Abstract
Steroids regulate cell proliferation, tissue development, and cell signaling via two pathways: a nuclear receptor mechanism and genome-independent signaling. Sperm activation, egg maturation, and steroid-induced anesthesia are executed via the latter pathway, the key components of which remain unknown. Here, we present characterization of the human sperm progesterone receptor that is conveyed by the orphan enzyme α/β hydrolase domain–containing protein 2 (ABHD2). We show that ABHD2 is highly expressed in spermatozoa, binds progesterone, and acts as a progesterone-dependent lipid hydrolase by depleting the endocannabinoid 2-arachidonoylglycerol (2AG) from plasma membrane. The 2AG inhibits the sperm calcium channel (CatSper), and its removal leads to calcium influx via CatSper and ensures sperm activation. This study reveals that progesterone-activated endocannabinoid depletion by ABHD2 is a general mechanism by which progesterone exerts its genome-independent action and primes sperm for fertilization.
According to the conventional model of steroid signaling, steroid hormones act through their corresponding genomic receptors to regulate gene expression in development, metabolism, and reproduction (1, 2). The time scale of these signaling events ranges from hours to days (2). However, there is a much faster pathway that alters ion channel activity through steroid activation of membrane receptors. Nongenomic signaling of the steroid hormone progesterone (P4) occurs on a time scale of seconds and is required for distinct physiological events, such as oocyte maturation (3) and human sperm cell activation (4, 5), and P4 likely triggers anesthesia in rodents (6). Many tissues have both nuclear and membrane progesterone receptors, which complicates identification of the latter. Because sperm are transcriptionally silent cells and, therefore, lack the nuclear progesterone receptor effect, we used human spermatozoa as a cellular model to identify the nongenomic progesterone receptor.
Progesterone is a major component of follicular fluid and is released by ovaries and cumulus cells that surround the oocyte. Nanomolar concentrations of P4 act through the nongenomic P4 receptor pathway and cause robust elevation of sperm cytoplasmic [Ca2+] (7–9) through the activation of the cation channel, CatSper (10–14). This rise in intracellular [Ca2+] leads to changes in sperm motility, known as hyperactivation, and primes sperm for acrosomal exocytosis (4, 5, 15). Here, we show that the orphan enzyme α/β hydrolase domain–containing protein 2 (ABHD2) serves as a sperm progesterone receptor. It functions as a lipid hydrolase by removing endogenous CatSper inhibitors upon association with P4. Thus, this unconventional lipid-signaling event is responsible for sperm activation and is vital for sperm fertility.
Serine hydrolases are involved in CatSper activation by progesterone
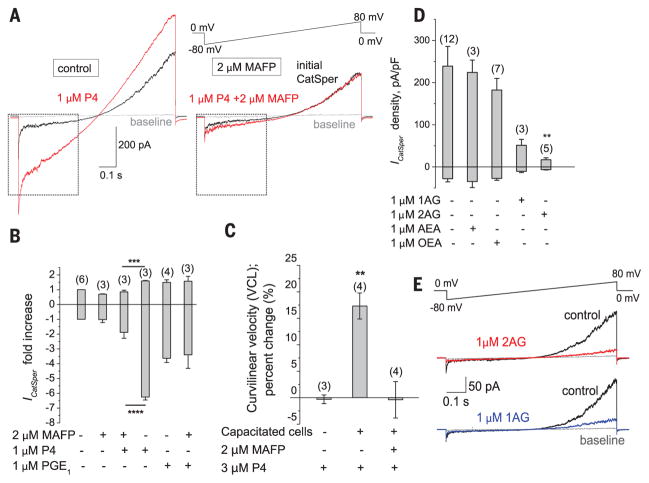
The CatSper complex comprises seven subunits that together form the sperm-specific cation channel expressed exclusively within the flagellum (11–14, 16, 17). Functional expression of CatSper in heterologous systems has not been achieved; therefore, characterization of this channel is limited to sperm. Spermatozoa are transcriptionally silent and, therefore, lack conventional P4 genomic signaling. By applying the patch-clamp technique in whole-cell mode (13, 18) and depleting sperm from all hydrophilic intracellular and extracellular second messengers, we have shown that CatSper is activated by P4 in a nongenomic manner (7). This activation could take place under two scenarios: Either P4 binds directly to CatSper or P4 binds to a separate target, which initiates lipid signaling within the sperm plasma membrane and ultimately leads to CatSper activation. To test the latter, we pharmacologically inhibited sperm metabolic serine hydrolases (mSHs), enzymes that participate in metabolism of phospholipids and endocannabinoids (19). Recording P4-induced channel activation in the absence or presence of the irreversible mSH inhibitor, methyl arachidonyl fluorophosphanate (MAFP), resulted in complete removal of P4-dependent activation of CatSper (Fig. 1, A and B) but showed no effect on basal CatSper activity (Fig. 1B and fig. S1, A and B). Moreover, MAFP treatment ablated P4-induced motility changes, which further supports the idea that physiological P4 effects are a result of indirect activation of CatSper (Fig. 1C and fig. S1C). Note that MAFP had no appreciable influence on prostaglandin E1 (PGE1)–stimulated CatSper activation, which indicates a potential direct effect of PGE1 on CatSper (Fig. 1B and fig. S1D).
Fig. 1. Inhibition of sperm mSHs removes P4-dependent CatSper activation.
(A) (Left) Representative ICatSper recorded from control human spermatozoa in the absence (black) and presence (red) of P4. (Right) ICatSper recorded from human spermatozoa pretreated with MAFP, an irreversible serine hydrolase inhibitor, for 30 min in the absence or presence of P4. (B) Fold increase of ICatSper in response to P4, PGE1, and MAFP. (C) Human capacitated spermatozoa do not hyperactivate in response to P4 if pretreated with MAFP, as recorded with computer-aided sperm analyses (CASA). (D) Arachidonoylglycerols are inhibitors of ICatSper. ICatSper densities are obtained at +80 mV (positive scale) or −80 mV (negative scale). (E) Representative ICatSper in the absence (black) or presence of the corresponding endocannabinoid. Data are represented as means ± SEM; numbers in parentheses indicate the number of experiments; and ****P < 0.0001, ***P < 0.001, **P < 0.005.
Regulation of CatSper by endocannabinoids
Lysophospholipids and endocannabinoids were previously found to be mSH substrates (19). We have therefore assessed these lipids for their involvement in CatSper regulation. Neither lysophosphatidylinositol nor lysophosphatidylethanolamine affected ICatSper (fig. S2); thus, their involvement in CatSper activation is unlikely. The family of endocannabinoids includes anandamide (AEA), oleamide (OEA), and monoacylglycerols: 2-arachidonoylglycerol (2AG) and 1-arachidonoylglycerol (1AG). Application of either AEA or OEA had no effect (Fig. 1D and fig. S2), whereas 1AG and 2AG (AGs) inhibited ICatSper (Fig. 1, D and E, and fig. S3A). The compounds differ, however, in their rate of CatSper inactivation: 2AG acts twice as fast as 1AG (fig. S3, B to D). When applied extracellularly, 2AG inhibits ICatSper with a median inhibitory concentration (IC50) of 350 nM for outward currents and 670 nM for inward currents (fig. S3, E and F). Intracellular application of 2AG could not mimic this effect (fig. S4, A and B), which highlights the importance of an extracellular pool of this lipid in the mechanism of action. Extracellular glycerol did not ablate ICatSper or its regulation by P4, which indicated that hydrophobic tails of AGs are likely responsible for their effect on ICatSper (fig. S4, B and C).
To investigate whether AGs are endogenous CatSper regulators, we performed selective lipid extraction from sperm plasma membrane followed by unbiased identification of lipids by liquid chromatography–mass spectrometry (LC-MS). To selectively extract lipids, we developed a cyclodextrin-based protocol that allows removal of steroids and single-tailed lipids, such as AGs, while leaving multitailed lipids in the membrane. We determined that spermatozoa initially have relatively large amounts of AGs and that, after normalization to internal standards and taking into account the dimensions of human sperm flagellum (45 μm by 0.5 μm, with an average volume of 8.8 fl), the concentration of AGs in a spermatozoon could reach at least 0.7 μM. Upon P4 treatment, AG content decreases to that observed in cell-free controls (figs. S4D and S5). Our results show that AGs are detected in the sperm plasma membrane and that their content is drastically reduced upon P4 stimulation. Thus, P4 appears to act through mSHs to deplete AGs in plasma membrane, which leads to CatSper activation.
P4 receptor identification
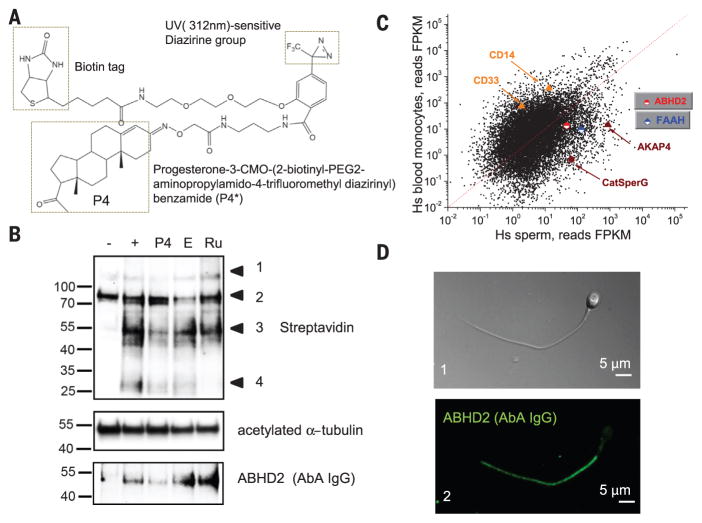
On the basis of our previous work using cell-impermeable P4 to activate ICatSper (7), the P4 target is localized to the outer leaflet of the plasma membrane. This suggests that the P4 receptor is either a peripheral membrane protein or a transmembrane protein with mSH activity. The P4 receptor is not anchored by glycosylphosphatidylinositol (GPI), because removing GPI-anchored proteins from sperm by treating them with phosphoinositide phospholipase C does not remove ICatSper P4 sensitivity (fig. S6, A and B). In order to identify human spermatozoa’s P4 binding partner, we designed a P4 analog (P4*) that is active toward CatSper, and upon activation with ultraviolet (UV) light, this analog covalently binds to its neighboring molecules (Fig. 2A; fig. S6, B and C; and fig. S7). The same compound contains a biotin tag that allows for pull-down. Next, we treated human spermatozoa with P4*, followed by UV exposure, streptavidin-assisted purification, and binding-protein identification using LC and tandem MS (LC-MS/MS) (Fig. 2B). To determine the specificity of P4* binding, mock-irradiated cells, as well as cells treated in the presence of excess unlabeled P4, were included as negative controls. To triage P4*-positive bands, we used estrogen and progestin Ru486, which do not interfere with P4 activation of CatSper (7). Pull-down experiments revealed four distinct biotin-positive bands (Fig. 2B, bands 1 to 4), although only a single ~50 kD band was present in positive controls and absent or reduced from all negative controls. MS analysis of this specific protein band resulted in 1233 unique peptides that correspond to 169 proteins (database S1). We did not identify any CatSper subunits from our six independent pull-down experiments, which suggests that the sperm P4 receptor is not a component of CatSper. Next, we searched for the candidate molecules that specifically bind P4*. We based this search on five criteria applicable to proteins identified by our pull-down: (i) the candidate should be present in all pull-down experiments except for negative controls; (ii) it should be either a transmembrane protein with an extracellular domain, or a protein tightly associated with the membrane, through a linkage distinct from GPI-anchor; and (iii) the P4 target should function independently from CatSper with mSH activity, as the P4 effect on CatSper is fully reversible in the absence of exogenous energy sources (7, 8). The enzymatic changes imposed on mSH substrates can be removed in an energy-independent manner, unlike the effect of other serine protease or amidases. Also, (iv) the P4 target should be expressed in sperm on the level of mRNA and protein to exclude epididymal-derived proteins, as testicular CatSper retains P4 sensitivity (8). Finally, (v) the P4 receptor should localize to the flagellum, because flagellar CatSper is P4 sensitive (7).
Fig. 2. Photoreactive progesterone analog P4* activates ICatSper and associates with P4 receptor.
(A) P4* consists of an active group (progesterone), a diazirine–UV-sensitive moiety, and a biotin tag that allows for pull-down of bound proteins. CMO, (O-carboxymethyl)oxime; PEG, polyethylene glycol. (B) Western blot from P4* binding experiments resolves biotinylated (P4*-bound) proteins. Lanes are labeled −, mock-irradiated cells; +, UV-treated cells; P4, P4 (20 μM); E, estrogen (0.5 μM); and Ru, Ru486 (0.5 μM). Acetylated α-tubulin (middle) staining was used as the loading control. (Bottom) The blot was probed with AbA. One experiment out of six independent pull-downs is shown. (C) ABHD2 is present in the human sperm transcriptome. mRNA sequencing (mRNA-seq) reads from human blood monocytes and sperm cells. FPKM, fragments per kilobase of transcript per million mapped reads. Red line indicates expected number of sequencing reads for genes with similar expression levels in the two samples. CatSperG and AKAP4 indicate sperm-specific transcripts; CD14 and CD33 indicate monocyte-specific transcripts. Transcripts for FAAH and abhd2 are also shown. (D) Immunostaining of human spermatozoa with AbA and a corresponding differential interference contrast (DIC) image of the same cell.
Selection of the candidates
Based on the three initial criteria, top candidates were identified: fatty-acid amide hydrolase 1 (FAAH), ABHD2, and prostatic acid phosphatase, which has a transmembrane domain containing splice isoform (TmPAP) (fig. S8). The first two candidates are members of a large family of mSHs, whereas the last candidate is an acid-activated phosphatase with hydrolase activity. We ruled out TmPAP involvement by exposing ICatSper to two extracellular pHs, 6.6 and 7.4 (fig. S2), and observed no activity differences between these recordings, which suggested that activation of TmPAP is not sufficient for CatSper stimulation. In addition, activity of recombinantly expressed TmPAP is not altered by P4 or by addition of TmPAP-specific antibodies (fig. S9). To test FAAH involvement, we recorded ICatSper from spermatozoa before and after treatment with PF750, an irreversible selective FAAH inhibitor. This treatment had no effect upon ICatSper (fig. S2). If FAAH is the P4 target, its native substrate, AEA, would mimic P4 action. However, AEA does not change ICatSper (Fig. 1D and fig. S2), which suggests that neither activation nor inhibition of FAAH affects CatSper and so rules out FAAH involvement in P4-dependent CatSper activation.
ABHD2 is present in sperm and acts as a P4-activated lipid hydrolase
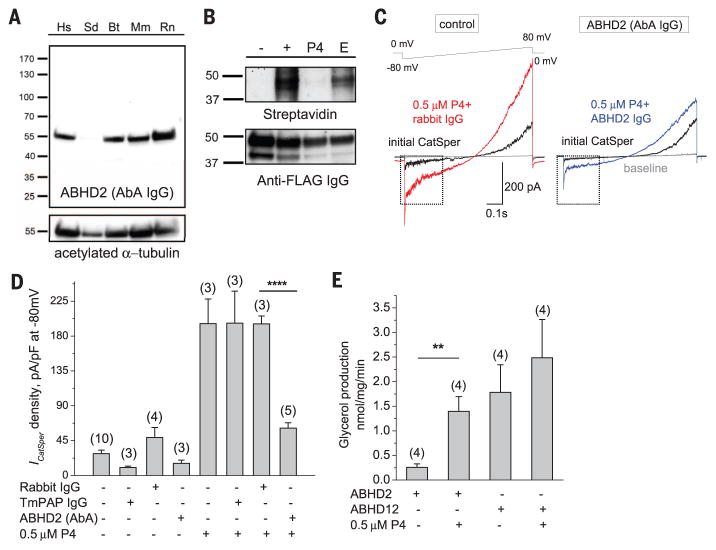
Our last candidate, ABHD2, is a member of a superfamily of mSHs with unknown activity. In pull-down experiments, ABHD2 was specifically detected in all lanes, except for negative controls (Fig. 2B). We confirmed that ABHD2 meets our last two requirements: It is functionally expressed in sperm at the level of mRNA and protein and is confined to the same cellular compartment as the CatSper (Fig. 2, B to D, and figs. S10 and S11A). Antibodies (Abs) against ABHD2 (AbAs) detected the protein in spermatozoa of various mammalian species: humans, boar, bovine, mouse, and rat (Fig. 3A and fig. S11B). ABHD2 transcripts are abundant in sperm, as evidenced by unbiased RNA deep sequencing of human spermatozoa and reverse transcription polymerase chain reaction experiments (Fig. 2C, fig. S11A, and database S2). To confirm that ABHD2 binds P4, we have carried out a P4* binding assay using recombinantly expressed ABHD2 (figs. S10 and S11C). Indeed, positive streptavidin staining revealed that ABHD2 binds P4* specifically and in a UV-dependent manner, and this binding is competed off by excess unlabeled P4 (Fig. 3B). Additionally, when human spermatozoa were incubated with AbAs, the result was a significant decrease of the CatSper activation by P4, whereas sperm treated with nonspecific antibodies retained their P4 sensitivity (Fig. 3, C and D, and figs. S9C and S11D). These results indicate that human ABHD2 is expressed in the sperm flagellum and is actively involved in P4-dependent signaling. Next, we explored human ABHD2’s enzymatic activity. Because our lipid MS data showed a P4-dependent decrease in sperm AGs, we examined whether recombinant ABHD2 could hydrolyze AGs. Such hydrolysis results in the formation of glycerol and arachidonic acid (AA) (20), so we monitored free glycerol production as an indicator of ABHD2 activity. As controls, we used mock-transfected cells or recombinant ABHD12 (fig. S11E), another mSH with known activity toward AGs (20). ABHD2 revealed modest hydrolase activity unless supplemented with 0.5 μM P4 (Fig. 3E). P4 significantly enhanced ABHD2 activity, whereas it had no significant effect on ABHD12 (Fig. 3E). We also tested whether ABHD2 is responsible for P4-induced calcium influx in sperm flagella. Indeed, inactivation of ABHD2 with protein-specific antibodies or sperm exposure to MAFP both abolished P4-activated calcium influx into sperm flagella (Fig. 4, A and B, and movies S1 to S6).
Fig. 3. ABHD2 is expressed in human spermatozoa and acts as a P4-dependent lipid hydrolase.
(A) ABHD2 expression in sperm from various mammalian species: Hs, humans; Sd, boar; Bt, bovine; Mm, mouse; and Rn, rat. Acetylated α-tubulin was used as the loading control. (B) Western blot from P4*-binding experiments of recombinant ABHD2 expressed in human embryonic kidney 293 (HEK293) cells resolves biotinylated proteins. Lane labeling is as in Fig. 2B. FLAG staining was used as the loading control for recombinant ABHD2 expression. (C) AbA prevents P4 activation of human ICatSper. Representative ICatSper treated with nonspecific IgG (left) or ABHD2 immunoglobulin G (IgG) (right). ICatSper values were recorded from control sperm or sperm treated with 1:100 dilutions of either nonspecific IgG or AbA for ~30 min. (D) ICatSper densities extracted from ramps shown in (C). (E) Monoacylglycerol hydrolysis by recombinant ABHD2 and ABHD12 in the presence or absence of 0.5 μM P4. Data are represented as means ± SEM; numbers in parentheses indicate number of experiments; and ****P < 0.0001, ***P < 0.001, **P < 0.005.
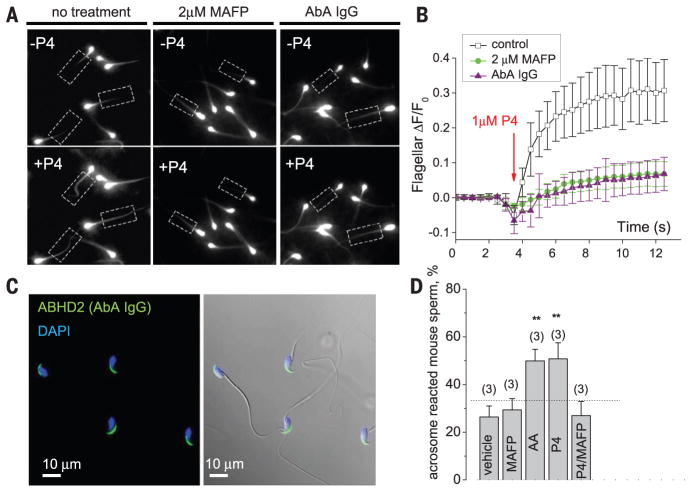
Fig. 4. ABHD2 regulates Ca2+ influx in human flagellum and is involved in mouse acrosome reaction.
(A) Representative images from calcium imaging experiments before and after P4 treatment with regions of interest (ROIs) indicated. (B) Preincubation with either MAFP or AbA impeded P4-induced calcium influx. ΔF/F, the change in intensity from the original intensity before stimulation. Between 40 and 60 sperm flagella were assessed per condition. (C) Immunostaining of mouse cauda epididymal spermatozoa with AbA shows acrosomal localization. (Right) An overlay with DIC images. Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI). (D) Percentage of AR murine spermatozoa in response to indicated treatments. Live, capacitated mouse spermatozoa were incubated with 3 μM P4, 3 μM AA, 2 μM MAFP, or vehicle (ethanol), after which they were imaged for the presence of intact acrosome. Data are represented as means ± SEM; numbers in parentheses indicate number of experiments; and **P < 0.005.
ABHD2 is present in rodent acrosome
ABHD2 expression was also detected in mouse epididymal sperm cells (Fig. 4C). Our report (7) showed that murine CatSper is insensitive to P4 and, therefore, may lack ABHD2-dependent regulation. Murine and human ABHD2 proteins share a high degree of sequence similarity; however, their subcellular localizations in sperm are different. Unlike that in humans, murine ABHD2 is restricted to the acrosomal region and is absent from the flagellum (Fig. 4C). This may explain the absence of CatSper P4 sensitivity in these species, whereas P4-induced acrosome reaction (AR) is retained (15). On the basis of the ABHD2 localization in acrosome, it is possible that P4 activation of ABHD2 and consequent AA release could be required for AR. Indeed, both P4 and AA stimulate mouse AR to similar levels, whereas MAFP blocks the P4 effect (Fig. 4D and fig. S12). This suggests that mouse AR is dependent on mSH action and that ABHD2 may play a role in this process. Note that 2AG also inhibits mouse ICatSper but with less efficacy than it inhibits human ICatSper (fig. S13). Murine sperm gradually lose 2AG content as they travel through the epididymis (21), and high activity of monoacylglycerol AG hydrolase, the lipase MAGL, contributes to low resting AG levels (22). Thus, murine CatSper is no longer inhibited by endogenous AGs and, therefore, does not require P4 stimulation. In contrast, human spermatozoa retain substantial amounts of AGs, which keep CatSper shut and, thus, require ABHD2 to eliminate AGs upon P4 exposure. As ABHD2 degrades AGs and activates CatSper, the human sperm membrane must also have a feedback mechanism for CatSper inactivation. Indeed, the sperm plasma membrane constantly produces AGs in order to keep CatSper closed. P4 application produced prolonged CatSper activation that slowly returns to basal levels when P4 is removed from the external solution (fig. S14). The rate of this “washout” matches precisely the rate of 2AG inhibition (fig. S3D), which supports the idea that human sperm cells constantly make AGs and that slow “washout” of P4 activation reflects the time-dependent replenishment of 2AG (figs. S3D and S14).
Human ABHD2 is a 425–amino acid molecule with a predicted N-terminal transmembrane domain, followed by an extracellularly facing domain. ABHD2 was identified as a candidate protein from our pull-down experiments. This fulfills two of our five requirements for the sperm nongenomic P4 receptor. ABHD2 participates in bioactive lipid signaling by hydrolyzing AGs in a P4-dependent manner, so they act as true mSHs. ABHD2 is functionally expressed in sperm and is confined to the flagellum alongside the CatSper channel. Taking into account that (i) ABHD2 is the only candidate molecule that meets all five search requirements and (ii) modulation of its function by pharmacological perturbation disrupts P4 signaling in sperm and impairs sperm activation, we conclude that the serine hydrolase ABHD2 is the nongenomic progesterone receptor of sperm.
Discussion
Metabolic serine hydrolases acting as monoacylglycerol lipases convert AGs into glycerol and AA. By hydrolyzing AGs in a P4-dependent manner, ABHD2 releases CatSper from AG inhibition, which liberates AA (fig. S15). During sperm transit through the epididymis, the plasma membrane undergoes lipid remodeling, which results in reduced levels of AA (23) and altered sperm motility (24), perhaps by means of modification of the ion channels’ function. Indeed, we found that application of AA briefly activates CatSper, whereas prolonged incubation in 3 μM AA results in CatSper inactivation and loss of P4 sensitivity (fig. S16, A and B). In accordance with our model, an overabundance of AA should negatively regulate ABHD2 activity; therefore, continuous P4 application will result in a considerable accumulation of AA in the outer leaflet of the sperm plasma membrane, which would ultimately lead to CatSper desensitization. However, prolonged P4 exposure does not cause CatSper desensitization (7). It is possible then, that during exposure to P4, liberated AA diffuses into the inner membrane leaflet or is released into the extracellular medium. Indeed, under our experimental conditions, any compound released from the plasma membrane will be removed by continuous perfusion. It is possible that AA removal from the outer leaflet in vivo could be achieved by either fatty acid transporters or chelation by albumin, which is abundant in the female reproductive tract.
Both murine and human CatSper are upregulated by intracellular alkalinization (7, 13); however, P4 exposure is also required for full human CatSper activation. This is explained by the fact that ejaculated human spermatozoa retain a substantial amount of 2AG; therefore, P4 activation of ABHD2 is needed for 2AG clearance. That MAFP-treated cells still respond to PGE1 points to an intriguing possibility that PGE1 may stimulate CatSper by allosteric activation of CatSper or may compete with 2AG directly for the binding site. Prostaglandins are derivatives of AA and are structurally similar to the 2AG tail; however, such a hypothesis requires additional experimental confirmation.
Regulation of male and female fertility by endocannabinoids has been explored previously, with particular focus on anandamide (25), whereas the role of 2AG in reproductive tissues is not well investigated. This study fills the gap by showing an important role of 2AG in sperm activation. It is possible that the P4-ABHD2-endocannabinoid axis could also regulate female reproduction by a similar mechanism. In fact, the expression of endocannabinoid system components was previously linked with genomic steroid activity (26, 27). However, our results indicate that progesterone can affect the level of endocannabinoids by binding ABHD2 and directly activating its hydrolase activity. The P4 binding site for this protein seems to be unique and does not resemble the classical steroid-binding pocket (28) on the basis of sequence similarity. Furthermore, competitive ligands of classical P4 signaling have no effect on the non-genomic action (Fig. 2B) (7).
In conclusion, we have characterized a novel function of ABHD2, which metabolizes endocannabinoids 1AG and 2AG only in the presence of progesterone. Moreover, we show that AGs act as endogenous inhibitors of CatSper. Upon P4 stimulation, ABHD2 hydrolyzes these endocannabinoids and that, together with intracellular alkalinization, leads to CatSper opening and triggers sperm hyperactivation, which makes spermatozoa fertile. Characterization of P4-dependent endocannabinoid hydrolysis reveals the missing link in nongenomic steroid signaling that regulates sperm activation. In addition, our model suggests that AGs are continuously produced to block CatSper, unless progesterone stimulates their hydrolysis by ABHD2 contradicting the “on-demand synthesis model” of endocannabinoid activity, whereby these lipids act only upon stimulus-dependent release from their precursors. Identification of ABHD2 as the nongenomic progesterone receptor of sperm provides a new target for developing novel contraceptives. Given the widespread expression of ABHD2 (29), the progesterone-ABHD2 axis is likely a critical component of the nongenomic action of this steroid in other tissues.
Supplementary Material
Acknowledgments
The authors thank L. Kohlstaedt for protein mass spectrometry and peptide identification. This work was supported by NIH R01GM111802 to P.V.L. and J.F.S; R21HD081403, Pew Scholars, and Alfred P. Sloan Awards to P.V.L.; NIH R01HD068914 to Y.K.; and NIH R01AR059385 to D.M.B. The Vincent J. Proteomics and Mass Spectrometry Laboratory at University of California (UC) Berkeley, is supported by NIH S10 Instrumentation grant S10RR025622. The combined QB3/Chemistry Mass Spectrometry Facility at UC Berkeley is supported by NIH S10 grant 1S10OD020062-01. The authors thank R. Heald (UC Berkeley) for critical reading of the manuscript, D. Julius (UC San Francisco) for help with next-generation sequencing, and M. Okabe (Osaka University, Japan) and J.-J. Chung (Yale University) for transgenic mice used in this study. P.V.L., M.R.M., and Y.K. are inventors on a patent application filed by UC Berkeley related to usage and application of ABHD2. B6D2-Tg(CAG/Su9-DsRed2,Acr3-EGFP) RBGS002Osb transgenic mice were transferred to UC Berkeley under material transfer agreement 13-176 with Osaka University. M.R.M. and P.V.L. conceived the project, designed the experiments, and wrote the manuscript. M.R.M. performed most of the experiments. P.V.L. led the study, helped with pilot experiments, generated transcriptomes, and provided financial support for the project. A.T.I. performed lipid mass spectrometry, and lipid analysis and identification. N.M. helped with pilot experiments. R.S. helped with recombinant protein binding experiments. Y.K. provided support for the transcriptome construction and P4* synthesis. E.O.G. generated libraries for the transcriptomes. J.F.S. helped with computer-aided sperm analysis experiments. R.Z.H. and D.M.B. helped with calcium imaging. All authors discussed the results and commented on the manuscript.
Footnotes
REFERENCES AND NOTES
- 1.Evans RM. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lösel R, Wehling M. Nat Rev Mol Cell Biol. 2003;4:46–55. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 3.Revelli A, Massobrio M, Tesarik J. Endocr Rev. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- 4.Blackmore PF, Beebe SJ, Danforth DR, Alexander N. J Biol Chem. 1990;265:1376–1380. [PubMed] [Google Scholar]
- 5.Baldi E, et al. J Androl. 1991;12:323–330. [PubMed] [Google Scholar]
- 6.Poisbeau P, et al. Front Cell Neurosci. 2014;8:174. doi: 10.3389/fncel.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lishko PV, Botchkina IL, Kirichok Y. Nature. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- 8.Smith JF, et al. Proc Natl Acad Sci USA. 2013;110:6823–6828. doi: 10.1073/pnas.1216588110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strünker T, et al. Nature. 2011;471:382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- 10.Quill TA, Ren D, Clapham DE, Garbers DL. Proc Natl Acad Sci USA. 2001;98:12527–12531. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren D, et al. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson AE, et al. Proc Natl Acad Sci USA. 2003;100:14864–14868. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirichok Y, Navarro B, Clapham DE. Nature. 2006;439:737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 14.Lishko PV, et al. Annu Rev Physiol. 2012;74:453–475. doi: 10.1146/annurev-physiol-020911-153258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osman RA, Andria ML, Jones AD, Meizel S. Biochem Biophys Res Commun. 1989;160:828–833. doi: 10.1016/0006-291x(89)92508-4. [DOI] [PubMed] [Google Scholar]
- 16.Chung JJ, et al. Cell. 2014;157:808–822. doi: 10.1016/j.cell.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi H, et al. Proc Natl Acad Sci USA. 2007;104:1219–1223. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lishko P, Clapham DE, Navarro B, Kirichok Y. Methods Enzymol. 2013;525:59–83. doi: 10.1016/B978-0-12-397944-5.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long JZ, Cravatt BF. Chem Rev. 2011;111:6022–6063. doi: 10.1021/cr200075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blankman JL, Simon GM, Cravatt BF. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cobellis G, et al. Biol Reprod. 2010;82:451–458. doi: 10.1095/biolreprod.109.079210. [DOI] [PubMed] [Google Scholar]
- 22.Catanzaro G, et al. Mol Cell Endocrinol. 2011;343:88–92. doi: 10.1016/j.mce.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Rejraji H, et al. Biol Reprod. 2006;74:1104–1113. doi: 10.1095/biolreprod.105.049304. [DOI] [PubMed] [Google Scholar]
- 24.Lenzi A, et al. Mol Hum Reprod. 2000;6:226–231. doi: 10.1093/molehr/6.3.226. [DOI] [PubMed] [Google Scholar]
- 25.Di Marzo V, Bifulco M, De Petrocellis L. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 26.Grimaldi P, et al. Cell Mol Life Sci. 2012;69:4177–4190. doi: 10.1007/s00018-012-1074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maccarrone M, Bari M, Di Rienzo M, Finazzi-Agrò A, Rossi A. J Biol Chem. 2003;278:32726–32732. doi: 10.1074/jbc.M302123200. [DOI] [PubMed] [Google Scholar]
- 28.Williams SP, Sigler PB. Nature. 1998;393:392–396. doi: 10.1038/30775. [DOI] [PubMed] [Google Scholar]
- 29.Miyata K, et al. Biochem Biophys Res Commun. 2005;329:296–304. doi: 10.1016/j.bbrc.2005.01.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.