Abstract
Background
Memory-based alterations are among the hallmark symptoms of posttraumatic stress disorder (PTSD) and may be associated with the integrity of the hippocampus. However, neuroimaging studies of hippocampal volume in individuals with PTSD have yielded inconsistent results, raising the possibility that various moderators, such as genetic factors, may influence this association. We examined whether the catechol-O-methyltransferase (COMT) Val158Met polymorphism, which has previously been shown to be associated with hippocampal volume in healthy individuals, moderates the association between PTSD and hippocampal volume.
Methods
Recent war veterans underwent structural MRI on a 3 T scanner. We extracted volumes of the right and left hippocampus using FreeSurfer and adjusted them for individual differences in intracranial volume. We assessed PTSD severity using the Clinician-Administered PTSD Scale. Hierarchical linear regression was used to model the genotype (Val158Met polymorphism) × PTSD severity interaction and its association with hippocampal volume.
Results
We included 146 white, non-Hispanic recent war veterans (90% male, 53% with diagnosed PTSD) in our analyses. A significant genotype × PTSD symptom severity interaction emerged such that individuals with greater current PTSD symptom severity who were homozygous for the Val allele showed significant reductions in left hippocampal volume.
Limitations
The direction of proposed effects is unknown, thus precluding definitive assessment of whether differences in hippocampal volume reflect a consequence of PTSD, a pre-existing characteristic, or both.
Conclusion
Our findings suggest that the COMT polymorphism moderates the association between PTSD and hippocampal volume. These results highlight the role that the dopaminergic system has in brain structure and suggest a possible mechanism for memory disturbance in individuals with PTSD.
Introduction
Memory disturbances manifested in the form of intrusive trauma memories, flashbacks and deficits in episodic memory are among the hallmark symptoms of posttraumatic stress disorder (PTSD).1 These memory alterations have often been described as devoid of spatiotemporal context and other properties that are the defining features of episodic memory.2 Accordingly, given its role in memory encoding and consolidation and its modulatory influence on hypothalamic–pituitary–adrenal (HPA) axis function, the hippocampus has long been a focus of research on the etiology and maintenance of PTSD.3 Recent meta-analyses have found evidence for PTSD-associated reductions in hippocampal volume,4–7 though results across studies have not been uniform,8–18 suggesting that various moderating variables, including genetic factors, may influence the association between PTSD and hippocampal volume.
The rs4680 functional variant (Val158Met polymorphism) of the catechol-O-methyltransferase (COMT) gene is the most widely studied COMT single nucleotide polymorphism (SNP), and because of its role in catecholamine regulation, it has garnered substantial interest from investigators studying psychiatric conditions ranging from PTSD19–21 to obsessive–compulsive disorder22 and schizophrenia.23 The rs4680 variant generates an amino acid change from a valine (val) to a methionine (met) at codon 158 (Val158Met), leading to a 4-fold reduction in enzyme activity in Met carriers. Higher enzyme activity in Val carriers is putatively associated with lower dopamine availability.24 Importantly, COMT is highly expressed in the hippocampus.25,26 Recent studies of healthy individuals suggest that Val carriers have smaller hippocampal volumes than Met carriers,27,28 possibly owing to alterations in the neural correlates of memory function.
Although the role of COMT in the hippocampus has to date been largely underexplored, recent evidence suggests that the Val158Met polymorphism modulates dopamine metabolism in the hippocampus.29 Furthermore, this modulation of dopamine has downstream effects on hippocampally dependent tasks, such as delayed reward alternation and spatial novelty preference, with a positive association between putative levels of dopamine availability and memory performance.29 More broadly, dopamine availability is known to be involved in facilitating long-term potentiation (LTP) in the hippocampus, which is an important mechanism underlying memory encoding.30 In humans, COMT enzyme inhibition by the drug tolcapone produces episodic memory enhancement,31 as does administration of the dopamine agonist L-DOPA.32 In addition, the Met allele, which is presumed to be associated with higher levels of dopamine, is associated with better episodic memory than the Val allele.33 Given the central role that memory alterations play in PTSD34,35 and the evidence that the Val158Met polymorphism is associated with PTSD symptoms,19,20,36 we examined whether the Val158Met polymorphism moderated the association between PTSD symptom severity and hippocampal volume.
Methods
Participants
We recruited veterans of Operations Enduring Freedom, Iraqi Freedom and New Dawn for participation in this study as part of the Translational Research Center for Traumatic Brain Injury and Stress Disorders (TRACTS) at the Jamaica Plain Division of the VA Boston Healthcare System. Individuals were excluded if their medical history included vascular disease, cardiac conditions, seizures, cognitive disorders not due to traumatic brain injury (TBI), moderate or severe TBI, homicidal or suicidal ideation requiring intervention, or a current psychotic disorder (e.g., schizophrenia, bipolar disorder) based on a DSM-IV diagnosis.37 For MRI acquisition, individuals were excluded if they had any metal implant, shrapnel, aneurysm clip, or pacemaker, or if they were pregnant. We obtained approval for the study from all institutional review boards and regulatory committees, and informed consent was obtained from all participants before the procedure.
MRI acquisition and processing
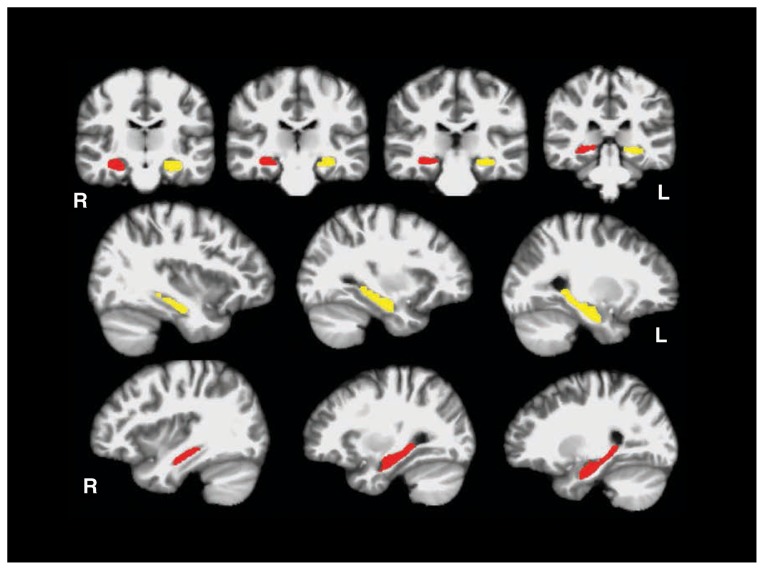
Participants were scanned on a Siemens 3 T TIM Trio located at the Jamaica Plain Division of VA Boston Healthcare System. Two 3-dimensional (3D) magnetization-prepared rapid gradient-echo (MP-RAGE) scans were acquired in the sagittal plane and averaged to create a single high contrast-to-noise image (voxel size 1 mm3, inversion time [TI] 1000 ms, repetition time [TR] 2530 ms, echo time [TE] 3.32 ms). Automated cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis suite (version 5.0), which is available for download online (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications.38–49 This processing includes motion correction and averaging50 of multiple volumetric T1-weighted images, removal of nonbrain tissue using a hybrid watershed/surface deformation procedure,51 automated Talairach transformation, segmentation of the subcortical white matter and deep grey matter volumetric structures (including the hippocampus).42,46 We used the ENIGMA pipeline to generate and inspect hippocampal volumes. This pipeline mandates careful inspection of each participant’s data to ensure segmentation accuracy (for examples using this pipeline, see van Erp and colleagues52 and Hibar and colleagues53). Automated FreeSurfer hippocampal volumes have been shown to have high overlap and correlation with expert hand-drawn volumes of the hippocampus.42,54,55 For illustration purposes, Figure 1 shows an example of the automated hippocampal volumes, as defined by FreeSurfer.
Fig. 1.
Automated segmentation of the hippocampus. Coronal and sagittal views showing the right (red) and left (yellow) hippocampal regions of interest as defined by FreeSurfer.
DNA genotyping
A trained phlebotomist collected peripheral blood samples from each participant. We isolated DNA from peripheral blood samples on a Qiagen AutoPure instrument with Qiagen reagents and samples normalized using PicoGreen assays (Invitrogen). Samples were run on Illumina HumanOmni2.5–8 BeadChips, which analyze approximately 2.5 million SNPs per sample, including rs4680 (Val158Met). Arrays were scanned using an Illumina iScan System according to the manufacturer’s protocol. All participants had genome-wide call rates > 98%, indicating generally good-quality DNA and results. The call rate for rs4680 was 100%. Details regarding the elimination of participants and genotype-based confirmation of ancestry and sex are presented in Appendix 1, available at jpn.ca.
Clinical and cognitive assessment
We assessed PTSD using the Clinician-Administered PTSD Scale (CAPS),56 which is the gold-standard structured diagnostic interview for the assessment of the disorder. The CAPS assessment was performed by doctoral-level psychologists with advanced training in psychological assessment. On this interview, each DSM-IV PTSD criterion is assessed with 2 CAPS subitems: 1 that reflects the frequency of the symptom on a 0–4 scale and 1 that reflects the intensity of the symptom on a 0–4 scale. The 2 subitems can be combined to reflect symptom severity. We derived a score for each individual using the total score for current PTSD symptoms. The presence of current and past mood and substance use disorders were assessed using the Structured Clinical Interview Scale for DSM-IV disorders (SCID).
Premorbid IQ was estimated using the Wechsler Test of Adult Reading (WTAR).57 This measure contains 50 irregularly spelled words of increasing difficulty to pronounce. The ability to perform this test remains unchanged following disease and injury.
Statistical analysis
Statistical analyses were performed using SPSS software version 22 (IBM Corp.). Hippocampal volumes were adjusted based on participants’ FreeSurfer-derived intracranial volume (ICV) to control for variation in head size using the following formula: adjusted volume = raw volume − (βICV −mean ICV), where β is the regression coefficient when the raw volume is regressed against ICV and mean ICV is the group mean.
Demographic differences among genotype groups were examined using 1-way analysis of variance (ANOVA) or χ2 tests where appropriate. We examined the association between PTSD severity, genotype and hippocampal volume using hierarchical linear regression. Covariates, including age (mean-centred to improve interpretability), major depression diagnosis, lifetime substance use disorder, handedness and probable mild TBI (assessed by the VA TBI screen), were entered in step 1. To further examine the potential impact of mild TBI, we repeated the regression model removing 69 individuals with mild TBI. The Val158Met genotype (coded additively; there was no evidence of a nonlinear trend using a polynomial regression approach: Val/Val = 0, Val/Met = 1, and Met/Met = 2) and current PTSD symptom severity (CAPS score) were entered in step 2. The genotype × PTSD symptom severity interaction was entered in step 3. These models were run separately for the adjusted volumes of the left and right hippocampus. The α for significance was adjusted using Bonferroni correction for the 2 hemispheres (p = 0.05 ÷ 2 = 0.025).
To evaluate the role of additional potential confounders, including education (highest grade completed) and population stratification (see Appendix 1 for a detailed description), we ran post hoc analyses with the potential confounder added to step 1 of the 3-stage models as previously described. To examine whether sex influenced the results, we repeated the analyses after removing the women from the data set.
Results
Sample characteristics
We included 146 (90% male) white, non-Hispanic veterans in our analyses. Group demographic characteristics by Val-158Met genotype are shown in Table 1. The groups did not differ significantly in age, sex, WTAR-estimated IQ, number of lifetime mood disorders or substance use diagnoses, CAPS score, duration of symptoms, or probable mild TBI. However, the groups differed in education, with the Met homozygote group having more education than the Val/Met group (p = 0.032).
Table 1.
Sample characteristics
| Group; mean ± SD or no. (%) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characterisitc | Total (n = 146) | Val/Val (n = 45) | Val/Met (n = 64) | Met/Met (n = 37) | Comparison statistic | p value |
| Age, yr | 31 ± 8 | 32 ± 8.1 | 31 ± 8.4 | 32 ± 8.9 | F2,143 = 0.26 | 0.77 |
| Male sex | 135 (92.5) | 43 (95.6) | 58 (90.6) | 34 (91.9) | χ22 = 0.95 | 0.62 |
| Education, yr | 14 ± 2 | 14 ± 2.3 | 14 ± 1.8 | 15 ± 1.8 | F2,143 = 3.51 | 0.032§ |
| WTAR estimated IQ* | 103 ± 11.5 | 104 ± 10.9 | 102 ± 11.4 | 104 ± 12.4 | F2,141 = 0.33, | 0.72 |
| CAPS total score | 44 ± 28.6 | 47 ± 27.7 | 45 ± 29.2 | 38 ± 28.6 | F2,143 = 1.05 | 0.35 |
| Symptom duration, mo† | 58 ± 60.5 | 63 ± 62.8 | 56 ± 68.6 | 54 ± 38.8 | F2,82 = 0.15 | 0.87 |
| Lifetime mood disorder diagnosis | 48 (32.9) | 16 (35.6) | 21 (32.8) | 11 (29.7) | χ22 = 0.31 | 0.86 |
| Lifetime substance use diagnosis | 93 (63.7) | 32 (71.1) | 41 (70.7) | 20 (58.8) | χ22 = 2.56 | 0.28 |
| Probable TBI | 69 (47) | 23 (51) | 28 (44) | 18 (49) | χ22 = 0.61 | 0.74 |
| L hippocampal vol, mm3‡ | 4355 ± 365 | 4378 ± 386 | 4410 ± 330 | 4232 ± 380 | F2,143 = 3.0 | 0.05 |
| R hippocampal vol, mm3‡ | 4456 ± 389 | 4413 ± 416 | 4524 ± 354 | 4391 ± 404 | F2,143 = 1.78 | 0.17 |
CAPS = Clinician-Administered PTSD Scale; L = left; PTSD = posttraumatic stress disorder; R = right; SD = standard deviation; TBI = traumatic brain injury; WTAR = Wechsler Test of Adult Reading.
Information missing for 2 participants.
Symptom duration available only for individuals with PTSD.
Adjusted for intracranial volume.
The Met/Met group had higher education than the Val/Met group.
Moderating effect of COMT Val158Met
Hierarchical linear regression showed no significant overall model effect in steps 1 and 2 for the left hippocampus (Table 2). After entering the genotype × PTSD symptom severity interaction in the third step, the overall model was significant (F8,137 = 2.69, p = 0.009, R2 = 0.14), with a significant F change (F1,137 = 5.43, p = 0.022, ΔR2 = 0.03). Further examination of the interaction term revealed that individuals homozygous for the Val allele showed reduced left hippocampal volume with increasing PTSD symptom severity (β = −0.49, p = 0.007; Fig. 2A). There was no association between left hippocampal volume and PTSD symptom severity for either the Val/Met group (β = −0.03, p = 0.80; Fig. 2B) or the Met/Met homozygous group (β = −0.01, p = 0.90; Fig. 2C). Using a categorical PTSD variable did not change the pattern of results; the moderating effect of COMT Val158Met on the association between PTSD and left hippocampal volume remained significant.
Table 2.
Summary of hierarchical regression analysis for variables predicting left hippocampal volume
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Variable | B | SE(B) | β | p value | B | SE(B) | β | p value | B | SE(B) | β | p value |
| Age | −5.14 | 3.62 | −0.12 | 0.16 | −5.74 | 3.59 | −0.13 | 0.11 | −6.17 | 3.54 | −0.14 | 0.09 |
| Handedness | 211.6 | 82 | 0.22 | 0.011 | 241.8 | 82.63 | 0.25 | 0.004 | 238.9 | 81.34 | 0.24 | 0.004 |
| Depression | −74.13 | 46.96 | −0.13 | 0.12 | −57.71 | 47.54 | −0.10 | 0.23 | −74.98 | 47.38 | −0.13 | 0.12 |
| Substance use | 56.22 | 64.51 | 0.08 | 0.39 | 59.4 | 65.33 | 0.08 | 0.37 | 83.17 | 65.12 | 0.11 | 0.20 |
| Possible TBI | 10.43 | 61.5 | 0.01 | 0.87 | 33.89 | 63.16 | 0.05 | 0.59 | 45.50 | 62.37 | 0.06 | 0.47 |
| PTSD symptoms | — | — | — | — | −1.68 | 1.17 | −0.13 | 0.15 | −4.93 | 1.81 | −0.39 | 0.007 |
| Val/Met | — | — | — | — | −74.34 | 39.87 | −0.15 | 0.06 | −213.6 | 71.51 | −0.44 | 0.003 |
| PTSD × Val/Met | — | — | — | — | — | — | — | — | 3.31 | 1.42 | 0.41 | 0.021 |
| R2 | — | 0.07 | — | — | — | 0.10 | — | — | — | 0.14 | — | — |
| F for Δ R2 | — | 2.08 | — | — | — | 2.49 | — | — | — | 5.43 | — | — |
PTSD = posttraumatic stress disorder; SE = standard error; TBI = traumatic brain injury.
Fig. 2.
Partial regression plots accounting for covariates. (A) Val/Val homozygotes showed reduced hippocampal volume with increasing traumatic stress. (B, C) This pattern was not observed in Met carriers. PTSD = posttraumatic stress disorder.
For the right hippocampus, there were no significant main effects or an interaction effect. For Val/Val homozygotes, there was no association between PTSD symptom severity and right hippocampal volume (β = −0.20, p = 0.27). This was also true for the Val/Met group (β = −0.09, p = 0.52) and Met/Met homozygotes (β = −0.26, p = 0.16).
Post hoc models for potential confounding variables
None of the post hoc models that included additional covariates modified the estimates of the main effects in step 2 or the genotype × PTSD symptom severity interaction in step 3. After removing women from the analysis, the F change for the genotype × PTSD symptom severity interaction in step 3 remained significant (ΔF1,126 = 5.49, p = 0.025, ΔR2 = 0.04). The results also remained significant after removing all individuals with probable mild TBI (ΔF1,69 = 6.41, p = 0.014, ΔR2 = 0.07), despite the reduced sample size (n = 77). Taken together, these results suggest that the primary genotype × PTSD symptom severity interaction finding was not explained by several other variables, including mild TBI or the small number of women included in the study.
Discussion
The primary finding from our study was that Val homozygotes with severe PTSD symptomatology exhibited reduced left hippocampal volume in comparison to carriers of the Met allele, suggesting that putatively lower dopamine availability in Val carriers may interact with traumatic stress to negatively affect hippocampal structure. This effect was observed after adjusting for a number of potentially confounding variables, including mood and substance use disorders, suggesting a unique imaging genetic association with hippocampal atrophy in individuals with PTSD. These results are notable given that prior studies of the association between PTSD and hippocampal volume have yielded inconsistent findings and suggest that examination of genetic variation could improve specificity of the association between traumatic stress and the structure of the hippocampus.
Leading views of hippocampal alterations in individuals with PTSD have often emphasized the effect of glucocorticoids58 and glutamate59 in mediating the association between psychological stress and hippocampal structure. However, recent studies have shown that both dopamine and glutamate are required for hippocampally mediated processes, such as long-term potentiation (LTP),30,60,61 which is associated with neurogenesis in the hippocampus, both in terms of proliferation of progenitor cells and the survival of new cells.62 This, combined with the evidence that stress inhibits dopamine-modulated LTP63,64 and that dopamine dysregulation is associated with neurodegeneration65 points to the possible involvement of dopamine-mediated processes in the association between PTSD and hippocampal structure.
The findings raise plausible implications for understanding memory disturbance in individuals with PTSD. One implication of smaller COMT-associated hippocampal volume may be dysfunction in hippocampal–prefrontal cortex connections that support episodic memory. Consistent with this, Bertolino and colleagues66 found that reduced hippocampal–prefrontal cortex coupling was associated with poorer memory retrieval in Val carriers. Further, Schott and colleagues67 found that Val carriers had less functional connectivity between the hippocampus and prefrontal cortex during memory encoding. Recent evidence suggests that COMT Val carriers with PTSD show reduced volume in the anterior cingulate cortex.68 Thus, Val allele–associated reductions in volume in this region and the hippocampus may lead to dysfunctional connectivity among regions that support episodic memory. Though speculative, it is conceivable that under conditions of traumatic stress, lower dopamine availability creates vulnerability for memory impairments in individuals with PTSD.
The present findings may also have bearing on the function of the hippocampal–ventral tegmental area (VTA) loop, which has been identified as a pathway that subserves memory under conditions of novelty.69 The VTA, which is the seat of dopaminergic cell bodies of the mesocorticolimbic dopamine system, has connections to and from the hippocampus and has been associated with facilitation of fear extinction.70 Disturbances in this pathway have been linked to enhanced fear generalization in clinical samples.71 One implication from these studies is that dopamine-associated hippocampal function is critical for the encoding of distinct memory representations. It is possible that higher dopamine degradation —putatively associated with Val homozygosity — is a catalyst in dopaminergic pathway dysfunction that leads to difficulty in distinguishing related yet novel cues from the previously encoded trauma episode. A potential consequence of such pattern separation failures may be reactivation of trauma memories in individuals with PTSD. Additional research is necessary to examine how Val158Met may regulate dopamine in the hippocampal–VTA loop and whether alterations in this circuit have implications for brain structure in individuals with PTSD.
In the present study, the Val158Met × PTSD effect was observed unilaterally in the left hippocampus. Although 2 prior meta-analytic studies of hippocampal volume in individuals with PTSD reported greater reduction in left hippocampal volume,5,6,2 other meta-analyses did not find such effects.4,7 The present results contribute to this body of literature by suggesting that the left-lateralized effects may be influenced by Val homozygosity. Left-lateralized medial temporal lobe dysfunction has been consistently associated with verbal memory deficits.72 Moreover, poor verbal memory performance has consistently been associated with PTSD.73 Thus, it is possible that the left-lateralized reductions in volume are related to the verbal memory dysfunction observed in individuals with PTSD.
Limitations
Our study findings and conclusions should be considered in the context of its limitations. First, the cross-sectional nature of these data precludes strong conclusions regarding the direction of the proposed effects. Thus, we are unable to determine whether observed differences in hippocampal volume reflect a consequence of PTSD, a pre-existing characteristic, or both, or the impact of PTSD chronicity. Furthermore, our hypotheses about the downstream functional consequences of hippocampal volume differences and the influence of dopamine on these processes should be considered preliminary, and would benefit from future studies that directly examine hippocampal function and neurocognition. Finally, we note that the findings from this study should be considered provisional until they can be replicated in additional samples. These limitations notwithstanding, the present study should provide impetus for future research investigating the association between PTSD, dopamine and hippocampally based memory systems.
Conclusion
The results of our study suggest that individuals with severe PTSD symptoms who are carriers of the Val/Val genotype are more likely to show reductions in hippocampal volume than Met carriers. These findings advance our understanding of structural hippocampal alterations in individuals with PTSD by identifying a novel molecular mechanism by which exposure to environmental stress is associated with atrophy in a brain region that is thought to be critical in the etiology and maintenance of PTSD. The integration of neuroimaging and genetic approaches as reported in this study can be used to direct future refinement of theories of memory-based alterations in individuals with PTSD.
Acknowledgements
This research was supported in part by National Institute of Mental Health grant R21MH102834, VA SPiRe award I21RX001594, and the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research and Development Traumatic Brain Injury Center of Excellence (B9254-C). This work was also supported by a Career Development Award to E.J. Wolf from the United States Department of Veterans Affairs, Clinical Sciences Research and Development Program. M. Verfaellie is supported by the Department of Veterans Affairs Clinical Science Research and Development Service. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Competing interests: None declared.
Contributors: J. Hayes, M. Logue, R. McGlinchey, W. Milberg and M. Miller designed the study. D. Salat, R. McGlinchey, W. Milberg and M. Miller acquired the data, which J. Hayes, M. Logue, A. Reagan, E. Wolf, N. Sadeh, J. Spielberg, E. Sperbeck, S. Hayes, M. Verfaellie, A. Stone, S. Schichman and M. Miller analyzed. J. Hayes, M. Logue and A. Reagan wrote the article, which all authors reviewed and approved for publication.
References
- 1.Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewin CR, Gregory JD, Lipton M, et al. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol Rev. 2010;117:210. doi: 10.1037/a0018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17:337–47. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Kitayama N, Vaccarino V, Kutner M, et al. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Karl A, Schaefer M, Malta LS, et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–31. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 6.O’Doherty DC, Chitty KM, Saddiqui S, et al. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res Neuroimaging. 2015;232:1–33. doi: 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- 8.De Bellis MD, Hall J, Boring AM, et al. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related post-traumatic stress disorder. Biol Psychiatry. 2001;50:305–9. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 9.De Bellis MD, Keshavan MS, Shifflett H, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–78. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 10.Bonne O, Brandes D, Gilboa A, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–51. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrion VG, Weems CF, Eliez S, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–51. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 12.Schuff N, Neylan TC, Lenoci MA, et al. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50:952–9. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fennema-Notestine C, Stein MB, Kennedy CM, et al. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:1089–101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 14.Pederson CL, Maurer SH, Kaminski PL, et al. Hippocampal volume and memory performance in a community-based sample of women with posttraumatic stress disorder secondary to child abuse. J Trauma Stress. 2004;17:37–40. doi: 10.1023/B:JOTS.0000014674.84517.46. [DOI] [PubMed] [Google Scholar]
- 15.Golier JA, Yehuda R, De Santi S, et al. Absence of hippocampal volume differences in survivors of the Nazi Holocaust with and without posttraumatic stress disorder. Psychiatry Res Neuroimaging. 2005;139:53–64. doi: 10.1016/j.pscychresns.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Freeman T, Kimbrell T, Booe L, et al. Evidence of resilience: neuroimaging in former prisoners of war. Psychiatry Res Neuroimaging. 2006;146:59–64. doi: 10.1016/j.pscychresns.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Jatzko A, Rothenhöfer S, Schmitt A, et al. Hippocampal volume in chronic posttraumatic stress disorder (PTSD): MRI study using two different evaluation methods. J Affect Disord. 2006;94:121–6. doi: 10.1016/j.jad.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Yehuda R, Golier JA, Tischler L, et al. Hippocampal volume in aging combat veterans with and without post-traumatic stress disorder: relation to risk and resilience factors. J Psychiatr Res. 2007;41:435–45. doi: 10.1016/j.jpsychires.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Boscarino JA, Erlich PM, Hoffman SN, et al. Association of FKBP5, COMT and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSD. Psychiatry Res. 2011;188:173–4. doi: 10.1016/j.psychres.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolassa I-T, Kolassa S, Ertl V, et al. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase Val 158 Met polymorphism. Biol Psychiatry. 2010;67:304–8. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Boscarino JA, Erlich PM, Hoffman SN, et al. Higher FKBP5, COMT, CHRNA5, and CRHR1 allele burdens are associated with PTSD and interact with trauma exposure: implications for neuropsychiatric research and treatment. Neuropsychiatr Dis Treat. 2012;8:131. doi: 10.2147/NDT.S29508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pooley EC, Fineberg N, Harrison P. The met158 allele of catechol-O-methyltransferase (COMT) is associated with obsessive-compulsive disorder in men: case–control study and meta-analysis. Mol Psychiatry. 2007;12:556–61. doi: 10.1038/sj.mp.4001951. [DOI] [PubMed] [Google Scholar]
- 23.Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Lipska BK, Halim N, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–21. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong J, Shu-Leong H, Tao X, et al. Distribution of catechol-O-methyltransferase expression in human central nervous system. Neuroreport. 1998;9:2861–4. doi: 10.1097/00001756-199808240-00033. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto M, Weickert CS, Akil M, et al. Catechol-O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116:127–37. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- 27.Taylor WD, Züchner S, Payne ME, et al. The COMT Val158Met polymorphism and temporal lobe morphometry in healthy adults. Psychiatry Res Neuroimaging. 2007;155:173–7. doi: 10.1016/j.pscychresns.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerasa A, Cherubini A, Quattrone A, et al. Met158 variant of the catechol-O-methyltransferase genotype is associated with thicker cortex in adult brain. Neuroscience. 2010;167:809–14. doi: 10.1016/j.neuroscience.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 29.Laatikainen LM, Sharp T, Bannerman D, et al. Modulation of hippocampal dopamine metabolism and hippocampal-dependent cognitive function by catechol-O-methyltransferase inhibition. J Psychopharmacol. 2012;26:1561–8. doi: 10.1177/0269881112454228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011;34:536–47. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apud JA, Mattay V, Chen J, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32:1011–20. doi: 10.1038/sj.npp.1301227. [DOI] [PubMed] [Google Scholar]
- 32.Knecht S, Breitenstein C, Bushuven S, et al. Levodopa: faster and better word learning in normal humans. Ann Neurol. 2004;56:20–6. doi: 10.1002/ana.20125. [DOI] [PubMed] [Google Scholar]
- 33.De Frias CM, Annerbrink K, Westberg L, et al. COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav Genet. 2004;34:533–9. doi: 10.1023/B:BEGE.0000038491.06972.8c. [DOI] [PubMed] [Google Scholar]
- 34.Hayes JP, LaBar K, McCarthy G, et al. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. J Psychiatr Res. 2011;45:660–9. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brohawn KH, Offringa R, Pfaff DL, et al. The neural correlates of emotional memory in posttraumatic stress disorder. Biol Psychiatry. 2010;68:1023–30. doi: 10.1016/j.biopsych.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Valente NLM, Vallada H, Cordeiro Q, et al. Catechol-O-methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. J Mol Neurosci. 2011;43:516–23. doi: 10.1007/s12031-010-9474-2. [DOI] [PubMed] [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington (DC): APA; 2000. [Google Scholar]
- 38.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 39.Dale AM, Sereno M. Improved localization of cortical activity by combining EEG and MEG and MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5:162–76. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- 40.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. Medical Imaging. IEEE Transactions on. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 42.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 43.Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 44.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 45.Fischl B, Sereno MI, Tootell RB, et al. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 47.Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–94. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 48.Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–43. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 49.Ségonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–29. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 50.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53:1181–96. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ségonne F, Dale A, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 52.van Erp TG, Hibar D, Rasmussen J, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–53. doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hibar DP, Westlye L, van Erp T, et al. Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry. 2016 doi: 10.1038/mp.2015.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morey RA, Petty CM, Xu Y, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–66. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mulder ER, de Jong RA, Knol DL, et al. Hippocampal volume change measurement: quantitative assessment of the reproducibility of expert manual outlining and the automated methods Free-Surfer and FIRST. Neuroimage. 2014;92:169–81. doi: 10.1016/j.neuroimage.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 56.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 57.Wechsler D. Wechsler Test of Adult Reading: WTAR. Psychological Corporation; 2001. [Google Scholar]
- 58.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 59.Moghaddam B, Bolinao ML, Stein-Behrens B, et al. Glucocortcoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res. 1994;655:251–4. doi: 10.1016/0006-8993(94)91622-5. [DOI] [PubMed] [Google Scholar]
- 60.Bailey CH, Giustetto M, Huang Y-Y, et al. Is heterosynaptic modulation essential for stabilizing hebbian plasiticity and memory. Nat Rev Neurosci. 2000;1:11–20. doi: 10.1038/35036191. [DOI] [PubMed] [Google Scholar]
- 61.O’Carroll CM, Morris RG. Heterosynaptic co-activation of glutamatergic and dopaminergic afferents is required to induce persistent long-term potentiation. Neuropharmacology. 2004;47:324–32. doi: 10.1016/j.neuropharm.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Bruel-Jungerman E, Davis S, Rampon C, et al. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26:5888–93. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rocher C, Spedding M, Munoz C, et al. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex. 2004;14:224–9. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- 64.Jay TM, Rocher C, Hotte M, et al. Plasticity at hippocampal to prefrontal cortex synapses is impaired by loss of dopamine and stress: importance for psychiatric diseases. Neurotox Res. 2004;6:233–44. doi: 10.1007/BF03033225. [DOI] [PubMed] [Google Scholar]
- 65.Caudle WM, Richardson JR, Wang MZ, et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–48. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bertolino A, Rubino V, Sambataro F, et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry. 2006;60:1250–8. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- 67.Schott BH, Seidenbecher CI, Fenker DB, et al. The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J Neurosci. 2006;26:1407–17. doi: 10.1523/JNEUROSCI.3463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schulz-Heik RJ, Schaer M, Eliez S, et al. Catechol-O-methyltransferase Val158Met polymorphism moderates anterior cingulate volume in posttraumatic stress disorder. Biol Psychiatry. 2011;70:1091–6. doi: 10.1016/j.biopsych.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 69.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–13. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 70.Menezes J, Alves N, Borges S, et al. Facilitation of fear extinction by novelty depends on dopamine acting on D1-subtype dopamine receptors in hippocampus. Proc Natl Acad Sci U S A. 2015;112:E1652–8. doi: 10.1073/pnas.1502295112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cha J, Carlson JM, DeDora DJ, et al. Hyper-reactive human ventral tegmental area and aberrant mesocorticolimbic connectivity in over-generalization of fear in generalized anxiety disorder. J Neurosci. 2014;34:5855–60. doi: 10.1523/JNEUROSCI.4868-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee TM, Yip JT, Jones-Gotman M. Memory deficits after resection from left or right anterior temporal lobe in humans: a meta-analytic review. Epilepsia. 2002;43:283–91. doi: 10.1046/j.1528-1157.2002.09901.x. [DOI] [PubMed] [Google Scholar]
- 73.Uddo M, Vasterling JJ, Brailey K, et al. Memory and attention in combat-related post-traumatic stress disorder (PTSD) J Psychopathol Behav Assess. 1993;15:43–52. [Google Scholar]