Abstract
Varicella-zoster virus (VZV), an alphaherpesvirus that causes chicken pox (varicella) and shingles (herpes zoster), is a medically important pathogen that causes considerable morbidity and, on occasion, mortality in immunocompromised patients. Herpes zoster can afflict the elderly with a debilitating condition, postherpetic neuralgia, triggering severe, untreatable pain for months or years. The lipid envelope of VZV, similar to all herpesviruses, contains numerous glycoproteins required for replication and pathogenesis.
Purpose of Review
To summarize the current knowledge about VZV glycoproteins and their roles in cell entry, replication and pathogenesis.
Recent Findings
The functions for some VZV glycoproteins are known, such as gB, gH and gL in membrane fusion, cell-cell fusion regulation, and receptor binding properties. However, the molecular mechanisms that trigger or mediate VZV glycoproteins remains poorly understood.
Summary
VZV glycoproteins are central to successful replication but their modus operandi during replication and pathogenesis remain elusive requiring further mechanistic based studies.
Keywords: Varicella zoster virus, glycoprotein, receptor, fusion, replication, pathogenesis
Introduction
Varicella Zoster Virus (VZV) is a pathogenic human alphaherpesvirus that causes varicella (chickenpox), a vesicular exanthum in children, and zoster (shingles), a severe exanthema that is typically restricted to a single dermatome in adults [1]. VZV is both lymphotropic and neurotropic but initiates a primary infection at the mucosal epithelium via respiratory droplets or vesicular fluid from infected individuals [2]. A T cell-associated viremia disseminates VZV to the skin in the host leading to the formation of the characteristic vesicles [3]. Neurons in the skin that connect to sensory nerve ganglia subsequently become infected leading to a latent reservoir of VZV that persists for the life of the host. Waning immunity to VZV renders the host susceptible to reactivation of VZV from latently infected neurons, causing zoster [4–7]. In herpes zoster afflicted patients 27–73% will develop postherpetic neuralgia (PHN), which is age dependent [7]. PHN is refractory to treatment and characterized by severe pain that can last from days to months and, in 48% of patients over the age of 70, it can last for more than a year.
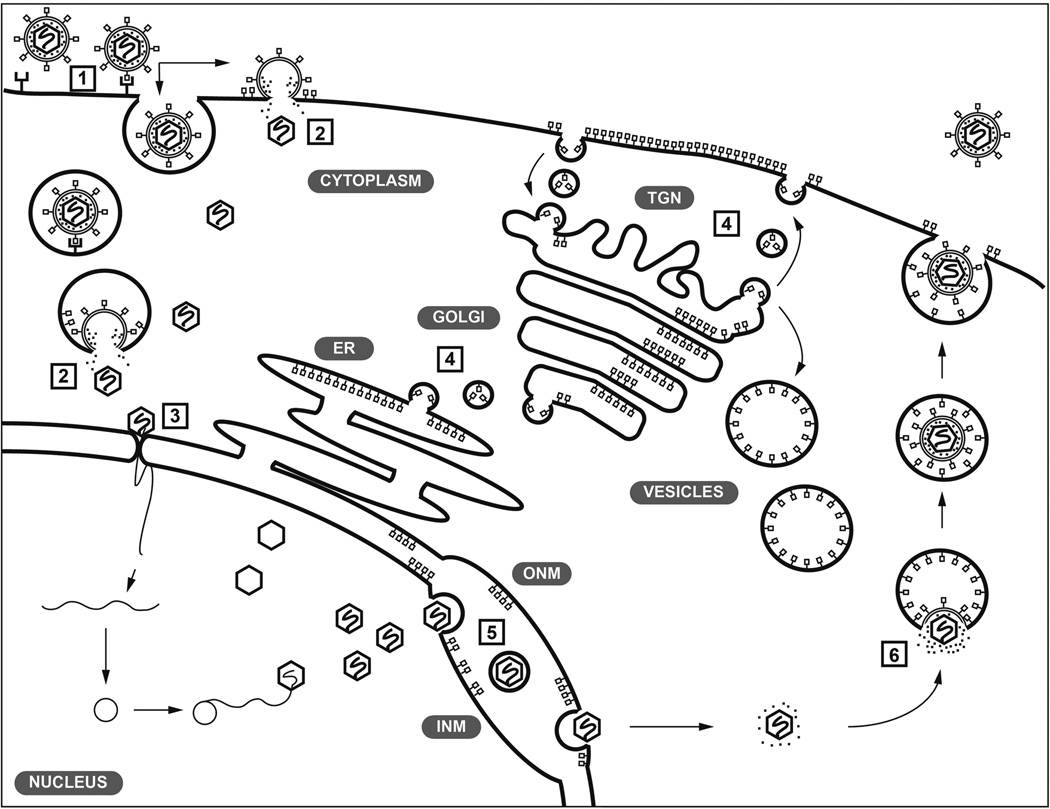
The double stranded DNA genome of VZV encodes 70 known unique open reading frames (ORFs), of which 11 encode for glycoproteins (Table 1). These membrane bound proteins are glycosylated through N- or O-linked sugars and many are known to be incorporated into the envelope of VZV virions [8]. There are some important differences between VZV and other members of the Alphaherpesvirinae. In addition to the glycoproteins encoded by VZV, herpes simplex viruses (HSV) encode three other glycoproteins, gD (encoded by unique short 6 (US6)), gG, (US4) and gJ (US5), which might account for the different tropisms of HSV and VZV [9]. Presently, it is recognized that herpesvirus glycoproteins use canonical cellular processes for their synthesis and posttranslational modifications and are synthesized in the endoplasmic reticulum (ER), shuttled to the Golgi and trafficked to the cell surface via vesicles. The cell surface glycoproteins are then endocytosed and trafficked to the trans-Golgi, the site of VZV particle secondary envelopment (Figure 1). The primary role of glycoproteins on the virion membrane is currently thought to be to direct interactions with cell surface proteins to enable attachment, uptake and entry of VZV into cells to initiate the replication cycle [9]. In addition to glycosylation, glycoproteins can undergo further posttranslational modifications, such as phosphorylation and ubiquitination that might also function in VZV replication [10–15].
Table 1.
Glycoproteins encoded by the VZV genome.
| Glycoprotein | ORF | Length | Mw (kDa)A |
Require d |
Heterodime r |
|
|---|---|---|---|---|---|---|
| NT | AA | |||||
| ORFS/L | ORFS/L | 474 | 157 | 19–30 | NoB | |
| ORF5 | gK | 1023 | 340 | 40 | Yes | ORF39 |
| ORF9A | gN | 264 | 87 | 7 | NoB | gM |
| ORF14 | gC | 1776 | 591 | 100 | No | |
| ORF31 | gB | 2796 | 931 | 130 | Yes | |
| ORF37 | gH | 2496 | 841 | 118 | Yes | gL |
| ORF39 | ORF39 | 723 | 240 | 30 | NoB | gK |
| ORF50 | gM | 1308 | 435 | 50 | NoB | gN |
| ORF60 | gL | 483 | 160 | 17 | Yes | gH |
| ORF67 | gI | 1065 | 354 | 65 | NoB | gE |
| ORF68 | gE | 1872 | 623 | 110 | Yes | gI |
Glycosylated form.
Severe replication defect in cell culture
Figure 1.
Model of the localization and trafficking of VZV glycoproteins during infection. VZV is presumed to use glycoproteins to bind cell surface receptors during attachment (1). The gB/gH-gL complex fuses the virion membrane with cell membranes either in endocytic vesicles or directly with the plasma membrane (2). The capsid is released into the cytoplasm and docks with a nuclear pore where the dsDNA genome is injected into the cell nucleus to initiate replication (3). Glycoproteins are synthesized in the endoplasmic reticulum (ER) and trafficked to the Golgi during maturation, exocytosed then endocytosed and trafficked to the trans-Golgi network (TGN) (4). Some glycoproteins, including gB, are trafficked to the nuclear envelope (inner nuclear membrane, INM; outer nuclear membrane, ONM) and might have roles in egress from the nucleus (5). Glycoproteins are trafficked from the TGN for incorporation into nascent virus particles (6). Adapted from [100].
VZV is a human host restricted pathogen, making it challenging to investigate pathogenesis. Mutagenesis to create recombinant viruses is useful strategy to determine the requirements of ORFs and protein motifs needed for VZV replication [16–21]. Replication studies in cell culture have been a valuable approach to determine the effects that mutations have on VZV infection and propagation in vitro. However, the effects of VZV mutations might not be apparent in cell culture creating additional challenges in deciphering how VZV causes disease. The application of human xenografts in SCID mice to assess recombinant mutants in vivo has proven very informative for understanding the roles of glycoproteins in skin and neuronal pathogenesis [13, 19,20, 22–33]. This review will outline the known contributions of the VZV glycoproteins in viral entry, replication and pathogenesis. These functions also make the VZV glycoproteins important targets of humoral and cell-mediated immunity.
Glycoproteins that Function in Virion Attachment
For most alphaherpesviruses, including herpes simplex virus (HSV) and pseudorabies virus (PRV), high titer purified virions can be generated reproducibly, which enables the production of non-infectious virus particles that lack specific glycoproteins using recombinant DNA methodologies [34–39]. This approach allows for the identification of glycoproteins that have a role in virion attachment and entry. Due to the extremely cell associated nature of VZV, generating reproducible, purified, high titer cell free virus has proven to be elusive, precluding the use of this approach to study VZV glycoproteins in entry directly. However, deletions of gB, gE, gH, gK, gL and ORF39 from VZV cosmids or bacterial artificial chromosomes (BACs) have been demonstrated to inactivate VZV [17, 19, 20, 25, 40]. It is uncertain whether these individual glycoproteins are directly involved in virion attachment to cell surface receptors, or whether effects of their deletion result from impeding other steps of viral replication.
It has been inferred from experimental evidence with other herpesviruses and cell based fusion assays that the VZV glycoproteins gB, gH and gL are required for attachment and entry [41–44]. The myelin associated glycoprotein (MAG; Siglec4) was demonstrated to bind directly to gB using an Ig expression system [45]. Importantly, MAG has a high amino acid identity compared to the paired Ig-like type-2 receptor α (PILRα) protein that binds to herpes simplex virus (HSV) gB, suggesting that gB of the alphaherpesviruses might bind to a similar class of cell surface proteins. Transient transfection of MAG in fibroblasts enhanced both VZV and HSV infection supporting the hypothesis of a conserved role for cell surface protein homologs similar to MAG and PILRα. Another factor implicated in the binding of VZV gB to MAG is the involvement of sialic acids, which are a diverse group of monosaccharides found on N- or O-linked glycans [46]. The loss of the sialic acid binding site on MAG reduces cell entry of VZV in MAG transfected cells and gB/gH-gL mediated cell-cell fusion [46]. However, MAG is unlikely to be the sole receptor for gB binding and fusion as cells lacking MAG are still susceptible to VZV infection.
In addition to gB, the stable gH-gL heterodimer is also necessary for membrane fusion required for virion entry [19]. Importantly, the crystal structure of gH-gL bound to human monoclonal antibodies that neutralize VZV has recently been resolved [47]. The VZV gH-gL heterodimer was found to have a structure more comparable to HSV gH-gL than EBV gH-gL [47–49]. While the function of gH is not yet clear, it is thought that these monoclonal antibodies inhibit the binding of gH to surface proteins that would otherwise lead to the activation of gB, which has been proposed for the gB/gH-gL homologs of other herpesviruses [50, 51]. A study performed with a mouse monoclonal antibody against gH demonstrated the effectiveness of neutralizing VZV infection by targeting VZV gH-gL [30, 52]. Virus titers and pathologic changes due to lesion formation were reduced in human skin xenografts when the host mice were treated with the anti-gH monoclonal antibody (mAb) 206. Human derived mAbs 24, 94 and RC that bind to gH-gL have all been shown to have VZV neutralizing properties, providing further evidence for the importance of gH-gL in VZV infection, [47, 53, 54]. Integrins are expressed on the cell surface and have been reported to bind gH of other herpesviruses [55–58]. Integrins might also have a role in VZV attachment and entry because inhibition of the αV subunit was found to reduce cell fusion and limit virus propagation in cell culture [59]. Whether the neutralizing capability of mAbs 24, 94, 206 and RC against VZV was due to the prevention of virion binding via gH-gL with cell surface integrins requires further investigation.
Critically, the gB/gH-gL complex is highly conserved throughout the Herpesviridae, reinforcing the concept that these glycoproteins are the core components of VZV envelope fusion during cell entry. Conspicuously absent in VZV compared to other alphaherpesviruses, including HSV and PRV, is gD, which is reported to be the glycoprotein necessary for receptor binding and priming of the gB/gH-gL fusion complex [44, 60, 61]. In addition, gC has also been linked with virion attachment in HSV, PRV and other alphaherpesviruses [62, 63]. Importantly, gC is not required for VZV virions to bind with cell surface receptors because recombinant VZV ΔgC and ORF14stop mutants can be propagated in cell culture without deleterious effects on replication kinetics [20, 64]. Similar data have been obtained with the closely related herpesvirus, simian varicella virus [65]. However, gC does have a functional role in VZV propagation due to the poor replication of the ΔgC mutant in human skin tissue, suggesting an indirect role for gC in tissue tropism [20, 27]. Despite this important characteristic the function of gC remains unknown. Unlike other glycoproteins, gC transcription does not occur until very late in VZV and is considered a true late protein as production and accumulation of gC during VZV infection occurs late in the replication cycle [66, 67]. HSV gC binds heparin sulfate and antibodies that target this binding region neutralize HSV [68]. However, equivalent studies have not been performed with VZV gC.
VZV gE forms a stable heterodimer with gI but also binds at least one cellular protein, insulin degrading enzyme (IDE)[69]. Initially, IDE was identified to be important for infection, with VZV replication substantially diminished in IDE knockdown cells. However, in subsequent studies it has been demonstrated that gE interaction with IDE is not required for entry into cell types tested, including differentiated human T cells [24]. Another cellular protein implicated in VZV entry was the cation independent mannose-6-phosphate receptor encoded by the IGF2R gene [70, 71]. Mannose-6-phosphate is a sugar found on all glycoproteins and the cation independent mannose-6-phosphate receptor is involved in VZV infection as demonstrated by a knockdown of IGF2R using RNAi, which significantly reduced VZV replication [70]. Curiously, this effect was predominantly associated with cell-free virus as the absence of IGF2R had little effect on the spread of cell-associated VZV. Although these studies implicate IDE and IGFR2 in VZV replication these are likely to have roles post entry. IDE binds to a gE precursor found in the endoplasmic reticulum and IGFR2 has roles in lysosomal biogenesis [72, 73]. This suggests that the roles of IDE and IGFR2 are independent of virion attachment to the cell surface and function in the VZV replication cycle post entry.
The gM-gN heterodimer is also incorporated into VZV particles, but it is uncertain whether the heterodimer is required for virion attachment [18]. Disruption of ORF50[gM] and ORF9A[gN], which prevents expression of gM and gN, yielded VZV mutants that replicated poorly with reduced plaque sizes in cell culture [18]. These mutants contrast with the ΔORF50 and ΔORF9A deletions made by Zhang et al 2010, which inactivated VZV. The differences in phenotype are attributed to the complete deletions ORF50 or ORF9A in the mutant viruses, which also disrupted two essential ORFs, ORF8 and ORF51 that overlapped with ORF9 and ORF50 [20]. A pair of substitutions in gM, V42P and G301M, prevents gM maturation and disrupts the interaction between gM and gN. Incorporation of gM[V42P/G301M] into the VZV genome yielded a virus that has reduced propagation in melanoma cells and human embryonic lung fibroblasts [18]. This supports the notion that the heterodimer forms a functional unit but whether this complex is involved directly in virion attachment and entry into melanoma cells, fibroblasts or alters pathogenesis is not clear.
Studies investigating the functions of VZV gK and ORF39 are limited but extrapolation of data from the alphaherpesviruses HSV and PRV suggest that gK and ORF39 form a complex [74–76]. Corroborating the findings in other herpesviruses, truncation mutants of VZV gK or a ΔgK virus showed gK is indispensable for replication [17, 20]. Although gK is incorporated into the virion it is not clear whether gK has a functional role in the attachment of virions to the cell or cell entry via fusion. A study on the trafficking of VZV gK and ORF39 implies that additional VZV proteins are required for the localization during infection because transient transfection of the glycoproteins alone or together leads to accumulation in the ER, whereas during infection they reach the Golgi [77]. Deleting ORF39 was lethal in a study that sequentially knocked out each open reading frame from a VZV BAC [20]. Since this is the only ΔORF39 reported and confirmation is needed since the gM deletion was also lethal in contrast to previous studies. The HSV homolog of ORF39 is UL20, which also associates with gK. Moreover, recent studies that used an HSV gKΔ31–68 mutant demonstrated that gK is implicated in neurotropism [78]. Whether VZV gK has similar roles as a neurotropic factor needs to be investigated.
Glycoproteins that Drive Membrane Fusion
The core glycoproteins that drive membrane fusion during the entry of herpesviruses are gB, gH and gL [79]. Unlike HSV, which requires gD for membrane fusion and cell entry, gB and gH-gL are necessary and sufficient for VZV membrane fusion [19, 45]. A substantial amount of literature implicates herpesvirus gB homologues to be the fusogen, whereas the gH-gL heterodimer has been linked to the activation of gB [79]. Homology modelling of VZV gB using the crystal structure of HSV gB strongly suggests that VZV gB also forms a trimeric protein with five extracellular domains [28]. Importantly, the predicted fusion loops in domain II of VZV gB have been demonstrated to be functional. The two substitutions, gB[W180G] or gB[Y185G], eliminated membrane fusion but did not affect the surface expression of gB [13]. Importantly, these fusion defective mutants inactivate VZV [28]. Membrane fusion driven by gB is regulated by the cytoplasmic domain (gBcyt). A carboxyl-terminal truncation mutant, VZV gB-36, a 36 amino acid deletion in the cytoplasmic domain, replicates with an exaggerated syncytial phenotype [80]. Importantly, the frequency of intact virions at the surface of infected cells was reported to be considerably reduced for VZV gB-36 at four days post infection. Similar to this VZV truncation mutant, single amino acid substitutions within the cytoplasmic domain have profound effects on gB mediated cell-cell fusion. A hyperfusion phenotype, a significantly increased propensity to induce fusion in a cell-based assay, was identified when the tyrosine residue at position 881 (gB-Y881) was substituted with a large aromatic sidechain [13]. The gB-Y881 residue was also demonstrated to be phosphorylated, and central to a predicted immunoreceptor tyrosine-base inhibition motif (ITIM), suggesting that this residue was important for fusion regulation. In contrast to gB-Y881F, substitutions with phosphomimetic residues aspartic acid (gB[Y881D]) or glutamic acid (gB[Y881E]) eliminated fusion and significantly reduced cell surface expression, suggesting that Y881 might act as a molecular switch for gB trafficking or localization.
Mutagenesis studies of VZV gH have identified domains that play important roles in membrane fusion. Antibodies that bind to the N-terminus of VZV gH prevent fusion and also have virus neutralizing properties. The anti-gH mAb 206 has potent virus neutralizing properties and is very effective at preventing cell-cell fusion [30, 47, 52, 59]. The mAb 206 epitope has been mapped to a region in the extreme N-terminus of gH [19, 47]. Human mAbs 94 and RC have neutralizing capability similar to mAb 206 [47]. These antibodies bind a similar region in gH-gL, leading to the proposal that gH has a site of vulnerability targeted by the human adaptive humoral immune response to VZV infection. Similar to VZV gB, the cytoplasmic domain of gH also has a mechanism to regulate cell-cell fusion. This critical function is related to the length of the gH cytoplasmic domain rather than any specific sequence or motifs [81]. The regulation of cell-cell fusion by both gB and gH are vital to the effective propagation of VZV.
VZV Glycoprotein Mutagenesis and the Effects on Replication and Pathogenesis
Targeted mutagenesis of ORFs encoding VZV glycoproteins using cosmids or BACs is either lethal, thereby defining an essential role in replication, or the change is compatible with replication, permitting further study of the role of the glycoprotein or its subdomains in cultured cells in vitro and in xenografts in the SCID mouse model in vivo (Table 2). With respect to gB, mutagenesis of the furin cleavage site and the cytoplasmic domain of gB have revealed some surprising nuances in its role in VZV skin pathogenesis. Although not apparent in cell culture, both the gB-Δ491RSRR494 and gB-491GSGG494 mutants decreased the titers of VZV recoverable from human skin xenografts, implying that the cleavage of gB is required for efficient replication in the tissue microenvironment [28]. A more surprising finding was the effect of hyperfusion on VZV pathogenesis via the loss of fusion regulation by the gBcyt. It had long been thought that syncytia formation was beneficial to the spread of VZV, especially in human skin where multinucleated cells are prominent within lesions [82]. However, the hyperfusion inducing mutation gB[Y881F] was severely detrimental to VZV propagation in human skin tissue [13]. VZV replication was detected but titers were either low or undetectable at 10 and 21 days post inoculation. These data demonstrate that the regulation of gB induced membrane fusion is an important requirement for VZV pathogenesis in human tissue. The unfavorable effects of hyperfusion was confirmed with gH truncation mutants gH-TL and gH-Δ834–841, which are hyperfusogenic and propagate also poorly in differentiated human skin in vivo [81].
Table 2.
Recombinant VZV glycoprotein mutants and their effect on replication in cell culture, human skin and dorsal root ganglia.
| ORF [Glycoprotein] |
Mutation | Phenotype | Reference | ||
|---|---|---|---|---|---|
| Cell culture | Skin | DRG | |||
| ORFS/L | ΔTMD | WT in MeWo | - | - | [21] |
| ORF5[gK] | ΔORF5 | Lethal | N/A | N/A | [17, 20] |
| ΔNgK | Lethal | N/A | N/A | ||
| ΔCgK | Lethal | N/A | N/A | ||
| ΔCgK5251 | Lethal | N/A | N/A | ||
| ORF9A[gN] | ΔgNA | Lethal | N/A | N/A | [20] |
| ORF14[gC] | ΔgC | WT | Reduced | N/A | [20] |
| ORF14stop | WT | - | - | [64] | |
| ORF31[gB] | ΔgB | Lethal | N/A | N/A | [20] |
| W180G | Lethal | N/A | N/A | [28] | |
| Y185G | Lethal | N/A | N/A | ||
| 491GSGG494 | WT | Reduced | - | ||
| 491ΔRSRR494 | WT | Reduced | - | ||
| Δ36 (gB-36) | Hyperfusion | - | - | [80] | |
| Y881F | Hyperfusion | Reduced | - | [13] | |
| Y920F | WT | WT | - | ||
| Y881/920F | Hyperfusion | Reduced | - | ||
| ORF37[gH] | ΔgH | Lethal | N/A | N/A | [19, 20] |
| αX | WT | Reduced | - | [19] | |
| S42A | WT | WT | - | ||
| N45A | WT | WT | - | ||
| S47A | WT | Reduced | - | ||
| S47T | WT | WT | - | ||
| T127A | WT | WT | - | ||
| C327A | WT | WT | - | ||
| T351A | WT | WT | - | ||
| C647A | Small Plaque | Reduced | - | ||
| S694F | - | - | - | ||
| C703A | Small Plaque | Reduced | - | ||
| S694F/C724A | - | - | - | ||
| C727A | Small Plaque | Reduced | - | ||
| S694F/C727A | WT | Reduced | - | ||
| T751A | Small Plaque | WT | - | ||
| α8 | Lethal | N/A | N/A | ||
| α9 | Lethal | N/A | N/A | ||
| α12 | Lethal | N/A | N/A | ||
| α14 | Lethal | N/A | N/A | ||
| C540A | Lethal | N/A | N/A | ||
| C575A | Lethal | N/A | N/A | ||
| C724A | Lethal | N/A | N/A | ||
| 781FPNG784 | Lethal | N/A | N/A | ||
| ORF37[gH] | gH-TL (834 stop) | Hyperfusion | Reduced | - | [81] |
| Δ834–841 | Hyperfusion | Reduced | - | ||
| 834StopV5 | Hyperfusion | - | - | ||
| gH-V5 | WT | WT | - | ||
| Y835A | WT | WT | - | ||
| Y835F | WT | WT | - | ||
| ORF39 | ΔORF39 | Lethal | N/A | N/A | [20] |
| ORF50[gM] | ΔgMB | Lethal | N/A | N/A | [20] |
| gMim[V42P,G301M] | Small plaque | - | - | [18] | |
| ORF50AS(−) | WT | - | - | ||
| ORF60[gL] | ΔgL | Lethal | N/A | N/A | [20] |
| ORF67[gI] | ΔgI | Small Plaque | Reduced | Reduced | [16, 20, 25, 31] |
| Δ37–167 | Small Plaque | - | - | [29] | |
| Δ105–125 | Small Plaque | Reduced | - | ||
| C83A | WT | - | - | ||
| C95A | Small Plaque | Reduced | - | ||
| C106A | WT | WT | - | ||
| C200A | WT | - | - | ||
| N116A | WT | WT | - | ||
| ORF68[gE] | ΔgE | Lethal | N/A | N/A | [16, 20] |
| Insertion G16 | Lethal | N/A | N/A | [22] | |
| Insertion P27 | Reduced | Reduced | - | ||
| Insertion Y51 | WT | WT | - | ||
| Insertion G90 | WT | - | - | ||
| Insertion I146 | WT | - | - | ||
| Insertion P187 | WT | - | - | ||
| S31A | WT | Reduced | - | ||
| S49A | WT | WT | - | ||
| ΔP27-Y51 | WT | - | - | ||
| ΔY51-P187 | Reduced | Not recovered | - | ||
| ΔP27–P187 | Lethal | N/A | N/A | ||
| ΔCys208–236 | Reduced in HELFs | Reduced | Delayed | [23, 32] | |
| ΔP27-G90 | Small Plaque | Reduced | - | [24] | |
| ΔY51-G90 | Small Plaque | WT | - | ||
| Δ32–71 | Small Plaque | - | - | [84] | |
| Δ163–208 | Lethal | N/A | N/A | ||
| Y582G (YAGL) | Lethal | N/A | N/A | [26] | |
| gEΔcyt | Lethal | N/A | N/A | ||
| Y569A (AYRV) | WT | WT | WT | [26, 32] | |
| 593AEAADA598 | Increased | Increased | WT | ||
Also deletes a portion of ORF8.
WT – Wild type, not different from parental virus. N/A – Not applicable. Not tested (−)
Also deletes a portion of ORF51.
WT – Wild type, not different from parental virus. N/A – Not applicable. Not tested (−)
Functional domains within VZV gH required for replication were defined by mutagenesis of the gH ectodomain, targeting four alpha helices (α8, α9, α12, α14), three cysteine residues (C540A, C575A, C724A), which are conserved in HSV gH, and the amino acid motif, FPNG, also conserved in HSV gH [19]. The alpha helices are all located in domain II of VZV gH, as were C540 and C575, which form a disulfide bond, and the mutations very likely disrupted the structure and function of gH. Alanine substitutions of the FPNG motif in domain III completely disrupted gH trafficking. Five additional VZV gH mutants, one in domain I (αX) and three in domain III (C647A, C703A and C727A) yielded viable viruses but all had reduced replication in human skin xenografts at 10 and 21 days post infection. The αX mutation was of particular interest because the mutant retained wild type-like replication in cell culture, implicating the N-terminal region of gH, which contains the mAb 206 epitope, in skin tropism [19, 47]. Each of the viable cysteine mutants replicated poorly in cell culture and, as expected, were markedly attenuated in human skin, which was attributable to the reduced fusion capability of these mutants as a consequence of destabilizing domain III of gH.
Compared to other alphaherpesviruses, VZV gE has a unique N-terminus comprised of 188 unique amino acids [22]. A large deletion mutant in this N-terminus, gE[ΔP27–P187], inactivates VZV, which is attributed to the inability of this mutant to traffic to the plasma membrane [22]. The site for gE binding to IDE is within this region although its precise location has not been resolved. The two gE deletion mutants, gE[Δ32–71], which has a small plaque phenotype, and gE[ΔP27-G90], which replicates poorly in human skin, are unable to bind IDE [24, 83, 84]. However, the two mutants gE[ΔP27-Y51] and gE[ΔY51-P90] both bind IDE at levels similar to wild type gE but only the gE[ΔP27-Y51] has reduced replication in skin [24]. This suggested that the gE[Δ32–71] region might contain a protein conformation that is not fully disrupted by the gE[ΔP27-Y51] or gE[ΔY51-P90] mutations. Critically, the binding of IDE to VZV gE is not required for neurotropism because the gE[Δ27–90] mutant successfully replicated in neurons [32]. Importantly, the unique N-terminal region of gE does not have domain for gI binding, which is required for propagation in human skin xenografts [25, 29]. However, the cysteine rich region between residues 208–236 is necessary for heterodimer formation between gE and gI [23]. The gE[ΔCys] mutant, which has residues 208–236 deleted, replicates poorly in cell culture and skin xenografts but its phenotype was not as severe as the ΔgI mutant [23, 25, 29]. In addition, the gE[ΔCys] mutant propagated in DRG but at a reduced rate compared to intact VZV [31, 32]. A single point mutation, gI[C95A], disrupted the structure of gI and had a similar small plaque phenotype as the ΔgI VZV with limited replication in skin xenografts [29]. It has been proposed that gI is required for incorporation of gE in to the virion. However, gE was incorporated into particles in ΔgI VZV infected melanoma cells. These data suggest that gI has functions in VZV replication that are independent of gE.
In addition to gB and gH, the cytoplasmic domain of gE (gEcyt) and its involvement in pathogenesis has been scrutinized. The gEcyt has been shown to contain important functional motifs. A YXXΦ motif, gE[582YAGL585], mediates endocytosis, gE[568AYRV571] enables trans-Golgi network targeting, and the gE[593SXSTXT598] acid cluster is phosphorylated [85–88]. A gE[Y582G] substitution is lethal as is the gEΔcyt [26]. In addition, the gE[AYRV], which has reduced skin virulence, and gE[SSTT], which is a mutant that prevents phosphorylation in the cytoplasmic domain of gE but does not affect replication in skin xenografts, were both able to replicate in DRG at similar levels to wild-type virus demonstrating that these motifs are not required for neurovirulence [26, 32].
VZV Glycoproteins Associated with Vaccination and the Immune Response
Vaccines are available to prevent both varicella and herpes zoster [89]. Varicella vaccines have been very successful in reducing the prevalence of varicella amongst children. Two doses are recommended, one at 12–15 months and one a 4–6 years of age is ~98% effective at preventing chicken pox [90]. Zostavax® is the only licensed vaccine available for herpes zoster and protects 51% of individuals over the age of 60 against shingles and 67% against post herpetic neuralgia, a very painful sequelae of herpes zoster [91]. Although successful in significantly reducing severe sequelae, there was limited success in complete protection against herpes zoster, which is thought to be related to limitations in boosting the immune response in the elderly.
The glycoprotein based enzyme linked immunosorbent assays (gpELISAs) are widely used to assess antibody levels to VZV in serum. The gpELISA relies on lentil/lectin-affinity purified glycoproteins from MRC5 cells infected with VZV and a control antigen from mock-infected MRC5 cells [92]. Based on the gpELISA, VZV vaccine efficiency is >95% in seronegative children if IgG titers are ≥ 5 gpELISA units at 6 weeks post vaccination and immunity to varicella is >90% after 6 years [93–95]. However, in elderly subjects that received a herpes zoster vaccine ≥ 10 years after originally being vaccinated, serum responses to VZV did not increase above baseline titers using the gpELISA after a single dose [96]. Importantly, booster vaccination enhances cell mediated immunity, which has been shown to decline after the age of 50, but a correlation between serum antibodies and protective cell mediated immunity is not apparent in this demographic [97, 98]. A new vaccine based on recombinant gE formulated with the liposome-based AS01B adjuvant system was demonstrated to be very effective in the elderly [99]. In recipients over the age of 70 vaccine efficacy was >96%, considerably greater than the current vaccine for herpes zoster [91]. Moreover, this subunit vaccine is safe for immunocompromised HIV and autologous hematopoietic cell transplant patients for whom live attenuated vaccines such as Zostavax are not recommended. This glycoprotein-based vaccine appears to be very promising.
Conclusions
Glycoproteins are critical for VZV entry and replication, which makes them potential targets for antiviral drug candidates. At present, generating effective pharmaceuticals that target VZV glycoproteins is challenging because of the vacuum in knowledge about their molecular mechanisms during VZV infection. More information about the structures and functions of VZV glycoproteins is also relevant to improving VZV vaccines to prevent primary and recurrent infections. Humoral and cell-mediate immunity induced by the live attenuated varicella and zoster vaccines includes responses directed against major VZV glycoproteins but how these responses contribute to protection from disease is not known. These questions are of particular interest given the recent report that a vaccine based on recombinant gE formulated with the liposome-based AS01B adjuvant system was highly effective against zoster in the elderly [99]. Gaining a better structural understanding of glycoprotein topology holds significant promise since it will aid in mapping critical functional domains which will then make developing drugs and vaccines that target specific molecular functions of VZV glycoproteins more feasible.
Acknowledgments
This work was supported by NIH grants AI102456 and AI20459.
Stefan Oliver reports grants from NIH, during the conduct of the study.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Edward Yang and Ann Arvin declare they have no conflicts of interest.
References
Recently published papers of particular interest have been highlighted as:
• Of importance
•• Of major importance
- 1.Arvin AM, Gilden D. Varicella-Zoster Virus. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 6th. Vol. 2. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013. pp. 2015–2184. [Google Scholar]
- 2.Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12:197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moffat J, Ku CC, Zerboni L, Sommer M, Arvin A. VZV: pathogenesis and the disease consequences of primary infection. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: 2007. [PubMed] [Google Scholar]
- 4.Arvin AM, Moffat JF, Sommer M, Oliver S, Che X, Vleck S, Zerboni L, Ku CC. Varicella-zoster virus T cell tropism and the pathogenesis of skin infection. Curr Top Microbiol Immunol. 2010;342:189–209. doi: 10.1007/82_2010_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zerboni L, Reichelt M, Arvin A. Varicella-zoster virus neurotropism in SCID mouse-human dorsal root ganglia xenografts. Curr Top Microbiol Immunol. 2010;342:255–276. doi: 10.1007/82_2009_8. [DOI] [PubMed] [Google Scholar]
- 6.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Neurological disease produced by varicella zoster virus reactivation without rash. Curr Top Microbiol Immunol. 2010;342:243–253. doi: 10.1007/82_2009_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kost RG, Straus SE. Postherpetic neuralgia--pathogenesis, treatment, and prevention. N Engl J Med. 1996;335:32–42. doi: 10.1056/NEJM199607043350107. [DOI] [PubMed] [Google Scholar]
- 8.Edson CM, Hosler BA, Poodry CA, Schooley RT, Waters DJ, Thorley-Lawson DA. Varicella-zoster virus envelope glycoproteins: biochemical characterization and identification in clinical material. Virology. 1985;145:62–71. doi: 10.1016/0042-6822(85)90201-6. [DOI] [PubMed] [Google Scholar]
- 9.Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: 2007. [PubMed] [Google Scholar]
- 10.Gabel CA, Dubey L, Steinberg SP, Sherman D, Gershon MD, Gershon AA. Varicella-zoster virus glycoprotein oligosaccharides are phosphorylated during posttranslational maturation. J Virol. 1989;63:4264–4276. doi: 10.1128/jvi.63.10.4264-4276.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu Rev Microbiol. 1990;44:59–80. doi: 10.1146/annurev.mi.44.100190.000423. [DOI] [PubMed] [Google Scholar]
- 12.Kenyon TK, Cohen JI, Grose C. Phosphorylation by the varicella-zoster virus ORF47 protein serine kinase determines whether endocytosed viral gE traffics to the trans-Golgi network or recycles to the cell membrane. J Virol. 2002;76:10980–10993. doi: 10.1128/JVI.76.21.10980-10993.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oliver SL, Brady JJ, Sommer MH, Reichelt M, Sung P, Blau HM, Arvin AM. An immunoreceptor tyrosine-based inhibition motif in varicella-zoster virus glycoprotein B regulates cell fusion and skin pathogenesis. Proc Natl Acad Sci U S A. 2013;110:1911–1916. doi: 10.1073/pnas.1216985110. An ITIM motif was identified in VZV gB that is phosphorylated and has regulatory properties that limits gB/gH-gL mediated membrane fusion. A tyrosine residue central to the ITIM was proposed to act as a phosphorylation dependent molecular switch for gB trafficking and localization.
- 14.Olson JK, Bishop GA, Grose C. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J Virol. 1997;71:110–119. doi: 10.1128/jvi.71.1.110-119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calistri A, Sette P, Salata C, Cancellotti E, Forghieri C, Comin A, Gottlinger H, Campadelli-Fiume G, Palu G, Parolin C. Intracellular trafficking and maturation of herpes simplex virus type 1 gB and virus egress require functional biogenesis of multivesicular bodies. J Virol. 2007;81:11468–11478. doi: 10.1128/JVI.01364-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallory S, Sommer M, Arvin AM. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J Virol. 1997;71:8279–8288. doi: 10.1128/jvi.71.11.8279-8288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo C, Suen J, Sommer M, Arvin A. Characterization of Varicella-Zoster virus glycoprotein K (open reading frame 5) and its role in virus growth. J Virol. 1999;73:4197–4207. doi: 10.1128/jvi.73.5.4197-4207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadaoka T, Yanagi T, Yamanishi K, Mori Y. Characterization of the varicella-zoster virus ORF50 gene, which encodes glycoprotein M. J Virol. 2010;84:3488–3502. doi: 10.1128/JVI.01838-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vleck SE, Oliver SL, Brady JJ, Blau HM, Rajamani J, Sommer MH, Arvin AM. Structure-function analysis of varicella-zoster virus glycoprotein H identifies domain-specific roles for fusion and skin tropism. Proc Natl Acad Sci U S A. 2011;108:18412–18417. doi: 10.1073/pnas.1111333108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Selariu A, Warden C, Huang G, Huang Y, Zaccheus O, Cheng T, Xia N, Zhu H. Genome-wide mutagenesis reveals that ORF7 is a novel VZV skin-tropic factor. PLoS Pathog. 2010;6:e1000971. doi: 10.1371/journal.ppat.1000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koshizuka T, Ota M, Yamanishi K, Mori Y. Characterization of varicella-zoster virus-encoded ORF0 gene--comparison of parental and vaccine strains. Virology. 2010;405:280–288. doi: 10.1016/j.virol.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Berarducci B, Ikoma M, Stamatis S, Sommer M, Grose C, Arvin AM. Essential functions of the unique N-terminal region of the varicella-zoster virus glycoprotein E ectodomain in viral replication and in the pathogenesis of skin infection. J Virol. 2006;80:9481–9496. doi: 10.1128/JVI.00533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berarducci B, Rajamani J, Reichelt M, Sommer M, Zerboni L, Arvin AM. Deletion of the first cysteine-rich region of the varicella-zoster virus glycoprotein E ectodomain abolishes the gE and gI interaction and differentially affects cell-cell spread and viral entry. J Virol. 2009;83:228–240. doi: 10.1128/JVI.00913-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berarducci B, Rajamani J, Zerboni L, Che X, Sommer M, Arvin AM. Functions of the unique N-terminal region of glycoprotein E in the pathogenesis of varicella-zoster virus infection. Proc Natl Acad Sci U S A. 2010;107:282–287. doi: 10.1073/pnas.0912373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moffat J, Ito H, Sommer M, Taylor S, Arvin AM. Glycoprotein I of varicella-zoster virus is required for viral replication in skin and T cells. J Virol. 2002;76:8468–8471. doi: 10.1128/JVI.76.16.8468-8471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moffat J, Mo C, Cheng JJ, Sommer M, Zerboni L, Stamatis S, Arvin AM. Functions of the C-terminal domain of varicella-zoster virus glycoprotein E in viral replication in vitro and skin and T-cell tropism in vivo. J Virol. 2004;78:12406–12415. doi: 10.1128/JVI.78.22.12406-12415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moffat JF, Zerboni L, Kinchington PR, Grose C, Kaneshima H, Arvin AM. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J Virol. 1998;72:965–974. doi: 10.1128/jvi.72.2.965-974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliver SL, Sommer M, Zerboni L, Rajamani J, Grose C, Arvin AM. Mutagenesis of varicella-zoster virus glycoprotein B: putative fusion loop residues are essential for viral replication, and the furin cleavage motif contributes to pathogenesis in skin tissue in vivo. J Virol. 2009;83:7495–7506. doi: 10.1128/JVI.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver SL, Sommer MH, Reichelt M, Rajamani J, Vlaycheva-Beisheim L, Stamatis S, Cheng J, Jones C, Zehnder J, Arvin AM. Mutagenesis of varicella-zoster virus glycoprotein I (gI) identifies a cysteine residue critical for gE/gI heterodimer formation, gI structure, and virulence in skin cells. J Virol. 2011;85:4095–4110. doi: 10.1128/JVI.02596-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vleck SE, Oliver SL, Reichelt M, Rajamani J, Zerboni L, Jones C, Zehnder J, Grose C, Arvin AM. Anti-glycoprotein H antibody impairs the pathogenicity of varicella-zoster virus in skin xenografts in the SCID mouse model. J Virol. 2010;84:141–152. doi: 10.1128/JVI.01338-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zerboni L, Arvin A. Investigation of varicella-zoster virus neurotropism and neurovirulence using SCID mouse-human DRG xenografts. J Neurovirol. 2011;17:570–577. doi: 10.1007/s13365-011-0066-x. [DOI] [PubMed] [Google Scholar]
- 32.Zerboni L, Berarducci B, Rajamani J, Jones CD, Zehnder JL, Arvin A. Varicella-zoster virus glycoprotein E is a critical determinant of virulence in the SCID mouse-human model of neuropathogenesis. J Virol. 2011;85:98–111. doi: 10.1128/JVI.01902-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zerboni L, Reichelt M, Jones CD, Zehnder JL, Ito H, Arvin AM. Aberrant infection and persistence of varicella-zoster virus in human dorsal root ganglia in vivo in the absence of glycoprotein I. Proc Natl Acad Sci U S A. 2007;104:14086–14091. doi: 10.1073/pnas.0706023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ligas MW, Johnson DC. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rauh I, Weiland F, Fehler F, Keil GM, Mettenleiter TC. Pseudorabies virus mutants lacking the essential glycoprotein gII can be complemented by glycoprotein gI of bovine herpesvirus 1. J Virol. 1991;65:621–631. doi: 10.1128/jvi.65.2.621-631.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baghian A, Huang L, Newman S, Jayachandra S, Kousoulas KG. Truncation of the carboxy-terminal 28 amino acids of glycoprotein B specified by herpes simplex virus type 1 mutant amb1511-7 causes extensive cell fusion. J Virol. 1993;67:2396–2401. doi: 10.1128/jvi.67.4.2396-2401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai P, Homa FL, Person S, Glorioso JC. A genetic selection method for the transfer of HSV-1 glycoprotein B mutations from plasmid to the viral genome: preliminary characterization of transdominance and entry kinetics of mutant viruses. Virology. 1994;204:312–322. doi: 10.1006/viro.1994.1536. [DOI] [PubMed] [Google Scholar]
- 38.Husak PJ, Kuo T, Enquist LW. Pseudorabies virus membrane proteins gI and gE facilitate anterograde spread of infection in projection-specific neurons in the rat. J Virol. 2000;74:10975–10983. doi: 10.1128/jvi.74.23.10975-10983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peeters B, de Wind N, Broer R, Gielkens A, Moormann R. Glycoprotein H of pseudorabies virus is essential for entry and cell-to-cell spread of the virus. J Virol. 1992;66:3888–3892. doi: 10.1128/jvi.66.6.3888-3892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mo C, Lee J, Sommer M, Grose C, Arvin AM. The requirement of varicella zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology. 2002;304:176–186. doi: 10.1006/viro.2002.1556. [DOI] [PubMed] [Google Scholar]
- 41.Haan KM, Lee SK, Longnecker R. Different functional domains in the cytoplasmic tail of glycoprotein B are involved in Epstein-Barr virus-induced membrane fusion. Virology. 2001;290:106–114. doi: 10.1006/viro.2001.1141. [DOI] [PubMed] [Google Scholar]
- 42.Muggeridge MI. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J Gen Virol. 2000;81:2017–2027. doi: 10.1099/0022-1317-81-8-2017. [DOI] [PubMed] [Google Scholar]
- 43.Pertel PE. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J Virol. 2002;76:4390–4400. doi: 10.1128/JVI.76.9.4390-4400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pertel PE, Fridberg A, Parish ML, Spear PG. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology. 2001;279:313–324. doi: 10.1006/viro.2000.0713. [DOI] [PubMed] [Google Scholar]
- 45.Suenaga T, Satoh T, Somboonthum P, Kawaguchi Y, Mori Y, Arase H. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc Natl Acad Sci U S A. 2010;107:866–871. doi: 10.1073/pnas.0913351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suenaga T, Matsumoto M, Arisawa F, Kohyama M, Hirayasu K, Mori Y, Arase H. Sialic Acids on Varicella-Zoster Virus Glycoprotein B Are Required for Cell-Cell Fusion. J Biol Chem. 2015;290:19833–19843. doi: 10.1074/jbc.M114.635508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xing Y, Oliver SL, Nguyen T, Ciferri C, Nandi A, Hickman J, Giovani C, Yang E, Palladino G, Grose C, Uematsu Y, Lilja AE, Arvin AM, Carfi A. A site of varicella-zoster virus vulnerability identified by structural studies of neutralizing antibodies bound to the glycoprotein complex gHgL. Proc Natl Acad Sci U S A. 2015;112:6056–6061. doi: 10.1073/pnas.1501176112. The first crystal structure of a VZV glycoprotein is resolved. The gH-gL heterodimer bound to naturally occuring human VZV neutralizing antibodies demonstrates that the immune system can target this vital entry complex.
- 48.Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat Struct Mol Biol. 2010;17:882–888. doi: 10.1038/nsmb.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuura H, Kirschner AN, Longnecker R, Jardetzky TS. Crystal structure of the Epstein-Barr virus (EBV) glycoprotein H/glycoprotein L (gH/gL) complex. Proc Natl Acad Sci U S A. 2010;107:22641–22646. doi: 10.1073/pnas.1011806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atanasiu D, Cairns TM, Whitbeck JC, Saw WT, Rao S, Eisenberg RJ, Cohen GH. Regulation of herpes simplex virus gB-induced cell-cell fusion by mutant forms of gH/gL in the absence of gD and cellular receptors. MBio. 2013;4 doi: 10.1128/mBio.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Jardetzky TS, Longnecker R. The large groove found in the gH/gL structure is an important functional domain for Epstein-Barr virus fusion. J Virol. 2013;87:3620–3627. doi: 10.1128/JVI.03245-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montalvo EA, Grose C. Neutralization epitope of varicella zoster virus on native viral glycoprotein gp118 (VZV glycoprotein gpIII) Virology. 1986;149:230–241. doi: 10.1016/0042-6822(86)90124-8. [DOI] [PubMed] [Google Scholar]
- 53.Birlea M, Owens GP, Eshleman EM, Ritchie A, Traktinskiy I, Bos N, Seitz S, Azarkh Y, Mahalingam R, Gilden D, Cohrs RJ. Human anti-varicella-zoster virus (VZV) recombinant monoclonal antibody produced after Zostavax immunization recognizes the gH/gL complex and neutralizes VZV infection. J Virol. 2013;87:415–421. doi: 10.1128/JVI.02561-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki K, Akahori Y, Asano Y, Kurosawa Y, Shiraki K. Isolation of therapeutic human monoclonal antibodies for varicella-zoster virus and the effect of light chains on the neutralizing activity. J Med Virol. 2007;79:852–862. doi: 10.1002/jmv.20838. [DOI] [PubMed] [Google Scholar]
- 55.Gianni T, Salvioli S, Chesnokova LS, Hutt-Fletcher LM, Campadelli-Fiume G. alphavbeta6- and alphavbeta8-integrins serve as interchangeable receptors for HSV gH/gL to promote endocytosis and activation of membrane fusion. PLoS Pathog. 2013;9:e1003806. doi: 10.1371/journal.ppat.1003806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheshenko N, Trepanier JB, Gonzalez PA, Eugenin EA, Jacobs WR, Jr, Herold BC. Herpes simplex virus type 2 glycoprotein H interacts with integrin alphavbeta3 to facilitate viral entry and calcium signaling in human genital tract epithelial cells. J Virol. 2014;88:10026–10038. doi: 10.1128/JVI.00725-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108:407–419. doi: 10.1016/s0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Huang DY, Huong SM, Huang ES. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat Med. 2005;11:515–521. doi: 10.1038/nm1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang E, Arvin AM, Oliver SL. A role for the alphaV integrin subunit in Varicella Zoster Virus-mediated fusion and infection. J Virol. 2016 doi: 10.1128/JVI.00792-16. The requirement for integrins in VZV gB/gH-gL mediated fusion is demonstrated for the first time. A novel fusion assay was developed that provides a reporter signal combined with an shRNA knockdown cassette on the same vector.
- 60.Johnson DC, Ligas MW. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988;62:4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karger A, Mettenleiter TC. Glycoproteins gIII and gp50 play dominant roles in the biphasic attachment of pseudorabies virus. Virology. 1993;194:654–664. doi: 10.1006/viro.1993.1305. [DOI] [PubMed] [Google Scholar]
- 62.Mettenleiter TC, Zsak L, Zuckermann F, Sugg N, Kern H, Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J Virol. 1990;64:278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laquerre S, Argnani R, Anderson DB, Zucchini S, Manservigi R, Glorioso JC. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J Virol. 1998;72:6119–6130. doi: 10.1128/jvi.72.7.6119-6130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen JI, Seidel KE. Absence of varicella-zoster virus (VZV) glycoprotein V does not alter growth of VZV in vitro or sensitivity to heparin. J Gen Virol. 1994;75(Pt 11):3087–3093. doi: 10.1099/0022-1317-75-11-3087. [DOI] [PubMed] [Google Scholar]
- 65.Gray WL, Byrne BH. Characterization of the simian varicella virus glycoprotein C, which is nonessential for in vitro replication. Arch Virol. 2003;148:537–545. doi: 10.1007/s00705-002-0945-9. [DOI] [PubMed] [Google Scholar]
- 66.Storlie J, Carpenter JE, Jackson W, Grose C. Discordant varicella-zoster virus glycoprotein C expression and localization between cultured cells and human skin vesicles. Virology. 2008;382:171–181. doi: 10.1016/j.virol.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Storlie J, Jackson W, Hutchinson J, Grose C. Delayed biosynthesis of varicella-zoster virus glycoprotein C: upregulation by hexamethylene bisacetamide and retinoic acid treatment of infected cells. J Virol. 2006;80:9544–9556. doi: 10.1128/JVI.00668-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adamiak B, Trybala E, Mardberg K, Johansson M, Liljeqvist JA, Olofsson S, Grabowska A, Bienkowska-Szewczyk K, Szewczyk B, Bergstrom T. Human antibodies to herpes simplex virus type 1 glycoprotein C are neutralizing and target the heparan sulfate-binding domain. Virology. 2010;400:197–206. doi: 10.1016/j.virol.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 69.Li Q, Ali MA, Cohen JI. Insulin degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell. 2006;127:305–316. doi: 10.1016/j.cell.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen JJ, Zhu Z, Gershon AA, Gershon MD. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell. 2004;119:915–926. doi: 10.1016/j.cell.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Zhu Z, Gershon MD, Ambron R, Gabel C, Gershon AA. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose 6-phosphate and heparin. Proc Natl Acad Sci U S A. 1995;92:3546–3550. doi: 10.1073/pnas.92.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carpenter JE, Jackson W, de Souza GA, Haarr L, Grose C. Insulin-degrading enzyme binds to the nonglycosylated precursor of varicella-zoster virus gE protein found in the endoplasmic reticulum. J Virol. 2010;84:847–855. doi: 10.1128/JVI.01801-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olson LJ, Peterson FC, Castonguay A, Bohnsack RN, Kudo M, Gotschall RR, Canfield WM, Volkman BF, Dahms NM. Structural basis for recognition of phosphodiester-containing lysosomal enzymes by the cation-independent mannose 6-phosphate receptor. Proc Natl Acad Sci U S A. 2010;107:12493–12498. doi: 10.1073/pnas.1004232107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foster TP, Chouljenko VN, Kousoulas KG. Functional and physical interactions of the herpes simplex virus type 1 UL20 membrane protein with glycoprotein K. J Virol. 2008;82:6310–6323. doi: 10.1128/JVI.00147-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Foster TP, Melancon JM, Olivier TL, Kousoulas KG. Herpes simplex virus type 1 glycoprotein K and the UL20 protein are interdependent for intracellular trafficking and trans-Golgi network localization. J Virol. 2004;78:13262–13277. doi: 10.1128/JVI.78.23.13262-13277.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dietz P, Klupp BG, Fuchs W, Kollner B, Weiland E, Mettenleiter TC. Pseudorabies virus glycoprotein K requires the UL20 gene product for processing. J Virol. 2000;74:5083–5090. doi: 10.1128/jvi.74.11.5083-5090.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Govero J, Hall S, Heineman TC. Intracellular localization of varicella-zoster virus ORF39 protein and its functional relationship to glycoprotein K. Virology. 2007;358:291–302. doi: 10.1016/j.virol.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 78.Jambunathan N, Charles AS, Subramanian R, Saied AA, Naderi M, Rider P, Brylinski M, Chouljenko VN, Kousoulas KG. Deletion of a Predicted beta-Sheet Domain within the Amino Terminus of Herpes Simplex Virus Glycoprotein K Conserved among Alphaherpesviruses Prevents Virus Entry into Neuronal Axons. J Virol. 2016;90:2230–2239. doi: 10.1128/JVI.02468-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat Rev Microbiol. 2011;9:369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heineman TC, Hall SL. Role of the varicella-zoster virus gB cytoplasmic domain in gB transport and viral egress. J Virol. 2002;76:591–599. doi: 10.1128/JVI.76.2.591-599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang E, Arvin AM, Oliver SL. The cytoplasmic domain of varicella-zoster virus glycoprotein H regulates syncytia formation and skin pathogenesis. PLoS Pathog. 2014;10:e1004173. doi: 10.1371/journal.ppat.1004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.King DF, King LA. Giant cells in lesions of varicella and herpes zoster. Am J Dermatopathol. 1986;8:456–458. doi: 10.1097/00000372-198610000-00023. [DOI] [PubMed] [Google Scholar]
- 83.Li Q, Krogmann T, Ali MA, Tang WJ, Cohen JI. The amino terminus of varicella-zoster virus (VZV) glycoprotein E is required for binding to insulin-degrading enzyme, a VZV receptor. J Virol. 2007;81:8525–8532. doi: 10.1128/JVI.00286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ali MA, Li Q, Fischer ER, Cohen JI. The insulin degrading enzyme binding domain of varicella-zoster virus (VZV) glycoprotein E is important for cell-to-cell spread and VZV infectivity, while a glycoprotein I binding domain is essential for infection. Virology. 2009;386:270–279. doi: 10.1016/j.virol.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olson JK, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Z, Hao Y, Gershon MD, Ambron RT, Gershon AA. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yao Z, Jackson W, Grose C. Identification of the phosphorylation sequence in the cytoplasmic tail of the varicella-zoster virus Fc receptor glycoprotein gpI. J Virol. 1993;67:4464–4473. doi: 10.1128/jvi.67.8.4464-4473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gershon AA. Varicella-zoster vaccine. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: 2007. [PubMed] [Google Scholar]
- 90.American Academy of Pediatrics Committee on Infectious D. Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine 2-dose varicella immunization schedule. Pediatrics. 2007;120:221–231. doi: 10.1542/peds.2007-1089. [DOI] [PubMed] [Google Scholar]
- 91.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 92.Hammond O, Wang Y, Green T, Antonello J, Kuhn R, Motley C, Stump P, Rich B, Chirmule N, Marchese RD. The optimization and validation of the glycoprotein ELISA assay for quantitative varicella-zoster virus (VZV) antibody detection. J Med Virol. 2006;78:1679–1687. doi: 10.1002/jmv.20754. [DOI] [PubMed] [Google Scholar]
- 93.Li S, Chan IS, Matthews H, Heyse JF, Chan CY, Kuter BJ, Kaplan KM, Vessey SJ, Sadoff JC. Inverse relationship between six week postvaccination varicella antibody response to vaccine and likelihood of long term breakthrough infection. Pediatr Infect Dis J. 2002;21:337–342. doi: 10.1097/00006454-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 94.Shinefield H, Black S, Digilio L, Reisinger K, Blatter M, Gress JO, Brown ML, Eves KA, Klopfer SO, Schodel F, Kuter BJ. Evaluation of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children. Pediatr Infect Dis J. 2005;24:665–669. doi: 10.1097/01.inf.0000172902.25009.a1. [DOI] [PubMed] [Google Scholar]
- 95.Shinefield HR, Black SB, Staehle BO, Matthews H, Adelman T, Ensor K, Li S, Chan I, Heyse J, Waters M, Chan CY, Vessey SJ, Kaplan KM, Kuter BJ Kaiser Permanente Medical Team for V. Vaccination with measles, mumps and rubella vaccine and varicella vaccine: safety, tolerability, immunogenicity, persistence of antibody and duration of protection against varicella in healthy children. Pediatr Infect Dis J. 2002;21:555–561. doi: 10.1097/00006454-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 96.Levin MJ, Schmader KE, Pang L, Williams-Diaz A, Zerbe G, Canniff J, Johnson MJ, Caldas Y, Cho A, Lang N, Su SC, Parrino J, Popmihajlov Z, Weinberg A. Cellular and Humoral Responses to a Second Dose of Herpes Zoster Vaccine Administered 10 Years After the First Dose Among Older Adults. J Infect Dis. 2016;213:14–22. doi: 10.1093/infdis/jiv480. [DOI] [PubMed] [Google Scholar]
- 97.Sadaoka K, Okamoto S, Gomi Y, Tanimoto T, Ishikawa T, Yoshikawa T, Asano Y, Yamanishi K, Mori Y. Measurement of varicella-zoster virus (VZV)-specific cell-mediated immunity: comparison between VZV skin test and interferon-gamma enzyme-linked immunospot assay. J Infect Dis. 2008;198:1327–1333. doi: 10.1086/592219. [DOI] [PubMed] [Google Scholar]
- 98.Vermeulen JN, Lange JM, Tyring SK, Peters PH, Nunez M, Poland G, Levin MJ, Freeman C, Chalikonda I, Li J, Smith JG, Caulfield MJ, Stek JE, Chan IS, Vessey R, Schodel FP, Annunziato PW, Schlienger K, Silber JL. Safety, tolerability, and immunogenicity after 1 and 2 doses of zoster vaccine in healthy adults >/=60 years of age. Vaccine. 2012;30:904–910. doi: 10.1016/j.vaccine.2011.11.096. [DOI] [PubMed] [Google Scholar]
- 99.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barbera J, Vesikari T, Watanabe D, Weckx L, Zahaf T, Heineman TC Group ZOES. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 100.Chouljenko DV, Kim IJ, Chouljenko VN, Subramanian R, Walker JD, Kousoulas KG. Functional hierarchy of herpes simplex virus 1 viral glycoproteins in cytoplasmic virion envelopment and egress. J Virol. 2012;86:4262–4270. doi: 10.1128/JVI.06766-11. [DOI] [PMC free article] [PubMed] [Google Scholar]