Abstract
Objective:
To evaluate the association between sleep duration and the risk of incident dementia and brain aging.
Methods:
Self-reported total hours of sleep were examined in the Framingham Heart Study (n = 2,457, mean age 72 ± 6 years, 57% women) as a 3-level variable: <6 hours (short), 6–9 hours (reference), and >9 hours (long), and was related to the risk of incident dementia over 10 years, and cross-sectionally to total cerebral brain volume (TCBV) and cognitive performance.
Results:
We observed 234 cases of all-cause dementia over 10 years of follow-up. In multivariable analyses, prolonged sleep duration was associated with an increased risk of incident dementia (hazard ratio [HR] 2.01; 95% confidence interval [CI] 1.24–3.26). These findings were driven by persons with baseline mild cognitive impairment (HR 2.83; 95% CI 1.06–7.55) and persons without a high school degree (HR 6.05; 95% CI 3.00–12.18). Transitioning to sleeping >9 hours over a mean period of 13 years before baseline was associated with an increased risk of all-cause dementia (HR 2.43; 95% CI 1.44–4.11) and clinical Alzheimer disease (HR 2.20; 95% CI 1.17–4.13). Relative to sleeping 6–9 hours, long sleep duration was also associated cross-sectionally with smaller TCBV (β ± SE, −1.08 ± 0.41 mean units of TCBV difference) and poorer executive function (β ± SE, −0.41 ± 0.13 SD units of Trail Making Test B minus A score difference).
Conclusions:
Prolonged sleep duration may be a marker of early neurodegeneration and hence a useful clinical tool to identify those at a higher risk of progressing to clinical dementia within 10 years.
A small number of studies have reported associations between both long1,2 and short2 self-reported sleep duration and an increased risk of incident dementia. However, it is unclear whether abnormal sleep duration serves as a risk factor for or an early marker of neurodegeneration. Sleep may provide a restorative function, removing metabolic waste from the brain and preventing the accumulation of β-amyloid,3 a hallmark of Alzheimer disease (AD). On the other hand, sleep disorders may also emerge as a result of atrophy to brain regions involved in sleep and wakefulness4 or as a consequence of mood disturbances,5,6 which are common in dementia.
The aim of the current study was to examine if long (>9 hours) or short (<6 hours) self-reported sleep duration was associated with the risk of incident all-cause dementia or incident AD dementia. We also examined the association of sleep duration with brain volume on MRI and cognitive ability. Using serial assessments of self-reported sleep duration, a mean of 13 years apart, we examined whether sleep duration was likely to be a risk factor for or an early biological marker of dementia.
METHODS
Sample.
The Framingham Heart Study (FHS) involves a series of ongoing community-based prospective cohort studies conducted in Framingham, Massachusetts. The study commenced in 1948 with the enrollment of 5,209 original cohort participants who have been examined approximately every 2 years. Recruitment and characterization of the original cohort have been described previously.7 Between 1971 and 1975, the offspring of the original cohort and their spouses were enrolled into an offspring cohort.8 Participants of the offspring cohort have been assessed approximately once every 4 years with the most recent examination concluding in 2014.
We used the 20th original cohort examination (1986–1990) and the 7th offspring examination (1998–2001) as a baseline from which we examined the risks of incident all-cause dementia and clinical AD over 10 years. We also examined neuropsychological performance and brain volume on MRI using cross-sectional data in offspring study participants. The study design is summarized in figure 1 (see figure e-1 at Neurology.org for detailed information about the selection of study participants). For the analysis of incident dementia, we excluded persons younger than 60 years at baseline, persons with prevalent dementia at baseline, and persons without follow-up for dementia. For the analysis of neuropsychological function and brain volume, we excluded persons with prevalent stroke or dementia at the time of outcome assessment but did not restrict the analysis to a certain age group.
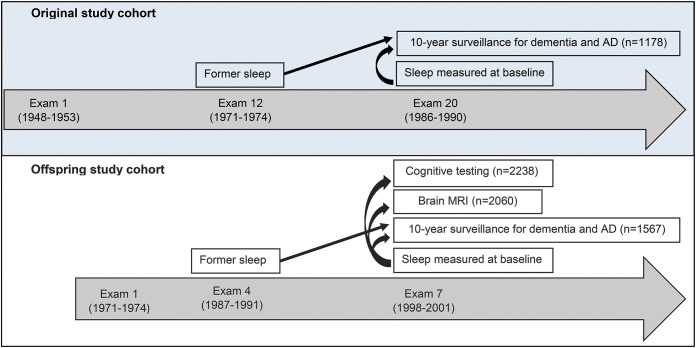
Figure 1. Overview of the study design.
For the analysis of incident dementia, both the original and offspring study participants were studied together. Sleep was related to cross-sectional cognitive performance and brain MRI in the offspring cohort only. After exclusion of persons with missing covariates, the final analysis sample was 2,457 for analysis of incident dementia. Sample sizes depict data for sleep duration measured at baseline. AD = Alzheimer disease.
Standard protocol approvals, registrations, and patient consents.
All participants provided written informed consent and the study was approved by the institutional review board and Boston University Medical Center.
Self-reported total sleep time.
Self-reported sleep duration was obtained as part of the Physical Activity Index (PAI) questionnaire. Participants responded to the question “How many hours do you typically sleep?” Responses were recorded to the nearest integer. Participants of the original cohort completed the questionnaire at examinations 12 (1971–1974) and 20 (1986–1990) whereas offspring cohort participants completed the questionnaire at examinations 4 (1987–1991) and 7 (1998–2001). We examined sleep duration at baseline using the most current administration of the PAI, former sleep duration using the first administration of the PAI, and change in sleep duration by comparing the 2 time points. Former sleep was only examined in the sample of participants with available baseline sleep data.
Outcomes: Incident dementia.
Participants in the FHS are under continued surveillance for incident dementia (see appendix e-1 for details). A diagnosis of dementia was made in accordance with the DSM-IV, and a diagnosis of clinical AD based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association for definite, probable, or possible AD.9
Outcomes: Total cerebral brain volume on MRI.
We used MRI to measure total cerebral brain volume (TCBV), defined as the ratio of total brain parenchymal volume above the tentorium to total intracranial volume, which therefore adjusted for head size. Since intracranial volume reflects the largest brain size achieved during life, TCBV provides a proxy for global brain atrophy. MRIs were obtained with a Siemens (Munich, Germany) Magnetom 1T or 1.5T field strength machine using a T2-weighted double spin-echo coronal imaging sequence of 4-mm contiguous slices from nasion to occiput. The imaging parameters, measurement protocols, and reproducibility of these measurements have been described previously.10
Outcomes: Neuropsychological function.
Trail Making Test A and B and Logical Memory Delayed from the Wechsler Memory Scales were completed as part of a neuropsychological test battery administered by trained research assistants and neuropsychologists. As is common practice, we subtracted the Trails A from the Trails B score to derive Trails B–A, an outcome reflecting executive function and processing speed. Logical Memory was included as a measure of verbal recall memory. Consistent with our previous publications, Trail Making Test scores were log transformed and standardized, and directionality was reversed such that higher scores reflect superior performance.11
Statistical analyses.
All analyses were conducted in SAS (SAS Institute, Cary, NC). Cox regression models were used to relate self-reported sleep duration to the incidence of dementia and AD whereas linear regressions were used to examine the associations of sleep duration with TCBV and cognitive scores. Sleep duration was examined as a 3-level variable: <6 hours (short sleep duration), 6–9 hours (reference), and >9 hours (long sleep duration) and related to all outcome measures. However, for incident dementia, we observed a threshold effect of sleeping ≤9 hours vs sleeping >9 hours. Thus, we modeled sleep as a dichotomous variable (≤9 hours vs >9 hours) in further models involving incident dementia but as a 3-level variable for models involving TCBV and cognitive scores. Change in sleep duration between the 2 assessments of the PAI was examined by grouping participants in 1 of the following 4 categories: sleep duration ≤9 hours at both former sleep and baseline sleep time points (reference), sleep duration >9 hours at both former sleep and baseline sleep time points, change from ≤9 hours (former sleep) to >9 hours (baseline sleep), and change from >9 hours (former sleep) to ≤9 hours (baseline sleep). Results are expressed as hazard ratios (HR) and 95% confidence intervals (CIs). Statistical models involving incident dementia were adjusted for age, sex, education, homocysteine, and the presence of an APOE ε4 allele. These covariates were chosen because they are among the strongest predictors of dementia in our sample.12,13 Statistical models for TCBV and cognition included an additional adjustment for the time delay between the measurement of sleep duration and the outcome and age squared in the case of TCBV (given that associations between age and TCBV are nonlinear).10
Interactions and sensitivity analysis.
For the outcome of all-cause dementia, we explored for interactions with potential effect-modifying variables such as education (no high school degree vs at least a high school degree), age, sex, and APOE ε4 allele status. We then performed a sensitivity analysis, stratifying results according to mild cognitive impairment (MCI) status at baseline, using the criteria defined by Petersen et al.14 The aim of this sensitivity analysis was to examine whether sleep duration was likely to be a risk factor for dementia in those without MCI or whether sleep duration was likely to be an early marker of neurodegeneration in those with MCI. Results were considered significant if p < 0.05, except tests of interaction, which were considered statistically significant if p < 0.10.
RESULTS
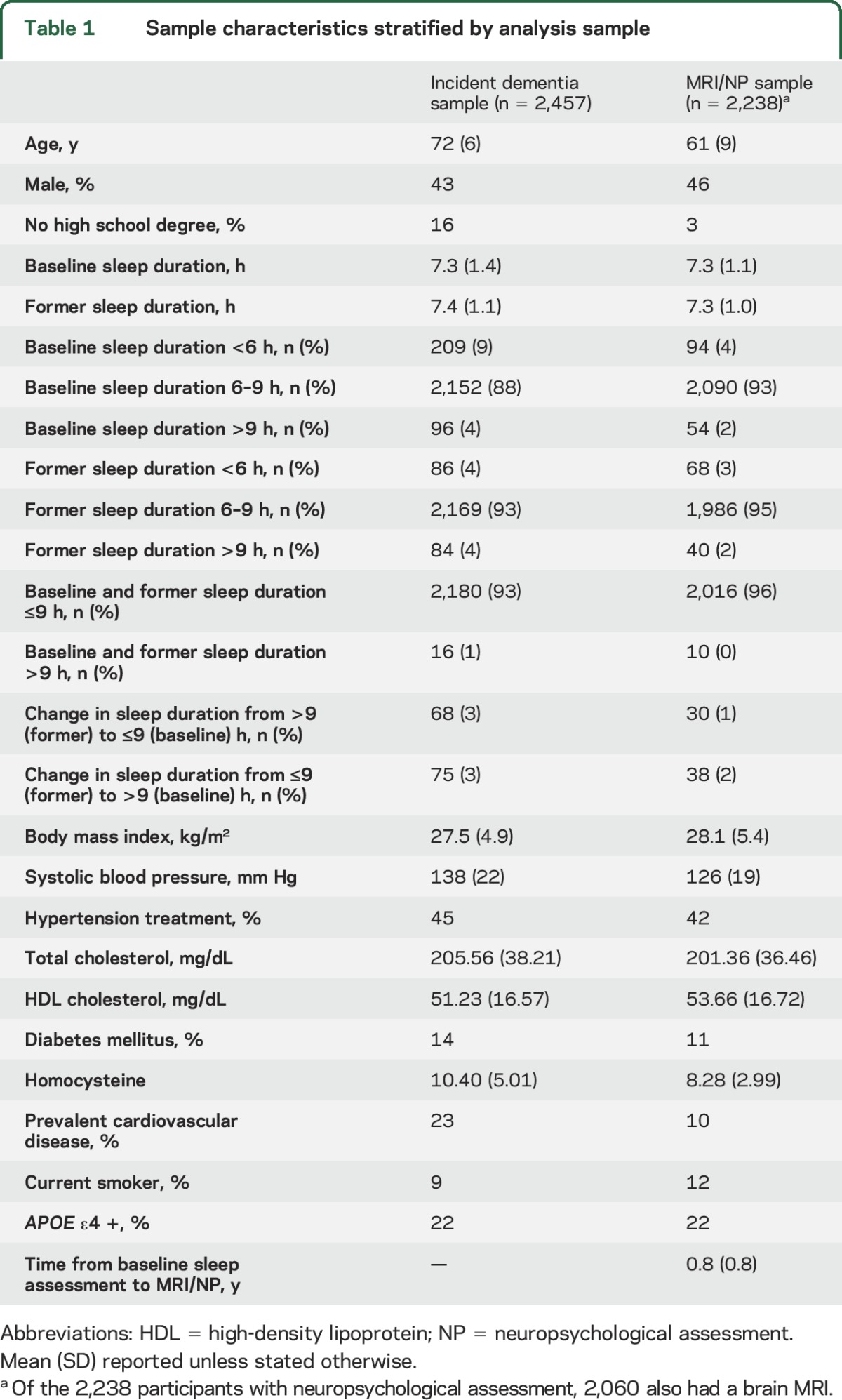
Baseline characteristics are displayed in table 1. There were 234 (10%) cases of all-cause dementia, 181 (8%) of which were clinically consistent with AD. The mean TCBV value was 79% (SD 3), the mean Trail Making Test B–A score was 51 seconds (SD 52), and the mean number of words correctly recalled for Logical Memory Delayed was 11 (SD 4).
Table 1.
Sample characteristics stratified by analysis sample
Sleep duration, incident dementia, and brain aging.
When directly comparing those sleeping >9 hours to the remainder of the sample, long sleep duration was a significant predictor of all-cause dementia (table 2, figure 2). There was a statistical trend observed between long sleep duration and an increased risk of AD (p = 0.05). Whereas former sleep duration was not associated with the risk of dementia, those who transitioned to sleeping >9 hours (from former to baseline time points) were at an increased risk of all-cause dementia. A statistical trend also suggested a possible association between transitioning to sleeping >9 hours and an increased risk of AD.
Table 2.
Self-reported sleep duration and the adjusted risk of incident dementia
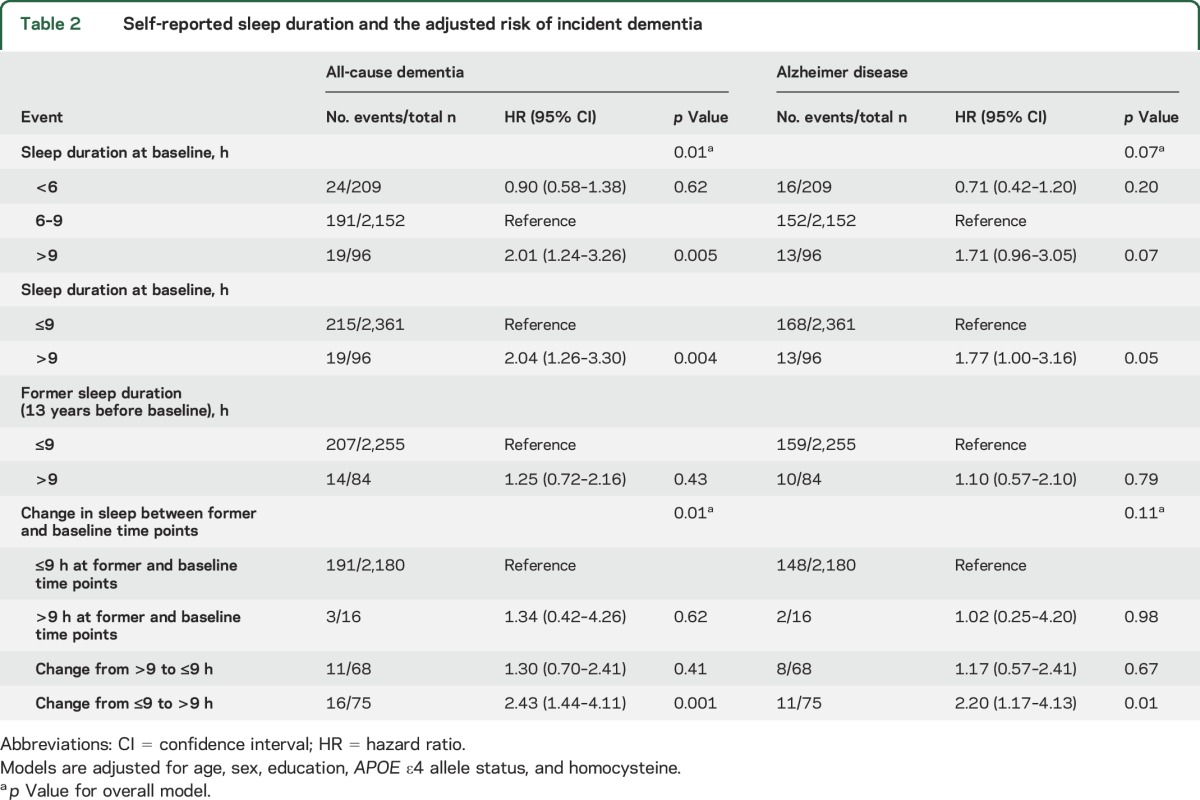
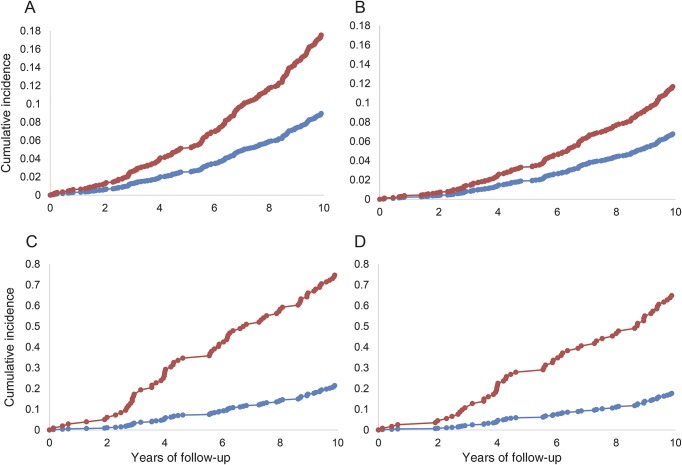
Figure 2. Cumulative incidence of dementia by total sleep time.
Cumulative incidence of (A) all-cause dementia by hours of sleep in the whole sample, (B) Alzheimer disease (AD) dementia by hours of sleep in the whole sample, (C) all-cause dementia by hours of sleep in persons without a high school degree, (D) AD dementia by hours of sleep in persons without a high school degree. Blue lines denote ≤9 hours sleep and red lines denote >9 hours sleep. Incidence curves are adjusted for age, sex, and cohort.
Both short (β ± SE, −0.22 ± 0.10 SD unit score difference; p = 0.03) and long (β ± SE, −0.41 ± 0.13 SD unit score difference; p = 0.001) sleep duration were associated cross-sectionally with poorer performance on Trail Making Test B–A (6–9 hours of sleep used as the reference; p for trend, <0.0001). Long sleep duration was associated cross-sectionally with smaller TCBV (β ± SE, −1.08 ± 0.41 mean units of TCBV difference; p = 0.009), whereas short sleep duration was associated with larger TCBV (β ± SE, 1.10 ± 0.31 mean units of TCBV difference; p = 0.0003; 6–9 hours of sleep used as the reference; p for trend, <0.0001). We regressed TCBV and Trail Making Test scores on age and compared differences in means attributable to sleeping >9 hours (vs 6–9 hours). The difference in Trail Making Test B–A scores and TCBV associated with sleeping >9 hours (vs 6–9 hours) was equivalent to approximately 12 and 5 years of brain aging, respectively. These estimates are intended to provide some clinical context but are based on small numbers and are not intended to be precise. There were no associations between sleep duration and Logical Memory performance (p for trend = 0.61).
Interactions.
In our model predicting incident all-cause dementia, we observed an interaction between baseline sleep duration and education (p = 0.003). Specifically, among participants without a high school degree (table e-1 and figure 2), sleeping >9 hours at baseline was associated with approximately a 600% increase in the risk of both incident all-cause dementia (HR 6.05; 95% CI 3.00–12.18) and AD (HR 6.00; 95% CI 2.73–13.16). No other interactions were observed.
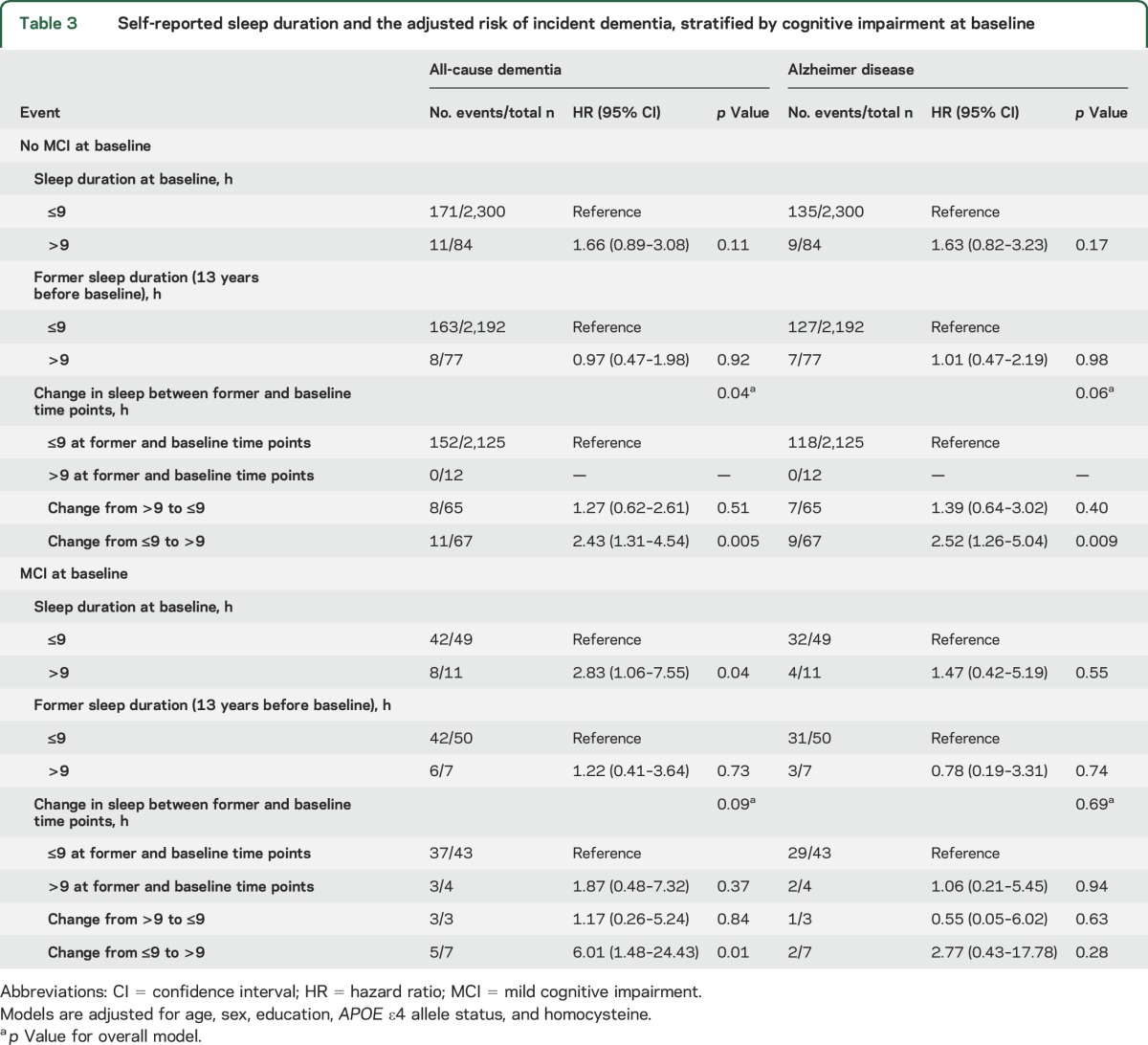
Sensitivity analysis.
Long sleep duration at baseline was only associated with an increased risk of all-cause dementia in those with MCI (n = 60; table 3). In contrast, transitioning to becoming a long sleeper was associated with incident all-cause dementia in both those with and without MCI at baseline, although the effect was more pronounced in those with MCI. In post hoc analysis, we included additional adjustments for medical comorbidities, including prevalent cardiovascular disease (comprising coronary heart disease, congestive heart failure, or stroke), prevalent diabetes, prevalent atrial fibrillation, and depression (available as an adjustment only for brain volumes and neuropsychological outcomes). The additional adjustments did not change the observed pattern of results (tables e-2 and e-3).
Table 3.
Self-reported sleep duration and the adjusted risk of incident dementia, stratified by cognitive impairment at baseline
DISCUSSION
In this large community-based study spanning 2 generations of participants, persons reporting sleeping more than 9 hours vs 9 hours or less at baseline had a higher risk of incident all-cause dementia. Persons reporting sleeping more than 9 hours compared to 6–9 hours also displayed lower cross-sectional brain volume and poorer cross-sectional processing speed and executive function. Associations between long sleep duration at baseline and dementia were driven by persons with MCI and persons without a high school degree. Whereas long sleep duration in the past was not associated with dementia risk, transitioning to >9 hours of sleep over a mean period of 13 years leading up to baseline was associated with an increased risk of dementia across the whole sample. Collectively, these results suggest that long sleep duration serves as an early biological marker of neurodegeneration, especially in those with low educational attainment.
The association between sleep duration and incident dementia has not been well-studied. Sleep duration as measured using polysomnography was not associated with the risks of MCI or dementia in a prospective study of 298 women followed for a median of 4.7 years.15 Consistent with the present results, the Neurological Disorders in Central Spain Study showed that those who slept 9 or more hours displayed a greater than 2-fold risk of incident dementia.1 In this previous study, short sleep duration was not associated with the risk of dementia in the fully adjusted statistical model. However, follow-up for dementia was limited to a mean of 3.2 years, meaning that results may have been driven by reverse causality. Researchers using the Survey on Elders' Health showed that sleep duration ≥9 hours was associated with incident amnestic cognitive impairment over 1 year in women.16 However, some participants may have already had cognitive impairment at baseline given that participants were eligible for inclusion with Mini-Mental State Examination scores as low as 22 at baseline. More recently, the Women's Health Initiative observed a V-shaped curve, with persons having <6 hours or >8 hours of sleep showing an increased risk of dementia/MCI as a combined outcome.2
While some studies demonstrate an association between long sleep duration and dementia, the temporal association between the 2 events was in need of further clarification. We extend previous findings by demonstrating that transitioning from sleeping ≤9 hours to more than >9 hours, over a mean period of 13 years, was associated with a higher risk of incident dementia. In our study, long sleep duration in the past was not associated with an increased risk of dementia and sleep duration at baseline was only associated with the risks of dementia in those with MCI. Collectively, our findings suggest that transitioning to being a long sleeper is an early marker of neurodegeneration. Thus, interventions to reduce sleep duration in long sleepers are unlikely to mitigate clinical dementia risk.
The cross-sectional association between short sleep duration and poorer cognition was expected given that performance on the Trail Making Test is dependent on cognitive processes, such as attention, which are impaired under conditions of sleep deprivation.17,18 Thus, the mechanisms linking short sleep duration to poorer cognitive function may be independent of any neurodegenerative disease. In support of the present findings, other studies have shown that prolonged sleep is associated with poorer cognitive function.19,20 Associations between prolonged sleep duration and poorer cognition may be driven by early neurodegeneration, a finding supported by the fact that long sleep duration was also cross-sectionally associated with smaller TCBV in the present study, a proxy for global brain atrophy. The association between short sleep duration and larger TCBV was surprising, and the underlying mechanisms are unclear. A previous study demonstrated that each hour of reduced sleep duration was associated with an annual increase in brain ventricular volume by 0.59%, indicating the progression of cortical atrophy.21
Mechanisms underpinning prolonged sleep in early dementia may extend to neurodegenerative changes in brain regions involved in the regulation of sleep and wakefulness including the projections between the suprachiasmatic nucleus, pineal gland, and retina.22 This may lead to circadian dysfunction, misalignment, and the altered production and secretion of melatonin.22,23 Levels of the wake-promoting neuropeptide hypocretin-1 have also been reported to be reduced in AD, as are the levels of hypocretin-1 neurons.24 Sleep duration may also be indirectly linked to dementia. For example, psychiatric comorbidities such as anxiety and depression are common in dementia and are associated with sleep disturbances, as are the medications used to treat these disorders.5,6
Whereas excessive sleep duration appeared to be a marker for early neurodegeneration in the present study, other facets of sleep may predispose to the pathology of AD and dementia. For example, current interest surrounds the role of sleep in the clearance of metabolic waste from the brain. Forcing sleep deprivation upon mice predisposed to dementia exacerbates the accumulation of Aβ25,26 and p-tau26 and overnight sleep in humans has been shown to reduce Aβ levels in CSF.27 Using polysomnography to examine sleep objectively, Yaffe et al.15 demonstrated that sleep-disordered breathing was associated with a higher risk of developing MCI or dementia. Thus, even if long sleep duration is a marker for early neurodegeneration, sleep pathologies or specific aspects of sleep architecture may contribute differently to the risk of dementia.
The present study is not without limitations. Our sensitivity analysis stratified results by MCI status at baseline. However, we cannot exclude the possibility that persons without MCI may have had subclinical disease below the threshold for MCI. As we did not correct for multiple comparisons, we cannot exclude the possibility that some of the reported results are attributable to chance. Our study relied on self-report to estimate total sleep duration. Although self-reported sleep has been shown to reflect objective assessments of sleep accurately,28 reporting may be influenced by recall bias. Moreover, time in bed may not always accurately reflect total sleep time, and our study does not differentiate between overnight sleep duration and time spent napping throughout the day. Moreover, we are unable to determine the biology underlying long sleep duration. The use of polysomnography would help clarify whether long sleep duration was associated with sleep fragmentation, including sleep efficiency, prolonged sleep latency, or frequent nocturnal arousals.
The advantage of using self-report to estimate sleep time is that the information is easy to collect, increasing the applicability of our results to general practice. Patients reporting long sleep duration and cognitive complaints to their primary care provider could be triaged for further dementia screening, without the need for overnight sleep studies. As our findings were driven by those with low educational attainment, we identified a rather select subgroup that may warrant further screening for dementia. Thus, self-reported sleep duration may be a useful clinical tool to help predict persons at risk of progressing to clinical dementia within 10 years. Our results also suggest a possible role for cognitive reserve, with higher educational attainment potentially protecting against clinical dementia in the presence of long sleep duration.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the Framingham Heart Study participants for their participation and Kendra Plourde (Boston University School of Medicine) for helping tabulate the results.
GLOSSARY
- AD
Alzheimer disease
- CI
confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- FHS
Framingham Heart Study
- HR
hazard ratio
- MCI
mild cognitive impairment
- PAI
Physical Activity Index
- TCBV
total cerebral brain volume
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Study concept and design: Drs. Westwood, Beiser, Seshadri, and Pase. Acquisition of data: Drs. DeCarli, Auerbach, and Seshadri. Analysis/interpretation of data: Drs. Beiser, Himali, Pase, and Seshadri. Draft of the manuscript: Dr. Pase and N. Jain. Critical revision of the manuscript for important intellectual content: Dr. Westwood, N. Jain, Drs. Beiser, Himali, DeCarli, Auerbach, Pase, and Seshadri. Obtained funding: Dr. Seshadri. Administrative, technical, or material support: Drs. Beiser, Pase, and Seshadri. Supervision: Drs. Seshadri, Beiser, and Pase.
STUDY FUNDING
The Framingham Heart Study is supported by the National Heart, Lung, and Blood Institute (contract no. N01-HC-25195 and no. HHSN268201500001I) and by grants from the National Institute on Aging (R01 AG054076, R01 AG049607, R01 AG033193, U01 AG049505, U01 AG052409) and the National Institute of Neurological Disorders and Stroke (NS017950 and UH2 NS100605). Prof. DeCarli directs the UC Davis Alzheimer's Disease Center with funding from the NIH (P30 AG010182). Dr. Pase is funded by an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (APP1089698).
DISCLOSURE
A. Westwood, A. Beiser, N. Jain, and J. Himali report no disclosures relevant to the manuscript. C. Decarli is a consultant to Novartis on a clinical trial of LCZ696 for heart failure. S. Auerbach, M. Pase, and S. Seshadri report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Benito-León J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol 2009;16:990–997. [DOI] [PubMed] [Google Scholar]
- 2.Chen JC, Espeland MA, Brunner RL, et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimers Dement 2016;12:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu YH, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer's disease. Sleep Med 2007;8:623–636. [DOI] [PubMed] [Google Scholar]
- 5.Krystal AD. Psychiatric disorders and sleep. Neurol Clin 2012;30:1389–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Argyropoulos SV, Wilson SJ. Sleep disturbances in depression and the effects of antidepressants. Int Rev Psychiatry 2005;17:237–245. [DOI] [PubMed] [Google Scholar]
- 7.Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health 1951;41:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study: design and preliminary data. Prev Med 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 9.McKhann G, Drachman D, Folstein M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 10.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 11.Pase MP, Himali JJ, Mitchell GF, et al. Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: the Framingham Third Generation Cohort Study. Hypertension 2016;67:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes DE, Beiser AS, Lee A, et al. Development and validation of a brief dementia screening indicator for primary care. Alzheimers Dement 2014;10:656–665. e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. New Engl J Med 2002;346:476–483. [DOI] [PubMed] [Google Scholar]
- 14.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 15.Yaffe K, Laffan AM, Harrison S, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potvin O, Lorrain D, Forget H, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep 2012;35:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat 2007;3:553–567. [PMC free article] [PubMed] [Google Scholar]
- 18.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull 2010;136:375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimäki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep 2011;34:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx Aging Study. Behav Sleep Med 2007;5:39–56. [DOI] [PubMed] [Google Scholar]
- 21.Lo JC, Loh KK, Zheng H, Sim SKY, Chee MWL. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep 2014;37:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skene DJ, Swaab DF. Melatonin rhythmicity: effect of age and Alzheimer's disease. Exp Gerontol 2003;38:199–206. [DOI] [PubMed] [Google Scholar]
- 23.Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer Disease. Exp Mol Med 2015;47:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fronczek R, van Geest S, Frolich M, et al. Hypocretin (orexin) loss in Alzheimer's disease. Neurobiol Aging 2012;33:1642–1650. [DOI] [PubMed] [Google Scholar]
- 25.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009;326:1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Abeta and pTau in a mouse model of Alzheimer's disease. Brain Res 2013;1529:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA. Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol 2014;71:971–977. [DOI] [PubMed] [Google Scholar]
- 28.Biddle DJ, Robillard R, Hermens DF, Hickie IB, Glozier N. Accuracy of self-reported sleep parameters compared with actigraphy in young people with mental ill-health. Sleep Health 2015;1:214–220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.