not only enhance the characterization of these conditions but also provide new insight into the regulatory processes that support fatigue as a protective element. Physiological integration and the closed loop regulation spanning broadly across the immune, autonomic, endocrine systems as well as at the level of intracellular signalign and metabolic function may force a paradigm shift in our approach to complex illness as illustrated in Figure 2. Indeed this type of integrative network-based analysis may be required to progress to the next chapter in our understanding of the biology of fatigue.
Figure 2.
Diagrammatic representation of the hypothesis whereby two complex and very distinct illnesses CFS/ME and cancer-related fatigue occupy distinct energy-minimal equilibrium points characterized by alternative configurations of the neuroendocrine-immune biomarker and symptom association networks.
ACKNOWLEDGEMENT
This work was supported by grants from the NIAAA: R21AA016635 (PI MA Fletcher); NIAID: R01AI065723 (PI MA Fletcher); CFIDS Assoc. of America: (PI N Klimas and PI G Broderick); US Department of Defense CDMRP W81XWH-09-2-0071 (PI N Klimas), CDMRP W81XWH-10-1-0774 (PI G Broderick); Veterans’ Affairs Administration Merit Awards (PI N Klimas); NIAID: UO1 AI459940 (PI N Klimas)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albright F, Light K, Light A, Bateman L, Cannon-Albright LA. Evidence for a heritable predisposition to Chronic Fatigue Syndrome. BMC Neurol. 2011 May 27;11:62. doi: 10.1186/1471-2377-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit. Rev. Clin. Lab Sci. 2011;48:155–170. doi: 10.3109/10408363.2011.599831. [DOI] [PubMed] [Google Scholar]
- Al-shair K, Kolsum U, Dockry R, Morris J, Singh D, Vestbo J. Biomarkers of systemic inflammation and depression and fatigue in moderate clinically stable COPD. Respiratory Res. 2011 Jan 5;12:3. doi: 10.1186/1465-9921-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspler AL, Bolshin C, Vernon SD, Broderick G. Evidence of inflammatory immune signalign in chronic fatigue syndrome: a pilot study of gene expression in peripheral blood. Behav. Brain Funct. 2008 Sep 26;4:44. doi: 10.1186/1744-9081-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraniuk JB, Casado B, Maibach H, Clauw DH, Pannell LK, Hess SA. Chronic fatigue syndrome – related proteome in human cerebrospinal fluid. BMC Neurol. 2005 Dec 5;:22. doi: 10.1186/1471-2377-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Lazovic J, Krady JK, Mauger DT, Rothstein RP, Smith MB, Levison SW. Interleukin-1 and the interleukin-1 type 1 receptor are essential for the progressive neurodegeneration that ensues subsequent to a mild hypoxic/ischemic injury. J. Cereb. Blood Flow Metab. 2005;25:17–29. doi: 10.1038/sj.jcbfm.9600002. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi A, Vernon SD, Broderick G. Model-Based Therapeutic Correction of Hypothalamic-Pituitary-Adrenal Axis Dysfunction. PLoS Comput. Biol. 2009 Jan;5(1):e1000273. doi: 10.1371/journal.pcbi.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou-Holaigah I, Rowe PC, Kan J, Calkins H. The relationship between neurally mediated hypotension and the chronic fatigue syndrome. J.A.M.A. 1995;274:961–967. [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Boncler M, Watala C. Regulation of cell function by isoforms of C-reactive protein: a comparative analysis. Acta Biochim. Pol. 2009;56:17–31. [PubMed] [Google Scholar]
- Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav. Immun. 2007;21:863–871. doi: 10.1016/j.bbi.2007.03.013. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-κB and decreased glucocorticoid signalign in breast cancer survivors with persistent fatigue. Brain Behav. Immun. 2011a;25:147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J. Clin. Oncol. 2011b;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley TJ, Chervin RD. Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment. SLEEP. 2010;33:1061–1067. doi: 10.1093/sleep/33.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling R. Robust signalign networks of the adipose secretome. Trends Endocrinol. Metab. 2009;20:1–7. doi: 10.1016/j.tem.2008.08.006. Review. [DOI] [PubMed] [Google Scholar]
- Brenu EW, van Driel ML, Staines DR, Ashton KJ, Ramos SB, Keane J, Klimas NG, Marshall-Gradisnik SM. Immunological abnormalities as potential biomarkers in Chronic Fatigue Syndrome/MyalgicEncephalomyelitis. J. Transl. Med. 2011;28(9):81. doi: 10.1186/1479-5876-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick G, Craddock RC, Whistler T, Taylor R, Klimas N, Unger ER. Identifying illness parameters in fatiguing syndromes using classical projection methods. Pharmacogenomics. 2006;7:407–419. doi: 10.2217/14622416.7.3.407. [DOI] [PubMed] [Google Scholar]
- Broderick G, Fuite J, Kreitz A, Vernon SD, Klimas N, Fletcher MA. A formal analysis of cytokine networks in Chronic Fatigue Syndrome. Brain Behav. Immun. 2010;24:1209–1174. doi: 10.1016/j.bbi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick G, Kreitz A, Fuite J, Fletcher MA, Vernon SD, Klimas N. A pilot study of immune network remodeling under challenge in Gulf War Illness. Brain Behav Immun. 2011a;25(2):302–313. doi: 10.1016/j.bbi.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Broderick G, Klimas NG, Fletcher MA, Efroni S. From cytokines to cells to gene expression: an integrative approach to the study of gulf war illness systems biology. Presentation to the Research Advisory Committee on Gulf War Veterans Illnesses; June 27–28; Washington, D.C.. 2011b. http://www.va.gov/RAC-GWVI/Minutes_June_2011.asp. [Google Scholar]
- Byrnes A, Jacks A, Dahlman-Wright K, Evengard B, Wright FA, Pedersen NL, Sullivan PF. Gene expression in peripheral blood leukocytes in monozygotic twins discordant for chronic fatigue: no evidence of a biomarker. PLoS One. 2009 Jun 5;4(6):e5805. doi: 10.1371/journal.pone.0005805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers BM, van de Sande MI, DeMeirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles AC, Speight N, Vallings R, Bateman L, Baumgarten-Austrheim B, Bell DS, Carlo-Stella N, Chia J, Darragh A, Jo D, Lewis D, Light AR, Marshall-Gradisbik S, Mena I, Mikovits JA, Miwa K, Murovska M, Pall ML, Stevens S. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011;270:327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse K. US Open: Venus Williams describes fights with fatigue. New York Times, Sports. 2011 Friday, September 2. [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav. Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Davis MC, Zautra AJ, Younger J, Motivala SJ, Attrep J, Irwin MR. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: implications for fatigue. Brain Behav. Immun. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron IS, Schaefer CF, Buetow KH. Identification of key processes underlying cancer phenotypes using biologic pathway analysis. PLoS One. 2007 May 9;2(5):e425. doi: 10.1371/journal.pone.0000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evengård B, Klimas N. Chronic fatigue syndrome: Probable pathogenesis and possible treatments. Drugs. 2002;62:2433–2446. doi: 10.2165/00003495-200262170-00003. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Alfano CM, Bennett JM, Povoski SP, Lipari AM, Agnese DM, Yee LD, Carson WE, 3rd, Farrar WB, Malarkey WB, Kiecolt-Glaser JK. Fatigue and herpesvirus latency in women newly diagnosed with breast cancer. Brain Behav. Immun. 2012;26:394–400. doi: 10.1016/j.bbi.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-de-Las-Peñas C, Cantarero-Villanueva I, Fernández-Lao C, Ambite-Quesada S, Díaz-Rodríguez L, Rivas-Martínez I, Moral-Avila RD, Arroyo-Morales M. Influence of catechol-o-methyltransferase genotype (Val158Met) on endocrine, sympathetic nervous and mucosal immune systems in breast cancer survivors. Breast. 2012;21:199–203. doi: 10.1016/j.breast.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Flachenecker P, Bihler I, Weber F, Gottschalk M, Toyka KV, Rieckmann P. Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Mult. Scler. 2004;10:165–169. doi: 10.1191/1352458504ms991oa. [DOI] [PubMed] [Google Scholar]
- Fletcher MA, Zeng XR, Barnes Z, Levis S, Klimas NG. Plasma cytokines in women with chronic fatigue syndrome. J. Transl. Med. 2009 Nov 12;7:96. doi: 10.1186/1479-5876-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher MA, Zeng XR, Maher K, Levis S, Hurwitz B, Antoni M, Broderick G, Klimas NG. Biomarkers in chronic fatigue syndrome: evaluation of natural killer cell function and dipeptidyl peptidase IV/CD26. PLoS One. 2010a May 25;5(5):e10817. doi: 10.1371/journal.pone.0010817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher MA, Rosenthal M, Antoni M, Ironson G, Zeng XR, Barnes Z, Harvey JM, Hurwitz B, Levis S, Broderick G, Klimas NG. Plasma neuropeptide Y: a biomarker for symptom severity in chronic fatigue syndrome. Behav. Brain Funct. 2010b Dec 29;6:76. doi: 10.1186/1744-9081-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton D, Kerr J, Harrison TJ, Kellam P. Assessment of a 44 gene classifier for the evaluation of chronic fatigue syndrome from peripheral blood mononuclear cell gene expression. PLoS One. 2011 Mar 30;6(3):e16872. doi: 10.1371/journal.pone.0016872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuite J, Vernon SD, Broderick G. Neuroendocrine and immune network re-modeling in chronic fatigue syndrome: an exploratory analysis. Genomics. 2008;92:393–399. doi: 10.1016/j.ygeno.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study.International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Heitz V, Engert V, Schad T, Schürmeyer TH, Ehlert U. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30:188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Glaser R, Padgett DA, Litsky ML, Baiocchi RA, Yang EV, Chen M, Yeh PE, Klimas NG, Marshall GD, Whiteside T, Herberman R, Kiecolt-Glaser J, Williams MV. Stress-associated changes in the steady-state expression of latent Epstein-Barr virus: Implications for chronic fatigue syndrome and cancer. Brain Behav. Immun. 2005;19:91–103. doi: 10.1016/j.bbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Gold SM, Krüger S, Ziegler KJ, Krieger T, Schulz KH, Otte C, Heesen C. Endocrine and immune substrates of depressive symptoms and fatigue in multiple sclerosis patients with comorbid major depression. J. Neurol. Neurosurg. Psychiatry. 2011;82:814–818. doi: 10.1136/jnnp.2010.230029. [DOI] [PubMed] [Google Scholar]
- Graff LA, Walker JR, Russell AS, Bissonnette R, Bernstein CN. Fatigue and quality of sleep in patients with immune-mediated inflammatory disease. J. Rheumatol. Suppl. 2011;88:36–42. doi: 10.3899/jrheum.110902. [DOI] [PubMed] [Google Scholar]
- Graff LA, Vincent N, Walker JR, Clara I, Carr R, Ediger J, Miller N, Rogala L, Rawsthorne P, Lix L, Bernstein CN. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm. Bowel Dis. 2011;17:1882–1889. doi: 10.1002/ibd.21580. [DOI] [PubMed] [Google Scholar]
- Harboe E, Tjensvoll AB, Vefring HK, Gøransson LG, Kvaløy JT, Omdal R. Fatigue in primary Sjögren's syndrome--a link to sickness behavior in animals? Brain Behav. Immun. 2009;23:1104–1108. doi: 10.1016/j.bbi.2009.06.151. [DOI] [PubMed] [Google Scholar]
- Hartkamp A, Geenen R, Kruize AA, Bossema ER, Godaert GL, Bootsma H, Bijlsma JW, Derksen RH. Serum dehydroepiandrosteronesulphate levels and laboratory and clinical parameters indicating expression of disease are not associated with fatigue, well-being and functioning in patients with primary Sjögren's syndrome. Clin. Exp. Rheumatol. 2011;29:18–21. [PubMed] [Google Scholar]
- Heesen C, Nawrath L, Reich C, Bauer N, Schulz KH, Gold SM. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J. Neurol. Neurosurg. Psychiatry. 2006;77:34–39. doi: 10.1136/jnnp.2005.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoogmoed D, Fransen J, Bleijenberg G, van Riel P. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology. 2010;49:1294–1302. doi: 10.1093/rheumatology/keq043. [DOI] [PubMed] [Google Scholar]
- Hurwitz BE, Coryell VT, Parker M, Martin P, Laperriere A, Klimas NG, Sfakianakis GN, Bilsker MS. Chronic fatigue syndrome: illness severity, sedentary lifestyle, blood volume and evidence of diminished cardiac function. Clin. Sci. (Lond) 2009;118:125–135. doi: 10.1042/CS20090055. [DOI] [PubMed] [Google Scholar]
- Jammes Y, Steinberg JG, Delliaux S, Brégeon F. Chronic fatigue syndrome combines increased exercise-induced oxidative stress and reduced cytokine and Hsp responses. J. Intern. Med. 2009;266:196–206. doi: 10.1111/j.1365-2796.2009.02079.x. [DOI] [PubMed] [Google Scholar]
- Jason LA, Richman JA, Rademaker AW. A community-based study of chronic fatigue syndrome. Arch. Intern. Med. 1999;159:2129–2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- Jelsness-Jørgensen LP, Bernklev T, Henriksen M, Torp R, Moum BA. Chronic fatigue is more prevalent in patients with inflammatory bowel disease than in healthy controls. Inflamm. Bowel Dis. 2011;17:1564–1572. doi: 10.1002/ibd.21530. [DOI] [PubMed] [Google Scholar]
- Kerr JR, Petty R, Burke B, Gough J, Fear D, Sinclair LI, Mattey DL, Richards SC, Montgomery J, Baldwin DA, Kellam P, Harrison TJ, Griffin GE, Main J, Enlander D, Nutt DJ, Holgate ST. Gene expression subtypes in patients with chronic fatigue syndrome/myalgic encephalomyelitis. J. Infect. Dis. 2008;197:1171–1184. doi: 10.1086/533453. [DOI] [PubMed] [Google Scholar]
- Kwak SM, Choi YS, Yoon HM, Kim DG, Song SH, Lee YJ, Yeom CH, Koh SJ, Park J, Lee MA, Suh SY. The relationship between interleukin-6, tumor necrosis factor-{alpha}, and fatigue in terminally ill cancer patients. Palliat.Med. 2011;26:275–282. doi: 10.1177/0269216311406991. [DOI] [PubMed] [Google Scholar]
- Lattie E, Antoni MH, Fletcher MA, Penedo F, Czaja S, Lopez C, Perdomo D, Sala A, Nair S, Fu S, Klimas N. Stress management skills, neuroimmune processes and fatigue levels in persons with chronic fatigue syndrome. Brain Behav. Immun. 2012 doi: 10.1016/j.bbi.2012.02.008. (in press,). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallukka T, Sarlio-Lähteenkorva S, Roos E, Laaksonen M, Rahkonen O, Lahelma E. Working conditions and health behaviours among employed women and men: The Helsinki Health Study. Prev. Med. 2004;38:48–56. doi: 10.1016/j.ypmed.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Landmark-Høyvik H, Reinertsen KV, Loge JH, Kristensen VN, Dumeaux V, Fosså SD, Børresen-Dale AL, Edvardsen H. The genetics and epigenetics of fatigue. PMR. 2010;5:456–465. doi: 10.1016/j.pmrj.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Lee HM, Sugino H, Nishimoto N. Cytokine networks in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010;2010:676284. doi: 10.1155/2010/676284. Epub 2010 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Iser JH, Sparrow MP, Newnham ED, Headon BJ, Gibson PR. Thiopurines, a previously unrecognised cause for fatigue in patients with inflammatory bowel disease. J. Crohns Colitis. 2009;3:196–199. doi: 10.1016/j.crohns.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Light AR, White AT, Hughen RW, Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J. Pain. 2009;10:1099–1112. doi: 10.1016/j.jpain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Bateman L, Jo D, Hughen RW, Vanhaitsma TA, White AT, Light KC. Gene expression alterations at baseline and following moderate exercise in patients with Chronic Fatigue Syndrome and Fibromyalgia Syndrome. J. Intern. Med. 2012;271:64–81. doi: 10.1111/j.1365-2796.2011.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, Sadler GR, Parker BA, Ancoli-Israel S. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav. Immun. 2012 Mar 2; doi: 10.1016/j.bbi.2012.02.001. [Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loge J, Ekeberg O, Kaasa S. Fatigue in the general Norwegian population: normative data and associations. J. Psychosom. Res. 1998;45:53–65. doi: 10.1016/s0022-3999(97)00291-2. [DOI] [PubMed] [Google Scholar]
- Maes M, Coucke F, Leunis JC. Normalization of the increased translocation of endotoxin from gram negative enterobacteria (leaky gut) is accompanied by a remission of chronic fatigue syndrome. Neuro. Endocrinol. Lett. 2007;28:739–744. [PubMed] [Google Scholar]
- Maloney EM, Boneva RS, Lin JM, Reeves WC. Chronic fatigue syndrome is associated with metabolic syndrome: results from a case-control study in Georgia. Metabolism. 2010;59:1351–1357. doi: 10.1016/j.metabol.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Horai R, Sudo K, Iwakura Y. IL-1 plays an important role in lipid metabolism by regulating insulin levels under physiological conditions. J. Exp. Med. 2003;198:877–888. doi: 10.1084/jem.20030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J, Ross GL, Price H, Mortensen N, Evans J, Castell LM. Biochemical markers for post-operative fatigue after major surgery. Brain Res. Bull. 2003;60:125–130. doi: 10.1016/s0361-9230(03)00021-2. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Kim JH, Bardwell W, Hong S, Dimsdale JE. Predictors of fatigue in obstructive sleep apnea. Sleep Breath. 2008;12:397–399. doi: 10.1007/s11325-008-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Fujita M. Fluctuation of serum vitamin E (alpha-tocopherol) concentrations during exacerbation and remission phases in patients with chronic fatigue syndrome. Heart Vessels. 2010;25:319–323. doi: 10.1007/s00380-009-1206-6. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Mao X, Collins KA, Kelly C, Andrade G, Nestadt P, Levine SM, Mathew SJ, Shungu DC. Increased ventricular lactate in chronic fatigue syndrome measured by 1H MRS imaging at 3.0. T. II: comparison with major depressive disorder. N.M.R. Biomed. 2010;23:643–650. doi: 10.1002/nbm.1512. [DOI] [PubMed] [Google Scholar]
- Myhill S, Booth NE, McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int. J. Clin. Exp. Med. 2009;2:1–16. [PMC free article] [PubMed] [Google Scholar]
- Orre IJ, Murison R, Dahl AA, Ueland T, Aukrust P, Fosså SD. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain Behav. Immun. 2009;23:868–874. doi: 10.1016/j.bbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Orre IJ, Reinertsen KV, Aukrust P, Dahl AA, Fosså SD, Ueland T, Murison R. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J. Psychosom. Res. 2011;71:136–141. doi: 10.1016/j.jpsychores.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Papadopoulos AS, Cleare AJ. Hypothalamic-pituitary-adrenal axis dysfunction in chronic fatigue syndrome. Nat. Rev. Endocrinol. 2011;8:22–32. doi: 10.1038/nrendo.2011.153. [DOI] [PubMed] [Google Scholar]
- Prins J, van der Meer J, Bleijenberg G. Chronic Fatigue Syndrome. Lancet. 2006;28:367(9507):346–355. doi: 10.1016/S0140-6736(06)68073-2. [DOI] [PubMed] [Google Scholar]
- Radice D, Redaelli A. Breast cancer management: quality-of-life and cost considerations. Pharmacoeconomics. 2003;21:383–396. doi: 10.2165/00019053-200321060-00003. Review. [DOI] [PubMed] [Google Scholar]
- Raison CL, Lin JM, Reeves WC. Association of peripheral inflammatory markers with chronic fatigue in a population-based sample. Brain Behav. Immun. 2009;23:327–337. doi: 10.1016/j.bbi.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Rajeevan MS, Smith AK, Dimulescu I, Unger ER, Vernon SD, Heim C, Reeves WC. Glucocorticoid receptor polymorphisms and haplotypes associated with chronic fatigue syndrome. Genes Brain Behav. 2007;6:167–176. doi: 10.1111/j.1601-183X.2006.00244.x. [DOI] [PubMed] [Google Scholar]
- Reinertsen KV, Grenaker-Alnæs GI, Landmark-Høyvik H, Loge JH, Wist E, Kristensen VN, Fosså SD, Edvardsen H. Fatigued breast cancer survivors and gene polymorphisms in the inflammatory pathway. Brain Behav. Immun. 2011 Apr 7; doi: 10.1016/j.bbi.2011.04.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Reyes M, Nisenbaum R, Hoaglin DC. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Arch. Intern. Med. 2003;163:1530–1536. doi: 10.1001/archinte.163.13.1530. [DOI] [PubMed] [Google Scholar]
- Rezaieyazdi Z, Sahebari M, Hatef MR, Abbasi B, Rafatpanah H, Afshari JT, Esmaily H. Is there any correlation between high sensitive CRP and disease activity in systemic lupus erythematosus? Lupus. 2011;20:1494–1500. doi: 10.1177/0961203311418706. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Charler ML, Papadopoulos A, Wessely S, Chalder T, Cleare AJ. Does hypocortisolism predict a poor response to cognitive behavioral therapy in chronic fatigue syndrome? Psychol. Med. 2010;40:515–522. doi: 10.1017/S0033291709990390. [DOI] [PubMed] [Google Scholar]
- Robinson M, Gray SR, Watson MS, Kennedy G, Hill A, Belch JJ, Nimmo MA. Plasma IL-6, its soluble receptors and F2-isoprostanes at rest and during exercise in chronic fatigue syndrome. Scand. J. Med. Sci. Sports. 2010;20:282–290. doi: 10.1111/j.1600-0838.2009.00895.x. [DOI] [PubMed] [Google Scholar]
- Robson-Ansley P, Cockburn E, Walshe I, Stevenson E, Nimmo M. The effect of exercise on plasma soluble IL-6 receptor concentration: a dichotomous response. Exerc. Immunol. Rev. 2010;16:56–76. [PubMed] [Google Scholar]
- Schifitto G, Deng L, Yeh TM, Evans SR, Ernst T, Zhong J, Clifford D. Clinical, laboratory, and neuroimaging characteristics of fatigue in HIV-infected individuals. J. Neurovirol. 2011;17:17–25. doi: 10.1007/s13365-010-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav. Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Schutzer SE, Angel TE, Liu T, Schepmoes AA, Clauss TR, Adkins JN, Camp DG, Holland BK, Bergquist J, Coyle PK, Smith RD, Fallon BA, Natelson BH. Distinct cerebrospinal fluid proteomes differentiate post-treatment lyme disease from chronic fatigue syndrome. PLoS One. 2011 Feb 23;6(2):e17287. doi: 10.1371/journal.pone.0017287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry CL, Kramer JM, York JM, Freund GG. Behavioral recovery from acute hypoxia is reliant on leptin. Brain Behav. Immun. 2009;23:169–175. doi: 10.1016/j.bbi.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MN, Heim CM, Nater UM, Marques AH, Sternberg EM. Neuroendocrine and immune contributors to fatigue. PM R. 2010;2:338–346. doi: 10.1016/j.pmrj.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence VA, Kennedy G, Belch JJ, Hill A, Khan F. Low-grade inflammation and arterial wave reflection in patients with chronic fatigue syndrome. Clin. Sci. (Lond) 2008;114:561–566. doi: 10.1042/CS20070274. [DOI] [PubMed] [Google Scholar]
- Stebbings S, Herbison P, Doyle TC, Treharne GJ, Highton J. A comparison of fatigue correlates in rheumatoid arthritis and osteoarthritis: disparity in associations with disability, anxiety and sleep disturbance. Rheumatology (Oxford) 2010;49:361–367. doi: 10.1093/rheumatology/kep367. [DOI] [PubMed] [Google Scholar]
- Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol. 2010;29:333–337. doi: 10.1037/a0018836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Harding S, Sorenson M, Jason LA, Reynolds N, Brown M, Maher K, Fletcher MA. The associations between basal salivary cortisol levels and illness symptomatology in chronic fatigue syndrome. J. Appl. Biobehavioral Res. 2009;13:157–160. doi: 10.1111/j.1751-9861.2008.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, Mobley GM, Liao Z. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiationtherapy. Brain Behav. Immun. 2010;24:968–974. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Evengård B, Natelson BH, Jason LA. Preface and mini-review. In: Kuratsune H, editor. Fatigue Science for Human Health. New York: Springer; 2008. pp. 5–11. [Google Scholar]
- White AT, Light AR, Hughen RW, Vanhaitsma TA, Light KC. Differences in metabolite-detecting, adrenergic, and immune gene expression after moderate exercise in patients with chronic fatigue syndrome, patients with multiple sclerosis, and healthy controls. Psychosom. Med. 2012;74:46–54. doi: 10.1097/PSY.0b013e31824152ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AT, Light AR, Hughen RW, Bateman L, Martins TB, Hill HR, Light KC. Severity of symptom flare after moderate exercise is linked to cytokine activity in chronic fatigue syndrome. Psychophysiology. 2010;47:615–624. doi: 10.1111/j.1469-8986.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler T, Fletcher MA, Lonergan W, Zeng XR, Lin JM, Laperriere A, Vernon SD, Klimas NG. Impaired immune function in Gulf War Illness. BMC Med. Genomics. 2009;2:12. doi: 10.1186/1755-8794-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]