Abstract
In rural communities, high rates of diabetes and its complications are compounded by limited access to health care and scarce community resources. We systematically reviewed the evidence for the impact of diabetes self-management education interventions designed for patients living in rural areas on glycemic control and other diabetes outcomes. Fifteen studies met inclusion criteria. Ten were randomized controlled trials. Intervention strategies included in-person diabetes (n=9) and telehealth (n=6) interventions. Four studies demonstrated between group differences for biologic outcomes, four studies demonstrated changes in behavior, and three studies demonstrated changes in knowledge. Intervention dose was associated with improved A1c or weight loss in two studies and session attendance in one study. Interventions that included collaborative goal-setting were associated with improved metabolic outcomes and self-efficacy. Telehealth and face-to-face diabetes interventions are both promising strategies for rural communities. Effective interventions included collaborative goal-setting. Intervention dose was linked to better outcomes and higher attendance.
Keywords: Diabetes self-management, Rural, Telehealth, Systematic review
Introduction
Type 2 diabetes mellitus (T2DM) is an epidemic in the USA, with 21 million people diagnosed with the disease and another 8.1 million residents undiagnosed [1]. Levels of morbidity and mortality attributable to diabetes remain high; diabetes ranks seventh on the list of leading causes of death in the USA [2]. Costs related to diabetes are also high and continue to rise [3–5]. Though diabetes is a nationwide problem, its burden is heterogeneously distributed across the country [6]. Notably, diabetes is 17 % more prevalent in rural communities than in urban settings [7]. This statistic is particularly sobering since there are half as many physicians available to care for patients in rural areas of the USA compared to those in urban areas [8]. Individuals living with T2DM in rural areas face multiple challenges, including lack of access to diabetes education [8] and clinical services [9], limited cell phone coverage and internet access [10], limited transportation and long travel distances [5], as well as higher rates of poverty [11]. These challenges in turn contribute to suboptimal diabetes management and higher rates of diabetes-related complications [9, 12•].
Creative strategies are needed to promote diabetes self-management in rural communities. The effectiveness of diabetes self-management education (DSME) on improving diabetes care and glycemic control has been demonstrated previously [13–15]. In a review published by Norris et al. in 2002, DSME was associated with improvement in knowledge, frequency, and accuracy of self-monitoring of blood glucose, self-reported dietary habits, and glycemic control, particularly in the short term (<6 months) [14•]. However, due to the challenges referenced above, individuals in rural areas often fail to receive DSME [12•]. Studies have reported numerous challenges related to the availability and sustainability of DSME in rural areas [16–18]. More recently, guidelines from the American Diabetes Association (ADA) have proposed that in addition to education for diabetes self-management, individuals need ongoing support in order to sustain their efforts [19]. Thus, efforts to promote DSME in rural areas should also include a focus on provision of support for self-management.
Despite increased recognition of diabetes as a major problem in rural areas, research focused on diabetes care in rural areas is limited. A review by Massey et al., published in 2010 [18], identified several promising strategies to increase diabetes education and support in rural areas including telephone hotlines, telemedicine, web-based education, and community health worker interventions. However, the study identified a limited number of rigorous studies with comparable health outcomes [18]. We conducted a systematic review to examine the scientific evidence for interventions specifically designed to provide education and/or support for patients living with type 2 diabetes mellitus (T2DM) in rural areas and their impact on glycemic control and other diabetes-related outcomes.
Methods
Search Strategy
We searched PubMed and CINAHL using the term [rural population] combined with terms: [diabetes education, diabetes “self-management”, diabetes “self-management” education, OR diabetes “self-management” support] from January 2004 to June 24, 2014. We supplemented this search by performing a backwards search of all the references of articles that met inclusion criteria.
Eligibility/Exclusion Criteria
Only rigorously designed studies that described interventions with measured outcomes, a control or comparison group, and a sample size greater than 50 (N>50) implemented to improve diabetes management among adult patients with T2DM in rural areas were included. Two authors reviewed the abstracts of identified articles, and studies were excluded if authors determined that eligibility criteria were not clearly met. If either reviewer could not exclude the study based on the abstract, the full article was reviewed by the two authors to assess eligibility.
Data Abstraction
Two authors abstracted the data from the articles using predetermined tables. Since selected outcomes differed notably between studies, data were not abstracted for meta-analysis. Abstraction forms included author, year of publication, study design, program duration, length to follow-up, program description, intervention strategy, description of study population, study setting, behavioral theory, measured outcomes, retention rate, and significant results. Intervention strategies were categorized as “in-person diabetes self-management education (DSME)” or “telehealth,” defined by the Health Resources and Services Administration as “the use of electronic information and telecommunication technologies to support long-distance clinical health care, patient and professional health-related education, public health and health administration [using] technologies [which] include videoconferencing, the internet…and terrestrial and wireless communications.” Studies were further categorized according to the type of individual delivering the intervention and whether or not the intervention was group based.
Results
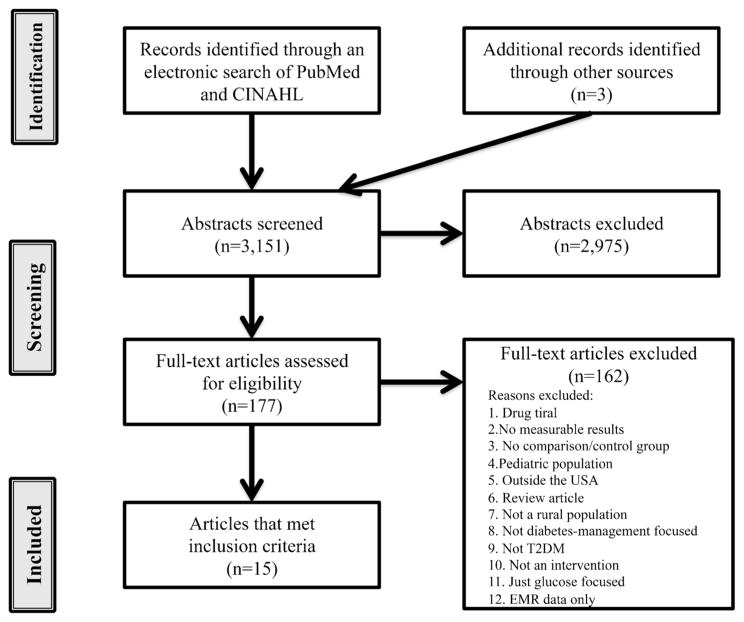
An electronic literature search was conducted using both PubMed and CINAHL databases which yielded a total of 3151 articles. The PubMed search identified 1512 articles and the remaining 1639 from the CINAHL search. Two additional articles were identified through backward searches. After review of the abstracts, 177 articles were selected and read in totality. Of these articles, fifteen met inclusion criteria and were included in the analysis (Fig. 1). Two studies evaluated the same intervention; Shea et al. reported outcomes for the entire sample (rural and urban) at 12 months [20], Izquierdo et al. reported outcomes on the rural subset of participants at 12 and 24 months [21]. Because outcomes and length of follow-up differed between the two, the studies are described separately for purposes of clarity in the current review. Intervention designs are described in Table 1; outcome measures and results are described in Table 2.
Fig. 1.
Summary of electronic search and details of excluded articles
Table 1.
Selected details of included articles categorized by intervention strategy
| Strategy | Author (year) | Format | Professional delivering program | Program length | Program description | Behavioral theory |
|---|---|---|---|---|---|---|
| Telehealth | Ciemins E (2011) | Videoconferences | NP CDE RD |
3 years | Telehealth intervention delivering DSME. Patients traveled to their primary care clinic for videoconferences. Control group received in-person education and was urban dwelling. | None mentioned |
| Davis RM (2010) | Videoconferences | CDE RD NE/NCM |
12 months | Evidence-based DSME1 videoconferences delivered by health professionals. Patients traveled to their primary care clinic for videoconferences. Three sessions were delivered in-person, 13 sessions delivered via videoconference. Control group received usual care. | Health belief model, trans-theoretical model | |
| Hawkins SY (2010) | Videophone calls | NP | 6 months | Videophones installed using a landline connection were used to deliver a DSME and motivational interviewing intervention. Intervention groups received weekly 15-min video calls from a NP for 3 months followed by 15-min monthly video calls for 3 months, with emphasis on diabetes self-management support. Control group received scripted 5-min monthly video calls from an RN. Both groups received educational handouts in the mail that were discussed during video calls. | Motivational interviewing, stages of change model, social cognitive theory | |
| Izquierdo R (2010) | One-on-one videoconferences | RD NE/NCM |
2 years | Telemedicine units were installed in participants’ homes to allow 30-min videoconference calls with a dietician every other month. A NCM made home visits to help with self-management techniques and problem solving. | None mentioned | |
| McIlhenny CV (2011) | Individual sessions | NE/NCM | 6 months | Web portal designed to make evidence-based diabetes information available to rural patients. Intervention group received one-on-one education and hands-on instructions on portal use while control group received a handout on portal use. | None mentioned | |
| Shea S (2009) | One-on-one videoconferences, secure messaging to NCM | NCM | 2–6 years | Telemedicine units were installed in participants’ homes to allow videoconferencing with NCM and remote monitoring of blood glucose and blood pressure. The unit also allowed participants access to their own clinical data and a tailored education web page in English/Spanish created by the American Diabetes Association. | None mentioned | |
| In-person DSME | Anderson-Loftin W (2005) | Group classes | CDE RD NE/NCM |
6 months | Diabetes education program for rural African Americans consisting of four group classes in dietary strategies, five support groups, and weekly telephone follow-up. Control group received usual care. | None mentioned |
| Bray P (2005) | Group classes | PharmD MD NE/NCM |
6 months | Diabetes education and support intervention for rural African Americans involving redesign to implement evidence-patient management in a primary care clinic. Intervention included regular planned care visits with a nurse and four 2-hour visits consisting of measuring vital signs and blood glucose, a group educational session, and individual evaluations by a physician. | Chronic care model | |
| Brown SA (2005) | Group classes | NE/NCM RD CHW/PA |
1 year | Latino patients randomly assigned to either “compressed” or “extended” interventions; extended intervention consisted of 52 contact hours over 12 months, compressed intervention consisted of 22 contact hours over 12 months. | None mentioned | |
| Brown SA (2011) | Group classes plus NCM | NE/NCM RD CHW/PA |
8 weeks | Evaluation of adding a NCM to a previously studied DSME program for Hispanic Americans. Control group received DSME program only. | None mentioned | |
| Kattelmann KK (2010) | Group classes | RD | 6 months | The intervention group received six 2-hour nutrition education sessions based on the Medicine Wheel Model for Nutrition. Control group received usual care from primary care provider. Culturally tailored support group sessions were offered. | None mentioned | |
| Mayer-Davis EJ (2004) | Group and individual classes | RD | 12 months | Comparison of two DMSE interventions with a control group receiving usual care. “Intensive-lifestyle” intervention (16 sessions over 1 year) vs. “reimbursable-lifestyle” intervention (eight sessions over 1 year). | None mentioned | |
| Samuel-Hodge CD (2009) | Group sessions | RD CHW/PA |
12 months | Special intervention (SI) involving an 8-month intensive phase (one individual visit, 12 group sessions, monthly phone contacts, and three encouragement postcards) followed by a 4-month reinforcement phase consisting of monthly phone contacts) vs. minimal intervention (MI) group receiving only educational pamphlets in the mail. | Social cognitive theory, stages of change model, adult learning theory | |
| Sixta CS (2008) | Group classes | CHW | 10 weeks | CHW-led DSME intervention group vs. control group receiving usual care. Weekly 90-min classes were held in Spanish. CDEs were available to answer any unresolved questions that participants had. | None mentioned | |
| Skelly AH (2009) | In-home education sessions with a nurse | NE/NCM | 9 months | Symptom-focused intervention involving four 1-hour educational sessions focusing on symptoms of hyperglycemia, hypoglycemia, tingling and pain in feet, and prevention of cardiovascular symptoms vs. attentional control intervention consisting of four 1-hour-long educational sessions focused on weight management and dietary sodium and fat control. The results of a telephone follow-up “booster,” involving four 15-min phone calls to randomly chosen symptom-based intervention participants in the 12 weeks following the intervention were examined to see if they had an impact on maintenance of glycemic control and habits formed during the intervention. | Symptom-focused model |
DSME diabetes self-managment education, NP nurse practioner, RN registered nurse, CDE certified diabetes educator, RD registered dietician, NE nurse educator, NCM nurse case manager, CHW community health worker, PA peer advisor
Table 2.
Measured outcomes and results categorized by diabetes self-management intervention strategy of selected articles for patients with type 2 diabetes mellitus living in rural areas
| Strategy | Author (Year) | Study design | Sample | Setting |
|---|---|---|---|---|
| Telehealth | Ciemins E (2011) | Pre-post test (N=206) | Age ≥21 years, with at least one uncontrolled vascular risk factor (HbA1c, LDL-C, or BP) | Primary care clinics (5) in Montana |
| Davis RM (2010) | RCT (N=165) | Age 35+ years with HbA1C >7 % from three community centers | Health center in South Carolina | |
| Hawkins SY (2010) | RCT (N=66) | Rural-dwelling adults with T2DM, age 60+ years without cognitive impairment | Rural USA | |
| Izquierdo R (2010) | RCT (N=890) | Rural-dwelling Medicare beneficiaries age ≥55 with diabetes living in a medically underserved area | Upstate New York | |
| McIlhenny CV (2011) | Pre-post test (N=98) | Adults from two rural medical clinics; one received intervention the other control | Pennsylvania | |
| Shea S (2009) | RCT (N=1665) | Medicare beneficiaries, age ≥55 years living in underserved areas (890 rural dwelling) | New York | |
| In-person DSME | Anderson-Loftin W (2005) | Pre-post test (N=97) | High-risk African American adult patients from three primary care practices | South Carolina |
| Bray P (2005) | Controlled trial (N=160) | African American adults with T2MD, HbA1c >7 %, BP >135/85 mmHg, or high risk of end-organ damage | Rural North Carolina | |
| Brown SA (2005) | Group RCT (N=216) | Mexican Americans aged 35–70 years | Starr County, US-Mexico border | |
| Brown SA (2011) | Pre-post-test (N=83) | Low-income Mexican Americans aged 35–70 years | Starr County, US-Mexico border | |
| Kattelmann KK (2010) | RCT (N=114) | Northern plains Indians, aged 18–65 years | Cheyenne River Sioux Reservation, South Dakota | |
| Mayer-Davis EJ (2004) | Three-arm RCT (N=187) | Age 45+ years with BMI ≥25 kg/m2 | Two primary care clinics in South Carolina | |
| Samuel-Hodge CD (2009) | RCT (N=201) | African American with phone and living within 50 miles participating church | Churches Chapel Hill, NC | |
| Sixta CS (2008) | RCT (N=131) | Mexican Americans aged ≥18 years with T2DM | Community health center in Webb County, Texas | |
| Skelly AH (2009) | RCT (N=180) | African American women age >50 years with phone, HbA1c >7 %, and T2DM for more than 1 year | Rural southeast |
| Strategy | Length of follow-up (Retention) | Outcomes
|
Results | |
|---|---|---|---|---|
| Behavior and knowledge | Biologic | |||
| Telehealth | 2 years (66 %), 3 years (38 %) | DSM behaviors; knowledge; self-efficacy | HbA1c, LDL-C, BP | Improvement in HbA1c, LDL-C, and BP as well as knowledge, self-care and self-efficacy in both groups at 3 years. No between group differences for any outcome but BP, which favored intervention. |
| 6 months (90 %), 12 months (82 %) | None mentioned | HbA1c, LDL-C | Improvement in HbA1c for telehealth group vs. control at 6 months (P<.003) and 12 months (P<.004). LDL-C reduced at 12 months for telehealth vs. control (P=0.02) | |
| 6 months (85 %) | Self-efficacy; diabetes knowledge | HbA1c, lipids, BP, BMI | Improvement in HbA1c in both groups (P<.05), no significant changes from baseline for BP or lipids; self-efficacy (P=.002) and knowledge (P=.023) improved in intervention group. For intervention, individuals with higher self-efficacy had greater improvements in HbA1c than those with lower self-efficacy. | |
| 12 months (84 %) 24 months (33 %) | Diet; exercise; diet and exercise knowledge | BMI, waist circumference | Diet and exercise knowledge and behaviors improved (P=.002) from baseline for intervention compared to control. WC decreased in women in the intervention group compared to women in usual care (P=.02). | |
| 6 months (41 %) | DSM behaviors; diabetes knowledge; quality of life | Weight, HbA1c, FBG | Improvements in knowledge and glucose self-monitoring for intervention vs. control (P=.001). No difference between groups for HbA1c or quality of life. | |
| 12 months (85 %) | None mentioned | HbA1c, lipids, BP | Reductions favoring intervention included HbA1c 0.18 % (P=.006), SBP 3.4 (P=.001) and DBP 1.9 (P<.001) and LDL-C 9.5 mg/dl (P<.001). Effect sizes similar for rural vs. urban regions. | |
| In-person DSME | 6 months (67 %) | Dietary behavior | Hb1Ac, lipids, BMI | Improvements in BMI (P=.009) and dietary habits (P=.005) for intervention vs. control. HbA1c and lipids improved in both groups with no between- group differences. |
| 12 months (all patients included for analysis) | Frequency of physician visits | HbA1c, BP, weight | Significant reduction in HbA1c in intervention group compared to control (P<.05); no change in weight or BP from baseline. Increased adherence to recommended office visits. | |
| 12 months (95 %) | Knowledge | HbA1c, FBG | HbA1c improved in both groups; no between-group differences in HbA1c, FBG, BMI, or knowledge. Those in “extended” intervention who attended more sessions had greater reduction in HbA1c and improved FBG. | |
| 3 months, 6 months (NR) | Diet; physical activity; glucose monitoring | HbA1c, FBG, lipids, BMI | Both intervention and control groups had improvements in diet, PA, and FBG with no between-group differences. Trend for individuals with more contacts to have higher education and attendance rates. | |
| 6 months (91 %) | Dietary adherence and satiety; physical activity | HbA1c, FBG, lipids, BMI, weight, BP | Within-group improvement in weight and BMI for the intervention group from baseline to completion, but no between-group differences in any measured outcomes. No change in HbA1c. | |
| 12 months (81 %) | None mentioned | HbA1c, weight loss | Intensive group lost weight (2.2 kg, P<.003), 49 % of intensive group lost ≥2 kg vs. 25 % of the usual-care participants (P<.05); HbA1c improved in both groups but no between-group differences. Weight loss was greater for “high attenders” compared to usual care. | |
| 8 months (87 %), 12 months (85 %) | Quality of life; knowledge | HbA1c | HbA1c reduction (P=.009) in special intervention (SI) group vs. minimal intervention (MI) at 8 months, with attenuation at 12 months. Knowledge improved in SI; quality of life improved in SI and worsened for MI at 8 (P=.004) and 12 months (P=.015). | |
| (74 %) | Knowledge; health beliefs | HbA1c | Improvement in knowledge for intervention vs. control (P=.0035). No significant changes in HbA1c from baseline in either group. | |
| 3, 6, and 9 months (90.6 %) | Self-care; symptom distress; quality of life | HbA1c | Improvements in HbA1c, symptom distress, and quality of life and self-care in all groups, with no differences between study arms. | |
HbA1c glycated hemoglobin, LDL-C low density lipoprotein cholesterol, BP blood pressure, DSM diabetes self-managment, DSME diabetes self-management education, RCT randomized controlled trial, T2DM type 2 diabetes mellitus, BMI body mass index, FBG fasting blood glucose, WC waist circumference, FHQ food habits questionnaire
Intervention Design and Delivery
Studies differed in the design of their interventions including the strategies used, the duration, and the delivery format (Table 1). The application of behavioral theories varied across studies for intervention design, implementation, and evaluation.
Interventions were categorized as telehealth (n=6) [22–24, 21, 25, 20] or in-person DSME (n=9) [26–34]. Telehealth interventions used technology, such as videoconferencing, telephone calls, or the Internet, to deliver an intervention from a remote site. In-person DSME programs included group or individual sessions. The average retention for interventions which required participants to travel to the intervention site was 72 % in contrast to the average retention rate of 80 % for interventions which were delivered in the participant’s home, whether by phone or in-person.
The length of interventions varied from 8 weeks to 2 years; however, all programs followed patients for at least 6 months to assess the impact of the intervention. Interventions that included more patient contact hours tended to show improved outcomes, supporting a “dosage effect” for diabetes education [31, 32].
An interdisciplinary team of healthcare professionals delivered most interventions. Nine interventions included registered dieticians (RD) and nutritionists as part of the healthcare team, and nine interventions included nurses, acting as nurse educators or nurse case managers. Certified diabetes educators (CDE) were part of the healthcare delivery team in three interventions [26, 22, 23]. Two studies included nurse practitioners [22, 24]; one intervention was led by nurse practitioners assisted by a team of CDEs and RDs [22]. All four interventions that involved community health workers (CHWs) or peer advisors were designed specifically for African American or Latino study populations [28, 29, 32, 33]. The roles of these individuals varied greatly. In a study by Samuel-Hodge and colleagues, church diabetes advisors, individuals who either had T2DM or had lived with someone diagnosed with the disease for over 2 years, provided social and practical support in an intervention designed for African Americans living in rural North Carolina [32]. The role of the church diabetes advisor was to assist the RD in facilitating educational sessions and to provide support to the participants through regular phone calls during which the church diabetes advisors followed up on the participant’s progress toward behavioral goals. Two studies recruiting Latinos residing on the Mexican American border in Starr County, Texas, implemented a community health worker model [28, 29]. Community health workers (CHWs) assisted nurse case managers and RDs by establishing weekly phone contact with participants, arranging DSME meeting locations, organizing supplies, providing transportation if needed, recording attendance at DSME meetings, and assisting RDs with food preparation. Another study recruiting Latinos in Webb County, Texas, implemented an intervention where CHWs delivered weekly DSME classes assisted by CDEs [33]. Of the four studies that included CHWs, one demonstrated between group differences in HbA1c and quality of life [32], two reported improvements in fasting blood sugar or HbA1c in both intervention and control groups [28, 29], and one reported no biologic improvements in either group [33].
Behavioral Theory
Five of the 15 studies mentioned a specific behavior theory or conceptual framework to guide their interventions, including health belief model, social cognitive theory, stages of change model, adult learning theory, symptom-focused model, and the chronic care model [27, 23, 24, 32, 34]. Two of these studies, using the chronic care model and a combination of the health belief model with the trans-theoretical model, respectively, demonstrated statistically significant improvements in glycemic control in the intervention arm compared to control [27, 23]. Hawkins and colleagues used a combination of social cognitive theory with the trans-theoretical model and motivational interviewing and observed improved glycemic control for both study arms, with specific improvements in self-efficacy in the intervention group [24]. Samuel-Hodge and colleagues used a combination of social cognitive theory, the trans-theoretical model, and adult learning theory and found a transient improvement in glycemic control for the “intensive” intervention that attenuated over time; however, improvements in quality of life were also observed and were sustained over the follow-up period of 12 months [32]. Skelly and colleagues used two separate theories, social cognitive theory versus a symptom-focused model [34] and observed improvements in both arms (skills-based vs. symptom-based interventions).
Cultural Context
Six studies specifically included culturally relevant strategies [26, 28–30, 32, 33]. For example, the intervention implemented by Anderson-Loftin, et al. was designed around cultural food preferences, ethnic beliefs, and learning methods that worked best for the local African American community [26]. Samuel-Hodge et al. used churches as locations for the intervention’s meetings and as a social support system for the participants [32]. For interventions implemented in Starr County by Brown et al. (2005, 2011), an entirely bilingual healthcare team was engaged, and part of the intervention involved educating family members of the Latino patients to provide social support [28, 29]. Of the six studies that incorporated culturally relevant strategies into their intervention, two demonstrated significant between group differences in biologic outcomes; Anderson-Loftin observed improvements in BMI while Samuel-Hodge observed improvement in HbA1c [26, 32]. Kattelmann observed within group improvements in weight and BMI for the intervention group [30]. Brown and colleagues observed improvements in biologic outcomes (HbA1c and FBG) in both intervention and control with no significant between group differences [28, 29].
Social Support
An element of social support for diabetes self-management, defined as supportive strategies that went beyond the provision of information only, was incorporated into ten interventions; the remaining five interventions were primarily educational. Five [23, 24, 21, 32, 20] of the ten interventions included collaborative goal-setting and individual motivation while five interventions [29, 28, 26, 27, 30] incorporated support group sessions. All five interventions that used a collaborative goal-setting model resulted in improved metabolic control, though Hawkins et al. [24] saw significant improvements for intervention and control conditions. In addition to improving metabolic control, the intervention also contributed significantly to improved patient self-efficacy in the intervention group compared to the control group [24]. Of the five interventions that included support groups, one [27] significantly improved HbA1c levels in intervention compared with control, and another saw improvements in diet and BMI for intervention group only, but improved A1c for both groups [26].
Study Design and Outcomes
Ten of the 15 studies analyzed randomized controlled trials (RCTs) (Table 2) [28, 23, 24, 21, 30–32, 20, 33, 34]. Five RCTs compared the intervention group to a control group receiving usual care [23, 21, 30, 20, 33]. Samuel-Hodge et al. [32] designed an RCT to assess the efficacy of a program with less contact hours compared to a previously tested, time-intensive intervention; the control group received the intensive intervention program rather than usual care. Brown et al. (2005) compared an extended intervention (52 contact hours) to a compressed intervention (22 contact hours) in a group-randomized trial [28]. Mayer-Davis et al. conducted a three-way comparison of and “intensive lifestyle” intervention, a “reimbursable lifestyle” intervention, and usual care [31]. Skelly et al. and Hawkins et al. each employed an attention control arm with specified health-related activities and education [24, 34]. A pre-posttest design with a comparison group was used in five studies [26, 27, 29, 22, 25]. Four of these included control groups living in rural areas receiving usual care [26, 27, 29, 25]. The fifth pre-posttest design study was designed to assess the feasibility and efficacy of a telehealth intervention delivered via videoconferencing; the intervention group was rural and received the telehealth program while participants in an urban, traditional face-to-face DSME course served as the control group [22].
Health Indicators
Biologic indicators of health assessed included HbA1c, blood pressure, weight/BMI, waist circumference, blood glucose, and lipids. The most common outcome, HbA1c, was reported in 14 studies. Of these, only three studies reported statistically significant improvements in HbA1c levels when comparing intervention to control [27, 23, 20]. Samuel-Hodge et al. found an improvement for the “intensive” intervention compared to the “minimal” intervention at 8 months, but the result attenuated at 12 months. Five studies demonstrated reductions in HbA1c among both control and intervention groups, but no differences between groups [26, 28, 22, 24, 34]. Brown, et al. (2005) found a dosage effect correlating reductions in HbA1c to the number of intervention meetings attended by the participant was observed [28]. No significant differences in HbA1c from baseline were observed in five studies [29–31, 25, 33]. Fasting blood glucose (FBG) was a measured outcome of four studies. Brown and colleagues observed decreases in blood glucose from baseline in both the extended and compressed intervention groups [29]. No study reported between group differences in FBG levels.
Lipid profiles were measured in seven studies; low-density lipoprotein cholesterol (LDL-C) was the most commonly measured lipid. Of the seven studies, two observed significant reductions in LDL-C at 12 months between the intervention and usual care [23, 20]. Ciemins et al. observed similar improvements in LDL-C for both groups [22] as did an additional two studies; however, results in the latter two failed to reach statistical significance [26, 24]. The four remaining studies reported no significant changes in lipids [29, 24, 26, 30].
Changes in weight or body mass index (BMI) were measured in eight studies [26, 27, 29, 24, 21, 30, 31, 25]. Anderson-Loftin reported significant reductions in weight/ BMI between the intervention group and usual care groups [26]. Two studies observed significant decrease in weight from baseline for the intervention group but found no statistically significant differences between groups [30, 31]. No significant changes in weight or BMI were observed in the other five studies [27, 29, 24, 21, 25].
Blood pressure was reported in five studies [27, 22, 24, 30, 20]. Of these, only Shea et al. reported significant improvements in BP for intervention compared to control [20]. Ciemins et al. reported improvements in blood pressure for both the telehealth and face-to-face DSME intervention groups [22]. The remaining three studies reported no significant changes in blood pressure [24, 27, 30].
In summary, intervention effects on biologic outcomes were mixed. There were few studies that demonstrated between group differences in HbA1c (3 of 13), lipids (2 of 7), BMI (1 of 8), or BP (1 of 5).
Diabetes Knowledge
Seven studies reported on the effect of DSME interventions on diabetes or health behavior knowledge. Four studies demonstrated improvements in knowledge in the intervention group compared with control [21, 25, 32, 33]. Ciemins et al. reported improved diabetes-related knowledge for both the telehealth intervention group and face-to-face DSME control groups [22]. Hawkins et al. saw an increase in knowledge for the intervention group, but it was not significant. Another study reported no statistically significant differences between groups but did observe a dosage effect correlating the number of intervention sessions attended to greater diabetes knowledge [28].
Health Behaviors
Eight studies reported outcomes for diabetes-related behaviors [26, 27, 29, 22, 21, 30, 25, 34]. Four of these demonstrated improved behavioral measures when comparing intervention to control [26, 27, 21, 25]. Izquierdo et al. reported improvements in both diet and exercise behaviors for the intervention group compared to control [21]. Anderson-Loftin et al. used the food habits questionnaire (FHQ) to assess fat intake and found significant a decrease in FHQ scores for intervention arm participants [26]. Bray et al. found an increase in the frequency of office visits (to recommended levels) for patients with high HbA1c in a randomly selected subset of the intervention group [27]. McIlhenny demonstrated an improvement in glucose-self monitoring but not in other behaviors for intervention compared to control [25]. Three studies demonstrated improvements in some measure of diabetes self-care for both intervention and control groups [29, 22, 34], and one did not observe behavioral improvements in either the intervention group or control group [30].
Discussion
In this systematic review of self-management interventions for adults with type 2 diabetes living in rural areas, we found examples of both telehealth interventions and face-to-face interventions that resulted in improved behavioral, biologic, and diabetes knowledge-related outcomes in adults with T2DM living in rural areas. The most frequently measured outcome to assess metabolic control was HbA1c; though few studies demonstrated significant differences between intervention and control groups. Several other studies tested different “interventions” against one another (rather than against usual care) and reported improvements in both arms [22, 32, 34]. For example, Ciemins et al. observed similar improvements in HbA1c, LDL-C, and BP in a rural telehealth intervention group and in an urban in-person DSME control group [22]. These comparable improvements in HbA1c suggest that telehealth and in-person DSME strategies both have the potential to be effective in a rural population. Distances were associated with lower retention and greater number of contacts was associated with higher attendance and improved glycemic and/or weight loss control [28, 31]. Interventions based on theory, such as stages of change model and social cognitive theory, improved metabolic control, as did interventions that incorporated collaborative goal setting.
In rural communities, where access to care is limited by a number of providers, distance to providers, and lack of transportation and community resources [35], telehealth presents a unique way to improve diabetes self-management using fewer resources. However, even among telehealth interventions, study designs varied. Some required patients to travel to their primary care clinic to access videoconferencing technologies [22, 23]. While these interventions were successful in improving metabolic control in the study population, traveling may not be feasible for some patients. We observed that requiring patients to travel to the intervention was associated with lower retention rates. In contrast, Hawkins et al. used a videophone technology that connects to a landline telephone jack [24]. This home-based intervention does not rely on Internet access that may be limited for some patients in rural areas [10], yet it provides the video and audio connections that provide a similar experience to videoconferencing. This may serve as a promising strategy moving forward, especially for patients who are not able to leave their homes or who lack access to a primary care clinic or the Internet.
We observed that interventions based on behavior theories uniformly produced significant improvements in metabolic control while interventions that did not mention use of behavior theories did not always yield positive results. Of the five studies that were theory based, two resulted in improved glycemic control for intervention compared to usual care. The other three studies saw improvements in both study arms [24, 32, 34]. It is well documented that programs designed to influence health behavior, in this case, diabetes self-management, are more likely to benefit participants when the intervention itself is grounded in theory [36]. The theory in turn helps identify targets for change, informs evaluation, and can provide a roadmap for future refinement and dissemination [36].
The Robert Wood Johnson Foundation reports collaborative goal-setting, motivation, social support, and regular access to primary care as resources needed for self-management [37]. We found that interventions incorporating collaborative goal-setting and motivational support were more likely to be associated with positive outcomes than purely educational interventions. However, interventions that included support groups did not meet with the same uniform level of success. One explanation for the varying success of interventions including support groups could be inconsistent attendance for participants required to travel to a support-group meeting location. Another possible explanation for this may be that these interventions relied primarily on educational sessions to impact participant behavior. A 2013 systematic review of efforts to improve diabetes care for rural patients in Organization for Economic Cooperation and Development (OECD) countries found that though patient-education interventions were frequently implemented, they were less effective at improving diabetes-related outcomes than interventions that targeted the healthcare delivery system or had multiple targets [38]. For interventions where peer social support was provided, it may have fallen short of helping patients identify goals and practice diabetes-related behaviors in real time. Hawkins et al. cite continuous access to supportive resources and emphasis on behavior-change theories as key elements that may be missing in the traditional DSME approach [24]. Thus, while patient knowledge is an important component of diabetes self-management, the ongoing supportive role healthcare providers and peers can play in helping patients develop and maintain behavior-change goals is pivotal.
The limitations of this study include challenges comparing data from different studies, potential publication bias, and inability to ensure that all relevant literature was identified. The interventions reviewed were of varying lengths, focused on a variety of outcomes, and were designed for various cultural groups, making comparison across studies difficult. While we hope that the spectrum of studies included serves to inform the reader, it precluded meta-analysis of this data. One possible reason for lack of significant improvement in HbA1c and BMI/weight in shorter interventions may be inadequate length to follow up for these long-term measures of metabolic control.
Conclusions and Future Directions
Rural communities must contend with high rates of diabetes in the face of limited access to health services and diabetes education, long distances, and scarce community resources. Health systems often have the professional expertise necessary to provide diabetes self-management education and are increasingly employing creative strategies to take that expertise into rural communities. This review identified examples of both in-person DSME and telehealth interventions that have the potential to be effective for patients with T2DM living in rural areas. Interventions delivered in a participant’s home may facilitate patient participation particularly in communities with long distances. Interventions that include motivational support and collaborative goal-setting informed by behavior theories may be more likely to improve metabolic control. Future studies are needed to examine the comparative effectiveness of implementing these strategies in real world settings, with attention to not only health outcomes but also patient-centered outcomes and cost effectiveness.
Acknowledgments
The project described was supported by the University of Alabama at Birmingham’s (UAB) Diabetes Research Center [Award Number P30 DK-079626] and the UAB Obesity Training Program [Award Number T32 DK-062710] from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. Funding was also provided by grants from the American Diabetes Association [191605] (Dr. Cherrington) and the Agency for Healthcare RQ [K12 HS019465] (PI Saag/Project PI Dr. Cherrington).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Morgan Griesemer Lepard, Alessandra L. Joseph, April A. Agne, and Andrea L. Cherrington declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Prevention. CfDCa, editor. National Diabetes Statistics Report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 2.Hoyert DLXJ. Deaths: preliminary data for 2011. Hyattsville, MD: National Center for Health Statistics; 2012. National vital statistics reports. [PubMed] [Google Scholar]
- 3.Seaquist ER. Addressing the burden of diabetes. JAMA. 2014;311(22):2267–8. doi: 10.1001/jama.2014.6451. [DOI] [PubMed] [Google Scholar]
- 4.Zhuo X, Zhang P, Hoerger TJ. Lifetime direct medical costs of treating type 2 diabetes and diabetic complications. Am J Prev Med. 2013;45(3):253–61. doi: 10.1016/j.amepre.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Jessee BT, Rutledge CM. Effectiveness of nurse practitioner coordinated team group visits for type 2 diabetes in medically under-served Appalachia. J Am Acad Nurse Pract. 2012;24(12):735–43. doi: 10.1111/j.1745-7599.2012.00764.x. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention, editor. Report MaMW. Jan 14, 2011. CDC health disparities and inequalities report—United States, 2011. [Google Scholar]
- 7.Maez L, Erickson L, Naumuk L. Diabetic education in rural areas. Rural Remote Health. 2014;14(2):2742. [PubMed] [Google Scholar]
- 8.Siminerio LMPG, Zgibor JC. Implementing the chronic care model for improvements in diabetes care and education in a rural primary care practice. Diabetes Educ. 2005;31(2):225–34. doi: 10.1177/0145721705275325. [DOI] [PubMed] [Google Scholar]
- 9.Andrus MR, Kelley KW, Murphey LM, Herndon KC. A comparison of diabetes care in rural and urban medical clinics in Alabama. J Community Health. 2004;29(1):29–44. doi: 10.1023/b:johe.0000007443.96138.03. [DOI] [PubMed] [Google Scholar]
- 10.Skillman SM, Andrilla CH, Patterson DG, Fenton SH, Ostergard SJ. Health information technology workforce needs of rural primary care practices. J Rural Health Off J Am Rural Health Assoc Natl Rural Health Care Assoc. 2014 doi: 10.1111/jrh.12081. [DOI] [PubMed] [Google Scholar]
- 11.Service ER, editor. Rural poverty & wellbeing: poverty overview. Washington, DC: United States Department of Agriculture; 2014. [Google Scholar]
- 12•.Hale NL, Bennett KJ, Probst JC. Diabetes care and outcomes: disparities across rural America. J Community Health. 2010;35(4):365–74. doi: 10.1007/s10900-010-9259-0. This manuscript describes geographic disparities in diabetes-related complications (rural versus urban) using a nationally representative sample in the United States. [DOI] [PubMed] [Google Scholar]
- 13.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288(15):1909–14. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 14•.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159–71. doi: 10.2337/diacare.25.7.1159. This classic manuscript provides the evidence supporting the beneficial impact of diabetes self-management education on glycemic control and diabetes care. [DOI] [PubMed] [Google Scholar]
- 15.Strine TW, Okoro CA, Chapman DP, Beckles GL, Balluz L, Mokdad AH. The impact of formal diabetes education on the preventive health practices and behaviors of persons with type 2 diabetes. Prev Med. 2005;41(1):79–84. doi: 10.1016/j.ypmed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Balamurugan A, Rivera M, Jack L, Jr, Allen K, Morris S. Barriers to diabetes self-management education programs in underserved rural Arkansas: implications for program evaluation. Prev Chronic Dis. 2006;3(1):A15. [PMC free article] [PubMed] [Google Scholar]
- 17.Cherrington A, Martin MY, Hayes M, Halanych JH, Wright MA, Appel SJ, et al. Intervention mapping as a guide for the development of a diabetes peer support intervention in rural Alabama. Prev Chronic Dis. 2012;9:E36. doi: 10.5888/pcd9.110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massey CN, Appel SJ, Buchanan KL, Cherrington AL. Improving diabetes care in rural communities: an overview of current initiatives and a call for renewed efforts. Clin Diabetes. 2010;28(1):20–7. doi: 10.2337/diaclin.28.1.20. [DOI] [Google Scholar]
- 19.Vassilev IRA, Kennedy A, Koetsenruijter J. The influence of social networks on self-management support: a metasynthesis. BMC Public Health. 2014;14(719):1–12. doi: 10.1186/1471-2458-14-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shea S, Weinstock RS, Starren J, Teresi J, Palmas W, Field L, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc. 2006;13(1):40–51. doi: 10.1197/jamia.M1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izquierdo RLC, Meyer S, et al. Telemedicine intervention effects on waist circumference and body mass index in the IDEATel project. Diabetes Technol Ther. 2010;12(3):213–20. doi: 10.1089/dia.2009.0102. [DOI] [PubMed] [Google Scholar]
- 22.Ciemins E, Coon P, Peck R, Holloway B, Min SJ. Using telehealth to provide diabetes care to patients in rural Montana: findings from the promoting realistic individual self-management program. Telemed J e-health Off J Am Telemed Assoc. 2011;17(8):596–602. doi: 10.1089/tmj.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis RM, Hitch AD, Salaam MM, Herman WH, Zimmer-Galler IE, Mayer-Davis EJ. TeleHealth improves diabetes self-management in an underserved community: diabetes TeleCare. Diabetes Care. 2010;33(8):1712–7. doi: 10.2337/dc09-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkins SY. Improving glycemic control in older adults using a videophone motivational diabetes self-management intervention. Res Theory Nurs Pract. 2010;24(4):217–32. doi: 10.1891/1541-6577.24.4.217. [DOI] [PubMed] [Google Scholar]
- 25.McIlhenny CVGB, Knee DR, Demuth BR, Roberts JB. Using technology to deliver healthcare education to rural patients. Rural Remote Health. 2011;11(4):1798. [PubMed] [Google Scholar]
- 26.Anderson-Loftin W, Barnett S, Bunn P, Sullivan P, Hussey J, Tavakoli A. Soul food light: culturally competent diabetes education. Diabetes Educ. 2005;31(4):555–63. doi: 10.1177/0145721705278948. [DOI] [PubMed] [Google Scholar]
- 27.Bray PTD, Wynn JD, Cummings DM, Whetstone L. Confronting disparities in diabetes care: the clinical effectiveness of redesigning care management for minority patients in rural primary care practices. J Rural Health. 2005;21(3):317–21. doi: 10.1111/j.1748-0361.2005.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 28.Brown SA, Blozis SA, Kouzekanani K, Garcia AA, Winchell M, Hanis CL. Dosage effects of diabetes self-management education for Mexican Americans: the Starr County Border Health Initiative. Diabetes Care. 2005;28(3):527–32. doi: 10.2337/diacare.28.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown SAGA, Winter M, Silva L, Brown A, Hanis CL. Integrating education group support and case management for diabetic Hispanics. Ethn Dis. 2011;2(1):20–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Kattelmann KK, Conti K, Ren C. The Medicine Wheel nutrition intervention: a diabetes education study with the Cheyenne River Sioux Tribe. J Am Diet Assoc. 2010;110(5 Suppl):S44–51. doi: 10.1016/j.jada.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Mayer-Davis EJ, D’Antonio AM, Smith SM, Kirkner G, Martin SL, Parra-Medina D, et al. Pounds off with empowerment (POWER) a clinical trial of weight management strategies for black and white adults with diabetes who live in medically underserved rural communities. Res Pract. 2004;94(10):1736–42. doi: 10.2105/ajph.94.10.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuel-Hodge CD, Keyserling TC, Park S, Johnston LF, Gizlice Z, Bangdiwala SI. A randomized trial of a church-based diabetes self-management program for African Americans with type 2 diabetes. Diabetes Educ. 2009;35(3):439–54. doi: 10.1177/0145721709333270. [DOI] [PubMed] [Google Scholar]
- 33.Sixta CS, Ostwald S. Texas-Mexico border intervention by promotores for patients with type 2 diabetes. Diabetes Educ. 2008;34(2):299–309. doi: 10.1177/0145721708314490. [DOI] [PubMed] [Google Scholar]
- 34.Skelly AHCJ, Leeman J, Soward A, Burns D. Controlled trial of nursing interventions to improve health outcomes of Older African American women with type 2 diabetes. Nurs Res. 2009;58(6):410–8. doi: 10.1097/NNR.0b013e3181bee597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoodt G, Lengerich EJ. Reducing the burden of chronic disease in rural North Carolina. N C Med J. 1993;54(10):532–5. [PubMed] [Google Scholar]
- 36.Glanz K, Rimer B, Lewis F, editors. Health behavior and health education: theory, research, and practise. 3. San Francisco: Jossey-Bass; 2002. [Google Scholar]
- 37.Fisher EB, Brownson CA, O’Toole ML, Shetty G, Anwuri VV, Fazzone P, et al. The Robert Wood Johnson Foundation Diabetes Initiative: demonstration projects emphasizing self-management. Diabetes Educ. 2007;33(1):83–4. 6–8, 91–2. doi: 10.1177/0145721706297454. passim. [DOI] [PubMed] [Google Scholar]
- 38.Ricci-Cabello I, Ruiz-Perez I, Nevot-Cordero A, Rodriguez-Barranco M, Sordo L, Goncalves DC. Health care interventions to improve the quality of diabetes care in African Americans: a systematic review and meta-analysis. Diabetes Care. 2013;36(3):760–8. doi: 10.2337/dc12-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]