Abstract
Mechanical thrombectomy using the stent retriever has been proven to be effective for select patients with acute ischemic stroke. We evaluated our early experience using the device after its approval in 2014 in Japan, with a special emphasis on the occlusion site. Fifty consecutive endovascular revascularization procedures for treating anterior acute large vessel occlusion were performed using the Trevo ProVue as the first-line device at our institute between April 2015 and March 2016. Focusing on the involvement of the M1–M2 bifurcation with deployment or retrieval of the stent retriever, we regarded the middle cerebral artery M1 mid-portion as the boundary and divided the cases into proximal (n = 26) and distal (n = 24) groups. We assessed the overall clinical outcome and compared the outcome between the two groups. Among 50 patients (median age, 80 years; National Institutes of Health Stroke Scale score (NIHSS) score, 20), successful (modified Thrombolysis in Cerebral Infarction score (TICI) 2b or 3) or complete revascularization (TICI 3) was achieved in 41 patients (82%; 88% in the proximal group vs 75% in the distal group, P = 0.28) and in 27 patients (54%; 73% vs 33%, P = 0.01), respectively. Symptomatic intracranial hemorrhage occurred in three patients (6%; 4% vs 8%, P = 0.60). A good outcome (mRS score 0 to 2) was obtained in 25 patients at 90 days (50%; 54% vs 46%, P = 0.78). Mechanical thrombectomy using the Trevo ProVue was safe and effective in patients with acute cerebral artery occlusion, especially for proximal occlusions. The efficacy of the procedure for distal occlusions was somewhat inferior to those for proximal occlusions, which might be resolved by next generation devices.
Keywords: acute ischemic stroke, endovascular treatment, mechanical thrombectomy, stent retriever
Introduction
Recent randomized controlled trials (RCTs) have established a clinical benefit of mechanical thrombectomy for adequately selected patients with acute cerebral artery occlusion in the anterior circulation.1–5) These studies all used the stent retriever as the main device, which was considered to be one reason for the procedure’s clinical benefit. In Japan, the stent retriever was approved for use in performing mechanical thrombectomy in July 2014. Two types of stent retriever device were approved: the Trevo ProVue (Stryker, Kalamazoo, MI) and the Solitaire-FR (eV3/Covidien, Irvine, CA). Although a large number of patients treated by endovascular revascularization before the approval of mechanical thrombectomy has been reported, there have been few reports of mechanical thrombectomy using the stent retriever in the real-world setting in Japan.6)
In major studies including RCTs, the target vessels were mainly proximal artery occlusions. There are limited reports concerning mechanical thrombectomy using the stent retriever for distal artery occlusions, such as the M2 portion of the middle cerebral artery (MCA).7–9) Achieving satisfactory revascularization of small caliber and highly tortuous distal vessels using the stent retriever might be difficult and there is a potential risk of complications such as subarachnoid hemorrhage. Even for distal M1 occlusions, the stent retriever is usually deployed from the M2 with the stent retriever flexed, which could result in insufficient expansion of the stent strut or could cause straightening of the frail curve of the M1–M2 bifurcation. Therefore, problems, such as insufficient expansion of the stent strut or the straightening of the vessels, may occur in deployment and retrieval of the stent retriever near or through the M1–M2 bifurcation. For this reason, a novel technique or device that overcomes these barriers is needed. Conversely, there seems to be no technical difference in the deployment or retrieval of the stent retriever between the occlusions of the proximal M1 and the terminus of the internal carotid artery (ICA) that involves the robust curve of the M1-IC bifurcation.
There were two goals for this study. First, we sought to report our early experiences with using the Trevo ProVue stent retriever in Japan for mechanical thrombectomy in 50 consecutive patients with acute anterior cerebral artery occlusions after its approval in Japan. We also assessed whether the efficacy and safety of mechanical thrombectomy using the stent retriever differed depending on the involvement of the M1–M2 bifurcation with deployment or retrieval of the stent retriever.
Materials and Methods
Patient selection
Between April 2015 and March 2016 at our institute, 50 consecutive endovascular revascularizations were performed to treat acute cerebral artery occlusion in the anterior circulation. The target vessels included acute large vessel occlusions in the anterior circulation, including the ICA as well as the M1 and M2 portion of the MCA. Our selection criteria for patients receiving endovascular revascularization included any neurological deficit and <8 h from symptom onset when known or <24 h from the time the patient was last seen to be well for those when the time of symptom onset was unknown. There were no restrictions on age of the patient as the inclusion criteria. Both computed tomography (CT) and magnetic resonance imaging (MRI) were performed immediately after admission. Patients with a score of ≥6 for the Alberta Stroke Program Early Computed Tomography Score (ASPECTS) or on ASPECTS-diffusion weighted imaging (DWI) were candidates for endovascular revascularization. Intravenous tissue plasminogen activator (IV tPA) was administered according to the Japanese Guidelines for the Management of Stroke.10) In cases treated by IV tPA, endovascular treatment was performed if a subsequent diagnostic angiography did not show recanalization after the initiation of IV tPA. Informed consent was obtained from each patient or family member before administering IV tPA and performing the endovascular procedure.
Endovascular procedures
All procedures were performed with minimal conscious sedation (dexmedetomidine or pentazocine). The Trevo ProVue stent retriever, which is a one-size stent retriever, was used as the first-line device for performing mechanical thrombectomy. The standard endovascular procedures performed at our institute were as follows. An intravenous heparin bolus (2000 to 5000 U) was given to elevate the activated clotting time to the range of 1.5- to 2.5-fold above baseline values. A nine French balloon guide catheter was navigated into the ICA via transfemoral access. A Trevo Pro18MC microcatheter with a Transend-EX ST microguidewire (both from Stryker), which was shaped to a J shape taking care not to perforate the vessel, was navigated into the portion distal to the occluded site crossing the clot. Simultaneous angiography from the guiding catheter and the microcatheter was performed to determine the length of the clot. The Trevo ProVue stent retriever was deployed using the “Push and Fluff technique”.11) For a short clot lesion with vascular tortuosity, the Trevo stent retriever was deployed using the “Half-Trevo technique,” our novel technique for deployment. This technique involves the least amount of deployment that is sufficient to cover the clot with the purpose of minimizing damage to the vessel (Fig. 1). If a maximum of three passes of the Trevo device failed to revascularize the vessel, additional endovascular procedures, including percutaneous transluminal angioplasty (PTA), aspiration by Penumbra catheter (Penumbra Inc., Alameda, CA), mechanical disruption with a microcatheter and microguidewire, and intra-arterial thrombolysis using urokinase, were attempted. When extracranial ICA stenosis was confirmed, and there was difficulty with crossing the devices for mechanical thrombectomy, carotid artery stenting (CAS) was performed before mechanical thrombectomy. Just after the procedure, an 8Fr Angio-Seal (St. Jude Medical, Inc., Saint Paul, MN) was used for hemostasis at the femoral artery puncture site in all cases. The heparin was not reversed with protamine except in the case of the patients with intracranial hemorrhage on post-procedural CT.
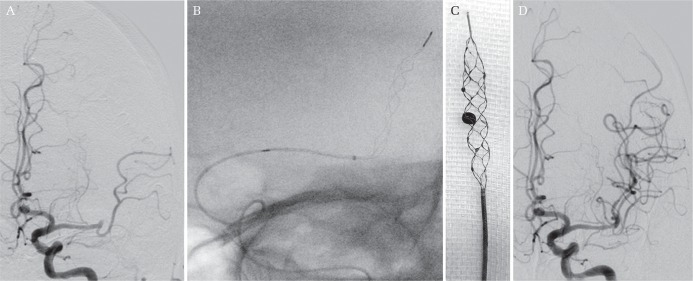
Fig. 1.
Half-Trevo technique. A: Initial angiography showing left M2 occlusion. B: After deployment of the Trevo ProVue, fluoroscopy demonstrated satisfactory configuration of the stent retriever wall, although the unsheathing length of the microcatheter was half the size of the whole stent retriever component. This partial deployment of the stent retriever, the “Half-Trevo technique,” could shorten the length of stent retriever deployment at a tortuous vessel with a short clot lesion. This technique might contribute to the relief of stress placed on tortuous vessels. C: After retrieval of the Trevo ProVue, the clot was removed with the stent retriever. D: After mechanical thrombectomy, angiography showed successful revascularization.
Postoperative treatment
Just after the endovascular procedures, intravenous anticoagulation therapy was not performed except in patients that were treated by additional stenting for carotid stenosis or intracranial artery stenosis, or had demonstrated the least acute ischemic infarction on diffusion weighted imaging (DWI) upon admission. After assessment of hemorrhagic changes on CT and infarction on DWI the next day, oral anticoagulants were given for cardiogenic embolism if there were no hemorrhagic changes without massive infarction. For atherosclerotic lesions, most of which were treated with PTA or CAS, oral antiplatelets were given.
Outcome assessment
Successful recanalization was defined as modified Thrombolysis in Cerebral Infarction score (TICI) 2b or 3. A good outcome was defined as a modified Rankin Scale score (mRS) of 0 to 2 at 90 days. Routine post-procedural imaging included CT immediately and at 24 h after treatment and MRI at 24 h and 7 days after treatment. Symptomatic intracranial hemorrhage was defined as subarachnoid hemorrhage or intracerebral hemorrhage combined with an increased National Institutes of Health Stroke Scale score (NIHSS) of ≥4 points from baseline within 24 h of treatment.
Comparison of the occluded site
We hypothesized that there are technical problems in the deployment or retrieval of the stent retriever while treating distal artery occlusions near or through the M1–M2 bifurcation compared to more proximal occlusions. To assess whether the effectiveness and safety of mechanical thrombectomy differ depending on the involvement of the M1–M2 bifurcation, we defined the M1 mid-portion as the midpoint of the sphenoidal segment (M1) of the MCA from the carotid bifurcation to the genu at the limen insula described by Krayenbühl et al., and regarded it as the boundary between the proximal and distal portions and divided the cases into a proximal group (intracranial ICA, and proximal M1) and a distal group (distal M1, and M2).12) We compared the outcome between the two groups.
Statistical analysis
Descriptive statistics are presented as the median with interquartile range (IQR), and were compared using Welch’s two-sample t-test for continuous variables and Wilcoxon rank sum test for discrete variables. The proportion of patients with each parameter was compared using Fisher’s exact test. Probability values of P < 0.05 were considered to be statistically significant. Statistical analysis was performed with free open-source software (R3.1.1; R Foundation for Statistical Computing; http://www.r-project.org ).
Results
Preprocedural findings
The baseline characteristics of the 50 patients analyzed in this study (24 men, 26 women; median age, 80 years [IQR, 72–86]) are summarized in Table 1. Good activity (mRS 0–2) before onset was confirmed in 45 patients (90%). The median baseline NIHSS was 20 and ASPECTS was 8. The median time from onset or last known well time to admission was 143 min. Nine patients (18%) received IV tPA before undergoing endovascular procedures. The occlusion sites were intracranial ICA in 13 (26%), proximal M1 in 13 (26%), distal M1 in eight (16%), and M2 in 16 (32%) patients. Among 13 patients with the intracranial ICA occlusion, 11 patients had occlusions in the ICA terminus involving the proximal MCA, and the remaining two patients had occlusions in the pure distal ICA from the cavernous to supraclinoid segment.
Table 1.
Characteristics, treatment results, and the outcomes of all patients
| All (n = 50) | Proximal group (n = 26) | Distal group (n = 24) | P value† | |
|---|---|---|---|---|
| Age‡ | 80 (72–86) | 81 (71–87) | 79 (73–85) | 0.81 |
| Male | 24 (48%) | 12 (46%) | 12 (50%) | 1.00 |
| mRS 0–2 before onset | 45 (90%) | 21 (81%) | 24 (100%) | 0.051 |
| Baseline NIHSS‡ | 20 (11–23) | 22 (15–26) | 12 (8–20) | 0.003* |
| Baseline ASPECTS‡ | 8 (7–9) | 8 (7–9) | 8 (7–9) | 0.90 |
| Occlusion site | ||||
| Intracranial ICA | 13 (26%) | 13 (50%) | – | – |
| Proximal M1 | 13 (26%) | 13 (50%) | – | – |
| Distal M1 | 8 (16%) | – | 8 (33%) | – |
| M2 | 16 (32%) | – | 16 (67%) | – |
| IV tPA | 9 (18%) | 4 (15%) | 5 (21%) | 0.72 |
| Onset to admission, min‡ | 143 (86–322) | 135 (92–263) | 146 (63–366) | 0.87 |
| Admission to groin puncture‡ | 90 (84–108) | 89 (82–101) | 94 (88–116) | 0.51 |
| Groin puncture to successful revascularization, min‡ | 41 (28–53) | 41 (26–47) | 45 (29–65) | 0.13 |
| Groin puncture to sheath removal, min‡ | 66 (50–95) | 54 (46–74) | 81 (59–105) | 0.010* |
| CAS | 2 (4%) | 1 (4%) | 1 (4%) | 1.00 |
| Trevo device deployment | 48 (96%) | 26 (100%) | 22 (92%) | 0.23 |
| Total passes of Trevo‡ | 2 (1–2) | 2 (1–2) | 2 (1–2) | 0.96 |
| Cases using Half-Trevo technique | 14 (28%) | 3 (12%) | 11 (46%) | 0.011* |
| Cases needing additional intracranial procedures | 23 (46%) | 8 (31%) | 15 (63%) | 0.046 |
| Percutaneous transluminal angioplasty | 9 (18%) | 4 (15%) | 5 (21%) | 0.72 |
| Mechanical disruption | 8 (16%) | 2 (7%) | 6 (25%) | 0.13 |
| Aspiration by Penumbra catheter | 6 (12%) | 3 (12%) | 3 (13%) | 1.00 |
| Intra-arterial thrombolysis using urokinase | 6 (12%) | 1 (4%) | 5 (21%) | 0.09 |
| Successful revascularization (TICI 2b or 3) | 41 (82%) | 23 (88%) | 18 (75%) | 0.28 |
| Complete revascularization (TICI 3) | 27 (54%) | 19 (73%) | 8 (33%) | 0.010* |
| Distal embolisms | 6 (12%) | 3 (12%) | 3 (13%) | 1.00 |
| Symptomatic intracranial hemorrhage | 3 (6%) | 1 (4%) | 2 (8%) | 0.60 |
| mRS at 90 days | 3 (1–4) | 2 (1–4) | 3 (2–4) | 0.53 |
| mRS 0–2 at 90 days | 25 (50%) | 14 (54%) | 11 (46%) | 0.78 |
| mRS 6 at 90 days | 2 (4%) | 2 (8%) | 0 (0%) | 0.49 |
ASPECTS: Alberta Stroke Program Early Computed Tomography Score, CAS: carotid artery stenting, ICA: internal carotid artery, IV tPA: intravenous tissue plasminogen activator, M1: M1 portion of middle cerebral artery, M2: M2 portion of middle cerebral artery, mRS: modified Rankin Scale score, NIHSS: National Institutes of Health Stroke Scale score, PTA: percutaneous transluminal angioplasty, TICI: modified Thrombolysis In Cerebral Infarction score,
Statistically significant,
P-value of the difference between the proximal and distal groups,
Median (interquartile range).
An intracranial proximal artery occlusion (proximal group) was observed in 26 patients (52%) and the remaining 24 patients (48%) were diagnosed with a distal artery occlusion (distal group). There was no significant difference in baseline characteristics between the proximal and distal groups except for the baseline NIHSS (22 in the proximal group vs 12 in the distal group, P = 0.003). Although not statistically significant, there were fewer patients who had good activity (mRS 0–2) before onset in the proximal group compared with the distal group (81% in the proximal group vs 100% in the distal group, P = 0.051).
Endovascular procedures
Endovascular procedures are summarized in Table 1. CAS was performed in a total of two patients (4%): before the intracranial procedure in a patient and after the intracranial procedure in the other patient. The Trevo device was used in 48 patients (96%), but not in patients in whom navigation of the microcatheter was difficult or in those with an aneurysm proximal to the occluded site. The median number of total passes of the Trevo device was two. An intracranial procedure other than the Trevo device was needed in 23 patients (46%). Successful revascularization (TICI 2b or 3) was achieved in 41 patients (82%). Complete revascularization (TICI 3) was achieved in 27 patients (54%). The median time from a groin puncture to successful revascularization was 41 min. The median procedure time from groin puncture to sheath removal was 66 min. Distal embolism during the procedure occurred in six patients (12%). Symptomatic intracranial hemorrhage occurred in three patients (6%). There was only one (2%) complication associated with the femoral artery puncture. One patient presented with severe retroperitoneal hemorrhage on the next day after the procedure and required blood transfusion.
There were significant differences in the results of the endovascular treatment between the proximal and distal groups in relation to the median procedure time, additional endovascular procedures, deployment with the “Half-Trevo technique”, and the rate of complete revascularization (TICI 3). The median procedure time from groin puncture to sheath removal was 54 min in the proximal group and 81 min in the distal group (P = 0.010). Additional intracranial procedures beyond the Trevo device were needed in eight patients (31%) in the proximal group and in 15 patients (63%) in the distal group (P = 0.046). In the proximal group, PTA and aspiration by Penumbra were the frequent additional procedures and used in four patients (15%) and three patients (12%), respectively. In the distal group, mechanical disruption, PTA, and intra-arterial thrombolysis using urokinase were the frequent additional procedures and used in six patients (25%), in five patients (21%), and five patients (21%), respectively. The Trevo was deployed using the “Half-Trevo technique” in three patients (12%) in the proximal group and in 11 patients (46%) in the distal group (P = 0.011). Complete revascularization (TICI 3) was achieved in 19 patients (73%) in the proximal group and eight patients (33%) in the distal group (P = 0.010). Conversely, there were no significant differences between the proximal and distal groups in terms of the rate of successful revascularization (TICI 2b or 3) (88% vs 75%, respectively, P = 0.28) and symptomatic intracranial hemorrhage (4% vs 8%, respectively, P = 0.60).
Outcomes
At 90 days after treatment, the median mRS was three (IQR, 1–4) in all 50 patients. A good outcome (mRS 0 to 2) was obtained in 25 patients (50%) and two patients (4%) had died (mRS 6). There was no significant difference between the proximal and distal groups in terms of good outcome (54% vs 46%, respectively, P = 0.78) or death (8% vs 0%, respectively, P = 0.49).
Discussion
This study analyzed our early experience in Japan of performing mechanical thrombectomy using the Trevo ProVue stent retriever. In doing so, it revealed that the procedure was effective and safe for patients with acute ischemic stroke caused by anterior large vessel occlusion. For distal occlusions, however, the efficacy of the device in achieving complete recanalization was inferior to the efficacy of the device used for treating proximal occlusions.
In this study, the rate of successful revascularization (TICI 2b or 3) was 82%, the rate of symptomatic intracranial hemorrhage was 6%, and a good outcome (mRS 0 to 2) was obtained in 50% of all patients. These results are comparable with those of recent RCTs (Table 2).1–5) In a reflection of real-world practice outside RCTs, the median age of the patients in the present study was considerably higher than those in the RCTs, and baseline NIHSS and ASPECTS in the present study were slightly worse than those in the RCTs. The proportions of specific occluded sites in our study were almost similar to those in the RCTs, but the proportion of M2 occlusion was higher in our study. The rate of IV tPA in our study was considerably low (18%) compared with the RCTs. Time from onset to groin puncture, time from onset to revascularization, and the rate of symptomatic intracranial hemorrhage in the present study were comparable with those in the RCTs. In general, the results of our early experience using the stent retriever after its approval in Japan appear to be acceptable and comparable with those in recent RCTs.
Table 2.
Comparison with RCTs
| Our study (n = 50) | RCTs | |||||
|---|---|---|---|---|---|---|
| MR CLEAN1 (n = 233) | ESCAPE2 (n = 165) | EXTEND-IA3 (n = 35) | SWIFT PRIME4 (n = 98) | REVASCAT5 (n = 103) | ||
| Age* | 80 | 66 | 71 | 69 | 65 | 66 |
| Baseline NIHSS* | 20 | 17 | 16 | 17 | 17 | 17 |
| Baseline ASPECTS* | 8 | 9 | 9 | Not reported | 9 | 7 |
| Occlusion site | ||||||
| ICA | 26% | 26% | 28% | 31% | 17% | 26% |
| M1 | 52% | 66% | 68% | 51% | 68% | 65% |
| M2 | 32% | 8% | 4% | 17% | 14% | 10% |
| IV tPA | 18% | 87% | 73% | 100% | 100% | 68% |
| Onset to groin puncture, min* | 246 | 260 | 200 | 210 | 224 | 269 |
| Onset to revascularization, min* | 276 | 332 | 241 | 248 | 252 | 355 |
| Successful revascularization (TICI 2b or 3) | 82% | 59% | 72% | 86% | 83% | 66% |
| Symptomatic intracranial hemorrhage | 6% | 8% | 4% | 0% | 0% | 2% |
| mRS 0–2 at 90 days | 50% | 33% | 53% | 71% | 60% | 44% |
| mRS 6 at 90 days | 4% | 21% | 10% | 9% | 9% | 18% |
ASPECTS: Alberta Stroke Program Early Computed Tomography Score, ICA: internal carotid artery, IV tPA: intravenous tissue plasminogen activator, M1: M1 portion of middle cerebral artery, M2: M2 portion of middle cerebral artery, mRS: modified Rankin Scale score, RCT: randomized controlled trial, NIHSS: National Institutes of Health Stroke Scale score, TICI: modified Thrombolysis In Cerebral Infarction score,
median.
In previous major studies including RCTs, the targets were mainly proximal artery occlusions. The highly effective reperfusion evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration, a recent meta-analysis of pooled data from five RCTs (MR CLEAN, ESCAPE, EXTEND-IA, SWIFT-PRIME, and REVASCAT), reported that the treatment effect favoring endovascular thrombectomy over control was most obvious in the patients with ICA occlusions and was also obvious in the patients with M1 occlusions; on the other hand, the effect was not obvious in the patients with M2 occlusions although these trails included only a small number of the patients with M2 occlusions.13) In accordance with the results of the HERMES, the current study showed that mechanical thrombectomy could contribute greatly to the outcome of the patients with the proximal occlusions because 54% of the patients with ICA and proximal M1 occlusions had reached a good outcome (mRS score 0 to 2) at 90 days despite of their higher baseline NIHSS scores. There were only three case series of mechanical thrombectomy using the stent retriever for M2 occlusion, involving more than 50 patients.7–9) These case series reported that the rate of successful revascularization (TICI 2b or 3) was 77% to 82% and the rate of symptomatic intracranial hemorrhage was 0% to 9% for M2 occlusion, which were comparable with the results for M1 occlusion in each study.
However, problems with the deployment and retrieval of the stent retriever near or through the M1–M2 bifurcation may occur. For this reason, a novel technique or device that overcomes these barriers is needed. The use of the stent retriever near or through the frail curve of the M1–M2 bifurcation could result in insufficient expansion of the stent strut or the straightening of the vessels, which might produce unfavorable results, such as losing the retrieved clot and subarachnoid hemorrhage. In the current study, we regarded distal M1 occlusions as the same entity as M2 occlusions because the stent retriever is usually deployed from the M2 in treating the distal M1 occlusion and might have similar problems associated with the M1–M2 bifurcation. Conversely, there seems to be no technical difference in the use of the stent retriever between the occlusions of the proximal M1 and the terminalis of the ICA that involves the robust curve of the M1-IC bifurcation. Therefore, we divided the cases into the proximal and distal groups according to the involvement of the M1–M2 bifurcation with the deployment or retrieval of the stent retriever, and evaluated the difference between the groups. We found significant differences in the rates of complete recanalization (73% in the proximal group vs 33% in the distal group, P = 0.010), additional endovascular procedures (31% vs 63%, P = 0.046), and the procedural time from groin puncture to sheath removal (54 min vs 81 min, P = 0.010) between the groups. The rates of good outcome at 90 days after treatment were similar between the groups; however, the device’s efficacy for treating distal occlusions was considered to be somewhat inferior since the rate of patients with good activity before onset in the proximal group was less than that in the distal group, although the difference did not reach statistical significance (81% in the proximal group vs 100% in the distal group, P = 0.051). Moreover, proximal M1 occlusions originally had an unfavorable anatomical factor that they usually involved the lenticulostriate perforators, contrary distal M1 occlusions.14) The reason why the considerably favorable outcome could be obtained in the proximal group against these disadvantages might be that we could achieve early revascularizations with high rates of complete recanalization and that our classification of the proximal M1 was different from the previous report.14) The reason for the poor results of treating distal occlusions could be technical problems in using the stent retriever near or through the M1–M2 bifurcation, as was suggested in our hypothesis. However, contrary to our hypothesis, there was no significant difference in the rate of the symptomatic hemorrhage between the two groups.
The Trevo ProVue is a closed-cell stent retriever thrombectomy device, which is characterized by fully radiopaque strut that is radially oriented for promotion of clot integration. The Solitaire is the most commonly used stent retriever in the recent RCTs; however, its strut is not radiopaque. We used the Trevo ProVue as the first-line thrombectomy device since the visibility of the strut may contribute to the efficacy and safety in the development and retrieval of the stent retriever. Haussen et al. reported that “Push and Fluff technique” utilizing the visibility and design was effective in the clot retrieval.11) In the current study, we also used our novel technique for deployment, the “Half-Trevo technique”. This technique involves the least amount of deployment that is sufficient to cover the clot with the purpose of minimizing damage to the vessel for a short clot lesion with vascular tortuosity. The Trevo could be deployed partially because of its cell design and be well visualized (Fig. 1). This “Half-Trevo technique,” which enables shortening the length of stent retriever deployment, might contribute to the relief of stress placed on tortuous vessels. However, the Trevo ProVue we used was a one-sized device with a diameter of 4 mm and a length of 20 mm and its diameter might be too large for distal vessels. Our results suggest the need for improved devices for distal occlusions to achieve a more satisfactory revascularization. It has been reported that the use of the Trevo XP 3 × 20 mm retriever (“Baby Trevo”) is feasible for distal artery occlusions.15) This device has recently been approved in Japan and is expected to be effective for small and tortuous distal vessels.
There are some limitations to this study. First, this study involved a retrospective analysis of a case series containing variable baseline characteristics. Second, the small sample size resulted in lower power for the detection of significant differences between the proximal and distal groups. A further larger scale study is needed to clarify the present insights regarding treating distal occlusions.
Conclusions
Our single-center initial experience showed that mechanical thrombectomy using the Trevo ProVue was safe and effective in patients with acute cerebral artery occlusion, especially for proximal occlusions. Although safety was guaranteed by careful procedures, such as the least deployment of the stent retriever, the efficacy in treating the distal occlusions was somewhat inferior to those for proximal occlusions, which might be resolved by next generation devices.
Footnotes
Conflicts of Interest Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. All authors who are members of The Japan Neurosurgical Society (JNS) have completed the self-reported Conflicts of Interest Disclosure Statement Forms through the website for JNS members.
References
- 1). Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW, MR CLEAN Investigators : A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372: 11– 20, 2015. [DOI] [PubMed] [Google Scholar]
- 2). Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD, ESCAPE trial investigators : Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372: 1019– 1930, 2015. [DOI] [PubMed] [Google Scholar]
- 3). Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM, EXTEND-IA Investigators : Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372: 1009– 1018, 2015. [DOI] [PubMed] [Google Scholar]
- 4). Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R, SWIFT PRIME Investigators : Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372: 2285– 2295, 2015. [DOI] [PubMed] [Google Scholar]
- 5). Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A, REVASCAT Trial Investigators : Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 372: 2296– 2306, 2015. [DOI] [PubMed] [Google Scholar]
- 6). Hayakawa M, Yamagami H, Sakai N, Matsumaru Y, Yoshimura S, Toyoda K, JR-NET study group : Endovascular treatment of acute stroke with major vessel occlusion before approval of mechanical thrombectomy devices in Japan: Japanese registry of neuroendovascular therapy (JR-NET) and JR-NET 2. Neurol Med Chir (Tokyo) 54: 23– 31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Munich SA, Hall SL, Cress MC, Rangel-Castilla L, Snyder KV, Hopkins LN, Siddiqui AH, Levy EI: To Treat or Not to Treat M2 Occlusions? The Question (and Answer) From a Single Institution. Neurosurgery 79: 428– 436, 2016. [DOI] [PubMed] [Google Scholar]
- 8). Flores A, Tomasello A, Cardona P, de Miquel MA, Gomis M, Garcia Bermejo P, Obach V, Urra X, Martí-Fàbregas J, Cánovas D, Roquer J, Abilleira S, Ribó M, Catalan Stroke Code and Reperfusion Consortium Cat-SCR : Endovascular treatment for M2 occlusions in the era of stentrievers: a descriptive multicenter experience. J Neurointerv Surg 7: 234– 237, 2015. [DOI] [PubMed] [Google Scholar]
- 9). Coutinho JM, Liebeskind DS, Slater LA, Nogueira RG, Baxter BW, Levy EI, Siddiqui AH, Goyal M, Zaidat OO, Davalos A, Bonafé A, Jahan R, Gralla J, Saver JL, Pereira VM: Mechanical thrombectomy for isolated M2 occlusions: A post hoc analysis of the STAR, SWIFT, and SWIFT PRIME studies. AJNR Am J Neuroradiol 37: 667– 672, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Shinohara Y, Yamaguchi T: Outline of the Japanese guidelines for the management of stroke 2004 and subsequent revision. Int J Stroke 3: 55– 62, 2008. [DOI] [PubMed] [Google Scholar]
- 11). Haussen DC, Rebello LC, Nogueira RG: Optimizating clot retrieval in acute stroke: The push and fluff technique for closed-cell stentrievers. Stroke 46: 2838– 2842, 2015. [DOI] [PubMed] [Google Scholar]
- 12). Krayenbühl H, Yaşargil MG, Huber P, Bosse G: Cerebral Angiography, London, Thieme, 1982, pp 55– 56 [Google Scholar]
- 13). Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG, HERMES collaborators : Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387: 1723– 1731, 2016. [DOI] [PubMed] [Google Scholar]
- 14). Behme D, Kowoll A, Weber W, Mpotsaris A: M1 is not M1 in ischemic stroke: the disability-free survival after mechanical thrombectomy differs significantly between proximal and distal occlusions of the middle cerebral artery M1 segment. J Neurointerv Surg 7: 559– 563, 2015. [DOI] [PubMed] [Google Scholar]
- 15). Haussen DC, Lima A, Nogueira RG: The Trevo XP 3 × 20 mm retriever (‘Baby Trevo’) for the treatment of distal intracranial occlusions. J Neurointerv Surg 8: 295– 299, 2016. [DOI] [PubMed] [Google Scholar]