Abstract
Since the discovery of the non–image-forming visual system, tremendous research efforts have been dedicated to understanding its mechanisms and functional roles. Original functions associated with the melanopsin system include the photoentrainment of circadian sleep-wake cycles and the pupillary light reflex. Recent findings, however, suggest a much broader involvement of this system in an array of physiologic responses to light. This newfound insight into the underlying function of the non–image-forming system has revealed the many connections to human pathology and attendant disease states, including seasonal affective disorder, migraine, glaucoma, inherited mitochondrial optic neuropathy, and sleep dysregulation of aging. In this review, the authors discuss in detail the clinical implications of the melanopsin system.
The melanopsin signaling system plays a vital role in non–image-forming visual functions, which are physiologic responses to light that do not require the construction of images by the visual system. Since the discovery of the photopigment melanopsin in 1998, strides in laboratory research have etched a modern framework of the system's basic structure and function.1–5 This sensory system has been linked to several important roles, among them photoentrainment of circadian rhythms, acute control of locomotor activity, sleep regulation, suppression of melatonin biosynthesis, and the pupillary light reflex (PLR) (table).3,6–8 Additional functions have emerged recently and include modulation of vision,8–13 neonatal light aversion,14,15 associative learning,16 metabolic regulation,17 and vascular development (table).18
Table.
Clinical implications of the melanopsin signaling system and associated literature
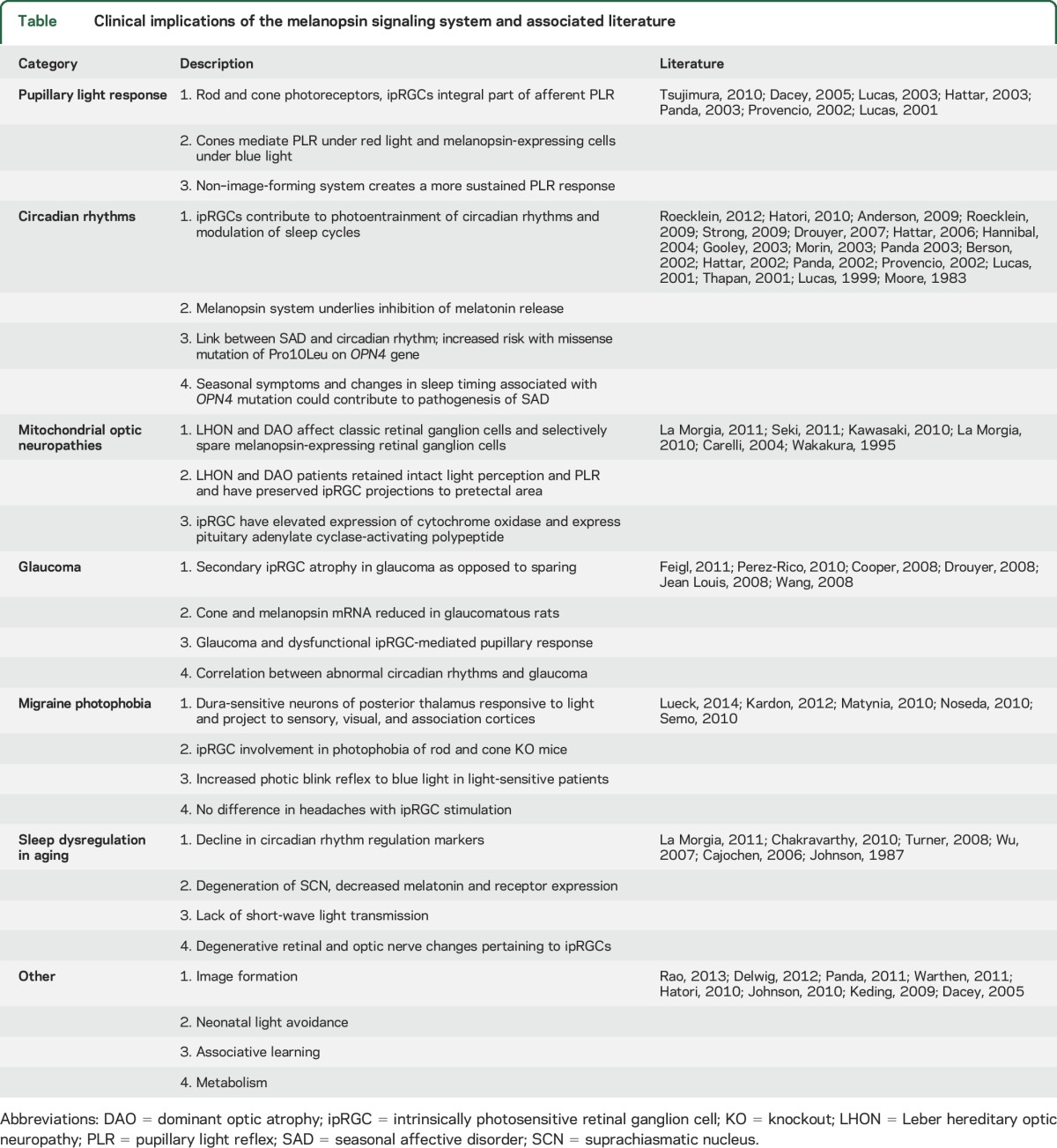
The basic functional unit of the non–image-forming visual system is the intrinsically photosensitive retinal ganglion cell (ipRGC). These cells express the melanopsin photopigment (HUGO gene symbol OPN4), and thereby are directly photosensitive.19 ipRGCs comprise only about 3,000 of the approximate 1.5 million retinal ganglion cells in the human retina; they are sparsely distributed among the classically defined retinal ganglion cells that are involved in vision.10,20,21 Interestingly, the ipRGC signal transduction cascade shares multiple similarities with invertebrate rhabdomeric phototransduction,22,23 which may hint at primordial neurobiological origins. As such, it is not surprising that the newly proposed functions of the nonvisual photoreceptive system are seemingly rooted in more primitive ones that have been conserved over time and across species. The substantial progress in refining the elegance of the non–image-forming visual system has afforded exciting avenues of investigation into pathophysiology and treatment of disease.
The focus of this review is to detail the clinical implications of the non–image-forming visual system. We discuss previously reported clinical associations and the historical underpinnings and research that have brought these developments into light (table). In addition, we critically assess future areas of research of the melanopsin system that may inform clinical care. Given its diverse functions and apparent clinical role, the modern researcher and clinician will benefit from an understanding of the non–image-forming visual system.
PUPILLARY LIGHT RESPONSE
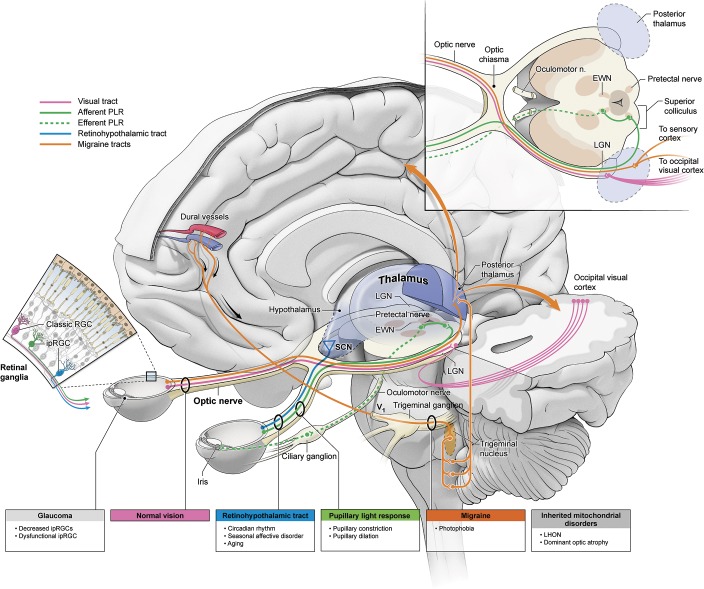
A primary function of the melanopsin system bears special relevance to clinicians. The PLR is a widely used diagnostic test to assess brainstem function. This response is mediated by an autonomic reflex arc that controls pupillary constriction and dilation via the sphincter pupillae (parasympathetic) and dilator pupillae (sympathetic) iris muscles, respectively.24 Retinal inputs project to the olivary pretectal nucleus in the midbrain and journey through the Edinger-Westphal nucleus (EWN).19,25,26 The EWN subsequently transmits parasympathetic signals for pupillary constriction to the ciliary ganglion along the third cranial nerve, ultimately innervating the sphincter pupillae (figure). This reflex was described in the cat with electrical stimulation in 193327 and 197828 and in primates with neuroanatomical tracing29 and electrophysiology in 199530 and 1997.31 Furthermore, Hattar et al.3 in 2002 and Morin et al.32 in 2003 showed ipRGC projections extending to the olivary pretectal nucleus in mice and hamsters, respectively.
Figure. Schematic of clinical implications of non–image-forming visual system.
EWN = Edinger-Westphal nucleus; ipRGC = intrinsically photosensitive retinal ganglion cell; LGN = lateral geniculate nucleus; LHON = Leber hereditary optic neuropathy; PLR = pupillary light reflex; RGC = retinal ganglion cell; SCN = suprachiasmatic nucleus.
In 1927, Keeler33 recognized that the PLR persisted in visually blind mice lacking rods and cones, suggesting that a yet-to-be-discovered, non-rod, non-cone class of photoreceptors mediates this response. Lucas et al.26 in 2001 confirmed that mutant mice lacking both rod and cone photoreceptors retained the PLR, albeit requiring higher levels of light to elicit a standard response. In addition, Lucas et al.19,26 demonstrated a peak spectral sensitivity of the PLR in the blue wavelengths (λmax = 479 nm). The subsequent spectral characterization of ipRGCs, also showing a peak sensitivity at 480 nm, strongly implicated ipRGCs as subserving the PLR in blind mice.19,34,35 Indeed, the critical role of ipRGCs in the PLR was proven through the generation of mice lacking these novel photoreceptive cells. These animals, despite having a competent visual system, show no PLR and exhibit a fully dilated pupil, even at ambient light levels equivalent to sunlight at noon.19,34–38
Interestingly, mice that lack only the melanopsin photopigment (Opn4−/−) but retain the ipRGCs that would normally harbor this photopigment continue to exhibit a robust PLR.19,34,35 If these mice are crossed with Keeler's rodless, coneless mice, generating offspring that lack rods, cones, and the melanopsin protein, the PLR is abolished in the progeny. The abolished PLR in these mice lacking rods, cones, and the melanopsin protein phenocopies the abolished PLR in mice that possess a full complement of rods and cones but lack ipRGCs.19,34,35 Taken together, these findings indicate that rods, cones, and ipRGCs are the photoreceptive elements that initiate the PLR.
Importantly, these findings demonstrate that the ipRGCs also serve as the sole conduit by which rod- and cone-derived information feeds into the central circuitry driving the PLR. Specifically, rod- and cone-driven retinal circuits activate ipRGCs that project to the olivary pretectal nucleus, ultimately mediating the PLR. Therefore, ablating the ipRGCs also negates the input of rods and cones. As such, even visually competent mice that possess a full array of visual photoreceptors fail to exhibit a PLR in the absence of ipRGCs.35–38
Analyses of the various animal models mentioned above have revealed that the rods and cones are largely responsible for the PLR at low (scotopic) and moderate (mesopic) light levels, while the contribution of the intrinsic photosensitivity of ipRGCs is not apparent until very high (photopic) light levels are achieved.5,10,19,34 Even at scotopic light levels, however, the ipRGCs are necessary to convey rod-initiated signals to the olivary pretectal nuclei. At higher light levels, the rod and cone photoreceptors desensitize through multiple processes including photopigment bleaching and adaptation mechanisms. By contrast, ipRGCs require high irradiances to depolarize, and activation persists, even after the termination of the stimulus.2,39 If ipRGCs are rendered insensitive to light by knocking out melanopsin, then the pupil constricts at high light levels, but never to the extent observed in wild-type mice despite continued exposure to high ambient light levels.5,10,19,34,35 Therefore, ipRGCs convey rod- and cone-driven PLR at low and moderate light levels while also functioning to maintain constriction of the iris at high illuminance, thereby protecting the retina from photodamage.40
CIRCADIAN RHYTHMS
Since 2002, ipRGCs have been shown to contribute to photoentrainment of circadian rhythms and modulation of sleep cycles.2,3,5,7 Preliminary literature revealed that Opn4 knockout mice had a subtle but significant impairment in circadian photoentrainment but ultimately could still entrain.41,42 Subsequent studies indicated that mice whose ipRGCs had been ablated, yet retained a fully competent visual system, could not photoentrain, lending credence to the prospect that rods, cones, and ipRGCs contribute to this pathway.34,35 ipRGCs project to brain sites that are consistent with a potential role in circadian rhythm modulation. These projections primarily innervate the suprachiasmatic nucleus (SCN) via the retinohypothalamic tract and the intergeniculate leaflet via the optic tract (figure).2,3,43 SCN ablation experiments in the 1980s44 demonstrated that the SCN was likely the site of the master circadian clock. Subsequent transplant studies conclusively proved this role.45,46 In single-cell recordings, SCN neurons were shown to be modulated by illumination of the eyes.47,48 The axons of ipRGCs are the cellular elements that comprise the retinohypothalamic tract, a subset of optic nerve axons that convey light information from the eyes to the SCN. ipRGCs also send lesser projections to extra-SCN sites, which previously have been implicated in stabilizing circadian rhythms and regulating sleep/wake cycles.43 These sites include the ventral subparaventricular zone, intergeniculate leaflet of the lateral geniculate nucleus, and the ventrolateral preoptic nucleus.6,20,32,43,49 Other work has demonstrated the melanopsin system's role underlying inhibition of melatonin release from the pineal gland.35,50–53 Mice lacking rods and cones remain capable of photosuppressing elevated nocturnal melatonin levels; however, mice devoid of rods and cones and null for melanopsin fail to photoregulate melatonin biosynthesis.35,53 In human studies, blue wavelengths (446–479 nm) that coincide with the spectral range of melanopsin activation have proven to be most effective in decreasing plasma melatonin when compared to other wavelengths.52,54,52,55,56
Recent evidence suggests loss of ipRGC function as a possible mechanism for known circadian rhythm dysfunction in Alzheimer disease (AD). In a 2016 study by La Morgia et al.,57 postmortem analysis of retinas and optic nerves of patients with AD showed histochemical evidence of ipRGC loss and ipRGC morphologic abnormalities when compared to normal controls. The ipRGCs in these patients were shown to be directly affected by amyloid deposition, which is well-characterized in Alzheimer pathogenesis. Furthermore, clinical studies in the intensive care unit (ICU) setting have shown that ill patients notice changes in room lighting and that cycling of light during day and night subjectively improves patient mood and sleep and supports their natural circadian rhythms.58 Circadian regulation of not only sleep but also cortisol release, immune modulation, the inflammatory reflex, and vitamin D absorption have all been described and may contribute to improved outcomes in ICU patients.59 Current trials are underway to study the effect of light cycling on overall ICU patient outcomes.59
Proper circadian rhythm function is necessary for overall health and its dysfunction has been implicated in cognitive loss in several psychiatric and neurodegenerative disorders.60 As studies such as the above arise and continue to link ipRGCs to circadian rhythm dysregulation in many disease states, the importance of the melanopsin non–image-forming visual system will continue to emerge in describing disease pathogenesis.
SEASONAL AFFECTIVE DISORDER
Given melanopsin's role in photoentrainment of circadian rhythms and other nonvisual photoresponses to light, there was strong consideration for its potential involvement in seasonal affective disorder (SAD). SAD represents a common psychiatric disorder in about 5% of the US population.e1,e2 This disorder is characterized by yearly depressive episodes (usually in the fall and winter) when there is a seasonal limitation of sunlight. Outside of antidepressants and cognitive-behavioral therapy, light therapy has been shown to provide successful treatment for about half of SAD cases.e3,e4 Although the pathophysiology of SAD remains unknown, numerous hypotheses have pointed to dysregulated photoentrainment or wintertime insensitivity to light, long before the discovery of the non–image-forming visual system. More specifically, the original hypothesis held that SAD occurs when circadian rhythms are out of phase with regular sleep-wake cycles (e.g., phase shift).e5 Another theory described the depressive periods of SAD as secondary to a longer duration of melatonin release.e6 Finally, a third hypothesis proposed that patients with SAD do not have normal compensatory retinal sensitivity for lack of light during the winter season.e3,e7 Despite some evidence for aspects of each of the above theories, none completely accounts for SAD pathophysiology.
Once melanopsin and ipRGCs were shown to play a critical role in circadian regulation, discovering a link between melanopsin and SAD became an obvious research pursuit. In 2009, it was reported that among a cohort of 130 patients with SAD, those homozygous for a missense proline-to-leucine polymorphism (P10L) had a 5.6-fold increased risk of SAD when compared to age-matched non-SAD controls.e8 Just 3 years later, it was reported that healthy individuals with the P10L polymorphism had seasonal changes in sleep timing, which were characterized by an earlier bedtime during short days and a later bedtime during longer days.e9 Although a direct mechanism arising from this genotype has not been shown, these data suggest that seasonal symptoms and changes in sleep timing associated with the P10L melanopsin polymorphism could contribute to the pathogenesis of SAD.e9 Treatment of SAD with light therapy further delineates a role for the non–image-forming system. Several studies using light sources enriched in the blue wavelengths have suggested that wavelengths around 470 nm are responsible for the efficacious treatment seen with bright light therapy in patients with SAD.e10,e11 Further investigation into the melanopsin photosensory system will help to unearth a clearer picture of SAD pathogenesis and help guide its treatment.
SLEEP DYSREGULATION IN AGING
Retinal ganglion cell loss with aging has been reported in humans and in animal models.e12–e14 Age-associated sleep changes have also been well-documented, specifically with impaired non-REM sleep and increased frequency of awakening.e15 Only recently, however, has a connection emerged between the two. Several studies have shown disruption of circadian rhythms within aging populations. In 2006, Cajochen et al.e16 showed a decline in circadian rhythm regulation markers in the aging population. The circadian phase markers used were core body temperature, melatonin, and cortisol secretion as well as sleep-wake cycle shortening. The underlying mechanisms of these changes are still undetermined but hormonal alterations associated with aging provide a popular and plausible explanation. Several groups reported a reduction in vasopressin and vasoactive intestinal peptide expressing neurons associated with degenerative changes of the SCN.e17,e18 Similarly, decreased melatonin production from the pineal gland, along with melatonin receptor expression, was also noted in older patients.e17,e18 Another possibility is the lack of short-wave light transmission as being associated with precataract formation in the elderly.e19 Among these theories rests an idea of degenerative retinal and optic nerve changese13,e18,e20 and more specifically as it pertains to ipRGCs.e18 In postmortem analyses of elderly patients, La Morgia et al.e18 found an age-related loss of ipRGCs. Going forward, further substantiated ipRGC involvement in age-related sleep dysregulation could aid in understanding and treating circadian disorders in this population.
Two studies have shown a direct link between the non–image-forming visual system and sleep circuitry, suggesting further potential involvement of ipRGCs in sleep pathology. Altimus et al.e21 in 2008 demonstrated that acute light directly modulates sleep in mice through both rod-cone and melanopsin signaling through ipRGCs. Furthermore, in a separate study, Lupi et al.e22 in 2008 elaborated this circuitry and showed melanopsin-dependent activation of sleep-promoting neurons in the ventrolateral preoptic area and the superior colliculus. As melanopsin's regulation of sleep is further elaborated, its role in the mechanism of sleep dysregulation found in SAD, aging, and other diseases will be understood.
INHERITED MITOCHONDRIAL OPTIC NEUROPATHIES
Inherited mitochondrial optic neuropathies, including Leber hereditary optic neuropathy (LHON) and dominant optic atrophy (DAO), constitute predominant causes of inherited vision loss.e23–e27 Both conditions begin in the early decades of life and progress invariably to diverse visual dysfunction of acuity and color perception of varying severity.e23–e27 These diseases manifest secondary to mutations in genes for mitochondrial proteins. LHON exhibits a mitochondrial inheritance pattern while DAO is an autosomal disease.e23–e27 Of note, both LHON and DAO have been shown to affect classic retinal ganglion cells and to selectively spare the melanopsin-expressing retinal ganglion cells.e28 La Morgia et al.21,e18 showed that patients with LHON and patients with DAO retained melatonin suppression by light. In postmortem studies, patients with LHON and patients with DAO had preserved ipRGC and axon projections to the pretectal area. These findings echoed prior observations wherein patients with LHON and patients with DAO retained intact light perceptione28 and PLR,e29 particularly under blue light.e30,e31 This sparing mechanism is currently unknown, though speculation holds that ipRGCs are “metabolically robust,” have elevated expression of cytochrome c oxidase,21 and express the neuroprotective peptide pituitary adenylate cyclase-activating polypeptide, all of which potentially play a role in their resilience.e32,e33 LHON and DAO present a model for studying ipRGC involvement in other optic neuropathies.
GLAUCOMA
Glaucoma, the foremost optic neuropathy characterized by intraocular hypertension, has been shown to initially affect arcuate retinal nerve fibers and then reliably progress to retinal ganglion cell perikarya. Unlike LHON and DAO, recent evidence suggests secondary ipRGC atrophy in glaucoma as opposed to sparing (figure).e34 In 2008, Drouyer et al.e35 employed a rat model of chronic intraocular hypertension and found that rats with elevated pressures required more time to adjust to shifted light-dark cycles and showed more variability in activity. Further, cone and melanopsin mRNA was significantly reduced in glaucomatous rats. Wang et al.e36 used retrograde labeling to identify retinal ganglion cells and melanopsin-containing retinal ganglion cells from projections to the superior colliculus. These authors illustrated a decreased number of ipRGCs in comparison to normal rats.e36 Furthermore, in humans, Beatrix et al.e37 showed that patients with glaucoma have dysfunctional ipRGC-mediated pupillary response. In a recent prospective case-controlled study, Perez-Rico et al.e38 compared patients with advanced glaucoma to age-matched controls and showed a significantly decreased melatonin suppression to blue light in the glaucomatous group. Finally, preliminary data from Cooper at al.e39 suggest a correlation between abnormal circadian rhythms and advanced glaucoma. ipRGC destruction in glaucoma provides an important model to study ipRGC function and potentially provides insight into symptomatic treatment of glaucoma in the future.
PHOTOPHOBIA IN MIGRAINE
A more recent development in the melanopsin system's involvement in human disease is that of photosensitivity in headache and migraine. The original hypothesis stemmed from observation that blind patients with intact light perception exhibited worse photophobia and migraine exacerbation when compared to their enucleated counterparts.e28,e40 Since this observation, some animal studies have purportedly ratified this claim. Assuming the trigeminovascular pathwaye41 for migraine pathophysiology, Noseda et al.e40 sought to identify the location and modulators of second-order neurons in this path. These authors identified dura-sensitive neurons in the rat posterior thalamic nuclear group using single-cell recordings. These neurons were shown to project to sensory, visual, and association cortices and abutted axons emanating from ipRGCs (figure). Most neurons of the posterior thalamus that were active during dural stimulation were also responsive to ocular illumination, suggesting a modulatory role for the projecting ipRGCs. Furthermore, preliminary data from a different laboratory also suggested ipRGC involvement in photophobia of rod- and cone-deficient mice.e42,e43 Human studies, on the other hand, have been less convincing. Kardon et al.e44 loosely supported this thesis by demonstrating an increased photic blink reflex to blue light as opposed to red light in light-sensitive patients. Lueck et al.,e45 on the other hand, challenged this view in a prospective, randomized controlled trial. These authors used multifocal pupillographic objective perimetry with blue and yellow light to examine pupillary response and headache severity in patients with migraine. There was no statistically significant difference in severity or mean number of headaches with ipRGC stimulation. They did, however, show a difference in pupillary response as influenced by recent attacks but this does not necessarily support the above hypothesis.e45 Insofar as photophobia is such a common finding in patients with migraine headaches, further research will serve to elucidate a connection to the non–image-forming visual system.
OTHER CLINICAL IMPLICATIONS
Our understanding of the cellular elements of the nonvisual system is relatively recent, and as such, several preliminary clinical correlations have been postulated based on animal studies and remote clinical observations. Recent evidence suggests a role for the non–image-forming system in vision proper.8,11,e46–e48 Several animal studies have traced ipRGC innervation to the lateral geniculate nucleus. In mouse studies, Ecker et al.e47 used a Cre-dependent melanopsin reporter to map ipRGC projections and reported that beyond the already known innervations, these cells also projected to the lateral geniculate nucleus (LGN) as well as other, previously unknown hypothalamic structures.13,e23 In 2005, Dacey et al.10 described a giant melanopsin-expressing ganglion cell in primate retina. This cell is intrinsically photosensitive, exhibits a complete dynamic range of irradiance coding, is strongly activated by rods and cones, and projects to the LGN.10 These studies provided preliminary evidence of image-forming integration through the non–image-forming system. Further investigation addressing ipRGC's involvement in and remodeling of the visual system can help target future therapeutic strategies for vision loss.
The melanopsin system also appears to play a role in neonatal light avoidance. During development in humans and rodents, melanopsin is expressed before birthe49 and the retinohypothalamic tract is already elaborated and functional.e50 Although the role of this early expression and development is not completely clear, this seems to be important in immediate postnatal light avoidance. Johnston et al.14 in 2010 provided electrophysiologic evidence of light-induced activity in the ipRGCs of P6 pups without any crosstalk from the image-forming system. In this study, they showed that P9 pups turn away upon introduction of light, presumably to find their way back to their dark nest.14 In a similar experiment, the same group subsequently showed that this mechanism was in fact a response to light as an aversive stimulus and activated regions in the posterior thalamus and central amygdala (thought to be responsible for aversion).15 Finally, a study by Rao et al.18elucidated the ipRGC's role (via light stimulation) in prenatal regression of ocular hyaloid vasculature. Mice that were either null for melanopsin or those dark-reared from late gestation showed persistent, overgrown hyaloid vasculature within the eye postnatally. These prenatal and immediately postnatal studies underscore the melanopsin system's importance not only in behavior but also in normal ocular development. Further studies could contribute to our understanding of vascular anomalies in the eye or brain18 and could shed further light on childhood blindness and postnatal behavior.
Finally, a role for melanopsin has emerged in metabolic regulation. Typically, mice fed a ketogenic diet exhibit a 5%–7% loss of body mass over the first week of the diet, eventually stabilizing at that reduced body weight even if maintained on the diet. By contrast, melanopsin-null mice exhibit continued weight loss, never exhibiting any stabilization of body mass, even after 5 weeks on the ketogenic diet.17 The mechanism leading to this phenotype remains unknown. Although weight loss is greater in melanopsin-null mice maintained in a red:dark light:dark cycle than in wild-type controls, the weight loss phenotype is also observed in mice maintained in constant darkness.17 These data suggest a potential role for the non–image-forming system in the regulation of metabolism and point toward a wide range of clinical implications.
DISCUSSION
As insights into the non–image-forming visual system continue to accumulate, its clinical role becomes abundantly clear. Since its discovery, the melanopsin system has been implicated in the pupillary light response, seasonal affective disorder, sleep dysregulation of aging, migraine, glaucoma, and inherited mitochondrial optic neuropathy. Furthermore, molecular studies and animal models have broadened these proposed functions and have unearthed new possible roles for the non–image-forming visual system in associative learning, metabolic control, and image formation. Over the last 13 years, knowledge of this system and its clinical role has grown exponentially and points to an encouraging path of inquiry ahead.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- DAO
dominant optic atrophy
- EWN
Edinger-Westphal nucleus
- ICU
intensive care unit
- ipRGC
intrinsically photosensitive retinal ganglion cell
- LGN
lateral geniculate nucleus
- LHON
Leber hereditary optic neuropathy
- PLR
pupillary light reflex
- SAD
seasonal affective disorder
- SCN
suprachiasmatic nucleus
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Ksendzovsky: study concept and design, analysis and interpretation, drafting and revision of the manuscript. I.J. Pomeraniec, Dr. Zaghloul, Dr. J. Provencio: analysis and interpretation, drafting and revision of the manuscript. Dr. I. Provencio: study concept and design, analysis and interpretation, drafting and revision of the manuscript, study supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Benarroch EE. The melanopsin system: phototransduction, projections, functions, and clinical implications. Neurology 2011;76:1422–1427. [DOI] [PubMed] [Google Scholar]
- 2.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002;295:1070–1073. [DOI] [PubMed] [Google Scholar]
- 3.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 2002;295:1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci 2000;20:600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina: this mesh of cells may explain how some blind mice can still tell day from night. Nature 2002;415:493. [DOI] [PubMed] [Google Scholar]
- 6.Dijk DJ, Archer SN. Light, sleep, and circadian rhythms: together again. PLoS Biol 2009;7:e1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev 2010;90:1547–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown TM, Gias C, Hatori M, et al. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol 2010;8:e1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen AE, Storchi R, Martial FP, et al. Melanopsin-driven light adaptation in mouse vision. Curr Biol 2014;24:2481–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005;433:749–754. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt TM, Alam NM, Chen S, et al. A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron 2014;82:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keding SR, Hatori M, Le H, Panda S. Comprehensive labelling of melanopsin expressing retinal ganglion cells and mapping their central projection in mouse. Mol Biol Cell 2009;20(suppl). Abstract 656. [Google Scholar]
- 13.Panda S, Keding SR, Le HD, Gibbs D, Fitzpatrick J, Hatori M. Comprehensive analysis of the central projections of melanopsin-expressing retinal ganglion cell in mice. 2011. ARVO Abstract. [Google Scholar]
- 14.Johnson J, Wu V, Donovan M, et al. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci USA 2010;107:17374–17378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delwig A, Logan AM, Copenhagen DR, Ahn AH. Light evokes melanopsin-dependent vocalization and neural activation associated with aversive experience in neonatal mice. PLoS One 2012;7:e43787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warthen DM, Wiltgen BJ, Provencio I. Light enhances learned fear. Proc Natl Acad Sci USA 2011;108:13788–13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayturk DG, Castrucci AM, Carr DE, Keller SR, Provencio I. Lack of melanopsin is associated with extreme weight loss in mice upon dietary challenge. PLoS One 2015;10:e0127031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao S, Chun C, Fan J, et al. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature 2013;494:243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 2003;299:245–247. [DOI] [PubMed] [Google Scholar]
- 20.Hannibal J, Fahrenkrug J. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res 2004;316:99–113. [DOI] [PubMed] [Google Scholar]
- 21.La Morgia C, Ross-Cisneros FN, Sadun AA, et al. Melanopsin retinal ganglion cells are resistant to neurodegeneration in mitochondrial optic neuropathies. Brain 2010;133:2426–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isoldi MC, Rollag MD, Castrucci AM, Provencio I. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc Natl Acad Sci USA 2005;102:1217–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu X, Kumbalasiri T, Carlson SM, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature 2005;433:745–749. [DOI] [PubMed] [Google Scholar]
- 24.Markwell E, Feigl B, Zele A. Intrinsically photosensitive melanopsin retinal ganglion cell contributions to the pupillary light reflex and circadian rhythm. Clin Exp Optom 2010;93:137–149. [DOI] [PubMed] [Google Scholar]
- 25.Pickard GE, Smeraski CA, Tomlinson CC, et al. Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. J Neurosci 2002;22:2701–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci 2001;4:621–626. [DOI] [PubMed] [Google Scholar]
- 27.Ranson SW. Cutaneous sensation. Science 1933;78:395–399. [DOI] [PubMed] [Google Scholar]
- 28.Hultborn H, Mori K, Tsukahara N. The neuronal pathway subserving the pupillary light reflex. Brain Res 1978;159:255–267. [DOI] [PubMed] [Google Scholar]
- 29.Gamlin PD, Clarke RJ. The pupillary light reflex pathway of the primate. J Am Optometric Assoc 1995;66:415–418. [PubMed] [Google Scholar]
- 30.Gamlin PD, Zhang H, Clarke RJ. Luminance neurons in the pretectal olivary nucleus mediate the pupillary light reflex in the rhesus monkey. Exp Brain Res 1995;106:169–176. [DOI] [PubMed] [Google Scholar]
- 31.Kourouyan HD, Horton JC. Transneuronal retinal input to the primate Edinger-Westphal nucleus. J Comp Neurol 1997;381:68–80. [DOI] [PubMed] [Google Scholar]
- 32.Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: bifurcation and melanopsin immunoreactivity. J Comp Neurol 2003;465:401–416. [DOI] [PubMed] [Google Scholar]
- 33.Keeler CE. Iris movements in blind mice. Am J Physiol 1927;81:107–112. [Google Scholar]
- 34.Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 2003;424:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panda S, Provencio I, Tu DC, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science 2003;301:525–527. [DOI] [PubMed] [Google Scholar]
- 36.Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS One 2008;3:e3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 2008;453:102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hatori M, Le H, Vollmers C, et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS One 2008;3:e2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren EJ, Allen CN, Brown RL, Robinson DW. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur J Neurosci 2006;23:2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsujimura S, Ukai K, Ohama D, Nuruki A, Yunokuchi K. Contribution of human melanopsin retinal ganglion cells to steady-state pupil responses. Proc Biol Sci 2010;277:2485–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panda S, Sato TK, Castrucci AM, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 2002;298:2213–2216. [DOI] [PubMed] [Google Scholar]
- 42.Ruby NF, Brennan TJ, Xie X, et al. Role of melanopsin in circadian responses to light. Science 2002;298:2211–2213. [DOI] [PubMed] [Google Scholar]
- 43.Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol 2006;497:326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore RY. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc 1983;42:2783–2789. [PubMed] [Google Scholar]
- 45.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science 1990;247:975–978. [DOI] [PubMed] [Google Scholar]
- 46.Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 1996;382:810–813. [DOI] [PubMed] [Google Scholar]
- 47.Drouyer E, Rieux C, Hut RA, Cooper HM. Responses of suprachiasmatic nucleus neurons to light and dark adaptation: relative contributions of melanopsin and rod-cone inputs. J Neurosci 2007;27:9623–9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong KY, Graham DM, Berson DM. The retina-attached SCN slice preparation: an in vitro mammalian circadian visual system. J Biol Rhythms 2007;22:400–410. [DOI] [PubMed] [Google Scholar]
- 49.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci 2003;23:7093–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hatori M, Panda S. The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol Med 2010;16:435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucas RJ, Freedman MS, Lupi D, Munoz M, David-Gray ZK, Foster RG. Identifying the photoreceptive inputs to the mammalian circadian system using transgenic and retinally degenerate mice. Behav Brain Res 2001;125:97–102. [DOI] [PubMed] [Google Scholar]
- 52.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol 2001;535:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science 1999;284:505–507. [DOI] [PubMed] [Google Scholar]
- 54.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 2001;21:6405–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brainard GC, Sliney D, Hanifin JP, et al. Sensitivity of the human circadian system to short-wavelength (420-nm) light. J Biol Rhythms 2008;23:379–386. [DOI] [PubMed] [Google Scholar]
- 56.Revell VL, Barrett DC, Schlangen LJ, Skene DJ. Predicting human nocturnal nonvisual responses to monochromatic and polychromatic light with a melanopsin photosensitivity function. Chronobiol Int 2010;27:1762–1777. [DOI] [PubMed] [Google Scholar]
- 57.La Morgia C, Ross-Cisneros FN, Koronyo Y, et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol 2016;79:90–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castro R, Angus DC, Rosengart MR. The effect of light on critical illness. Crit Care 2011;15:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engwall M, Fridh I, Johansson L, Bergbom I, Lindahl B. Lighting, sleep and circadian rhythm: an intervention study in the intensive care unit. Intensive Crit Care Nurs 2015;31:325–335. [DOI] [PubMed] [Google Scholar]
- 60.Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci 2010;11:589–599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.