Abstract
Background
Increased attention bias toward drug-related cues over non–drug-related intrinsically pleasant reinforcers is a hallmark of drug addiction. In this study we used the late positive potential (LPP) to investigate whether such increased attention bias toward drug-related relative to non–drug-related cues changes over a protracted period of reduced drug use in treatment-seeking individuals with a cocaine use disorder (CUD).
Methods
Treatment-seeking individuals with CUD and matched healthy controls passively viewed a series of pleasant, neutral and drug-related pictures while their event-related potentials were recorded at baseline (≤ 3 weeks after treatment initiation) and at 6-month follow-up (only CUD).
Results
We included 19 treatment-seeking individuals with CUD and 18 matched controls in our analyses. The results showed a reversal in attention bias (i.e., LPP amplitude) from baseline (i.e., drug > pleasant) to follow-up (i.e., pleasant > drug) driven by an increased attentional engagement with pleasant pictures; this LPP reversal was paralleled by a concomitant reduction in self-reported wanting and craving for cocaine in the CUD group. Furthermore, reduced attention bias toward drug-related cues (relative to pleasant cues) was correlated with longer duration of abstinence at baseline, and the extent of its longitudinal reversal was correlated with decreased craving at follow-up, providing support for abstinence as a putative mechanism of this bottom–up attentional change.
Limitations
A limited sample size and the use of the same set of pictures at baseline and follow-up were the major limitations of this study.
Conclusion
Results collectively indicate that, by tracking with drug abstinence, LPP in response to drug-related relative to pleasant cues may serve as an indicator of clinical progress in treatment-seeking individuals with CUD.
Introduction
Drug addiction is a neuropsychiatric disorder characterized by attentional abnormalities whereby enhanced attention is afforded to drugs and drug-related cues at the expense of other (including intrinsically pleasant) reinforcers (e.g., food, sex or money).1,2 This attention bias toward drug-related cues is posited to result from conditioning to drug-related cues, which become excessively and motivationally salient through habitual use.1,3,4 The underlying mechanism invokes changes to mesolimbic dopamine transmission, associated with an increased incentive salience that is automatically attributed to drug-related cues.5,6 Ultimately, this drug-related attention bias influences drug-seeking behaviour, which in turn is associated with increased craving7–9 and relapse susceptibility.10–13 Abstinence from drug use improves cognitive and affective functioning, including attentional bias/dysregulation, in drug-addicted individuals.14
To objectively quantify attention to salient cues, several prior studies have used the late positive potential (LPP), an event-related potential (ERP) component that marks motivated attention to emotionally salient stimuli.15,16 Specifically, increased LPP amplitude in response to drug-related compared with neutral cues has consistently been shown across all substance use disorders17–23 and has further been linked with cue-induced craving.24 Importantly, cross-sectional studies have shown that the LPP amplitude to drug-related cues is decreased25,26 while that to non–drug-related cues is increased19,27 after a period of reduced drug use (3 d to about 1 yr). Moreover, prospective neuroimaging studies modelling relapse vulnerability and future drug use have shown that reduced attention toward drug-related cues11,12,28–30 and increased attention toward non–drug-related reinforcers31–33 predicted longer abstinence durations in individuals with substance use disorders. Although these studies are highly informative, many have been limited to the cross-sectional effects of shorter-term19 or longer-term abstinence25,31 relying mostly on self-reported follow-up assessments.12,28–30,32,33 An exception is a longitudinal behavioural study that reported reduced attention bias to drug-related cues (assessed via an emotional Stroop task) in heroin-addicted individuals after 3 weeks of heroin abstinence.11 Thus, a systematic within-subjects longer-term longitudinal investigation that examines the effects of drug use reduction/abstinence on attention bias to drug-related compared with non–drug-related reinforcers is lacking.
The goal of the present study was to examine the effects of reduced drug use on LPP-indexed attention bias (to drug-related v. alternative pleasant stimuli) using a longitudinal pre–post design in initially abstinent treatment-seeking individuals with cocaine use disorders (CUD). We hypothesized that, compared with baseline, at 6-month follow-up (during which drug use was substantially reduced or eliminated) individuals with CUD would show decreased LPPs in response to drug-related images and increased LPPs to pleasant images, reflecting a reversal in relative attention bias. Furthermore, given prior reports, we predicted that this attention bias modulation would track with reduced drug craving and longer abstinence duration.
Methods
Participants
We recruited treatment-seeking individuals with CUD for participation in our study. They all completed the study procedures twice: at baseline after detoxification (≥ 3 wk after last drug use) and then again 6 months later. For comparison, we recruited matched healthy controls for scanning; unlike individuals with CUD who were scanned twice, controls were scanned only once (at baseline). To be included in the study, all participants were required to be native English speakers and free of sustained/maintenance medications for more than 30 days prior and throughout the study. Exclusion criteria were history of head trauma or loss of consciousness (> 30 min) or other neurologic diseases of central origin (including seizures), current medical diseases that required hospitalization or regular monitoring, positive urine screens for psychoactive drugs or their metabolites (phencyclidine, benzodiazepines, cannabis, opiates, barbiturates and inhalants) other than cocaine, and verbal intelligence score more than 2 standard deviations below normal. Depression, ascertained via the Beck Depression Inventory,34 and cigarette smoking status were assessed at baseline in all participants and at follow-up in those with CUD. All participants were fully informed of all study procedures and risks, and they provided written informed consent in accordance with the Stony Brook University Institutional Review Board and the associated treatment facility’s Institutional Review Board. A detailed outline of recruitment sources is presented in Appendix 1, available at jpn.ca.
Our sample size, albeit relatively small, is consistent with the anticipated effect sizes in a longitudinal study examining attention bias in individuals with CUD. Specifically, prior cross-sectional studies assessing attention to salient cues in using versus abstaining individuals with addition19,25–27 found effect sizes (Cohen d) that averaged 0.9. We determined that to detect effect sizes of this magnitude in a longitudinal study with 2 time points (baseline and follow-up) at a significance level of p < 0.05 and 80% power we would need a 10 participants per time point.
Diagnostic interview
We conducted a clinical diagnostic interview with all participants at baseline, consisting of the Structured Clinical Interview for DSM-IV axis I disorders;38,39 the Addiction Severity Index,40 a semistructured interview instrument that assesses the severity as well as recent and lifetime history of alcohol- and drug-related problems as they relate to 7 problem areas (medical, employment, legal, alcohol, other drug use, family-social functioning and psychological status); the 18-item Cocaine Selective Severity Assessment Scale,41 designed to evaluate cocaine abstinence/withdrawal signs and symptoms (i.e., sleep impairment, anxiety, energy levels, craving, and depressive symptoms) within 24 hours of the interview; the 5-item Severity of Dependence Scale;42 and the 5-item Cocaine Craving Questionnaire.43 This interview determined that individuals with CUD met the criteria for current cocaine dependence or cocaine dependence in partial or sustained remission.
Abstinence assessment in individuals with CUD
To characterize our treatment-seeking sample, we tracked participants’ abstinence from baseline to follow-up using a 3-tiered system.35 First, we made monthly phone calls to all individuals with CUD and their designated collaterals (e.g., family member, counselor); for those participants who remained in residential care for the duration of the 6-month study, we consulted the treatment centre (Samaritan Village) directly. At follow-up, participants completed the Timeline Follow-back Calendar (CL-90),36 a retrospective calendar that assesses drug use (including number of days and amount used) in the past 90 days. Finally, also at follow-up, we collected retrospective data regarding last date of cocaine use. Participants were informed about the importance of reliable self-reporting, and they had no incentive for denial or biased reporting since neither inclusion into the study nor compensation were contingent on abstinence. Nevertheless, there was a discrepancy between 1 participant self-report (reporting abstinence) and a collateral report (reporting probable cocaine use); as a conservative measure, we coded this participant as having used cocaine.
Study procedures
On each visit, participants underwent electroencephalogram (EEG) recordings as they passively viewed a set of 120 pictures. These pictures were selected from the International Affective Picture System37 and included 30 pleasant, 30 unpleasant and 30 neutral pictures. The fourth picture category (30 pictures) depicted drugs and individuals preparing, using or simulating use of cocaine, as previously described.18,19 In accordance with our a priori hypothesis, in this study we report only the results in response to pictures from pleasant, drug and neutral categories (Fig. 1A–C). Details regarding the normative valence and arousal ratings of these pictures and steps involved in EEG signal acquisition and preprocessing are presented in Appendix 1. The number of artifact-free trials used to create averaged ERPs for each condition is included in Appendix 1, Table S1.
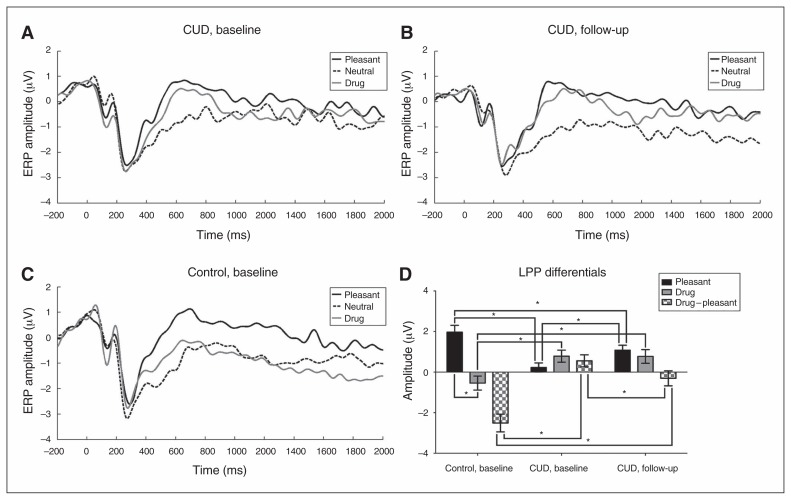
Fig. 1.
Grand averaged event-related potential (ERP) waveforms at the C1, Cz, C2, CP1, CPz and CP2 electrodes for pleasant, neutral and drug images for individuals with cocaine use disorders (CUD) at (A) baseline and (B) 6-month follow-up and for (C) healthy controls at baseline. (D) Bar graphs of late positive potential (LPP) differentials between controls and treatment-seeking individuals with CUD for pleasant (relative to neutral), drug (relative to neutral) and the drug – pleasant direct contrast. *p < 0.05.
Picture ratings
Immediately following EEG recordings, participants rated each picture on its evocation of cocaine liking (e.g., “Rate how much you like (or do not like) cocaine in response to this picture”) and wanting (e.g., “Rate how much you want (or do not want) cocaine in response to this picture”). These ratings were collected with a computerized version of the Self-Assessment Manikin38 (SAM), for which participants chose the numbers 1 through 9 (9 corresponded to most liking/wanting and 1 corresponded to least liking/wanting) that appeared below the SAM characters.
Attention bias contrasts
The ERP time series in response to neutral pictures were subtracted from those in response to pleasant and drug-related pictures (for normalization purposes). From these difference waveforms, LPP amplitudes were scored as the averaged activity from 400 ms to 2000 ms at the C1, Cz, C2, CP1, CPz and CP2 electrodes. The longer time window (1600 ms) for LPP quantification was chosen as the hypothesis is specific to the overall attention in response to salient cues (during the entire presentation) and the longitudinal changes in attention bias from baseline to 6-month follow-up. Similar or even longer time windows have previously been used to quantify LPP amplitude.16,39,40 Hereafter, these differential scores are referred to as pleasant (i.e., pleasant – neutral) and drug (i.e., drug – neutral) LPP scores. Moreover, differential scores for the drug pictures relative to the pleasant pictures were also created, as done previously18,30 as a parameter of attention bias to 2 salient reinforcers; a similar contrast, evaluating drug-taking behaviour in the presence of another concurrently available salient nondrug alternative, is used in drug choice procedures across species41–43 and mirrors the addiction diagnostic criterion of using the drug of choice to the exclusion of other activities.44
Statistical analysis
We analyzed longitudinal effects in individuals with CUD using a pictures (pleasant v. drug) × time (baseline v. follow-up) 2 × 2 repeated-measures analysis of variance (ANOVA), in which both pictures and time were entered as within-group factors. As a targeted test of our “reallocation of attention” hypothesis, we also examined the a priori–defined differential (drug – pleasant) scores using a paired t test (baseline v. follow-up). Subsequently, to ascertain whether effects reflected a normalization of function, healthy controls at baseline were compared with individuals with CUD at baseline and separately with individuals with CUD at follow-up on LPP amplitudes using pictures (pleasant v. drug) × group (CUD v. control) 2 × 2 mixed-model analyses of covariance (ANCOVAs), controlling for the effects of depression. Controlling for depression is theoretically justified because severe depression is a comorbid symptom in individuals with CUD;45 its severity (assessed via BDI) was expectedly significantly higher in individuals with CUD than in controls in the present sample. Analyses with pleasant and drug picture ratings were undertaken to complement the electrocortical results using the same analytical approach. In examining all omnibus effects in the ANOVAs, we applied the Greenhouse–Geisser correction for cases in which sphericity was not met. To localize their source, all significant interactions were followed by independent and paired t tests.
In addition to these ANOVAs, Spearman rank correlation analyses examined associations between the LPP amplitude for each picture category with respective self-reported liking and wanting ratings for cocaine. To specifically assess the hypothesized competition for attentional resources (assessed via ERPs) and its behavioural correlates (assessed via ratings), Spearman correlations examined associations between the LPP amplitude in response to drug-related cues with subjective ratings of pleasant pictures and vice versa. Finally, to test for possible relevance of our main variables to CUD symptomology, Spearman correlations examined associations between differential LPP scores and ratings (i.e., drug – pleasant for both variables) with clinical variables.
Secondary analyses were conducted to investigate the longitudinal stability of our pleasant – neutral and drug – neutral contrasts. We tested whether the raw LPP amplitude in response to neutral pictures also changed from baseline to follow-up using a paired t test. Additionally, to test whether the degree of change in attention bias was affected by a lapse in abstinence, we conducted a pictures (pleasant v. drug) × time (baseline v. follow-up) × lapse (lapsed v. abstinent) mixed ANOVA, with pictures and time as within-subjects factors and lapse as a between-subjects factor. Finally, we investigated the impact of cigarette smoking status on LPP amplitudes using an independent t test, comparing smokers and nonsmokers across diagnosis at baseline. The results of these secondary analyses are reported in Appendix 1.
Results
Participants
We included 19 treatment-seeking individuals with CUD (8 women, mean age 41.7 ± 7.4 yr) and 18 matched healthy controls (5 women, mean age 43.2 ± 6.2 yr) in our study (Table 1). At baseline, the groups differed on depression (t26.36 = 2.08, p = 0.048) and cigarette smoking status (11 of 19 individuals with CUD v. 3 of 18 controls were current smokers; χ21 = 6.68, p = 0.029).
Table 1.
Demographic and drug use characteristics in treatment-seeking individuals with cocaine use disorders at baseline and follow-up and in healthy controls at baseline
| Group, time point; mean ± SD* | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | CUD, baseline (n = 19) | CUD, follow-up (n = 19) | Control, baseline† (n = 18) | Statistical test |
| Demographic | ||||
| Age, yr | 41.68 ± 7.4 | 42.31 ± 7.6 | 43.20 ± 6.2 | t = −0.67 |
| Sex, male/female | 11/8 | — | 13/5 | χ2 = 0.83 |
| Education, yr | 12.63 ± 2.9 | — | 13.08 ± 1.4 | t = −0.62 |
| Nonverbal IQ‡ | 9.58 ± 3.4 | — | 11.12 ± 2.2 | t = −1.62 |
| Depression: Beck Depression Inventory II§ | 7.00 ± 8.1 | 5.40 ± 7.5 | 2.28 ± 4.4 | F = 2.08 |
| Drug use | ||||
| Cigarette smokers, current/past or nonsmoker§ | 11/8 | — | 3/15 | χ2 = 6.68 |
| Daily cigarettes in current smokers | 5.27 ± 3.6 | 6.91 ± 6.4 | 4.67 ± 5.0 | F = −1.24 |
| Age at onset of cocaine use, yr | 25.53 ± 9.5 | — | — | — |
| Duration of cocaine use, yr | 14.00 ± 7.8 | — | — | — |
| Duration of current abstinence, d | 143.88 ± 169.23 | 183.12 ± 136.16 | — | Z = −1.34 |
| Frequency of cocaine use (last 30 d), d/wk | 0.53 ± 1.7 | 0.06 ± 0.2 | — | Z = 1.14 |
| Cocaine Craving Questionnaire score (0–45) | 7.6 ± 4.8 | 7.0 ± 8.8 | — | Z = 0.27 |
ANOVA = analysis of variance; CUD = cocaine use disorder; SD = standard deviation.
Unless indicated otherwise.
Healthy controls completed the passive viewing of images only once.
Determined using the Wechsler Abbreviated Scale of Intelligence, Matrix Reasoning Scale.
p < 0.05; χ2 tests were used for categorical variables; Mann–Whitney U for all drug-related variables (continuous non-normally distributed variables) and ANOVAs for all comparisons between the 3 groups.
Among participants with CUD, median abstinence at baseline was 75 days, and median abstinence at 6-month follow-up was 210 days. The average time between scanning sessions across all participants was 197.3 ± 27.4 days. Of the 19 individuals with CUD, 11 met the criteria for current cocaine dependence, 6 for cocaine dependence in partial remission, and 2 for cocaine dependence in sustained remission. Comorbidities included marijuana use disorder (n = 4), ecstasy abuse (n = 1), alcohol use disorder (n = 5), opiate use disorder (n = 1), antisocial personality disorder (n = 4) and posttraumatic stress disorder (n = 1). All other comorbidities were in partial or sustained remission. Between baseline and follow-up, 12 individuals with CUD remained abstinent (259 ± 117.5 d abstinent) whereas 7 had lapsed (1–5 instances of cocaine use) between the 2 sessions (74.7 ± 73.6 d abstinent). Despite any instances of drug use, both the abstinent and the lapsed individuals showed a significant reduction in frequency of recent cocaine use from baseline (0.83 d/wk v. 1.43 d/wk) to follow-up (0 d/wk v. 0.29 d/wk; Z = 2.63, p = 0.036).
Longitudinal LPP comparisons in individuals with CUD
In individuals with CUD, the repeated-measures ANOVA revealed a significant pictures × time interaction (F1,18 = 7.084, p = 0.016), while the main effects did not reach significance (all p > 0.10). Follow-up t tests showed that although LPPs in response to drug-related cues were not different from baseline to follow-up (all p > 0.9), the LPPs in response to pleasant cues significantly increased from baseline to follow-up (t18 = 2.383, p = 0.028). Further highlighting this finding, the a priori planned comparison for the drug – pleasant LPPs revealed a significant decrease from baseline to follow-up (t18 = 2.66, p = 0.016). These results indicate a significant change in attention bias: whereas drug-related cues elicited higher LPP amplitude than pleasant cues at baseline, at follow-up this difference was reversed (Table 2; Fig. 1B).
Table 2.
Late positive potential amplitudes and task-related liking and wanting ratings for cocaine for each condition in healthy controls at baseline and in individuals with cocaine use disorders at baseline and 6-month follow-up
| Group, time point; mean ± SD | |||
|---|---|---|---|
|
|
|||
| Measure | Control, baseline | CUD, baseline | CUD, follow-up |
| Pleasant LPP, μV*† | 1.12 ± 1.79 | −0.28 ± 2.00 | 0.19 ± 1.54 |
| Drug LPP, μV*† | −0.41 ± 1.03 | 0.27 ± 1.90 | −0.12 ± 1.44 |
| Neutral LPP, μV | −1.63 ± 1.80 | −0.51 ± 1.75 | −0.89 ± 1.44 |
| Drug – pleasant LPP, μV*†‡ | −1.54 ± 1.50 | 0.56 ± 1.29 | −0.31 ± 1.61 |
| Pleasant ratings | |||
| Liking*† | 1.45 ± 0.96 | 2.92 ± 1.71 | 2.82 ± 1.76 |
| Wanting | 2.55 ± 3.40 | 2.28 ± 1.47 | 2.77 ± 1.69 |
| Drug ratings | |||
| Liking*† | 1.33 ± 0.96 | 3.38 ± 1.75 | 3.55 ± 2.32 |
| Wanting | 2.56 ± 3.33 | 3.56 ± 1.69 | 3.59 ± 2.38 |
| Neutral ratings | |||
| Liking | 1.60 ± 1.11 | 2.34 ± 1.73 | 2.45 ± 1.59 |
| Wanting | 1.67 ± 2.08 | 1.98 ± 1.41 | 2.39 ± 1.57 |
| Drug – pleasant ratings | |||
| Liking† | −0.17 ± 0.33 | 0.45 ± 1.91 | 0.73 ± 1.37 |
| Wanting*† | −0.06 ± 0.16 | 1.27 ± 1.55 | 0.82 ± 1.48 |
CUD = cocaine use disorder; LPP = late positive potential; SD = standard deviation.
Significant difference between controls and CUD at baseline (p < 0.05).
Significant difference between controls at baseline and CUD at follow-up (p < 0.05).
Significant difference in CUD between baseline and follow-up (p < 0.05).
LPP group comparisons
At baseline, the mixed ANCOVA revealed a significant main effect of pictures (F1,36 = 13.62, p = 0.001; pleasant > drug) and a significant pictures × group interaction (F1,36 = 34.37, p < 0.001). Follow-up t tests revealed that compared with healthy controls at baseline, individuals with CUD at baseline showed significantly higher LPPs in response to drug cues (t35 = 2.92, p = 0.006) and significantly lower activity in response to pleasant cues (t35 = 4.33, p < 0.001). Furthermore, within-group analyses revealed marginally higher LPP amplitudes to drug cues compared with pleasant cues in individuals with CUD at baseline (t18 = 1.88, p = 0.08) and significantly higher LPP amplitudes to pleasant cues compared with drug cues in healthy controls (t18 = 5.75, p < 0.001; Table 2 and Fig. 1B).
A similar pattern of results emerged when we compared individuals with CUD at follow-up with healthy controls at baseline. The mixed ANOVA revealed a significant main effect of pictures (F1,35 = 24.45, p < 0.001; pleasant > drug) and a significant pictures × group interaction (F1,35 = 14.98, p < 0.001). Follow-up independent t tests revealed that individuals with CUD had higher LPPs in response to drug-related cues (t35 = 2.73, p = 0.010) and lower LPPs in response to pleasant cues (t35 = 2.12, p = 0.040) at follow-up than healthy controls at baseline. However, unlike at baseline when drug LPPs marginally exceeded pleasant LPPs in individuals with CUD, within-group analyses at follow-up showed that the mean LPP for pleasant cues was higher (albeit not significantly) than the LPP for drug cues, similar to healthy controls (Table 2 and Fig. 1B).
Longitudinal rating comparisons in individuals with CUD
In individuals with CUD, the repeated-measures ANOVA revealed a significant main effect of pictures for wanting ratings (F1,13 = 11.272, p = 0.005; drug > pleasant) but not for liking ratings (p = 0.25). All other interactions and main effects did not reach significance (all p > 0.1). Similarly, the a priori planned comparison between baseline and follow-up for the drug – pleasant ratings did not reveal significant differences for either rating scale (both p > 0.05).
Rating group comparisons
Liking
At baseline, the mixed ANCOVA revealed only a significant main effect of group (F1,29 = 16.421, p < 0.001; CUD > control) and no other main effects or interactions (all p > 0.05). These results show that individuals with CUD at baseline provided higher liking ratings than healthy controls at baseline for both cocaine and pleasant pictures.
A similar pattern emerged at follow-up, as the mixed ANOVA revealed a significant main effect of group (F1,28 = 10.340, p = 0.003; CUD > control) and a significant pictures × group interaction (F1,28 = 5.329, p = 0.029); the other main effect did not reach significance (p = 0.59). Follow-up t tests showed that individuals with CUD at 6-month follow-up provided higher liking ratings for cocaine than healthy controls at baseline as they viewed drug (t18.652 = 3.407, p = 0.003) and pleasant (t21.690 = 2.664, p = 0.014) pictures. Paired t tests revealed that although healthy controls showed no significant difference in liking of cocaine while viewing drug and pleasant images (p = 0.17), individuals with CUD at follow-up showed higher liking of cocaine while viewing drug images compared with pleasant images (t14 = 3.984, p = 0.001).
Wanting
At baseline, the mixed ANCOVA revealed a significant main effect of pictures (F1,31 = 5.031, p = 0.032; drug > pleasant) and a significant pictures × group interaction (F1,33 = 11.670, p = 0.002), while the main effect of group did not reach significance (p = 0.67). Follow-up t tests to further dissect the interaction showed higher wanting for cocaine while viewing drug images than pleasant images (t17 = 3.494, p = 0.003).
Similarly, at follow-up the mixed ANOVA revealed a significant main effect of pictures (F1,30 = 5.225, p = 0.030; drug > pleasant) and a significant pictures × group interaction (F1,30 = 5.052, p = 0.032), while the main effect of group did not reach significance (p = 0.35). Follow-up t tests to further dissect the interaction showed that at follow-up individuals with CUD showed marginally higher wanting of cocaine while viewing drug images than pleasant images (t14 = 2.149, p = 0.05).
Correlations
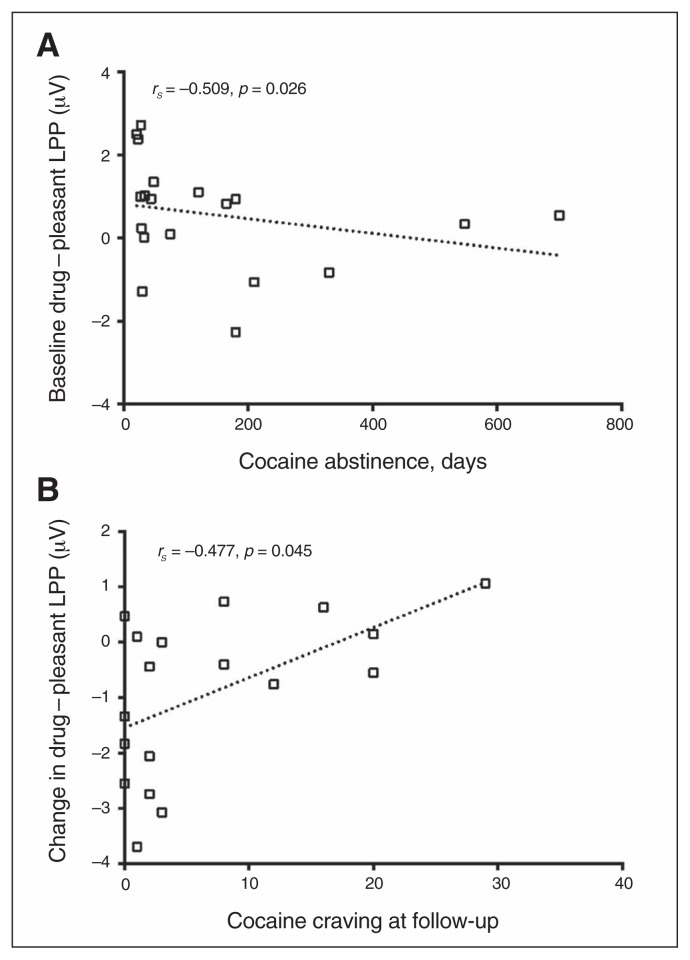
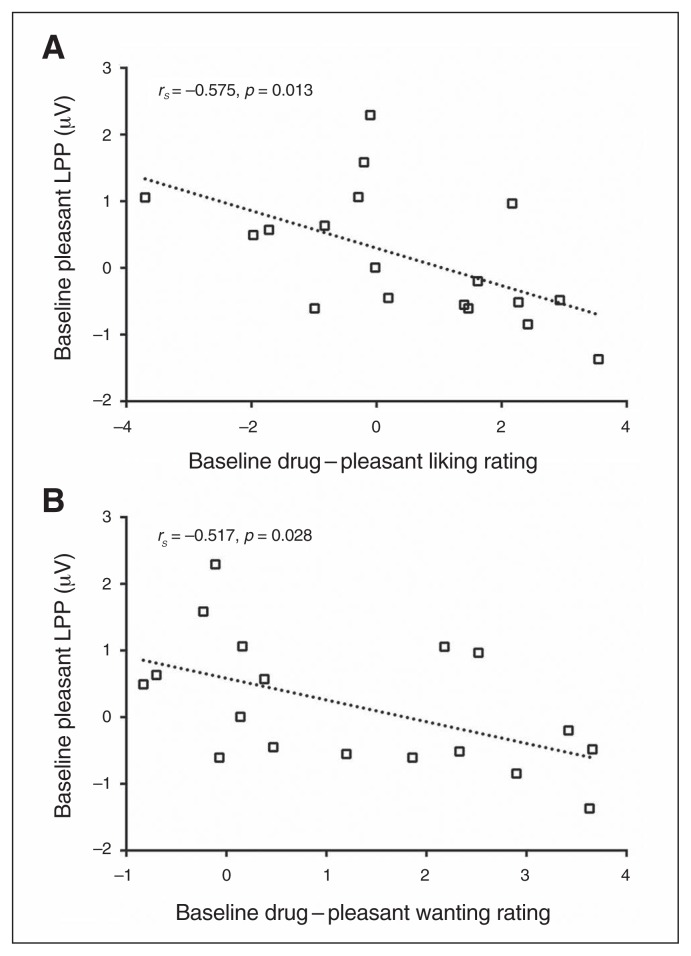
Spearman rank correlation analyses revealed that drug – pleasant LPPs negatively correlated with days of abstinence in individuals with CUD at baseline (rs = −0.509, p = 0.026), such that stronger response to drug-related cues relative to pleasant cues was associated with shorter abstinence (i.e., at baseline; Fig. 2A). The longitudinal change in these drug – pleasant cue LPPs between baseline and follow-up [(follow-up drug – pleasant) – (baseline drug – pleasant)] positively correlated with cocaine craving at follow-up (rs = 0.477, p = 0.045), such that a greater decrease in response to drug-related cues relative to pleasant cues between baseline and follow-up was associated with lower cocaine craving at follow-up (Fig. 2B). Furthermore, at baseline, LPPs elicited in response to pleasant cues were negatively correlated with self-reported liking and wanting for cocaine as individuals with CUD viewed drug relative to pleasant cues (liking: rs = −0.575, p = 0.013; wanting: rs = −0.517, p = 0.028), such that the more responsive individuals with CUD were to pleasant cues, the less they liked (Fig. 3A) and wanted (Fig. 3B) cocaine as they viewed drug-related relative to pleasant cues.
Fig. 2.
(A) The correlation between baseline drug (relative to pleasant) late positive potential (LPP) and duration of abstinence before the first scan shows that treatment-seeking individuals with cocaine use disorders (CUD) who had abstained from drug use for a longer time had greater modulated attention toward pleasant stimuli over drug-related cues. (B) The correlation between the change in drug (relative to pleasant) LPPs from baseline to 6-month follow-up and cocaine craving at the 6-month follow-up session shows a decreased motivated attention toward drug-related cues in association with decreased craving at follow-up in individuals with CUD.
Fig. 3.
The correlation between baseline pleasant late positive potential (LPP) and self-reported (A) liking and (B) wanting of cocaine in response to drug-related pictures (relative to pleasant pictures) shows that treatment-seeking individuals with cocain use disorders (CUD) who had an increased motivated attention for pleasant cues concurrently had a decreased liking and wanting of cocaine in response to drug-related cues.
The results outlining the effects of confounding factors are presented in Appendix 1.
Discussion
The present longitudinal study shows that the hyposensitivity to non–drug-related positive reinforcers in initially abstinent, treatment-seeking, drug-addicted individuals is ameliorated by sustained reduction of drug use to the extent that the attention bias reverses to favour pleasant non–drug-related reinforcers. Specifically, results demonstrated that at baseline, the attention allocated toward drug-related cues (i.e., the LPP amplitude) was higher than that toward pleasant cues, suggesting increased attention bias toward drug-related cues for individuals with CUD; those with shorter abstinence duration showed higher motivated attention toward drug-related than toward pleasant cues. However, after longer periods of abstinence and/or significantly reduced drug use, the direction of this attention bias reversed such that, in a direct contrast (drug > pleasant), increased motivated attention was allocated toward pleasant cues relative to drug-related cues; the individuals with CUD who showed a greater reversal toward this pattern (pleasant > drug) also reported decreased cocaine craving at follow-up, suggesting that the reversal in attention bias is associated with recovery from clinical symptomology.
The present study first confirms and extends previous findings of increased motivated attention toward drug-related cues7,8,17,19,20,24 compared with other non–drug-related reinforcers30,46 in individuals with CUD, as evident from increased LPP amplitude as well as increased cocaine wanting while viewing drug-related cues versus pleasant cues. Moreover, the association between the increased liking and wanting for cocaine while viewing drug-related cues and decreased LPP in response to pleasant cues further highlights the notion that chronic drug use reflects the excessive attribution of motivational value to the drug of abuse and its associated cues at the expense of other non–drug-related rewards, consistent with the impaired Response Inhibition and Salience Attribution (iRISA) model of drug addiction.1,3
Interestingly, however, following significantly reduced drug use (i.e., at follow-up), results showed reversal of this attention bias such that more attention was afforded to pleasant than to drug-related cues in individuals with CUD, and the extent of this reversal increased with reduced self-reported craving at the follow-up visit. By directly contrasting drug-related cues with pleasant cues, the observed correlation between the shift in attention bias and lower craving is consistent with prior reports, especially that of a meta-analysis that showed an association between greater attention bias toward drug-related cues and increased self-reported cravings across various substances of abuse (e.g., tobacco, cocaine, alcohol, cannabis, heroin and caffeine).47 Thus, those who showed greater craving at 6-month follow-up and lower longitudinal change in attention bias as well as greater liking for cocaine while viewing drug-related compared with pleasant pictures at follow-up may be at a greater risk for relapse. Prior studies reported associations between increased attention bias toward drug-related cues and susceptibility to relapse.11,12,29–33 That relapse status did not have a significant impact on our results is not surprising because in the present study all individuals with CUD showed a significant reduction of drug use (in both abstinent and relapsed subgroups); however, additional longitudinal studies with longer follow-up are required.
Of note, this reversal in attention bias with decreased drug use at follow-up was driven by increased responsiveness to pleasant cues rather than a decrease in reactivity to drug-related cues, which suggests that maladaptations mediated by chronic drug use that led to hyposensitivity to non–drug-related reinforcers are perhaps more plastic than those that led to hypersensitivity to drug-related cues. This conclusion is further supported by similar results from a prior behavioural study in smokers showing that smokers who maintained abstinence for at least 3 months demonstrated recovery in sensitivity to natural reinforcers compared with current cigarette smokers.48 Notably, the LPP amplitude in response to neutral pictures did not change significantly from baseline to follow-up (Appendix 1). This result is critical as LPP amplitude for pleasant and drug conditions were extracted from pleasant – neutral and drug – neutral contrasts, respectively, and shows that the effects were not driven by the modulation in LPP amplitude to neutral pictures.
Unlike some previous reports,25,49 our results did not show a longitudinal decrease in attention afforded to drug-related cues. The lack of attention modulation in response to drug-related cues has been reported in studies comparing treatment-seeking and non–treatment-seeking individuals with CUD,50 satiated and abstinent chronic smokers,51 and short- and long-term abstinent heroin users.52 These studies suggest that even after longer durations of reduced drug use, responsiveness to drug-related cues stays elevated perhaps owing to long-term pairing of the cues (i.e., pipes of crack-cocaine, other paraphernalia) with the actual drugs. This inability to divert attention away from drug-related cues has been interpreted to mark enhanced personal relevance and could reflect a strong desire to avoid drug-related cues,53 potentially suggesting an increased motivation to quit/remain abstinent in the present study. Moreover, preclinical and some human studies have shown that cue-induced craving (i.e., craving induced in response to a drug-related cue) incubates during early abstinence time windows54,55 before declining at longer abstinence durations in an inverted U-shaped trajectory.56,57 Thus, it can be inferred that abstinence durations of individuals with CUD at baseline and at follow-up in the present study were situated on either side of the peak cue-induced craving on the inverted U-shaped curve and therefore resulted in no apparent change in motivated attention to drug-related cues that precedes the craving sensation. However, follow-up studies with assessments at varying abstinence durations are required to adequately delineate the trajectory of motivated attention to drug-related cues in abstaining individuals with CUD.
Limitations
Limitations of the present study include the following. First, our sample size was not large, particularly given the heterogeneity in relapse status. Accordingly, future studies should include a larger sample size to further elucidate the impact of abstinence and relapse on attention bias in treatment-seeking individuals (possibly while also examining changes in attention bias over various periods of reduced drug use). Another limitation of the study was the use of the same stimuli in both sessions, which might have promoted habituation. However, we consider this possibility unlikely, as there was an increase in attention to pleasant cues at follow-up in individuals with CUD, and response to neutral stimuli did not change.
Conclusion
Our study showed that a 6-month period of reduced drug use in individuals with CUD resulted in a reversal in attention bias: whereas at baseline individuals with CUD exhibited more attention to drug-related than to pleasant cues, at follow-up they showed increased attention to pleasant compared with drug-related cues. The associations between this attention bias (drug > pleasant) at baseline and duration of abstinence and between its change (pleasant > drug) at follow-up and self-reported craving suggest that attention bias in addicted individuals could be malleable and may signal clinically meaningful and beneficial outcomes of longer-term abstinence and/or response to treatment. These results therefore further speak to the potential utility of EEG in providing clinically relevant markers of treatment course and its efficacy in drug addiction. Our findings can be directly translated to treatment paradigms, where there could be more emphasis on attaining and cultivating response to nondrug rewards while maintaining abstinence, thereby enhancing the salience of non–drug-related reinforcers to reduce craving and ultimately prevent relapse.
Acknowledgements
The authors gratefully thank Anna B. Konova, Tom Maloney, Patricia Woicik and Federico d’Oleire Uquillas for their help with data acquisition and analysis.
Footnotes
Funding: This study was supported by grants from the National Institute on Drug Abuse (F32DA033088 to M.A.P., 1K01DA037452 to S.J.M., R01DA023579 to R.Z.G., P50DA016556 to R.S.) and from the National Institute on Mental Health (R01MH090134 to N.A.K.).
Competing interests: None declared.
Contributors: M. Parvaz, S. Moeller, R. Sinha, N. Alia-Klein and R. Goldstein designed the study. M. Parvaz acquired and analyzed the data, which S. Moeller, P. Malaker and R. Goldstein also analyzed. M. Parvaz, S. Moeller and P. Malaker wrote the article, which all authors reviewed and approved for publication.
References
- 1.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hester R, Luijten M. Neural correlates of attentional bias in addiction. CNS Spectr. 2014;19:231–8. doi: 10.1017/S1092852913000473. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 5.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 6.Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci U S A. 2011;108:E255–64. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosse RB, Johri S, Kendrick K, et al. Preattentive and attentive eye movements during visual scanning of a cocaine cue: correlation with intensity of cocaine cravings. J Neuropsych Clin N. 1997;9:91–3. doi: 10.1176/jnp.9.1.91. [DOI] [PubMed] [Google Scholar]
- 8.Franken IH, Kroon LY, Wiers RW, et al. Selective cognitive processing of drug cues in heroin dependence. J Psychopharmacol. 2000;14:395–400. doi: 10.1177/026988110001400408. [DOI] [PubMed] [Google Scholar]
- 9.Field M, Mogg K, Mann B, et al. Attentional biases in abstinent alcoholics and their association with craving. Psychol Addict Behav. 2013;27:71–80. doi: 10.1037/a0029626. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter KM, Schreiber E, Church S, et al. Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict Behav. 2006;31:174–81. doi: 10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Marissen MA, Franken IH, Waters AJ, et al. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–12. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 12.Waters AJ, Shiffman S, Sayette MA, et al. Attentional bias predicts outcome in smoking cessation. Health Psychol. 2003;22:378–87. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox WM, Hogan LM, Kristian MR, et al. Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug Alcohol Depen. 2002;68:237–43. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 14.Garavan H, Brennan KL, Hester R, et al. The neurobiology of successful abstinence. Curr Opin Neurobiol. 2013;23:668–74. doi: 10.1016/j.conb.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schupp HT, Flaisch T, Stockburger J, et al. Emotion and attention: event-related brain potential studies. Prog Brain Res. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- 16.Hajcak G, Olvet DM. The persistence of attention to emotion: brain potentials during and after picture presentation. Emotion. 2008;8:250–5. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- 17.van de Laar MC, Licht R, Franken IH, et al. Event-related potentials indicate motivational relevance of cocaine cues in abstinent cocaine addicts. Psychopharmacology (Berl) 2004;177:121–9. doi: 10.1007/s00213-004-1928-1. [DOI] [PubMed] [Google Scholar]
- 18.Moeller SJ, Hajcak G, Parvaz MA, et al. Psychophysiological prediction of choice: relevance to insight and drug addiction. Brain. 2012;135:3481–94. doi: 10.1093/brain/aws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunning JP, Parvaz MA, Hajcak G, et al. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users — an ERP study. Eur J Neurosci. 2011;33:1716–23. doi: 10.1111/j.1460-9568.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franken IH, Dietvorst RC, Hesselmans M, et al. Cocaine craving is associated with electrophysiological brain responses to cocaine-related stimuli. Addict Biol. 2008;13:386–92. doi: 10.1111/j.1369-1600.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 21.Minnix JA, Versace F, Robinson JD, et al. The late positive potential (LPP) in response to varying types of emotional and cigarette stimuli in smokers: a content comparison. Int J Psychophysiol. 2013;89:18–25. doi: 10.1016/j.ijpsycho.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asmaro D, Carolan PL, Liotti M. Electrophysiological evidence of early attentional bias to drug-related pictures in chronic cannabis users. Addict Behav. 2014;39:114–21. doi: 10.1016/j.addbeh.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Franken IH, Stam CJ, Hendriks VM, et al. Neurophysiological evidence for abnormal cognitive processing of drug cues in heroin dependence. Psychopharmacology (Berl) 2003;170:205–12. doi: 10.1007/s00213-003-1542-7. [DOI] [PubMed] [Google Scholar]
- 24.Franken IH, Hulstijn KP, Stam CJ, et al. Two new neurophysiological indices of cocaine craving: evoked brain potentials and cue modulated startle reflex. J Psychopharmacol. 2004;18:544–52. doi: 10.1177/0269881104047282. [DOI] [PubMed] [Google Scholar]
- 25.Littel M, Franken IH. The effects of prolonged abstinence on the processing of smoking cues: an ERP study among smokers, ex-smokers and never-smokers. J Psychopharmacol. 2007;21:873–82. doi: 10.1177/0269881107078494. [DOI] [PubMed] [Google Scholar]
- 26.Vollstädt-Klein S, Loeber S, von der Goltz C, et al. Avoidance of alcohol-related stimuli increases during the early stage of abstinence in alcohol-dependent patients. Alcohol Alcohol. 2009;44:458–63. doi: 10.1093/alcalc/agp056. [DOI] [PubMed] [Google Scholar]
- 27.Aguilar de Arcos F, Verdejo-Garcia A, Ceverino A, et al. Dysregulation of emotional response in current and abstinent heroin users: negative heightening and positive blunting. Psychopharmacology (Berl) 2008;198:159–66. doi: 10.1007/s00213-008-1110-2. [DOI] [PubMed] [Google Scholar]
- 28.Cox WM, Pothos EM, Hosier SG. Cognitive-motivational predictors of excessive drinkers’ success in changing. Psychopharmacology (Berl) 2007;192:499–510. doi: 10.1007/s00213-007-0736-9. [DOI] [PubMed] [Google Scholar]
- 29.Janes AC, Pizzagalli DA, Richardt S, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–9. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lubman DI, Yucel M, Kettle JW, et al. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch Gen Psychiatry. 2009;66:205–12. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- 31.Heinz A, Wrase J, Kahnt T, et al. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol Clin Exp Res. 2007;31:1138–47. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- 32.Versace F, Engelmann JM, Robinson JD, et al. Prequit fMRI responses to pleasant cues and cigarette-related cues predict smoking cessation outcome. Nicotine Tob Res. 2014;16:697–708. doi: 10.1093/ntr/ntt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Versace F, Lam CY, Engelmann JM, et al. Beyond cue reactivity: blunted brain responses to pleasant stimuli predict long-term smoking abstinence. Addict Biol. 2012;17:991–1000. doi: 10.1111/j.1369-1600.2011.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck AT, Steer RA, Ball R, et al. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 35.Moeller SJ, Beebe-Wang N, Woicik PA, et al. Choice to view cocaine images predicts concurrent and prospective drug use in cocaine addiction. Drug Alcohol Depend. 2013;130:178–85. doi: 10.1016/j.drugalcdep.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl. 1994;12:112–8. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- 37.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instructional Manual Technical Report A-8. Gainsville, FL: University of Florida; 2008. [Google Scholar]
- 38.Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 39.Parvaz MA, Macnamara A, Goldstein RZ, et al. Event-related induced frontal alpha as a marker of lateral prefrontal cortex activation during cognitive reappraisal. Cogn Affect Behav Neurosci. 2012;12:730–40. doi: 10.3758/s13415-012-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajcak G, Dunning JP, Foti D. Motivated and controlled attention to emotion: time-course of the late positive potential. Clin Neurophysiol. 2009;120:505–10. doi: 10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 41.Banks ML, Hutsell BA, Schwienteck KL, et al. Use of preclinical drug vs. food choice procedures to evaluate candidate medications for cocaine addiction. Curr Treat Options Psychiatry. 2015;2:136–50. doi: 10.1007/s40501-015-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foltin RW. Food and cocaine self-administration by baboons: effects of alternatives. J Exp Anal Behav. 1999;72:215–34. doi: 10.1901/jeab.1999.72-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moeller SJ, Stoops WW. Cocaine choice procedures in animals, humans, and treatment-seekers: Can we bridge the divide? Pharmacol Biochem Behav. 2015;138:133–41. doi: 10.1016/j.pbb.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, DC: 2013. [Google Scholar]
- 45.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–8. [PubMed] [Google Scholar]
- 46.Lubman DI, Allen NB, Peters LA, et al. Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. J Psychopharmacol. 2008;22:836–42. doi: 10.1177/0269881107083846. [DOI] [PubMed] [Google Scholar]
- 47.Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawkins L, Powell JH, Pickering A, et al. Patterns of change in withdrawal symptoms, desire to smoke, reward motivation and response inhibition across 3 months of smoking abstinence. Addiction. 2009;104:850–8. doi: 10.1111/j.1360-0443.2009.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ehrman RN, Robbins SJ, Bromwell MA, et al. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug Alcohol Depen. 2002;67:185–91. doi: 10.1016/s0376-8716(02)00065-0. [DOI] [PubMed] [Google Scholar]
- 50.Vadhan NP, Carpenter KM, Copersino ML, et al. Attentional bias towards cocaine-related stimuli: relationship to treatment-seeking for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:727–36. doi: 10.1080/00952990701523722. [DOI] [PubMed] [Google Scholar]
- 51.McClernon FJ, Hiott FB, Huettel SA, et al. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–7. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Preller KH, Wagner M, Sulzbach C, et al. Sustained incentive value of heroin-related cues in short- and long-term abstinent heroin users. Eur Neuropsychopharmacol. 2013;23:1270–9. doi: 10.1016/j.euroneuro.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci. 2014;18:635–41. doi: 10.1016/j.tics.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimm JW, Hope BT, Wise RA, et al. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bedi G, Preston KL, Epstein DH, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–11. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu L, Grimm JW, Dempsey J, et al. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 2004;176:101–8. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- 57.Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8:e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]