Abstract
Background
Similarities between behavioral and substance addictions exist. However, direct neurobiological comparison between addictive disorders is rare. Determination of disorder-specificity (or lack thereof) of alterations within white-matter microstructures will advance understanding of the pathophysiology of addictions.
Methods
We compared white-matter microstructural features between individuals with gambling disorder (GD; n=38), cocaine-use disorder (CUD; n=38) and healthy comparison (HC; n=38) participants, as assessed using diffusion-weighted magnetic resonance imaging (dMRI). To provide a more precise estimate of diffusion within regions of complex architecture (e.g., cortico-limbic tracts), analyses were conducted using a crossing-fiber model incorporating local-orientation modeling (tbss_x). Anisotropy estimates for primary and secondary fiber orientations were compared using ANOVAs corrected for multiple comparisons across space using threshold-free cluster enhancement (pFWE<.05).
Results
A main effect of group on anisotropy of secondary fiber orientations within the left internal capsule, corona radiata, forceps major and posterior thalamic radiation, involving reduced anisotropy among GD and CUD participants in comparison to HC participants. No differences in anisotropy measures were found between GD and CUD individuals.
Conclusions
This is the first study to compare diffusion indices directly between behavioral and substance addictions and the largest dMRI study of GD. Our findings indicate similar white-matter microstructural alterations across addictions that cannot be attributed solely to exposure to drugs or alcohol and thus may be a vulnerability mechanism for addictive disorders.
Keywords: behavioral addiction, substance-use disorder, diffusion tensor imaging (DTI), impulsivity, alcohol-use disorder, pathological gambling
Introduction
Neuroimaging data suggest similarities between behavioral and substance addictions which may relate to disease etiology (1–9). However, direct comparisons between addiction subtypes are needed to confirm this hypothesis and to advance pathophysiological understanding of disorder subtypes via identification of unique versus shared neurobiological characteristics (10), as is consistent with ongoing transdiagnostic research efforts (11–13).
Alterations within white-matter (WM) tracts, as assessed using diffusion-weighted magnetic resonance imaging (dMRI), have been reported among individuals with gambling disorder (GD) (1–3) and with substance addictions including cocaine-use disorder (CUD) (6, 7, 14), consistent with theories of common mechanisms of addictions (3). In addition, recent studies indicate neural functional similarities between CUD and gambling disorder GD (4, 9). However, the extent to which neural structural alterations are truly shared across addictive disorders has not been assessed previously. Identification of common and distinct neural structural features of addiction subtypes may be used to guide development of novel intervention based on known brain features, particularly as dMRI measures have been shown to link to neurocognition and behavior and may be sensitive to behavioral and pharmacological interventions (3, 15–17). Therefore, this study tests the hypothesis of shared WM tissue alterations between behavioral and substance addictions, via comparison of dMRI measures from individuals with GD, individuals with CUD and healthy comparison (HC) individuals.
All previous dMRI studies of GD (1–3) and CUD (6, 7, 14, 15, 18–25) have utilized tensor-derived indices of diffusion such as fractional anisotropy (FA), mean diffusivity (MD), parallel (‘axial’) and perpendicular (‘radial’) diffusion. Interpretation of these measures is relatively straightforward within the context of anatomical structures with uniform fiber orientations (26), such as the corpus callosum (27). However, within the context of more complex WM architecture - i.e., when a given voxel contains fibers of different orientations, as in most areas of the brain (28) - interpretation of these measures is more ambiguous, making it difficult to make appropriate inferences with respect to underlying biology (26, 29–33). This limitation has been recognized for years (28, 33, 34), and it was recently estimated that up to 90% of WM voxels contain crossing fibers (28).
To address the ambiguity of tensor-derived measures, newer analytic approaches allow for estimation of diffusion for multiple fiber orientations per voxel and provide more precise estimates of diffusion within regions of complex fiber architecture (26, 30, 31, 35); for example, cortico-limbic and association tracts implicated in reward-processing and addiction vulnerability such as the corticospinal tracts, corona radiata and thalamic radiations (36, 37). Reduced FA within these and other regions of complex fiber organization have been reported among individuals with GD and CUD, albeit not consistently across studies; e.g., (2, 3, 14, 18, 19). Estimation of multiple diffusion orientations for regions of complex fiber architecture in addicted populations may therefore be helpful in reconciling existing data.
We compared dMRI measures between individuals with GD, individuals with CUD, and HC individuals using the crossing fiber model proposed by Behrens and colleagues (35). We hypothesized that individuals with GD and individuals with CUD would exhibit reductions in anisotropy measures when compared to HC participants but would not differ from one another on these measures. Specifically, we anticipated that, as in earlier studies of GD and CUD separately (1–3, 14, 19, 20, 24), individuals with addictions would have reduced anisotropy within WM tracts including the genu, splenium, internal capsules, thalamic radiations, corona radiata and superior longitudinal fasciculus.
Given effects of chronic alcohol-use on WM (38, 39), previous dMRI studies have either excluded GD participants with a history of alcohol-use disorders (AUDs) (3) or else tried to control for possible effects of AUD histories post-hoc (1, 2). These approaches have significant benefits (e.g., diagnostic specificity), yet - given the high co-occurrence rates of AUDs among individuals with GD (40, 41) may risk limiting generalizability of findings to real-world clinical populations. In order to allow for comparisons related to AUDs, we included equivalent numbers of GD and CUD individuals with and without histories of AUDs (approximately 50% per patient group). Based on previous findings of reduced FA within the corpus genu among individuals with AUDs (1, 38, 39, 42, 43), we anticipated that reductions in anisotropy measures within the genu would be greater among patients with a history of AUDs, versus those without.
Elevated rates of impulsivity have been reported among individuals with a range of addictions, and this has been hypothesized as a shared vulnerability marker across addictions and other disorders (44, 45). Thus, a third aim of this study was to assess the relationship between impulsivity and WM characteristics across diagnoses. We anticipated replicating previous findings of negative associations between frontal WM and self-reported impulsivity among individuals with GD and CUD within the anterior corpus callosum (e.g., genu) (1, 7).
Methods
Participants
GD, CUD and HC individuals who participated in dMRI protocols as part of ongoing fMRI research projects in conjunction with the Center for Excellence in Gambling Research, the Psychotherapy Development Center and the Clinical Neuroscience Research Unit at Yale University’s Department of Psychiatry were considered for inclusion in this study. To increase signal-to-noise for dMRI analyses (46), only participants with two complete dMRI acquisitions (acquired during the same scanning session) of good quality (based on visual inspection by two separate researchers) were considered for inclusion in this study. Other inclusion criteria included a DSM-IV diagnosis of pathological gambling (GD participants only) or cocaine-use disorder (CUD participants only), as assessed via structured clinical interview (SCI-PG (47) and SCID (48)). Exclusion criteria for GD and CUD participants included contraindication to MRI scanning (including head trauma) and a history of psychotic symptoms. Additional exclusion criteria for HC participants included any past or current Axis-I disorder with the exception of nicotine dependence and any current or past psychotropic medication exposure. Based on these criteria, 38 individuals with GD were selected for study inclusion. Thirty-eight HC and 38 CUD comparison participants were further selected for study inclusion based on their demographic and clinical information in relation to GD participants (details below and in Table 1), for a final sample of 114 individuals.
Table 1.
Demographic and clinical characteristics of participants (n=114)
| Gambling Disorder (n=38) | Cocaine-Use Disorder (n=38) | Healthy Comparison (n=38) | ||||
|---|---|---|---|---|---|---|
| mean (SD) | mean (SD) | mean (SD) | F | p | df | |
| Age (years) | 38.26 (11.76) | 42.45 (6.02) | 38.11 (10.90) | 2.36 | 0.10 | 111 |
| Education (years) | 13.11 (1.57) | 12.37 (1.10) | 14.34 (1.91) | 15.48 | <.001a | 111 |
| n (%) | n (%) | n (%) | x2 | p | df | |
| Gender (male) | 28 (73.7) | 25 (65.8) | 28 (73.7) | 0.77 | 0.68 | 2 |
| Tobacco smoker | 20 (52.6) | 30 (78.9) | 6 (15.8) | 30.61 | <.001 | 2 |
| Alcohol-use disorderb, c | 21 (55.3) | 19 (50.0) | -- | 0.21 | 0.65 | 1 |
| Cannabis-use disorderc | 10 (26.3) | 11 (28.9) | -- | 0.07 | 0.80 | 1 |
| Opioid-use disorderc | 3 (7.9) | 2 (7.4) | -- | 0.05 | 0.94 | 1 |
| Major Depressionc | 7 (18.4) | 9 (23.7) | -- | 0.32 | 0.57 | 1 |
| Anxiety Disordersc | 4 (10.5) | 2 (5.3) | -- | 0.72 | 0.40 | 1 |
Post-hoc comparisons indicated significantly fewer years of education among both gambling-disorder (GD) and cocaine-use-disorder (CUD) groups, in comparison to healthy comparison (HC) participants. Gambling disorder and cocaine-use disorder groups did not differ in years of education.
Two GD and 3 CUD participants met criteria for a current alcohol-use disorder; all other alcohol-use disorders were remitted.
Healthy comparison participants were excluded for Axis-I disorders except for possible nicotine dependence. Statistics shown for comparison of GD versus CUD groups.
HC and CUD groups were group-matched to GD participants as closely as possible for years of education, age and gender. However, years of education was significantly higher among HC participants in comparison to both patient groups. This variable was included as a regressor-of-no-interest in all subsequent analyses.
CUD participants were matched to GD participants as closely as possible for occurrences of major depression, anxiety disorders, and alcohol-, tobacco-, cannabis-, and opioid-use disorders. This matching was successful for all variables with the exception of tobacco-smoking which was significantly higher among CUD participants (χ2=5.85, df=2, p=0.02). Frequencies of other disorders were equivalent across patient groups (Table 1). Following tobacco-use disorder, AUDs were the most prevalent co-occurring disorders among patient groups (~50%); however, AUDs were remitted in all individuals with the exception of two GD and three CUD individuals. Three participants were taking psychotropic medication. One GD participant was taking sertraline, buspirone and hydroxyzine, one GD participant was taking sertraline, and one CUD participant was taking trazadone.
Data acquisition
Diffusion-weighted images were acquired between 2006 and 2014. Due to an equipment upgrade in 2009, data acquisition was performed using two 3T Seimens Trio scanners. Sixty individuals (22 GD, 21 CUD, 17 HC) were scanned on one system and 54 individuals (16 GD, 17 CUD, 21 HC) were scanned on the other, and these frequencies did not differ between participant groups (χ2=1.48, df=2, p=0.48). Forty contiguous slices parallel to the AC-PC line were acquired with the following parameters: TR = 7400 ms; TE = 115; B values = 0, 1000s/mm2; bandwidth = 1396 Hz/px; directions = 32 [+0]; slice thickness=3.0mm; averages = 2. dMRI data from five GD participants were included in a previous publication (1).
Image analysis
Pre-processing and diffusion estimates
dMRI data were analyzed in FSL using recommended tract-based spatial statistics (TBSS) procedures (26, 30, 49): dMRI acquisitions (two per participant) were corrected for gradient coil eddy currents and head movement using affine registration (46, 49). These acquisitions were averaged prior to brain extraction, fitting of the diffusion tensor and calculation of FA using dti_fit. Multiple diffusion estimates (one for each fiber orientation) per voxel were obtained using a partial volume model, as operationalized in FSL’s Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques for Crossing Fibers (BEDPOSTX) (26, 35). This method uses Markov Chain Monte Carlo sampling to estimate probability distributions of diffusion parameters and provides voxelwise modeling of multiple fiber orientations in the form of partial volume estimates (PVEs; one per orientation) (26, 35, 46). PVEs corresponding to the primary and secondary fiber orientations (PVE1 and PVE2, respectively) for each voxel were incorporated in TBSS analyses using tbss_x (26). This included alignment of FA images to a common space (FMRIB58_FA) using nonlinear registration (FNIRT) prior to affine transformation to MNI152 space. Standard space FA images were concatenated, averaged and thinned to create the mean FA skeleton (49). Individual participant aligned FA, PVE1 and PVE2 data were projected onto the mean skeleton for voxelwise statistical analyses.
ROI analysis of partial volume estimates
The corpus genu and corticospinal tracts were selected as a priori regions-of-interest (ROIs) to assess the effect of the partial volume model on anisotropy estimates within regions characterized by relatively uniform fiber orientations versus those characterized by crossing fibers, respectively (27, 50). Details of ROIs are provided in the Supplemental Materials.
Between-group comparisons
One-way analyses of variance (ANOVAs) were conducted to compare GD, CUD and HC participants on anisotropy measures (FA, PVE1, PVE2). Separate t-tests were then used for group-wise comparisons (GD vs. HC; CUD vs. HC; GD vs. CUD) of anisotropy measures identified as significantly different across the three groups. All statistical models included years-of-education and scanner as variables of no interest and were conducted using FSL’s ‘randomise’ with 5000 permutations. Resultant statistical maps were family-wise-error (FWE) corrected for multiple comparisons using FSL’s threshold-free-cluster-enhancement (TFCE) and considered significant at α=.05.
Analyses related to other clinical variables
In order to test our second and third hypotheses, diffusion measures for the corpus genu ROI were entered into SPSS for analyses related to AUDs and impulsivity (further details below). Values for clusters identified as significantly different across groups were also extracted for post-hoc analyses related to these variables. Additional post-hoc analyses were conducted to determine effects of tobacco-use and other drug-use on diffusion measures.
AUDs
Post-hoc analyses related to AUDs were conducted as follows. First, within-group GLMs (GDAUD- versus GDAUD+; CUDAUD- versus CUDAUD+) of diffusion indices were conducted to determine the effects of AUDs within these groups. Next, between-group comparisons excluding all individuals with an AUD history were conducted to determine whether between-group findings remained significant after excluding these individuals.
Impulsivity
Self-reported impulsivity was assessed using the Barratt Impulsiveness Scale (BIS-11) (51). Correlational analyses were conducted in SPSS using Pearson’s r.
Results
Comparison of PVEs in regions of uniform versus crossing fibers
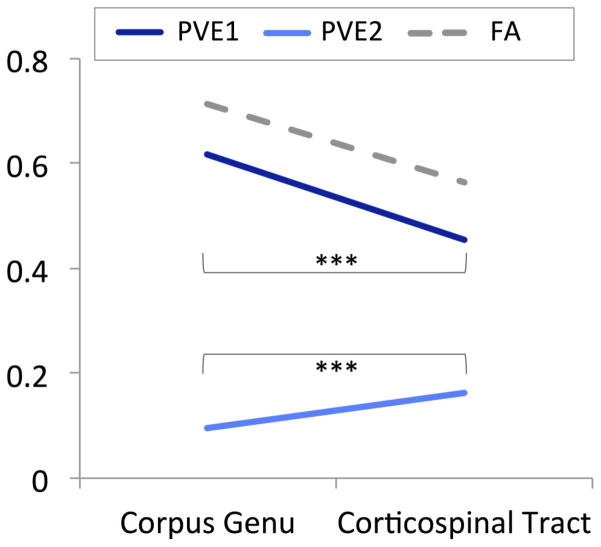
As shown in Figure 1, PVEs for primary fibers were significantly higher within a region of uniform fiber orientations (corpus genu) than within a region of crossing fiber orientations (corticospinal tract; t=41.54(113), p<.001), whereas the opposite pattern was observed for secondary fiber PVEs (t(113)=−20.54, p<.001).
Figure 1. Partial volume estimates (PVEs) for primary and secondary fibers in the corpus genu and corticospinal tract.
Figure one shows partial volume estimates (PVEs) for a region of uniform fiber orientations (corpus genu) and for a region associated with crossing fibers (corticospinal tract). Across all participants (n=114), PVEs for primary fibers (PVE 1) were decreased and PVEs for secondary fibers (PVE 2) were increased in the corticospinal tract in comparison to the corpus genu. For reference, fractional anisotropy (FA) values are also shown. ***p<.001
Voxelwise group comparisons
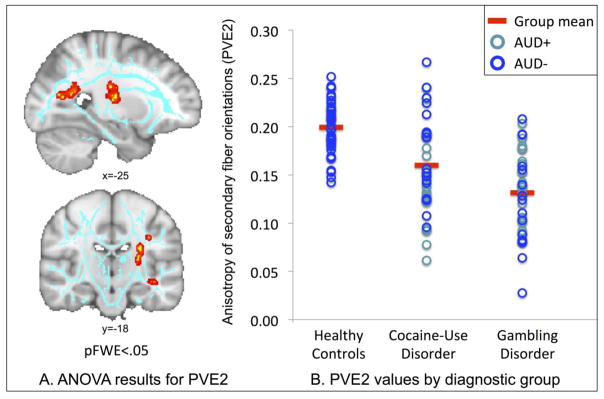
There was no main effect of group on FA or on primary fiber PVEs, as assessed using voxelwise ANOVAs corrected for multiple comparisons across space (pFWE<.05; for reference, uncorrected results for FA are shown in Supplemental Figure S1). There was a significant main effect of group on secondary fiber PVEs within two clusters involving decreased anisotropy among GD and CUD individuals in comparison to HC participants (Figure 2, Table 2). The first cluster included the left forceps major and extended laterally into regions including the posterior thalamic radiations and posterior corona radiata. The second cluster primarily included the posterior limb of the left internal capsule and extended into the superior corona radiata. These findings remained significant after controlling for tobacco-smoking and other (non-alcohol) substance use (details in Supplement).
Figure 2. ANOVA results for secondary fiber partial volume estimates (PVE2).
Panel A shows main effect of diagnostic group on PVE2 values within the forceps major and internal capsule. The mean FA skeleton is shown in light blue. Significant clusters (pFWE<.05) were ‘thickened’ using tbss_fill and for display purposes and are shown in red-yellow. Images shown in radiological convention (left=right). Panel B shows individual partial volume estimates for secondary fibers (PVE2) within the forceps major cluster. For reference, different markers are used to indicate patients with and without lifetime alcohol-use disorders (AUDs), with light blue indicating patients positive for AUDs (AUD+) and dark blue indicating patients negative for AUDs (AUD−).
Table 2.
Tract-based spatial statistics results for anisotropy of secondary fibers (PVE2)
| Main Effect of Diagnostic Group (ANOVA) | k | x | y | z | F value | pFWE |
| Forceps major/L posterior thalamic radiation (including optic radiation)/L posterior corona radiata | 321 | −28 | −62 | 18 | 9.8 | 0.018 |
| L internal capsule (posterior limb)/L external capsule/L superior corona radiata | 163 | −27 | −19 | 23 | 11.6 | 0.023 |
| Between-Group Comparisons (t-tests) | k | x | y | z | t-value | pFWE |
| Gambling Disorder < Healthy Comparison | ||||||
| Forceps major/L & R corona radiata (anterior, posterior & superior)/L & R internal capsule (anterior & posterior limbs; retrolenticular parts)/L & R superior longitudinal fasciculus/L posterior thalamic radiation (including optic radiation)/L & R cerebral peduncle/L & R external capsule | 31251 | −24 | −15 | 9 | 4.71 | 0.003 |
| R superior longitudinal fasciculus | 107 | 49 | −33 | 40 | 2.73 | 0.047 |
| Cocaine-Use Disorder < Healthy Comparison | ||||||
| L superior longitudinal fasciculus/L corona radiata (anterior & superior)/L sagittal stratum (include inferior longitidinal fasciculus and inferior fronto-occipital fasciculus)/L internal capsule (anterior & posterior limbs; retrolenticular part)/L external capsule/L cerebral peduncle/L posterior thalamic radiation (including optic radiation) | 7248 | −39 | −22 | −7 | 4.49 | 0.021 |
| Forceps major/R internal capsule (retrolenticular part)/R posterior corona radiata/R posterior thalamic radiation (including optic radiation) | 1956 | 50 | −10 | −5 | 4.25 | 0.036 |
| R superior longitudinal fasciculus/R corona radiata (anterior & superior)/R internal capsule (anterior limb) | 1147 | 42 | 6 | 19 | 4.09 | 0.039 |
| R anterior corona radiata | 613 | 38 | 29 | 13 | 4.26 | 0.033 |
| R internal capsule (anterior limb) | 166 | 12 | 10 | 1 | 4.47 | 0.040 |
Clusters significant at pFWE<.05, k>100. Anatomical labels derived from the JHU ICBM-DTI-81 White-Matter and JHU White-Matter Tractography Atlas Labels in FSL. There were no significant differences in PVE2-values between GD and CUD participants.
In order to further understand the nature of the identified main effect, group-wise comparisons of secondary PVEs across the mean skeleton were conducted (Table 2, Supplemental Figure S2). These analyses confirmed findings from the ANOVA of decreased anisotropy of secondary fibers within regions including the posterior thalamic radiations, corona radiata, forceps major and internal and external capsules among both GD and CUD groups when compared to HC participants. The analyses further indicated reductions among GD and CUD versus HC groups within regions including the superior longitudinal fasciculus and cerebral peduncles. No significant differences in anisotropy measures were found between GD and CUD groups.
Relationship to AUD histories
Following exclusion of GD and CUD patients with a history of AUDs (n=40), between-group differences in secondary fiber PVEs within the identified clusters remained significant (internal capsule: F(2,69)=14.72, p<.001; forceps major: F(2,69)=19.23, p<.001). No significant effect of AUDs on secondary fiber PVEs within either cluster were found among GD (internal capsule: F(1,35)=0.30, p0.59; forceps major: F(1,35)=0.30, p=0.59) or CUD (internal capsule: F(1,35)=0.22, p=0.64; forceps major: F(1,35)=0.30, p=0.87) participants.
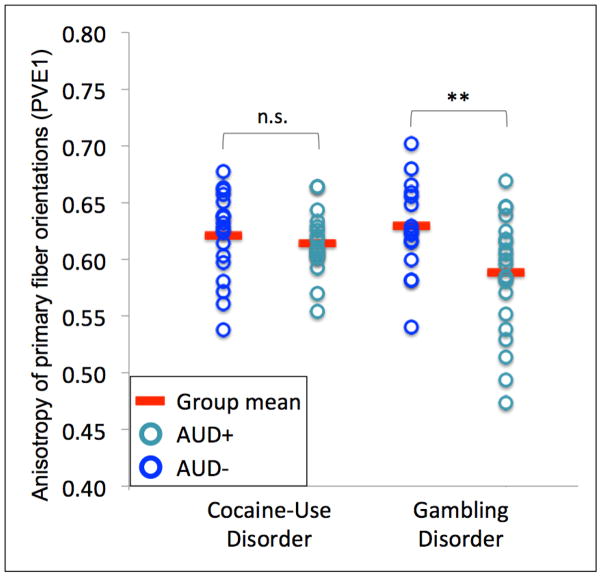
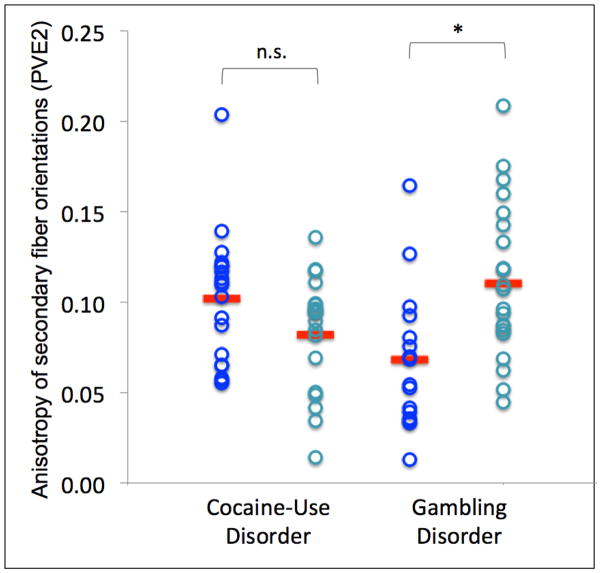
Within the corpus genu ROI, primary fiber PVEs were decreased (F(1,35)=7.52, p=0.01) and secondary fiber PVEs were increased (F(1,35)=5.93, p=0.02) among GD participants with a history of AUDs in comparison to those without (Figure 3). There were no significant differences in PVEs between CUD participants with and without AUDs (primary fibers: F(1,35)=0.41, p=0.53; secondary fibers: F(1,35)=0.07, p=0.80; Figure 3).
Figure 3. Anisotropy of primary (PVE1) and secondary (PVE2) fibers for cocaine-use disorder (CUD) and gambling disorder (GD) patients with and without lifetime alcohol-use disorders (AUDs).
Figure 3 shows individual partial volume estimates for primary fibers (PVE1; left) and secondary fibers (PVE2; right) within the corpus genu. No differences in anisotropy were found among CUD patients with and without lifetime AUDs. Primary fiber PVEs were decreased and secondary fiber PVEs were increased among GD patients with a history of AUDs in comparison to those without.
AUD+ = lifetime alcohol-use-disorder; AUD− = no lifetime alcohol-use-disorder; n.s.=not significant; **p≤.01; *p<.05
Relationship to impulsivity
There were no significant associations between FA or PVEs within the corpus genu and BIS-11 scores within any of the three participant groups or across the sample as a whole. Across all participants, secondary fiber PVEs within the internal capsule and forceps major clusters were negatively associated with BIS-11 scores (r(1,110)=−0.35, p<.001; r(1,110)=−0.44, p<.001). However, these associations were not significant when assessed within diagnostic groups separately (i.e., no significant association between BIS-11 scores and anisotropy measures among CUD, GD or HC groups).
Discussion
dMRI data from 114 individuals were analyzed to identify shared versus unique characteristics of GD and CUD. Given recent advancements in tractography algorithms and local-orientation modeling (30), analyses were conducted using tbss_x (26, 35). This method provides diffusion estimates of primary and secondary fiber orientations in the form of PVEs to ensure that diffusion measurements correspond to the same fiber populations across individuals (26). As anticipated, and consistent with known anatomy (26, 27, 50, 52), secondary PVEs were significantly lower within regions of uniform fiber orientations (genu) than within those characterized by crossing fibers (corticospinal tract). Consistent with our primary hypothesis, individuals with GD and CUD had reduced anisotropy within cortico-limbic tracts when compared to HC participants, but did not differ from one another on anisotropy within these regions. Notably, these alterations were observed within regions of complex WM architecture (internal capsule, forceps major, thalamic radiation) (50, 52) and were found for secondary (non-dominant) and not primary fiber orientations.
Areas identified as differentiating GD and CUD from HC groups included portions of cortico-striatal and parietal-occipital tracts previously implicated in reward-processing and addiction vulnerability; e.g., the internal capsule, corona radiata, and forceps major (36, 37, 53–55). The internal capsule separates the thalamus and caudate from the putamen and runs adjacent to the ventral striatum (52, 56) and has been implicated previously in GD (2) as well as in other candidate behavioral addictions; e.g., internet gaming disorder (57, 58). FA within this region has been positively associated with reward-related nucleus accumbens activity in healthy adults (53). Thus, it is possible that reduced internal capsule anisotropy may be a neural structural mechanism for blunted ventral striatal activity previously reported in both GD and CUD populations(4, 59, 60), or for alterations in ventral striatal functional connectivity recently reported in both GD and CUD individuals (9).
The forceps major crosses the splenium of the corpus callosum to form interhemispheric parietal-occipital connections (61). Recent multimodal data implicate the forceps major in default-mode and dorsal-attention network alterations among individuals with attention-deficit/hyperactivity disorder (62). Thus, altered anisotropy in this region may relate to alterations in these networks. However, multi-modal, transdiagnostic research is needed to test these hypotheses as well as to determine whether associations between dMRI and fMRI measures are similar between behavioral and substance addictions.
Contrary to expectations, anisotropy estimates (FA, PVE1, PVE2) for the corpus genu were not different across participant groups. Previous studies have yielded somewhat equivocal findings for this region, with decreases in FA reported in some (1, 2, 7, 25) but not all (3, 18) prior studies of GD and CUD. In comparison, reduced FA within the genu among individuals with AUDs has been relatively consistently reported; reviewed in (42). Consistent with this, PVEs for primary fibers were reduced among GD patients who had a history of AUDs, in comparison to those without. Somewhat surprisingly, secondary PVEs within this region were increased among GD participants (although these values were still low, as would be anticipated in an area of mostly uniform fiber orientations) (26). The genu is primarily comprised of relatively uniformly oriented, densely packed fibers (63, 64); thus, reductions in primary fiber anisotropy in this region likely reflect reduced fiber density (e.g., as opposed to changes in myelination) (63). The meaning of increased secondary fiber PVEs within the genu remains to be determined; however, one hypothesis is that this may relate to aberrant compensatory development of non-dominant fibers (65).
When examined separately within diagnostic groups, self-reported impulsivity (BIS-11 total score) was not associated with anisotropy measures (FA, PVE1, PVE2). After combining diagnostic groups, correlational analyses indicated a negative association between anisotropy within the internal capsule and forceps major clusters across all participants. This finding is consistent with dimensional and transdiagnostic concepts of impulsivity (45), and of psychiatry more generally (11, 66), but does not support the hypothesis that microstructural WM characteristics are related to individual variability in impulsivity within GD or CUD diagnoses per se.
No differences in internal capsule or forceps major anisotropy were observed between GD and CUD groups, suggesting that alterations in these regions may be a common feature of behavioral and substance addictions. Consistent with this notion, GD and CUD participants with and without lifetime AUDs did not differ in anisotropy in these regions, nor did GD patients with and without histories of substance abuse or dependence. These data suggest that reduced secondary fiber anisotropy within striatal and parietal-occipital regions is a shared feature of addiction subtypes possibly related to disease etiology. Further research (e.g., multimodal imaging) is needed to determine the precise functional significance of these findings. Given that behavioral and pharmacological interventions have been linked to changes in dMRI measures (16, 17), further studies might investigate how treatments and treatment outcomes relate to WM-pathway differences among individuals with addictions. However, as only three of the participants in this study were currently receiving medications, we were not able to explore medication-by-group effects. We are also unable to exclude possible influences of prior behavioral treatments on WM characteristics in this sample.
Strengths, limitations and conclusions
To our knowledge, this is the largest dMRI study of a behavioral addiction (1–3) and the first to directly compare dMRI measures between substance and behavioral addictions. Further strengths of this study include the use of an optimized crossing fiber model, allowing for direct comparison of the same fiber populations across individuals (26). These strengths should nonetheless be considered within the context of some limitations. Our groups were not well matched for years of education or tobacco-smoking. Although we controlled statistically for these variables, we cannot exclude the possibility that some findings may relate to between-group differences on these measures. We did not have a biological measure of drug use at the time of scanning for all participants; thus, we cannot exclude the possibility that acute cocaine intoxication may have influenced our study findings. To our knowledge, no prior studies have assessed the effects of acute cocaine on dMRI measures, and this will be an important area for further research. A further limitation of this study is the absence of a common measure of addiction severity which would have allowed us to explore relationships between neuroimaging measures and illness severity, as has been done in functional MRI studies; e.g., (9, 67).
This is nonetheless the first attempt to identify common WM-related features between behavioral and substance addictions. Findings of shared but not unique factors between individuals with GD and CUD support the hypothesis of a common disease etiology across addictive disorders. However, large-scale longitudinal research studies (e.g., NIDA’s Adolescent Brain Cognitive Development (ABCD) initiative) are needed to directly test this hypothesis.
Supplementary Material
Acknowledgments
This study was supported by R01 DA019039, R01 DA020908, R01 AA017539, P20 DA027844, P50 DA09241, T32 DA022975, T32 DA007238, the National Center for Responsible Gaming and CASAColumbia. The views presented in the manuscript are not necessarily those of the funding agencies who did not have input into the content of the manuscript outside of funding the proposed research.
Footnotes
Monica Solornzano, Stephen Healy, Scott Bullock and Alex Goggletino assisted with data collection and literature review.
Financial disclosures: Dr. Carroll has received grant support from NIH and is a member of CBT4CBT LLC, which makes CBT4CBT available to qualified clinical providers and organizations on a commercial basis. Dr. Carroll works with Yale University to manage any potential conflicts of interest. Dr. Potenza has: consulted for and advised Somaxon, Boehringer Ingelheim, Lundbeck, Ironwood, Shire, INSYS and RiverMend Health; received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories, Ortho-McNeil, Oy-Control/Biotie, Glaxo-SmithKline, Pfizer and Psyadon pharmaceuticals; participated in surveys, mailings, or telephone consultations related to drug addiction, impulse control disorders or other health topics; consulted for law offices and the federal public defender’s office in issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; given academic lectures in grand rounds, CME events and other clinical/scientific venues; and generated books or chapters for publishers of mental health texts. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yip SW, Lacadie C, Xu J, Worhunsky P, Fulbright R, Constable RT, Potenza MN. Reduced genual corpus callosal white matter integrity in pathological gambling and its relationship to alcohol abuse or dependence. World J Biol Psychiatry. 2013;14(2):198–38. doi: 10.3109/15622975.2011.568068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joutsa J, Saunavaara J, Parkkola R, Niemelä S, Kaasinen V. Extensive abnormality of brain white matter integrity in pathological gambling. Psychiatry Res Neuroimaging. 2011;194(3):340–346. doi: 10.1016/j.pscychresns.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Mohammadi B, Hammer A, Miedl SF, Wiswede D, Marco-Pallares J, Herrmann M, Munte TF. Intertemporal choice behavior is constrained by brain structure in healthy participants and pathological gamblers. Brain structure & function. 2015 doi: 10.1007/s00429-015-1093-9. [DOI] [PubMed] [Google Scholar]
- 4.Worhunsky PD, Malison RT, Rogers RD, Potenza MN. Altered neural correlates of reward and loss processing during simulated slot-machine fMRI in pathological gambling and cocaine dependence. Drug Alcohol Depend. 2014;145:77–86. doi: 10.1016/j.drugalcdep.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balodis IM, Potenza MN. Anticipatory Reward Processing in Addicted Populations: A Focus on the Monetary Incentive Delay Task. Biol Psychiatry. 2015;77(5):434–444. doi: 10.1016/j.biopsych.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim K, Choi SJ, Pomara N, Wolkin A, Rotrossen JP. Reduced frontal white matter integrity in cocaine dependence: A controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- 7.Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: Diffusion tensor imaging. Neuropsychopharmacol. 2005;30(3):610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- 8.Grant JE, Odlaug BL, Chamberlain SR. Reduced cortical thickness in gambling disorder: a morphometric MRI study. Eur Arch Psychiatry Clin Neurosci. 2015;265(8):655–61. doi: 10.1007/s00406-015-0592-2. [DOI] [PubMed] [Google Scholar]
- 9.Contreras-Rodriguez O, Albein-Urios N, Vilar-Lopez R, Perales JC, Martinez-Gonzalez JM, Fernandez-Serrano MJ, et al. Increased corticolimbic connectivity in cocaine dependence versus pathological gambling is associated with drug severity and emotion-related impulsivity. Addict Biol. 2015 doi: 10.1111/adb.12242. [DOI] [PubMed] [Google Scholar]
- 10.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA psychiatry. 2015;72(4):305–15. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 12.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171(4):395–7. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 13.Insel TR, Wang PS. Rethinking mental illness. JAMA. 2010;303(19):1970–1971. doi: 10.1001/jama.2010.555. [DOI] [PubMed] [Google Scholar]
- 14.Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend. 2011;114(2–3):159–68. doi: 10.1016/j.drugalcdep.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, EED, FWP, KMC, BJR, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacol. 2010;35(7):1541–1549. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci. 2009;1169:385–94. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harsan LA, Steibel J, Zaremba A, Agin A, Sapin R, Poulet P, et al. Recovery from chronic demyelination by thyroid hormone therapy: myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. J Neurosci. 2008;28(52):14189–201. doi: 10.1523/JNEUROSCI.4453-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, et al. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69(7):684–92. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA, et al. Diffusion Tensor Imaging and Decision Making in Cocaine Dependence. PLoS ONE. 2010;5(7):e11591. doi: 10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viswanath H, Velasquez KM, Savjani R, Molfese DL, Curtis K, Molfese PJ, et al. Interhemispheric insular and inferior frontal connectivity are associated with substance abuse in a psychiatric population. Neuropharmacology. 2015;92:63–8. doi: 10.1016/j.neuropharm.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Ma L, Steinberg JL, Keyser-Marcus L, Ramesh D, Narayana PA, Merchant RE, et al. Altered white matter in cocaine-dependent subjects with traumatic brain injury: A diffusion tensor imaging study. Drug Alcohol Depend. 2015;151:128–34. doi: 10.1016/j.drugalcdep.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero MJ, Asensio S, Palau C, Sanchez A, Romero FJ. Cocaine addiction: diffusion tensor imaging study of the inferior frontal and anterior cingulate white matter. Psychiatry Res. 2010;181(1):57–63. doi: 10.1016/j.pscychresns.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Ma L, Hasan KM, Steinberg JL, Narayana PA, Lane SD, Zuniga EA, et al. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend. 2009;104(3):262–7. doi: 10.1016/j.drugalcdep.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim K, Wozniak J, Mueller B, Franc D, Specker S, Rodriguez C, et al. Brain macrostructural and miscrostructural abnormalities in cocaine dependence. Drug & Alcohol Dependence. 2008;92(1–3):164–72. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Valdes I, Lai LY, et al. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Pyschiatry Res Neuroimaging. 2007;154:253–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Jbabdi S, Behrens TE, Smith SM. Crossing fibres in tract-based spatial statistics. Neuroimage. 2010;49(1):249–56. doi: 10.1016/j.neuroimage.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 27.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44(4):625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 28.Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp. 2013;34(11):2747–66. doi: 10.1002/hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 30.Reveley C, Seth AK, Pierpaoli C, Silva AC, Yu D, Saunders RC, et al. Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proc Natl Acad Sci U S A. 2015;112(21):E2820–8. doi: 10.1073/pnas.1418198112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumgartner T, Nash K, Hill C, Knoch D. Neuroanatomy of intergroup bias: A white matter microstructure study of individual differences. NeuroImage. 2015;122:345–354. doi: 10.1016/j.neuroimage.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–54. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 33.Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61(5):1255–60. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- 34.Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL. Analysis of partial volume effects in diffusion-tensor MRI. Magn Reson Med. 2001;45(5):770–80. doi: 10.1002/mrm.1105. [DOI] [PubMed] [Google Scholar]
- 35.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White Matter Integrity Predicts Delay Discounting Behavior in 9- to 23-Year-Olds: A Diffusion Tensor Imaging Study. Journal of Cognitive Neuroscience. 2009;21(7):1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobus J, Thayer RE, Trim RS, Bava S, Frank LR, Tapert SF. White matter integrity, substance use, and risk taking in adolescence. Psychol Addict Behav. 2013;27(2):431–42. doi: 10.1037/a0028235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfefferbaum A, Sullivan E. Microstructural but Not Macrostructural Disruption of White Matter in Women with Chronic Alcoholism. NeuroImage. 2002;15:708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- 39.Pfefferbaum A, Sullivan EV. Disruption of Brain White Matter Microstructure by Excessive Intracellular and Extracellular Fluid in Alcoholism: Evidence from Diffusion Tensor Imaging. Neuropsychopharmacol. 2004;30(2):423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- 40.Lorains FK, Cowlishaw S, Thomas SA. Prevalence of comorbid disorders in problem and pathological gambling: systematic review and meta-analysis of population surveys. Addiction. 2011;106(3):490–8. doi: 10.1111/j.1360-0443.2010.03300.x. [DOI] [PubMed] [Google Scholar]
- 41.Petry NM, Stinson FS, Grant BF. Co-morbidity of DSM-IV pathological gambling and other psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005;66:564–574. doi: 10.4088/jcp.v66n0504. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan EV, Harris RA, Pfefferbaum A. Alcohol’s effects on brain and behavior. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2010;33(1–2):127–43. [PMC free article] [PubMed] [Google Scholar]
- 43.Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In Vivo Detection and Functional Correlates of White Matter Microstructural Disruption in Chronic Alcoholism. Alcoholism: Clinical & Experimental Research. 2000;24(8):1214–1221. [PubMed] [Google Scholar]
- 44.Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience and biobehavioral reviews. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci. 2012;16(1):81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU, et al. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. Neuroimage. 2011;55(3):880–90. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grant J, Steinberg MA, Kim SW, Rounsaville BJ, Potenza MN. Preliminary validity and reliability testing of a structured clinical interview for pathological gambling (SCI-PG) Psychiatry Res. 2004;128:79–88. doi: 10.1016/j.psychres.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 48.First M, Gibbon M, Spitzer R, Williams J. User’s Guide to the structured clinical interview for DSM-IV axis disorders (SCID-I, Version 2.0) 1995 [Google Scholar]
- 49.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 50.Lee D-H, Park JW, Park S-H, Hong C. Have You Ever Seen the Impact of Crossing Fiber in DTI?: Demonstration of the Corticospinal Tract Pathway. PLoS ONE. 2015;10(7):e0112045. doi: 10.1371/journal.pone.0112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patton J, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 52.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford University Press; Cary, NC, USA: 2009. [Google Scholar]
- 53.Koch K, Wagner G, Schachtzabel C, Schultz CC, Gullmar D, Reichenbach JR, et al. Association between white matter fiber structure and reward-related reactivity of the ventral striatum. Hum Brain Mapp. 2014;35(4):1469–76. doi: 10.1002/hbm.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monnig MA, Thayer RE, Caprihan A, Claus ED, Yeo RA, Calhoun VD, Hutchison KE. White matter integrity is associated with alcohol cue reactivity in heavy drinkers. Brain and behavior. 2014;4(2):158–70. doi: 10.1002/brb3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu J, Kober H, Carroll KM, Rounsaville BJ, Pearlson GD, Potenza MN. White matter integrity and behavioral activation in healthy subjects. Hum Brain Mapp. 2012;33(4):994–1002. doi: 10.1002/hbm.21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greenberg BD, Gabriels LA, Malone DA, Jr, Rezai AR, Friehs GM, Okun MS, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15(1):64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinstein A, Lejoyeux M. New developments on the neurobiological and pharmaco-genetic mechanisms underlying internet and videogame addiction. Am J Addict. 2015;24(2):117–25. doi: 10.1111/ajad.12110. [DOI] [PubMed] [Google Scholar]
- 58.Lin F, Zhou Y, Du Y, Qin L, Zhao Z, Xu J, Lei H. Abnormal white matter integrity in adolescents with internet addiction disorder: a tract-based spatial statistics study. PLoS One. 2012;7(1):e30253. doi: 10.1371/journal.pone.0030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Potenza MN. Diminished Frontostriatal Activity During Processing of Monetary Rewards and Losses in Pathological Gambling. Biol Psychiatry. 2012;71(8):749–757. doi: 10.1016/j.biopsych.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bustamante JC, Barros-Loscertales A, Costumero V, Fuentes-Claramonte P, Rosell-Negre P, Ventura-Campos N, et al. Abstinence duration modulates striatal functioning during monetary reward processing in cocaine patients. Addict Biol. 2014;19(5):885–94. doi: 10.1111/adb.12041. [DOI] [PubMed] [Google Scholar]
- 61.Abercrombie H, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, Perlman SB, Turski PA, Krahn DD, Benca RM, Davidson RJ. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998;9:3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- 62.Kessler D, Angstadt M, Welsh RC, Sripada C. Modality-Spanning Deficits in Attention-Deficit/Hyperactivity Disorder in Functional Networks, Gray Matter, and White Matter. The Journal of Neuroscience. 2014;34(50):16555–16566. doi: 10.1523/JNEUROSCI.3156-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, et al. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27(45):12132–8. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598(1–2):143–53. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- 65.Lee SK, Mori S, Kim DJ, Kim SY, Kim SY, Kim DI. Diffusion tensor MR imaging visualizes the altered hemispheric fiber connection in callosal dysgenesis. AJNR. American journal of neuroradiology. 2004;25(1):25–8. [PMC free article] [PubMed] [Google Scholar]
- 66.Casey BJ, Oliveri ME, Insel T. A Neurodevelopmental Perspective on The Research Domain Criteria (RDoC) Framework. Biol Psychiatry. 2014;76(5):350–353. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 67.van Holst RJ, Chase HW, Clark L. Striatal connectivity changes following gambling wins and near-misses: Associations with gambling severity. NeuroImage Clin. 2014;5:232–9. doi: 10.1016/j.nicl.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.