Abstract
Background
Human genetic studies have indicated that mutations in calcium/calmodulin-dependent serine protein kinase (CASK) result in X-linked mental retardation and autism-spectrum disorders. We aimed to establish a mouse model to study how Cask regulates mental ability.
Methods
Because Cask encodes a multidomain scaffold protein, a possible strategy to dissect how CASK regulates mental ability and cognition is to disrupt specific protein–protein interactions of CASK in vivo and then investigate the impact of individual specific protein interactions. Previous in vitro analyses indicated that a rat CASK T724A mutation reduces the interaction between CASK and T-brain-1 (TBR1) in transfected COS cells. Because TBR1 is critical for glutamate receptor, ionotropic, N-methyl-d-aspartate receptor subunit 2B (Grin2b) expression and is a causative gene for autism and intellectual disability, we then generated CASK T740A (corresponding to rat CASK T724A) mutant mice using a gene-targeting approach. Immunoblotting, coimmunoprecipitation, histological methods and behavioural assays (including home cage, open field, auditory and contextual fear conditioning and conditioned taste aversion) were applied to investigate expression of CASK and its related proteins, the protein–protein interactions of CASK, and anatomic and behavioural features of CASK T740A mice.
Results
The CASK T740A mutation attenuated the interaction between CASK and TBR1 in the brain. However, CASK T740A mice were generally healthy, without obvious defects in brain morphology. The most dramatic defect among the mutant mice was in extinction of associative memory, though acquisition was normal.
Limitations
The functions of other CASK protein interactions cannot be addressed using CASK T740A mice.
Conclusion
Disruption of the CASK and TBR1 interaction impairs extinction, suggesting the involvement of CASK in cognitive flexibility.
Introduction
Intellectual disability, also known as mental retardation, is one of the most common neurologic disorders in developed countries, with a prevalence around 2%–3%.1 Compared with girls, boys are more susceptible to mental retardation. Large-scale analyses suggest that defects in X-linked genes are the primary cause of the sex bias in mental retardation.2,3 Calcium/calmodulin-dependent serine protein kinase (CASK) is one of the X-linked genes associated with syndromic mental retardation.4–9 CASK mutations identified from patients are widely distributed across the entire gene, and no hot mutation spot in CASK linked to the disorder has been identified.7,10 The phenotypes of patients with CASK mutations also vary.10 It has been reported that CASK mutation may cause neonatal death in boys.4 In some patients, typical progressive microcephaly with pontine and cerebral hypoplasia (MICPCH) develops.4,10 In others, mild to severe mental retardation with or without nystagmus has been reported.10 In some cases, autism-spectrum disorders (ASD) develop in patients with CASK mutations.10 These human genetic studies indicate that CASK is a critical gene for brain development and function.
CASK is a versatile molecule. It is widely distributed in various tissues, though its expression level is around 3-fold higher in the brain than other tissues.11 In neurons, CASK proteins are widely present in axons, dendrites, synapses, somata and even the nuclei of neurons.12–14 It acts as a multi-domain scaffold protein with several different protein–protein binding domains to interact with more than 2 dozen proteins.15 CASK and its interacting proteins control a variety of cellular functions, including synaptic targeting of ion channels and synaptic adhesion molecules, dendritic spine formation, axonal outgrowth and differentiation and transcriptional control.7,15–17 As in humans, Cask is an essential gene in mice because knockout or insertion mutations of Cask in male mice results in neonatal death within 1–2 days after birth.18,19 Mice carrying the floxed allele of Cask exhibit reduced CASK expression and smaller body size,18 further suggesting that CASK regulates brain development as well as that of other tissues. Because of lethality and smaller body size, it is either impossible or inappropriate to use knockout or floxed mutant mice to analyze the cognitive functions of CASK. Thus, although genetic studies indicate the critical role of CASK in brain function, an appropriate Cask animal model for studies of learning and memory is still lacking.
Among various CASK-interacting proteins, T-Brain-1 (TBR1) — a brain-specific T-box transcription factor14,20 —controls neuronal migration and axonal projection of the cerebral cortex and amygdala.21–24 Interaction with TBR1 likely contributes to the role of CASK in development. In addition to neurodevelopment, TBR1 also serves as an immediate early gene in response to synaptic stimulation to upregulate glutamate receptor, ionotropic, N-methyl-d-aspartate receptor subunit 2B (Grin2b, also known as Nmdar2b) expression in mature neurons.25 Human genetic studies have indicated that TBR1 is a high-confidence risk factor for ASD and intellectual disability.26–31 Because interaction with CASK increases the transcriptional activity of TBR1 to upregulate Grin2b expression,14,24,32–34 the TBR1–GRIN2B pathway is likely involved in the function of CASK in regulating intellectual ability. Our previous study showed that the Thr724 (T724) residue of rat CASK protein is the protein kinase A (PKA) phosphorylation site. The T724 residue is located in the C-terminal guanylate kinase domain (i.e., the interacting domain for TBR1).14 The rat CASK T724A mutation noticeably reduces interactions between CASK and TBR1.35,36 Since TBR1 is specifically expressed in the brain, disruption of the interaction between CASK and TBR1 only impairs the neuronal function of CASK. Thus, it may serve as a model to explore the role of CASK in learning and memory. To investigate this possibility, here we generated CASK T740A (corresponding to T724A of the rat protein) knock-in mutant mice. By analyzing the mutant mice, we found that CASK is critical for extinction of associative memory.
Methods
CASK T740A knock-in mouse construction
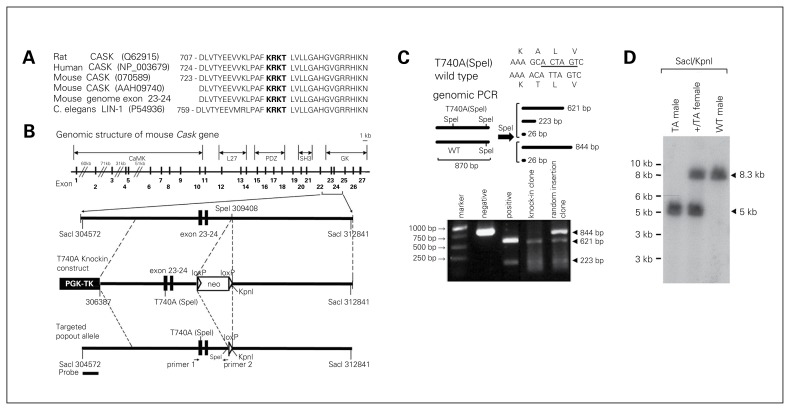
Genomic DNA fragments of the Cask gene were polymerase chain reaction (PCR)–amplified from purified 129/SvJ genomic DNA, subcloned into vector osdupdel (a gift from Dr. Hsin-Fang Yang-Yen of the Institute of Molecular Biology, Academia Sinica, Taiwan) and sequence-verified based on the genomic clone ID 12361 (Mouse Genome Informatics, www.informatics.jax.org/). We determined the exon number based on the mouse Cask gene with accession number NM_009806. The 5′ arm of the targeting construct contains exon 23 and 24 of the mouse Cask gene. Residue T740 is located in exon 23. We used the QuickChange method (Strata-gene) to make the T740A(SpeI) double mutation in the genomic fragment. In addition to the T740A mutation, a silence mutation near the T740A site was also created to generate an additional SpeI site. The T740A knock-in construct was then electroporated into RW4 embryonic stem (ES) cells by the Transgenic Core Facility of the Institute of Molecular Biology, Academia Sinica, Taiwan. We used G418 resistance to preliminarily screen the transfected ES cells. Two methods were applied for genotyping: genomic PCR with primers Cask-308565 (5′-AATGGCACTATGAATTAAATCACA-3′) and Cask-a-309434 (5′-AGAGGTTGATAATATGAAATAG-3′) followed by SpeI digestion, and Southern blotting using a DNA fragment corresponding to nucleotides 304355–305104 as a probe to hybridize SacI and KpnI double-digested genomic DNA fragments.
Antibodies
The following antibodies were used in this study: anti-β-ACTIN (A2228, mouse) and anti-β-TUBULIN (T4026) from Sigma, anti-GAPDH (sc-25778), anti-GRIN2B (AB1557P, rabbit, Millipore Chemicon), anti-PARKIN (AB5112, Cell Signalling), anti-VCP (612183, mouse, BD Transduction Laboratories), and anti-VELI-3 (51–5600, Zymed). CASK, CASK interacting nucleosome assembly protein (CINAP), Munc-18 interacting protein 1 (MINT1), TBR1, VELI-2, PSD-95 and SAP-97 rabbit polyclonal antibodies have been described previously.14,32,37–39
Tissue extraction and subcellular fractionation
Mouse whole brains were homogenized in 5 mL of HEPES buffer containing protease and phosphatase inhibitors (4 mM HEPES, pH 7.4, 320 mM sucrose, 5 mM ethylenediamine-tetraacetic acid [EDTA], 5 mM sodium pyrophosphate, 30 mM β-glycerophosphate, 30 mM sodium fluoride, 1μg/mL leupeptin, 5μg/mL aprotinin and 1 mM phenylmethyl-sulfonyl fluoride [PMSF]). Total homogenate (H) was centrifuged at 800g for 10 min to remove the crude nuclear fraction (P1). The supernatant was then centrifuged at 9200g for 15 min to yield the crude synaptosomal fraction (P2). The supernatant was further centrifuged at 165 000g for 2 h to result in cytosol (S3) and light membrane (P3) fractions. The P2 pellets were resuspended in 250 μL HEPES buffer and added into 2.25 mL hypotonic buffer (HEPES buffer without sucrose). After 30 min of incubation on ice, the synaptosomal membrane fraction LP1 was collected by centrifugation at 25 000g for 20 min at 4°C. Equal protein amounts of each fraction were analyzed with immunoblotting.
Animals and behaviour test
CASK T740A mice were maintained in a C57BL/6 background by backcrossing with wild type (WT) C57BL/6 mice for more than 10 generations. Mice were housed in group cages of 4–5 mice each in an air-conditioned vivarium and maintained on a 12-hour light/dark cycle (light on at 8 am) with free access to food and water (except for mice subjected to conditioned taste aversion [CTA]). We used 3-month-old male mice for behavioural tests, which were performed between 10 am and 6 pm. All procedures adhered to the guidelines for care and use of experiment animals of Academic Sinica, Taiwan. Monitoring of mouse behaviours in home cages and the open field were performed as described previously.40
Conditioned taste aversion
We performed CTA as described previously.24,40 Briefly, during the 7-day pretraining period (days −7 to −1), mice were deprived of water in their home cages and put into the experimental cages to receive their daily water for 15 min every day. On the day of training (D0), mice were presented with a sucrose-lithium chloride (LiCl) pairing, where they were first offered a sucrose solution (pleasant, novel taste; 100 mM, 15 min) and then they were given an intraperitoneal injection of LiCl (malaise-inducing agent; 0.15 or 0.1 M, 20 μL/g of body weight). Finally, they were returned to their home cages and observed for diarrhea. Animals that received NaCl instead of LiCl served as controls. Two days after training, mice were presented with 1 bottle of water and 1 bottle of sucrose solution simultaneously for a 15-min period in the experimental cages. Sucrose and water intakes were recorded to measure sucrose preference, where the sucrose preference index = sucrose intake ÷ (sucrose + water intake). A sucrose preference of less than 50% indicated memory of the CTA.
Fear conditioning
The schemes of auditory and contextual fear conditioning are summarized in the Results section. Briefly, the size of the chambers (both box A and box B) was 15 × 15 × 20 cm. In both auditory and contextual fear conditioning, 3 footshocks (unconditioned stimulus; each 0.6 mA for 2 s) were applied at training day 0 to induce a fear response. After 3 footshocks, mice were allowed to stay in the conditioning chamber for at least 1 min more to record the response after stimulation. For auditory fear conditioning, a tone of 2000 Hz, 80 dB for 18 s was used as the conditioned stimulus. The average freezing percentage in response to the first 5 tone stimulations at training day 1 represented fear memory. The average freezing percentage in response to the last 5 tone stimulations at training day 2 was recognized as the level of extinction. For contextual fear conditioning, mice were placed back in box A for 10 min every day from days 1–6. We measured the average freezing percentage of the entire 10 min. The response at day 1 was contextual fear memory. The responses for days 2–6 indicated the levels of extinction. The freezing responses of mice were videotaped and analyzed using FreezeScan 2.0 (CleverSys Inc.).
Statistical analysis
Data are presented as means with standard errors of the mean (SEM) based on the number of animals or samples analyzed. For each experiment, statistical comparisons were performed with a 2-tailed unpaired t test, a 2-way analysis of variance (ANOVA) and a post hoc Bonferroni test, or repeated-measures 2-way ANOVA and a post hoc Bonferroni test using GraphPad Prism 5.0 or Sigmastat 3.5. The results of statistical analyses are summarized in Appendix 1, Table S1, available at jpn.ca.
Results
Generation of CASK T740A mutant mice
Through amino acid sequence alignment, we found that the T724 PKA site of the rat CASK protein is conserved among humans, rats, mice and Caenorhabditis elegans (Fig. 1A). In mice, the T740 residue corresponds to the T724 residue of rat CASK proteins (Fig. 1A). We then introduced the T740A mutation into exon 23 of the mouse Cask gene using a traditional gene targeting approach (Fig. 1B). Without changing the codon, an additional SpeI enzyme digestion site was also generated directly adjacent to the T740A site (Fig. 1C). Genomic PCR amplification followed by SpeI digestion provided an easy way to distinguish WT and T740A alleles (Fig. 1C). The genotypes of mutant mice were also confirmed by Southern blotting (Fig. 1D).
Fig. 1.
Generation of calcium/calmodulin-dependent serine protein kinase (CASK) T740A knock-in mutant mice. (A) Alignment of the amino acid sequence flanking residue T724 of rat CASK. The conserved protein kinase A (PKA) site KRKT is shown in bold. Note that residue T740 of mouse CASK corresponds to residue T724 of rat CASK. (B) Schematic representations of the mouse Cask gene, CASK T740A targeting vector and CASK T740A mutant allele after removal of the Neo cassette. The codon for residue T740 is located in exon 23. In addition to the T740A mutation, an additional SpeI site was also generated directly adjacent to the T740A codon, indicated as T740A(SpeI). The corresponding positions of (C) primers used for genomic polymerase chain reaction (PCR) and (D) the probe used for Southern blotting are also indicated. (C) Genomic PCR for embryonic stem cell screening. The PCR products were digested with SpeI to distinguish wild-type (WT) and T740A(SpeI) alleles. (D) Genomic Southern blot of heterozygous (+/TA) female, T740A (TA) mutant and WT male mice.
CASK T740A mutation reduces the interaction of CASK with TBR1 and CINAP
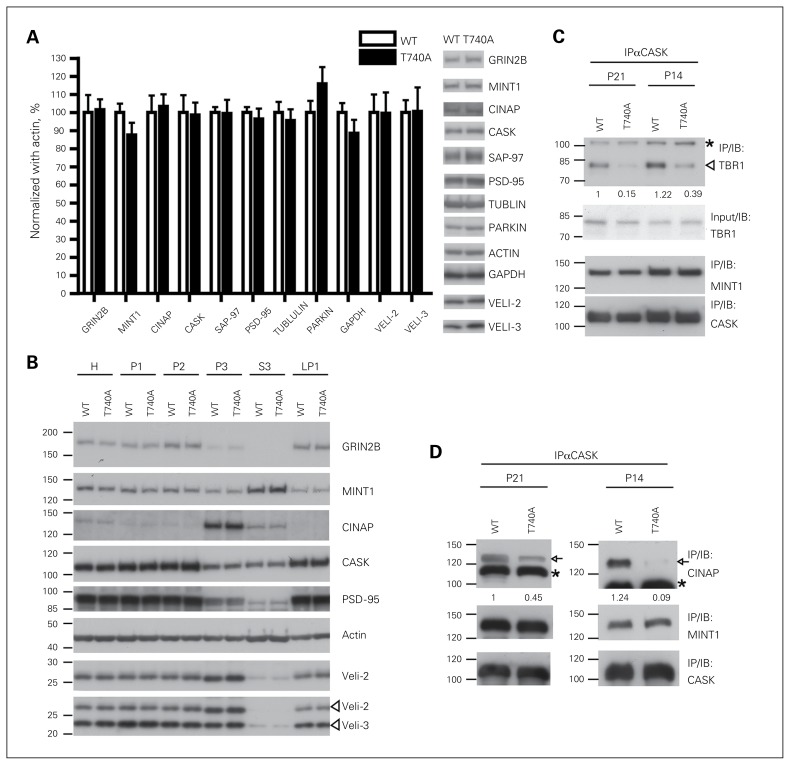
In general, both female CaskT740A/T740A and male CaskT740A/y mutant mice were healthy and fertile. For this report, only male CaskT740A/y mutant mice (referred to as CASK T740A mice hereafter) were used in the following biochemical, anatomic and behavioural assays. We first examined whether the T740A mutation influences expression or subcellular distribution of CASK itself and its interacting proteins. Total homogenates prepared from adult WT and T740A mutant brains were probed with a series of antibodies recognizing CASK interacting proteins or related molecules. We found that the total protein levels of examined proteins, including CASK itself, were not changed in T740A mutant brains (Fig. 2A). By analyzing a series of biochemical fractions, we found that the CASK T740A mutation did not alter the subcellular distribution of its interacting proteins or of CASK itself (Fig. 2B). We then investigated whether, similar to the in vitro system using the rat Cask construct,35,36 the mouse CASK T740A mutation attenuates the interaction between CASK and TBR1 in the brain. Because TBR1 is highly expressed at the early stage, we performed coimmunoprecipitation from postnatal days 14 and 21 (P14 and P21) mouse brains using CASK antibody. In both ages, the T740A mutation greatly reduced interactions between CASK and TBR1 by 68%–85% (Fig. 2C). In addition to TBR1, the guanylate kinase domain of CASK also interacts with CINAP.32 We found that the CASK T740A mutant also precipitated less CINAP from both P14 and P21 brains (Fig. 2D). In contrast to TBR1 and CINAP, interactions between CASK and MINT1 — a protein interacting with the N-terminal CaMK domain of CASK — was not affected by the CASK T740A mutation (Fig. 2C, Fig. 2D). These analyses suggest that the T740A mutation reduces the interaction of CASK with TBR1 and CINAP, but has no obvious effect on other CASK interacting proteins.
Fig. 2.
Calcium/calmodulin-dependent serine protein kinase (CASK) T740A mutation reduces CASK–T-brain-1 (TBR1) and CASK–CASK interacting nucleosome assembly protein (CINAP) interactions. (A) Expressions of CASK and several CASK-associated proteins are not influenced by the CASK T740A mutation. Immunoblotting using cortex and hippocampus extracts of CASK T740A mutant and wild-type (WT) adult mice was performed. The levels of examined proteins were normalized with ACTIN. Data represent means ± standard errors of the mean (SEM); n = 4. (B) Subcellular distributions of CASK and CASK-related proteins are not altered by the CASK T740A mutation. (C, D) Cortical and hippocampal extracts of P14 and P21 mouse brains were solubilized and subjected to immunoprecipitation (IP) using CASK antibody. The precipitates were then analyzed by immunoblotting (IB) using TBR1, CINAP, MINT1 and CASK antibodies. The input levels of TBR1 are also indicated. Representative data of 3 independent coimmunoprecipitation experiments are shown in (C and D). *Crossreactive protein species. The relative intensities of coprecipitated TBR1 and CINAP are indicated. H = total homogenate; LP1 = lyzed synaptosomal membrane fraction; P1 = crude nuclear fraction; P2 = crude synaptosomal fraction; P3 = light membrane fraction; S3 = soluble cytosolic fraction.
GRIN2B expression is slightly lower in the amygdala of CASK T740A mice
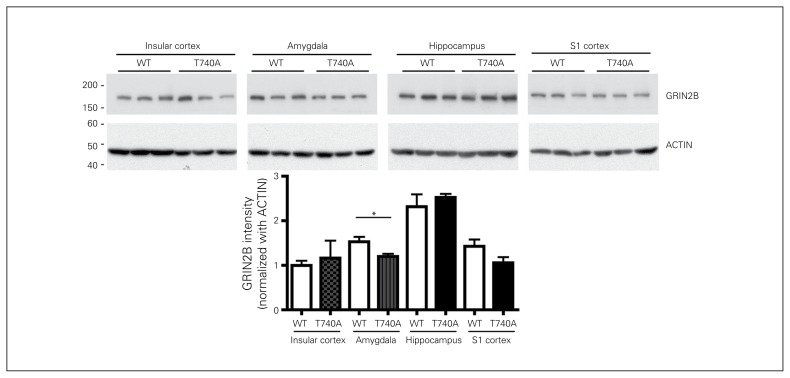
Our previous study showed that the CASK–TBR1–CINAP complex regulates Grin2b expression.33 Since the interaction of CASK with TBR1 and CINAP was reduced in CASK T740A mouse brains, we wondered whether Grin2b expression is altered by the T740A mutation. Because TBR1 is specifically expressed in the cerebral cortex, hippocampus and amygdala, we then examined GRIN2B levels in these brain regions. In the insular cortex, sensory cortex and hippocampus, there were no obvious differences in GRIN2B levels. However, in the amygdala, GRIN2B levels were reduced by 20% in CASK T740A mice (Fig. 3). These results are consistent with our previous observation that the amygdala is the most sensitive brain region to Tbr1 deficiency.24 This biochemical analysis also suggests that the function of the amygdala is likely affected by the CASK T740A mutation.
Fig. 3.
Glutamate receptor, ionotropic, N-methyl-d-aspartate receptor subunit 2B (GRIN2B) levels are reduced in the amygdala of calcium/calmodulin-dependent serine protein kinase (CASK) T740A mice. Extracts of the insular and sensory (S1) cortex, hippocampus and amygdala were analyzed with immunoblotting using GRIN2B and ACTIN antibodies. The GRIN2B protein levels were normalized with ACTIN. *p < 0.05; n = 3. WT = wild type.
CASK T740A mice are generally healthy and normal in home cages and the open field
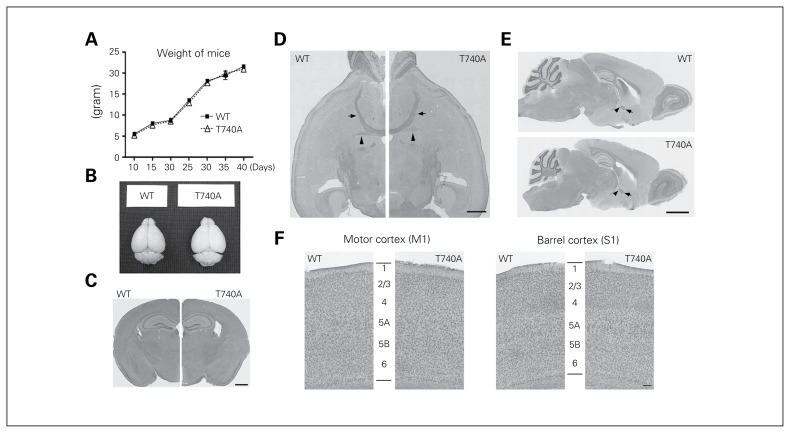
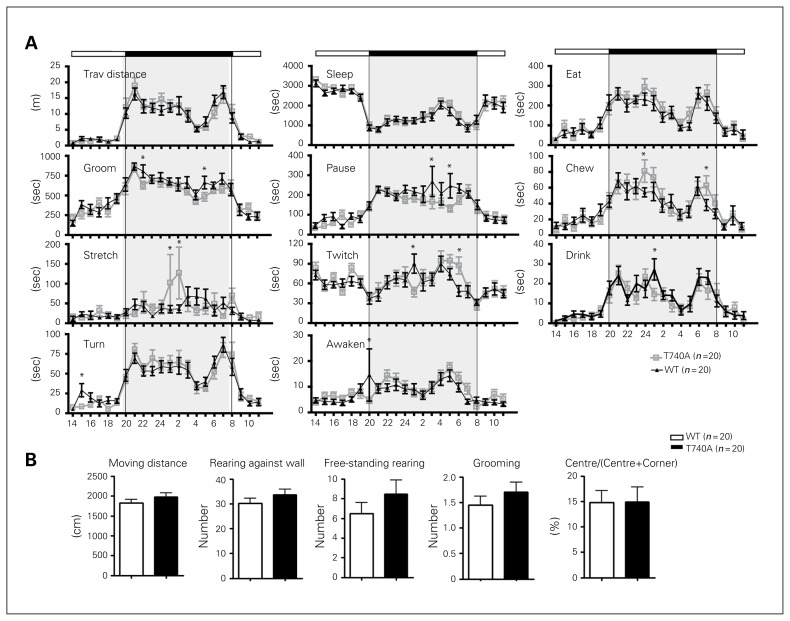
The appearance of CASK T740A mice and their brains were normal and comparable to WT littermates (Fig. 4A and B). The histological features of CASK T740A brains were also normal (Fig. 4C and F). Even the posterior part of the anterior commissure, the structure specifically missing in Tbr1+/− mice, was still present in CASK T740A mice (Fig. 4D and E). Neuronal lamination was also normal in both motor and sensory cortices (Fig. 4F). A series of behavioural paradigms were then applied to analyze behaviours of CASK T740A mice. In home cages, T740A mutant mice were generally comparable with WT littermates (Fig. 5A). In an open field, total moving distance, numbers of rearing and grooming events and the percentage of time spent in the central area were comparable between WT and CASK T740A mice (Fig. 5B). Thus, the daily behaviours in home cages and the locomotion, anxiety and exploratory activity in the open field were normal in CASK T740A mice.
Fig. 4.
Body weight and brain sizes of calcium/calmodulin-dependent serine protein kinase (CASK) T740A mice are generally normal. (A) Body weight of wild-type (WT) littermates and CASK T740A mice was measured from P10 to P40. n = 20. (B) Appearance of CASK T740A mouse brain is also normal. (C–E) Histological features of CASK T740A mouse brains revealed by hematoxylin and eosin staining are also similar to those of WT brains in (C) coronal, (D) horizontal and (E) sagittal views. Arrowheads indicate the posterior part of the anterior commissure; arrows point to the anterior part of the anterior commissure. (F) Higher magnification images of cortical M1 and S1 regions revealed by crystal violet stain. The 6-layer structure of the cerebral cortex is labelled. Scale bar: (C, D) 1 mm, (E) 2 mm, (F) 0.1 mm.
Fig. 5.
Calcium/calmodulin-dependent serine protein kinase (CASK) T740A mice are generally normal in home cages and the open field. (A) Home cage behaviours. A total of 11 different behaviours were analyzed. Shaded boxes represent the dark cycle (8 pm to 8 am). (B) Open field behaviours. The behaviours of animals in the open field were monitored for 5 min. Total moving distance, the numbers of rearing against the walls, free-standing rearing and grooming events, and the percentage of time spent in the central area in the open field are shown. Animal numbers for each genotype are indicated. *p < 0.05. WT = wild type.
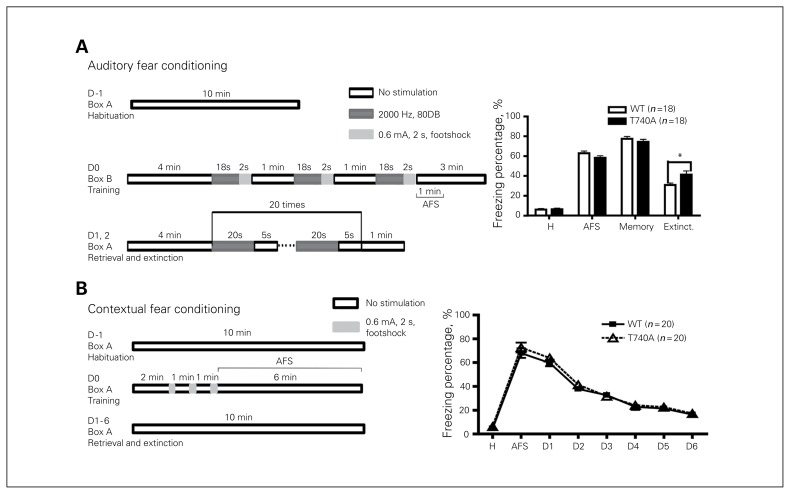
CASK T740A mutation impairs extinction of cued fear conditioning but not contextual fear conditioning
Fear memory of CASK T740A mice was then measured using auditory and contextual fear conditioning (Fig. 6). In both paradigms, basal freezing responses during habituation at day −1 were comparable between T740A and WT mice (Fig. 6A and B). At training day 0, the responses right after footshock stimulation (AFS) were also similar in these 2 kinds of mice (Fig. 6A and D). When fear memory was retrieved at day 1, the day immediately after training, fear memory of both auditory and cued fear conditioning was still not altered by the CASK T740A mutation (Fig. 6A and B). We then continued to investigate whether the CASK T740A mutation had any impact on fear extinction. After memory retrieval, mice were repeatedly exposed to conditioned stimulation in the absence of footshock to induce fear extinction. For auditory fear extinction, CASK T740A mice had a noticeably higher freezing response than WT littermates (Fig. 6A), suggesting the impairment of fear extinction in CASK T740A mice. In contrast, contextual fear extinction was normal in CASK T740A mice (Fig. 6B). These results suggest that auditory fear extinction is particularly sensitive to the CASK T740A mutation.
Fig. 6.
Calcium/calmodulin-dependent serine protein kinase (CASK) T740A mice show an extinction defect in (A) auditory fear conditioning, but not (B) contextual fear conditioning. The fear conditioning schemes are listed on the left. The freezing percentages during the periods of habituation (H), right after 3 footshock stimulations (AFS), retrieval (memory) and extinction (extinct.) are shown. For contextual fear conditioning, the average freezing percentage at D1 indicates memory; the percentages at D2–D6 indicate the levels of cognitive extinction. The only difference between wild-type (WT) and CASK T740A mice is extinction of auditory fear conditioning. Sample sizes are indicated. *p < 0.05.
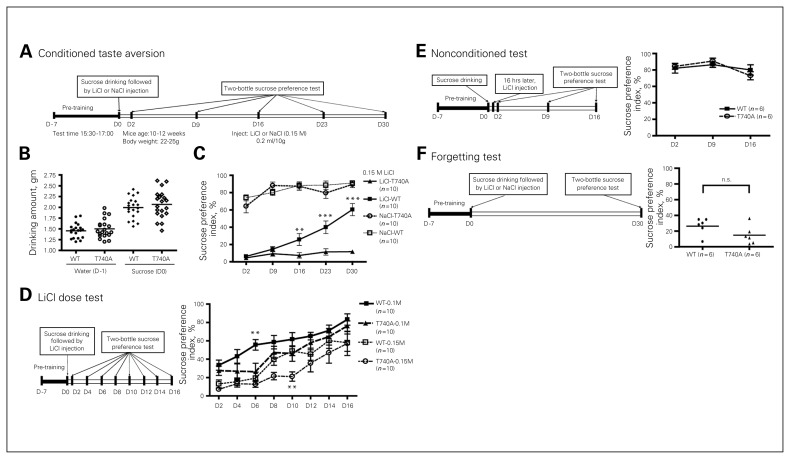
Extinction of conditioned taste aversion is also inhibited in CASK T740A mice
To further explore the role of CASK in extinction of associative memory, CASK T740A mice were further analyzed using CTA (Fig. 7A), a well-known amygdala-dependent memory assay. The amounts of water consumed on pretraining day 1 (day −1) and sucrose on the training day (day 0) were similar between WT littermates and CASK T740A mice (Fig. 7B), suggesting that their sucrose preferences were similar. After induction of an aversion response with LiCl, both WT and T740A mice showed very low levels of sucrose intake at day 2 when both water and sucrose were presented as a 2-bottle preference test (Fig. 7C), indicating that sucrose intake was successfully conditioned by LiCl-induced aversion. These results suggest that, similar to auditory fear conditioning, CASK T740A mice exhibited normal learning and memory in the CTA test. Mice were then subjected to a 2-bottle sucrose preference test every week to investigate the role of the CASK T740A mutation in extinction of CTA (Fig. 7C). For WT littermates, the sucrose preference index gradually increased when the sucrose solution was repeatedly presented in the absence of LiCl-induced aversion (Fig. 7C). At day 30, the index was higher than 50%, indicating an extinction of taste aversion in WT mice. In contrast, sucrose preference in CASK T740A mice remained at a very low level (about 10%) even at day 30 (Fig. 7C). For mice injected with NaCl, sucrose preference was not affected in either WT or CASK T740A mice (Fig. 7C). Together, these results suggest that it is difficult to extinguish CTA in CASK T740A mice.
Fig. 7.
Calcium/calmodulin-dependent serine protein kinase (CASK) T740A mice show an extinction deficit in conditioned taste aversion (CTA). (A) CTA paradigm. (B) Amounts of water and sucrose consumed at training day −1 (D −1) and on training day (D0). Both mutant and wild-type (WT) mice preferred sucrose before the assay. (C) CASK T740A mice do not extinguish old memories in a CTA test. The injection dosage of LiCl and NaCl is 0.15 M. (D) Dosage effect of LiCl in CTA. Mice were treated with 0.1 M or 0.15 M LiCl. (E, F) Mutant and WT mice exhibit no differences in nonconditioned tests and forgetting. Sample sizes are indicated. *p < 0.05; **p < 0.01.
To further investigate whether the fear extinction defect of CASK T740A mice was dependent on stimulation strength, we modified the protocol by comparing the effect of 0.15 M with 0.1 M LiCl and performing the 2-bottle sucrose preference test every other day after training (Fig. 7D). For the group injected with 0.15 M LiCl, WT mice noticeably increased their sucrose preference at day 8. By day 10, the sucrose preference of WT mice had reached around 50%, indicating extinction of CTA (Fig. 7D). In contrast, CASK T740A mice noticeably increased their sucrose preference until day 12, but reached 50% sucrose preference only at day 16 (Fig. 7D). For the group injected with 0.1 M LiCl, extinction of CTA was faster than that of animals treated with 0.15 M LiCl (Fig. 7D). In WT mice, extinction was successfully achieved at day 6 (Fig. 7D), reflecting a weaker memory strength induced by 0.1 M LiCl. For CASK T740A mice injected with 0.1 M LiCl, sucrose preferences were higher than 50% at day 12, which was faster than CASK T740A mice injected with 0.15 M LiCl. However, this response was still slower than that of WT mice injected with 0.1 M LiCl (Fig. 7D). Together, extinction of CTA in CASK T740A mice was much slower than that of WT mice, although the response was still dose-dependent.
A nonconditioned test was also performed by injecting LiCl 16 h after sucrose intake (Fig. 7E). Delayed administration of LiCl did not impair the sucrose preferences of WT or CASK T740A mice (Fig. 7E). Taken together, these results suggest that, compared with WT littermates, CASK T740A mice have a specific defect in memory extinction.
To further rule out the possibility that the differences between WT littermates and CASK T740A mice were due to a difference in memory strength (e.g., WT mice forget faster than CASK T740A mice), we modified the CTA protocol. Instead of testing sucrose preferences every week, the sucrose preference test was performed only at day 30 after LiCl injection (Fig. 7F). For this assay, the sucrose preferences of both WT littermates and CASK T740A mice were much lower than 50% and were comparable (Fig. 7F), indicating that memory of CTA remained in both WT and CASK T740A mice 30 days after learning. These data suggest that faster extinction in WT mice was unlikely to be due to forgetting the experience. It also suggests that the behavioural differences shown in Fig. 7C were due to the defect in extinction.
Discussion
To our knowledge, we have generated in this study the first Cask point mutation mice that exhibit impairment of specific protein–protein interactions of CASK. Because the mutant mice are generally healthy, this line serves as an appropriate genetic model to explore the mental functions of CASK. Based on our analyses, the most dramatic phenotype of CASK T740A mice is defective cognitive extinction, though memory is not affected by the CASK T740A mutation. Extinction of conditioned taste aversion and auditory fear conditioning, but not contextual fear conditioning, are specifically affected by the CASK T740A mutation. It is interesting to point out that deletion of Tbr1 (Tbr1−/−) — a critical transcriptional factor for brain development — results in severe morphological defects in the cerebral cortex and amygdala and neonatal death. However, the CASK T740A mutation does not lead to morphological alteration or developmental defects. Even the posterior part of the anterior commissure is still present in CASK T740A mice, suggesting that reduction of the interactions between CASK and TBR1 is not critical for brain development.
In addition to development, Tbr1 acts as an immediate early gene to upregulate Grin2b expression.25 Because GRIN2B is critical for memory as well as extinction of CTA,41–43 taken together with our data, it seems possible that the interaction between CASK and TBR1 regulates Grin2b expression and thus contributes to cognitive extinction. A slight reduction of GRIN2B levels in the amygdala of CASK T740A mice supports this speculation. A lower expression level of Grin2b might impair the relearning process and thereby interfere with extinction of associative memory. Note that memory of CTA is disrupted in Tbr1+/− mice.24 Perhaps CASK T740 phosphorylation by protein kinase A (PKA) is specifically involved in the TBR1–GRIN2B pathway during extinction. Because old memories are more difficult to extinguish in CASK T740A mice, it also implies that they lack cognitive flexibility, which may be relevant to autistic behaviours in patients.10 Although the corresponding CASK T740A mutation has not yet been identified in patients with CASK deficiency, our study unexpectedly reveals that PKA phosphorylation of CASK plays a role in extinction. In our previous study, d-cycloserine — a GRIN2B coagonist — is able to ameliorate memory impairments caused by Tbr1 haploinsufficiency.24 If Grin2b deficiency is indeed the key to extinction defects caused by the CASK T740A mutation, it should be possible to test whether d-cycloserine can also ameliorate cognitive extinction defects in CASK T740A mice in the future.
In addition to TBR1, CASK T740A mutation also reduces the interaction between CASK and CINAP. Our previous study indicated that CINAP influences the transcriptional activity of TBR1 through the interaction with CASK.32 Knockdown of CINAP in cultured neurons reduces the activity of the Grin2b promoter.32 However, Cinap knockout does not noticeably alter Grin2b expression in mouse brains.40 It seems possible that in the TBR1–CASK–CINAP complex, TBR1 is more critical for regulating Grin2b expression. CASK and CINAP likely play a moderate role in controlling Grin2b expression. Alternatively, it is also possible that other nucleosome assembly proteins compensate for the Cinap deficit.40 More investigations are required to examine these possibilities.
Limitations
CASK interacts with multiple proteins in neurons to control different neuronal responses or activity. Here, the CASK T740A mutation disrupts only interactions between CASK and TBR1 and CINAP. To further explore the contribution of the other protein–protein interactions of CASK in cognition, more genetic models need to be established. Since the CRISPR-Cas system is available to rapidly edit genomes to create mutant mice, it is now possible to perform a larger-scale experiment to generate more mutant mice for more functional characterization.
Conclusion
Using CASK T740A mice, we show that a T740A mutation reduces interactions between CASK and TBR1 and CINAP in mouse brains and provide evidence that CASK is critical for extinction of associative memory.
Acknowledgements
T.-N. Huang is supported by a postdoctoral fellowship of Academia Sinica. Y.-P. Sueh is supported by grants from Academia Sinica (AS-103-TP-B05) and the Ministry of Science and Technology (MOST 103-2321-B-001-002 and 104-2321-B-001-050).
Footnotes
Competing interests: Both authors have a patent pending for “A pharmaceutical composition for treating autism-spectrum disorders which comprises clioquinol” in Korea and the United States. Y.-P. Hsueh has a patent pending for “Increase of protein synthesis ameliorates synaptopathy-related neurological disorders” in Taiwan and the United States.
Contributors: Both authors designed the study. T.-N. Huang acquired the data, which both authors analyzed. Both authors wrote and reviewed the article and approved the final version for publication.
References
- 1.Leonard H, Wen X. The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment Retard Dev Disabil Res Rev. 2002;8:117–34. doi: 10.1002/mrdd.10031. [DOI] [PubMed] [Google Scholar]
- 2.Lehrke R. Theory of X-linkage of major intellectual traits. Am J Ment Defic. 1972;76:611–9. [PubMed] [Google Scholar]
- 3.Lehrke RG. X-linked mental retardation and verbal disability. Birth Defects Orig Artic Ser. 1974;10:1–100. [PubMed] [Google Scholar]
- 4.Najm J, Horn D, Wimplinger I, et al. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet. 2008;40:1065–7. doi: 10.1038/ng.194. [DOI] [PubMed] [Google Scholar]
- 5.Piluso G, D’Amico F, Saccone V, et al. A missense mutation in CASK causes FG syndrome in an Italian family. Am J Hum Genet. 2009;84:162–77. doi: 10.1016/j.ajhg.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarpey PS, Smith R, Pleasance E, et al. A systematic, large-scale re-sequencing screen of X-chromosome coding exons in mental retardation. Nat Genet. 2009;41:535–43. doi: 10.1038/ng.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsueh YP. Calcium/calmodulin-dependent serine protein kinase and mental retardation. Ann Neurol. 2009;66:438–43. doi: 10.1002/ana.21755. [DOI] [PubMed] [Google Scholar]
- 8.Hackett A, Tarpey PS, Licata A, et al. CASK mutations are frequent in males and cause X-linked nystagmus and variable XLMR phenotypes. Eur J Hum Genet. 2010;18:544–52. doi: 10.1038/ejhg.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moog U, Kutsche K, Kortum F, et al. Phenotypic spectrum associated with CASK loss-of-function mutations. J Med Genet. 2011;48:741–51. doi: 10.1136/jmedgenet-2011-100218. [DOI] [PubMed] [Google Scholar]
- 10.Moog U, Bierhals T, Brand K, et al. Phenotypic and molecular insights into CASK-related disorders in males. Orphanet J Rare Dis. 2015;10:44. doi: 10.1186/s13023-015-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–94. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsueh YP, Yang FC, Kharazia V, et al. Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J Cell Biol. 1998;142:139–51. doi: 10.1083/jcb.142.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsueh YP, Sheng M. Regulated expression and subcellular localization of syndecan heparan sulfate proteoglycans and the syndecan-binding protein CASK/LIN-2 during rat brain development. J Neurosci. 1999;19:7415–25. doi: 10.1523/JNEUROSCI.19-17-07415.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsueh YP, Wang TF, Yang FC, et al. Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature. 2000;404:298–302. doi: 10.1038/35005118. [DOI] [PubMed] [Google Scholar]
- 15.Hsueh YP. The role of the MAGUK protein CASK in neural development and synaptic function. Curr Med Chem. 2006;13:1915–27. doi: 10.2174/092986706777585040. [DOI] [PubMed] [Google Scholar]
- 16.Kuo TY, Hong CJ, Hsueh YP. Bcl11A/CTIP1 regulates expression of DCC and MAP1b in control of axon branching and dendrite outgrowth. Mol Cell Neurosci. 2009;42:195–207. doi: 10.1016/j.mcn.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Kuo TY, Hong CJ, Chien HL, et al. X-linked mental retardation gene CASK interacts with Bcl11A/CTIP1 and regulates axon branching and outgrowth. J Neurosci Res. 2010;88:2364–73. doi: 10.1002/jnr.22407. [DOI] [PubMed] [Google Scholar]
- 18.Atasoy D, Schoch S, Ho A, et al. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci U S A. 2007;104:2525–30. doi: 10.1073/pnas.0611003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laverty HG, Wilson JB. Murine CASK is disrupted in a sex-linked cleft palate mouse mutant. Genomics. 1998;53:29–41. doi: 10.1006/geno.1998.5479. [DOI] [PubMed] [Google Scholar]
- 20.Bulfone A, Smiga SM, Shimamura K, et al. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 1995;15:63–78. doi: 10.1016/0896-6273(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 21.Huang T-N, Hsueh Y-P. Brain-specific transcriptional regulator T-brain-1 controls brain wiring and neuronal activity in autism spectrum disorders. Front Neurosci. 2015;9:406. doi: 10.3389/fnins.2015.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hevner RF, Shi L, Justice N, et al. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–66. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 23.Remedios R, Huilgol D, Saha B, et al. A stream of cells migrating from the caudal telencephalon reveals a link between the amygdala and neocortex. Nat Neurosci. 2007;10:1141–50. doi: 10.1038/nn1955. [DOI] [PubMed] [Google Scholar]
- 24.Huang TN, Chuang HC, Chou WH, et al. Tbr1 haploinsufficiency impairs amygdalar axonal projections and results in cognitive abnormality. Nat Neurosci. 2014;17:240–7. doi: 10.1038/nn.3626. [DOI] [PubMed] [Google Scholar]
- 25.Chuang HC, Huang TN, Hsueh YP. Neuronal excitation upregulates Tbr1, a high-confidence risk gene of autism, mediating Grin2b expression in the adult brain. Front Cell Neurosci. 2014;8:280. doi: 10.3389/fncel.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Rubeis S, He X, Goldberg AP, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–15. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Roak BJ, Vives L, Fu W, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–22. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Traylor RN, Dobyns WB, Rosenfeld JA, et al. Investigation of TBR1 hemizygosity: four individuals with 2q24 microdeletions. Mol Syndromol. 2012;3:102–12. doi: 10.1159/000342008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burrage LC, Eble TN, Hixson PM, et al. A mosaic 2q24.2 deletion narrows the critical region to a 0.4 Mb interval that includes TBR1, TANK, and PSMD14. Am J Med Genet A. 2013;161A:841–4. doi: 10.1002/ajmg.a.35751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamdan FF, Srour M, Capo-Chichi JM, et al. De novo mutations in moderate or severe intellectual disability. PLoS Genet. 2014;10:e1004772. doi: 10.1371/journal.pgen.1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palumbo O, Fichera M, Palumbo P, et al. TBR1 is the candidate gene for intellectual disability in patients with a 2q24.2 interstitial deletion. Am J Med Genet A. 2014;164A:828–33. doi: 10.1002/ajmg.a.36363. [DOI] [PubMed] [Google Scholar]
- 32.Wang GS, Hong CJ, Yen TY, et al. Transcriptional modification by a CASK-interacting nucleosome assembly protein. Neuron. 2004;42:113–28. doi: 10.1016/s0896-6273(04)00139-4. [DOI] [PubMed] [Google Scholar]
- 33.Wang TF, Ding CN, Wang GS, et al. Identification of Tbr-1/CASK complex target genes in neurons. J Neurochem. 2004;91:1483–92. doi: 10.1111/j.1471-4159.2004.02845.x. [DOI] [PubMed] [Google Scholar]
- 34.Chuang HC, Huang TN, Hsueh YP. T-Brain-1 — a potential master regulator in autism spectrum disorders. Autism Res. 2015;8:412–426. doi: 10.1002/aur.1456. [DOI] [PubMed] [Google Scholar]
- 35.Huang TN, Hsueh YP. CASK point mutation regulates protein-protein interactions and NR2b promoter activity. Biochem Biophys Res Commun. 2009;382:219–22. doi: 10.1016/j.bbrc.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Huang TN, Chang HP, Hsueh YP. CASK phosphorylation by PKA regulates the protein-protein interactions of CASK and expression of the NMDAR2b gene. J Neurochem. 2010;112:1562–73. doi: 10.1111/j.1471-4159.2010.06569.x. [DOI] [PubMed] [Google Scholar]
- 37.Hong CJ, Hsueh YP. CASK associates with glutamate receptor interacting protein and signaling molecules. Biochem Biophys Res Commun. 2006;351:771–6. doi: 10.1016/j.bbrc.2006.10.113. [DOI] [PubMed] [Google Scholar]
- 38.Kim E, Niethammer M, Rothschild A, et al. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–8. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 39.Kim E, Sheng M. Differential K+ channel clustering activity of PSD-95 and SAP97, two related membrane-associated putative guanylate kinases. Neuropharmacology. 1996;35:993–1000. doi: 10.1016/0028-3908(96)00093-7. [DOI] [PubMed] [Google Scholar]
- 40.Chung WC, Huang TN, Hsueh YP. Targeted deletion of CASK-interacting nucleosome assembly protein causes higher locomotor and exploratory activities. Neurosignals. 2011;19:128–41. doi: 10.1159/000327819. [DOI] [PubMed] [Google Scholar]
- 41.Rosenblum K, Berman DE, Hazvi S, et al. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J Neurosci. 1997;17:5129–35. doi: 10.1523/JNEUROSCI.17-13-05129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yasoshima Y, Morimoto T, Yamamoto T. Different disruptive effects on the acquisition and expression of conditioned taste aversion by blockades of amygdalar ionotropic and metabotropic glutamatergic receptor subtypes in rats. Brain Res. 2000;869:15–24. doi: 10.1016/s0006-8993(00)02397-0. [DOI] [PubMed] [Google Scholar]
- 43.Welzl H, D’Adamo P, Lipp HP. Conditioned taste aversion as a learning and memory paradigm. Behav Brain Res. 2001;125:205–13. doi: 10.1016/s0166-4328(01)00302-3. [DOI] [PubMed] [Google Scholar]