Abstract
Background
22q11.2 Microdeletion syndrome (22q11DS) is associated with elevated rates of autism spectrum disorders (ASDs), although the diagnosis is controversial. In order to determine whether there is a biological substrate of ASD in 22q11DS, we examined neurocognitive and structural neuroanatomic differences between those with 22q11DS and an ASD diagnosis (22q11DS-ASD+) and those with 22q11DS without ASD (22q11DS-ASD−); we then determined whether these differences were better characterized within a categorical or dimensional framework.
Methods
We collected multiple neurocognitive measures and high-resolution T1-weighted scans on 116 individuals (29 22q11DS-ASD+, 32 22q11DS-ASD−, 55 typically developing controls) between 6 and 26 years of age. Measures of subcortical volume, cortical thickness (CT), and surface area were extracted using the FreeSurfer image analysis suite. Group differences in neurocognitive and neuroanatomic measures were assessed; regression analyses were then performed to determine whether a categorical or dimensional measure of ASD was a better predictor of neurocognitive impairment and/or neuroanatomic abnormalities observed in 22q11DS-ASD+.
Results
In comparison to 22q11DS-ASD−, 22q11DS-ASD+ participants exhibited decreased bilateral hippocampal CT and decreased right amygdala volumes. Those with 22q11DS-ASD+ also showed slowed processing speed and impairments in visuospatial and facial memory. Neurocognitive impairments fit a dimensional model of ASD, whereas reductions in parahippocampal CT were best explained by a categorical measure of ASD.
Conclusions
A combination of categorical and dimensional measures of ASD may provide the most comprehensive understanding of ASDs in 22q11DS.
Keywords: velocardiofacial syndrome, cortical thickness, amygdala, parahippocampus, social behavior, dimensional measures
Introduction
Autism spectrum disorders (ASD) are characterized by marked impairments in social interactions and both verbal and non-verbal communication, as well as repetitive behaviors or restricted interests. However, ASD is also associated with remarkable heterogeneity at both the phenotypic (1) and genetic level (2–4), which may be why it has been so difficult to identify replicable cognitive and biological substrates of the disorder. Given that multiple rare copy number variants (CNVs) have been found to play a significant role in ASD pathogenesis (5–10), an alternative strategy is to focus on highly penetrant genetic subtypes of the disorder. Indeed, up to 25% of individuals diagnosed with ASD have an identifiable genetic etiology (11). Examination of cognition and neuroanatomy in a homogenous subgroup with a well-known etiology may provide us with insights that are otherwise obscured due to both genetic and phenotypic heterogeneity in ASD.
22q11.2 Microdeletion Syndrome (22q11DS) offers one potential model disorder in which to investigate this question, as rates of ASD are substantially elevated in this syndrome; a recent meta-analysis concluded that 22q11DS is associated with a >8-fold increase in risk for ASD (12). However, the reported prevalence of categorical ASD diagnosis in 22q11DS is highly variable across studies, ranging from 0–50% (13–18).
Some argue that this variability is attributable to methodological differences across studies (18); others speculate that ASD diagnosis in 22q11DS is actually a misdiagnosis of prodromal psychosis, given that 25–30% of patients with 22q11DS develop psychotic illness in adolescence or adulthood (19). Still others have suggested that ASD diagnosis in 22q11DS is capturing a generalized social impairment that differs from idiopathic autism (20). On the other hand, core autism behaviors, such as impairments in joint attention, gestural communication, initiating conversation, and circumscribed interests, are recognized as characteristic phenotypes in 22q11DS individuals, independent of ASD diagnosis (21). These findings suggest that a dimensional explanation of ASD, or a “broader autism phenotype”, may be more applicable to the 22q11DS population.
This concept is not specific to 22q11DS. Population twin and family studies support a dimensional view of autistic-like behaviors, suggesting that autism is at the extreme tail of a continuous distribution (22–25). Furthermore, studies have found that empirically derived clusters are more likely to map onto severity of ASD symptoms (26–28), rather than the classification approach used by DSM-IV and ICD-10. However, no studies have yet addressed the question of whether neurocognitive and neuroanatomic abnormalities in 22q11DS are better explained by a categorical vs. a dimensional approach to ASD-relevant traits. This question is highly relevant to the Research Domain Criteria framework (RDoC, 29), which posits that to accelerate progress in identifying disease mechanisms underlying ASD, it may be more informative to examine autistic symptoms based on dimensions and observable behaviors (30; 31).
Prior studies have not observed differences in overall cognitive abilities (Full-Scale intelligence quotient; FSIQ) between 22q11DS patients with an ASD diagnosis (22q11DS-ASD+) compared to those who do not have ASD (22q11DS-ASD−; 13; 21; 32). However, greater ASD symptom severity was associated with poorer performance on measures of verbal knowledge (vocabulary) and coding (processing speed) in patients with 22q11DS (32). Additionally, a previous investigation from our group did not find differences in a theory of mind measure (Animations task) between those with 22q11DS-ASD+ vs. those without ASD (33); nevertheless, poorer performance was associated with greater impairment on a dimensional measure of reciprocal social behavior, the Social Responsiveness Scale (34). These findings suggest that dimensional measures may yield more precision and power when testing hypotheses regarding ASD-related neurocognitive deficits in 22q11DS.
In addition, given increasing recognition of mental disorders as biological disorders of the brain (29), there may be distinct structural neuroanatomic alterations that characterize 22q11DS patients with ASD. To our knowledge, there is only one published study to investigate this; these authors found increased amygdala volumes in 22q11DS-ASD+ children vs. 22q11DS-ASD−, with all other regions of interest being statistically similar between the two groups (14). However, it is important to examine cortical volume in its constituent parts, cortical thickness (CT) and surface area (SA), given that these two measures are believed to have distinct neurodevelopmental origins (35).
Here, we sought to determine whether there are distinct neurocognitive or biological substrates associated with categorically defined ASD diagnosis in 22q11DS or, alternatively, whether a dimensional approach better characterizes the findings. Specifically, we investigated whether: 1) those with 22q11DS-ASD+ vs. 22q11DS-ASD−differ in terms of social and non-social cognition measures; 2) we could identify patterns of neuroanatomic alteration specific to 22q11DS-ASD+; 3) any observed categorical differences could be better explained by dimensional measures of autism-associated symptomatology in 22q11DS.
Given the central role of face recognition and social cognition deficits in idiopathic ASD (36–40), we hypothesized that those with 22q11DS-ASD+ would show worse performance on measures of emotion recognition and face memory. Furthermore, based on findings in idiopathic ASD, we hypothesized that those with 22q11DS-ASD+ would show increased CT relative to 22q11DS-ASD− in brain regions associated with social cognition, including the orbitofrontal cortex and fusiform gyrus (41; 42), as well as alterations in amygdala volume (43). Finally, based on the premise that dimensional, quantitative traits may map more closely to the disease biology than categorical diagnoses, we hypothesized that dimensional measures would account for more variance in the neurocognitive and neuroanatomic measures than categorical ASD diagnosis in 22q11DS.
Methods
Participants
The overall sample consisted of 116 individuals (29 22q11DS-ASD+, 32 22q11DS-ASD−, 55 typically developing controls) between 6 and 26 years of age (Table 1), recruited from an ongoing longitudinal study. Fifty percent of the participants (22q11DS=27, Controls=32) were included in a previous structural MRI publication (5), which investigated neuroanatomic measures associated with psychotic symptoms in 22q11DS. Study procedures were approved by the UCLA Institutional Review Board and performed in accordance with the Declaration of Helsinki. All subjects or their legal guardians provided written informed consent, after study procedures were fully explained. 22q11DS participants had a molecularly confirmed diagnosis of 22q11.2 deletion, and controls did not meet criteria for any major mental disorder based on information gathered during structured clinical interview (see 5; 44 for more information regarding subject ascertainment procedures). Exclusion criteria included history of a neurological or medical disorder (independent of 22q11.2 deletion) that could affect CNS function, insufficient fluency in English (i.e., could not validly complete research measures), and substance or alcohol abuse and/or dependence within the past six months.
Table 1.
Demographic and clinical characteristics of study participants
| 22q11DS- ASD+ Participants (n=29) |
22q11DS-ASD− Participants (n=32) |
Typically Developing Controls (n=55) |
||
|---|---|---|---|---|
|
Age (years, +/− SD) [Age range, years] |
14.34 (5.70) [6–26] |
13.78 (5.35) [6–25] |
12.87 (4.93) [6–26] |
p=.45 |
|
Participant Education (years,+/− SD) |
6.72 (4.41) | 6.47 (4.72) | 7.15 (5.16) | p=.81 |
| Gender (N, % female) | 11 (38%) | 18 (56%) | 26 (47%) | p=.36 |
| Mean WASI IQ (+/− SD) | 76.7 (11.8) | 81.5 (14.0) | 110.2 (20.4) |
p= 9.0121e- 15a |
22q-ASD+ and 22q11DS-ASD− did not significantly differ on measures of WASI IQ (t=1.5, p=.2).
Autism Spectrum Diagnosis
For the children, a diagnosis of ASD was based on the Autism Diagnostic Observation Schedule (ADOS, (45)) and the Autism Diagnostic Interview-Revised (ADI-R, (46)), further described in supplemental text, whereas for adult participants a SCID interview (47) with an additional developmental disorders module (48) were administered to establish a diagnosis of ASD based on DSM-IV criteria (49).
Dimensional ASD Measures
The ADI-R social interaction score, ADI-R communication language score, and ADI-R repetitive behavior score, ADOS severity score, Social Responsiveness Scale (SRS), Repetitive Behavior Scale-Revised (RBS-R), and Short Sensory Profile were used as dimensional measures of ASD (see Supplemental Text for details).
MRI Acquisition
All scanning was carried out on an identical Siemens 3 Tesla (Tim Trio) MRI scanner with a 12-channel head coil at the Brain Mapping Center at UCLA (22q11DS=30, controls=28) or at the Center for Cognitive Neuroscience (22q11DS=31, controls=27). Measures of brain structure were obtained with high-resolution structural MRI (see Supplemental Text for scan parameters).
Neurocognitive Measures
Supervised clinical psychology doctoral students or Ph.D. staff administered a neuropsychological battery assessing multiple domains of cognitive functioning, detailed in Supplementary Table S1.
sMRI Image Processing
The FreeSurfer image analysis suite (version 5.0, http://surfer.nmr.mgh.harvard.edu) surface-based processing pipeline was used to derive CT and SA measures for the structural data. Freesurfer is a well-validated processing protocol (50; 51), further described in the Supplemental Text.
Statistical Analyses
Overview
Statistical analyses were performed using SPSS software v.22 (IBM, Chicago, Illinois) and R (R Core Team, 2015). To compare demographic variables between the three groups (controls, 22q11DS-ASD+, 22q11DS-ASD−) we performed a univariate ANOVA for continuous variables (age, years of participant education) and chi-squared tests for categorical variables (sex). To examine differences on dimensional measures of ASD symptoms, we conducted t-tests between 22q11DS-ASD+ and 22q11DS-ASD−and calculated effect sizes (Cohen’s d). To ensure that there were no cross-scanner differences, we conducted a univariate analysis of covariance (ANCOVA) for each neuroanatomic measurement, with scanner location as the between-group factor and group, age, sex, and intracranial volume (for volume and surface area) as covariates. Analyses testing for group differences in cognition and neuroanatomy are detailed in Supplemental Text.
Comparison of categorical and dimensional measures in 22q11DS
We then tested whether variance in the neurocognitive and neuroanatomic measures that differentiated between 22q11DS-ASD+ and 22q11DS-ASD− were better explained by categorical (diagnosis) or dimensional measures of ASD (ADI-R communication-language score, ADI-R social interaction score, ADI-R repetitive-behavior, ADOS total score, total SRS score, total RBS-R score, total SSP score). We conducted separate regression analyses in the entire 22q11DS group (N= 61) with the neuroanatomic or neurocognitive measure as the dependent variable. Individual categorical and dimensional measures were each entered into the model separately. For each analysis, age and gender, along with categorical or dimensional ASD measure, were simultaneously entered into the regression model. We used Aikaike’s criterion (AIC), a commonly used measure for model selection, to determine the model with the best fit (52). AIC estimates the model fit relative to other models and attempts to balance model fit and complexity by penalizing for additional parameters (52). The smaller the AIC value in reference to the other models, the better the fit. A suggested “rule of thumb” for comparing AIC values is that is that if the AIC value decreases by 2 or less, this is weak evidence for improved model fit. A decrease in 4–7 points is moderate evidence of improved model fit, while a decrease in 10+ points is strong evidence for preferring one model over another (53). See Supplemental Text for secondary analyses of psychotic symptoms.
Results
Overview
As shown in Table 1, 22q11DS-ASD+, 22q11DS-ASD− and control groups were matched on all demographic factors (all p-values .36 or greater). Group differences on dimensional measures of ASD are reported in Supplementary Table S2. As anticipated, there were significant differences between 22q11DS-ASD+ and 22q11DS-ASD on all dimensional measures, with 22q11DS-ASD+ having greater impairment and/or severity of symptoms, with greatest differences observed in the total ADOS score (d=−1.58). There were no between-scanner differences in volume, CT, or SA that survived multiple comparison correction (Supplementary Tables S3 and S4).
Neurocognitive Impairments in 22q11DS-ASD+
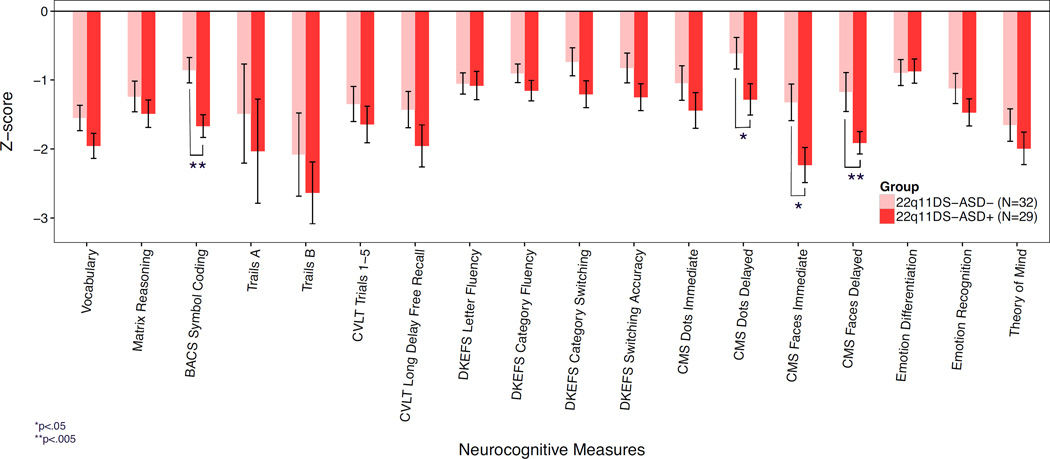
All 21 neurocognitive measures showed a statistically significant main effect of group (q<.05; see Table 2). Both 22q11DS-ASD+ and 22q11DS-ASD− had significantly lower Full Scale IQ (FSIQ) estimates in comparison to the control group; however, there was not a significant difference in FSIQ between 22q11DS-ASD+and 22q11DS-ASD− (F(1,60)=1.9, p=.18; Table 1). In comparison to both 22q11DS-ASD− and controls, 22q11DS-ASD+ exhibited significantly impaired performance on measures of immediate (p=.004, ηp2=.08) and delayed facial memory (p=.01, ηp2=.07), processing speed (p=.001, ηp2=.14) and delayed visuospatial memory (p=.02; ηp2=.07, Figure 1). However, individuals with 22q11DS-ASD+ did not significantly differ from 22q11DS-ASD− on social cognition tasks examining emotion identification, emotion differentiation, and Theory of Mind (all p>.12).
Table 2.
Comparison of neurocognitive task performance in 22q11DS participants with an autism spectrum diagnosis (22q11DS-ASD+) to 22q11DS participants without ASD (22q11DS-ASD−), and healthy controls (CTL). Cells highlighted in yellow indicate a statistically significant difference (p<.05) between 22q11DS-ASD+ vs. 22q11DS-ASD− in the pairwise comparisons.
| Neuropsychological Construct Measured |
F | FDR q value |
↑ or ↓ in 22q11DS- ASD+ vs. 22q11DS- ASD- |
↑ or ↓ in 22q11DS- ASD+ vs. CTL |
|---|---|---|---|---|
| Verbal Knowledge | ||||
| Vocabulary | 31.4 | 1.4E-10 | ↓ | |
|
Nonverbal Reasoning Matrix Reasoning |
18.7 | 2.2E-07 | ↓ | |
| Processing Speed | ||||
| BACS Symbol Coding | 16.9 | 8.2E-07 | ↓ | ↓ |
| Trails A | 4.6 | 0.01 | ↓ | |
|
Set Switching Trails B |
16.7 | 8.3E-07 | ↓ | |
| Verbal Memory | ↓ | |||
| CVLT Trials 1–5 | 19.0 | 2.2E-07 | ↓ | |
| CVLT Short delay free recall | 18.8 | 2.2E-07 | ↓ | |
| CVLT Short delay cued recall | 16.6 | 8.3E-07 | ↓ | |
| CVLT Long delay free recall | 20.9 | 7.9E-08 | ↓ | |
| CVLT Long delay cued recall | 18.9 | 2.2E-07 | ↓ | |
| Verbal Fluency | ↓ | |||
| DKEFS-Letter Fluency | 10.7 | 7.3E-05 | ↓ | |
| DKEFS-Category Fluency | 12.2 | 2.3E-05 | ↓ | |
| DKEFS-Category Switching | 7.5 | 0.001 | ↓ | |
| DKEFS-Switching Accuracy | 7.9 | 0.0007 | ↓ | |
| Visuopatial Memory | ||||
| Dots Immediate | 11.3 | 4.7E-05 | ↓ | |
| Dots Delayed | 9.3 | 0.0002 | ↓ | ↓ |
| Facial Memory | ||||
| Faces Immediate | 22.5 | 3.1E-08 | ↓ | ↓ |
| Faces Delayed | 20.0 | 1.4E-07 | ↓ | ↓ |
| Emotion Processing | ||||
| Penn Emotion Recognition Task | 16.7 | 8.3E-07 | ↓ | |
| Penn Emotion Differentiation Task | 7.4 | 0.001 | ↓ | |
|
Theory of Mind The Awareness of Social Inference Task |
25.4 | 1.9E-08 | ↓ |
Figure 1.
Performance across neurocognitive domains in 22q11DS individuals with an autism spectrum disorder diagnosis (22q11DS-ASD+) vs. those without autism spectrum disorder (22q11DS-ASD−). Z-scores created using the control mean are presented for 22q11DS-ASD+ and 22q11ASD-. In comparison to 22q11DS-ASD−, those with 22q11DS-ASD+ had significant impairments in processing speed (BACS symbol coding), delayed visuospatial memory (CMS dots delayed), and immediate and delayed face memory (CMS Faces Immediate, CMS Faces delayed).
Subcortical volume and cortical thickness alterations in 22q11DS-ASD+
Global neuroanatomic measures are reported in Supplementary Table S5. Fourteen subcortical volumes, 38 CT measures, and 41 SA measures showed a statistically significant main effect of group (q<.05; see Tables 3 and 4).
Table 3.
Comparison of subcortical volumes and white matter regions in 22q11DS participants with ASD (22q11DS-ASD+) to 22q11 DS participants without ASD (22q11DS-ASD−), and healthy controls. Cells highlighted in yellow indicate a statistically significant difference (p<.05) between22q11DS-ASD+ vs. 22q11DS-ASD− in the pairwise comparisons. Cells highlighted in gray were not examined in post-hoc analyses because the significance in the overall F-test (ANCOVA) was q > .05.
| Region of Interest |
Hemi- sphere |
F | FDR q- value |
Effect Size |
↑ or ↓ in 22q11DS -ASD+ vs. 22q11DS -ASD- |
↑ or ↓ in 22q11DS -ASD+ vs. CTL |
↑ or ↓ in 22q11DS -ASD- VS. CTL |
|---|---|---|---|---|---|---|---|
| Cerebellum (white matter) |
L | 26.1 | 0.000000009 | 0.32 | ↓ | ↓ | |
| R | 26.3 | 0.000000009 | 0.33 | ↓ | ↓ | ||
| Cerebellum (cortex) |
L | 18.0 | 0.000002 | 0.25 | ↓ | ↓ | |
| R | 17.6 | 0.000002 | 0.24 | ↓ | ↓ | ||
| Caudate | L | 4.8 | 0.02 | 0.08 | ↑ | ↑ | |
| R | 7.2 | 0.003 | 0.11 | ↑ | ↑ | ||
| Putamen | L | 2.4 | 0.13 | 0.04 | |||
| R | 5.6 | 0.01 | 0.09 | ↓ | ↓ | ||
| Hippocampus | L | 4.6 | 0.02 | 0.08 | ↓ | ||
| R | 12.3 | 0.0001 | 0.18 | ↓ | ↓ | ||
| Amygdala | L | 4.7 | 0.02 | 0.08 | ↓ | ↓ | |
| R | 7.0 | 0.003 | 0.11 | ↓ | ↓ | ||
| Nucleus accumbens area |
L | 2.7 | 0.11 | 0.05 | |||
| R | 11.4 | 0.0002 | 0.17 | ↑ | ↑ | ||
| Ventral diencephalon |
L | 5.5 | 0.01 | 0.09 | ↓ | ↓ | |
| R | 3.9 | 0.04 | 0.07 | ↓ | |||
| Thalamus | L | 0.7 | 0.56 | 0.01 | |||
| R | 0.9 | 0.45 | 0.01 | ||||
| Pallidum | L | 2.7 | 0.11 | 0.05 | |||
| R | 3.2 | 0.06 | 0.06 | ||||
| Corpus callosum (posterior) |
N/A | 3.1 | 0.07 | 0.05 | |||
| Corpus callosum (mid- posterior) |
N/A | 0.3 | 0.8 | 0.01 | |||
| Corpus callosum (central) |
N/A | 1.1 | 0.4 | 0.02 | |||
| Corpus callosum (mid- anterior) |
N/A | 0.3 | 0.7 | 0.01 | |||
| Corpus callosum (anterior) |
N/A | 11.2 | 0.0002 | ↑ | ↑ |
↓ or ↑ indicates measures that showed a statistical significance in the results (p-values <.05).
Table 4.
Comparison of cortical thickness and surface area in 22q11DS-ASD+, 22q11DS-ASD− and typically developing controls (CTL). Cells highlighted in yellow indicate a statistically significant difference (p<.05) between 22q11DS-ASD+ vs. 22q11 DS-ASD- in the pairwise comparisons. Cells highlighted in gray were not examined in post-hoc analyses because the significance in the overall F-test (ANCOVA) was q > .05.
| Cortical Thickness | Surface Area | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region of Interest |
Hemisphere | F | FDR q-value |
Effect size |
↑ or ↓ in 22q11DS-ASD+ vs. 22q11DS-ASD− |
↑ or ↓ in 22q11DS-ASD+ vs. CTL |
↑ or ↓ in 22q11DS-ASD− vs. CTL |
F | FDR q-value |
Effect size |
↑ or ↓ in 22q11DS-ASD+ vs. 22q11DS-ASD− |
↑ or ↓ in 22q11DS-ASD+ vs. CTL |
↑ or ↓ in 22q11DS-ASD− vs. CTL |
| Banks of Superior Temporal Sulcus |
L | 0.5 | 0.7 | 0.009 | 3.7 | 0.05 | 0.06 | ||||||
| R | 1.5 | 0.3 | 0.03 | 11.4 | 0.0002 | 0.17 | ↓ | ↓ | |||||
| Caudal Anterior Cingulate |
L | 4.4 | 0.02 | 0.08 | ↓ | 17.9 | 0.000001 | 0.25 | ↓ | ↓ | |||
| R | 3.5 | 0.06 | 0.06 | 10.0 | 0.0004 | 0.16 | ↓ | ↓ | |||||
| Caudal Middle Frontal |
L | 9.1 | 0.0007 | 0.15 | ↑ | ↑ | 0.6 | 0.6 | 0.01 | ||||
| R | 5.8 | 0.007 | 0.1 | ↑ | ↑ | 0.04 | 1.0 | 0.001 | |||||
| Cuneus | L | 4.5 | 0.02 | 0.08 | ↑ | ↑ | 44.3 | 1.3E-12 | 0.45 | ↓ | ↓ | ||
| R | 2.4 | 0.1 | 0.04 | 38.8 | 1.4E-11 | 0.42 | ↓ | ↓ | |||||
| Entorhinal | L | 0.9 | 0.5 | 0.02 | 2.6 | 0.1 | 0.05 | ||||||
| R | 2.2 | 0.2 | 0.04 | 1.3 | 0.3 | 0.02 | |||||||
| Fusiform | L | 0.4 | 0.7 | 0.007 | 16.1 | 0.000005 | 0.23 | ↓ | ↓ | ||||
| R | 2.9 | 0.09 | 0.05 | 18.2 | 0.000002 | 0.25 | ↓ | ↓ | |||||
| Inferior Parietal |
L | 3.7 | 0.05 | 0.06 | 3.4 | 0.06 | 0.06 | ↓ | ↓ | ||||
| R | 9.3 | 0.0007 | 0.15 | ↑ | ↑ | 3.8 | 0.009 | 0.10 | ↓ | ↓ | |||
| Inferior Temporal |
L | 0.6 | 0.6 | 0.01 | 29.8 | 1.5E-09 | 0.36 | ↓ | ↓ | ||||
| R | 2.0 | 0.2 | 0.04 | 13.0 | 0.00005 | 0.19 | ↓ | ↓ | |||||
| Isthmus Cingulate |
L | 0.6 | 0.6 | 0.01 | 0.8 | 0.5 | 0.01 | ||||||
| R | 0.6 | 0.6 | 0.01 | 0.2 | 0.9 | 0.003 | |||||||
| Lateral Occipital |
L | 1.2 | 0.4 | 0.02 | 10.8 | 0.0002 | 0.17 | ↓ | ↓ | ||||
| R | 1.8 | 0.2 | 0.03 | 7.3 | 0.003 | 0.12 | ↓ | ↓ | |||||
| Lateral Orbitofrontal |
L | 5.8 | 0.009 | 0.1 | ↑ | ↑ | 0.9 | 0.5 | 0.02 | ||||
| R | 5.1 | 0.02 | 0.09 | ↑ | 1.0 | 0.4 | 0.02 | ||||||
| Lingual | L | 5.9 | 0.007 | 0.1 | ↑ | 23.9 | 3.5E-08 | 0.31 | ↓ | ↓ | |||
| R | 7.7 | 0.003 | 0.13 | ↑ | ↑ | 30.0 | 1.5E-09 | 0.36 | ↓ | ↓ | |||
| Medial Orbitofrontal |
L | 4.6 | 0.02 | 0.08 | ↑ | 2.7 | 0.1 | 0.05 | |||||
| R | 10.8 | 0.0002 | 0.17 | ↑ | ↑ | 0.6 | 0.6 | 0.01 | |||||
| Middle Temporal |
L | 1.3 | 0.4 | 0.02 | 10.5 | 0.0003 | 0.16 | ↓ | ↓ | ||||
| R | 4.8 | 0.02 | 0.08 | ↑ | ↑ | 6.8 | 0.005 | 0.11 | ↓ | ↓ | |||
| Parahippocampal | L | 11.6 | 0.0002 | 0.18 | ↓ | ↓ | 1.8 | 0.2 | 0.03 | ||||
| R | 8.3 | 0.002 | 0.13 | ↓ | ↓ | ↓ | 3.7 | 0.05 | 0.06 | ||||
| Paracentral | L | 10.0 | 0.0004 | 0.16 | ↑ | ↑ | 4.6 | 0.02 | 0.08 | ↓ | ↓ | ||
| R | 8.8 | 0.001 | 0.14 | ↑ | ↑ | 3.8 | 0.05 | 0.07 | |||||
| Pars Opercularis |
L | 15.4 | 0.000007 | 0.22 | ↑ | ↑ | 0.4 | 0.7 | 0.007 | ||||
| R | 5.9 | 0.009 | 0.10 | ↑ | ↑ | 0.8 | 0.5 | 0.01 | |||||
| Pars Orbitalis |
L | 5.3 | 0.01 | 0.09 | ↑ | ↑ | 0.8 | 0.5 | 0.02 | ||||
| R | 2.0 | 0.2 | 0.04 | 0.1 | 0.9 | 0.002 | |||||||
| Pars Triangularis |
L | 17.6 | 0.000002 | 0.24 | ↑ | ↑ | 3.5 | 0.05 | 0.06 | ||||
| R | 2.7 | 0.1 | 0.05 | 6.6 | 0.005 | 0.11 | ↓ | ↓ | |||||
| Pericalcarine | L | 2.1 | 0.2 | 0.04 | 20.6 | 3.2E-07 | 0.28 | ↓ | ↓ | ||||
| R | 6.7 | 0.005 | 0.11 | ↓ | ↑ | 22.1 | 1.2E-07 | 0.29 | ↓ | ↓ | |||
| Postcentral | L | 8.3 | 0.001 | 0.133 | ↑ | ↑ | 8.0 | 0.003 | 0.13 | ↓ | ↓ | ||
| R | 5.4 | 0.01 | 0.09 | ↑ | ↑ | 15.7 | 0.000007 | 0. 22 | ↓ | ↓ | |||
| Posterior Cingulate |
L | 1.0 | 0.5 | 0.02 | 2.5 | 0.1 | 0.04 | ||||||
| R | 1.9 | 0.2 | 0.03 | 4.2 | 0.04 | 0.07 | ↓ | ↓ | |||||
| Precentral | L | 4.9 | 0.02 | 0.08 | ↑ | ↑ | 1.1 | 0.4 | 0.02 | ||||
| R | 8.0 | 0.003 | 0.13 | ↑ | ↑ | 0.9 | 0.5 | 0.02 | |||||
| Precuneus | L | 3.7 | 0.05 | 0.06 | 24.2 | 3.1E-08 | 0.23 | ↓ | ↓ | ||||
| R | 9.0 | 0.0007 | 0.14 | ↓ | ↑ | 16.6 | 3.9E-06 | 0.23 | ↓ | ↓ | |||
| Rostral Anterior Cingulate |
L | 1.6 | 0.3 | 0.03 | 10.1 | 0.0004 | 0.16 | ↓ | ↓ | ||||
| R | 1.9 | 0.2 | 0.03 | 8.7 | 0.001 | 0.14 | ↓ | ↓ | |||||
| Rostral Middle Frontal |
L | 14.0 | 0.00002 | 0.21 | ↑ | ↑ | 18.6 | 1.3E-06 | 0.25 | ↓ | ↓ | ||
| R | 16.0 | 0.000005 | 0.23 | ↑ | ↑ | 27.3 | 6.3E-09 | 0.33 | ↓ | ↓ | |||
| Superior Frontal |
L | 7.8 | 0.003 | 0.13 | ↓ | ↑ | 4.2 | 0.04 | 0.07 | ↓ | ↓ | ||
| R | 6.1 | 0.005 | 0.10 | ↑ | 2.4 | 0.1 | 0.04 | ||||||
| Superior Parietal |
L | 2.1 | 0.2 | 0.04 | 33.2 | 2.8E-10 | 0.38 | ↓ | ↓ | ||||
| R | 8.0 | 0.003 | 0.13 | ↑ | ↑ | 26.5 | 9.1E-09 | 0.33 | ↓ | ↓ | |||
| Superior Temporal |
L | 3.6 | 0.05 | 0.06 | 0.7 | 0.6 | 0.01 | ||||||
| R | 0.2 | 0.9 | 0.003 | 7.5 | 0.003 | 0.12 | ↓ | ↓ | |||||
| Supram arginal |
L | 10.9 | 0.0002 | 0.17 | ↑ | ↑ | 2.4 | 0.1 | 0.04 | ||||
| R | 11.8 | 0.0001 | 0.18 | ↑ | ↑ | 1.4 | 0.3 | 0.02 | |||||
| Frontal Pole |
L | 7.3 | 0.003 | 0.12 | ↑ | ↑ | 1.0 | 0.5 | 0.02 | ||||
| R | 0.6 | 0.6 | 0.01 | 0.3 | 0.7 | 0.006 | |||||||
| Temporal Pole |
L | 5.1 | 0.02 | 0.09 | ↑ | ↑ | 9.3 | 0.0007 | 0.15 | ↓ | ↓ | ||
| R | 0.4 | 0.7 | 0.008 | 5.9 | 0.009 | 0.10 | ↓ | ↓ | |||||
| Transverse Temporal |
L | 0.1 | 0.9 | 0.002 | 2.1 | 0.2 | 0.04 | ||||||
| R | 1.2 | 0.4 | 0.02 | 3.0 | 0.08 | 0.05 | |||||||
| Insula | L | 10.5 | 0.0003 | 0.16 | ↑ | ↑ | 3.7 | 0.05 | 0.06 | ||||
| R | 8.2 | 0.001 | 0.13 | ↑ | ↑ | 3.8 | 0.05 | 0.07 | |||||
↓ or ↑ indicates measures that showed a statistical significance in the results (p-values <.05).
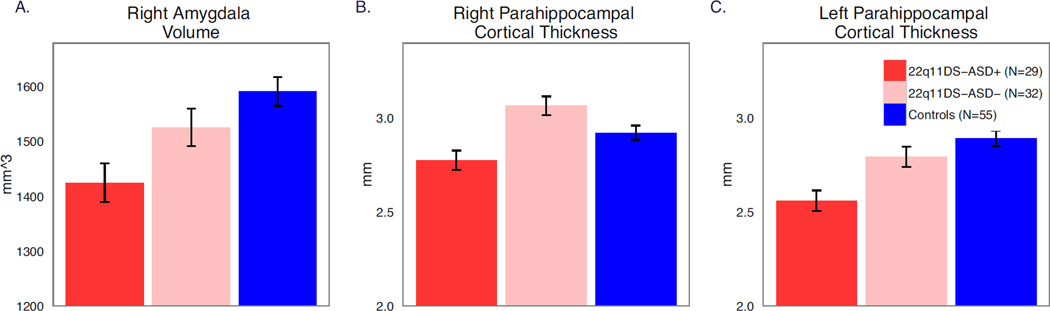
22q11DS-ASD+ had significantly decreased right amygdala volume compared to both 22q11DS-ASD− (p=.04, ηp2=.03) and controls (p=.001, ηp2=.18, Figure 3A). No other subcortical regions differed between 22q11DS-ASD+ and 22q11DS-ASD− (Table 4). However, both 22q11DS groups had volumetric reductions relative to controls in multiple subcortical regions, including bilateral regions of the cerebellum, right hippocampus and putamen, left amygdala and ventral diencephalon. In comparison to controls, both patient groups showed increases in right nucleus accumbens volume, and in bilateral regions of the caudate, consistent with previous findings (54–57).
22q11DS-ASD+ demonstrated significantly decreased CT in parahippocampal cortices bilaterally compared to both 22q11DS-ASD− (left: p=.003, ηp2=.14, right: p=0.00009, ηp2=.21) and controls (left: p=0.000005, ηp2=.25, right: p=.03, ηp2=.07; Figure 2B–C). CT in the right caudal anterior cingulate was also significantly decreased in 22q11DS-ASD+ compared to controls (p=. 006, ηp2=0.08), but there was not a significant difference between 22q11DS patients with and without ASD (p=0.29, ηp2=0.01), nor between 22q11DS-ASD− and controls (p=0.09, ηp2=0.02). Both patient groups showed increased CT in comparison to controls in frontal, insular, and parietal areas, largely replicating previous findings (5; 58; 59).
Figure 2.
Marginal means (after correcting for age and gender) for right amygdala volume (A) and bilateral parahippocampal thickness (B,C) in those with 22q11DS and autism spectrum disorder diagnosis (22q11DS-ASD+, red), 22q11DS without an autism spectrum disorder diagnosis (22q11DS-ASD−, pink), and typically developing controls (blue).Those with 22q11DS and autism spectrum disorder diagnosis (22q11DS-ASD+) exhibited reduced amygdala volumes (A), and reduced right (B) and left (C) cortical thickness in the parahippocampal regions in comparison in 22q11DS-ASD− and typically developing controls.
No SA measures significantly differentiated between 22q11DS-ASD+ and 22q11DS-ASD−. Both patient groups showed decreased SA in widespread brain regions in comparison to controls (54), with greatest effect sizes seen for the left cuneus and bilateral superior parietal and inferior temporal regions, in line with our previous findings in a smaller sample (5).
Within the 22q11DS group, no significant age*group (ASD+ vs. ASD-) interactions were observed for any brain structures that showed a main effect of group.
Comparison of categorical and dimensional ASD symptom measures in 22q11DS
Regression statistics for the best-fitting models are presented in Supplementary Table S6; AIC scores are presented in Supplementary Table S7. For most cognitive measures (processing speed, immediate and delayed face memory), as well as right amygdala volume, the best-fitting model was a dimensional one, including ADI-R communication-language score as a predictor (accounting for 19–46% of the variance in these measures). For delayed visuospatial memory, the best fitting model was also dimensional but included ADOS score as a predictor, accounting for 34% of the variance in performance. In contrast, for bilateral parahippocampal CT, the best fitting model emerged when ASD categorical diagnosis was entered as a predictor, accounting for 17 and 30% of the variance in CT, respectively. Based on the guidelines suggested by Burnham & Anderson (53), all findings that were best explained by a dimensional over a categorical model showed strong evidence for a dimensional measure as the better fit. Evidence was equally strong that a categorical measure of ASD is a better fit for right parahippocampal CT. Findings were similar for left parahippocampal CT, though evidence that the categorical measure of ASD is a better fit is less strong.
The above results remained stable after removing 22q11DS individuals with a psychotic disorder diagnosis (see Supplementary Tables S8–S9 and Supplemental Text for additional analyses of psychotic symptoms).
Discussion
This study was, to our knowledge, the first to investigate autism-associated intermediate cognitive and neuroanatomic phenotypes in 22q11DS, with the goal of determining whether these deficits are better characterized within a categorical or dimensional framework. These analyses revealed that, in comparison to individuals with 22q11DS without an ASD diagnosis (22q11DS-ASD−), individuals with 22q11DS and ASD (22q11DS-ASD+) exhibited impaired performance on facial memory and had slower processing speed, with the greatest magnitude of deficit observed for processing speed. Follow-up analyses revealed that deficits in these measures were better explained by a dimensional measure of ASD (ADI-R communication-language). When comparing neuroanatomic deficits in 22q11DS-ASD+ vs. 22q11DS-ASD−, we found that those with 22q11DS-ASD+ had reduced amygdala volumes and reduced cortical thickness (CT) in bilateral parahippocampal regions in comparison to 22q11DS-ASD−. When compared against multiple dimensional measures, a categorical diagnosis of ASD best explained the variability in parahippocampal CT. Our findings suggest that categorical and dimensional approaches may provide complementary information in understanding impairments associated with ASD symptomatology in 22q11DS.
Group differences in cognitive measures
Similar to previous investigations, we did not observed global cognitive impairments in 22q11DS-ASD+ vs. 22q11DS-ASD− (13; 21; 32). While the domains in which we observed selective deficits in 22q11DS-ASD+ (facial memory and processing speed) are not explicitly measures of social cognition, it is likely that these impairments contribute to social aspects of the ASD phenotype. For example, slower processing speed is associated with lower adaptive functioning in those with idiopathic ASD (60) and lower social functioning in other clinical populations (61; 62). Furthermore, impairments in processing speed and face memory have been previously identified in idiopathic ASD(37; 60; 63; 64).
Contrary to our hypothesis, social cognition measures (emotion recognition, ToM) did not differentiate between 22q11DS individuals with and without ASD. However, the 22q11DS group as a whole was impaired on social cognition measures, in line with previous literature (2; 4). These results suggest that the social impairments observed in 22q11DS-ASD+ may be driven by more basic neurocognitive functions (processing speed, face memory), whereas deficits in emotion recognition and perspective-taking (perhaps arising from different underlying etiologies) are more broadly characteristic of 22q11DS.
Neuroanatomic substrates of 22q-ASD+
Alterations in neuroanatomic measures revealed a distinct phenotype for ASD within 22q11DS. We found that 22q11DS-ASD+ individuals had reduced right amygdala volumes compared to both 22q11DS-ASD− and controls. The amygdala is implicated in multiple cognitive functions important for social interaction, including salience and novelty detection (65; 66), processing of emotional stimuli (67; 68) and judgments of when a stimulus changes in value or reward (69), functions found to be impaired in idiopathic ASD (70–72). While we did not find that emotion identification was differentially impaired in those with 22q11DS and ASD, future studies are warranted to examine other processes in which the amygdala is implicated (i.e. salience, novelty detection, and reward processing) in 22q11DS.
The only prior study to examine structural neuroimaging measures in relation to ASD diagnosis in 22q11DS found larger right amygdala volumes in 22q11DS-ASD+ compared to 22q11DS-ASD−(14). Although our studies converge in terms of region and laterality affected, our results point in the opposite direction (14). This discrepancy in directionality may be due to methodology differences [i.e., hand tracing (14) vs. semi-automated (current analysis)], the age ranges studied [ages 6–16 (14) vs. 6–25 years (current analysis)], or may also be related to the crucial role of neurodevelopment in ASD. Multiple studies have found increased amygdalar volumes in children with idiopathic ASD relative to controls (73–75), with no observed differences in older youth (76; 77) but reduced amygdalar volumes in adults with ASD (78; 79). While our analyses did not indicate differential effects of age on amygdalar volume in 22q11DS-ASD+, we were likely limited in our power to detect such differences. Thus, future larger scale longitudinal studies are needed to examine age-related neurodevelopmental changes in 22q11DS.
We also found decreased CT in bilateral parahippocampal regions in 22q11DS-ASD+. Altered volume and thickness of the parahippocampal region has also been found in individuals with idiopathic ASD (41; 80–82). Like many higher-order cortical regions, the parahippocampal cortex has been associated with a range of cognitive functions, with the two most prominent processes being episodic memory (83; 84) and visuospatial processing (85; 86). Aminoff et al (2013) suggested that the parahippocampus’ primary role is mediating contextual associations (87), which is crucial for successfully engaging in successful social behaviors. For example, comprehension of sarcasm, a situation in which understanding context is of paramount importance, has been associated with parahippocampal volume (88).
Intriguingly, contrary to our initial hypothesis, we did not observe differences in CT in brain regions involved in social interactions, such as the orbitofrontal cortex, in those with 22q11DS and ASD. However, these findings are consistent with the absence of a detectable behavioral difference between 22q11DS-ASD+ and 22q11DS-ASD− on social cognition measures. Interestingly, we previously found that increased CT in medial orbitofrontal cortex was associated with increased psychotic symptom severity (5); therefore, alterations in these regions in 22q11DS may be driven by other pathological conditions, not ASD. Future studies will need to examine to what extent neuroanatomic measures can distinguish between multiple psychiatric conditions in 22q11DS.
Categorical vs. dimensional approach
The recently proposed Research Domain Criteria (RDoC) project proposes that measures based on dimensions and observable behaviors may be more informative than current diagnostic systems about mechanisms underlying the development of psychiatric disorders (30; 31). We examined this hypothesis by comparing whether categorical or dimensional measures of autistic symptoms better accounted for the variability in neurocognitive and neuroanatomic patterns in 22q11DS. For the neurocognitive measures (processing speed, facial memory, visuospatial memory), a dimensional measure always explained the variability in the data better than the categorical measure, suggesting that dimensional measures are more sensitive to individual differences in neurocognition in 22q11DS.
Furthermore, individual differences in several measures (processing speed, facial memory, and right amygdala volume) were best explained by a dimensional measure of communication and language abilities (ADI-R communication-language score). These results suggest a direct link between a specific circuit-based behavioral dimension of autism and downstream cognitive processes, implying possible avenues for intervention. Also, reduced amygdala volumes were specifically associated with dimensionally measured communication and language impairments in 22q11DS-ASD+. Both human and non-human primate studies have found a significant link between amygdala volume and social network sizes (89; 90), providing preliminary evidence that a larger amygdala supports the increased demands that are required when one has more social interactions.
A number of factors may account for variability across studies in the percentage of 22q11DS individuals diagnosed with ASD , including differences in ascertainment approaches, intellectual functioning, and/or assessment tool choice (13–18). In line with the shift towards a dimensional approach to psychopathology (29; 31), it has also been suggested that multiple symptom domains better explain clinical phenotypes observed in 22q11DS, with social-cognitive deficits as one of these domains (91). As such, classification based on ASD symptoms only may be misleading, given that alterations in multiple, different biological pathways likely lead to similar clinical profiles. Though our findings need to be replicated, our results offer promising new information regarding sub-type identification in 22q11DS. In line with recent work on “biotypes” in schizophrenia and bipolar disorder (92), it will be important to continue to examine behavioral and brain-based measures across traditional diagnostic categories, in both 22q11DS and idiopathic ASD. Our work identifies relevant measures to be used in future studies.
While the RDoC approach has the potential to overcome many limitations of our current diagnostic nosology (29–31), one major challenge that arises when applying a dimensional approach is choosing appropriate statistical methods. The majority of statistical approaches commonly applied in psychiatry research were designed to test categorical differences between groups. Thus, development of novel methodological approaches is necessary to utilize RDoC to its fullest potential.
Limitations
There are several limitations to this study. Given our wide age range (6–26 years old), our sample size was likely not sufficiently powered to detect differential effects of age between 22q11DS-ASD+ and 22q11DS-ASD−. In addition, our effects may be primarily driven by specific age epochs. We attempted to control for this by co-varying for age in all analyses; however, we acknowledge that this design cannot address nonlinear effects of age across this age range, which likely encompasses distinct developmental stages. Furthermore, we were not powered to examine sex differences in the two 22q11DS groups; given the strong male bias in idiopathic ASD prevalence (93), this will be an important topic to pursue in the future. Notably, however, there is not a male bias for ASD in 22q11DS, or other neurogenetic syndromes highly penetrant for ASD(13). Finally, other automated amygdala parcellation schemes are more highly correlated with volumetric hand-tracing of the amygdala (left: 0.83, right: 0.79) than Freesurfer’s automatic amygdala parcellation, although the bivariate correlations between hand-tracing and the Freesurfer amygdala parcellation were still high (left: 0.56, right:0.56) (94). Thus, in future studies the use of multiple methods to determine amygdala volumes should be considered. Finally, our sample was cross-sectional; given consistent evidence that brain structural alterations in idiopathic ASD change across development (95), longitudinal studies of cognitive and neuroanatomic trajectories are warranted.
Future Directions
This study was an initial inquiry into whether dimensional or categorical measures of ASD best explain individual differences in 22q11DS. Our results suggest that a combination of dimensional and categorical variables may provide us with the most comprehensive understanding of ASD symptomatology in 22q11DS. Future studies examining the extent to which the impairments observed in 22q11DS-ASD+overlap with impairments in idiopathic ASD are warranted to confirm the generalizability of our findings to the broader ASD population. Studies that utilize this approach to investigate other spectra of psychopathology in 22q11DS are also warranted. Furthermore, continued study of a homogenous genetic population that presents with varying forms of dimensionally measured psychopathology offers a valuable framework for translational neuroscience investigations across species.
Supplementary Material
Acknowledgments
We thank the participants and their families for being a part of our research. We would also like to acknowledge the following funding sources: R01MH085953 Neurodevelopment and Psychosis in the 22q11.2 Deletion Syndrome and U01MH087626 Brain-Behavior and Genetic Studies of the 22q11DS (Carrie E. Bearden), T32NS048004 NINDS Training Grant (Maria Jalbrzikowski), and Heyler Research Award (Maria Jalbrzikowski).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Georgiades S, Szatmari P, Boyle M, Hanna S, Duku E, Zwaigenbaum L, et al. Investigating phenotypic heterogeneity in children with autism spectrum disorder: a factor mixture modeling approach. J Child Psychol Psychiatry. 2013;54:206–215. doi: 10.1111/j.1469-7610.2012.02588.x. [DOI] [PubMed] [Google Scholar]
- 2.Gur RE, Yi JJ, McDonald-McGinn DM, Tang SX, Calkins ME, Whinna D, et al. Neurocognitive development in 22q11.2 deletion syndrome: comparison with youth having developmental delay and medical comorbidities. Molecular Psychiatry. 2014 doi: 10.1038/mp.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeste SS, Geschwind DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat Rev Neurol. 2014;10:74–81. doi: 10.1038/nrneurol.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell LE, McCabe KL, Melville JL, Strutt PA, Schall U. Social cognition dysfunction in adolescents with 22q11.2 deletion syndrome (velo-cardio-facial syndrome): relationship with executive functioning and social competence/functioning. J Intellect Disabil Res. 2015;59:845–859. doi: 10.1111/jir.12183. [DOI] [PubMed] [Google Scholar]
- 5.Jalbrzikowski M, Jonas R, Senturk D, Patel A, Chow C, Green MF, Bearden CE. Structural abnormalities in cortical volume, thickness, and surface area in 22q11.2 microdeletion syndrome: Relationship with psychotic symptoms. Neuroimage Clin. 2013;3:405–415. doi: 10.1016/j.nicl.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012:1–6. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron. 2015;87:1215–1233. doi: 10.1016/j.neuron.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helsmoortel C, Vulto-van Silfhout AT, Coe BP, Vandeweyer G, Rooms L, van den Ende J, et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet. 2014;46:380–384. doi: 10.1038/ng.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krumm N, Turner TN, Baker C, Vives L, Mohajeri K, Witherspoon K, et al. Excess of rare, inherited truncating mutations in autism. Nat Genet. 2015;47:582–588. doi: 10.1038/ng.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JA, Penagarikano O, Belgard TG, Swarup V, Geschwind DH. The emerging picture of autism spectrum disorder: genetics and pathology. Annu Rev Pathol. 2015;10:111–144. doi: 10.1146/annurev-pathol-012414-040405. [DOI] [PubMed] [Google Scholar]
- 12.Richards C, Jones C, Groves L, Moss J, Oliver C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2:909–916. doi: 10.1016/S2215-0366(15)00376-4. [DOI] [PubMed] [Google Scholar]
- 13.Vorstman JAS, Morcus MEJ, Duijff SN, Klaassen PWJ, Heineman-de Boer JA, Beemer FA, et al. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45:1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- 14.Antshel KM, Aneja A, Strunge L, Peebles J, Fremont WP, Stallone K, et al. Autistic spectrum disorders in velo-cardio facial syndrome (22q11.2 deletion) J Autism Dev Disord. 2007;37:1776–1786. doi: 10.1007/s10803-006-0308-6. [DOI] [PubMed] [Google Scholar]
- 15.Niklasson L, Rasmussen P, Oskarsdóttir S, Gillberg C. Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Research in Developmental Disabilities. 2009;30:763–773. doi: 10.1016/j.ridd.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Niklasson L, Rasmussen P, Oskarsdottir S, Gillberg C. Neuropsychiatric disorders in the 22q11 deletion syndrome. Genet Med. 2001;3:79–84. doi: 10.1097/00125817-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Schneider M, Debbané M, Bassett AS, Chow EWC, Fung WLA, van den Bree MBM, et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the international consortium on brain and behavior in 22q11.2 deletion syndrome. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2013.13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angkustsiri K, Goodlin-Jones B, Deprey L, Brahmbhatt K, Harris S, Simon TJ. Social impairments in chromosome 22q11.2 deletion syndrome (22q11.2DS): autism spectrum disorder or a different endophenotype? J Autism Dev Disord. 2013;44:739–746. doi: 10.1007/s10803-013-1920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliez S. Autism in children with 22q11.2 deletion syndrome. J Am Acad Child Adolesc Psychiatry. 2007;46:433–434. doi: 10.1097/CHI.0b013e31802f5490. author reply 434-4. [DOI] [PubMed] [Google Scholar]
- 21.Kates WR, Antshel KM, Fremont WP, Shprintzen RJ, Strunge LA, Burnette CP, Higgins AM. Comparing phenotypes in patients with idiopathic autism to patients with velocardiofacial syndrome (22q11 DS) with and without autism. Am J Med Genet A. 2007;143A:2642–2650. doi: 10.1002/ajmg.a.32012. [DOI] [PubMed] [Google Scholar]
- 22.Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, et al. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry. 2006;163:294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- 23.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 24.Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:328–334. doi: 10.1002/ajmg.b.30810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundstrom S, Chang Z, Rastam M, Gillberg C, Larsson H, Anckarsater H, Lichtenstein P. Autism spectrum disorders and autistic like traits: similar etiology in the extreme end and the normal variation. Arch Gen Psychiatry. 2012;69:46–52. doi: 10.1001/archgenpsychiatry.2011.144. [DOI] [PubMed] [Google Scholar]
- 26.Eaves LC, Ho HH, Eaves DM. Subtypes of autism by cluster analysis. J Autism Dev Disord. 1994;24:3–22. doi: 10.1007/BF02172209. [DOI] [PubMed] [Google Scholar]
- 27.Sevin JA, Matson JL, Coe D, Love SR, Matese MJ, Benavidez DA. Empirically derived subtypes of pervasive developmental disorders: a cluster analytic study. J Autism Dev Disord. 1995;25:561–578. doi: 10.1007/BF02178188. [DOI] [PubMed] [Google Scholar]
- 28.Wiggins LD, Robins DL, Adamson LB, Bakeman R, Henrich CC. Support for a dimensional view of autism spectrum disorders in toddlers. J Autism Dev Disord. 2011;42:191–200. doi: 10.1007/s10803-011-1230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Insel TR. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on M ental Disorders. 2010:1–4. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 30.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14:29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuthbert BN. The RDoC framework: continuing commentary. World Psychiatry. 2014;13:196–197. doi: 10.1002/wps.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hidding E, Swaab H, De Sonneville LMJ, van Engeland H, Sijmens-Morcus MEJ, Klaassen PWJ, et al. Intellectual functioning in relation to autism and ADHD symptomatology in children and adolescents with 22q11.2 deletion syndrome. J Intellect Disabil Res. 2015;59:803–815. doi: 10.1111/jir.12187. [DOI] [PubMed] [Google Scholar]
- 33.Ho JS, Radoeva PD, Jalbrzikowski M, Chow C, Hopkins J, Tran W-C, et al. Deficits in Mental State Attributions in Individuals with 22q11.2 Deletion Syndrome (Velo-Cardio-Facial Syndrome) Autism Res. 2012;5:407–418. doi: 10.1002/aur.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Constantino JN, CP G. Social Responsiveness Scale (SRS) Los Angeles: Western Psychological Services; (n.d.) [Google Scholar]
- 35.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neuroscience & Biobehavioral Reviews. 2012;36:1060–1084. doi: 10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Weigelt S, Koldewyn K, Kanwisher N. Face recognition deficits in autism spectrum disorders are both domain specific and process specific. PLoS ONE. 2013;8:e74541. doi: 10.1371/journal.pone.0074541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- 39.Harms MB, Martin A, Wallace GL. Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychol Rev. 2010;20:290–322. doi: 10.1007/s11065-010-9138-6. [DOI] [PubMed] [Google Scholar]
- 40.Senju A. Atypical development of spontaneous social cognition in autism spectrum disorders. Brain Dev. 2013;35:96–101. doi: 10.1016/j.braindev.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Ecker C, Ginestet C, Feng Y, Johnston P, Lombardo MV, Lai M-C, et al. Brain Surface Anatomy in Adults With Autism. JAMA Psychiatry. 2013;70:59. doi: 10.1001/jamapsychiatry.2013.265. [DOI] [PubMed] [Google Scholar]
- 42.Dziobek I, Bahnemann M, Convit A, Heekeren HR. The role of the fusiform-amygdala system in the pathophysiology of autism. Arch Gen Psychiatry. 2010;67:397–405. doi: 10.1001/archgenpsychiatry.2010.31. [DOI] [PubMed] [Google Scholar]
- 43.Groen W, Teluij M, Buitelaar J, Tendolkar I. Amygdala and hippocampus enlargement during adolescence in autism. J Am Acad Child Adolesc Psychiatry. 2010;49:552–560. doi: 10.1016/j.jaac.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 44.Jalbrzikowski M, Carter C, Senturk D, Chow C, Hopkins JM, Green MF, et al. Schizophrenia Research. Schizophrenia Research. 2013;142:99–107. doi: 10.1016/j.schres.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lord C, Risi S, Lambrecht L, Cook EHJ, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 46.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 47.First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 48.First MB, Spitzer RL, Williams J. Structured clinical Interview for DSM-IV axis I disorders clinician version SCID-I. Developmental disorder addition. American Psychiatric Association; 2009. [Google Scholar]
- 49.Association AP. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, D.C: (n.d.) [Google Scholar]
- 50.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 51.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 52.Akaike H. A new look at the statistical model identification. Automatic Control. 1974 [Google Scholar]
- 53.Burnham KP, Anderson DR. Multimodel Inference understanding AIC and multimode. Sociological methods & research. 2004:261–303. [Google Scholar]
- 54.Eliez S, Barnea-Goraly N, Schmitt JE, Liu Y, Reiss AL. Increased basal ganglia volumes in velo-cardio-facial syndrome (deletion 22q11.2) Biol Psychiatry. 2002;52:68–70. doi: 10.1016/s0006-3223(02)01361-6. [DOI] [PubMed] [Google Scholar]
- 55.Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Catani M, et al. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006;129:1218–1228. doi: 10.1093/brain/awl066. [DOI] [PubMed] [Google Scholar]
- 56.Kates WR, Burnette CP, Bessette BA, Folley BS, Strunge L, Jabs EW, Pearlson GD. Frontal and caudate alterations in velocardiofacial syndrome (deletion at chromosome 22q11.2) Journal of Child Neurology. 2004;19:337–342. doi: 10.1177/088307380401900506. [DOI] [PubMed] [Google Scholar]
- 57.Gothelf D, Penniman L, Gu E, Eliez S, Reiss AL. Developmental trajectories of brain structure in adolescents with 22q11.2 deletion syndrome: a longitudinal study. Schizophrenia Research. 2007;96:72–81. doi: 10.1016/j.schres.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmitt JE, Vandekar S, Yi J, Calkins ME, Ruparel K, Roalf DR, et al. Aberrant Cortical Morphometry in the 22q11.2 Deletion Syndrome. Biol Psychiatry. 2015;78:135–143. doi: 10.1016/j.biopsych.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schaer M, Debbané M, Bach Cuadra M, Ottet M-C, Glaser B, Thiran J-P, Eliez S. Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): a cross-sectional and longitudinal study. Schizophrenia Research. 2009;115:182–190. doi: 10.1016/j.schres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 60.Hedvall A, Fernell E, Holm A, Asberg Johnels J, Gillberg C, Billstedt E. Autism, processing speed, and adaptive functioning in preschool children. ScientificWorldJournal. 2013;2013:158263. doi: 10.1155/2013/158263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carrión RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Impact of Neurocognition on Social and Role Functioning in Individuals at Clinical High Risk for Psychosis. Am J Psychiatry. 2011:168. doi: 10.1176/appi.ajp.2011.10081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bachman P, Niendam TA, Jalbrzikowski M, Park CY, Daley M, Cannon TD, Bearden CE. Processing speed and neurodevelopment in adolescent-onset psychosis: cognitive slowing predicts social function. J Abnorm Child Psychol. 2012;40:645–654. doi: 10.1007/s10802-011-9592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hauck M, Fein D, Maltby N, Waterhouse L, Feinstein C. Memory for faces in children with autism. Child Neuropsychology. 1998;4:187–198. [Google Scholar]
- 64.Fried R, Joshi G, Bhide P, Pope A, Galdo M, Koster A, et al. A study of the neuropsychological correlates in adults with high functioning autism spectrum disorders. Acta Neuropsychiatr. 2016:1–10. doi: 10.1017/neu.2016.12. [DOI] [PubMed] [Google Scholar]
- 65.Ewbank MP, Barnard PJ, Croucher CJ, Ramponi C, Calder AJ. The amygdala response to images with impact. Social Cognitive and Affective Neuroscience. 2009;4:127–133. doi: 10.1093/scan/nsn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blackford JU, Buckholtz JW, Avery SN, Zald DH. A unique role for the human amygdala in novelty detection. NeuroImage. 2010;50:1188–1193. doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 68.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 69.Roesch MR, Calu DJ, Esber GR, Schoenbaum G. Neural correlates of variations in event processing during learning in basolateral amygdala. J Neurosci. 2010;30:2464–2471. doi: 10.1523/JNEUROSCI.5781-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Res. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, et al. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70:869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rump KM, Giovannelli JL, Minshew NJ, Strauss MS. The development of emotion recognition in individuals with autism. Child Dev. 2009;80:1434–1447. doi: 10.1111/j.1467-8624.2009.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim JE, Lyoo IK, Estes AM, Renshaw PF, Shaw DW, Friedman SD, et al. Laterobasal amygdalar enlargement in 6- to 7-year-old children with autism spectrum disorder. Arch Gen Psychiatry. 2010;67:1187–1197. doi: 10.1001/archgenpsychiatry.2010.148. [DOI] [PubMed] [Google Scholar]
- 74.Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nordahl CW, Scholz R, Yang X, Buonocore MH, Simon T, Rogers S, Amaral DG. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: a longitudinal study. Arch Gen Psychiatry. 2012;69:53–61. doi: 10.1001/archgenpsychiatry.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haznedar MM, Buchsbaum MS, Wei TC, Hof PR, Cartwright C, Bienstock CA, Hollander E. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- 77.Palmen SJMC, Durston S, Nederveen H, van Engeland H. No evidence for preferential involvement of medial temporal lobe structures in high-functioning autism. Psychol Med. 2006;36:827–834. doi: 10.1017/S0033291706007215. [DOI] [PubMed] [Google Scholar]
- 78.Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- 79.Rojas DC, Smith JA, Benkers TL, Camou SL, Reite ML, Rogers SJ. Hippocampus and amygdala volumes in parents of children with autistic disorder. Am J Psychiatry. 2004;161:2038–2044. doi: 10.1176/appi.ajp.161.11.2038. [DOI] [PubMed] [Google Scholar]
- 80.Ke X, Hong S, Tang T, Zou B, Li H, Hang Y, et al. Voxel-based morphometry study on brain structure in children with high-functioning autism. NeuroReport. 2008;19:921–925. doi: 10.1097/WNR.0b013e328300edf3. [DOI] [PubMed] [Google Scholar]
- 81.Jiao Y, Chen R, Ke X, Chu K, Lu Z, Herskovits EH. Predictive models of autism spectrum disorder based on brain regional cortical thickness. NeuroImage. 2010;50:589–599. doi: 10.1016/j.neuroimage.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang X, Si T, Gong Q, Qiu L, Jia Z, Zhou M, et al. Brain gray matter alterations and associated demographic profiles in adults with autism spectrum disorder: A meta-analysis of voxel-based morphometry studies. Aust N Z J Psychiatry. 2016 doi: 10.1177/0004867415623858. [DOI] [PubMed] [Google Scholar]
- 83.Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diana RA, Yonelinas AP, Ranganath C. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. J Cogn Neurosci. 2010;22:1808–1818. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mullally SL, Maguire EA. A new role for the parahippocampal cortex in representing space. J Neurosci. 2011;31:7441–7449. doi: 10.1523/JNEUROSCI.0267-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park S, Brady TF, Greene MR, Oliva A. Disentangling scene content from spatial boundary: complementary roles for the parahippocampal place area and lateral occipital complex in representing real-world scenes. J Neurosci. 2011;31:1333–1340. doi: 10.1523/JNEUROSCI.3885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends in Cognitive Sciences. 2013;17:379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rankin KP, Salazar A, Gorno-Tempini ML, Sollberger M, Wilson SM, Pavlic D, et al. Detecting sarcasm from paralinguistic cues: anatomic and cognitive correlates in neurodegenerative disease. NeuroImage. 2009;47:2005–2015. doi: 10.1016/j.neuroimage.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14:163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aggleton JB, Barton RA. Primate evolution and the amygdala. In: Aggleton JB, editor. The Amygdala: A Functional Analysis. Oxford University Press; (n.d.) [Google Scholar]
- 91.Baker K, Vorstman JAS. Is there a core neuropsychiatric phenotype in 22q11.2 deletion syndrome? Current Opinion in Neurology. 2012;25:131–137. doi: 10.1097/WCO.0b013e328352dd58. [DOI] [PubMed] [Google Scholar]
- 92.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers. Am J Psychiatry. 2016;173:373–384. doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Current Opinion in Neurology. 2013;26:146–153. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hanson JL, Suh JW, Nacewicz BM, Sutterer MJ, Cayo AA, Stodola DE, et al. Robust Automated Amygdala Segmentation via Multi-Atlas Diffeomorphic Registration. Front Neurosci. 2012;6:166. doi: 10.3389/fnins.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zielinski BA, Prigge MBD, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, et al. Longitudinal changes in cortical thickness in autism and typical development. Brain. 2014;137:1799–1812. doi: 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.