Abstract
Context
Sotos syndrome is a rare genetic disorder with a distinct phenotypic spectrum including overgrowth and learning difficulties. Here we describe a new case of Sotos syndrome with a 5q35 microdeletion, affecting the fibroblast growth factor receptor 4 (FGFR4) gene, presenting with infantile hypercalcemia.
Objective
We strived to elucidate the evanescent nature of the observed hypercalcemia by studying the ontogenesis of FGFR3 and FGFR4 – which are both associated with FGF23-mediated mineral homeostasis – in the developing human kidney.
Design
RT-qPCR and immunohistochemical analyses were used on archival human kidney samples to investigate expression of the FGFR signaling pathway during renal development.
Results
We demonstrated that renal gene and protein expression of both FGFRs increased during fetal development between the gestational ages (GA) of 14–40 weeks. Yet, FGFR4 expression increased more rapidly as compared to FGFR3 (slope: 0.047 vs. 0.0075, p = 0.0018). Moreover, gene and protein expression of the essential FGFR co-receptor, Klotho, also increased with a significant positive correlation between FGFR and Klotho mRNA expression during renal development. Interestingly, we found that perinatal FGFR4 expression (GA 38–40 weeks) was 7-fold higher as compared to FGFR3 (p=0.0035), while in adult kidney tissues, FGFR4 gene expression level was more than 2-fold lower compared to FGFR3 (p=0.0029), thus identifying a molecular developmental switch of FGFR isoforms.
Conclusion
We propose that the heterozygous FGFR4 deletion, as observed in the Sotos syndrome patient, leads to a compromised FGF23 signaling during infancy accounting for transient hypercalcemia. These findings represent a novel and intriguing view on FGF23-mediated calcium homeostasis.
Introduction
Sotos syndrome is an autosomal dominant disorder characterized by overgrowth, learning disability and distinctive facial features such as macrocephaly [1,2]. The syndrome results from mutations and deletions – mostly de novo – of the NSD1 gene located at chromosome 5, which encodes for the nuclear receptor-binding SET-domain containing protein 1 (NSD1). Little is known regarding the function of NSD1 and it remains unclear how NSD1 gene inactivation or NSD1 dysfunction may cause the Sotos phenotype [3]. Intragenic mutations are responsible for 80–85% of Sotos syndrome cases, while 5q35 microdeletions encompassing NSD1 cause 10–15% of the cases in Europe and the USA [4]. Of note, 5q35 microdeletions vary in size, from 0.4 to 5 Mb, and may affect genes flanking NSD1 [5]. Saugier-Veber et al. mentioned the occurrence of nephrocalcinosis in three patients with large deletions and suggested the presence of a predisposing gene in the deleted area [6]. This phenotype spectrum was confirmed by Kenny et al. who reported two unrelated cases of Sotos syndrome associated with nephrocalcinosis, one of whom had proven infantile hypercalcemia [7]. The authors suggested that the heterozygous deletion of SLC34A1 – encoding the main sodium phosphate cotransporter NPT2a – could explain the temporary hypercalcemia in these patients. However, hypercalcemia was absent in patients with a loss of function of NTP2a, as described by Magen et al. [8], in contrast to Npt2−/− mice that exhibited a slight but significant increase in serum calcium concentration compared to wild type mice [9]. Thus, the transient nature of the hypercalcemia remains unexplained in Sotos syndrome patients.
Another gene mapped to the deletion interval is FGFR4, encoding for the fibroblast growth factor receptor (FGFR) 4. The fibroblast growth factors (FGFs) are a large family of peptides that are involved in a myriad of biological processes, including development and mineral homeostasis [10]. Currently, seven FGF subfamilies have been identified encompassing 22 different FGFs. The FGF19 subfamily – containing FGF19, FGF21 and FGF23 – plays a key role in regulating energy and mineral metabolism [10]. Of interest, FGF23 is of the utmost importance in maintaining vitamin D, phosphate and calcium levels [11]. FGF23 is a ligand for several FGFRs (e.g. FGFR1, FGFR3 and FGFR4) and it has been demonstrated that Klotho forms a complex with these receptors resulting in an increased affinity selectively to FGF23 [10,12]. FGFR1 is implicated in FGF23 effects on NaPi-2a and NaPi-2c cotransporters [13], whereas FGFR3 and FGFR4 are linked to FGF23 effects on vitamin D and calcium levels [14]. Thus, there is a clear link between FGFR4-mediated FGF23 signaling and calcium homeostasis. Here, we describe a new Sotos syndrome case, due to a 5q35 microdeletion, with infantile hypercalcemia and we hypothesized that a heterozygous mutation in FGFR4 might be a possible explanation for the observed elevated calcium levels. To unravel the temporary characteristics of the perceived hypercalcemia, we studied FGFR3 and FGFR4 ontogenesis in the human developing kidney that has never reported to date.
Materials and methods
Ethics statement
Fetal samples were collected after parental informed and written consent and after declaration to the French Biomedical Agency (Decree 003812, 09/22/2006). Archival formol-fixed paraffin-embedded fetal and neonatal kidneys were selected from the collections of several departments of pathology according to the French legislation. Parental informed and written consents had been obtained at the time of tissue collection and were conserved in each department. Experimental use of these samples has been declared to the French Biomedical Agency (Decree 003812, 09/22/2006).
Human renal samples
Snap-frozen kidney samples from 18 fetuses, with a gestational age of 14–40 weeks, obtained from the Fetopathology Department of Robert-Debré University Hospital (Paris, France), were used for qPCR. For histology, a selection was made from thirty-seven archival tissue sections previously checked for quality and integrity by immunostaining with vimentin and low molecular weight cytokeratin [15].
Quantitative PCR
To study gene expression, total RNA (1 μg) was reverse-transcribed using 50 U MultiScribe Reverse Transcriptase (Life Technologies, Saint Aubin, France). Subsequently, cDNA was used for quantitative PCR performed with a StepOnePlus Real-Time PCR system using the TaqMan Universal PCR Master Mix (Life Technologies). GAPDH was used as housekeeping gene, and relative mRNA expression levels were calculated using the equation: (2−ΔCT)*100, or as fold change using the 2−ΔΔCT method. The following primer-probe sets were used: FGFR3, Hs00179829_m1; FGFR4, Hs01106908_m1; GAPDH, Hs99999905_m1; Klotho, Hs00183100_m1 (all obtained from Life Technologies).
Immunohistochemistry
Five-micrometer-thick tissue sections were deparaffinized and rehydrated in successive baths of xylene and graded alcohols. Afterwards, the slides were heated in sodium citrate buffer (pH 6) at 100 °C for 15 min. Endogenous peroxidase was blocked with 3 % (v/v) H2O2 for 30 min. Next, non-specific epitopes were blocked for 30 min at RT with 1% (w/v) bovine serum albumin in PBS containing 0.1% (v/v) Tween-20. Subsequently, the slides were incubated overnight at 4°C with rabbit-α-FGFR3 (1:1000; ab137084; Abcam, Cambridge, UK), rabbit-α-FGFR4 (1:500; ab41948; Abcam) or rabbit monoclonal-α-Klotho antibody (1:100; ab181373; Abcam) in blocking buffer. To reveal bound Ig, slides were incubated for 30 min at RT with the ImmPRESS anti-rabbit Ig kit (Vector, Peterborough, UK), and liquid DAB plus chromogene (Dako, Glostrup, Denmark) was used for visualization. Slides were counterstained with Mayer’s hematoxylin and mounted using Glycergel (Dako). Sections were studied via bright field microscopy (Olympus BX61) and images were taken by means of a Retiga-2000R Fast 1394 digital camera (QImaging, Surrey, Canada).
Statistics
Statistics were performed using GraphPad Prism 6.03 via one-way analysis of variance (ANOVA) followed by Bonferroni’s Multiple Comparison Test or an unpaired Student’s t-test. Differences between groups were considered to be statistically significant when p < 0.05. The software was also used to perform linear regression analyses and correlation analyses (Pearson and Spearman).
Results
Case report of a new Sotos syndrome patient with 5q35 microdeletion and infantile hypercalcemia
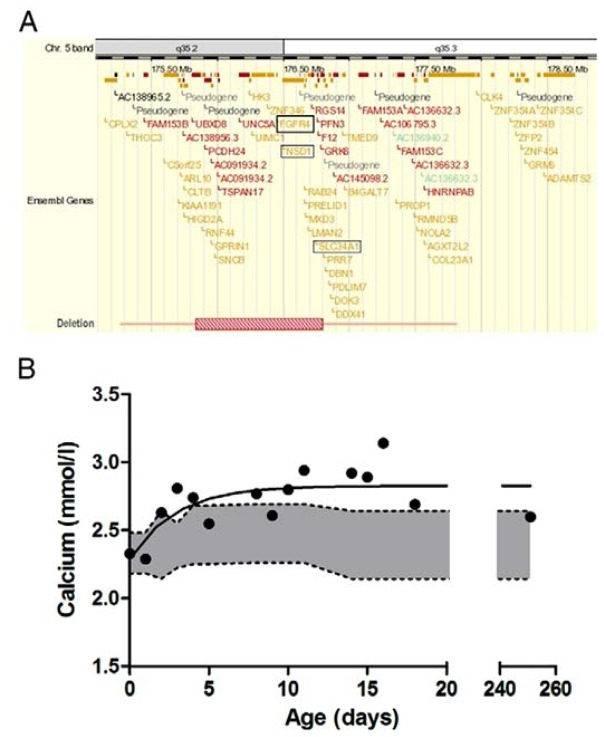
G was the fourth child of non-consanguineous parents. His birth weight was 3580 g after 39 weeks of uncomplicated pregnancy. A short period of artificial ventilation was required after birth due to respiratory distress. Because of dysmorphic features, Comparative Genomic Hybridization (CGH) array was performed, which revealed a microdeletion at 5q35, including both NSD1 and FGFR4, as shown in Fig. 1A. The deletion was absent in both parents, suggesting that it occurred de novo. In addition, hypercalcemia was observed during the first two weeks of life, as presented in Fig. 1B, and normalized afterwards. In the first weeks following birth from day 8–18, serum phosphate levels were slightly reduced ranging from 1.22–2.54 mmol/L with a median value of 1.92 (n=8) (reference range: 1.74–2.66 mmol/L) and returned to normal concentrations afterwards. The maximum phosphate reabsorption rate was decreased, possibly due to the heterozygous deletion of solute carrier family 34 (type II sodium/phosphate contransporter), member 1 gene (SLC34A1) also lost with the deletion (Fig. 1A) [16]. During the first 4 days, the child received intravenous nutrition and was then switched to breastfeeding. At 4 years of age, he was more extensively investigated and it was demonstrated that serum calcium and parathyroid hormone (PTH) levels were normal, whereas 1–25 dihydroxycholecalciferol (i.e. active vitamin D) was slightly elevated (86 ng/L, normal range: 20–80 ng/L). Nephrocalcinosis was absent on ultrasound scanning. We hypothesized that the heterozygous deletion in FGFR4 might be the possible explanation for the observed transient elevated calcium levels.
Figure 1. New Sotos syndrome case with infantile hypercalcemia.
(A) Extent of 5q35 microdeletion including both NSD1 and FGFR4. (B) Dots represent the calcium level per day during the first weeks of life. Grey area depicts the normal range of total serum calcium in healthy neonates, obtained from Roberton’s textbook of neonatology (4th ed., 2005, Elsevier Churchill Livingstone).
Expression profile of FGFRs during fetal renal development
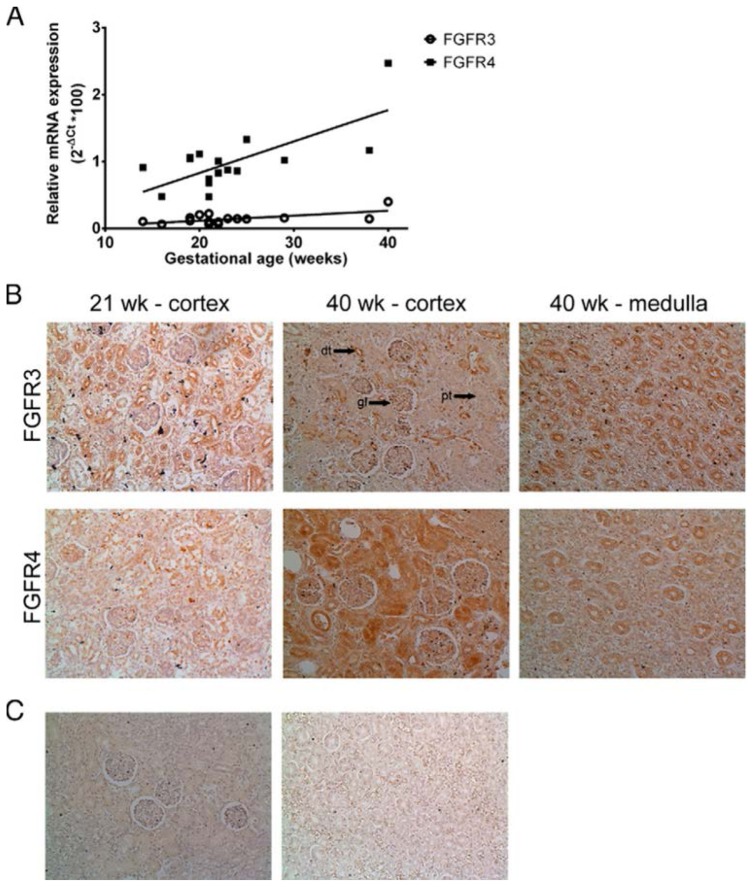
To elucidate the impact of the observed FGFR4 deletion as well as the transient infantile hypercalcemia, we studied the renal ontogenesis of FGFRs involved in calcium homeostasis. Fig. 2A demonstrates that the gene expression levels of both FGFR3 and FGFR4 in kidney samples significantly and positively correlate with gestational age (GA) with a calculated Pearson r of 0.63 (p = 0.0054) and 0.72 (p = 0.0008), respectively. Moreover, the expression level of both receptors steadily increases during renal development from 14 to 40 gestational weeks; yet there is a strikingly greater increase in FGFR4 gene expression as compared to FGFR3 (slope: 0.047 vs. 0.0075, p = 0.0018). An analogous immunochemical pattern was observed with light microscopy, revealing a similar amount of positively stained tubules at 21 weeks of gestation, while at a GA of 40 weeks, FGFR4 protein seems to be more abundantly present as compared to FGFR3 (Fig. 2B). Furthermore, in the fetal kidney, FGFR3 protein expression appears to be limited to the distal tubules, with some stainings in the glomeruli as well as the medulla (Fig. 2B). In contrast, FGFR4 was detected in both the proximal and distal tubules (especially at 40 gestational weeks; Fig. 2B) as well as the medulla (Fig. 2B), while the receptor was absent in the glomeruli.
Figure 2. Expression of FGFRs in the human fetal kidney.
(A)
FGFR gene expression was studied using RT-qPCR. Relative expression was calculated as (2−ΔCT)*100. Pearson correlation analysis revealed a significant association between GA and FGFR3 (
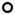 ; r = 0.63, p = 0.0054) and FGFR4 (■; r = 0.72, p = 0.0008) gene expression. (B) Immunostaining of FGFRs in the developing kidney. (C) Secondary antibody control for FGFR staining. Magnification, 20x. Arrows indicate a typical example of a glomerulus (gl), proximal tubule (pt) and distal tubule (dt).
; r = 0.63, p = 0.0054) and FGFR4 (■; r = 0.72, p = 0.0008) gene expression. (B) Immunostaining of FGFRs in the developing kidney. (C) Secondary antibody control for FGFR staining. Magnification, 20x. Arrows indicate a typical example of a glomerulus (gl), proximal tubule (pt) and distal tubule (dt).
Correlation between FGFR and Klotho expression
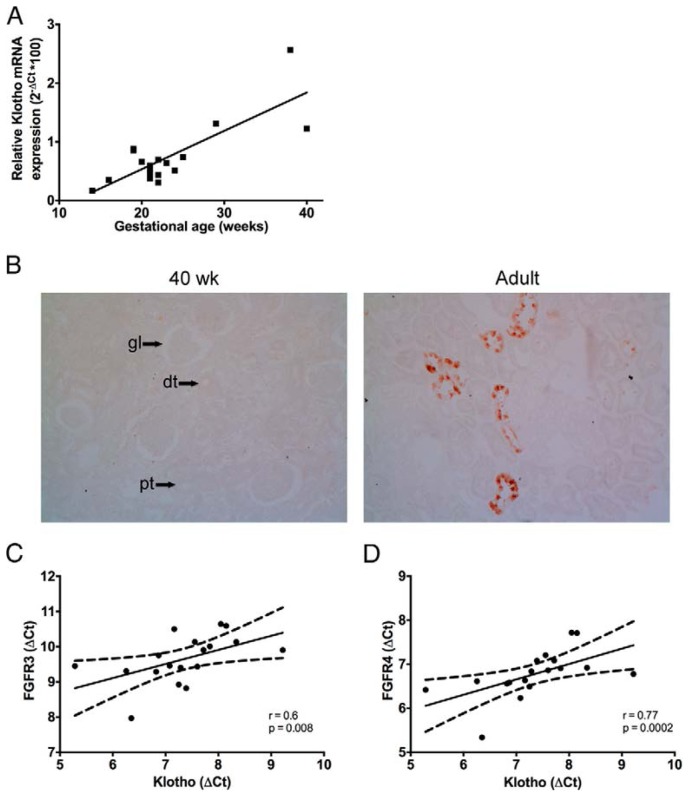
It has been previously reported that Klotho is an essential cofactor for FGF23 signaling [10,17]. As shown in Fig. 3A and 3B, Klotho mRNA and protein expression increases throughout renal development, with low mRNA levels in fetal kidneys, consistent with the lack of detectable Klotho protein in fetal and neonatal kidney samples, at variance with adult kidneys, in which Klotho protein is readily detected in renal distal tubules. A significant positive correlation was observed between Klotho mRNA and GA (calculated Pearson r = 0.80, p<0.0001). Moreover, the expression profile of Klotho mRNA significantly correlates with both FGFR3 (r = 0.6, p = 0.008, Fig. 3C) and FGFR4 (r = 0.77, p = 0.0002, Fig. 3D) as demonstrated with Spearman correlation analyses, consistent with the key role of Klotho acting as a renal co-receptor for FGF23.
Figure 3. Klotho expression in the developing kidney.
Klotho and FGFR gene expression were studied using RT-qPCR. (A) Relative Klotho expression was calculated as (2−ΔCT)*100. Pearson correlation analysis revealed a significant association between GA (gestational age) and Klotho (r = 0.80, p < 0.0001). (B) Immunostaining of Klotho in fetal (GA of 40 weeks) and adult kidney tissue (60 years of age). Magnification, 20x. Arrows indicate a typical example of a glomerulus (gl), proximal tubule (pt) and distal tubule (dt). (C) Positive correlation between Klotho and FGFR3 (Spearman r = = 0.6, p = 0.008). (C) Positive correlation between Klotho and FGFR4 (Spearman = 0.77, p = 0.0002).
Switch in FGFR expression profile during post-natal development
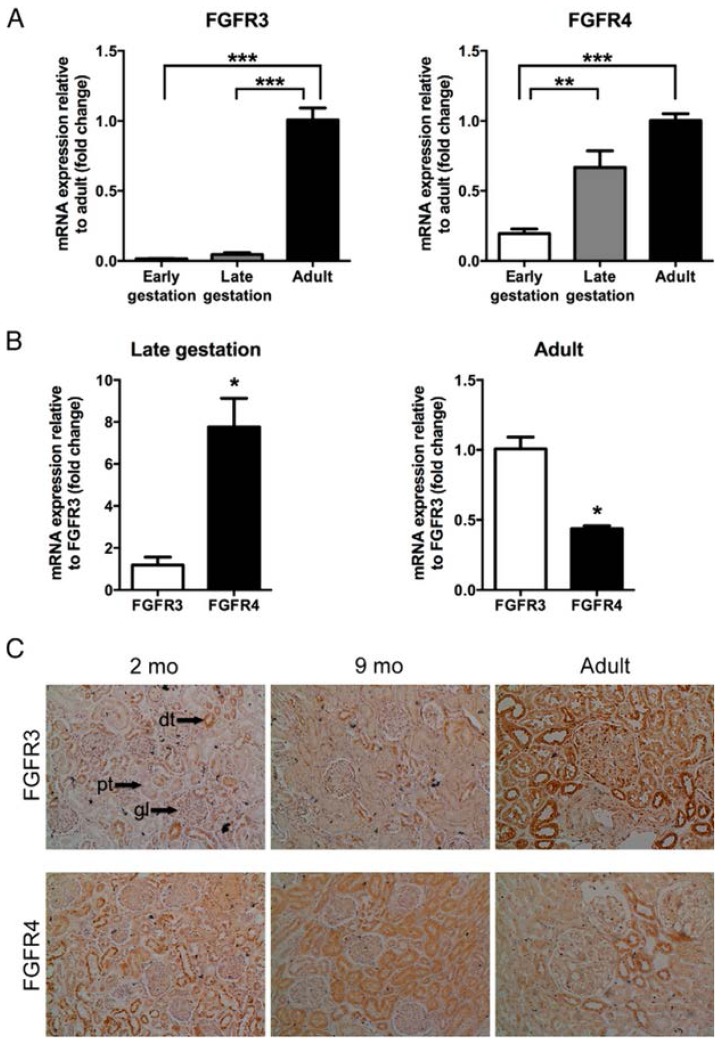
Next, we compared the FGFR expression profile in fetal, neonatal and adult kidney samples. As illustrated in Fig. 4A, renal FGFR3 gene expression was extremely low in both the early and late stage of gestation, with a significant 67-fold and 23-fold lower expression of the receptor as compared to the adult level (p<0.0001). In contrast, renal FGFR4 mRNA levels were only 5 times lower during early gestation as compared to the adult situation (p = 0.004), while there was no significant difference in renal FGFR4 expression in the late stage of development as compared to adult tissue. Interestingly, during late gestation, relative FGFR4 expression is 7-fold higher than that of FGFR3 (p = 0.0035, Fig. 4B), whereas in adults, renal FGFR3 expression level was significantly higher than that of FGFR4 (p = 0.0029, Fig. 4B). At the protein level, immunohistochemical studies revealed similar trend with more tubules positively stained for FGFR4 than for FGFR3 at 2 and 9 months of age. Moreover, FGFR3 and FGFR4 protein localization was similar in the neonatal kidney as compared to fetal kidney. Conversely, FGFR3 protein expression was markedly increased in the adult kidney (60 years old), and was detected in proximal and distal tubules as well as glomeruli, whereas there was a strong decline in the number of FGFR4 positive tubules (Fig. 4C). These findings indicate a major molecular switch in the respective contribution of both FGFRs in FGF23 signaling during human renal development.
Figure 4. Renal FGFR expression in fetal, neonatal and adult tissue.
FGFR gene expression was studied using RT-qPCR. (A) Expression of FGFRs in fetal (early GA of 14–16 weeks; late GA of 38–40 weeks) and adult renal tissues. Relative expression was calculated with the 2−ΔΔCT method. Data are expressed as fold change relative to adult values. Statistical analysis was performed via one-way ANOVA followed by Bonferroni’s Multiple Comparison Test. (B) FGFR4 expression in fetal (late, GA of 38–40 weeks) and adult tissue as compared to FGFR3. Relative expression was calculated with the 2−ΔΔCT method. Data are expressed as fold change relative to FGFR3 levels. Statistical analysis was performed via an unpaired Student’s t-test. Results are presented as mean ± SEM of two independent determinations performed in duplicate. *** = p < 0.0005, ** = p < 0.008, * = p < 0.004. (C) Immunostaining of FGFRs in neonatal and adult kidney tissue (60 years of age). Magnification, 20x. Arrows indicate a typical example of a glomerulus (gl), proximal tubule (pt) and distal tubule (dt).
Discussion
Sotos syndrome is a rare genetic disorder characterized by overgrowth, distinct facial features and learning disabilities. The clinical features of Sotos syndrome vary between cases and seem to be independent of genotype [3]. In addition, all features observed in microdeletion cases have also been reported in individuals with intragenic mutations [3]. Still, the individuals with 5q35 microdeletions are generally more likely to present with severe learning disabilities and less pronounced overgrowth as compared to patients with intragenic mutations [18]. Furthermore, there is a nascent amount of evidence that the phenotypic spectrum also encompasses impaired calcium homeostasis during infancy.
Here, we report a new Sotos syndrome case, due to a 5q35 microdeletion, with evanescent hypercalcemia. Since the deletion area included FGFR4, we hypothesized that dysfunction of this FGF23 receptor might account for the altered calcium levels. Endocrine calcium homeostasis is an intricate system involving several regulators and feedback loops. During hypocalcemia, PTH is stimulated causing active vitamin D levels to rise, which in turn augments intestinal calcium absorption as well as renal calcium retention [10]. In response to elevated serum calcium, osteocyte-produced FGF23 is released. Binding of FGF23 to the FGFR-Klotho complex – including either FGFR3 or FGFR4 – inhibits the synthesis of active 1,25 di(OH)-vitamin D3, both directly by reducing 1α-hydroxylase (CYP27B1) expression and increasing expression of the vitamin D degrading enzyme, 24–25 hydroxylase (CYP24) but also indirectly by decreasing PTH levels, resulting in a negative calcium balance [10,17]. Thus, changes in FGF23 signaling will directly impact calcium homeostasis. At present, both FGF23 pathway deficiency and renal FGFR ontogenesis have solely been studied in animal models and human data is lacking. Therefore, we studied the FGFR3 and FGFR4 expression profile in the developing human kidney.
Our results revealed a molecular switch in the FGFR expression profile during human renal development. These findings provide compelling evidence that heterozygous FGFR4 inactivation in humans, as observed in a 5q35 microdeletion Sotos syndrome case, might account for an impaired FGF23 signaling pathway during early life causing transient infantile hypercalcemia. This notion is corroborated by previous animal studies. For instance, Shimada et al. reported that calcium levels were elevated in Fgf23−/− mice [19], which is in accordance with the study by Yuan et al. [20], and an analogous discovery was made in Klotho deficient mice [21]. Hypercalcemia was also observed in Ffg23 and Klotho double knockout mice [22]. Moreover, Haenzi et al. described that the loss of Memo – a recently identified FGFR regulator – was associated with increased serum calcium [23]. Of interest, the only phenotypic feature that has been reported in Sotos syndrome cases with microdeletions is nephrocalcinosis [18]. Consistent with the transient hypercalcemia observed in the described case, as well as those previously reported [7,18], it is likely that alterations of calcium homeostasis might be a distinctive feature in Sotos syndrome associated with microdeletions. Moreover, Tatton-Brown et al. performed a microsatellite analysis in 33 microdeletion cases and detected a deletion of FGFR4 in 32 individuals [5], strengthening the hypothesis that a mutation in FGFR4, and subsequent impaired FGF23 signaling, is the culprit in infantile calcium imbalance.
Our study is one of the first to provide a detailed description of human renal FGFR ontogeny. Cancilla et al., demonstrated that FGFR3 and FGFR4 gene expression could be detected in the developing rat kidney and increased during development [24]. Furthermore, at embryonic day 20, FGFR3 protein expression was present in the distal tubules, whereas immunostaining was absent in the proximal tubules. In contrast, FGFR4 immunoreactivity was present in both distal and proximal tubules [24]. This expression pattern is similar to our observations. In addition, other studies have also demonstrated the expression of FGFR4 during renal development in rat and mice [25,26], and recently it was reported in a zebrafish study that fgf23 and αklotho were continuously expressed in the developing kidney [27]. Several decades ago, Partanen et al., reported the expression of both FGFR3 and FGFR4 in human fetal kidney (GA 17–18 weeks) and they described that organ-specific FGFR4 expression highly differs from other FGFRs [28]. In a follow-up study, they investigated the expression of FGFR4 in the developing mouse and, similar to our findings, they demonstrated that fgfr4 gene expression in the murine embryo steadily increased during development and was hardly detectable in newborn and 2 day old mice [29]. Clearly, temporal FGFR expression is conserved among species and is subjected to change during both early renal development as well as with aging.
The present study has some drawbacks; first of all, calcium homeostasis is a complex system involving a myriad of negative feedback loops and multiple hormones [10], but unfortunately, during the early clinical investigations serum levels of FGF23, soluble Klotho and active vitamin D have not been determined. Secondly, we could not get access to mRNA from early postnatal kidneys, limiting the latter part of our study solely to protein expression. Still, this study is the first to concisely describe postnatal serum calcium levels in an individual with Sotos syndrome, and owing to the availability of a unique and precious human kidney collection, we have been able to partially dissect and elucidate the expression profile of FGFRs during human renal development.
In conclusion, we have demonstrated the presence of transient infantile hypercalcemia in a 5q35 microdeletion case of Sotos syndrome, which included a deletion of FGFR4. Furthermore, we found that FGFR4 is highly expressed in fetal and neonatal kidney, whereas FGFR3 expression is highest in adult tissue. This indicates that there is a developmental switch in the contribution of both FGFRs to FGF23 signaling during aging, which could explain the fleeting hypercalcemia. These findings provide a novel and intriguing insight in FGF23-mediated calcium homeostasis.
Acknowledgments
This work was supported by the European Renal Association - European Dialysis and Transplant Association (ERA-EDTA; Fellowship no. ERA STF 132-2013; www.era-edta.org), and by fundings from Institut National de la Santé et de la Recherche Médicale and Université Paris Sud. Elena Levtchenko and Karel Allegaert are supported by the Fund for Scientific Research, Flanders, Belgium (FWO Vlaanderen; Grant Agreement 1801110N and 1800214N, respectively) and. The authors would like to thank Anne-Lise Delezoide (Robert-Debré University Hospital, Paris) for providing us with renal samples. The help of Jitske Jansen is also highly appreciated.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- 1.Sotos JF, Dodge PR, Muirhead D, Crawford JD, Talbot NB. Cerebral Gigantism in Childhood. A Syndrome of Excessively Rapid Growth and Acromegalic Features and a Nonprogressive Neurologic Disorder. N Engl J Med. 1964;271:109–116. doi: 10.1056/NEJM196407162710301. [DOI] [PubMed] [Google Scholar]
- 2.Cole TR, Hughes HE. Sotos syndrome: a study of the diagnostic criteria and natural history. J Med Genet. 1994;31:20–32. doi: 10.1136/jmg.31.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tatton-Brown K, Rahman N. Sotos syndrome. Eur J Hum Genet. 2007;15:264–271. doi: 10.1038/sj.ejhg.5201686. [DOI] [PubMed] [Google Scholar]
- 4.Baujat G, Cormier-Daire V. Sotos syndrome. Orphanet J Rare Dis. 2007;2:36. doi: 10.1186/1750-1172-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tatton-Brown K, Douglas J, Coleman K, Baujat G, Chandler K, Clarke A, Collins A, Davies S, Faravelli F, Firth H, Garrett C, Hughes H, Kerr B, Liebelt J, Reardon W, Schaefer GB, Splitt M, Temple IK, Waggoner D, Weaver DD, Wilson L, Cole T, Cormier-Daire V, Irrthum A, Rahman N Childhood Overgrowth Collaboration. Multiple mechanisms are implicated in the generation of 5q35 microdeletions in Sotos syndrome. J Med Genet. 2005;42:307–313. doi: 10.1136/jmg.2004.027755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saugier-Veber P, Bonnet C, Afenjar A, Drouin-Garraud V, Coubes C, Fehrenbach S, Holder-Espinasse M, Roume J, Malan V, Portnoi MF, Jeanne N, Baumann C, Héron D, David A, Gérard M, Bonneau D, Lacombe D, Cormier-Daire V, Billette de Villemeur T, Frébourg T, Bürglen L. Heterogeneity of NSD1 alterations in 116 patients with Sotos syndrome. Hum Mutat. 2007;28:1098–1107. doi: 10.1002/humu.20568. [DOI] [PubMed] [Google Scholar]
- 7.Kenny J, Lees MM, Drury S, Barnicoat A, Van’t Hoff W, Palmer R, Morrogh D, Waters JJ, Lench NJ, Bockenhauer D. Sotos syndrome, infantile hypercalcemia, and nephrocalcinosis: a contiguous gene syndrome. Pediatr Nephrol. 2011;26:1331–1334. doi: 10.1007/s00467-011-1884-z. [DOI] [PubMed] [Google Scholar]
- 8.Magen D, Berger L, Coady MJ, Ilivitzki A, Militianu D, Tieder M, Selig S, Lapointe JY, Zelikovic I, Skorecki K. A loss-of-function mutation in NaPi-IIa and renal Fanconi’s syndrome. N Engl J Med. 2010;362:1102–1109. doi: 10.1056/NEJMoa0905647. [DOI] [PubMed] [Google Scholar]
- 9.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503–533. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nabeshima Y. Discovery of alpha-Klotho unveiled new insights into calcium and phosphate homeostasis. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:125–141. doi: 10.2183/pjab/85.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M, Goetz R, Mohammadi M, Baum M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297:F282–291. doi: 10.1152/ajprenal.90742.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gattineni J, Twombley K, Goetz R, Mohammadi M, Baum M. Regulation of serum 1,25(OH)2 vitamin D3 levels by fibroblast growth factor 23 is mediated by FGF receptors 3 and 4. Am J Physiol Renal Physiol. 2011;301:F371–377. doi: 10.1152/ajprenal.00740.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinerie L, Viengchareun S, Delezoide AL, Jaubert F, Sinico M, Prevot S, Boileau P, Meduri G, Lombès M. Low renal mineralocorticoid receptor expression at birth contributes to partial aldosterone resistance in neonates. Endocrinology. 2009;150:4414–4424. doi: 10.1210/en.2008-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levtchenko E, Schoeber J, Jaeken J. Genetic disorders of renal phosphate transport. N Engl J Med. 2010;363:1774. doi: 10.1056/NEJMc1008407. author reply 1774–1775. [DOI] [PubMed] [Google Scholar]
- 17.Gattineni J, Baum M. Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatr Nephrol. 2010;25:591–601. doi: 10.1007/s00467-009-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tatton-Brown K, Douglas J, Coleman K, Baujat G, Cole TR, Das S, Horn D, Hughes HE, Temple IK, Faravelli F, Waggoner D, Turkmen S, Cormier-Daire V, Irrthum A, Rahman N Childhood Overgrowth Collaboration. Genotype-phenotype associations in Sotos syndrome: an analysis of 266 individuals with NSD1 aberrations. Am J Hum Genet. 2005;77:193–204. doi: 10.1086/432082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan Q, Sitara D, Sato T, Densmore M, Saito H, Schüler C, Erben RG, Lanske B. PTH ablation ameliorates the anomalies of Fgf23-deficient mice by suppressing the elevated vitamin D and calcium levels. Endocrinology. 2011;152:4053–4061. doi: 10.1210/en.2011-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 22.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23) -mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haenzi B, Bonny O, Masson R, Lienhard S, Dey JH, Dey JH, Kuro-o M, Hynes NE. Loss of Memo, a novel FGFR regulator, results in reduced lifespan. FASEB J. 2013 doi: 10.1096/fj.13-228320. [DOI] [PubMed] [Google Scholar]
- 24.Cancilla B, Ford-Perriss MD, Bertram JF. Expression and localization of fibroblast growth factors and fibroblast growth factor receptors in the developing rat kidney. Kidney Int. 1999;56:2025–2039. doi: 10.1046/j.1523-1755.1999.00781.x. [DOI] [PubMed] [Google Scholar]
- 25.Ford MD, Cauchi J, Greferath U, Bertram JF. Expression of fibroblast growth factors and their receptors in rat glomeruli. Kidney Int. 1997;51:1729–1738. doi: 10.1038/ki.1997.238. [DOI] [PubMed] [Google Scholar]
- 26.Cool SM, Sayer RE, van Heumen WR, Pickles JO, Nurcombe V. Temporal and spatial expression of fibroblast growth factor receptor 4 isoforms in murine tissues. Histochem J. 2002;34:291–297. doi: 10.1023/a:1023326524562. [DOI] [PubMed] [Google Scholar]
- 27.Mangos S, Amaral AP, Faul C, Juppner H, Reiser J, Wolf M. Expression of fgf23 and alphaklotho in developing embryonic tissues and adult kidney of the zebrafish, Danio rerio. Nephrol Dial Transplant. 2012;27:4314–4322. doi: 10.1093/ndt/gfs335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Partanen J, Makela TP, Eerola E, Korhonen J, Hirvonen H, Claesson-Welsh L, Alitalo K. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 1991;10:1347–1354. doi: 10.1002/j.1460-2075.1991.tb07654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korhonen J, Partanen J, Alitalo K. Expression of FGFR-4 mRNA in developing mouse tissues. Int J Dev Biol. 1992;36:323–329. [PubMed] [Google Scholar]