Abstract
Peripheral vascular disease is one of the major vascular complications in individuals suffering from diabetes and in the elderly that is associated with significant burden in terms of morbidity and mortality. Stem cell therapy is being tested as an attractive alternative to traditional surgery to prevent and treat this disorder. The goal of this study was to enhance the protective and reparative potential of marrow-isolated adult multilineage inducible (MIAMI) cells by incorporating them within a bio-inspired construct (BIC) made of 2 layers of gelatin B electrospun nanofibers. We hypothesized that the BIC would enhance MIAMI cell survival and engraftment, ultimately leading to a better functional recovery of the injured limb in our mouse model of critical limb ischemia compared to MIAMI cells used alone.
Our study demonstrated that MIAMI cell-seeded BIC resulted in a wide range of positive outcomes with an almost full recovery of blood flow in the injured limb, thereby limiting the extent of ischemia and necrosis. Functional recovery was also the greatest when MIAMI cells were combined with BICs, compared to MIAMI cells alone or BICs in the absence of cells. Histology was performed 28 days after grafting the animals to explore the mechanisms at the source of these positive outcomes. We observed that our critical limb ischemia model induces an extensive loss of muscular fibers that are replaced by intermuscular adipose tissue (IMAT), together with a highly disorganized vascular structure. The use of MIAMI cells-seeded BIC prevented IMAT infiltration with some clear evidence of muscular fibers regeneration.
INTRODUCTION
Peripheral vascular disease is one of the major vascular complications in individuals suffering from diabetes and in the elderly that is associated with significant morbidity and mortality [1–5]. The gold-standard treatment for severe peripheral vascular disease is surgery or endovascular revascularization [1, 2, 6]. However, a third of patients will not be suitable for surgery and most of them will die due to complications [1]. Understanding the behavior underlying the vascular pathophysiological alterations holds promise for reducing cardiovascular mortality in an aging population. Stem cell therapy associated with three-dimensional biologically-inspired constructs (BICs) represents a promising strategy for vascular protection and regeneration.
Current therapeutic angiogenesis strategies to revascularize the area of ischemia involve the administration (intravenous or intramuscular) of angiogenic growth factors (e.g. VEGF, bFGF, and Angiopoetin 1), the transplantation of cells (e.g. bone marrow mononuclear cells, marrow stromal cells (MSCs) or endothelial progenitor cells) or a combination of cell and cytokine therapy [7–13]. Tissue engineering approaches include the use of biomaterials and scaffolds for the localized delivery and/or protection of the administered molecules and cells [14–16]. The extracellular matrix (ECM), mainly formed by glycoproteins (largely collagens), proteoglycans, and hyaluronic acid, provides structural support to cells and regulates cellular and tissue functions, such as wound healing, cell migration and growth [17, 18]. Various techniques have been used to generate new scaffolds that closely mimic the ECM structure, chemical, and biological characteristics [8, 9, 19–21]. Scaffolds should provide some void volume to allow vascularization, new tissue formation and remodeling, and ultimately host tissue integration upon implantation. Also, scaffolds should provide support for either extraneously applied or endogenous cells to attach, grow and differentiate during both in vitro culture and in vivo implantation [22]. Recently, the fabrication of electrospun scaffolds has been investigated for the production of a 3D mesh with nanoscale fibers that resemble the ECM in scale and structure [8, 9, 23–27]. Electrospun scaffolds are made of a polymeric solution drawn from a needle tip by an electrostatic force generated from a high voltage power supply [8, 9, 23–25]. Fibers can be easily oriented with the use of different collection devices, generating scaffolds with random, aligned, gridded, tubular, or radial fibers [25, 27]. Fiber orientation is thought to control cell morphology, migration, and direction, and also to alter the mechanical signaling, diffusion, and adhesion of growth factors and cytokines [26, 28, 29]. Moreover, for therapeutic angiogenesis purposes, several groups have attempted to design drug-loaded and architecturally-relevant nanofibrous scaffolds in order to promote cellular behavior and mimicry of the native vascular tissue [8, 19, 25, 30].
In that aging and diabetes are the major risk factors for peripheral vascular disease, impaired angiogenesis and endothelial dysfunction likely contribute to the increased prevalence of peripheral vascular disease [31]. Indeed, patients with peripheral vascular disease have both higher mortality and an increased rate of limb amputation [32]. Elderly and diabetic patients have reduced capillary density and reduced angiogenesis as a result of ischemia [3, 4, 33–35]. In addition to reducing the endogenous angiogenic response, aging and diabetes may also limit the response to exogenous interventions such as angiogenic growth factors delivered in clinical trials [36]. Stem cell therapy represents another option to effectively reverse critical limb ischemia. We have isolated a developmentally immature and highly homogenous subpopulation of human MSCs termed marrow-isolated adult multilineage inducible (MIAMI) cells. MIAMI cells are extensively characterized and have a sustained expression of embryonic stem cells markers such as Oct4a, Rex1, Sox2, Nanog, SSEA-4, and TeRT, that distinguishes them from other marrow-derived cells previously described [37–42]. MIAMI cells expanded in a low oxygen tension environment are capable of preventing tissue damage and promoting tissue repair and functional recovery in animal models of cerebral ischemia, Parkinson’s disease, and critical limb ischemia [43–45]. MIAMI cells secrete cytokines involved in angiogenesis, cell survival, progenitor cell recruitment, and neuroprotection [44, 45].
In this study, we have synthetized gelatin-based tunable cellular sheets using a technique developed in our laboratory that can regulate vessel formation, maturation and spatial orientation of a progenitor cell population with a proangiogenic capability [45]. We have previously shown the benefit of MIAMI cell injection in our mouse animal model of critical limb ischemia [45]. In the present study, we hypothesized that using a construct made of 2 sheets of gelatin nanoscaffolds sandwiching MIAMI cells would enhance cell survival and engraftment, ultimately leading to a better functional recovery of the injured limb in our mouse model of peripheral vascular disease compared to MIAMI cells used alone.
MATERIALS AND METHODS
Reagents
Dulbecco’s modified Eagle medium-low glucose (DMEM-LG) and 0.25% trypsin-EDTA were from Invitrogen (Carlsbad, CA, USA). Phosphate-buffered saline from Thermo-Fisher Scientific (Waltham, MA, USA). Fetal bovine serum (FBS) was from HyClone (Logan, UT, USA). Penicillin/streptomycin, amphotericin B, ascorbic acid 2-phosphate, trypan blue, fibronectin, gelatin type B from bovine skin, and 1,1,1,3,3,3 hexafluoro-2-propanolol (HFIP) were from Sigma-Aldrich (St Louis, MO, USA). Von Willebrand factor (vWF) from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and anti-human mitochondria (hMito, Abcam, Cambridge, UK) was used as primary antibodies for immunofluorescence. Alexa Fluor 546 (Invitrogen, Carlsbad, CA, USA) was used as secondary antibody. Oil Red O (Sigma-Aldrich) was employed for staining lipid droplets to visualize adipose cells.
Human MIAMI cells
MIAMI cell were obtained following the University of Miami IRB protocol and expanded as previously described [37]. Briefly, human bone marrow-derived MIAMI cells from a 20-year-old-male donor (passage 2–3), were cultured in T175 (Nunc, Thermo Fisher Scientific) coated with 10ng/mL fibronectin (Sigma-Aldrich) in the presence of DMEM-LG with 3% FBS, 100 U/mL Penicillin, 1 mg/mL streptomycin, 100 μm ascorbic acid 2-phosphate and a mixed solution of essential fatty acids and incubated in a tri-gas incubator in a 100% humidified atmosphere of 3% O2 and 5% CO2 (low oxygen tension).
Human foreskin fibroblast (HFF)
Commercially available post-natal HFF were obtained from ATCC (Manassas, VA). Cells were cultured in T-75 flasks (Nunc) with DMEM-high glucose in the presence of 10% FBS in a 100% humidified atmosphere at 21% O2 and 5% CO2.
BIC fabrication
Gelatin B was dissolved in HFIP at 10% w/v, the solution was loaded into a 5mL syringe and electrospun using a custom-made apparatus [25] at 16 kV and 4 mL/hour feed rate from a 22G blunt needle at a 10 cm distance. A total of 1.0 mL was deposited onto an aluminum covered glass slide and left to dry for 1 hour under vacuum at room temperature. Crosslinking of gelatin B nanofibrous scaffolds was performed with 25% v/v glutaraldehyde vapor. Scaffolds were cross-linked for 4 hours at room temperature and then washed for 1 hour in 100 mM glycine solution to block any unreacted aldehyde groups.
Scanning electron microscopy analysis
Scaffold fiber diameters were characterized by SEM. Samples were sputter coated with gold and imaged under high vacuum using an FEI XL-30 Field Emission Environmental SEM. Twenty fiber diameters were measured from 3 regions of interest on the scaffold using the measure tool in ImageJ (NIH).
BIC seeding
Electrospun gelatin scaffolds were punched into disks (5 mm in diameter), sterilized with UV light for 2 hours and then rinsed 3 times with sterile PBS before use. BICs were placed into an ultra-low attachment 24 well-plate (Corning, NY, USA) and 1×105 MIAMI cells in 40 μL of expansion medium were seeded on a BIC. Subsequently, a second BIC was placed on top of the previous one onto which the MIAMI cells had been seeded, creating a double layer BIC which was then incubated at 37°C at low oxygen tension (3%) for 30 minutes. Incubation was then continued for another 3 hours after addition of 500 μL of expansion medium in the well (Fig. 1). Live/Dead staining (Thermo Fisher) was used on some control disks (which were not implanted) to confirm cell viability prior to implantation (data not shown). The overall thickness of the construct (double layer + cells) was approximately 0.5mm.
Figure 1. Experimental approach.
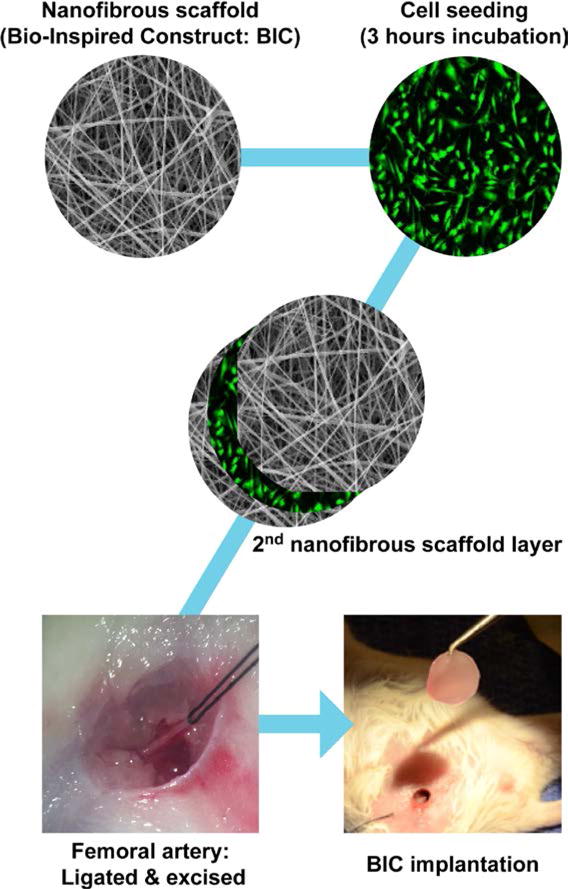
MIAMI cells were seeded within a double-layered electrospun gelatin BIC and incubated for 3 hours at low oxygen tension (3%) before implantation. Critical limb ischemia was induced by ligating the right femoral artery with 6-0 silk braided suture (Ethicon, Somerville, NJ, USA), which was subsequently excised above the saphenous branches along the deep femoral artery and below the external iliac artery. The BIC was then carefully grafted onto the lesion site, taking care not to separate the 2 nanofibrous scaffold layers.
Critical limb ischemia model and BIC implantation
All animals received humane care and were used in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, 2011 [46]. Immunocompetent male Balb/C mice 6–8 weeks old (body weight 20–24g; Taconic Biosciences, NY, USA) were used. Mice were anesthetized with an intraperitoneal injection of a combination of 100 mg/Kg ketamine and 4mg/Kg xylazine. Mice were placed on a 37°C heating pad and bepanthene ophthalmic ointment was applied to the eyes. A 5 mm longitudinal incision was made on the right hindlimb, at the inguinal crease to expose the femoral vessels. To generate a critical limb ischemia, the right femoral artery was ligated with 6-0 silk braided suture (Ethicon, Somerville, NJ, USA) and subsequently excised above the saphenous branches along the deep femoral artery and below the external iliac artery. The left hindlimb was kept intact as a non-ischemic control. The mice were divided into 4 groups (n = 10 mice/group): injection with i) PBS, ii) HFF, iii) BIC implantation, iv) MIAMI cells injection and v) implantation of MIAMI cells + BIC. Treatments were performed immediately after artery excision. A total of 1 × 105 MIAMI cells or HFF were resuspended in 100 μL PBS and injected into 4 different sites along the ischemic region of the adductor muscle for the groups ii) and iv). For group v), 1 × 105 MIAMI cells sandwiched in BIC (as described above) were placed in between the two ligations sites where the femoral artery had been excised (Fig. 1). Animals did not receive any immunosuppression. Analgesia was provided post-surgery using 0.1 mg/Kg buprenorphine injected subcutaneously.
Blood flow analysis
Hindlimb blood perfusion was measured with a Laser Speckle Contrast Imaging (LSCI; Perimed, NY, USA) apparatus. Mice were anesthetized with Ketamine and Xylazine as described above and blood flow was monitored before, immediately after surgery, and at day 7, 14 and 21 days’ post-surgery. Depilatory cream was used to remove hind limb hair, and the animals were kept on a heating pad at 37°C to minimize temperature variation. The imager was positioned at a distance of 30 cm from the targets and the animals were exposed for 1 minute. Two ROI were selected covering the inferior hind limbs (anatomically defined region of the lateral gastrocnemius and plantar foot). Perfusion was expressed as the ratio of blood flow measured in the ischemic limb versus that in the non-ischemic contralateral limb.
Functional assessment
Functional assessment was evaluated pre-operatively, immediately post-operatively after recovering from anesthesia, and at 7, 14, and 21 days after induction of critical limb ischemia. Ischemic damage and gait analysis were performed according to a modified ischemia score (Fig. 2), which allows detection of less severe levels of ischemia, and to the Tarlov Score [47], respectively. A blinded assessment was performed on all animals.
Figure 2. Modified ischemia score.
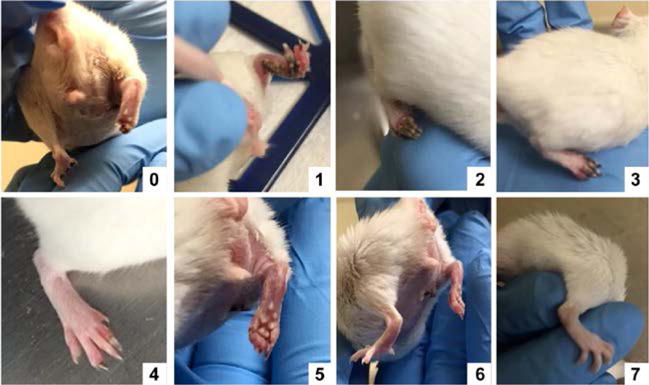
A modified ischemia score was used to detect less severe levels of ischemia. Grading was as follow: leg autoamputation (0), leg necrosis (1), foot necrosis (2), two or more toes discoloration (3), one toe discoloration (4), two or more nails discoloration (5), one nail discoloration (6), no sign of necrosis/ischemia (7)
Histological analysis
Muscles were processed for histology as previously described [45]. After fixation with 4% paraformaldehyde (PFA), samples were embedded in paraffin and cut at 5μm thickness. Longitudinal and transverse sections of the hind limbs were stained with hematoxylin and eosin to assess the regeneration of skeletal muscle.
Intermuscular adipose tissue (IMAT) infiltration analysis
Cryo-sections (8 μm thick) were used for Oil Red O staining. Staining was performed as previously described [37]. We evaluated the infiltration of adipose tissue by quantifying the area occupied by lipidic vesicles on transverse sections of adductor muscle stained with Oil Red O using the NIH ImageJ software (i.e., percent surface coverage). Eight fields of view per group were randomly selected, and the results were expressed as a mean ± standard deviation.
Immunofluorescence analysis
Cryo-sections of the adductor muscles were prepared from each specimen and immuno-stained with either von Willebrand factor (vWF), an endothelial marker or anti human mitochondria (hMito) antibody to specifically label cells of human origin (MIAMI cells) within the host tissue. Cryo-sections (8 μm) were used for immunofluorescence. An antifade mounting medium containing 4′6-diamidino-2-phennylindole (DAPI) was also used for the hMito staining.
Statistical analysis
Data analyses were performed using the Prism Graphpad software. Quantitative data are expressed as mean ± standard deviation (SD). Statistical analysis was done using a one-way analysis of variance (ANOVA) followed by a Tukey’s multiple comparison test. A value of p < 0.05 was considered statistically significant.
RESULTS
BIC morphology and biocompatibility
Using the electrospinning parameters described in the methods section (summarized in Fig. 3A), we obtained BICs with a narrow nanofiber size distribution and an average diameter of 350 nm (Fig. 3B,C). MIAMI cells seeded onto scaffolds remained viable and proliferated for an extended period of time. Confocal fluorescence microscopy showed that more than 90% of MIAMI cells primarily attached onto the scaffold, and then became elongated with a typical MIAMI cell morphology, as observed after 21 days (Fig. 3D).
Figure 3. BIC characterization and biocompatibility.
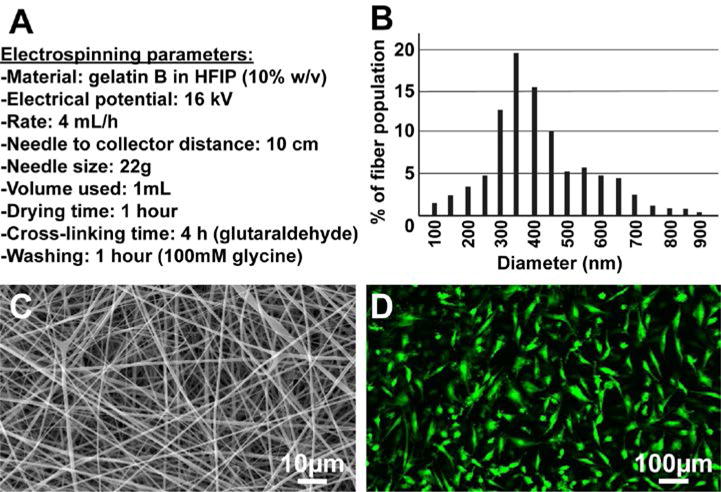
(A) summarizes the electrospinning parameters used to produce the gelatin electrospun nanofibers depicted in (C), with an average fiber diameter of 350 nm (B). MIAMI cells seeded onto scaffolds remained viable and proliferated for at least 21 days, adopting an elongated morphology typical of MIAMI cells (D, confocal microscopy).
MIAMI cells-seeded BICs improve blood perfusion
Laser Speckle Contrast Imaging (LSCI) was used to analyze blood flow in the injured and control limb. Results are expressed as the ratio between the blood perfusion in the injured limb and the uninjured (control) limb. After the femoral artery excision, the perfusion ratio decreased to 0.33±0.02 (67% flow reduction), which confirms the successful induction of critical limb ischemia. An almost complete perfusion recovery was observed within the first two weeks in mice treated with MIAMI cells-seeded BICs (Fig. 4). Mice treated with injected MIAMI cells alone showed some recovery, but to a lesser extent than what was observed with MIAMI cells-seeded BICs (Fig. 4). In contrast, mice treated with PBS, HFF, or scaffold only showed very limited recovery (Fig. 4). Overall, the perfusion ratio was significantly higher in mice treated with MIAMI cells-seeded BICs compared to MIAMI cells, PBS or BICs alone (Fig. 4).
Figure 4. MIAMI cells seeded on BICs exhibited the highest level of reperfusion.
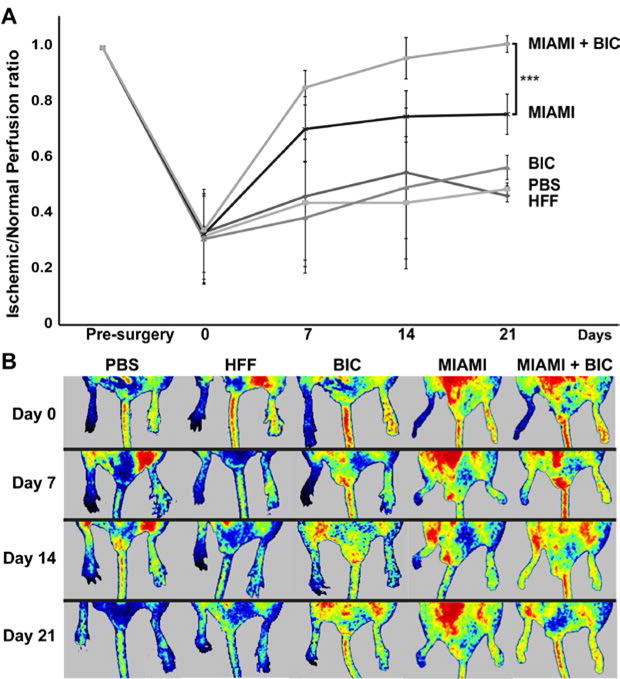
After the femoral artery excision, the perfusion ratio decreased to 0.33±0.02. An almost complete perfusion recovery was observed within the first two weeks in mice with MIAMI cells-seeded BICs. Mice treated with injected MIAMI cells alone showed some recovery, but to a lesser extent than what was observed with MIAMI cells-seeded BICs.Mice treated with PBS, HFF, or scaffold only showed very limited recovery (A). Representative LSCI images are depicted in (B).
MIAMI cells seeded on BICs improve ischemic score
We analyzed the degree of ischemia in the mice by using a modified ischemic score as described in the method section (Fig. 2). The degree of ischemia was well-correlated to the perfusion level of the limb described in the previous section. Briefly, after the femoral artery excision, the ischemia score decreased rapidly for the PBS, HFF and BIC alone groups (Fig. 5). MIAMI cells alone performed slightly better, while the MIAMI cells-seeded BIC group performed extremely well, with a score near 7, indicating very limited ischemia in the limbs of this animal group, which were also fully functional with a complete gripping ability (Fig. 5B).
Figure 5. MIAMI cells seeded on BICs improve ischemic score.
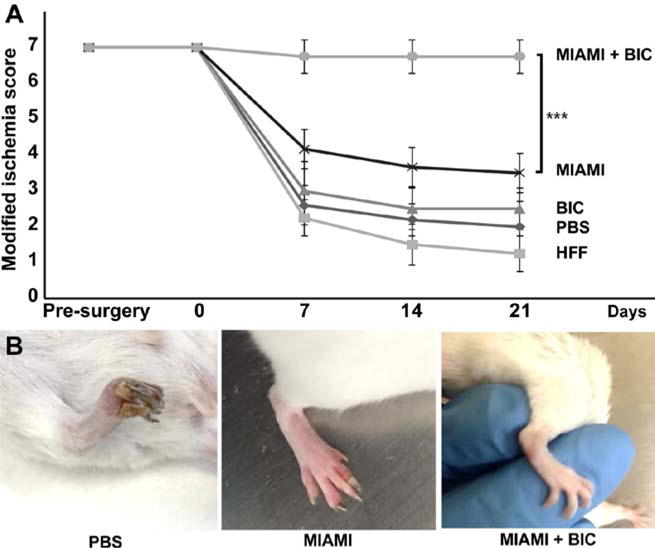
After the femoral artery excision, the ischemia score decreased rapidly for the PBS, HFF and BIC alone groups (A). MIAMI cells alone performed slightly better, while the MIAMI cells-seeded BIC group performed extremely well with a score near 7. These animals were also fully functional with a complete gripping ability (B).
MIAMI cells seeded on BICs promote functional recovery
Mice treated with MIAMI cells-seeded BICs had the best functional outcomes compared to all other treatments. At day 21, mice with MIAMI cells-seeded BICs showed a complete functional recovery, with a Tarlov score of 5.87 ± 0.35. In contrast, mice injected with MIAMI cells alone showed significantly less functional recovery, with a Tarlov score of 3.5 ± 0.54 (Fig. 6A). Interestingly, most of the effects were observed early, with a significantly different (p<0.01) Tarlov score of 4.87 ± 0.64 (MIAMI cells + BIC) vs. 2.66 ± 0.89 (MIAMI cells) at 7 days (Fig. 6A). In addition, for mice treated with PBS or HFF, the Tarlov score was 1.8±0.44 and 1.33±0.57, respectively. In these groups, the Tarlov score remained low during the entire course of the experiment (Fig. 6A). The gait of the animals was also analyzed using an ink pad, and clearly shows a fully functional leg in the MIAMI cell-seeded BICs condition compared to the MIAMI cells alone (missing steps) and PBS (dragging limb) conditions (Fig. 6B).
Figure 6. MIAMI cells-seeded BICs promote functional recovery.
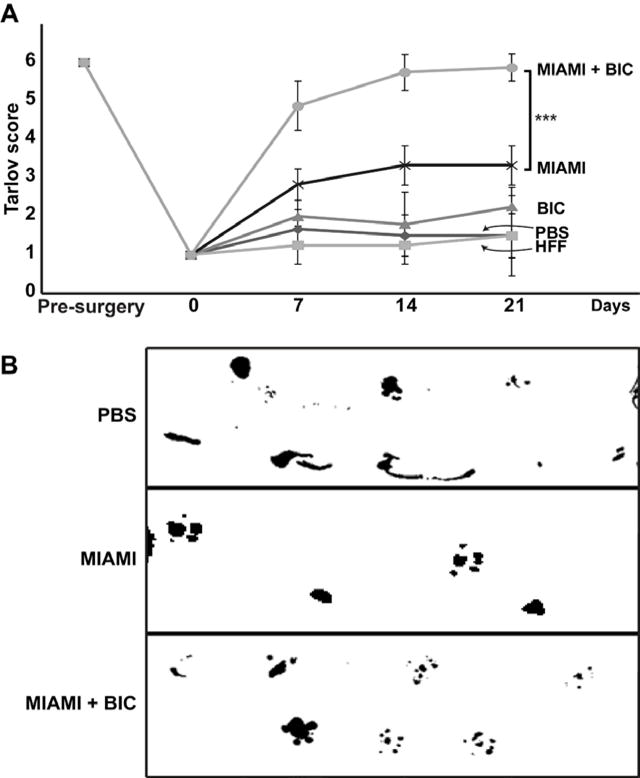
Mice treated with MIAMI cells-seeded BICs had the best functional outcomes compared to all other treatments. At day 21, mice with MIAMI cells-seeded BICs showed a complete functional recovery, with a Tarlov score of 5.87 ± 0.35. In contrast, mice injected with MIAMI cells alone showed significantly less functional recovery (Tarlov score 3.5 ± 0.54). Mice treated with PBS or HFF showed a very low Tarlov scores (1.8±0.44 and 1.33±0.57, respectively) (A). The gait of the animals was analyzed using an ink pad, and clearly shows a fully functional leg in the MIAMI cell-seeded BICs condition compared to the MIAMI cells alone (missing steps) and PBS (dragging limb) conditions (B).
MIAMI cells-seeded BICs protect and regenerate injured skeletal muscle
After 28 days, centro-nucleated myofibers, characteristic of regenerating fibers [48, 49] were strongly present in the adductor muscle of animals treated with MIAMI cells-seeded BICs, in conjunction with less loss of muscle tissue (Fig. 7). In this same group, the conformation of the fascicles (bundle of muscle fibers) was similar to normal skeletal muscle. A massive IMAT infiltration (20.3±3.1%), directly related to muscle dysfunction and perturbed regeneration [48, 49], was observed in animals treated with PBS, while this was more moderate (10.4±2.9%) in animals treated with MIAMI cells alone. Only scarce (up to 0.1±0.1%) IMAT infiltration was observed in animals treated with MIAMI cells-seeded BICs. Normal mice do not have any significantly detectable IMAT. Animals treated with BICs alone or HFF were histologically identical to the PBS-treated animals (data not shown). BICs were fully resorbed and undetectable at 28 days after implantation.
Figure 7. MIAMI cells-seeded BICs protect and regenerate the skeletal muscle after ischemic injury.
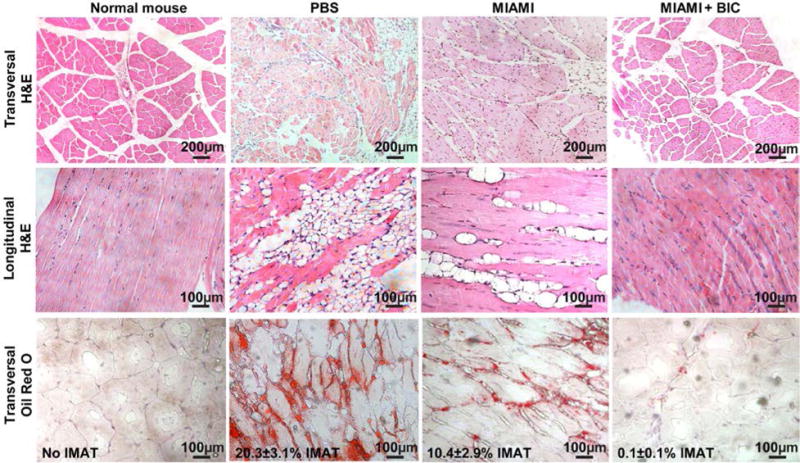
After 28 days, centro-nucleated myofibers (clearly observed on transversal and longitudinal H&E sections), characteristic of regenerating fibers, were strongly present in the adductor muscle of animals treated with MIAMI cells-seeded BICs, in conjunction with less loss of muscle tissue. Mice injected with PBS, HFF or BIC showed a very low number of regenerative myofibers. Oil Red O staining was performed on transversal section to confirm that the droplets observed on the H&E staining were indeed intramuscular adipose tissue (IMAT) infiltration. The percentage of IMAT is reported on the figure (third row).
MIAMI cells-seeded BICs preserve capillary density in the ischemic hind limb, with MIAMI cells detected in the adductor muscle after 28 days
Histological analysis of adductor muscles was performed on mice 28 days after surgery to visualize capillaries using vWF immunostaining (Fig. 8A–D) and to detect human MIAMI cells using human specific anti-mitochondria staining (Fig. 8F,G). The muscle architecture and capillary-to-fiber ratio were not significantly different between the normal tissue and the ones treated with MIAMI cells-seeded BICs (Fig. 8A,D,E). vWF staining was also detected in the control groups, but in a highly disorganized fashion (Fig. 8B), rendering the calculation of the capillary to muscle fiber ratio impossible. Injection of MIAMI cells alone (without BIC) did not preserve the muscle architecture as well as the MIAMI cells-seeded BICs, and the capillary to fiber ratio was significantly lower when the BICs were not used (Fig. 8E). Importantly, human MIAMI cells were detected in the adductor muscle of immunocompetent mice 28 days after injury, as observed using hMito staining (Fig. 8G) compared to normal mice that did not receive MIAMI cells (Fig. 8F).
Figure 8. MIAMI cells-seeded BICs preserve capillary density in the ischemic hind limb, with MIAMI cells detected in the adductor muscle after 28 days.
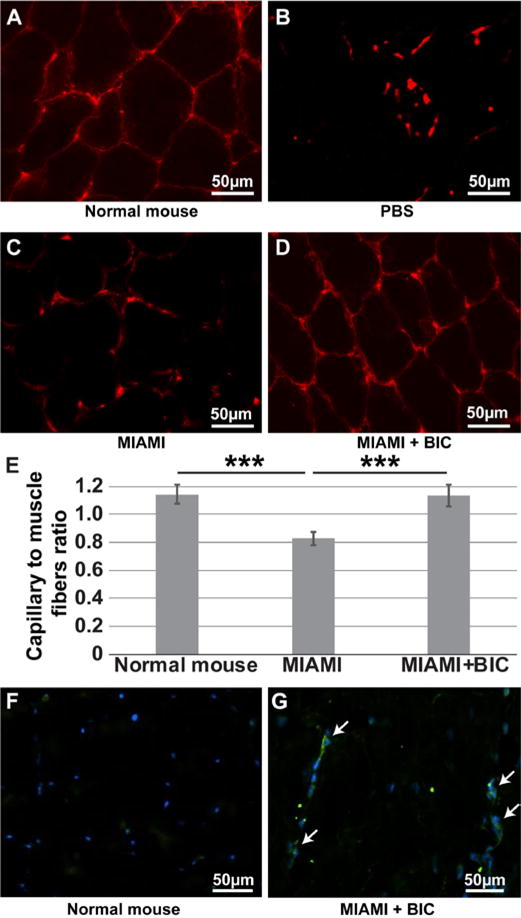
Immunostaining for vWF was used to reveal the presence of capillaries (A-D). The muscle architecture and capillary-to-fiber ratio was similar between the normal tissue and the ones treated with MIAMI cells-seeded BIC (A,D,E), while it was lower in the MIAMI group (C,E). vWF staining was also detected in the control groups (PBS), but in a highly disorganized fashion, so that calculation of the capillary to muscle fiber ratio was not possible (B). Human MIAMI cells were detected in the adductor muscle of immunocompetent mice 28 days after injury using hMito staining (G) compared to normal mice that did not receive MIAMI cells (F). White arrows point at MIAMI cells
DISCUSSION
Angiogenesis is needed during the normal development and maturation in early life of skeletal muscle, as well as during muscle regeneration. Upon an ischemic event, angiogenesis is a vital aspect in the development of collateral circulation to compensate for inadequate tissue perfusion. In this study we showed that mice from all groups had an ability for auto recovery, but which was insufficient to compensate for the critical ischemia created with the excision of the femoral artery. Indeed, mice treated with PBS or HFF had the poorest ischemic and perfusion scores, as well as least functional recovery compared to mice treated MIAMI cells-seeded BICs.
In a previous study we reported that MIAMI cells produce high levels of pro-angiogenic factors, and that an almost complete functional recovery can be achieved upon intramuscular injection of a high number of MIAMI cells (1×106) in a mouse model of critical limb ischemia [45]. The present study demonstrated that a 10-times smaller number (1×105) of MIAMI cells seeded onto BICs resulted in a number of positive outcomes with an almost complete recovery of blood flow in the injured limb, thereby limiting the extent of ischemia and necrosis. Overall, MIAMI cell-seeded BICs greatly improved blood flow, reduced ischemic damage to the skeletal muscle and improved function of the ischemic limb.
Attachment of cells to BICs is thought to have multiple benefits. After the induction of critical limb ischemia, the environment within the muscle is extremely hypoxic; hence, implanted cells need to be able to survive and secrete growth factors in low oxygen conditions. When cells are attached, they release factors that can act as potent stimulators of blood vessel formation, reduce inflammation, and prevent tissue damage. In addition, these secreted chemokines and growth factors can mobilize and attract circulating or local stem cells, known as satellite cells, which play a dominant role in muscle regeneration [50–57]. We hypothesized that BICs enhanced MIAMI cells survival, which could be one of the explanations for the positive results we obtained in this study while using a tenth of the cells we previously used to achieve similar results [45]. In that regard, it is important to note that human MIAMI cells were detected in the adductor muscle of the grafted immunocompetent mice as long as 28 days after implantation, which confirms the immune-privileged status of MIAMI cells as we reported previously [37, 58]. The surface of BICs also fully covers the injured area in a much more effective way than what can be obtained upon simply injecting MIAMI cells alone. Noteworthy, we achieved better results in this study when seeding MIAMI cells in between 2 layers of BICs, as depicted in Fig. 1, compared to when MIAMI cells were seeded and allowed to attach to a single layer of BIC (data not shown). The reason for this may be that the double-layered BICs provide better protection from the hostile environment to the MIAMI cells than the single-layer BICs do.
We observed that our critical limb ischemia model induces an extensive loss of muscular fibers that are replaced by intermuscular adipose tissue (IMAT). The use of MIAMI cells-seeded BICs prevented IMAT infiltration, with some clear evidence of muscular fiber regeneration. Mature skeletal muscle cells are one of the cell types generally lacking regenerative capacity, because they are not capable of mitotic division. Hence, when they are injured, their regeneration is from local undifferentiated cells that are mitotically quiescent until required for repair by being activated in response to physiological stimuli or pathological conditions such as ischemia to generate a new population of myoblasts [48, 49]. After ischemic injury, skeletal muscle passes through different coordinated stages, including degeneration, inflammation and regeneration processes [59]. These steps include recruitment of satellite cells, localized between the sarcolemma and the basal lamina of myofibers [48]. Recently, studies have reported the importance of muscle-resident mesenchymal stem cells in the regeneration process of skeletal muscle [60, 61]. In the skeletal muscle, the nuclei are positioned at the periphery of each myofiber. Centrally-positioned nuclei in transverse sections of the myofibers are considered to be a marker of ongoing myofiber repair (regenerative fibers). An important feature that can be observed in muscle longitudinal sections is nuclei located in the center of the fiber. We have shown that, after 28 days of treatment, mice treated with MIAMI cells-seeded BICs had a very high number of regenerative myofibers, in comparison with mice treated with PBS, HFF or MIAMI cells alone.
Necrosis, inflammation, and angiogenesis are required for muscle regeneration. However, after 28 days, we were not able to visualize any necrotic fibers or inflammation because these processes are mostly present during the first three weeks after injury [62]. Instead, we observed intermuscular adipose tissue infiltration (IMAT), which is correlated with the loss of muscle mass (due to early necrosis) and less functionality [63–66]. Our model led to loss of muscle mass, reduced physical capability, and disrupted functionality; with an accumulation of IMAT in the muscle affected. In this study, we showed the loss of functionality with consequent muscle atrophy in all the animals treated without cells and in animals injected with 1×105 cells, but without BICs. This correlates with the histological findings where we observed a higher accumulation of IMAT in all the control animals. In contrast, very little IMAT was observed in animals treated with MIAMI cells-seeded BICs. Importantly, most of the positive effects we observed (functionality, gait analysis, ischemic score, and blood perfusion) occurred during the first week after injury and implantation. Hence, it will be interesting to investigate earlier time points in the future to elucidate the protection and repair mechanisms in play after implantation of MIAMI cells-seeded BICs.
In summary, our tissue engineering strategy resulted in an extensive protection/repair of the critically injured limb, which would pave the way for future investigations using MIAMI cell-tissue engineering based strategies in humans, either in an autologous or allogeneic approach. Future investigations will make use of aligned nanofiber BICs to achieve a better directionality of the newly formed vessels as it is known that undirected vessel growth and disorganized vascular patterning can lead to immature vessel formation and poor network functionality [67–69].
Acknowledgments
We thank David Vazquez and N. Blanca Rodriguez for their assistance throughout this study as well as the entire GRECC staff. This study was supported by an NIH R21 grant (NIH-NHLBI R21 AG044624) to G.D.I. and F.A. and by a Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development) Merit Review award (BX000952) to P.C.S.
Footnotes
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
DISCLAIMER
The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Aranguren XL, Verfaillie CM, Luttun A. Emerging hurdles in stem cell therapy for peripheral vascular disease. J Mol Med. 2009;87(1):3–16. doi: 10.1007/s00109-008-0394-3. [DOI] [PubMed] [Google Scholar]
- 2.Aranguren XL, et al. Multipotent adult progenitor cells sustain function of ischemic limbs in mice. J Critical limb ischemian Invest. 2008;118(2):505–14. doi: 10.1172/JCI31153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holewski JJ, et al. Prevalence of foot pathology and lower extremity complications in a diabetic outpatient critical limb ischemianic. J Rehabil Res Dev. 1989;26(3):35–44. [PubMed] [Google Scholar]
- 4.Veves A, et al. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes. 1998;47(3):457–63. doi: 10.2337/diabetes.47.3.457. [DOI] [PubMed] [Google Scholar]
- 5.Association, A.D. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26(12):3333–41. doi: 10.2337/diacare.26.12.3333. [DOI] [PubMed] [Google Scholar]
- 6.De Sanctis JT. Percutaneous interventions for lower extremity peripheral vascular disease. Am Fam Physician. 2001;64(12):1965–72. [PubMed] [Google Scholar]
- 7.Cao L, Mooney DJ. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv Drug Deliv Rev. 2007;59(13):1340–50. doi: 10.1016/j.addr.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Layman H, et al. Synergistic angiogenic effect of codelivering fibroblast growth factor 2 and granulocyte-colony stimulating factor from fibrin scaffolds and bone marrow transplantation in critical limb ischemia. Tissue Eng Part A. 2011;17(1–2):243–54. doi: 10.1089/ten.TEA.2010.0270. [DOI] [PubMed] [Google Scholar]
- 9.Layman H, et al. Co-delivery of FGF-2 and G-CSF from gelatin-based hydrogels as angiogenic therapy in a murine critical limb ischemic model. Acta Biomater. 2009;5(1):230–9. doi: 10.1016/j.actbio.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Layman H, et al. Enhanced angiogenic efficacy through controlled and sustained delivery of FGF-2 and G-CSF from fibrin hydrogels containing ionic-albumin microspheres. J Biomater Sci Polym Ed. 2012;23(1–4):185–206. doi: 10.1163/092050610X546417. [DOI] [PubMed] [Google Scholar]
- 11.Hobo K, et al. Therapeutic angiogenesis using tissue engineered human smooth muscle cell sheets. Arterioscler Thromb Vasc Biol. 2008;28(4):637–43. doi: 10.1161/ATVBAHA.107.151829. [DOI] [PubMed] [Google Scholar]
- 12.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45(4):530–44. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phelps EA, Garcia AJ. Engineering more than a cell: vascularization strategies in tissue engineering. Curr Opin Biotechnol. 2010;21(5):704–9. doi: 10.1016/j.copbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmeliet P, Jain RK. Molecular mechanisms and critical limb ischemianical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovett M, et al. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15(3):353–70. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyahara Y, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12(4):459–65. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 17.Chien KR, Domian IJ, Parker KK. Cardiogenesis and the Complex Biology of Regenerative Cardiovascular Medicine. Science. 2008;322(5907):1494–1497. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- 18.Eble JA, Niland S. The Extracellular Matrix of Blood Vessels. Current Pharmaceutical Design. 2009;15(12):1385–1400. doi: 10.2174/138161209787846757. [DOI] [PubMed] [Google Scholar]
- 19.Bu X, et al. Properties of extracellular matrix-like scaffolds for the growth and differentiation of endothelial progenitor cells. J Surg Res. 2010;164(1):50–7. doi: 10.1016/j.jss.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Phelps EA, Garcia AJ. Update on therapeutic vascularization strategies. Regen Med. 2009;4(1):65–80. doi: 10.2217/17460751.4.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spadaccio C, et al. Drug releasing systems in cardiovascular tissue engineering. J Cell Mol Med. 2009;13(3):422–39. doi: 10.1111/j.1582-4934.2008.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17(Suppl 4):467–79. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montero RB, et al. Electrospun Gelatin Constructs with Tunable Fiber Orientation Promote Directed Angiogenesis. Open Journal of Regenerative Medicine. 2014;3:1–12. [Google Scholar]
- 24.Layman H, et al. Enhanced Angiogenic Efficacy through Controlled and Sustained Delivery of FGF-2 and G-CSF from Fibrin Hydrogels Containing Ionic-Albumin Microspheres. J Biomater Sci Polym Ed. 2011 doi: 10.1163/092050610X546417. [DOI] [PubMed] [Google Scholar]
- 25.Montero RB, et al. bFGF-containing electrospun gelatin scaffolds with controlled nano-architectural features for directed angiogenesis. Acta Biomater. 2012;8(5):1778–91. doi: 10.1016/j.actbio.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nisbet DR, et al. Review paper: a review of the cellular response on electrospun nanofibers for tissue engineering. J Biomater Appl. 2009;24(1):7–29. doi: 10.1177/0885328208099086. [DOI] [PubMed] [Google Scholar]
- 27.Kador KE, et al. Tissue engineering the retinal ganglion cell nerve fiber layer. Biomaterials. 2013;34(17):4242–50. doi: 10.1016/j.biomaterials.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7(3):211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 29.Knight B, et al. Visualizing muscle cell migration in situ. Curr Biol. 2000;10(10):576–85. doi: 10.1016/s0960-9822(00)00486-3. [DOI] [PubMed] [Google Scholar]
- 30.Hu X, et al. Preparation and cell affinity of microtubular orientation-structured PLGA(70/30) blood vessel scaffold. Biomaterials. 2008;29(21):3128–36. doi: 10.1016/j.biomaterials.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Avogaro A, et al. Endothelial dysfunction in type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2006;16(Suppl 1):S39–45. doi: 10.1016/j.numecd.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Ouriel K, Veith FJ. Acute lower limb ischemia: determinants of outcome. Surgery. 1998;124(2):336–41. discussion 341–2. [PubMed] [Google Scholar]
- 33.Parizkova J, et al. Body composition, aerobic capacity, and density of muscle capillaries in young and old men. J Appl Physiol. 1971;31(3):323–5. doi: 10.1152/jappl.1971.31.3.323. [DOI] [PubMed] [Google Scholar]
- 34.Coggan AR, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72(5):1780–6. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 35.Emanueli C, et al. Type-2 diabetic Lepr(db/db) mice show a defective microvascular phenotype under basal conditions and an impaired response to angiogenesis gene therapy in the setting of limb ischemia. Front Biosci. 2007;12:2003–12. doi: 10.2741/2205. [DOI] [PubMed] [Google Scholar]
- 36.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105(8):724–36. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Ippolito G, et al. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117(Pt 14):2971–81. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 38.D’Ippolito G, et al. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39(3):513–22. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 39.D’Ippolito G, et al. Sustained stromal stem cell self-renewal and osteoblastic differentiation during aging. Rejuvenation Res. 2006;9(1):10–9. doi: 10.1089/rej.2006.9.10. [DOI] [PubMed] [Google Scholar]
- 40.D’Ippolito G, et al. Isolation and characterization of marrow-isolated adult multilineage inducible (MIAMI) cells. Exp Hematol. 2006;34(11):1608–10. doi: 10.1016/j.exphem.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Tatard VM, et al. Neurotrophin-directed differentiation of human adult marrow stromal cells to dopaminergic-like neurons. Bone. 2007;40(2):360–73. doi: 10.1016/j.bone.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Rios C, et al. Low Oxygen Modulates Multiple Signaling Pathways, Increasing Self-Renewal, While Decreasing Differentiation, Senescence, and Apoptosis in Stromal MIAMI Cells. Stem Cells Dev. 2016 doi: 10.1089/scd.2015.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delcroix GJ, et al. The therapeutic potential of human multipotent mesenchymal stromal cells combined with pharmacologically active microcarriers transplanted in hemi-parkinsonian rats. Biomaterials. 2010;32(6):1560–73. doi: 10.1016/j.biomaterials.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 44.Garbayo E, et al. Neuroprotective properties of marrow-isolated adult multilineage-inducible cells in rat hippocampus following global cerebral ischemia are enhanced when complexed to biomimetic microcarriers. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahnemai-Azar A, et al. Human marrow-isolated adult multilineage-inducible (MIAMI) cells protect against peripheral vascular ischemia in a mouse model. Cytotherapy. 2011;13(2):179–92. doi: 10.3109/14653249.2010.515579. [DOI] [PubMed] [Google Scholar]
- 46.N.A.P. (US), editor. Animals, C.f.t.U.o.t.G.f.t.C.a.U.o.L. Guide for the Care and Use of Laboratory Animals. 8. Washington, D.C.: The National Academies Press; 2011. p. 248. [Google Scholar]
- 47.Westvik TS, et al. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J Vasc Surg. 2009;49(2):464–73. doi: 10.1016/j.jvs.2008.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–5. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gayraud-Morel B, Chretien F, Tajbakhsh S. Skeletal muscle as a paradigm for regenerative biology and medicine. Regen Med. 2009;4(2):293–319. doi: 10.2217/17460751.4.2.293. [DOI] [PubMed] [Google Scholar]
- 50.Aranguren XL, et al. In vitro and in vivo arterial differentiation of human multipotent adult progenitor cells. Blood. 2007;109(6):2634–42. doi: 10.1182/blood-2006-06-030411. [DOI] [PubMed] [Google Scholar]
- 51.Kinnaird T, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 52.Capoccia BJ, et al. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113(21):5340–51. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalka C, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97(7):3422–7. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moon MH, et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17(5–6):279–90. doi: 10.1159/000094140. [DOI] [PubMed] [Google Scholar]
- 55.Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 56.Subramaniyam V, et al. Bone marrow mobilization with granulocyte macrophage colony-stimulating factor improves endothelial dysfunction and exercise capacity in patients with peripheral arterial disease. Am Heart J. 2009;158(1):53–60 e1. doi: 10.1016/j.ahj.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 57.Tateishi-Yuyama E, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360(9331):427–35. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 58.D’Ippolito G, et al. Isolation and Characterization of Swine MIAMI Cells: A Valuable Animal Model for Adult Stem Cell Therapy. CellR4. 2014;2(5):e1215. [Google Scholar]
- 59.Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84-A(5):822–32. [PubMed] [Google Scholar]
- 60.Motohashi N, et al. Muscle CD31(−) CD45(−) side population cells promote muscle regeneration by stimulating proliferation and migration of myoblasts. Am J Pathol. 2008;173(3):781–91. doi: 10.2353/ajpath.2008.070902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takegahara Y, et al. Myotube formation is affected by adipogenic lineage cells in a cell-to-cell contact-independent manner. Exp Cell Res. 2014;324(1):105–14. doi: 10.1016/j.yexcr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 62.Y B, S S. Redefining the satellite cell as the motor of skeletal muscle regeneration. Journal of Science and Applications: Biomedicine. 2015;3(5) [Google Scholar]
- 63.Pagano AF, et al. Muscle Regeneration with Intermuscular Adipose Tissue (IMAT) Accumulation Is Modulated by Mechanical Constraints. PLoS One. 2015;10(12):e0144230. doi: 10.1371/journal.pone.0144230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kohrt WM, Holloszy JO. Loss of skeletal muscle mass with aging: effect on glucose tolerance. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):68–72. doi: 10.1093/gerona/50a.special_issue.68. [DOI] [PubMed] [Google Scholar]
- 65.Manini TM, et al. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Critical limb ischemian Nutr. 2007;85(2):377–84. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 66.Fatimi A, et al. The rheological properties of silated hydroxypropylmethylcellulose tissue engineering matrices. Biomaterials. 2008;29(5):533–43. doi: 10.1016/j.biomaterials.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 67.Bouta EM, et al. Biomaterial guides for lymphatic endothelial cell alignment and migration. Acta Biomater. 2011;7(3):1104–13. doi: 10.1016/j.actbio.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heath DE, Lannutti JJ, Cooper SL. Electrospun scaffold topography affects endothelial cell proliferation, metabolic activity, and morphology. J Biomed Mater Res A. 2010;94(4):1195–204. doi: 10.1002/jbm.a.32802. [DOI] [PubMed] [Google Scholar]
- 69.Hadjizadeh A, Doillon CJ. Directional migration of endothelial cells towards angiogenesis using polymer fibres in a 3D co-culture system. J Tissue Eng Regen Med. 2010;4(7):524–31. doi: 10.1002/term.269. [DOI] [PubMed] [Google Scholar]


