Abstract
The epidemiology of herpes zoster (HZ) in contemporary autologous hematopoietic cell transplant (HCT) recipients, and the impact of acyclovir/valacyclovir (ACV/VACV) prophylaxis, is not well described. In this observational study from 2002–2010, we retrospectively identified 1,000 varicella zoster virus (VZV) seropositive autologous HCT recipients with up to five years of follow up. The incidence of HZ and use of ACV/VACV prophylaxis were determined through review of medical records and mailed questionnaires. Risk factors for HZ were determined by multivariable Cox regression. Over a period of five years post-autologous HCT, 194 patients developed at least one HZ episode with a cumulative incidence of 21%; 159/194 (82%) were not on prophylaxis at the time of HZ. A second episode of HZ occurred in 31/194 (16%) patients. Patients taking ACV/VACV had reduced risk for HZ (adjusted hazard ratio [aHR], 0.59; 95% CI, 0.37–0.91), whereas those older than the median age (≥55.5 years) had increased risk (aHR 1.42, 95% CI 1.05–1.9). Disseminated VZV was reported in 8% and post-herpetic neuralgia in 13% of patients. We demonstrate a high burden of HZ late after autologous HCT, despite long-term antiviral prophylaxis. Improved prevention strategies are needed to provide sustained protection against HZ after autologous HCT.
Keywords: VZV, herpes zoster, varicella zoster virus, autologous, transplant
INTRODUCTION
Autologous hematopoietic cell transplantation (HCT) is a favored treatment option for many hematologic malignancies. The increasing use of novel chemotherapeutic regimens, T-cell depleting agents (e.g., CD34 selection, Thymoglobulin) and biologics, and maintenance therapies, along with an aging HCT population, poses a heightened risk for infections in this patient population1–5. Studies have reported herpes zoster (HZ) as a common infectious complication after autologous HCT in 16%-30% of patients, with most events occurring in the first year after HCT6–8.
In addition to the typical presentation of HZ with a painful dermatomal rash, HCT recipients often experience complications such as post-herpetic neuralgia, ocular disease, and potentially fatal disseminated disease in one-third or more of affected patients5,9–14. Given the significant advances in the treatment and supportive care for autologous HCT recipients over the past decade, including the increased use of novel drugs for maintenance therapy after HCT (e.g., bortezomib, lenalidomide)1,2, a contemporary understanding of the long-term risk of HZ in this population is important.
Long-term varicella zoster virus (VZV) prophylaxis with acyclovir/valacyclovir (ACV/VACV) has been shown to be safe and effective in allogeneic HCT recipients9,14–17. There is also evidence to support the utility of prolonged antiviral prophylaxis for up to two years after autologous HCT18. The 2009 international guidelines recommend ACV/VACV prophylaxis among VZV seropositive patients for one year post-allogeneic (B1 recommendation) and autologous (CII recommendation) HCT recipients19. The guidelines have a lower evidence rating in the setting of autologous HCT due to a limited number of studies19, and consequently, the duration of prophylaxis ranges widely between HCT centers20. Despite that the number of autologous HCTs exceeds that of allogeneic HCTs21, there is a relative lack of contemporary data regarding the epidemiology of HZ after autologous HCT and the impact of VZV prophylaxis in this population. Recent findings of substantially increased healthcare costs and utilization among cancer and HCT patients with HZ lends additional support to the need for such data22.
The purpose of this study was to determine the incidence of and risk factors for HZ over a 5-year period after autologous HCT in a large retrospective cohort of patients who were prescribed long-term ACV/VACV for VZV prophylaxis.
MATERIALS AND METHODS
Data Collection
Databases and available medical records were retrospectively reviewed for patient demographics and clinical characteristics, including details regarding HZ episodes and use of antiviral prophylaxis. In addition to patient follow up at our center, the Fred Hutchinson Cancer Research Center (Fred Hutch) Long Term Follow Up (LTFU) program prospectively sent post-HCT survey questionnaires to providers and patients followed outside of our center at 6 months post-HCT and then annually. Physician medical records were also requested. The patient survey includes a question asking whether the patient had ‘…chicken pox or a Herpes zoster or Varicella zoster (VZV) infection (shingles)’ and the area of the body involved since the prior questionnaire, as well as a section to list or select active medications. The physician survey includes a checkbox to indicate the diagnosis of ‘HZ/Shingles’ since the last follow up along with sections to document other medical complications. The survey did not inquire about microbiologic testing. Data obtained from medical records was given preference, followed by physician and then patient questionnaires. Report of HZ in any record was counted as an event in this study.
Herpes zoster, the primary endpoint, was classified as 1) localized, defined as the presence of lesions distributed in one or two contiguous dermatomes or as 2) disseminated, with lesions involving more than two dermatomes or any visceral or central nervous system involvement. Ocular involvement was classified as local disease for the purposes of this study. Diagnosis was at the discretion of the treating providers and may not have included microbiologic confirmation, as HZ is often a clinical diagnosis without additional microbiologic testing. Start and stop dates were collected from available records. If no clear date was indicated for the start of a HZ episode, the midpoint from date of last contact without notation of an event and date of first notation of the event was used. If the event was only indicated in physician or patient questionnaires, the midpoint from the dates covered by the questionnaires was used in the absence of additional data. Antiviral prophylaxis and maintenance therapy start and stop dates were abstracted using the same approach.
Patients
The study population included 1,000 consecutive patients who underwent autologous HCT at Fred Hutch between November 2002 and December 2010, including tandem autologous-autologous HCT recipients receiving transplants within 6 months (Figure S1). Patients were excluded if they received a planned tandem autologous-allogeneic HCT, were VZV seronegative, received a VZV vaccine without prior HZ or died within 30 days of HCT. Patients undergoing HCT for autoimmune diseases received regimens including CD34 selection and/or rabbit anti-thymocyte globulin in addition to conditioning with total body irradiation-based or high-dose chemotherapy.
Antiviral prophylaxis with ACV 800 mg by mouth twice daily or VACV 500 mg by mouth twice daily for one year after autologous HCT was routinely prescribed in this cohort per Fred Hutch guidelines. Most post-HCT maintenance therapy protocols, especially those using steroids or bortezomib, recommended continuation of ACV/VACV for 2 months beyond completion of maintenance therapy.
The Fred Hutch Institutional Review Board approved the study. Informed consent was signed by all participating patients in accordance with the declaration of Helsinki.
Statistical Analysis
The incidence rate of first HZ episode per person years and 1,000 person days after first autologous HCT were calculated, accounting for date of death or last date of patient contact recorded in our system. Cumulative incidence curves were used to estimate the probability of developing an initial or recurrent episode of HZ with death treated as a competing risk. The effect of ACV/VACV prophylaxis on the occurrence of first HZ episodes was evaluated in Cox regression models including demographic, HCT and clinical characteristics. Univariable analysis for risk factors associated with a recurrent HZ episode was also performed, but limited events precluded an adjusted analysis. ACV/VACV prophylaxis and maintenance therapy were analyzed as time-dependent variables. Absolute lymphocyte count (ALC) thresholds above and below the lower quartile (760 cells/µl) were used due to limited events at lower thresholds.
Variables with biological relevance or P value ≤0.2 in univariable analyses were considered for multivariable analyses. Statistical significance was defined as 2-sided P <0.05. SAS version 9.3 (SAS Institute, Cary, NC) was used for analyses.
RESULTS
Incidence of HZ
We retrospectively identified 1,000 consecutive VZV seropositive patients receiving an autologous HCT at our center from 2002 to 2010. Patient demographic and clinical characteristics are presented in Table 1. Patients were followed for a median of 39.7 months (interquartile range [IQR], 20.7–66.1 months) and a total of 3,778.1 person years. There were 359 deaths in this cohort during the study period at a median of 16.1 months (IQR, 7.3–37.9). Patients with 5 years of possible follow up had return of questionnaires or records for a median of 4 out of 5 years, suggesting good data capture for events spanning the follow up period. Additionally, patients on maintenance therapy or recurrent disease often returned or communicated with our center for continued follow up.
Table 1.
Clinical Characteristics of the Study Population
| Patient Characteristic | Overall no. (%) | Herpes zoster post-HCT | P Value | |
|---|---|---|---|---|
| No | Yes | |||
| Number | 1000 | 806 | 194 | |
| Median Age at HCT | 0.04 | |||
| ≤55.5 | 500 (50) | 416 (83.2) | 84 (16.8) | |
| ≥55.5 | 500 (50) | 390 (78) | 110 (22) | |
| Sex | 0.24 | |||
| Female | 376 (37.6) | 296 (78.7) | 80 (21.3) | |
| Male | 624 (62.4) | 510 (81.7) | 114 (18.3) | |
| Ethnicity | 0.54 | |||
| White | 850 (85.0) | 684 (80.5) | 166 (19.5) | |
| Non white | 94 (9.4) | 79 (84) | 15 (16) | |
| Unknown | 56 (5.6) | 43 (76.8) | 13 (23.2) | |
| Type of HCT | 0.07 | |||
| Single autologous HCT | 948 (94.8) | 759 (80.1) | 189 (19.9) | |
| Tandem autologous HCTs | 52 (5.2) | 47 (90.4) | 5 (9.6) | |
| Conditioning regimen | 0.99 | |||
| Myeloablative with TBI-based regimens |
165 (16.5) | 133 (80.6) | 32 (19.4) | |
| Myeloablative with chemotherapy | 835 (83.5) | 673 (80.6) | 162 (19.4) | |
| Status at HCT | 0.47 | |||
| Relapse | 727 (72.7) | 587 (80.7) | 140 (19.3) | |
| Remission | 208 (20.8) | 164 (78.8) | 44 (21.2) | |
| Unknown | 65 (6.5) | 55 (83.9) | 10 (16.1) | |
| Underlying Disease | 0.60 | |||
| Multiple myeloma | 347 (34.7) | 271 (78.1) | 76 (21.9) | |
| Hodgkin’s disease | 115 (11.5) | 95 (82.6) | 20 (17.4) | |
| Non-Hodgkin’s lymphoma | 414 (41.4) | 337 (81.4) | 77 (18.6) | |
| Autoimmune disease | 23 (2.3) | 18 (78.3) | 5 (21.7) | |
| Others* | 101 (10.1) | 85 (84.2) | 16 (15.8) | |
| HZ pre-HCT | 0.27 | |||
| No | 926 (92.6) | 750 (81) | 176 (19) | |
| Yes | 74 (7.4) | 56 (75.7) | 18 (24.3) | |
| Received Maintenance Therapy | 0.002 | |||
| No | 719 (71.9) | 597 (83.0) | 122 (17.0) | |
| Yes† | 281 (28.1) | 209 (74.4) | 72 (25.6) | |
| Type of Maintenance Therapy | 0.03 | |||
| Lenalidomide | 49 (4.9) | 38 (77.6) | 11 (22.4) | |
| Rituximab | 87 (8.7) | 63 (72.4) | 24 (27.6) | |
| Bortezomib | 23 (2.3) | 18 (78.2) | 5 (21.7) | |
| Other | 122 (12.2) | 90 (73.8) | 32 (26.2) | |
| None | 719 (71.9) | 597 (83) | 122 (17) | |
| Relapse | 0.98 | |||
| No | 819 (81.9) | 660 (80.6) | 159 (19.4) | |
| Yes§ | 181 (18.1) | 146 (80.7) | 35 (19.3) | |
| ALC at day 365 post-HCT¶ | 0.68 | |||
| Below Lower Quartile (<760 cells/µl) | 47 (24.4) | 34 (72.3) | 13 (27.7) | |
| Above Lower Quartile (≥760 cells/µl) | 146 (75.6) | 110 (75.3) | 36 (24.7) | |
| CD34 Selected HCT or ATG use | 0.79 | |||
| No | 967 (96.7) | 780 (80.7) | 187 (19.3) | |
| Yes | 33 (3.3) | 26 (78.8) | 7 (21.2) | |
| Duration of ACV prophylaxis after HCT | 0.02 | |||
| ≤3months | 179 (17.9) | 158 (88.3) | 21 (11.7) | |
| 3–6 months | 93 (9.3) | 78 (83.9) | 15 (16.1) | |
| 6–12 months | 380 (38.0) | 302 (79.5) | 78 (20.5) | |
| 12–24 months | 276 (27.6) | 210 (76.1) | 66 (23.9) | |
| >24 months | 72 (7.2) | 58 (80.6) | 14 (19.4) | |
| Recipient HSV serostatus | 0.29 | |||
| Positive | 779 (77.9) | 636 (81.6) | 143 (18.4) | |
| Negative | 220 (22.0) | 169 (76.8) | 51 (23.2) | |
| Equivocal | 1 (0.1) | 1 (100) | 0 (0.0) | |
| Recipient CMV serostatus | 0.25 | |||
| Positive | 547 (54.7) | 450 (82.3) | 97 (17.7) | |
| Negative | 451 (45.1) | 354 (78.5) | 97 (21.5) | |
| Equivocal | 2 (0.2) | 2 (100) | 0 (0.0) | |
| Follow up | 0.02 | |||
| Outside institution | 867 (86.7) | 709 (81.8) | 158 (18.2) | |
| SCCA/FHCRC | 133 (13.3) | 97 (72.9) | 36 (27.1) | |
Abbreviation: HCT, hematopoietic cell transplant; TBI, total body irradiation; HZ, herpes zoster; ALC, absolute lymphocyte count; ACV, acyclovir; HSV, herpes simplex; CMV, cytomegalovirus; SCCA, Seattle Cancer Care Alliance; FHCRC, Fred Hutchinson Cancer Research Center.
Other diseases included solid tumors (n=57), chronic inflammatory diseases (n=29), acute leukemia (n=10), and other lymphoproliferative diseases (n=5).
Patients receiving maintenance therapy had multiple myeloma (n=160), non-Hodgkin’s lymphoma (n=99), Hodgkin’s disease (n=8), and ‘other’ diseases (n=14).
Patients who relapsed had non-Hodgkin’s lymphoma (n=76), multiple myeloma (n=73), solid tumors (n=15), Hodgkin’s disease (n=14), and acute leukemia (n=3).
There were 193 patients with day 365 post-HCT ALC data available.
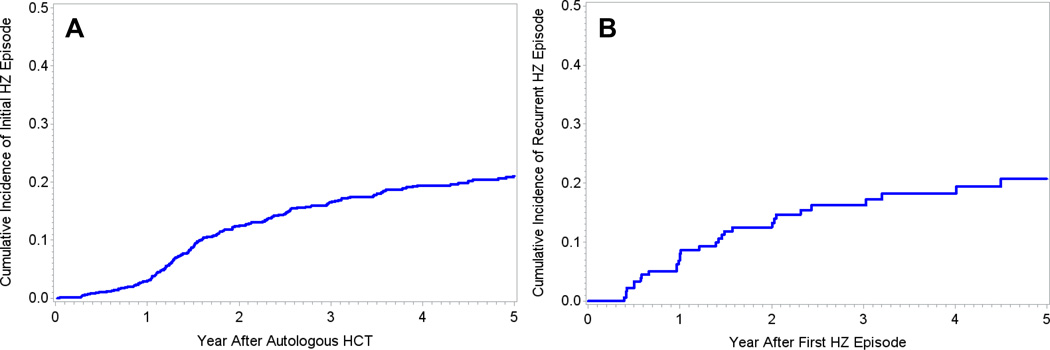
194 patients developed at least one HZ episode following autologous HCT with an overall cumulative incidence of 21% (95% confidence interval [CI], 18%-24%; Figure 1A). First HZ episodes occurred at a median of 19 months (IQR, 14.4–30.7; Table 2) after autologous HCT. Recurrence of HZ occurred in 31 of these patients (16%) at a median of 14.5 months after the first post-HCT HZ episode (IQR, 7.0–24.5; Table 2, Figure 1B). Time-to HZ did not differ for patients with disseminated disease (median of 18.2 months) versus localized disease (median of 19 months; p=0.4).
Figure 1. Cumulative incidence curves of time to HZ episode.
Panel A demonstrates the cumulative incidence of first HZ episodes in the entire cohort over time. 194 patients had an initial HZ episode within 5 years after autologous HCT with a cumulative incidence of 21% (95% CI, 18%-24%). Panel B demonstrates the cumulative incidence of recurrent HZ episodes over time. 31 patients had a recurrent episode of HZ after autologous HCT.
Table 2.
Clinical Characteristics of Herpes Zoster Episodes
| Clinical Findings | no. (%) |
|---|---|
| First HZ episode post-HCT | 194 |
| Months to event, median (IQR) | 19 (14.0–30.7) |
| Disseminated HZa | 16 (8.2) |
| Skin | 13 (81.3) |
| Visceral or CNS involvement | 4 (25) |
| Not reported | 2 (12.5) |
| Ocular disease | 8 (4.1) |
| Post-herpetic neuralgiab | 25 (12.9) |
| Recurrent HZ episode post-HCT | 31 |
| Months to event, median (IQR) | 14.5 (7.0–24.5) |
| Disseminated HZ | 0 |
| Post-herpetic neuralgia | 4 (12.9) |
Abbreviations: HZ, herpes zoster; HCT, hematopoietic cell transplantation; IQR, interquartile range; CNS, central nervous system
2 patients had GI disease, 1 had pulmonary disease, and 1 had CNS disease. 3 patients with visceral involvement also had skin involvement. The patients with GI or pulmonary disease has possible involvement by VZV based on clinical findings and response to acyclovir but did not have diagnostic confirmation. The patient with CNS disease had VZV identified by PCR in the CSF. Patients with disseminated disease based on skin findings had documentation of diffuse vesicular rashes in >2 dermatomes.
Post herpetic neuralgia was reported in 24% (9/37) of patients followed at our center and 10% (16/157) in patients who were followed at an outside institution.
The incidence rate of first HZ episode after autologous HCT per person years over the entire follow up period was 0.06 (95% CI, 0.05–0.08; Table 3). The highest incidence rate was 0.13 in the second year. Patients who had all their follow up with complete medical records at our center had a higher crude incidence of HZ (27.1%) than what was determined for patients outside of our center (18.2%), suggesting a potential underestimation of the overall incidence of HZ in this cohort.
Table 3.
Incidence Rate of First Herpes Zoster Episode Over 5 Years
| Incidence Rate (95% CI) | |||
|---|---|---|---|
| 1st year post-HCT | 2nd year post-HCT* | Overall period | |
| Per person years | 0.03 (0.02, 0.05) | 0.13 (0.10, 0.15) | 0.06 (0.05, 0.08) |
| Per 1,000 person days | 0.09 (0.08, 0.11) | 0.34 (0.31, 0.37) | 0.17 (0.14, 0.19) |
Abbreviations: CI, confidence interval; HCT, hematopoietic cell transplantation.
Incidence rate among one year survivors.
Risk Factors
At the time of first HZ episode, 159/194 patients (82%) were clearly documented as no longer receiving ACV/VACV prophylaxis. Of the remaining 35 patients, there was clear documentation that 23 patients were still taking ACV/VACV prophylaxis; the additional 12 patients had follow up questionnaires after their episode of HZ indicating that they were still taking prophylaxis. Seventeen of the 35 breakthrough cases occurred in the first year after HCT. The median time to first HZ episode after stopping ACV/VACV prophylaxis was 4 months (IQR, 1.7–7.3). Of the 194 patients who developed HZ, 69 patients received subsequent ACV/VACV prophylaxis for a median of 11.7 months (IQR, 3.6–24.5). Of the 31 patients with recurrent HZ, 15 had received ACV/VACV prophylaxis after their initial episode. Recurrent HZ occurred a median of 19.2 months (IQR, 5.1–35) after stopping ACV/VACV; 9 patients had documentation supporting development of recurrent HZ while on ACV/VACV.
Use of ACV/VACV was associated with a reduced risk for HZ in the univariable analysis (hazard ratio [HR] 0.62; 95% CI, 0.4–0.96; p=0.03; Table 4). In a multivariable model adjusting for sex, tandem autologous-autologous HCT, pre-HCT HZ, age, and use of maintenance chemotherapy, the effect of ACV/VACV prophylaxis remained protective for HZ (adjusted HR [aHR], 0.59; 95% CI, 0.37–0.91; p=0.02; Table 4). This effect was greater after adjusting for day 365 ALC among the subgroup who survived 1 year and had ALC data available (aHR, 0.14; 95% CI, 0.05–0.41; data not shown).
Table 4.
Univariable and Multivariable Cox Regression Models of Time to First HZ Episode Post-HCT
| Covariates | HR (95% CI) | P value | Adjusted HR (95% CI) |
P value |
|---|---|---|---|---|
| Median Age at HCT | ||||
| ≤55.5 | Ref | - | Ref | - |
| ≥ 55.5 | 1.42 (1.06–1.90) | 0.02 | 1.42 (1.05–1.90) | 0.02 |
| Sex | ||||
| Female | Ref | - | Ref | - |
| Male | 0.83 (0.62–1.11) | 0.22 | 0.81 (0.60–1.08) | 0.16 |
| Type of HCT | ||||
| Single autologous HCT | Ref | - | Ref | - |
| Tandem autologous HCTs | 0.44 (0.18–1.08) | 0.07 | 0.48 (0.20–1.18) | 0.11 |
| HZ Pre-HCT | ||||
| No | Ref | - | Ref | - |
| Yes | 1.54 (0.94–2.54) | 0.09 | 1.58 (0.96–2.61) | 0.07 |
|
Received maintenance therapy as time-dependent covariate |
||||
| No | Ref | - | Ref | - |
| Yes | 0.92 (0.57–1.49) | 0.74 | 0.98 (0.60–1.59) | 0.93 |
|
ACV/VACV prophylaxis post-HCT as time-dependent covariate |
||||
| No | Ref | - | Ref | - |
| Yes | 0.62 (0.40–0.96) | 0.03 | 0.59 (0.37–0.91) | 0.02 |
Abbreviations: ACV, acyclovir; VACV, valacyclovir; HCT, hematopoietic cell transplantation.
Age greater than the median of 55.5 years was also associated with increased risk for HZ (aHR, 1.42; 95% CI, 1.05–1.9; p=0.02). Patients who had a HZ episode pre-HCT (7.4%) had increased risk for HZ that did not meet statistical significance (aHR, 1.58; 95% CI, 0.96–2.61; Table 4). Among the subgroup of patients with data for ALC at day 365 post-HCT, those with values below the lower quartile (760 cells/µl) had increased risk for HZ that was not statistically significant (aHR, 1.63; 95% CI, 0.84–3.14; p=0.15; data not shown).
Patients who received maintenance therapy for their underlying disease post-HCT (28.1%) did not have an increased risk for HZ in an adjusted time-dependent analysis. We also compared patients receiving bortezomib maintenance therapy (n=23) to those receiving an alternative or no maintenance therapy and found no association with development of HZ (HR, 1.11; 95% CI, 0.15–7.97; p=0.92). However, the majority of patients (86%) were taking ACV/VACV prophylaxis while receiving maintenance therapy. Overall, patients on maintenance therapy received ACV/VACV prophylaxis for a significantly longer duration than patients not receiving maintenance therapy (median, 394 days vs 296 days, p<0.001).
Given that patients receiving T-cell depleted HCTs (CD34-selected or ATG use) for autoimmune or other diseases may have increased risk for HZ, we calculated the cumulative incidence of HZ among this specific group (n=33) compared to those without CD34 selected HCTs (n=967) and found no significant difference (Figure S2). There was also no significant difference in the cumulative incidence of HZ when stratified by underlying disease (Figure S3).
Relapse as a time-dependent covariate was not associated with HZ. No risk factors for the development of a recurrent HZ episode were apparent in the univariable analysis of this cohort (data not shown), so multivariable analyses were not performed.
Clinical Features
Clinical features of HZ episodes are reported in Table 2. Disseminated disease was reported in 16/194 (8.2%) patients during the first HZ episode after HCT. Disease manifestation was reported for 14 of these cases: 13 had skin involvement, 2 had presumptive gastrointestinal involvement, 1 had presumptive pulmonary involvement, and 1 had proven central nervous system involvement. Ocular involvement was documented in 8 patients. No patients died within 30 days of disseminated disease.
Post-herpetic neuralgia was common and reported in 24% of patients with well-documented follow up at our center compared to 10% in patients who left our center after HCT. Rates of post-herpetic neuralgia were similar after first and recurrent episodes of HZ. Among the 35 patients categorized as receiving ACV/VACV prophylaxis at the time of HZ, 20 patients had available documentation of appropriate response to first-line therapy for HZ (either intravenous ACV or increased doses of ACV or VACV) without the need for additional treatment.
DISCUSSION
We demonstrated a high burden of disease due to HZ in a cohort of 1,000 autologous HCT recipients, despite long-term antiviral prophylaxis. The use of ACV/VACV did reduce the development of HZ while being taken, but the cumulative incidence of disease remained high after discontinuation of antiviral prophylaxis.
The incidence rate of HZ over 5 years of follow up in our cohort (0.06 per person year) is approximately ten-fold higher than that reported in a large population-based study of immunocompetent adults prior to the introduction of the HZ vaccine (0.004 per person-year)24. The incidence of disseminated disease in our cohort was relatively high but still less than that reported in other studies9,25, possibly due to underreporting and the limitations of our study design as discussed below. The rate of post-herpetic neuralgia seen in the patients followed closely at our center was as high as that reported in immunocompetent patients ≥70 years old24. There was no apparent difference in the cumulative incidence of HZ among the subgroup of patients receiving T-cell depleted HCTs compared to the rest of the cohort, although the numbers were small.
Older age is a well-recognized risk factor for the development of HZ in the general population24,26,27 and was also associated with increased risk in this cohort of autologous HCT recipients. We did not find a strong association with ALC, a surprising finding given that T-cell immunity is important for controlling viral infections28. However, this analysis was limited by the smaller number of patients with available data at day 365 and our inability to use lower ALC thresholds due to sample size. A study in a Japanese cohort receiving a shorter duration of prophylaxis (median of 32 days) also did not find an association between ALC at day 30, 100, or 180 with HZ after autologous HCT 25. However, ALC may not adequately reflect T-cell function and the number of VZV-specific T-cells. Although there is only limited data showing a correlation between the recovery of VZV-specific T-cells with risk for HZ after autologous HCT, many studies have demonstrated the importance of T-cells for control VZV and the prolongation of VZV-specific T-cell recovery in the absence of viral reactivation post-allogeneic HCT29. Encouragingly, an adjuvanted VZV subunit vaccine in autologous HCT recipients was able to increase levels of VZV-specific T-cells compared to placebo30.
We did not find an association between receipt of bortezemib or any maintenance therapy and HZ. Although this association has been demonstrated with some agents (e.g. bortezomib)31, our center recommends continuing ACV/VACV prophylaxis during maintenance therapy, so the majority of patients receiving these treatments were on prolonged antiviral prophylaxis. We also did not find a clear association between a history of pre-HCT HZ and the risk for post-HCT HZ to suggest a benefit of a pre-HCT boosting of immunity, but this analysis was limited by small numbers.
The efficacy and safety of ACV/VACV prophylaxis for HZ after autologous and allogeneic HCT is well established9, although there is poor consensus on the dose and duration of ACV/VACV prophylaxis for HZ after autologous HCT16,17,19. Although acyclovir-resistant HZ has been described32, it is rare in HCT recipients33,34, and there was no clinical evidence for resistance in our patients who were taking ACV/VACV prophylaxis at the time of HZ based on subsequent response to these agents. VZV-specific T-cell reconstitution in allogeneic HCT recipients does not appear to be adversely affected by prolonged use of prophylactic ACV after HCT14, suggesting that the continued risk for HZ after discontinuation of antiviral prophylaxis likely reflects a prolonged immunodeficiency9. Our data demonstrate that despite such aggressive measures, breakthrough cases did occur. The cause of breakthrough cases may have been a result of imperfect adherence to medication recommendations or possibly a need for higher doses in high-risk patients. Adherence to chronic cancer medications for a year can be as low as 70–80%, and rates are lower for longer durations of therapy35. The number of breakthrough cases may have been overestimated due to the retrospective design and estimates of antiviral prophylaxis discontinuation date in some cases. We did not explore specific risk factors for breakthrough HZ given the limited events.
Our data suggest that continuing antiviral prophylaxis for HZ for longer durations, especially in older patients and those on maintenance therapy, may be beneficial. Our data also support the need for additional prophylaxis following a HZ event. These patients had relatively high recurrence rates, but many patients were not restarted on antiviral prophylaxis, and no guidelines address this issue. Alternative approaches to a durable antiviral prophylaxis strategy after autologous HCT are warranted given the high burden of late HZ, its associated morbidity, and increased healthcare utilization and costs among affected patients22. The currently available live attenuated varicella vaccines have been shown to be effective when given at two years post-HCT36, and guidelines allow the use of the zoster vaccine following autologous HCT in select recipients26. Investigational killed and subunit zoster vaccines, which demonstrated efficacy against HZ in immunocompromised hosts in early phases of development30,37, have the potential to provide long-term protection against HZ. Large phase III trials to examine the efficacy of vaccination in immunosuppressed populations including HCT recipients are currently ongoing (NCT01767467 and NCT01229267) and may provide an option for future prevention. Given the often-low rate of implementation of interventions such as vaccines, our real-world observational data will help to underscore the burden of illness and potentially improve vaccination acceptance.
The retrospective nature of this study with many patients followed at outside institutions introduced limitations. Incomplete return of patient and physician questionnaires and records limited identification of exact dates of HZ episodes, antiviral prophylaxis and maintenance therapy in some instances. The rate of potential breakthrough cases of HZ while on ACV/VACV prophylaxis without evidence for resistance was higher than expected and suggests non-adherence or overestimation of ACV/VACV use based on available records. This may have led to an underestimate of the effect size of ACV/VACV at preventing HZ. Diagnosis of HZ is typically a clinical diagnosis without microbiologic confirmation, especially in community practices. Although this could have led to an overestimation of the rate of HZ, clinical diagnosis is highly sensitive and specific for HZ38, and we do not think the lack of microbiologic diagnosis substantially impacted these results. Conversely, incomplete follow up or return of records may have led to an underestimate of the true incidence of HZ and its complications. This is supported by the higher observed rate of HZ in patients with complete follow up at our center’s continuity clinics (Table 1), although these patients may represent those with increased medical complexity requiring higher levels of care. Follow up data were not available for a full 5 years for patients receiving an autologous HCT from 2009–2010 because chart review for the study ended in 2013. Analyses accounted for differential patient follow up by censoring at the time of last contact with patients. Rates of post-herpetic neuralgia should be considered estimates given the lack of standardized approaches to diagnosis across different sites. We acknowledge that the data may not be generalizable to other centers due to the heterogeneity in conditioning and maintenance therapies, as well as differences in patient characteristics (e.g. relapsed disease at time of HCT).
Despite these limitations, the unique dataset established through prospective collection of patient data allowed for one of the largest and most comprehensive attempts to establish a baseline rate of HZ in a contemporary cohort of autologous HCT recipients receiving standardized prevention with up to five years of follow up. Our findings provide conservative estimates for endpoints in future clinical trials.
In conclusion, this study showed a significant burden of HZ and its complications in a contemporary cohort of autologous HCT recipients despite prolonged ACV/VACV prophylaxis. Although receipt of ACV/VACV was protective, there continued to be a high number of incident HZ cases after discontinuation of prophylaxis. Occurrence of HZ after the cessation of prolonged ACV/VCV remains an unresolved challenge, and additional strategies for prevention are needed. Prospective studies with documentation of prophylactic therapy use and compliance, along with confirmation of HZ diagnosis and outcomes, are important next steps in the field.
Supplementary Material
Acknowledgments
This work was supported by an investigator-initiated study grant from Merck and Co. Inc. (M.B.) and by the National Institutes of Health [1K23AI119133 to J.A.H., CA18029 to M.B. and HL093294 to M.B.]. Merck and Co. Inc. had an opportunity to review the design of the study but had no role in its conduct; collection, management, analysis, and interpretation of the data; preparation of the manuscript; or decision to submit the manuscript for publication. Merck Research Laboratories did have the opportunity to review and provide input on the final version of the manuscript.
Disclosures: F.S. received research support from Merck Research Laboratories. S.G. received research support from Merck Research Laboratories and Glaxo Smith Kline. J.A.H. received research support from Chimerix Inc. S.P. received research support from Chimerix Inc. and has served as a consultant and received support from Merck Research Laboratories. L.H. received research support from Millennium-Takeda. M.B. has served as a consultant and received research support from Merck Research Laboratories, Chimerix Inc., Glaxo Smith Kline, and Roche/Genentech in addition to consulting from Clinigen. I.L and K.L.L are or were employed by Merck Research Laboratories.
Abbreviations
- HCT
hematopoietic cell transplantation (HCT)
- HZ
herpes zoster
- VZV
varicella zoster virus
- ACV/VACV
acyclovir/valacyclovir
- LTFU
long term follow up
Footnotes
Previous presentation of the study: Abstract # 436 presented at the Infectious Disease Society of America (IDSA) on Oct. 09, 2014 at The Pennsylvania Convention Center, Philadelphia, USA.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119(4):940–948. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
- 3.Shank BR, Brown VT, Schwartz RN. Multiple myeloma maintenance therapy: a review of the pharmacologic treatment. J Oncol Pharm Pract. 2015;21(1):36–51. doi: 10.1177/1078155213514468. [DOI] [PubMed] [Google Scholar]
- 4.Rogers JE, Cumpston A, Newton M, Craig M. Onset and complications of varicella zoster reactivation in the autologous hematopoietic cell transplant population. Transpl Infect Dis. 2011;13(5):480–484. doi: 10.1111/j.1399-3062.2011.00655.x. [DOI] [PubMed] [Google Scholar]
- 5.Truong Q, Veltri L, Kanate AS, et al. Impact of the duration of antiviral prophylaxis on rates of varicella-zoster virus reactivation disease in autologous hematopoietic cell transplantation recipients. Ann Hematol. 2014;93(4):677–682. doi: 10.1007/s00277-013-1913-z. [DOI] [PubMed] [Google Scholar]
- 6.Schuchter LM, Wingard JR, Piantadosi S, Burns WH, Santos GW, Saral R. Herpes zoster infection after autologous bone marrow transplantation. Blood. 1989;74(4):1424–1427. [PubMed] [Google Scholar]
- 7.Vose JM, Kennedy BC, Bierman PJ, Kessinger A, Armitage JO. Long-term sequelae of autologous bone marrow or peripheral stem cell transplantation for lymphoid malignancies. Cancer. 1992;69(3):784–789. doi: 10.1002/1097-0142(19920201)69:3<784::aid-cncr2820690328>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Kamber S, Suter-Riniker F, Mueller BU, Taleghani BM, Betticher D, Zander T, Pabst TCZ. Varicella zoster virus reactivation after autologous SCT is a frequent event and associated with favorable outcome in myeloma patients. Bone Marrow Transpl. 2015;50(4):573–578. doi: 10.1038/bmt.2014.290. [DOI] [PubMed] [Google Scholar]
- 9.Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007;110(8):3071–3077. doi: 10.1182/blood-2007-03-077644. [DOI] [PubMed] [Google Scholar]
- 10.Locksley RM, Flournoy N, Sullivan KM, Meyers JD. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152(6):1172–1181. doi: 10.1093/infdis/152.6.1172. [DOI] [PubMed] [Google Scholar]
- 11.Han CS, Miller W, Haake R, Weisdorf D. Varicella zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant. 1994;13(3):277–283. [PubMed] [Google Scholar]
- 12.Arvin AM. Varicella-Zoster virus: pathogenesis, immunity, and clinical management in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2000;6(3):219–230. doi: 10.1016/s1083-8791(00)70004-8. [DOI] [PubMed] [Google Scholar]
- 13.Koc Y, Miller KB, Schenkein DP, et al. Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant. 2000;6(1):44–49. doi: 10.1016/s1083-8791(00)70051-6. [DOI] [PubMed] [Google Scholar]
- 14.Boeckh M, Kim HW, Flowers MED, Meyers JD, Bowden RA. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation--a randomized double-blind placebo-controlled study. Blood. 2006;107(5):1800–1805. doi: 10.1182/blood-2005-09-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamura K, Wada H, Yamasaki R, et al. Prophylactic role of long-term ultra-low-dose acyclovir for varicella zoster virus disease after allogeneic hematopoietic stem cell transplantation. Int J Infect Dis. 2014;19:26–32. doi: 10.1016/j.ijid.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Selby PJ, Powles RL, Easton D, et al. The prophylactic role of intravenous and long-term oral acyclovir after allogeneic bone marrow transplantation. Br J Cancer. 1989;59(3):434–438. doi: 10.1038/bjc.1989.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Kumar D, Messner HA, et al. Clinical efficacy of prophylactic strategy of long-term low-dose acyclovir for Varicella-Zoster virus infection after allogeneic peripheral blood stem cell transplantation. Clin Transplant. 22(6):770–779. doi: 10.1111/j.1399-0012.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 18.Klein A, Miller KB, Sprague K, DesJardin JA, Snydman DR. A randomized, double-blind, placebo-controlled trial of valacyclovir prophylaxis to prevent zoster recurrence from months 4 to 24 after BMT. Bone Marrow Transplant. 2011;46(2):294–299. doi: 10.1038/bmt.2010.99. [DOI] [PubMed] [Google Scholar]
- 19.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollack M, Heugel J, Xie H, et al. An international comparison of current strategies to prevent herpesvirus and fungal infections in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2011;17(5):664–673. doi: 10.1016/j.bbmt.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303(16):1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Chen S-Y, Burstin SJ, Levin MJ, Suaya JA. Cost of Herpes Zoster in Patients With Selected Immune-Compromised Conditions in the United States. Open Forum Infect Dis. 2016;3(2):ofw067. doi: 10.1093/ofid/ofw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pergam SA, Limaye AP. Varicella zoster virus (VZV) in solid organ transplant recipients. Am J Transplant. 2009;(9 Suppl 4):S108–S115. doi: 10.1111/j.1600-6143.2009.02901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 25.Mawatari M, Isoda A, Miyazawa Y, Sawamura M, Matsumoto M. A Japanese single-hospital observational trial with a retrospective case-control analysis of varicella zoster virus reactivation after autologous peripheral blood stem cell transplantation. Transpl Infect Dis. 2015;17(4):544–550. doi: 10.1111/tid.12406. [DOI] [PubMed] [Google Scholar]
- 26.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-5):1–30. quiz CE2-CE4. [PubMed] [Google Scholar]
- 27.Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61(5):310–316. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341–357. doi: 10.1007/82_2010_31. [DOI] [PubMed] [Google Scholar]
- 29.Distler E, Schnürer E, Wagner E, et al. Recovery of varicella-zoster virus-specific T cell immunity after T cell-depleted allogeneic transplantation requires symptomatic virus reactivation. Biol Blood Marrow Transplant. 2008;14(12):1417–1424. doi: 10.1016/j.bbmt.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Stadtmauer EA, Sullivan KM, Marty FM, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood. 2014;124(19):2921–2929. doi: 10.1182/blood-2014-04-573048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JW, Min CK, Mun YC, et al. Varicella-zoster virus-specific cell-mediated immunity and herpes zoster development in multiple myeloma patients receiving bortezomib- or thalidomide-based chemotherapy. J Clin Virol. 2015;73:64–69. doi: 10.1016/j.jcv.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Gueudry J, Boutolleau D, Gueudin M, et al. Acyclovir-resistant varicella-zoster virus keratitis in an immunocompetent patient. J Clin Virol. 2013;58(1):318–320. doi: 10.1016/j.jcv.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Hatchette T, Tipples GA, Peters G, Alsuwaidi A, Zhou J, Mailman TL. Foscarnet salvage therapy for acyclovir-resistant varicella zoster: report of a novel thymidine kinase mutation and review of the literature. Pediatr Infect Dis J. 2008;27(1):75–77. doi: 10.1097/INF.0b013e3181598315. [DOI] [PubMed] [Google Scholar]
- 34.Linnemann CC, Biron KK, Hoppenjans WG, Solinger AM. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS. 1990;4(6):577–579. doi: 10.1097/00002030-199006000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Hohneker J, Shah-Mehta S, Brandt PS. Perspectives on adherence and persistence with oral medications for cancer treatment. J Oncol Pract. 2011;7(1):65–67. doi: 10.1200/JOP.2010.000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Issa NC, Marty FM, Leblebjian H, et al. Live attenuated varicella-zoster vaccine in hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2014;20(2):285–287. doi: 10.1016/j.bbmt.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Mullane KM, Winston DJ, Wertheim MS, et al. Safety and immunogenicity of heat-treated zoster vaccine (ZVHT) in immunocompromised adults. J Infect Dis. 2013;208(9):1375–1385. doi: 10.1093/infdis/jit344. [DOI] [PubMed] [Google Scholar]
- 38.Sauerbrei a, Eichhorn U, Schacke M, Wutzler P. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14(1):31–36. doi: 10.1016/s1386-6532(99)00042-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.