Abstract
Interval timing is crucial for decision-making and motor control and is impaired in many neuropsychiatric disorders. Previous studies examined timing in various strains or genetically-altered mice, but not in parallel in male and female mice in the same experimental setting. We investigated timing and attention to time in male and female C57Bl/6J mice, when presented with gaps in the timed stimulus, novel auditory distracters presented during the un-interrupted timed stimulus, and gap+distracter combinations. No sex differences were found in regard to timing accuracy and precision. However, presentation of the gap+distracter combination over-reset timing in males but had a much smaller effect in females. The over-reset strategy was reported previously with emotional distracters (e.g., previously paired with footshock) but not with neutral distracters. These results reveal sex differences in attentional gating/switching or working memory for time.
Keywords: attention, C57Bl/6, interval timing, mouse, sex differences
Interval timing, or timing in the seconds-to-minutes range, is essential for rate estimation, planning and decision-making [1]. Deficits in interval timing are reported in many human central nervous system disorders, particularly in those associated with alterations in the dopaminergic system, as Parkinson’s Disease [2], Huntington’s Disease [3], Attention Deficit Hyperactivity Disorder [4], or schizophrenia [5]. While most interval timing studies in animals use rats as subjects, less is known about interval timing in mice. Mouse studies have been carried out in diverse strains or in genetically-engineered mice, using diverse timing paradigms, either only in males or only in females. This raises questions in interpretation, since differences in performance are reported in different strains, genders, and paradigms: fear conditioning [6], spatial memory, and social behavior [7]. We recently demonstrated that male C57Bl/6 mice -- the strain most used for behavioral studies -- shows accurate and scalar timing [8], and can be further used for genetic studies of timing [9]. Yet, to date, sex differences in attention to, and memory for time have not been investigated in a systematic manner in mice.
The peak-interval (PI) procedure is widely used to evaluate interval timing [10]. Changes in memory capacity and attention gating can be tested in the PI procedure by presenting unexpected events and observing changes in the timing of responses in trials with events relative to trials without events. For example, presentation of (dark) gaps in the timed visual signal delays rats’ responses with approximately the duration of the gap, suggesting that rats use a “stop” mode: they devote attentional resources to, and retain in working memory, the pre-gap interval, and resume timing after the gap where they left off before the gap [11]. However, presentation of novel neutral distracters during the uninterrupted to-be-timed stimulus considerably delays responding, suggesting rats fail to devote attentional resources to timing, do not retain the pre-gap interval in working memory, and restart timing after the distracter, using a “reset” mode [reviewed in 12]. Moreover, emotional distracters (e.g., previously paired with footshock) delay timing much longer than a reset [13, 14], suggesting that rats fail to switch attentional resources back to timing long after the end of the distracter, in an “over-reset” mode. To date, no “over-reset” has been reported following neutral distracters.
This study addresses two questions: To date no study investigated interval timing, attention to, and memory for time in male and female C57Bl/6J mice in the same experimental setting. Second, to date no study has evaluated the effect of gaps (interruptions in the timed stimulus), novel distracters (presented during the un-interrupted timed stimulus), and gap+distracter combinations. Six month-old male (n=9) and female (n=8) C57Bl/6J mice (Jackson Labs, Bar Harbor ME) were housed in groups of three or four in a temperature-controlled room under a 12-hr light-dark cycle. Mice were tested during the light period of the cycle. Mice were maintained at 85% of their ad libitum weight by restricting their access to food (Diet 5001, PMI Nutrition International, Brentwood MO). Water was given ad libitum in the home cages. Manipulations were performed in compliance with ethical standards for the treatment of animals, and approved by Utah State University IACUC Committee.
Training in the peak-interval (PI) procedure was conducted in two sets of operant chambers housed in a sound attenuating cubicles (Med Associates, St. Albans VT) [9]. Each chamber was equipped with two levers (only the left lever was used) and a foodcup between the levers on the front wall, and a house light and a 78-dB white noise generator/speaker on the opposing wall. Precision food pellets 20mg (BioServ, Frenchtown NJ) were delivered in the food cup according to the paradigm. Experiments were run in the silent box (no fan). The intensity of the white noise was measured with a sound-level meter (Realistic Radio Shack, Model 33–2050) from the center of the silent box.
Mice received 12 fixed-interval (FI) 20s sessions, followed by 16 PI sessions. Afterwards, mice received 4 test sessions which included FI, PI, gap, distracter, and gap+ distracter trials. Gap trials were similar to PI trials, except that 10s after the onset of the to-be-timed house light, the house light was turned off for 5s, and then turned back on for the remainder of the trial. Distracter trials were similar to PI trials, except that 10s after the onset of the to-be-timed house light, a novel 78-db white-noise stimulus was presented for 5s (during the un-interrupted house light). Gap+distracter trials were similar to gap trials, except that the 5-s noise was presented during the 5-s gap in visual signal.
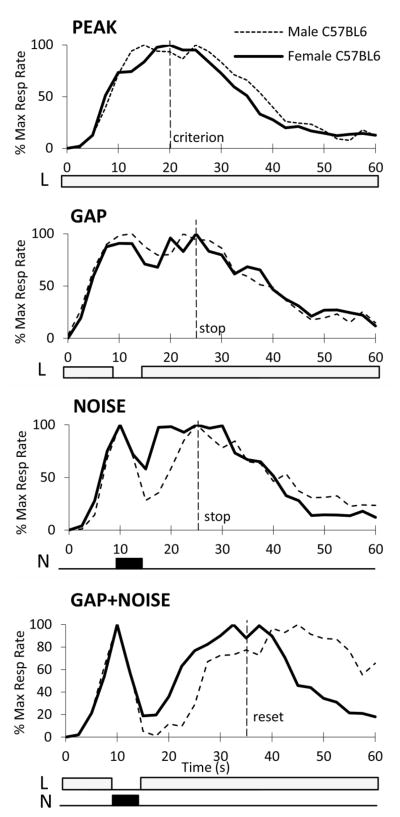
The average normalized response curves in the four trial types are shown in Fig. 1. Mice seem to have acquired the timing task as their responses peaked close to the 20-s criterion time in PI trials (“run” mode). Presentation of gaps and/or distracters delayed timing with varying intervals: A “stop” mode would result in a 5-s delay relative to the 20-s criterion, such that mice would peak at about 25s; a “reset” mode would result in restarting timing the 20-s criterion after the interrupting event, such that mice would peak about 35s. Fig. 1 indicates that presentation of gaps or distracters minimally delays timing in both male and female mice, consistent with a “stop” mode. Most interestingly, presentation of gap+distracter events delays timing considerably in female mice (between “stop” and “reset”) and “over-resets” timing in males. To evaluate these suggestions, timing accuracy and precision were estimated by averaging the individual response curves (in 2.5s bins) over the 4 test sessions, and fitting the individual curves in the window 15s to 55s by a Gaussian function (R2 = 0.89 ± 0.01) (Buhusi and Meck, 2000). Timing accuracy (peak time) and precision (width of response function) were submitted to mixed ANOVAs with between-subjects variable Sex (M, F) and within-subjects variables Gap (no gap, gap) and Distracter (no distracter, distracter). All statistical analyses were conducted with an alpha-level of 0.05.
Fig. 1. Interval timing in the PI procedure with distracters in male and female C57Bl/6J mice.
Average response curves (normalized to maximum response rate) in peak, gap, distracter, and gap+distracter trials. Vertical broken lines indicate the 20s criterion time, the stop time (25s), and the reset time (35s). Diagrams under each panel show the light-noise test stimulus. L = light to-be-timed stimulus; N = noise distracter.
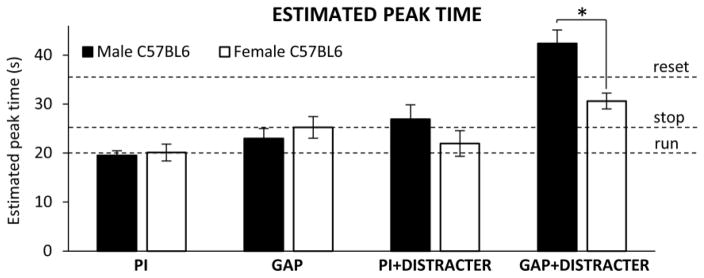
As shown in Fig. 2, no sex differences in response peak time were found in PI trials (F(1,15)=0.08, p>0.05). The estimated peak time was 19.6 ± 0.9s in male mice (not reliably different from the 20-s criterion time, t(8)=0.47, p>0.05), and 20.1 ± 1.7s in female mice (not reliably different from the 20-s criterion time, t(7)=0.07, p>0.05). These results suggest that all mice acquired the timing task (with no reliable sex differences in timing accuracy).
Fig. 2. Estimated timing accuracy and timing delay in male and female C57Bl/6J mice.
Average estimated peak time (± SEM) in PI, gap, distracter, and gap+distracter trials. The horizontal broken lines indicate the criterion time (20s), the stop time (25s), and the reset time (35s). A significant difference was found between male and female mice in gap+distracter trials only. * = p<0.05.
Analyses of timing precision (estimated width of the response function) failed to indicate any main effects or interactions (all Fs(1,15)<2.84, p>0.05), suggesting that irrespective of sex and interrupting event the response function was simply delayed relative to PI trials, with no changes in the shape of the response function. Analyses of timing accuracy (estimated response peak time), indicated a main effect of Gap (F(1,15)=23.24, p<0.01), and a main effect of Distracter (F(1,15)=23.79, p<0.01), suggesting that presentation of either visual gaps or noise distracters delays timing. No sex differences in response peak time were found in either gap (F(1,15)=0.57, p>0.05) or distracter trials (F(1,15)=1.53, p>0.05). In both male and female mice, response timing was reliably delayed relative to PI trials in both gap trials (F(1,15)=6.54, p<0.05) and distracter trials (F(1,15)=4.84, p<0.05). In neither males nor females the response peak time in gap or distracter trials was reliably different from the 25s “stop” mode (all ts(8)<0.98, p>0.05 for males; all ts(7)<1.15, p>0.05 for females).
Analyses of timing accuracy further indicated a Gap x Distracter interaction (F(1,15)=7.68, p<0.05), suggesting that presentation of gap+distracter events delays timing more than the individual events, and a Distracter x Sex interaction (F(1,15)=7.53, p<0.05), suggesting that distracters have differential effects in male and female mice. In females, the response function peaked at 30.7 ± 1.6s, reliably larger than the 25s “stop” mode (t(7)=3.49, p<0.01), but also reliably smaller than the 35s “reset” mode (t(7)=2.69, p<0.05). However, males peaked at 42.39 ± 2.7s, reliably larger than the 35s “reset” mode (t(8)=2.69, p<0.05). Therefore, results suggest that mice flexibly delay their timing when various distracters are presented: Mice use a “stop” mode during both gap and distracter trials, while in gap+distracter trials female mice use a stop/reset mode and male mice over-reset.
These results suggest that timing can be over-reset by neutral, novel gap+distracter events, a phenomenon difficult to explain by current timing theories. Interestingly, an over-reset strategy has been previously reported only when rodents were presented with emotionally-charged distracters (e.g., stimuli previously paired with footshock) [13, 14], but not by manipulating the intensity of a neutral noise distracter [15]. To date, this is the first study in which mice have been tested with various distracters, and the first report of an over-reset after an interruption by neutral stimuli.
Current timing models address the effect of distracters using stop and reset “modes”, which are more descriptive than mechanistic. Moreover, as long the over-reset mode was reported only when the distracter was paired with footshock [13, 14], it was largely ignored by timing theorists, as it could be explained by a non-timing phenomenon: freezing after a stimulus paired with footshock. For example, a description of the neurobiological mechanisms involved in interval timing is currently provided by the Striatal Beat-Frequency (SBF) model, which ascribes a role for detecting event durations to medium spiny neurons within the dorsal striatum [1, 16], which become entrained to fire in response to oscillating, coincident cortical inputs that become active at previously trained event durations [17]. However, our recent investigation of the SBF model failed to reveal a neurobiologically-plausible mechanism that would allow the SBF model to flexibly stop, reset, and over-reset in response to distracters [18], suggesting that currently the SBF model cannot flexibly address these phenomena in a neurobiologically-plausible manner. Nevertheless, the observation that males and females differ in regard to attentional processing during timing is compatible with the view that multiple brain regions or neural circuits are involved in the control of an internal clock [17].
The best current explanation of the effect of distracters in interval timing is provided by an attentional model [15] which proposes that presentation of a distracter event re-allocates attentional and memory resources in proportion to the discriminability of the event; in turn, the more resources are diverted from timing, the more timing is delayed. For example, the gap+distracter event resulted in a larger delay than the gap or distracter alone, supposedly because it was easier to discriminate (more salient) than each of the two. However, to date, this model explains the effect of distracters only in the stop-reset range. Our results suggest that this attentional model [12] should be extended to address the over-reset effect.
Sex-related differences have been reported for numerous behavioral and cognitive tasks, both in animals and in humans, suggesting differences in attentional processing and working memory. For example, women perform better than men in terms of accuracy and speed in visual attention tasks with auditory distracters, possibly reflecting differences in sensory gating functions [19], they require fewer resources than men in divided attention tasks [20], they outperform men in multitasking paradigms possibly due to an increased capacity to rapidly switch between different tasks [21], and show more working memory in delay-matching-to-sample tasks involving specific sensory modalities [22]. All these interpretations are consistent with our findings that female mice delay less, and male mice over-reset, after gap+disctrater events. Female mice may be disrupted less by auditory distracters, they may need fewer resources to process the distracter, may be able to more rapidly switch from processing the gap+distracter event to the timing task, or may have better working memory for time. Future studies should differentiate between these mechanisms contributing to sex differences in distractibility during interval timing tasks.
Many psychiatric disorders are characterized by skewed male-to-female ratios, thus evaluating sex differences in behavioral paradigms is important and relevant to pathology. The sex differences in distractibility and attention to time reported here may be relevant to disorders such as ADHD, Schizophrenia, and Autism, disorders characterized by an increased prevalence in men [23–25] and impairments in temporal processing and attention [26–28]. The source of the male bias is poorly understood [29]. As timing and attention to time rely on thalamo-cortical-striatal circuits modulated by dopamine [1], anatomical differences in dopaminergic circuits between males and females (due to the organizational effect of hormones [30, 31]), or acute effects of hormones on striatal dopamine release [32] could explain behavioral differences found in our study.
In turn, our findings call for a re-evaluation of interval timing studies in mouse models of disease. For example, while we investigated interval timing in the CHL1 model of Schizophrenia only in male C57Bl/6J mice [9], others have investigated interval timing in the D2OE mouse model of Schizophrenia only in female mice in a mixed background [33]. Contrasting the effects of these manipulations on interval timing can be made only after the sex and background variables have been fully accounted for. The present study suggests that the C57Bl/6J mice, the mouse strain most used for behavioral investigations does not display sex differences in timing accuracy and precision, but shows differences in attention to and memory for time, which may be relevant to pathology.
Highlights.
Timing was evaluated in male and female C57Bl/6J mice in the peak-interval procedure
Timing accuracy and precision did not differ between males and females
Mice stopped timing after visual gaps or auditory distracters
After gap+distracter female mice reset timing while male mice over-reset timing
This is first report of an over-reset of timing following neutral distracters in rodents
Acknowledgments
This work was supported by grant NS090283 from the National Institutes of Health to MB, and by a NARSAD Independent Investigator Award from the Brain and Behavior Research Foundation to CVB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6:755–65. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 2.Malapani C, Deweer B, Gibbon J. Separating storage from retrieval dysfunction of temporal memory in Parkinson’s disease. J Cog Neurosci. 2002;14:311–22. doi: 10.1162/089892902317236920. [DOI] [PubMed] [Google Scholar]
- 3.Paulsen JS, Zimbelman JL, Hinton SC, Langbehn DR, Leveroni CL, Benjamin ML, et al. fMRI Biomarker of Early Neuronal Dysfunction in Presymptomatic Huntington’s Disease. American Journal of Neuroradiology. 2004;25:1715–21. [PMC free article] [PubMed] [Google Scholar]
- 4.Barkley RA, Murphy KR, Bush T. Time perception and reproduction in young adults with attention deficit hyperactivity disorder. Neuropsychology. 2001;15:351–60. doi: 10.1037//0894-4105.15.3.351. [DOI] [PubMed] [Google Scholar]
- 5.Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009;71:345–53. doi: 10.1016/j.bandc.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith DR, Gallagher M, Stanton ME. Genetic background differences and nonassociative effects in mouse trace fear conditioning. Learn Mem. 2007;14:597–605. doi: 10.1101/lm.614807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, et al. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–63. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Buhusi CV, Aziz D, Winslow D, Carter RE, Swearingen JE, Buhusi MC. Interval timing accuracy and scalar timing in C57BL/6 mice. Behav Neurosci. 2009;123:1102–13. doi: 10.1037/a0017106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buhusi M, Scripa I, Williams CL, Buhusi CV. Impaired interval timing and spatial-temporal integration in mice deficient in CHL1, a gene associated with schizophrenia. Timing & Time Perception. 2013;1:21–8. doi: 10.1163/22134468-00002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buhusi CV, Meck WH. Timing Behavior. In: IPS, editor. Encyclopedia of Psychopharmacology. Berlin Heidelberg: Springer; 2010. pp. 1319–23. [Google Scholar]
- 11.Buhusi CV, Meck WH. Timing for the Absence of a Stimulus: The Gap Paradigm Reversed. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:305–22. doi: 10.1037//0097-7403.26.3.305. [DOI] [PubMed] [Google Scholar]
- 12.Buhusi CV, Meck WH. Relative time sharing: new findings and an extension of the resource allocation model of temporal processing. Philos Trans R Soc Lond B Biol Sci. 2009;364:1875–85. doi: 10.1098/rstb.2009.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown BL, Richer P, Doyere V. The effect of an intruded event on peak-interval timing in rats: isolation of a postcue effect. Behav Processes. 2007;74:300–10. doi: 10.1016/j.beproc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Matthews AR, He OH, Buhusi M, Buhusi CV. Dissociation of the role of the prelimbic cortex in interval timing and resource allocation: beneficial effect of norepinephrine and dopamine reuptake inhibitor nomifensine on anxiety-inducing distraction. Front Integr Neurosci. 2012;6:111. doi: 10.3389/fnint.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buhusi CV. Time-sharing in rats: effect of distracter intensity and discriminability. J Exp Psychol Anim Behav Process. 2012;38:30–9. doi: 10.1037/a0026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cognitive Brain Research. 2004;21:139–70. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Buhusi CV, Oprisan SA, Buhusi M. Clocks within Clocks: Timing by Coincidence Detection. Current opinion in behavioral sciences. 2016;8:207–13. doi: 10.1016/j.cobeha.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oprisan SA, Dix S, Buhusi CV. Phase resetting and its implications for interval timing with intruders. Behav Processes. 2014;101:146–53. doi: 10.1016/j.beproc.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomasi D, Chang L, Caparelli EC, Ernst T. Sex differences in sensory gating of the thalamus during auditory interference of visual attention tasks. Neuroscience. 2008;151:1006–15. doi: 10.1016/j.neuroscience.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Secer I, Yilmazogullari Y. Are attentional resources a mediator for sex differences in memory? International journal of psychology : Journal international de psychologie. 2016;51:117–22. doi: 10.1002/ijop.12117. [DOI] [PubMed] [Google Scholar]
- 21.Stoet G, O’Connor DB, Conner M, Laws KR. Are women better than men at multi-tasking? BMC Psychology. 2013;1:1–10. [Google Scholar]
- 22.Roddick KM, Schellinck HM, Brown RE. Olfactory delayed matching to sample performance in mice: sex differences in the 5XFAD mouse model of Alzheimer’s disease. Behav Brain Res. 2014;270:165–70. doi: 10.1016/j.bbr.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 23.Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Current opinion in neurology. 2013;26:146–53. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2012;9:490–9. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrath JJ. Variations in the incidence of schizophrenia: data versus dogma. Schizophrenia bulletin. 2006;32:195–7. doi: 10.1093/schbul/sbi052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falter CM, Noreika V, Wearden JH, Bailey AJ. More consistent, yet less sensitive: interval timing in autism spectrum disorders. Quarterly journal of experimental psychology. 2012;65:2093–107. doi: 10.1080/17470218.2012.690770. [DOI] [PubMed] [Google Scholar]
- 27.Penney TB, Meck WH, Roberts SA, Gibbon J, Erlenmeyer-Kimling L. Interval-timing deficits in individuals at high risk for schizophrenia. Brain Cogn. 2005;58:109–18. doi: 10.1016/j.bandc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Hwang-Gu SL, Gau SS. Interval timing deficits assessed by time reproduction dual tasks as cognitive endophenotypes for attention-deficit/hyperactivity disorder. PLoS One. 2015;10:e0127157. doi: 10.1371/journal.pone.0127157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waddell J, McCarthy MM. Sexual differentiation of the brain and ADHD: what is a sex difference in prevalence telling us? Current topics in behavioral neurosciences. 2012;9:341–60. doi: 10.1007/7854_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 2008;28:9525–35. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker JB. Sexual differentiation of motivation: a novel mechanism? Hormones and behavior. 2009;55:646–54. doi: 10.1016/j.yhbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neuroscience and biobehavioral reviews. 2000;24:137–41. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 33.Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–9. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]