Abstract
The ability to derive functional thyroid follicular cells from embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) would provide potential therapeutic benefit for patients with congenital or post-surgical hypothyroidism. Furthermore, understanding the process by which thyroid follicular cells develop will also provide great insight into the key steps that regulate the development of other tissues derived from endoderm. Here we review the advances in our understanding of the process of thyroid follicular cell development including the creation of two models that have allowed for the rescue of hypothyroid mouse recipients through the transplantation of thyroid follicular cells derived from mouse ESCs. Rapid progress in the field suggests that the same success should be achievable with human ESCs or iPSCs in the near future. Additionally, the availability of ESC or iPSC-derived thyroid follicular cell models will provide ideal systems to explore how genetic mutations, drugs or illness impact thyroid function in a cell-autonomous fashion.
Introduction
The development of novel modalities of thyroid hormone replacement have been considered for years given the issues with clinical efficacy of L-thyroxine replacement. Indeed, it is now clear that a significant number of patients requiring thyroid hormone replacement therapy do not feel well on replacement therapy. With the advent of stem cell technologies it is now possible to envision cellular replacement therapies for those with either congenital or post-surgical hypothyroidism who are dependent on exogenous replacement therapy. In contrast regenerative therapy may be difficult in patients with autoimmune thyroid disease given issues with tissue rejection and the likelihood that transplanted cells would be destroyed if autoimmunity is not first addressed. While the clinical merits of exogenous therapy versus the production of endogenous thyroid hormone by a transplanted gland can be debated it is clear that insights gained from a better understanding of follicular cell development may lead to clinical treatments for some that may be preferred over pharmacologic therapy. This is especially the case for children with congenital hypothyroidism whose genetic defects could potentially be corrected ex vivo in their cultured stem cells prior to transplantation of stem-cell derived thyroid follicular cells (1, 2). Additionally, cellular models that recapitulate follicular cell development will be able to provide key insight into developmental milestones as well as provide ex-vivo models to test the role of novel pathways or drugs on thyroid development and function. In the following article we will review the progress made in the development of functioning thyroid follicular cells from embryonic or induced pluripotent stem cells to date.
Early Thyroid Development
The fully developed thyroid gland contains two endocrine cell types. The minority component is made up of parafollicular cells (C cells) which secrete calcitonin and derive from the neural crest. The majority component within the thyroid gland are endodermally derived follicular epithelial cells and the focus of this review. Indeed, as will be discussed, the derivation of fully functioning thyroid tissue from embryonic stem cells in now way requires the presence of calcitonin-secreting C cells.
Beginning at E20 in humans and E8.5 in mice, the follicular cell development program begins in the anterior foregut endoderm as a small subset of endodermal progenitor cells commit to a thyroid cell fate through a process referred to as “lineage specification”. Follicular cell fate is defined by the co-expression of the transcription factors Nkx 2-1 and Pax8, the earliest known markers of thyroid lineage specification within developing endoderm (3–6). Indeed, while these transcription factors are expressed in other cell-types it is their co-expression at a unique developmental stage that first identifies the developing endodermal thyroid primordium and is required for normal, subsequent follicular cell development (7–9). What extrinsic factors cause the initial thyroid cell fate decision have not been completely defined but a role for factors released by the adjacent cardiac mesoderm has been implicated by some studies (10–15) and our recent work suggests combinatorial BMP and FGF signaling as two likely pathways that induce thyroid fate in the developing foregut endoderm of xenopus, mice, and humans. Close to the time of this cell fate decision additional transcription factors including Foxe1 and Hhex are expressed in the thyroid primordium, which together with Nkx2-1 and Pax8 form a core network of transcriptional regulators leading to differentiation, migration and maturation of the developing follicular cells (2, 6). The importance of these transcription factors is further underscored by the fact that mutations in Nkx2-1, Pax8 and Foxe1 lead to congenital hypothyroidism in humans (16–18). In addition to these key transcription factors, roles for the Hox genes and Eya1 gene have been implicated in thyroid development (19–21). While growth factors that induce FGF and BMP signaling appear to regulate the thyroid cell fate decision TSH which is a major growth factor for the gland is not required for lineage specification but does play a role in thyroid gland growth and function (22). Mice lacking a functional TSH receptor are hypothyroid but possess a hypoplastic gland that contains a normal structure (23–25). A similar phenotype is seen in humans with inactivating TSH receptor mutations (26, 27). Given the understanding of the transcriptional pathways employed in follicular cell development it became logical to try and re-create follicular cell development by activating these genes or pathways in vitro in cell-based models.
Thyroid Function in Embryonic Stem Cells – Initial Studies
Since the discovery of the pluripotency of embryonic stem cells (ESCs) there has been much focus on the derivation of specific cell types for potential therapeutic application. In the thyroid field initial studies focused on whether ESC-derived embryoid bodies, which contain the three germ layers of the embryo could express thyroid follicular genes. Lin et al developed embryoid bodies from a mouse ESC line which in the undifferentiated state expressed no follicular cell markers (28). After 6 days in culture they began to see the expression of the sodium iodide symporter (NIS), the thyrotropin stimulating hormone receptor (TSHr) and Pax8 which persisted in culture. They were able to validate TSHr expression using immunohistochemistry and subsequently by demonstrating an increase in cAMP production in response to recombinant TSH and an enhancement in Pax8, thyroid peroxidase (Tpo) and NIS gene expression (Figure 1). Although these early cells did not express thyroglobulin (Tg), a developmental marker of thyroid maturation known to occur earlier than Tpo or NIS in vivo, still this proof of concept study demonstrated that differentiating ESCs had the capability to express both thyroid follicular specific transcription factors and a subset of the genes required for thyroid hormone synthesis.
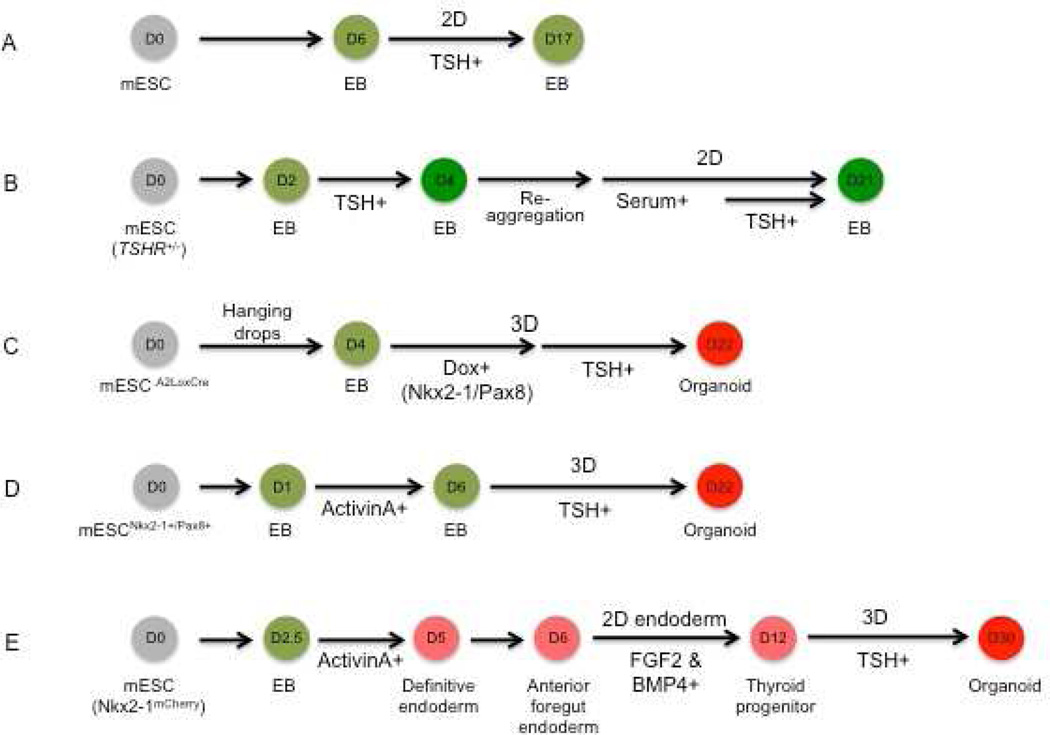
Figure 1. Schematic overview of mouse thyroid differentiation protocols.
(A) Lin et al (28) induced formation of embryoid bodies (EBs) for 6 days in suspension in the presence of serum. To form monolayers, EBs were re-plated on gelatin-coated dishes (2D) and treated with TSH in serum-free conditions. (B) Arufe et al (29) initiated EB formation with mESCs that carry GFP targeted to the TSHR locus (TSHR+/−) in serum-containing EB differentiation medium (EBDM) for 2 days followed by TSH treatment. GFP-positive cells were sorted and reaggregated in suspension to form EBs before replated on Matrigel-coated dishes. EBs were then further differentiated in EBDM and treated again with TSH. (C) Antonica et al (33) generated EBs using tetracycline-inducible mESC lines by culturing in hanging drops for 4 days. EBs were then embedded in Matrigel and re-plated followed by the sequential treatment with doxycycline (Dox) and TSH for 3 and 15 days, respectively. (D) Ma et al (47) cultured mESCs that stably express Nkx2-1 and Pax8 for one day in LIF and then removed mESC medium to induce EB formation. EBs were then treated with activin A for 5 days and re-plated in Matrigel embedded drops for further differentiation in the TSH-containing medium. (E) Kurmann et al (22) induced EB formation in complete serum-free differentiation medium for 2.5 days followed by definitive endoderm induction with activin A treatment for additional 2.5 days. After anteriorization of endoderm, Nkx2-1+ endoderm induction was performed in BMP4 and FGF2 containing thyroid specification medium for 6 days in a gelatin-coated 2D culture system. The sorted Nkx2-1mCherry+ cells on day 12 were embedded in Matrigel for 3D culture for further differentiation and maturation over 18 days.
To follow-up on this work this same group next used an ES cell line that expressed green fluorescent protein (GFP) under the control of the TSHr genomic locus on one allele (TSHrGFP) (29). These same ES cells had been used to generate a TSHr knockout mouse which had severe congenital hypothyroidism which was fatal by 4 weeks of life (24). Importantly, though hypothyroid these animals developed a hypoplastic thyroid with some thyroid follicles demonstrating that neither thyroid lineage specification nor initial follicular cell development requires TSH signaling. Importantly, these genetically altered ESCs functioned similarly in the undifferentiated state as wild-type ES cells and when differentiated into embryoid bodies upregulated both GFP and TSHr production indicating that the expression of GFP could be used as a marker of TSHr transcription. Next these investigators sorted day 4 TSHrGFP positive cells and cultured them in TSH and Matrigel to allow for a 3-dimensional structure to occur (30). Although the sorted cells and their progeny did not express thyroglobulin, after 21 days in culture these cells appeared to form thyroid follicle-like structures and demonstrated by immunohistochemistry correct spatial localization of both NIS and the TSHr (Figure 1). Furthermore, when cultured in the presence of TSH these cells could take up 125 I and this process was specifically inhibited by perchlorate. Finally, there was extensive induction in these cultured cells of Pax8, NIS, Tpo and the TSHr when compared to undifferentiated cells. Thus, further enhancement of the differentiation process was obtained by sorting the cells based on TSHrGFP expression as well as the use of Matrigel and TSH. Still the surprisingly rapid in vitro expression of TSHr, a marker that is not expressed in vivo until late in thyroid development, and the absence of Tg expression in this system, highlighted the ongoing challenges facing investigators attempting to achieve full follicular cell development from stem cells in vitro.
Finally, given that thyroid follicular cells could develop in the absence of a TSH signal, these investigators set out to understand what pathways delineated follicular cell development prior to the onset of TSH-signaling. To do this Ma et al employed activin A a known inducer of endoderm to determine if follicular cell development could be enhanced (31). Activin A was added to embryoid bodies and as expected caused a dramatic upregulation of endodermal markers such as Gata4 and FoxA2. However, the addition of TSH and IGF-1 to Activin A did not enhance the appearance of endoderm (32). Exposure of cells to 5 days of Activin A allowed for the enhanced detection of NIS in TSHrGFP positive cells even though TSHr expression was low and Tg was absent. After more prolonged exposure to Activin A increases in Pax8 was also seen. Interestingly, synergy between Activin A and TSH was not evident but the developed cells did respond to TSH as evidenced by cAMP production. These experiments confirmed the notion that endodermal development of follicular cells could occur independently of a TSH signal, but the exact paradigm for differentiating ESCs into endoderm competent to express all the markers or differentiation kinetics typical of developing thyroid follicular tissue (e.g. foregut endodermal Nkx2-1 and Pax8 expression prior to Tg or TSHr) was not yet established.
Follicular Cells Can be Derived From Overexpression of Nkx2-1 and Pax8
Because the approach was still unknown for differentiating ESCs into thyroid follicular cells via endoderm, the Costagliola group set out to determine if co-expression of Nkx2-1 and Pax8 could allow for follicular cells to develop from ESCs without needing to first optimize their differentiation into anterior foregut endoderm (33). In this way the specific steps needed to activate endodermal expression of both Nkx2.1 and Pax8 could be bypassed. To accomplish this recombinant mouse ESCs were engineered that contained doxycycline inducible integrated expression cassettes for either Nkx2.1, Pax8 or both (Figure 1). Importantly, this manipulation did not impair the pluripotency of these lines. Using these ES cells this group used doxycycline-induced forced over-expression of both transcription factors for three days at the embryoid body stage of development. Stunningly, induction of both Nkx2-1 and Pax8 at this time point were enough to induce the strong expression of follicular cell markers such as TSHr, NIS, Tg and Foxe1 as well as endogenous Nkx2.1 and endogenous Pax8, suggesting the presence of an auto-regulatory loop inducing their expression. Despite driving follicular cell development using this protocol the authors were unable to see the morphological development of thyroid follicles. To try and overcome this barrier and based on the role of TSH, described previously in follicular cell growth, the investigators altered their protocol to follow the transient induction of Nkx2-1 and Pax8 with TSH treatment. Importantly, Matrigel was included for 3 dimensional growth. Remarkably after a total of 22 days of culture, 60 percent of these cells co-expressed Nkx2.1 and Pax8 as well as expressing high levels of Tg, NIS, Tpo and Tshr. In addition these cells organized into follicular like structures and stained appropriately using immunohistochemistry for Tg, Nis and Nkx2-1 (Figure 1).
To determine whether the expression of both transcription factors was necessary, Antonica et al next used identical ESC lines but with only Nkx2-1 or Pax8 incorporated. Interestingly, the transient induced expression of Nkx2-1 but not Pax8 could induce some degree of follicular cell gene expression. However, despite inducing follicular cell specific genes the transient expression of Nkx2-1 followed by TSH exposure could not induce the 3-dimensional follicular structures seen with the co-expression of both transcription factors. This is likely due to the fact, at least in part, that Nkx2-1 expression alone was not able to induce a high enough level of expression of the TSHr to allow significant signaling and growth.
Because the transient expression of both Nkx2-1 and Pax8 followed by TSH treatment was able to induce the formation of anatomically correct follicular structures, Antonica et al next tested the ability of these cells to organify iodine, a critical step in thyroid hormone synthesis. Using immunohistochemistry with an antibody that recognizes iodine complexed to TG they were able to see Tg-Iodine complexes in the lumen of follicular units in culture. In addition, they determined that only cells exposed to transient co-expression of both transcription factors coupled to TSH treatment were able to take up and organify radioactive iodine (125I). Thus, taken together these data clearly demonstrated that the over-expression of Nkx2-1 and Pax8 in ES cells, coupled with TSH treatment, could induce functioning thyroid follicular cells in culture.
Based on these provocative results the authors next tested whether these cells could function in vivo. Using 131I to ablate all endogenous thyroid follicular tissue in mice, as published by Abel et al (34), hypothyroid mice were generated to serve as recipients of the ESC-derived follicles. Four weeks after the administration of 131I and the confirmation of severe hypothyroidism, mice received either ESCs that had been pre-treated in vitro with doxycycline (to over-express Nkx2-1 and Pax8) followed by TSH treatment or control ESCs that had no doxycycline exposure. All transplanted cells were placed underneath one kidney capsule. Four weeks after receiving the transplanted cells eight out of nine mice that received the treated ESCs had substantial recovery of their hypothyroidism with increased T4 secretion, decreased TSH levels and recovery from hypothyroid-induced hypothermia. To confirm that the transplanted cells were in fact responsible for the recovery from hypothyroidism, mice were imaged with 99m-Tc-pertechnetate (hereafter Tc) which is used clinically to image human thyroid tissue and is known to be taken into thyroid follicular cells through the sodium-iodide symporter (35). Transplanted mice exhibited markedly decreased Tc uptake in the region of the thyroid due to its ablation but displayed significant Tc uptake in the region of the kidney where the transplanted cells had grafted. Finally, to confirm that the transplanted cells could respond directly to TSH in vivo, recombinant TSH was administered to transplanted mice and a significant rise was seen in endogenous T4 production. Taken together these in vivo data confirmed that the transplanted ESC-derived follicles could fully function and rescue mice from hypothyroidism.
While the work of Antonica et al addressed the differentiation of mESCs into functional follicular cells they did not attempt the same experiments in human ESCs to determine if a similar process could be effective. Using lentiviral vectors expressing NKX2-1 and PAX8, Ma et al have recently tried to address this issue in a human ESC line (36). The human H9 line was successfully transduced with integrating lentiviral vectors that stably introduced expression cassettes for NKX2-1 tagged with mCherry, PAX8 tagged with GFP, or both. Under basal conditions forced over-expression of both transcription factors was able to stimulate the moderate expression of follicular cell-specific genes such as NIS and TSHr, but not Tg or Tpo. Interestingly, the co-expression of NKX2-1 and PAX8 did not abrogate the expression of the pluripotent markers OCT4, SOX2 and NANOG. Next these investigators tested activin A and TSH treatment of these cells, an approach that had been used previously with mouse ESCs. At the embryoid body stage the transfected line was exposed to activin A to further induce endoderm and then stimulated with TSH. This protocol resulted in moderate expression of some follicular cell genes, although TG and TPO levels were far below control tissue levels. Still this protocol allowed for the generation of a follicular structure that exhibited some degree of immunostaining for NIS and TG. Finally, when these differentiated cells were exposed to TSH, cAMP was produced and radioiodine uptake was seen. Transplantation of these cells was not attempted, however, from the work completed it appears that the forced over-expression of NKX2-1 and PAX8 can also promote some degree of thyroid-like gene expression in human ESCs in vitro.
The work of Antonica et al was a pivotal landmark in the field as this group demonstrated for the first time that functional thyroid follicular cells could be derived from pluripotent ESCs. Furthermore these cells could structurally organize into follicular units, organify iodine in culture, and fully function in vivo. However, despite proving that ESCs had the capability to be differentiated into functional thyroid tissue the process developed was entirely reliant on the forced over-expression of Nkx2.1 and Pax8. Clearly, further advancement in the field would require on an understanding of the biological pathways that could activate endogenous Nkx2.1 and Pax8 expression without the need for transgenic over-expression.
Derivation of Functional Thyroid Follicular Cells Using Developmental “Directed Differentiation” of ESCs and iPSCs
The embryonic development of the thyroid from primordial endoderm is a tightly controlled, unique outcome given that the adjacent developing endodermal gut tube also gives rise to the lung, liver, pancreas, and intestinal tract (37, 38). Thus, understanding the pathways that regulate endodermal fate in the developing embryo in vivo via the use of ESCs in vitro has the potential to reveal an efficient paradigm to generate thyroid follicular cells without the need for the overexpression of exogenous transcription factors. To begin to understand endodermal development with an initial focus on lung development, Longmire et al developed a murine ESC line in which a GFP cassette was inserted into the Nkx2-1 genomic locus on one allele (39). The targeting strategy used to create this line effectively replaced the coding sequence of one allele of Nkx2-1 causing haploinsufficiency of this transcription factor. However, this line created a valuable tool to sort and purify cells expressing Nkx2-1, thus providing access to candidate precursors for lung or thyroid epithelial cells given that within the developing endoderm in vivo, only lung and thyroid lineages are known to express Nkx2-1. After demonstrating that both this ESC line as well as a knock-in Nkx2-1GFP mouse colony made with this line indeed express GFP coincident with endogenous Nkx2-1 expression, the investigators were now in position to explore strategies to generate endodermal Nkx2-1+ progenitors potentially destined to become lung or thyroid, or ectodermal Nkx2-1+ progenitors potentially destined to become forebrain neural lineages (39).
Initial work using this cell line and others demonstrated issues with a paucity of Nkx2-1 expression, perhaps related to a lack of anterior-posterior patterning of the developing ESC-derived endoderm. To bypass this issue and given a described role for TGFβ and BMP (bone morphogenic protein) signaling in patterning of both the developing mouse embryo and human ESCs Longmire et al inhibited these signals with SB431542 (SB) and Noggin respectively early after endodermal induction (40). Using this approach with the Nkx2-1GFP line, ~20% of cells were found to express GFP. Further treatment of the cells with Wnt3a, FGF10, KGF, BMP4, FGF2 and heparin (WFKBE+F2/H) further induced cells that also expressed Pax8 (40). Finally, Longmire et al optimized their protocol by sorting GFP+ cells after WFKBE treatment and then treating further with FGF 2 and 10 followed by treatment with dexamethasone, IBMX, cAMP and KGF (DCI +K). Remarkably after 25 days of culture these cells demonstrated the expression of lung markers such as SPC (surfactant protein c) and CC10 (club cell 10) as well as thyroid follicular cell markers Tg and TSHr. Furthermore, these cells were able to assume lung alveolar morphology when seeded on a murine lung structure where cellular structures had been removed. Interestingly, when the maturation DCI +K media was replaced with TSH, sodium iodine and IGF-1 there was significant increase in the mRNA expression of follicular cell markers such as TSHr and Tg. Taken together these data demonstrated that it was possible to sequentially recapitulate in vivo sequences of endodermal development to re-capitulate lung and thyroid lineage specification followed by early follicular gene expression. Further work could then focus on the exact components required to firmly derive thyroid follicular cells.
To engineer a program that could enhance thyroid follicular cell development Kurmann et al first modified the protocol established by Longmire et al and determined that the specification of endodermal development with WFKBE+F2/H could be narrowed down just to BMP4 and FGF2 using the Nkx2-1GFP ESC line which produced maximal numbers of GFP+ cells that could be then be purified to express follicular cell markers (22). Next, because the Nkx2-1GFP ESC line likely is haploinsufficient for Nkx2-1, as discussed previously, Kurmann et al compared this line to a novel Nkx2-1 ESC line where a fluorochrome reporter gene (mCherry) had been inserted in the 3’ untranscribed region (UTR) of Nkx2-1 thus allowing for the expression of both endogenous Nkx2-1 alleles (41). While both lines showed similar Nkx2-1 lineage specification efficiencies, the Nkx2-1mCherry line showed a 2-fold elevation in Nkx2.1 mRNA expression and after sorting and further culturing for 25 days significantly increased expression levels of Pax8, Tg, Tpo, TSHr and NIS. This is consistent with expression of both alleles of Nkx2.1 being necessary for full thyroid development in humans and mice (16, 42, 43). Thus, the potential for successful derivation of functional thyroid follicular cells was expected to be enhanced by the use of the Nkx2-1mCherry line. A description of the differentiating protocol used by Kurmann et al is shown in Figure 1.
To determine if the same cells expressing Nkx2-1 were able to be precursors for both thyroid and lung epithelia or whether distinct Nkx2-1 groups developed during endodermal development, Kurmann et al used a second reporter system that would mark both Nkx2-1 and Pax8 and thus co-label cells that expressed both loci (39, 44). When exposed to the thyroid protocol a small subset of cells (5% of the Nkx2-1+ cells) co-expressed both labels. Remarkably, only this distinct subgroup (Nkx2-1+/Pax8+) was competent to express differentiated follicular cell markers but not lung markers. Thus, it is likely that the thyroid lineage is specified by a unique group of Nkx2-1 endodermal cells.
To conclusively determine the role of BMP and FGF signaling in thyroid lineage specification Kurmann et al employed a number of strategies to block either pathway or remove each BMP4 or FGF2 ligand. Importantly, the combinatorial actions of BMP4 and FG2 were required for the maximal induction of thyroid follicular cell lineage specification and gene expression. Furthermore, the actions of these ligands were independent of Wnt which was not required for thyroid specification. Since BMP and FGF actions are transmitted through signaling cascades that include: 1. SMADs (for BMP signaling) ; 2. p38 MAPK (for BMP signaling); 3. PI3-Kinase (for FGF2 signaling) and 4. MEK 1/2 (common to both BMP and FGF signaling) the investigators next utilized specific inhibitors of each of these pathways (45). Remarkably, the SMAD1/5/8-inhibitor dorsomorphin and the PI3-Kinase inhibitor LY294002 inhibited the emergence of Nkx2-1+ cells prior to sorting and overall follicular cell specific gene expression after 28 days of culture confirming the importance of BMP4 and FGF2 in thyroid lineage specification. To test whether these mechanisms revealed by the in vitro stem cell model were also those regulating thyroid development in vivo in embryos, developing mouse foreguts were isolated prior to the induction of Nkx2-1 in the thyroid field and treated with a BMP inhibitor. This treatment blocked the phosphorylation of SMAD 1/5 in the region of the developing thyroid and prevented the induction of Nkx2-1 and Pax8.
To determine if these pathways were conserved across species Xenopus embryos were employed as they undergo a similar process of thyroid development (46). Embryos were incubated in the presence or absence of inhibitors of the BMP or FGF signaling pathways. Almost all embryos that were treated with either inhibitor failed to exhibit thyroid specification. Furthermore, the addition of both FGF2 and BMP4 to Xenopus foregut explants successfully induced the development of follicular cell specific genes confirming the essential nature of these two signaling pathways. Importantly, FGF2 and BMP4 were able to induce thyroid fate even when only the endoderm of Xenopus embryos (dissected free of any contaminating mesoderm) was exposed to these growth factors, suggesting that these pathways acted intrinsically on foregut endoderm to induce thyroid fate, rather than acting secondarily through mesodermal or other neighboring developing tissues. Thus, Kurman et al had successfully identified the key components of a directed differentiation protocol that could potentially develop functioning thyroid follicular cells. The use of a sorting marker (Nkx2-1mCherry) also allowed the purification of unlimited numbers of endodermal Nkx2-1+ precursors.
With these reagents in hand Kurmann et al next set out to determine if ESC-derived thyroid follicular cells could actually function in vivo. Before doing this they further optimized their protocol to enhance thyroid follicular cell development and fitness. The role of TSH was first studied and found to have no effect on lineage specification before sorting Nkx2-1+ cells, consistent with published in vivo observations(25). However, during the maturation phase after sorting Nkx2-1 positive cells, TSH treatment further augmented the expression of genes important in thyroid hormone synthesis ie NIS and TSHr. Furthermore, the presence of insulin, IGF-1 and selenium was also included in this base medium in conjunction with FG2 and FG10. In the last few days of the protocol maturation media containing TSH and dexamethasone was included in addition to IBMX and KGF (see Figure 1). This final protocol was found to result in the highest degree of follicular cell expression. At this point most of the experiments performed had been in a 2 dimensional system. Given the history of thyroid follicular cell growth being enhanced by a 3D matrix such as Matrigel, Kurmann et al compared 2D culture conditions to 3D conditions after sorting of the cells (30, 33, 47). Strikingly, the addition of 3D Matrigel culture promoted the formation of follicular organoids that structurally resembled thyroid follicles and showed the presence of Tg in the lumen of cells co-expressing Nkx2-1 and Pax8. Furthermore, these organoids could be maintained in culture for at least 52 days and showed the ability to produce thyroxine both by immunolabelling and by ELISA which quantified the production of a small amount of T4 in the organoids in vitro.
To test functional capacity in vivo, Kurmann et al followed the paradigm of Antonica et al (33) and transplanted hypothyroid syngeneic mouse recipients (following radioactive iodine exposure) with ESCs that were differentiated with their protocol for 30 days vs. undifferentiated (day 0) ESCs. Approximately 2–3 million cells were transplanted under the left kidney capsule of each mouse. Beginning 2–4 weeks after transplantation both T4 and T3 levels started to rise in mice that had received the day 30 cells while mice receiving day 0 cells or those who underwent sham operations remained hypothyroid. By 8 weeks after transplantation, recipients of ESC-derived follicles had returned to a euthyroid state with normal thyroid hormone and TSH levels consistent with the transplanted cells being fully functional and responsive to TSH. The interpretation that a normal set point of TSH secretion had been established following transplantation was also confirmed in vivo by testing the direct response of the grafted cells to exogenously administered TSH. Imaging of the grafts in vivo revealed they remained strongly avid for Tc-99 pertechnetate and histological analysis of the grafts after several months revealed persistent follicular structures (see Figure 2) and expression of the mCherry reporter confirming these follicles derived from the transplanted cells. In contrast , mice that received control undifferentiated ESCs cells grew teratomas without evidence of any regeneration of thyroid follicular structure or function. Thus, using a protocol that recapitulates in vivo development ESC-derived thyroid follicles were capable of rescuing hypothyroid mice by regenerating in vivo thyroid function.
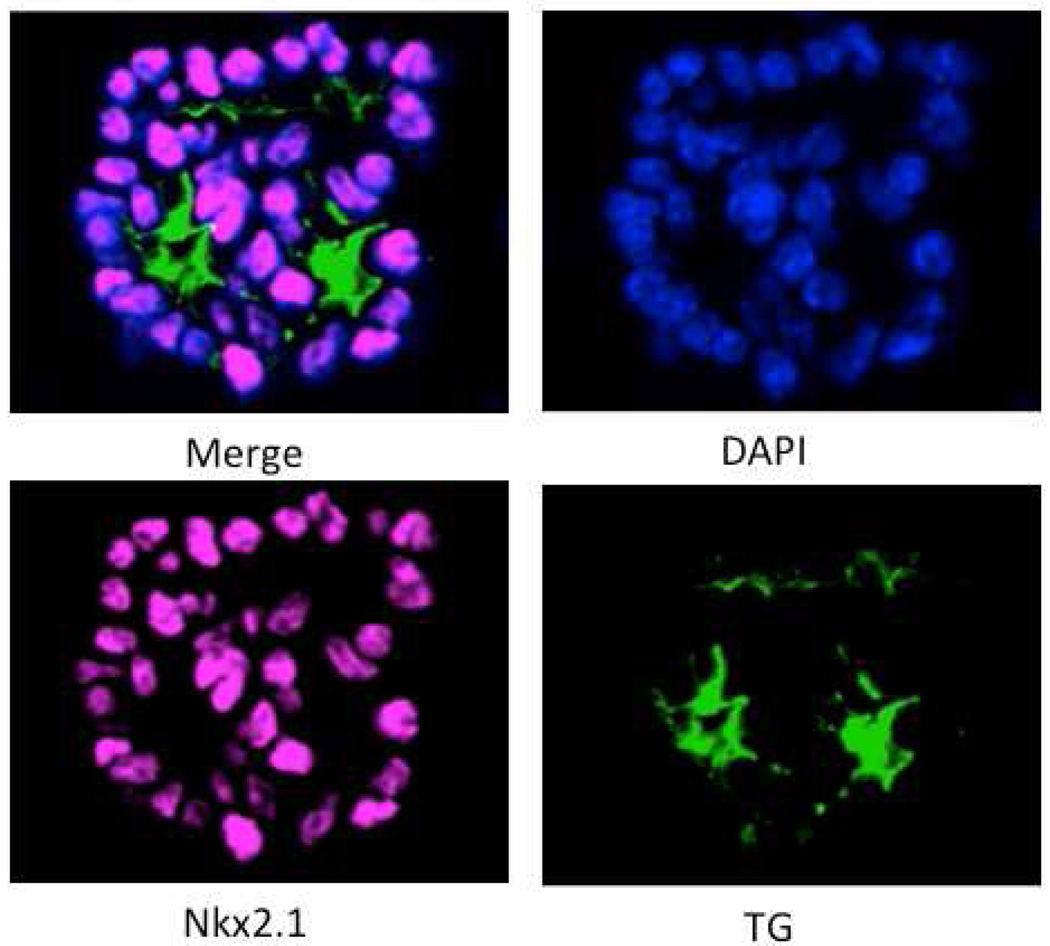
Figure 2. Transplanted Thyroid Organoids Fully Function in vivo.
Shown is immunostaining of differentiated Nkx2-1mCherry cells that have been used to from thyroid organoids in culture. The organoids were stained with DAPI or with antibodies directed against Nkx2-1 or Tg. The merged image shows Tg in the lumen of a thyroid follicular structure. Images by Anita Kurmann, MD
The last step for Kurmann et al was to explore the ability of their protocol to generate thyroid cells from human pluripotent cells. The investigators employed 3 separate types of human stem cell lines: normal human ESCs, normal induced pluripotent stem cells (iPSCs) and three separate iPSC lines this team engineered from three hypothyroid children carrying NKX2-1 point mutations known to result in haploinsufficiency (42, 48, 49). After endodermal induction with activin A anterior patterning was induced with inhibition of both TGFβ and BMP signaling followed by a variety of doses of BMP4 and FGF2 that resulted in the induction of NKX2.1 and PAX8 double positive cells in all lines tested. Addition of BMP4 or FGF2 alone resulted very rarely in the double positive expression of both transcription factors. Further treatment of these now double positive lines in the same thyroid specific medias used in the mouse protocol for 42 days in culture allowed for the induction of follicular cell specific genes including TG, TPO and TSHR as well as the persistence of NKX2-1 and PAX8 expression. These data suggest that, as in developing Xenopus and mouse embryos, the role of BMP4 and FGF2 in thyroid specification is evolutionarily conserved in human stem cell populations. Whether their protocol results in functional thyroid follicular cells, either in vitro or after transplantation in vivo remains unknown.
Summary and Conclusions
The development of regenerative therapy based on stem cell technology is becoming a reality as many protocols are becoming available for the development of differentiated cell types. In the thyroid field significant advances have been made in the last 15 years as has been detailed in this review. It now appears clear that protocols that allow for the rapid induction of Nkx2.1 and Pax8 in an endodermal environment will allow for the development of cells that can become functional thyroid follicular cells in vivo. However, the development of such cells is not a frequent event in ESC models or in vivo and successful generation of thyroid organoids currently requires the overexpression of Nkx2.1 and Pax8 or targeted alleles which allow for the sorting and purification of cells destined to become thyroid follicular cells. Still the advances made are striking and suggest that regenerative therapy for congenital or post-surgical hypothyroidism remains a distinct possibility. Given issues with therapeutic success or compliance in some patients with these disorders, regenerative therapy coupled with genome editing to correct mutations could be a preferred alternative to life-long pharmacologic thyroid hormone replacement therapy. Indeed, regenerative therapy would provide such patients with physiologic replacement of both T4 and T3 without a reliance on an external source.
While regenerative therapy for hypothyroidism remains a long-term goal the availability of differentiation protocols for the development of thyroid follicular cells should provide in the short-term key insight into endodermal and thyroid development. For example what are the signal(s) that delineate the development of a double positive Nkx2-1/Pax8 precursor from early endoderm destined to become a follicular cell versus a single positive Nkx2.1 cell destined to become lung epithelia? Additionally, how mechanistically do BMP/FGF signaling pathways specify thyroid development versus lung development and from where in vivo in the developing embryo do these signals emanate? Finally, the availability of thyroid organoids that are replicates of in vivo thyroid tissue will provide investigators with outstanding cell autonomous models to study the influence of drugs and toxins on thyroid dysfunction. Furthermore, these organoid model systems will be invaluable for elucidating other genetic modifiers of thyroid development given that only a small percentage of the causes of congenital hypothyroidism have been ascertained.
Acknowledgments
This work was supported by the NIH DK105029 (to ANH and DNK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev. 2004;25(5):722–746. doi: 10.1210/er.2003-0028. PubMed PMID: 15466939. [DOI] [PubMed] [Google Scholar]
- 2.De Felice M, Di Lauro R. Minireview: Intrinsic and extrinsic factors in thyroid gland development: an update. Endocrinology. 2011;152(8):2948–2956. doi: 10.1210/en.2011-0204. PubMed PMID: 21693675. [DOI] [PubMed] [Google Scholar]
- 3.Carre A, Rachdi L, Tron E, Richard B, Castanet M, Schlumberger M, et al. Hes1 is required for appropriate morphogenesis and differentiation during mouse thyroid gland development. PLoS One. 2011;6(2):e16752. doi: 10.1371/journal.pone.0016752. PubMed PMID: 21364918; PubMed Central PMCID: PMC3045378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies TF, Latif R, Minsky NC, Ma R. Clinical review: The emerging cell biology of thyroid stem cells. J Clin Endocrinol Metab. 2011;96(9):2692–2702. doi: 10.1210/jc.2011-1047. PubMed PMID: 21778219; PubMed Central PMCID: PMC3167664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113(4):1093–1104. doi: 10.1242/dev.113.4.1093. PubMed PMID: 1811929. [DOI] [PubMed] [Google Scholar]
- 6.Parlato R, Rosica A, Rodriguez-Mallon A, Affuso A, Postiglione MP, Arra C, et al. An integrated regulatory network controlling survival and migration in thyroid organogenesis. Dev Biol. 2004;276(2):464–475. doi: 10.1016/j.ydbio.2004.08.048. PubMed PMID: 15581879. [DOI] [PubMed] [Google Scholar]
- 7.Damante G, Tell G, Di Lauro R. A unique combination of transcription factors controls differentiation of thyroid cells. Progress in nucleic acid research and molecular biology. 2001;66:307–356. doi: 10.1016/s0079-6603(00)66033-6. Epub 2000/10/29. PubMed PMID: 11051768. [DOI] [PubMed] [Google Scholar]
- 8.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10(1):60–69. doi: 10.1101/gad.10.1.60. PubMed PMID: 8557195. [DOI] [PubMed] [Google Scholar]
- 9.Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet. 1998;19(1):87–90. doi: 10.1038/ng0598-87. PubMed PMID: 9590297. [DOI] [PubMed] [Google Scholar]
- 10.Celli G, LaRochelle WJ, Mackem S, Sharp R, Merlino G. Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J. 1998;17(6):1642–1655. doi: 10.1093/emboj/17.6.1642. PubMed PMID: 9501086; PubMed Central PMCID: PMC1170512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagman H, Liao J, Westerlund J, Andersson L, Morrow BE, Nilsson M. The 22q11 deletion syndrome candidate gene Tbx1 determines thyroid size and positioning. Hum Mol Genet. 2007;16(3):276–285. doi: 10.1093/hmg/ddl455. PubMed PMID: 17164259. [DOI] [PubMed] [Google Scholar]
- 12.Fagman H, Nilsson M. Morphogenesis of the thyroid gland. Mol Cell Endocrinol. 2010;323(1):35–54. doi: 10.1016/j.mce.2009.12.008. PubMed PMID: 20026174. [DOI] [PubMed] [Google Scholar]
- 13.Lania G, Zhang Z, Huynh T, Caprio C, Moon AM, Vitelli F, et al. Early thyroid development requires a Tbx1-Fgf8 pathway. Dev Biol. 2009;328(1):109–117. doi: 10.1016/j.ydbio.2009.01.014. PubMed PMID: 19389367; PubMed Central PMCID: PMC2705775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitelli F, Taddei I, Morishima M, Meyers EN, Lindsay EA, Baldini A. A genetic link between Tbx1 and fibroblast growth factor signaling. Development. 2002;129(19):4605–4611. doi: 10.1242/dev.129.19.4605. PubMed PMID: 12223416. [DOI] [PubMed] [Google Scholar]
- 15.Kameda Y, Ito M, Nishimaki T, Gotoh N. FRS2alpha is required for the separation, migration, and survival of pharyngeal-endoderm derived organs including thyroid, ultimobranchial body, parathyroid, and thymus. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238(3):503–513. doi: 10.1002/dvdy.21867. PubMed PMID: 19235715. [DOI] [PubMed] [Google Scholar]
- 16.Krude H, Schutz B, Biebermann H, von Moers A, Schnabel D, Neitzel H, et al. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. J Clin Invest. 2002;109(4):475–480. doi: 10.1172/JCI14341. PubMed PMID: 11854319; PubMed Central PMCID: PMC150790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trueba SS, Auge J, Mattei G, Etchevers H, Martinovic J, Czernichow P, et al. PAX8, TITF1, and FOXE1 gene expression patterns during human development: new insights into human thyroid development and thyroid dysgenesis-associated malformations. J Clin Endocrinol Metab. 2005;90(1):455–462. doi: 10.1210/jc.2004-1358. PubMed PMID: 15494458. [DOI] [PubMed] [Google Scholar]
- 18.Castanet M, Park SM, Smith A, Bost M, Leger J, Lyonnet S, et al. A novel loss-of-function mutation in TTF-2 is associated with congenital hypothyroidism, thyroid agenesis and cleft palate. Hum Mol Genet. 2002;11(17):2051–2059. doi: 10.1093/hmg/11.17.2051. PubMed PMID: 12165566. [DOI] [PubMed] [Google Scholar]
- 19.Manley NR, Capecchi MR. The role of Hoxa-3 in mouse thymus and thyroid development. Development. 1995;121(7):1989–2003. doi: 10.1242/dev.121.7.1989. PubMed PMID: 7635047. [DOI] [PubMed] [Google Scholar]
- 20.Manley NR, Capecchi MR. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Dev Biol. 1998;195(1):1–15. doi: 10.1006/dbio.1997.8827. PubMed PMID: 9520319. [DOI] [PubMed] [Google Scholar]
- 21.Xu PX, Zheng W, Laclef C, Maire P, Maas RL, Peters H, et al. Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid. Development. 2002;129(13):3033–3044. doi: 10.1242/dev.129.13.3033. PubMed PMID: 12070080; PubMed Central PMCID: PMC3873877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurmann AA, Serra M, Hawkins F, Rankin SA, Mori M, Astapova I, et al. Regeneration of Thyroid Function by Transplantation of Differentiated Pluripotent Stem Cells. Cell Stem Cell. 2015;17(5):527–542. doi: 10.1016/j.stem.2015.09.004. PubMed PMID: 26593959; PubMed Central PMCID: PMC4666682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beamer WJ, Eicher EM, Maltais LJ, Southard JL. Inherited primary hypothyroidism in mice. Science. 1981;212(4490):61–63. doi: 10.1126/science.7209519. PubMed PMID: 7209519. [DOI] [PubMed] [Google Scholar]
- 24.Marians RC, Ng L, Blair HC, Unger P, Graves PN, Davies TF. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci U S A. 2002;99(24):15776–15781. doi: 10.1073/pnas.242322099. PubMed PMID: 12432094; PubMed Central PMCID: PMC137792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Postiglione MP, Parlato R, Rodriguez-Mallon A, Rosica A, Mithbaokar P, Maresca M, et al. Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc Natl Acad Sci U S A. 2002;99(24):15462–15467. doi: 10.1073/pnas.242328999. PubMed PMID: 12432093; PubMed Central PMCID: PMC137739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abramowicz MJ, Duprez L, Parma J, Vassart G, Heinrichs C. Familial congenital hypothyroidism due to inactivating mutation of the thyrotropin receptor causing profound hypoplasia of the thyroid gland. J Clin Invest. 1997;99(12):3018–3024. doi: 10.1172/JCI119497. PubMed PMID: 9185526; PubMed Central PMCID: PMC508154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biebermann H, Schoneberg T, Krude H, Schultz G, Gudermann T, Gruters A. Mutations of the human thyrotropin receptor gene causing thyroid hypoplasia and persistent congenital hypothyroidism. J Clin Endocrinol Metab. 1997;82(10):3471–3480. doi: 10.1210/jcem.82.10.4286. PubMed PMID: 9329388. [DOI] [PubMed] [Google Scholar]
- 28.Lin RY, Kubo A, Keller GM, Davies TF. Committing embryonic stem cells to differentiate into thyrocyte-like cells in vitro. Endocrinology. 2003;144(6):2644–2649. doi: 10.1210/en.2002-0122. PubMed PMID: 12746328. [DOI] [PubMed] [Google Scholar]
- 29.Arufe MC, Lu M, Kubo A, Keller G, Davies TF, Lin RY. Directed differentiation of mouse embryonic stem cells into thyroid follicular cells. Endocrinology. 2006;147(6):3007–3015. doi: 10.1210/en.2005-1239. PubMed PMID: 16497809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin A, Valentine M, Unger P, Lichtenstein C, Schwartz AE, Friedman EW, et al. Preservation of functioning human thyroid organoids in the scid mouse: 1. System characterization. J Clin Endocrinol Metab. 1993;77(2):305–310. doi: 10.1210/jcem.77.2.8345031. PubMed PMID: 8345031. [DOI] [PubMed] [Google Scholar]
- 31.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131(7):1651–1662. doi: 10.1242/dev.01044. PubMed PMID: 14998924. [DOI] [PubMed] [Google Scholar]
- 32.Arufe MC, Lu M, Lin RY. Differentiation of murine embryonic stem cells to thyrocytes requires insulin and insulin-like growth factor-1. Biochem Biophys Res Commun. 2009;381(2):264–270. doi: 10.1016/j.bbrc.2009.02.035. PubMed PMID: 19232325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonica F, Kasprzyk DF, Opitz R, Iacovino M, Liao XH, Dumitrescu AM, et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491(7422):66–71. doi: 10.1038/nature11525. Epub 2012/10/12. PubMed PMID: 23051751; PubMed Central PMCID: PMC3687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abel ED, Boers ME, Pazos-Moura C, Moura E, Kaulbach H, Zakaria M, et al. Divergent roles for thyroid hormone receptor beta isoforms in the endocrine axis and auditory system. J Clin Invest. 1999;104(3):291–300. doi: 10.1172/JCI6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuckier LS, Dohan O, Li Y, Chang CJ, Carrasco N, Dadachova E. Kinetics of perrhenate uptake and comparative biodistribution of perrhenate, pertechnetate, and iodide by NaI symporter-expressing tissues in vivo. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2004;45(3):500–507. PubMed PMID: 15001694. [PubMed] [Google Scholar]
- 36.Ma R, Latif R, Davies TF. Human Embryonic Stem Cells Form Functional Thyroid Follicles. Thyroid. 2015 doi: 10.1089/thy.2014.0537. PubMed PMID: 25585054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardoso WV, Kotton DN. StemBook. Cambridge (MA): 2008. Specification and patterning of the respiratory system. [PubMed] [Google Scholar]
- 38.Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132(1):35–47. doi: 10.1242/dev.01570. PubMed PMID: 15576401. [DOI] [PubMed] [Google Scholar]
- 39.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10(4):398–411. doi: 10.1016/j.stem.2012.01.019. PubMed PMID: 22482505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green MD, Chen A, Nostro MC, d’Souza SL, Schaniel C, Lemischka IR, et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29(3):267–272. doi: 10.1038/nbt.1788. PubMed PMID: 21358635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilodeau M, Shojaie S, Ackerley C, Post M, Rossant J. Identification of a proximal progenitor population from murine fetal lungs with clonogenic and multilineage differentiation potential. Stem cell reports. 2014;3(4):634–649. doi: 10.1016/j.stemcr.2014.07.010. PubMed PMID: 25358791; PubMed Central PMCID: PMC4223706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carre A, Szinnai G, Castanet M, Sura-Trueba S, Tron E, Broutin-L’Hermite I, et al. Five new TTF1/NKX2.1 mutations in brain-lung-thyroid syndrome: rescue by PAX8 synergism in one case. Hum Mol Genet. 2009;18(12):2266–2276. doi: 10.1093/hmg/ddp162. PubMed PMID: 19336474. [DOI] [PubMed] [Google Scholar]
- 43.Pohlenz J, Dumitrescu A, Zundel D, Martine U, Schonberger W, Koo E, et al. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109(4):469–473. doi: 10.1172/JCI14192. PubMed PMID: 11854318; PubMed Central PMCID: PMC150877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouchard M, Souabni A, Busslinger M. Tissue-specific expression of cre recombinase from the Pax8 locus. Genesis. 2004;38(3):105–109. doi: 10.1002/gene.20008. PubMed PMID: 15048807. [DOI] [PubMed] [Google Scholar]
- 45.Gaarenstroom T, Hill CS. TGF-beta signaling to chromatin: how Smads regulate transcription during self-renewal and differentiation. Seminars in cell & developmental biology. 2014;32:107–118. doi: 10.1016/j.semcdb.2014.01.009. PubMed PMID: 24503509. [DOI] [PubMed] [Google Scholar]
- 46.Shifley ET, Kenny AP, Rankin SA, Zorn AM. Prolonged FGF signaling is necessary for lung and liver induction in Xenopus. BMC Dev Biol. 2012;12:27. doi: 10.1186/1471-213X-12-27. PubMed PMID: 22988910; PubMed Central PMCID: PMC3514138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma R, Latif R, Davies TF. Thyroid follicle formation and thyroglobulin expression in multipotent endodermal stem cells. Thyroid. 2013;23(4):385–391. doi: 10.1089/thy.2012.0644. Epub 2013/01/31. PubMed PMID: 23360087; PubMed Central PMCID: PMC3610443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamvas A, Deterding RR, Wert SE, White FV, Dishop MK, Alfano DN, et al. Heterogeneous pulmonary phenotypes associated with mutations in the thyroid transcription factor gene NKX2-1. Chest. 2013;144(3):794–804. doi: 10.1378/chest.12-2502. PubMed PMID: 23430038; PubMed Central PMCID: PMC3760742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christodoulou C, Longmire TA, Shen SS, Bourdon A, Sommer CA, Gadue P, et al. Mouse ES and iPS cells can form similar definitive endoderm despite differences in imprinted genes. J Clin Invest. 2011;121(6):2313–2325. doi: 10.1172/JCI43853. PubMed PMID: 21537085. [DOI] [PMC free article] [PubMed] [Google Scholar]