Abstract
Background
Acceptance of cannabis use is growing. However, prolonged use is associated with diminished psychosocial outcomes, potentially mediated by drug-induced cognitive impairments. Δ9-Tetrahydrocannabinol (THC) is the main psychoactive ingredient in cannabis, yet other phytocannabinoids in the plant, such as cannabidiol (CBD), have unique properties. Given that CBD can modulate the undesirable effects of THC, therapeutic agents, such as nabiximols, contain higher CBD:THC ratios than illicit marijuana. We tested the hypothesis that THC impairs a relevant cognitive function for long-term success, namely willingness to exert cognitive effort for greater rewards, and that CBD could attenuate such decision-making impairments.
Methods
Male Long–Evans rats (n = 29) performing the rat cognitive effort task (rCET) received acute THC and CBD, independently and concurrently, in addition to other cannabinoids. Rats chose between 2 options differing in reward magnitude, but also in the cognitive effort (attentional load) required to obtain them.
Results
We found that THC decreased choice of hard trials without impairing the animals’ ability to accurately complete them. Strikingly, this impairment was correlated with CB1 receptor density in the medial prefrontal cortex — an area previously implicated in effortful decision-making. In contrast, CBD did not affect choice. Coadministration of 1:1 CBD:THC matching that in nabiximols modestly attenuated the deleterious effects of THC in “slacker” rats.
Limitations
Only male rats were investigated, and the THC/CBD coadministration experiment was carried out in a subset of individuals.
Conclusion
These findings confirm that THC, but not CBD, selectively impairs decision-making involving cognitive effort costs. However, coadministration of CBD only partially ameliorates such THC-induced dysfunction.
Introduction
Cannabis is the world’s most widely used illicit drug, with North American estimates suggesting that 11% of adults experiment with the drug annually.1 While the psychoactive effects of cannabis are attributable to the agonistic actions of Δ9-tetrahydrocannabinol (THC) at the presynaptic CB1 receptor, around 70 other phytocannabinoids have been identified in the plant.2 One such compound, cannabidiol (CBD), has purported neuroprotective properties, and growing evidence that CBD can modulate the functional effects of THC has led to the development of medicinal cannabis extracts rich in CBD.3,4 In stark contrast, increasing concentrations of THC coincide with a concomitant decline in CBD levels in street cannabis.5,6 Concerns have been raised over this rising potency of cannabis, given that higher levels of THC may induce anxiety and psychosis, and because acute cannabis use is associated with cognitive impairments in the domains of learning, memory, reasoning and attention.7,8
Comparatively, the role of cannabinoid signalling in decision-related executive processes is unexplored. Crucial decisions in life require evaluating the costs associated with different options in light of the potential benefits obtained by those choices. Cannabis derivatives alter human choice behaviour in laboratory decision-making tasks involving delay or risk costs, and similar impairments have been observed in animal models following administration of THC or related cannabinoid agonists.9–12 While physical effort-based decision-making has recently been shown to be sensitive to cannabinoid receptor activation,10 it is unknown whether decisions involving cognitive effort costs are likewise susceptible. The distinction is important; these 2 forms of decision-making are subserved by dissociable neurobiological mechanisms, and cognitive costs are more representative of the effort costs faced in an industrialized society.13,14 Indeed, associations between cannabis use and impaired education, economic, and employment outcomes may reflect a fundamental deficit in effortful decision-making, whereby cannabis decreases the willingness to expend the greater cognitive load associated with lucrative prospects.15,16 However, issues of causation are difficult to resolve from clinical studies.
Our laboratory has validated a rodent cognitive effort task (rCET), wherein cognitive effort costs are varied by the amount of visuospatial attention required to complete low-reward or high-reward trials. Previous work indicates that the choice to apply cognitive effort is neurochemically dissociable from attentional ability and that baseline differences in the willingness to exert cognitive effort can critically determine drug response.17 Our goal was therefore to examine whether cannabinoid drugs differentially affected the animals’ performance on the rCET in natural “workers” and “slackers.” We initially evaluated the impact of a CB1 receptor inverse agonist rimonabant, the CB2 receptor antagonist AM 630, the fatty acid amide hydrolase (FAAH) inhibitor URB 597, and the CB1/CB2 receptor agonist WIN55212–2 (WIN) on rCET performance. We subsequently determined the impact of THC and CBD in isolation, as well as coadministered in ratios resembling those found in either street or medicinal cannabis.3,5,18 Finally, we analyzed CB1 receptor parameters ex vivo in brain regions previously implicated in effortful decision-making to determine if these were related to the behavioural effects of THC.
Methods
See Appendix 1, available at jpn.ca, for detailed experimental procedures.
Animals
We studied 32 male Long–Evans rats weighing 275–300 g at the start of the experiment. Rats were food-restricted to 14 g of rat chow per day and maintained at 85% of their free-feeding weight. Water was available ad libitum. Animals were paired housed in a climate-controlled colony room on a 12 h reverse light/dark cycle. Testing and housing were in accordance with the Canadian Council on Animal Care, and all experimental protocols were approved by the University of British Columbia Animal Care Committee.
The rCET
Task procedures have been described elsewhere17 (Appendix 1, Fig. S1). Briefly, animals were tested 5 days per week in 30-min sessions of no fixed trial limit. Levers were permanently designated to initiate either low-effort/low-reward (LR) or high-effort/high-reward (HR) trials, and these designations were counterbalanced across subjects. Animals began each trial by nose-poking in the illuminated food tray, thereby extending the levers. Pressing a lever would set the trial as LR or HR, at which point the levers would retract. After a 5-s intertrial interval (ITI), 1 of the 5 stimulus lights would briefly illuminate for a stimulus duration of 1.0 s on LR trials and 0.2 s on HR trials. Animals then had 5 s to nose-poke within the previously illuminated aperture (correct response) for a reward of 1 or 2 sugar pellets on LR and HR trials, respectively, at which point the tray light would reilluminate to signal the opportunity to start the next trial.
Trials went unrewarded for the following reasons: animals failed to make a lever response within 10 s (choice omission), animals nose-poked during the ITI (premature response, a measure of motor impulsivity19), animals nose-poked in another aperture (incorrect response), and animals failed to nose-poke any aperture within 5 s of stimulus illumination (response omission). All such outcomes led to a 5-s punishment time out, followed by tray light illumination to mark the next trial.
Pharmacological challenges
Once behavioural baseline was established, drugs were administered in the following order: the CB1 receptor inverse agonist rimonabant (0, 0.3, 1, 3 mg/kg), the CB2 receptor antagonist AM 630 (0, 5 mg/kg), the fatty acid amide hydrolase inhibitor URB 597 (0, 0.1, 0.3, 1.0 mg/kg), THC (dronabinol; 0, 0.3, 1, 2, 3 mg/kg), CBD (0, 5, 15 mg/kg), THC/CBD coadministration (0–0, 0–2, 2–0.2, 2–2 mg/kg) and the CB1 synthetic receptor agonist WIN 55, 212–2 (0, 1, 2, 3 mg/kg; see Appendix 1, Table S1, for a description of drugs used). All drugs were administered in a volume of 1 mL/kg via intra-peritoneal injection. Animals were given a minimum of 1 week drug-free testing between compounds to prevent carry-over effects.
All drugs were prepared fresh daily and administered according to a Latin-square within-subjects design. The injection schedule started with a baseline session, followed by a vehicle or drug injection session, and then by 1 or 2 nontesting days. Injections were administered 30 min before testing except for URB 597 injections, which were administered 45 min before testing. For the coadministration studies, THC and CBD were both injected in rapid succession 30 min before testing, with the order randomized across animals.
CB1 receptor radioligand-binding assay
Three weeks after the last drug challenge, animals were sacrificed by rapid decapitation, and the medial prefrontal cortex (mPFC) and nucleus accumbens (NAcc) were dissected. Samples from the top 8 workers and 8 slackers (highest and lowest HR choice, respectively) were processed for CB1 receptor radioligand binding, and maximal binding site density (Bmax) and binding affinity (Kd) values were determined as previously described.20 Four NAcc samples were not of adequate size, leaving 16 mPFC and 12 NAcc samples available for analysis.
Statistical analysis
All data were subjected to repeated-measures ANOVA with session or dose as within-subjects factors. Choice (2 levels: LR, HR) was also included as a within-subjects factor for appropriate variables: percent accuracy, percent response omissions, percent prematures, lever choice latency, latency to respond correctly and latency to collect reward. We also analyzed choice omissions and total trials completed. Three rats were excluded from the study following health complications. As per our previous report,17 rats that chose the HR option on more than 70% of trials were classified as “workers” (n = 17); those that chose HR for 70% of trials or less (n = 12) were classified as “slackers.” Groups proved stable across the experiment: at rCET baseline and all vehicle injections for rCET drug challenges, workers chose more HR trials than slackers (all F > 20.75, p < 0.001). This distinction was used as a between-subjects factor (group, 2 levels) in all ANOVAs.
Prior to experimental manipulation, rats were trained to behavioural stability, as demonstrated by a lack of significant session or group × session effects over 5 consecutive sessions. Any significant (p < 0.05) main effects or interactions were further analyzed via post hoc 1-way ANOVA or paired samples t tests with a Bonferroni correction for the number of comparisons made. We calculated Pearson correlations to assess associations between rCET performance and CB1 receptor properties. Any p values between 0.05 and 0.10 are reported as a statistical trend.
Results
See Appendix 1 for a complete analysis of rCET behaviour following drug challenge.
Effect of rimonabant
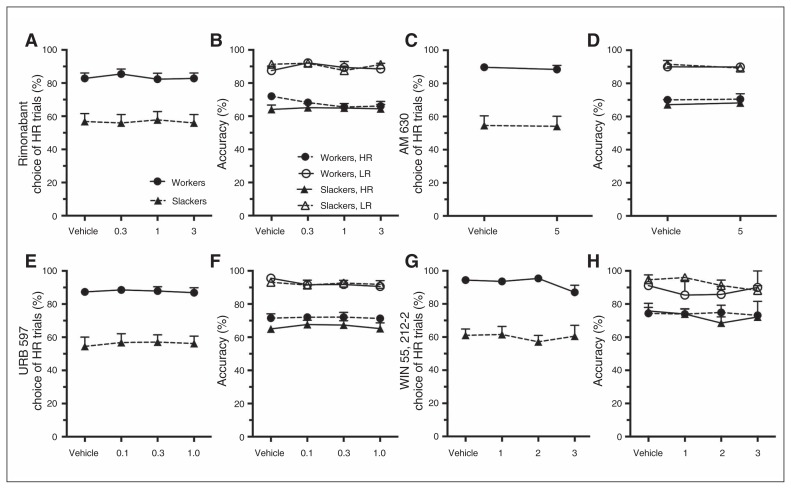
Baseline behaviour on the rCET has been discussed in detail previously, and so will only be briefly described here.17 As per previous reports, animals chose the HR trials more than LR trials following a vehicle injection (vehicle only — choice: F1,27 = 44.38, p < 0.001), with workers choosing a significantly higher proportion of HR trials than slackers (vehicle only — group: F1,27 = 20.75, p < 0.001). The CB1 receptor inverse agonist rimonabant had no effect on animals’ choice of HR or LR trials (dose: F3,81 = 0.48, p = 0.70; Fig. 1A).
Fig. 1.
(A–D) Rat cognitive effort task (rCET) performance following systemic administration of cannabinoid agents. The CB1 and CB2 receptor antagonists rimonabant and AM630 did not affect choice, accuracy, or premature responding. (E–F) Similarly, the FAAH inhibitor URB 597 did not affect rCET performance. (G–H) Although WIN 55, 212–2 increased premature responding for low-effort/low-reward (LR) trials across both groups (Appendix 1, Fig. S2D), this synthetic CB1 receptor agonist did not affect measures of choice or accuracy. Data are expressed as the mean (± standard error of the mean) percent for each variable. HR = high-effort/high-reward.
Animals were more accurate on LR trials than HR trials (vehicle only — choice: F1,25 = 76.89, p < 0.001), and as per previous reports, workers and slackers performed the rCET equally well (vehicle only — group/group × choice: all F < 1.46, p > 0.24). Thus the distinct choice profile of both groups was not driven by the animals’ ability to perform the task. Rimonabant had no effect on animals’ accuracy (Fig. 1B; all F < 1.65, p > 0.21).
Premature responding was generally higher for HR than LR trials (vehicle only — choice: F1,25 = 4.48, p = 0.044), and there was no difference in this measure between workers and slackers (group/group × choice: all F < 1.74, p > 0.20). Rimonabant did not influence animals’ rates of premature responding (Appendix 1, Fig. S2A; all F < 1.45, p > 0.24).
Rimonabant increased correct response latencies and response and choice omissions and decreased the number of completed trials for all animals (Appendix 1, Table S2).
Effect of AM630, URB 597 and WIN55212–2
AM 630, URB 597 and WIN had no effect on choice, accuracy, or premature responding (Fig. 1C–H), although there was a trend for the lowest dose of WIN to increase premature responding on LR trials across animals (LR trials — dose: F3,18 = 2.95, p = 0.06; vehicle v. 1.0 mg/kg: F1,7 = 10.046, p = 0.048; HR trials — dose/dose × group: all F < 0.58, p > 0.63; Appendix 1, Fig. S2D). URB 597 increased latencies to make a correct response on LR and HR trials across both groups, and WIN increased choice latencies and choice omissions and decreased the total number of trials completed, suggesting these doses were behaviourally active (Appendix 1, Table S3–S5).
Effect of THC administration
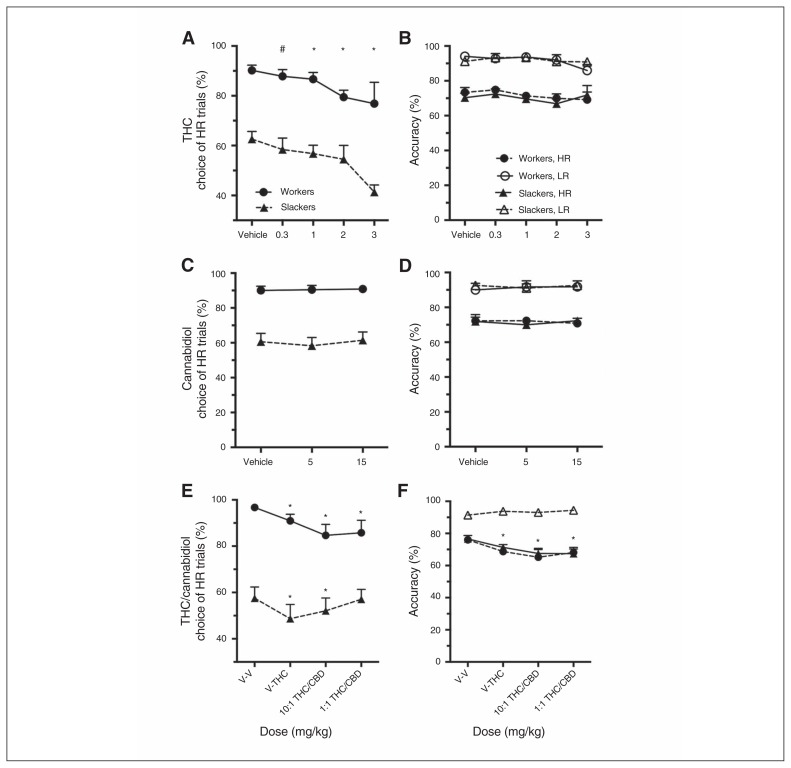
Regardless of baseline preference for the HR option, THC decreased choice of HR trials at all but the lowest dose tested (dose: F3,75 = 8.61, p < 0.001; vehicle v. 0.3 mg/kg: F1,28 = 5.17, p = 0.09; vehicle v. 1.0 mg/kg: F1,28 = 6.51, p = 0.048; vehicle v. 2.0 mg/kg: F1,26 = 20.24, p < 0.001; vehicle v. 3.0 mg/kg: F1,16 = 11.77, p = 0.004; dose × group: all F < 1.78, p > 0.16; Fig. 2A). This shift in choice was not due to an impaired ability to complete HR trials, as accuracy was not affected following the first 3 doses of THC (all Fs < 1.21, p > 0.32; Fig. 2B), and the trending attentional impairment at the 3 mg/kg dose was limited to workers only (dose × group: F1,11 = 4.78, p = 0.05; workers only: F1,4 = 14.85, p = 0.018; slackers only: F < 0.40, p > 0.55). Subsequent analysis revealed that this attentional impairment in workers was driven by reduced accuracy on easy LR (10.53% decline) relative to difficult HR trials (2.47% decline) at the 3 mg/kg dose. Administration of THC did not affect rates of premature responding for either trial type (Appendix 1, Fig. S2E).
Fig. 2.
(A–B) Rat cognitive effort task (rCET) performance following systemic administration of cannabinoids found in cannabis. At all doses, Δ9-tetrahydrocannabinol (THC) decreased selection of high-effort/high-reward (HR) trials in workers and slackers without affecting attentional ability or impulsivity. (C–D) Cannabidiol (CBD) did not affect rCET behaviour in isolation, (E) but partially attenuated THC-induced choice impairments in slacker rats when coadministered at a 1:1 THC:CBD ratio (V-V = 0–0 mg/kg, V-THC = 0–2.0 mg/kg, 10:1 THC:CBD = 2.0/0.2 mg/kg, 1:1 THC:CBD = 2.0/2.0 mg/kg). (E–F) The data are from a subset of the original cohort (top 9 workers and slackers, respectively), and as such the choice profiles at vehicle are more divergent. (F) Given the top workers rarely chose the low-effort/low-reward (LR) option, accuracy could not be calculated and for this reason is not displayed. Data are expressed as the mean (± standard error of the mean) percent for each variable. *p < 0.05; #p < 0.10.
Across groups, THC decreased the number of trials completed at the 2 mg/kg and 3 mg/kg doses (Appendix 1, Table S6). At the high THC doses this was accompanied by an increase in choice omissions and by a modest increase in response omissions. However, latencies to collect reward were unaffected at all doses (all F < 1.31, p > 0.28). Latencies to make an LR/HR choice were unchanged, and correct response latencies were affected only at the highest THC dose.
Effect of CBD and concurrent CBD/THC administration
Cannabidiol had no effect on any rCET measure (Fig. 2C and D and Appendix 1, Table S7). Given limited quantities of THC, the top 9 workers and 9 slackers (highest and lowest HR choice, respectively) were selected to receive 2mg/kg of THC alone and in combination with varying ratios of CBD. Of these, 1 slacker failed to initiate any trials on the rCET following injection and was removed from this analysis. As described previously, administration of 2 mg/kg of THC decreased choice of HR trials across groups (dose: F1,15 = 14.19, p = 0.002; dose × group: F1,15 = 0.303, p = 0.59). However, coadministration of THC/CBD had distinct effects on workers and slackers, as indicated by a significant dose × group interaction (F3,45 = 3.90, p = 0.015; dose: F3,45 = 6.25, p = 0.001; Fig. 2E). In workers, THC still decreased choice of HR trials when administered in combination with CBD at a 10:1 or 1:1 ratio (workers only — dose: F3,24 = 6.53, p = 0.002; vehicle v. 10:1 THC/CBD: F1,8 = 9.56, p = 0.045; vehicle v. 1:1 THC/CBD: F1,8 = 8.93, p = 0.051). Although the main effect of dose in slackers was only a trend (slackers only — dose: F3,21 = 2.84, p = 0.06), subsequent analysis revealed the effects of THC were attenuated in slackers when administered with CBD at a 1:1, but not 10:1, ratio (vehicle v. 10:1 THC/CBD: F1,7 = 11.39, p = 0.036; vehicle v. 1:1 THC/CBD: F1,7 = 0.023, p > 0.99). While this analysis is compromised by a smaller number of subjects and thus lower power, it suggests that CBD may partially ameliorate THC-induced decision-making impairments in select individuals. While THC previously had no effect on accuracy when administered alone, here THC administered alone or in combination with CBD impaired accuracy for HR trials (HR trials — dose: F3,32 = 4.91, p = 0.012; vehicle v. THC: F1,16 = 7.60, p = 0.042; vehicle v. 10:1 THC/CBD: F1,16 = 12.59, p = 0.010; vehicle v. 1:1 THC/CBD: F1,16 = 31.54, p < 0.001; dose × group: F3,32 = 0.13, p = 0.94; Fig. 2F). In contrast, THC alone or in combination with CBD had no effect on premature responding for LR or HR trials (Appendix 1, Fig. S1F and G).
In general, THC alone or in combination with CBD decreased the number of trials completed, increased the response omissions for HR trials and increased choice omissions across groups. Latencies (choice, correct and collect) were unaffected at all doses, and administration of CBD did not potentiate the behavioural effects of THC alone on any of these measures (Appendix 1, Table S8).
CB1 receptor binding
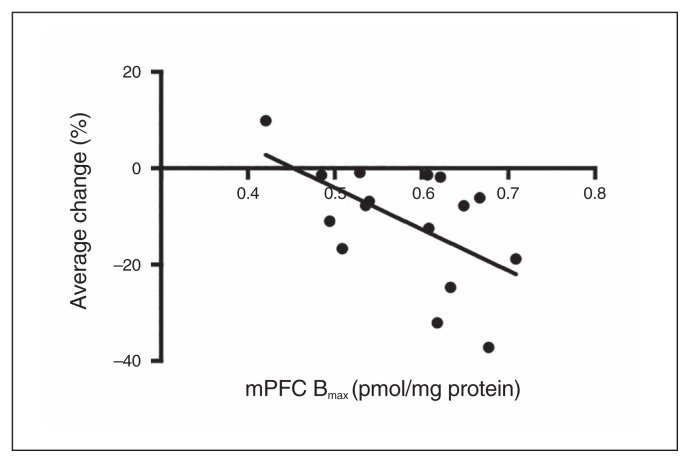
Workers and slackers did not differ in any measure of receptor binding (Appendix 1, Table S9). However, the choice shift induced by THC was correlated with mPFC Bmax values (r15 = −0.567, p = 0.022), such that greater sensitivity to THC’s effects on choice across the 4 doses was associated with higher CB1 receptor density in this region (Fig. 3). The mPFC Kd values (r15 = 0.04, p = 0.88) and binding parameters from NAcc samples (Bmax: r11 = 0.25, p = 0.41; Kd: r11 = 0.18, p = 0.56) were not related to THC-induced changes in behaviour.
Fig. 3.
CB1 receptor density in the medial prefrontal cortex (mPFC) is correlated with Δ9-tetrahydrocannabinol (THC)-induced choice impairments. The “average change” measure was derived by calculating the percent change in choice of the high-effort/high-reward (HR) option at each THC dose relative to vehicle, and then averaging across the doses to develop a general measure of THC sensitivity for each rat.
Discussion
Here we demonstrate a role for the cannabinoid system in decisions regarding the allocation of cognitive effort. Even low doses of THC decreased choice of HR trials across worker and slacker rats without affecting animals’ ability to perform the more demanding attentional challenge. Strikingly, the magnitude of this effect was correlated with CB1 receptor density in the mPFC area encompassing prelimbic and anterior cingulate cortices (ACC) — areas previously implicated in task performance.21,22 In contrast, CBD had no effect on performance, but partially attenuated THC’s choice effects in slackers when coadministered at a ratio akin to that found in cannabinoid therapeutics.3 Together, these data implicate the cannabinoid system in decision-making regarding the allocation of cognitive effort, but not necessarily in the performance of such cognitively demanding processes. These findings help to clarify the precise nature of the cognitive impairment caused by THC, which has been difficult to demonstrate objectively.23 Although our findings lend support to the hypothesis that CBD itself does not adversely affect cognitive function, its coadministration did not robustly negate the deleterious effects of THC, although benefit was observed in select individuals.
At higher doses THC decreased trials completed, increased omissions and increased latencies to make a correct response — all of which may suggest THC decreased motivation for sucrose reward. This explanation seems unlikely, however, as low doses of THC decreased choice of HR trials without affecting other variables. Also, THC did not alter the time rats took to collect sugar pellets on either trial type, suggesting rats were still as eager to obtain such rewards.19 Alternatively, THC may have impaired animals’ ability to associate the LR/HR trials with the assigned levers. If so, choice preferences should have moved to indifference, yet choice of HR in workers remained well above 50%, and slackers’ choice moved well below equivalence. Animals were therefore not indifferent to the relative outcomes of options despite their altered choice.
The decrease in preference for HR trials under THC cannot be attributed to an inability to complete HR trials, as THC did not affect HR accuracy. Similarly, cannabinoid receptor activation, via THC or WIN, did not disrupt attention on the 5-choice serial reaction time task, which differs from the rCET only in its lack of LR/HR options.9,24 However, when THC was administered to a subset of animals in the THC/CBD coadministration experiment, accuracy for HR trials was impaired at a dose that previously had no effect. Indeed, an analysis of HR trials from the original THC challenge revealed a trending decline in accuracy among animals included in the coadministration experiment (Appendix 1). Thus, some individuals may have exhibited attentional impairments at a higher dose of THC, but these effects were not robust when considered among a larger population and were not present at lower doses that nevertheless shifted choice away from options high in cognitive effort.
Additional cannabinoid drugs were tested on the rCET. CB1 and CB2 receptor antagonism did not affect measures of decision-making, attention, or impulsivity. The null effects observed with AM 630 are not surprising given the low density and functional activity of CB2 in the central nervous system.25 In contrast, the CB1 antagonist rimonabant reduced trials completed and increased omissions, possibly reflecting decreased motivation for food.26 Rimonabant also did not affect choice in a task involving delay costs, and when considered with the current data, it appears endocannabinoid signalling does not tonically regulate decision-making.9,24
URB 597 — an FAAH inhibitor — and the synthetic CB1 agonist WIN also did not affect choice, even though the latter drug had robust effects on trials completed and omission rates. These results seem difficult to reconcile with THC’s choice effects, given all of these agents increase ligand binding at the CB1 receptor. Such inconsistencies are likely related to the distinct pharmacodynamic profiles of these drugs. For example, THC and WIN have dissimilar chemical structures and thus differ in their binding at the CB1 receptor.27 THC is a partial agonist with a lower affinity and efficacy relative to WIN, hence its ability to activate the CB1 receptor will be influenced by the density and coupling efficiencies of these receptors in different brain regions.28–30 We are not the first group to show effects on decision-making following THC that are not replicated by a synthetic CB1 agonist: THC, but not WIN, increased HR choice in a task involving delay costs.9,24 Perhaps the most parsimonious explanation for the discrepancy between the effects of these agonists relates to their ability to recruit differential signalling cascades. While THC is a potent recruiter of the arrestin2 pathway, WIN and endogenous anandamide appear to signal through the more classical G-protein Gαi/o and Gβγ pathways.31 In future, it would be prudent to directly compare CB1 agonists that signal through these different mechanisms to better understand how cannabinoids with a common target produce unique effects on cognition.
The correlation observed between mPFC CB1 density and the magnitude of the THC-induced elevation in LR choice suggests that prefrontal CB1 receptors contribute to THC-induced cognitive laziness. Other experimental data likewise point to a plausible role for prefrontal CB1 receptors, particularly within the ACC, in mediating the deleterious effects of THC on effort-based cost–benefit decision-making.10 Inactivating the ACC decreased choice of HR trials on both the rCET used here, as well as a decision-making paradigm in which costs are physically rather than cognitively effortful.22,32 Stimulation of this region in humans can also elicit feelings of endeavour and “gearing up” for an effortful challenge.33 The ACC contains CB1 receptors localized on glutamatergic terminals, which regulate excitatory input into this structure.34 Thus THC may be dampening presynaptic glutamate release, thereby decreasing ACC neuron activity. Such a mechanism is in line with evidence that CB1 receptor antagonism increases Fos immunoreactivity in the cingulate cortex, suggesting CB1 agonism may negatively regulate excitation in this region.35
In stark contrast, CBD alone did not affect any behavioural rCET measure. However, when administered at a 1:1 ratio, CBD partially attenuated THC-induced cognitive laziness in slacker rats. This ratio models the composition of nabiximols — an oromucosal spray approved for the treatment of pain in multiple sclerosis.3 In contrast, the 10:1 THC:CBD ratio modelling street cannabis did not ameliorate the THC-induced shift in HR choice in either group. It must be noted that CBD’s reversal of THC-induced laziness in slackers is a modest effect, an issue underscored by the small number (8 slackers) used. However, other studies have also shown that CBD–THC interactions produce only modest physiologic and psychological changes36 that are sensitive to numerous factors, including the specific THC/CBD doses used, their ratio, and timing of coadministration.37
Understanding the mechanism by which CBD partially ameliorates THC’s effects may provide insight into why this effect is not as robust as expected. Recent in vitro evidence suggests that CBD may inhibit the effects of THC through negative allosteric modulation of the CB1 receptor,38 whereas human neuroimaging studies indicate that THC and CBD differentially influence brain activity while performing cognitive or emotional tasks.39–41 And while both additive36 and antagonistic18,42 associations have been reported between these agents in rodents, to date no studies have provided a direct mechanism as to how CBD moderates THC’s behavioural effects.
Limitations
We assessed the role of cannabinoids in male rats only, but a growing literature suggests that the sexes differ in cannabinoid signalling.43 Given the extensive training required to perform the rCET, we investigated a number of drugs in the same cohort. While it is possible these treatments may have affected CB1 receptor parameters, there is no evidence to suggest that changes in intracellular signalling caused by such acute injections should be evident after an extended time period. In any case, all rats contributing CB1 receptor data experienced the same treatments, and so this would not seriously confound our interpretation of the binding data. Finally, restrictions in the amount of THC available prevented us from carrying out the complete THC/CBD experiment in all rats.
Conclusion
Our findings raise interesting points given the state of cannabis use worldwide. THC concentrations in cannabis continue to grow at the expense of declining CBD levels.5,6 High THC concentrations have been previously linked to impaired executive functioning, and we specifically show these deficits extend to situations requiring cognitively effortful decision-making.44 Though our results suggest that THC affects decision-making acutely, it is interesting that prolonged cannabis use in humans is associated with negative socioeconomic outcomes.15,16,45 Although a chronic dosing experiment would be required to assess this association directly, we hypothesize that associations between THC and poorer life outcomes may be due to a drug-induced decrease in willingness to allocate cognitive effort, rather than impairments in fundamental cognitive abilities per se. Our findings also suggest that unlike THC, CBD does not adversely affect executive function, and as such its inclusion in medicinal cannabis is not of primary concern. And while CBD was able to modestly attenuate THC-induced cognitive laziness in a subset of individuals, it may not be the medical panacea some suggest it to be.46 Understanding how phytocannabinoids affect key cognitive abilities remains an important research priority, given the push for cannabis law reform that would see a rise in its medical and recreational use.
Acknowledgements
This work was supported by a discovery grant awarded to CAW from the Canadian Natural Sciences and Engineering Research Council (NSERC). CAW also receives salary support through the Michael Smith Foundation for Health Research and the Canadian Institutes of Health Research (CIHR) New Investigator Award program, and has consulted for Shire Pharmaceuticals on an unrelated matter. M. Silveira was supported by an NSERC Doctoral Research Award. This work was also supported by a CIHR grant to M. Hill, who is also the recipient of a Tier II Canada Research Chair for salary support.
Footnotes
Competing interests: M. Hill is a consultant for Pfizer on matters unrelated to this work. No other competing interests declared.
Contributors: M. Silveira, W. Adams and C. Winstanley designed the study. M. Silveira, W. Adams, M. Morena and M. Hill acquired the data, which M. Silveira and C. Winstanley analyzed. M. Silveira and C. Winstanley wrote the article, which all authors reviewed and approved for publication.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2014. [PubMed] [Google Scholar]
- 2.Elsohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–48. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Syed YY, McKeage K, Scott LJ. Delta-9-tetrahydrocannabinol/cannabidiol (Sativex®): a review of its use in patients with moderate to severe spasticity due to multiple sclerosis. Drugs. 2014;74:563–78. doi: 10.1007/s40265-014-0197-5. [DOI] [PubMed] [Google Scholar]
- 4.Niesink RJM, van Laar MW. Does cannabidiol protect against adverse psychological effects of THC? Front Psychiatry. 2013;4:130. doi: 10.3389/fpsyt.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swift W, Wong A, Li KM, et al. Analysis of cannabis seizures in NSW, Australia: cannabis potency and cannabinoid profile. PLoS One. 2013;8:e70052. doi: 10.1371/journal.pone.0070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgdorf JR, Kilmer B, Pacula RL. Heterogeneity in the composition of marijuana seized in California. Drug Alcohol Depend. 2011;117:59–61. doi: 10.1016/j.drugalcdep.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane NA, Schuster RM, Fusar-Poli P, et al. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev. 2013;23:117–37. doi: 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold JC, Boucher A, Karl T. The yin and yang of cannabis-induced psychosis: the actions of D 9-tetrahydrocannabinol and cannabidiol in rodent models of schizophrenia. Curr Pharm Des. 2012;18:5113–30. doi: 10.2174/138161212802884726. [DOI] [PubMed] [Google Scholar]
- 9.Wiskerke J, Stoop N, Schetters D, et al. Cannabinoid CB1 receptor activation mediates the opposing effects of amphetamine on impulsive action and impulsive choice. PLoS One. 2011;6:e25856. doi: 10.1371/journal.pone.0025856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khani A, Kermani M, Hesam S, et al. Activation of cannabinoid system in anterior cingulate cortex and orbitofrontal cortex modulates cost-benefit decision making. Psychopharmacology (Berl) 2015;232:2097–112. doi: 10.1007/s00213-014-3841-6. [DOI] [PubMed] [Google Scholar]
- 11.Lane SD, Cherek DR, Tcheremissine OV, et al. Acute marijuana effects on human risk taking. Neuropsychopharmacology. 2005;30:800–9. doi: 10.1038/sj.npp.1300620. [DOI] [PubMed] [Google Scholar]
- 12.Moreno M, Estevez AF, Zaldivar F, et al. Impulsivity differences in recreational cannabis users and binge drinkers in a university population. Drug Alcohol Depend. 2012;124:355–62. doi: 10.1016/j.drugalcdep.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt L, Lebreton M, Cléry-Melin M-L, et al. Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biol. 2012;10:e1001266. doi: 10.1371/journal.pbio.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosking JG, Floresco SB, Winstanley CA. Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology. 2015;40:1005–15. doi: 10.1038/npp.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fergusson DM, Boden JM. Cannabis use and later life outcomes. Addiction. 2008;103:969–76. doi: 10.1111/j.1360-0443.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- 16.Hyggen C. Does smoking cannabis affect work commitment? Addiction. 2012;107:1309–15. doi: 10.1111/j.1360-0443.2012.03796.x. [DOI] [PubMed] [Google Scholar]
- 17.Cocker PJ, Hosking JG, Benoit J, et al. Sensitivity to cognitive effort mediates psychostimulant effects on a novel rodent cost/benefit decision-making task. Neuropsychopharmacology. 2012;37:1825–37. doi: 10.1038/npp.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vann RE, Gamage TF, Warner J, et al. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of delta(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2008;94:191–8. doi: 10.1016/j.drugalcdep.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–80. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 20.Lee TTY, Hill MN. Age of stress exposure modulates the immediate and sustained effects of repeated stress on corticolimbic cannabinoid CB1 receptor binding in male rats. Neuroscience. 2013;249:106–14. doi: 10.1016/j.neuroscience.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Hosking JG, Cocker PJ, Winstanley CA. Prefrontal cortical inactivations decrease willingness to expend cognitive effort on a rodent cost/benefit decision-making task. Cereb Cortex. 2016;26:1529–38. doi: 10.1093/cercor/bhu321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosking JG, Cocker PJ, Winstanley CA. Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort. Neuropsychopharmacology. 2014;39:1558–67. doi: 10.1038/npp.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoeler T, Bhattacharyya S. The effect of cannabis use on memory function : an update. Subst Abuse Rehabil. 2013;4:11–27. doi: 10.2147/SAR.S25869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pattij T, Janssen MCW, Schepers I, et al. Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology (Berl) 2007;193:85–96. doi: 10.1007/s00213-007-0773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 26.Sink KS, Vemuri VK, Olszewska T, et al. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology (Berl) 2008;196:565–74. doi: 10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felder CC, Joyce KE, Briley EM, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–50. [PubMed] [Google Scholar]
- 28.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burkey TH, Quock RM, Consroe P, et al. delta 9-Tetrahydrocannabinol is a partial agonist of cannabinoid receptors in mouse brain. Eur J Pharmacol. 1997;323:R3–4. doi: 10.1016/s0014-2999(97)00146-5. [DOI] [PubMed] [Google Scholar]
- 30.Kearn CS, Greenberg MJ, DiCamelli R, et al. Relationships between ligand affinities for the cerebellar cannabinoid receptor CB1 and the induction of GDP/GTP exchange. J Neurochem. 1999;72:2379–87. doi: 10.1046/j.1471-4159.1999.0722379.x. [DOI] [PubMed] [Google Scholar]
- 31.Laprairie RB, Bagher AM, Kelly MEM, et al. Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J Biol Chem. 2014;289:24845–62. doi: 10.1074/jbc.M114.557025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudebeck PH, Walton ME, Smyth AN, et al. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–8. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 33.Parvizi J, Rangarajan V, Shirer WR, et al. The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron. 2013;80:1359–67. doi: 10.1016/j.neuron.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill EL, Gallopin T, Férézou I, et al. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol. 2007;97:2580–9. doi: 10.1152/jn.00603.2006. [DOI] [PubMed] [Google Scholar]
- 35.Singh ME, Verty N, Price I, et al. Modulation of morphine-induced Fos-immunoreactivity by the cannabinoid receptor antagonist SR 141716. Neuropharmacology. 2004;47:1157–69. doi: 10.1016/j.neuropharm.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Klein C, Karanges E, Spiro A, et al. Cannabidiol potentiates D9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl) 2011;218:443–57. doi: 10.1007/s00213-011-2342-0. [DOI] [PubMed] [Google Scholar]
- 37.Zuardi AW, Hallak JES, Crippa JAS. Interaction between cannabidiol (CBD) and D9-tetrahydrocannabinol (THC): influence of administration interval and dose ratio between the cannabinoids. Psychopharmacology (Berl) 2012;219:247–9. doi: 10.1007/s00213-011-2495-x. [DOI] [PubMed] [Google Scholar]
- 38.Laprairie RB, Bagher AM, Kelly MEM, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–74. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borgwardt SJ, Allen P, Bhattacharyya S, et al. Neural basis of delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry. 2008;64:966–73. doi: 10.1016/j.biopsych.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Fusar-Poli P, Crippa JA, Bhattacharyya S, et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66:95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- 42.Wright MJ, Vandewater S, Taffe M. Cannabidiol attenuates deficits of visuospatial associative memory induced by D(9) tetrahydrocannabinol. Br J Pharmacol. 2013;170:1365–73. doi: 10.1111/bph.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craft RM, Marusich J, Wiley JL. Sex differences in cannabinoid pharmacology: A reflection of differences in the endocannabinoid system? Life Sci. 2013;92:476–81. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110:19–35. doi: 10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- 45.Horwood LJ, Fergusson DM, Hayatbakhsh MR, et al. Cannabis use and educational achievement: findings from three Australasian cohort studies. Drug Alcohol Depend. 2010;110:247–53. doi: 10.1016/j.drugalcdep.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Welty TE, Luebke A, Gidal BE. Cannabidiol: promise and pitfalls. Epilepsy Curr. 2014;14:250–2. doi: 10.5698/1535-7597-14.5.250. [DOI] [PMC free article] [PubMed] [Google Scholar]