Abstract
Background
Abnormal maturation of brain connectivity is supposed to underlie the dysfunctional emotion regulation in patients with bipolar disorder (BD). To test this hypothesis, white matter integrity is usually investigated using measures of water diffusivity provided by MRI. Here we consider a more intuitive aspect of the morphometry of the white matter tracts: the shape of the fibre bundles, which is associated with neurodevelopment. We analyzed the shape of 3 tracts involved in BD: the cingulum (CG), uncinate fasciculus (UF) and arcuate fasciculus (AF).
Methods
We analyzed diffusion MRI data in patients with BD and healthy controls. The fibre bundles were reconstructed using Q-ball–based tractography and automated segmentation. Using Isomap, a manifold learning method, the differences in the shape of the reconstructed bundles were visualized and quantified.
Results
We included 112 patients and 82 controls in our analysis. We found the left AF of patients to be further extended toward the temporal pole, forming a tighter hook than in controls. We found no significant difference in terms of shape for the left UF, the left CG or the 3 right fasciculi. However, in patients compared with controls, the ventrolateral branch of the left UF in the orbitofrontal region had a tendency to be larger, and the left CG of patients had a tendency to be smaller in the frontal lobe and larger in the parietal lobe.
Limitations
This was a cross-sectional study.
Conclusion
Our results suggest neurodevelopmental abnormalities in the left AF in patients with BD. The statistical tendencies observed for the left UF and left CG deserve further study.
Introduction
Models of bipolar disorder (BD) assume that an altered neurodevelopment leads to a dysfunctional corticolimbic connectivity, causing an emotional dysregulation.1 The corticolimbic communication largely relies on structural brain connectivity, through white matter tracts, such as the uncinate fasciculus (linking prefrontal [orbitofrontal] cortices and temporal limbic regions, including the hippocampus and amygdala) and the cingulum.
Diffusion-weighted imaging (DWI) is widely used for the study of white matter changes in patients with psychiatric disorders.2 Voxel-based methods, such as tract-based spatial statistics (TBSS), can be used to perform whole brain studies.3 Alternatively, bundle-based methods rely on tractography to reconstruct white matter tracts before using them as regions of interest for quantification of their microstructural status.4 These methods focus on water diffusivity in the tracts using metrics such as fractional anisotropy (FA), mean diffusivity (MD) or volume of the tract.
A few investigations of white matter changes in patients with BD have been carried out,5 usually by assessing structural changes with FA, which gives information on the structural integrity of the white matter tracts. These studies reported changes of FA along the white matter of the frontal, occipital and limbic regions in patients with BD, including tracts such as the uncinate fasciculus (UF), the anterior thalamic radiations, the corpus callosum, the fornix, the cingulum (CG) and the arcuate fasciculus (AF). However, these results were not conclusive in terms of FA changes:5 although many studies found reduced FA in some of these tracts,6–8 several studies found no change in FA.4
In the present study, we used the volumetric information offered by bundle-based tractography methods to analyze the shape of the white matter bundles. Brain shapes are supposed to be strongly driven by neurodevelopmental factors.9,10 Since most of the brain shapes emerge early during ontogenesis, abnormal shape is often the hallmark of abnormal development.11 Our shape analysis methodology is derived from a technique initially developed for the study of the variability of cortical folding patterns.12 For each bundle, shape variability is projected into a simple 2-dimensional space obtained from an Isomap algorithm, a modern multidimensional scaling method13 (Fig. 1). This approach provides a powerful way to perform quantification of complex shapes, which has been illustrated by the discovery of a developmental feature of the central sulcus: the shape of the hand knob was found to be associated with innate handedness even after a forced switch of the writing hand at school.12 In the present study, we analyzed the shape of 3 of the main tracts involved in BD: the UF, AF and CG. We hypothesized that the shape of these 3 bundles would be altered in patients with BD compared with controls.
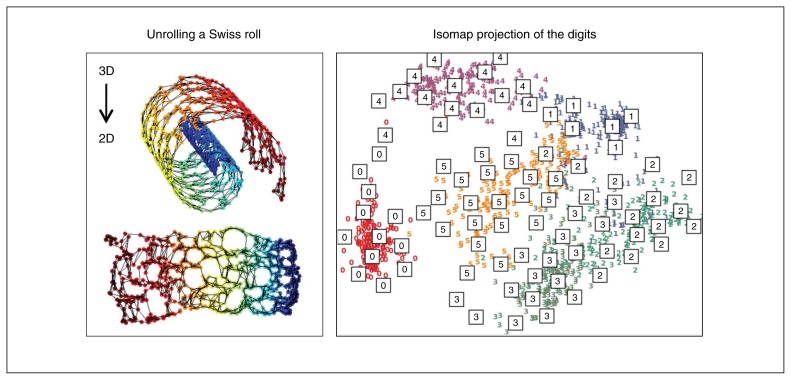
Fig. 1.
Isomap algorithm capacity. (Left) Isomap automatically finds the manifold of dimension 2 embedded in a Swiss roll (the manifold is an abstract mathematical space). (Right) Isomap creates a manifold of dimension 2 approximating the high-dimensional space of manually drawn digits (http://scikit-learn.org/).
Methods
Participants
Adult inpatients and outpatients with BD type I (DSM-IV-R) were recruited from 4 university-affiliated participating centres: APHP Henri Mondor hospitals, Créteil, France; Fernand Widal hospital, Paris, France; Western Psychiatric Institute and Clinic, UPMC, Pittsburgh, USA; and Central Institute for Mental Health, Mannheim, Germany. We recruited healthy controls with no personal or family history of Axis I mood disorders, schizophrenia or schizoaffective disorder via media announcements and registry offices. All participants were clinically assessed by trained raters (S.S., J.L., C.D., N.H., M.A.D., M.D. and A.V.) using the Diagnosis Interview for Genetic Study14 at the French sites and the Structured Clinical Interview for DSM-IV15 at the German and American sites, the Montgomery-Åsberg Depression Rating Scale16 or the Hamilton Rating Scale for Depression,17 the Young Mania Rating Scale18 and the National Adult Reading Test19 in all sites. Exclusion criteria for both patients and controls were history of neurologic disease or head trauma with loss of consciousness and MRI contraindications. Twenty-four of the patients (20.34%) had participated in a previous single-centre tractography study8 with different aims, DTI sequence and processing pipeline. The multicentre data set was used for a bundle-based FA analysis, which found a BD-associated decrease of FA in the corpus callosum, left CG and left AF.20 All participants were given full verbal and written information about the aims, methods and risks of the present study and were given the option to decline participation. They were invited to ask questions about the research and about the consent form, with replies contributing to ensuring their informed participation. Patients with enforced hospitalization or under conservatorship were not included in the study, nor were patients in an active psychotic state that may have interfered with their ability to give informed consent. Such processes were assessed through clinical examination and consultation with the medical team involved in the patients’ care, as appropriate. After a complete description of the study each participant gave written informed consent. The local ethics committees of each centre (the Ethical Committee of the Medical Faculty Mannheim, Heidelberg University; the Institutional Review Board of the University of Pittsburgh; and the Comité de Protection des Personnes Ile-de-France IX) approved the study protocol.
Data acquisition
To minimize between-sites bias, we obtained diffusion-weighted and T1-weighted images for all participants using the same hardware in the 3 MRI acquisition sites (Siemens, Magnetom TrioTim 3 T Syngo MR B17, 12-channel head-coil). The MRI protocol included a high-resolution T1-weighted acquisition (echo time [TE] 2.98 ms, repetition time [TR] 2300 ms, 160 slices, 1.0 × 1.0 × 1.1 mm) and a shared diffusion-weighted sequence along 41 directions (2.0 × 2.0 × 2.0 mm, b = 1000 s/mm2 plus a b = 0 image, TE 87 or 84 ms, TR 14000 ms, 60 or 64 axial slices, no gap, field of view 256 mm, number of excitations = 1, acquisition time 10.5 min). Two operators (J.H. and S.S.) blind to the diagnosis assessed the data for movement, susceptibility and noise artifacts. Participants with significant artifacts or movements and with missing information were consensually excluded from further analysis. For T1 images, we corrected the field inhomogeneity bias using an algorithm detailed previously.21
Whole brain tractography
Tractography and bundles segmentation involved a method we used in a previous multisite study.20,22 The diffusion-weighted MRI data were processed using Connectomist 2.0 and the T1-weighted MRI data were processed using Morphologist 2012 (www.brainvisa.info). An orientation distribution function (ODF) was computed at each voxel using an analytical Q-ball model, which improves tractography in complex white matter areas relative to classical diffusion tensor imaging (DTI) models with regards to crossing or kissing fibres.
For each participant, a T1-based tractography mask was computed in native space. We performed whole brain tractography using a regularized streamline deterministic algorithm.
Bundle segmentation
Whole brain tractograms were then compressed into clusters of streamlines with similar trajectories. Each cluster was finally labelled according to the distance between its centroid streamline and the centroids of the labelled bundles of a multisubject atlas after affine normalization into Talairach space. With regard to the Guevara atlas labels, the tracts of interest were defined as the UF, CG long tracts and AF long tracts. While other bundles of interest in patients with BD may be reconstructed using this processing pipeline, we decided to exclude the anterior thalamic radiations and the corpus callosum owing to the complexity of the associated fans of bundles and we excluded the fornix owing to its small diameter leading to frequent tractography issues. We visually checked segmentation for each participant.
Bundle sampling
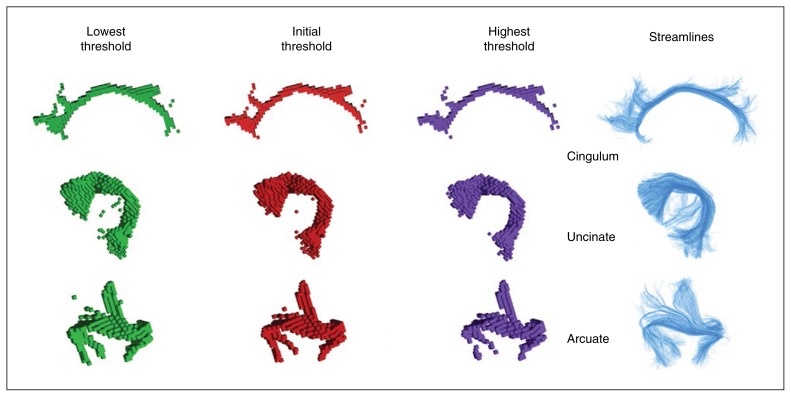
The manifold learning strategy initially designed to study the shape of cortical sulci12 was adapted to fibre bundles. First, we converted each fibre bundle into a streamline density map sampled at the diffusion acquisition resolution. This density map was thresholded at a value of 30 streamlines for a voxel of 2 mm in-plane resolution in order to get a list of voxels belonging to the core shape of the bundle. To check the robustness of the approach to the choice of this threshold, we reproduced each analysis with 4 additional thresholds, 24, 27, 33 and 36, leading to average changes of the bundle volume of −20%, −10%, +10% and +20%, respectively (Fig. 2). To control for the influence of brain size variability, we further normalized the voxel coordinates to standard Talairach reference space using a 9-parameter affine transformation. The bundles of the right hemisphere were finally flipped relative to the interhemispheric plane to allow asymmetry studies.
Fig. 2.
Sample for each bundle of interest (cingulum, uncinate, arcuate) with the streamline representations obtained from tractography. Three alternative voxel-based representations are obtained for each bundle with different thresholds on the number of streamlines crossing the voxels. The threshold used in our study was considered optimal, preventing spurious voxels including few streamlines to be selected (see the uncinate representation for a low threshold) and preserving the topology of the bundle (see the split representation of arcuate branches for a high threshold). Note that from the low to the right threshold, the diameter of the bundle core decreases without qualitative changes in the shape.
Shape quantification
For each bundle of interest, we used the Isomap algorithm13 to capture a low-dimensional approximation of the high-dimensional space spanned by the bundle shape. Isomap applies multidimensional scaling to a matrix of distances computed along the bundle manifold approximated as a nearest neighbour graph of the participants’ individual bundles. This graph is built from a similarity matrix where the similarity in shape between 2 individual bundles is coded by the average quadratic distance between their 3-dimensional (3-D) representations after pairwise alignment using an iterative closest point algorithm.23 We used the first 2 dimensions of the Isomap, which capture the 2 main shape variations. For each participant’s bundle, the 2 coordinates in the associated Isomap provide a quantification of its shape relative to these 2 principal kinds of variations. Hence, this approach allows a compact quantification of shape change, alleviating the problem of multiple comparisons disturbing voxel-based or deformation-based approaches. Since the volume of the bundles may vary extensively among participants in spite of the affine normalization to Talairach space, we assume that one of the dimensions shall be related to volume while the other embeds pure shape variations. We used correlations of each dimension with the volume of the tract to test our assumption. Bundle volume was estimated from a smooth 3-D meshed representation of the core shape.
Shape averaging
In order to clarify the shape features coded in each of the Isomap dimensions, local averages of the bundles are calculated at regularly spaced locations.23 This process results in a series of moving average shapes (MAS) capturing the shape evolution along the Isomap axis. For visualization, each MAS is translated in anteroposterior direction according to its Isomap coordinate. Hence a difference between 2 groups of participants relative to their coordinates in this Isomap axis has to be interpreted according to the regular gradual change of the average shapes along the axis. Note that the shape variations coded by the Isomap axis are specific to each bundle.
Statistical analysis
For each of the 12 Isomap dimensions, we used analysis of covariance (ANCOVA) to compare coordinates between patients with BD and healthy controls. Comparisons were performed independently for the left and the right hemispheres. The model included diagnosis (BD v. control) as a factor of interest, sex as a confounding factor, age as a confounding covariate, the site of inclusion as a confounding factor to control for potential site-specific effects, and FA as a confounding factor that could impact the behaviour of tractography. We considered results to be significant for p values below TBonferroni = 0.05 ÷ 12, to account for multiple testing. Simple asymmetry tests related to volume and Isomap coordinates were performed for the complete population using the Wilcoxon signed-rank test. We also used Pearson correlation to test if clinical variables (age at onset, depressive symptoms), bundle volume or FA were associated with shapes.
Results
Of the participants who met our inclusion criteria, 9 who had significant artifacts or movements and 1 patient with missing information were consensually dropped out from the initial sample, leading to a final sample of 194 participants (112 patients and 82 controls) for our analysis (Table 1 and Table 2).
Table 1.
Demographic and clinical characteristics of the sample
| Characteristic | Group; no. (%) or mean ± SD [range] | |
|---|---|---|
|
| ||
| BD, n = 118 | Control, n = 86 | |
| Scan site | ||
| Centre 1 | 24 (20.5) | 22 (25.6) |
| Centre 2 | 53 (45.3) | 26 (30.2) |
| Centre 3 | 40 (34.2) | 38 (44.2) |
| Male sex | 47 (40.2) | 41 (47.7) |
| Age at MRI, yr | 36.5 ± 10.4 [18–63] | 37.3 ± 11.22 [19–66] |
| Right-handedness | 110 (96.5) | 84 (97.7) |
| IQ* | 108.7 ± 10.58 | 107.39 ± 10.06 |
| Medication | ||
| Lithium | 39 (33.3) | — |
| Anticonvulsants | 64 (54.7) | — |
| Antipsychotic | 52 (44.4) | — |
| Antidepressant | 54 (46.2) | — |
| Age at onset, yr | 20.8 ± 8.0 | — |
| YMRS score†‡§ | 2.6 ± 3.7 | — |
| MADRS score†§ | 3.5 ± 5.4 | — |
| HAMD-17 score‡ | 9.9 ± 7.9 | — |
BD = bipolar disorder; HAMD-17= Hamilton Rating Scale for Depression; NART = National Adult Reading Test; MADRS = Montgomery–Asberg Depression Rating Scale; SD = standard deviation; YMRS = Young Mania Rating Scale.
Assessed using the NART.
Recorded at centre 1.
Recorded at centre 2.
Recorded at centre 3.
Table 2.
Comparison of shapes between patients with bipolar disorder and healthy controls
| Bundle | Df | Dim | Diagnosis | FA | Scanner | Age | Sex | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||
| F1 | p value | F1 | p value | F2 | p value | F1 | p value | F1 | p value | |||
| Left AF | 187 | 1 | 1.4 | 0.23 | 14.2 | < 0.001* | 2.1 | 0.13 | 5.1 | 0.026 | 0.8 | 0.78 |
| 2 | 15.1 | < 0.001* | 24.5 | < 0.001* | 1.9 | 0.16 | 3.5 | 0.06 | 0.3 | 0.61 | ||
| Right AF | 176 | 1 | 3.2 | 0.07 | 11.1 | 0.001† | 0.6 | 0.54 | 7.8 | 0.006† | 0.7 | 0.39 |
| 2 | 0.1 | 0.84 | 1.3 | 0.24 | 4.4 | 0.014 | 0 | 0.96 | 0.7 | 0.39 | ||
| Left CG | 187 | 1 | 0.4 | 0.54 | 48.6 | < 0.001* | 0.73 | 0.48 | 0 | 0.89 | 0.39 | 0.53 |
| 2 | 4.99 | 0.027† | 0 | 0.87 | 2.5 | 0.08 | 0.8 | 0.37 | 0.3 | 0.56 | ||
| Right CG | 187 | 1 | 0. | 0.91 | 32.4 | < 0.001* | 8.3 | < 0.001† | 0 | 0.99 | 0.8 | 0.91 |
| 2 | 3.5 | 0.06 | 1.5 | 0.22 | 10.7 | < 0.001* | 0.7 | 0.40 | 0 | 0.87 | ||
| Left UN | 187 | 1 | 4.4 | 0.036† | 30.3 | < 0.001* | 26.1 | < 0.001* | 4.9 | 0.029† | 2.8 | 0.10 |
| 2 | 1. | 0.31 | 0.5 | 0.48 | 7.7 | < 0.001† | 3.0 | 0.08 | 1.7 | 0.20 | ||
| Right UN | 187 | 1 | 2. | 0.16 | 3.0 | 0.08 | 39.7 | < 0.001* | 10.2 | 0.002† | 0.3 | 0.57 |
| 2 | 2.9 | 0.09 | 2.8 | 0.09 | 36.1 | < 0.001* | 0 | 0.97 | 0.4 | 0.51 | ||
AF = arcuate fasciculus; CG = cingulum; Df = degrees of freedom; Dim = dimension; FA = fractional anisotropy; UN = uncinate fasciculus.
Significant effect after Bonferroni correction for multiple tests (p < 4 × 10−4)
Trend toward significance after Bonferroni correction for multiple tests (4 × 10−4 < p < 0.05).
Uncinate fasciculus
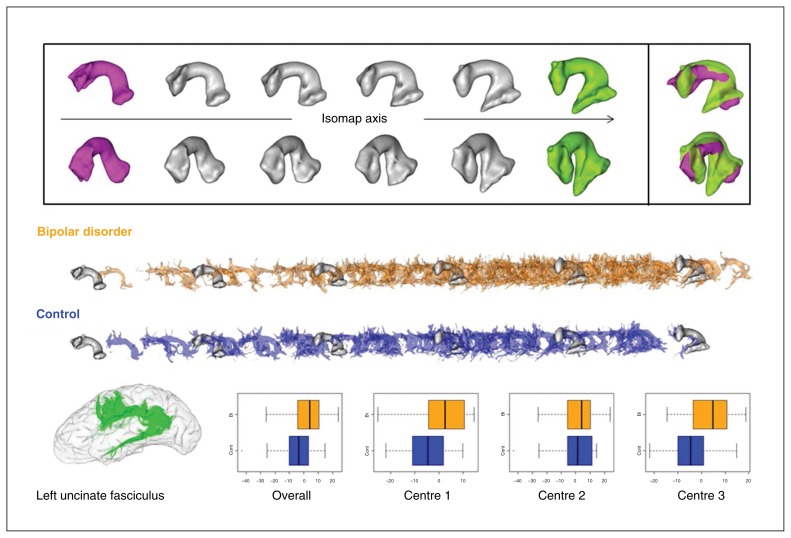
The UF was found in both hemispheres for all participants. It was highly asymmetric in volume in favour of the right hemisphere (left: 44 ± 29 cm3; right: 56 ± 27 cm3, p < 0.001). Patients with BD had a tendency to have a larger UF volume than controls in the left (48 ± 30 cm3 v. 40 ± 27 cm3, p = 0.05) and in the right hemisphere (58 ± 26 cm3 v. 53 ± 27 cm3, p = 0.10). The first dimension of the Isomap was correlated to the bundle volume (r = 0.4, p < 0.001) while the second dimension was not.
For the first dimension, Fig. 3 plots 6 MAS computed along the Isomap axis across the coordinate range including the left and right bundles. For the first dimension, as the volume of the bundle increased from the left to the right of the Isomap axis, the frontal horn assumed a more “fanned out” characteristic while the temporal horn shape remained relatively stable. At the right of the axis the frontal horn showed a Y shape induced by the emergence of the ventrolateral branch in the orbitofrontal region that did not exist in the left-most MAS. For the first dimension, patients with BD had a tendency to differ from controls in the left hemisphere (F1,187 = 4.4, p = 0.036). The ventrolateral branch of patients with BD had a tendency to be larger than in controls (Fig. 3). This tendency was qualitatively equivalent for the 4 alternative thresholds (Fig. 2). This coordinate also significantly depended on FA and scanning site (Table 2). The correlation between FA and the Isomap first coordinate was 0.43, which means that FA increases when the ventrolateral branch gets larger. No difference was found for the second dimension.
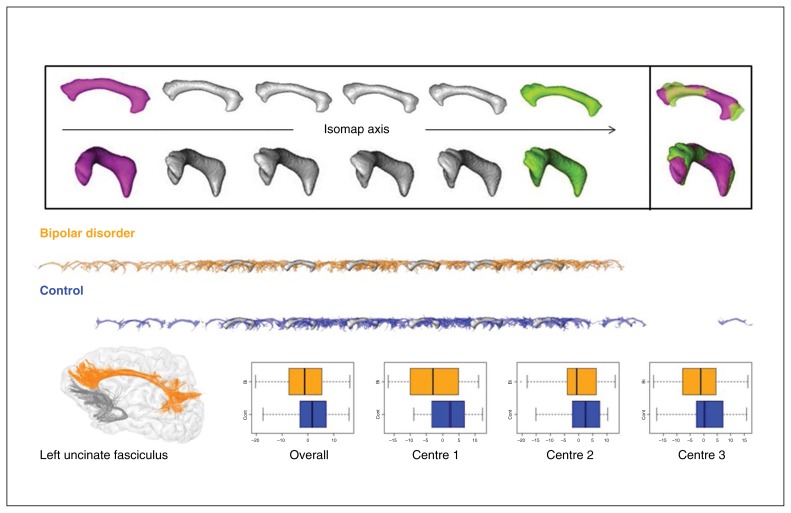
Fig. 3.
First dimension of the left uncinate fasciculus Isomap. (Top) Moving average shapes (MAS) along the Isomap observed with 2 different orientations (for each orientation, the frontal horn of the bundle is on the left). The extreme MAS (green and magenta) are combined to highlight the shape feature encoded by the Isomap. This dimension captures the volume variability of the bundles. From the left to the right of the Isomap axis, the volume increases, while the bundle becomes more “fanned out” in the frontal lobe. More particularly, the ventrolateral orbitofrontal branch emerges toward the right of the axis. (Middle) Individual bundles are superimposed over the same MAS along the Isomap axis to show their distributions for both groups. (Bottom) Uncinate fasciculus sample and overall and centre-based boxplots of the localization in the isomap after adjustment for fractional anisotropy, age and sex.
Cingulum
The CG was found to be highly asymmetric in volume in favour of the left hemisphere (left: 44 ± 19 cm3; right: 37 ± 15 cm3, p < 0.001). Patients with BD had a tendency to have a smaller CG volume than controls in the left hemisphere (42 ± 18 cm3 v. 47 ± 20 cm3, p = 0.10). The first dimension of the Isomap was correlated to the bundle volume (r = 0.6, p < 0.001) while the second dimension was not.
For the second dimension (Fig. 4), the shape feature captured by the Isomap was related to the different size of the frontal and the parietal extremities of the CG. From the left to the right of the Isomap axis, the volume of the frontal extremity increased while the volume of the parietal extremity decreased. Hemisphere asymmetry was observed where the left hemisphere was more toward the right side of this Isomap axis (p = 0.011). Patients with BD had a tendency to differ from controls in the left hemisphere (F1,187 = 4.99, p = 0.027). They had a tendency to have a smaller frontal extremity and a larger parietal extremity. This Isomap coordinate did not depend significantly on FA and scanning site. The tendency remained qualitatively equivalent for the 4 alternative thresholds (Fig. 2).
Fig. 4.
Second dimension of the cingulum fasciculus Isomap. (Top) Moving average shapes (MAS) along the Isomap axis observed with 2 different orientations (for each orientation, the frontal horn of the bundle is on the left). The extreme MAS (green and magenta) are combined to highlight the shape feature encoded by the Isomap. This dimension captures a pure shape feature. From the left to the right of the Isomap axis, the volume of the frontal extremity increases while the volume of the parietal extremity decreases. (Middle) Distributions along the Isomap axis of the left bundles of patients with bipolar disorder versus controls superimposed over the same MAS. (Bottom) Cingulum sample and the corresponding uncinate fasciculus and overall and centre-based boxplots of the localization in the isomap after adjustment for fractional anisotropy, age and sex.
Arcuate fasciculus
The left AF was found for all participants while the right AF did not pass our threshold on streamline density for 5 controls and 6 patients. In agreement with this observation, for the participants with adequate streamline density of AF in both hemispheres, we observed the well-known volume asymmetry toward the left hemisphere usually associated with language lateralization (left: 109 ± 51 cm3; right: 53 ± 52 cm3, p < 0.001). The first Isomap dimension of AF was strongly correlated to the bundle volume (r = 0.2, p < 0.001) and embedded a massive asymmetry (p < 0.001). While the correlation with volume was borderline for the second dimension (r = 0.1, p = 0.042), and asymmetry was straightforward (p < 0.001).
We observed that this second dimension captured a purer shape variation related to the size of the temporal horn toward the temporal pole. We found a significant effect of BD on this second dimension (F1,187 = 14.2, p < 0.001) that remained significant for the 4 alternative thresholds (Fig. 2). This Isomap coordinate also significantly depended on FA (F1,187 = 24.5, p < 0.001). The correlation between the second Isomap dimension and FA was 0.32, which means that FA increases when the temporal horn gets longer.
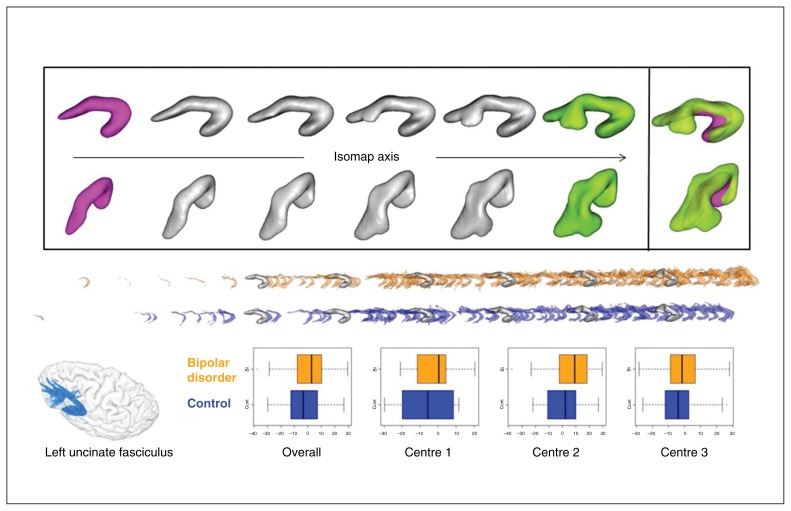
No difference was found between patients and controls for the first dimension, whereas for the second dimension, the left AF of the patients was more extended toward the temporal pole, and in general the left AF had a longer temporal horn than the right AF. Fig. 5 plots 6 MAS computed along the second dimension Isomap of AF across the range of coordinates including the participants’ left and right bundles. From this series of MAS, the shape feature could also be interpreted as a transition from a “looser” to a “tighter” arch or hook around the Sylvian fissure.
Fig. 5.
Second dimension of the arcuate fasciculus Isomap. (Top) Moving average shapes (MAS) along the Isomap axis observed with 2 different orientations (for each orientation, the frontal horn of the bundle is on the left). The extreme MAS (green and magenta) are combined to highlight the shape feature encoded by the Isomap. This dimension captures a pure shape feature: the extent of the development of the bundle toward the temporal pole. (Middle) Distributions along the Isomap axis of the left bundles of patients with bipolar disorder versus controls superimposed over the same MAS. (Bottom) Arcuate fasciculus sample and overall and centre-based boxplots of the localization in the Isomap after adjustment for fractional anisotropy, age and sex.
For the 3 relevant dimensions of these 3 bundles, we did not observe any effect of age at onset or depressive symptoms (all p > 0.10).
Discussion
To our knowledge, our paper reports the first tractography study exploring the potential of the whole bundle shapes to improve our understanding of white matter changes associated with BD. The most striking shape change observed in these patients relative to healthy controls was a significantly longer extension toward the temporal pole of the left AF.
The implication of AF in BD is not well understood despite several studies exhibiting FA abnormalities.2 A recent meta-analysis of DTI studies points to AF as one of the prominent regions of relevance to BD,2 which is consistent with the high statistical significance of our result. However, AF alterations are not yet integrated in recent models of BD.24 Changes in the AF are more consistently linked with models of schizophrenia.25 Our previous findings about FA with this data set,20 however, were located in the frontal part of the AF, in the anterior segment, whereas our findings about shape are in the temporal lobe. Hence, further investigation is required to explore the microstructure of the temporal horn. In our study, the extended streamlines detected in the patients with BD resulted from local changes of the microstructure beyond the scope of traditional methodologies. The design of refined methods taking potential shape change into account should further clarify the findings.
Regarding the UF, it is worth noting that the observed trend toward a global increase of the frontal lobe volume of the UF in patients (controlled for brain size variability by the normalization to Talairach space) is in agreement with previous results for the left UF using streamline count.4 This altered shape may result from abnormal developmental processes, such as defective neural pruning, as suggested by recent models of BD.24 Adolescence is a critical time of onset of BD, and this has been assumed to be related to abnormal white matter maturation.26 The UF is one of the main tracts linking prefrontal (orbitofrontal) cortices and temporal limbic regions, including the hippocampus and amygdala. In patients with BD, recent neural models assume a disrupted connectivity between these regions, leading to an inability of the prefrontal cortices to regulate an overreactive amygdala.1 Therefore, abnormal neurodevelopment of this fasciculus may be involved in disrupted or altered frontolimbic connectivity, contributing to aberrant interpretation, reaction and decision-making regarding emotional information.29
We also found a trend toward a smaller frontal extremity and a larger parietal extremity of the left CG in patients with BD than in controls. The CG contains fibres running from the subgenual and anterior cingulate frontal areas to retrosplenial temporal regions and the occipital lobe. It therefore links several parts of the limbic system and is involved in emotional regulation. It has been repeatedly found to be altered in MRI studies of BD,6,20,27–29 mostly left-sided.2,30 Many studies found a left-sided lateralization of this tract31,32 in healthy adults; loss of this asymmetry has been associated with higher neuroticism scores.33
Before the advent of tractography, in vivo assessment of white matter bundle shapes was beyond reach, except at the level of bottlenecks, such as the corpus callosum. Abnormalities of the shape of a midsagittal section of the corpus callosum have been demonstrated in patients with schizophrenia34 and BD.35 To our knowledge, the only previous work exploiting tractography to study white matter shape in a psychiatric syndrome was limited to the changes of the fan geometry of fibres passing through the corpus callosum in patients with schizophrenia.36
Linking our observations with the decrease of FA observed for the same tracts in the current literature is an open issue.6–8 A decrease of FA is usually associated with changes in the microstructural properties of the fibre pathways, such as decreased axonal diameter or density, reduced myelination, or decreased white matter coherence.37 Hence, the decrease of FA observed in patients with BD could be associated with either a lack of maturation, with a degenerative process akin to aging, or both.38 In the present study, however, we found that whatever the status of the participant, the longer temporal part of the AF correlated with higher global FA for the bundle. These correlations could mean that higher FA results in better tractography, or that larger bundles result in higher FA because of lower partial volume. More importantly, this observation could mean that FA changes associated with BD are probably heterogeneous across the bundle. Hence, taking into account bundle shape changes could shed some light on the inconsistencies in the literature on microstructure findings using parameters such as FA: shape differences could have a complex impact on the measurements using different methodologies.
To our knowledge, this report relies on the largest multicentre sample of patients with BD involved in a tractography study to date, thus reducing the risks associated with restrained statistical power and single-centre recruitment. Furthermore, the MRI acquisition protocol and hardware have been harmonized to reduce site-specific effects, and replication of the results with different parameters of the tractography method has been achieved. Finally, to our knowledge, this is the first systematic study of disease-related changes of the shape of fibre bundles ever reported.
Limitations
The findings described in this paper tend to support the malformation of the frontotemporal connectivity stemming from development. A common limitation of cross-sectional studies is the inability to determine whether the observed alterations precede the onset or develop during the course of the disease.38 However, white matter abnormalities have been reported in patients with BD at onset and in samples of unaffected relatives.27 Another limitation is the intersite effect in our results, as this is the case for most multisite neuroimaging studies. Intersite differences may arise from both technical factors (we attempted to limit these by homogenizing scanners, data acquisition and processing) and clinical differences between populations. Bipolar disorder is a highly heterogeneous condition, although we do not precisely know which clinical factors have a strong neurobiological basis. This is a clear limitation that has to be addressed in future studies. In spite of this limitation, our results were consistent across sites relative to displacements of means and quartiles between groups, supporting an effect shared across sites (Fig. 3, Fig. 4 and Fig. 5). Potential effect of medications was not investigated in this study; however, evidence suggests that medications are not a major confounder in imaging studies of white matter in patients with BD.39
Alternatively, shape modification may be associated with processes other than neurodevelopment (e.g., neuroprogressive effects), with medication usage, or with disease effects. We cannot rule them out in this cross-sectional study, but disease effects are not likely to yield larger tracts as seen here for the AF. Additionally, recent reviews suggest no effects or minimal effects of medication on DTI parameters.39
It is important to realize that the observed shape abnormalities can result either from an actual anatomic difference or from a different behaviour of tractography in the 2 populations. The main confounding factor that may modulate the shape of a fibre bundle resulting from tractography is the erroneous estimation of fibre directions in complex voxels, including crossing or kissing fibres.40 Therefore, some of the changes observed between patients and controls could result from changes in the neighbourhood of the tracts of interest. A lighter density of crossing fibres in patients with BD would simplify tractography and reveal some segments of the tracts usually lost in healthy controls. Further study involving not only deep white matter bundles, but also superficial U-fibre bundles will be required to tackle this issue. In the present study, the use of Q-ball imaging alleviated the risk of such mistakes relative to standard DTI, but further improvement of tractography methods, such as global tractography, would be of interest.40
Finally, another limitation of this study is the possible heterogeneity of the samples recruited and the different clinical tools used in the different centres. This limitation pertains to the multisite design of the study.
Conclusion
Our observation of an altered shape of the left AF suggests neurodevelopmental abnormalities in patients with BD. Future studies comparing patients with schizophrenia and patients with BD may enlighten the specificity of this finding and its link with psychosis. The differences found in the left AF support recent reports of predominantly left-sided changes in patients with BD. Links between disturbance of asymmetry and neurodevelopment in BD warrant further exploration, as do the statistical tendencies observed for the left UF and the left CG.
Acknowledgements
The authors thank all those who participated in this study and the personnel of participating centres for their help with data collection.
Footnotes
Funding: This work was supported by public funding from the Alliance pour les Sciences de la Vie et de la Santé (ITMO Neurosciences), the Agence Nationale pour la Recherche (ANR MNP VIP 2008 and ANR-11-IDEX-0004 Labex BioPsy), the Fondation pour la Recherche Médicale (Appel d’offres analyse bioinformatique pour la recherche en biologie 2014), the Deutsche Forschungsgemeinschaft (SFB636/C6 and We3638/3-1), and the NIMH R01 MH076971. S.Sarrazin is supported by a grant from the Agence Régionale de Santé Ile-de-France. The funders did not participate in the design and conduct of the study, in the collection, analysis or interpretation of the data; or in the preparation, review or approval of the manuscript.
Competing interests: S. Sarrazin declares travel expenses from Otsuka outside the submitted work. M. Phillips is a consultant with Roche.
Contributors: J. Houenou, D. Duclap, P. Le Corvoisier, M. Delavest, A. Versace, C. Poupon and M. Leboyer designed the study. J. Houenou, S. Sarrazin, J. Linke, C. Daban, N. Hamdani, M.-A. d’Albis, P. Le Corvoisier, F. Bellivier, J. Almeida, A. Versace, M. Phillips, M. Wessa and J.-F. Mangin acquired the data, which Z. Sun, J. Houenou, S. Sarrazin, P. Guevara, C. Poupon, M. Phillips and J.-F. Mangin analyzed. Z. Sun, J. Houenou and J.-F. Mangin wrote the article, which all authors reviewed and approved for publication.
References
- 1.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:33–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nortje G, Stein DJ, Radua J, et al. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J Affect Disord. 2013;150:192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Smith S, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Houenou J, Wessa M, Douaud G, et al. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Mol Psychiatry. 2007;12:1001–10. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- 5.Mahon K, Burdick KE, Szeszko PR. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev. 2010;34:533–54. doi: 10.1016/j.neubiorev.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emsell L, Leemans A, Langan C, et al. Limbic and callosal white matter changes in euthymic bipolar I disorder: an advanced diffusion magnetic resonance imaging tractography study. Biol Psychiatry. 2013;73:194–201. doi: 10.1016/j.biopsych.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 7.McIntosh AM, Munoz Maniega S, Lymer GK, et al. White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:1088–92. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 8.Versace A, Andreazza AC, Young LT, et al. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Mol Psychiatry. 2014;19:200–8. doi: 10.1038/mp.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sowell ER, Thompson PM, Mattson SN, et al. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002;12:856–65. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- 10.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–8. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- 11.Mangin JF, Jouvent E, Cachia A. In-vivo measurement of cortical morphology: means and meanings. Curr Opin Neurol. 2010;23:359–67. doi: 10.1097/WCO.0b013e32833a0afc. [DOI] [PubMed] [Google Scholar]
- 12.Sun Z, Kloppel S, Riviere D, et al. The effect of handedness on the shape of the central sulcus. Neuroimage. 2012;60:332–9. doi: 10.1016/j.neuroimage.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 13.Tenenbaum J, de Silva V, Langford J. A global geometric framework for nonlinear dimensionality reduction. Science. 2000;290:2319–23. doi: 10.1126/science.290.5500.2319. [DOI] [PubMed] [Google Scholar]
- 14.Nurnberger J, Blehar M, Kaufmann C, et al. Diagnostic interview for genetic-studies — rationale, unique features, and training. Arch Gen Psychiatry. 1994;51:849–59. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 15.First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research version. New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- 16.Montgomery S, Asberg M. New depression scale designed to be sensitive to change. B J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young R, Biggs J, Ziegler V, et al. Rating-scale for mania — reliability, validity and sensitivity. B J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 19.Nelson H. National adult reading test (NART) 2nd ed. Windsor: NFER-NELSON; 1991. [Google Scholar]
- 20.Sarrazin S, Poupon C, Linke J, et al. A multicenter tractography study of deep white matter tracts in bipolar I disorder: psychotic features and interhemispheric disconnectivity. JAMA Psychiatry. 2014;71:388–96. doi: 10.1001/jamapsychiatry.2013.4513. [DOI] [PubMed] [Google Scholar]
- 21.Mangin JF, editor. Entropy minimization for automatic correction of intensity nonuniformity. IEEE workshop on mathematical methods in biomedical image analysis. 2000 [Google Scholar]
- 22.Guevara P, Duclap D, Poupon C, et al. Automatic fiber bundle segmentation in massive tractography datasets using a multi-subject bundle atlas. Neuroimage. 2012;61:1083–99. doi: 10.1016/j.neuroimage.2012.02.071. [DOI] [PubMed] [Google Scholar]
- 23.Besl P, McKay N. A method for registration of 3-D Shapes. IEEE Trans Pattern Anal Mach Intell. 1992;14:239–56. [Google Scholar]
- 24.Strakowski SM, Adler CM, Almeida J, et al. The functional neuro-anatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–25. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leroux E, Delcroix N, Dollfus S. Left fronto-temporal dysconnectivity within the language network in schizophrenia: an fMRI and DTI study. Psychiatry Res. 2014;223:261–7. doi: 10.1016/j.pscychresns.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu LH, Zhou XJ, Keedy SK, et al. White matter microstructure in untreated first episode bipolar disorder with psychosis: comparison with schizophrenia. Bipolar Disord. 2011;13:604–13. doi: 10.1111/j.1399-5618.2011.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canales-Rodriguez EJ, Pomarol-Clotet E, Radua, et al. Structural abnormalities in bipolar euthymia: a multicontrast molecular diffusion imaging study. Biol Psychiatry. 2014;76:239–48. doi: 10.1016/j.biopsych.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Wang F, Jackowski M, Kalmar JH, et al. Abnormal anterior cingulum integrity in bipolar disorder determined through diffusion tensor imaging. Br J Psychiatry. 2008;193:126–9. doi: 10.1192/bjp.bp.107.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crow TJ, Chance SA, Priddle TH, et al. Laterality interacts with sex across the schizophrenia/bipolarity continuum: an interpretation of meta-analyses of structural MRI. Psychiatry Res. 2013;210:1232–44. doi: 10.1016/j.psychres.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 31.Takao H, Hayashi N, Ohtomo K. White matter asymmetry in healthy individuals: a diffusion tensor imaging study using tract-based spatial statistics. Neuroscience. 2011;193:291–9. doi: 10.1016/j.neuroscience.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 32.Gong G, Jiang T, Zhu C, et al. Side and handedness effects on the cingulum from diffusion tensor imaging. Neuroreport. 2005;16:1701–5. doi: 10.1097/01.wnr.0000183327.98370.6a. [DOI] [PubMed] [Google Scholar]
- 33.Madsen KS, Jernigan TL, Iversen P, et al. Cortisol awakening response and negative emotionality linked to asymmetry in major limbic fibre bundle architecture. Psychiatry Res. 2012;201:63–72. doi: 10.1016/j.pscychresns.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Whitford TJ, Savadjiev P, Kubicki M, et al. Fiber geometry in the corpus callosum in schizophrenia: evidence for transcallosal mis-connection. Schizophr Res. 2011;132:69–74. doi: 10.1016/j.schres.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walterfang M, Wood AG, Barton S, et al. Corpus callosum size and shape alterations in individuals with bipolar disorder and their first-degree relatives. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1050–7. doi: 10.1016/j.pnpbp.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 36.Mitelman SA, Nikiforova YK, Canfield EL, et al. A longitudinal study of the corpus callosum in chronic schizophrenia. Schizophr Res. 2009;114:144–53. doi: 10.1016/j.schres.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beaulieu C. The basis of anisotropic water diffusion in the nervous system — a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 38.Schneider MR, DelBello MP, McNamara RK, et al. Neuroprogression in bipolar disorder. Bipolar Disord. 2012;14:356–74. doi: 10.1111/j.1399-5618.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- 39.Hafeman DM, Chang KD, Garrett AS, et al. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- 40.Mangin JF, Fillard P, Cointepas Y, et al. Toward global tractography. Neuroimage. 2013;80:290–6. doi: 10.1016/j.neuroimage.2013.04.009. [DOI] [PubMed] [Google Scholar]