Abstract
Drugs of abuse alter synaptic connections in the ‘reward circuit’ of the brain, which leads to long-lasting behavioral changes that underlie addiction. Here we show that cadherin adhesion molecules play a critical role in mediating synaptic plasticity and behavioral changes driven by cocaine. We demonstrate that cadherin is essential for long-term potentiation (LTP) in the ventral tegmental area (VTA), and is recruited to the synaptic membrane of excitatory inputs onto dopaminergic neurons following cocaine-mediated behavioral conditioning. Furthermore, we show that stabilization of cadherin at the membrane of these synapses blocks cocaine-induced synaptic plasticity, leading to a significant reduction in conditioned place preference induced by cocaine. Our findings identify cadherins and associated molecules as targets of interest for understanding pathological plasticity associated with addiction.
Introduction
Drugs of abuse induce widespread alterations to the neural circuits that mediate reward learning in the brain. Cocaine exposure drives the strengthening of excitatory inputs onto dopaminergic neurons of the ventral tegmental area (VTA)1–4, and causes increased release of dopamine from the VTA onto corticolimbic structures, including the nucleus accumbens (NAc), the prefrontal cortex (PFC) and the dorsal striatum5,6. Drug-evoked synaptic plasticity in the VTA is believed to underlie behavioural changes that lead to addiction. The potentiation of excitatory inputs to dopaminergic neurons is increased following associative learning of reward-predicting cues7, and intact glutamatergic synapse function in the VTA is required for the formation of cocaine-induced conditioned place preference (CPP)8, indicating that plasticity at these synapses may contribute to the learned association between environmental cues and the rewarding effects of cocaine. Electrophysiological studies have shown that cocaine-induced potentiation of VTA synapses is mediated by the insertion of Ca2+-permeable, GluA2-lacking AMPA receptors (AMPARs) to the synaptic membrane9,10. In order to determine how drugs of abuse alter synapses within the reward circuit and cause behavioural changes underlying addiction, it is important to further understand the molecular mechanisms that mediate drug-induced plasticity at synapses in the VTA.
Cadherin adhesion molecules have been shown to play a critical role in synaptic plasticity underlying different forms of learning and memory. Cadherins mediate adhesion at synapses through homophilic trans interactions across the synaptic membrane and associate with AMPARs through direct and indirect interactions with both GluA1 and GluA2 AMPAR subunits11–13. In the hippocampus, cadherins are essential for long-term potentiation (LTP) and long-term depression (LTD). Following enhanced activity, cadherins are increasingly localized to the synaptic membrane14, leading to increased synapse stability15,16 and the stabilization of AMPAR at the synaptic membrane17. In contrast, during LTD the internalization of cadherin from the synaptic membrane is required for the removal of AMPARs from the postsynaptic membrane18. Disruption of trans-synaptic cadherin interaction in the hippocampus has been shown to abolish the acquisition of context-dependent memory formation20, while aberrant increases in cadherin stability at the membrane leads to impaired in behavioural flexibility on hippocampal-dependent tasks19.
Due to their role in regulating AMPAR trafficking and stability at synapses, cadherins are strong candidate molecules for mediating plasticity in the VTA underlying behavioural changes driven by drugs of abuse. In the context of addiction, genome-wide association studies have identified mutations in cadherin adhesion complex proteins as risk factors for substance abuse21. However, very little is known about the expression of cadherins in the VTA, and their potential function in synaptic plasticity in this region has not been examined.
In the present study, we show that cadherin plays a key role in synaptic plasticity in the VTA and behavioural changes driven by cocaine. We demonstrate that cadherins are widely expressed in dopaminergic neurons and are essential for long-term potentiation (LTP) of synapses in the VTA. We further demonstrate that recruitment of cadherin to excitatory inputs onto dopaminergic neurons is correlated with cocaine-mediated conditioned place preference (CPP). Finally, we demonstrate that stabilization of cadherin at the synaptic membrane of synapses onto dopaminergic neurons can completely block cocaine-induced changes in AMPAR localization and LTP, and greatly reduce behavioural conditioning driven by cocaine.
Results
Cadherins are expressed in dopaminergic neurons and required for LTP
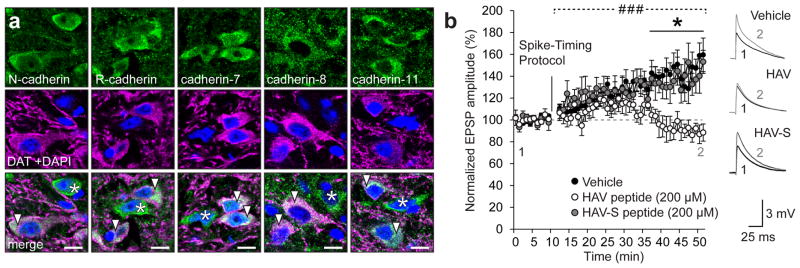
We first examined the expression of several classical cadherins and found that cadherins are widely expressed in both dopaminergic and non-dopaminergic neurons in the VTA (Fig. 1a, Supplementary Fig. 1). Nearly all dopaminergic cells were immunopositive for N-cadherin, R-cadherin, cadherin-7, cadherin-8, and cadherin-11, isoforms whose mRNA had previously been detected in this region22,23.
Figure 1. Cadherins are expressed in dopaminergic neurons and are essential for LTP in the VTA.
(a) VTA neurons co-immunostained for cadherins (green), dopamine transporter (DAT; magenta) and DAPI (blue). Arrowheads indicate neurons positive for both cadherin and DAT, asterisks indicate neurons which are positive for cadherin but not DAT. Scale bar = 10 μm. (b) Spike-timing-dependent LTP in the VTA was abolished by treatment with a peptide containing an HAV motif (His-Ala-Val) that disrupts N-cadherin extracellular interactions (### p<0.0001, significant interaction between peptide treatment and time, two-way RM ANOVA, F(78,624) = 4.037, *p< 0.05, Bonferroni’s test post hoc, n=8 cells/8 mice vehicle, 6 cells/6 mice HAV, 5 cells/5 mice HAV-S). Vehicle-only and scramble peptide (HAV-S) had no effect on LTP. Data shown as mean ± SEM.
We next investigated the role of cadherins in activity-induced potentiation of excitatory synapses onto dopaminergic neurons in the VTA. We used an HAV antagonistic peptide that blocks cadherin interactions in trans to reduce cadherin stability at the synaptic membrane. Indeed, it has previously been shown that disrupting cadherin trans interactions, significantly attenuates cadherin membrane stability24. Treatment of VTA slices with the HAV peptide abolished spike-timing-dependent (STD) LTP at excitatory synapses onto dopaminergic neurons (Fig. 1b). STD LTP is mediated by the insertion of Ca2+-permeable GluA1 homomers similar to potentiation of VTA synapses induced by cocaine9,25. As cadherin can stabilize AMPARs through its association with GluA111 and/or GluA212,13 subunits, this suggested that peptide treatment disrupted STD LTP by decreasing cadherin membrane stability and thus prevents the stabilization of newly-inserted GluA1 homomers at the synaptic membrane. We therefore next sought to investigate the relationship between cadherin localization and AMPAR trafficking in synaptic plasticity and behavioural conditioning driven by cocaine.
Cadherins are recruited to VTA synapses during cocaine CPP
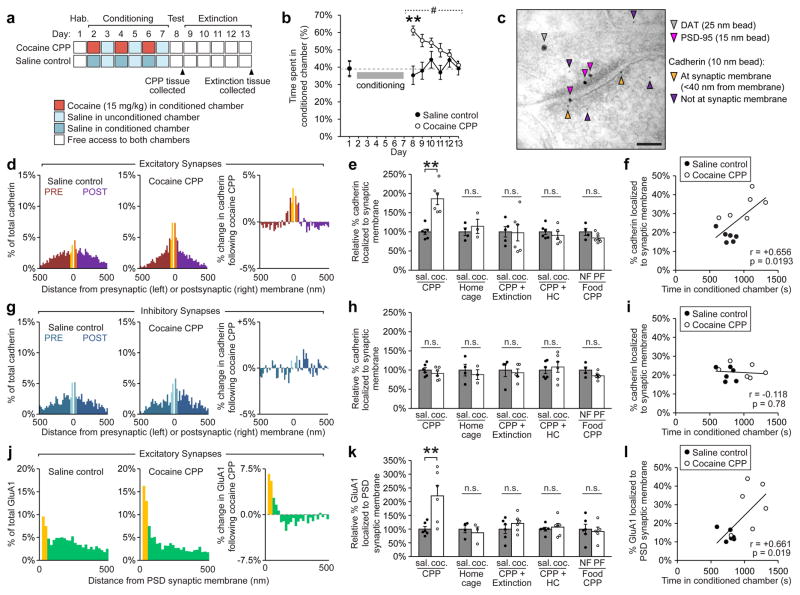
Drugs of abuse such as cocaine potentiate excitatory synapses onto dopaminergic neurons by promoting the insertion of Ca2+-permeable GluA1 homomers into synaptic membranes, which contributes to the formation and expression of drug-induced behaviours3,24,25. After demonstrating that cadherin is required for LTP at synapses in the VTA (Fig. 1b), we examined the role of cadherins in conditioned place preference (CPP), a behavioral assay that models the learned preference for previously neutral contextual cues driven by drugs of abuse26. In other brain regions, enhanced synaptic activity is associated with increased recruitment of cadherins to the synapse15,16 as well as increased stability of cadherin at the synaptic membrane18. Intact cadherin adhesion at synapses is also required for the acquisition of new memories20. We therefore hypothesized that the acquisition of cocaine-induced CPP is associated with increased insertion of cadherin to the synaptic membrane of VTA synapses. We used immunogold electron microscopy (EM) (validated in Supplementary Fig. 2) to examine nanometer-scale changes in the localization of cadherins and AMPAR subunits at synapses of the VTA following cocaine-induced CPP.
CPP was induced in a three-chamber apparatus (Fig. 2a), producing a robust increase in preference for the cocaine-paired, conditioned chamber (Fig. 2b). Mice were then sacrificed and the VTA isolated by microdissection for immunogold EM (Fig. 2c). We found that cocaine-induced CPP results in a striking redistribution of cadherin at excitatory synapses onto dopaminergic neurons in the VTA (Fig. 2d). Indeed, the proportion of cadherin localized to the synaptic membrane increased by 86% after CPP (number of immunolabelled beads within 40 nm of the synaptic membrane divided by total beads at the pre and postsynaptic compartments; Fig. 2e), though no changes in total levels of cadherin at synaptic compartments were detected (Supplementary Fig. 3). Moreover, analysis of individual mice demonstrated a strong positive correlation between the amount of cadherin at the synaptic membrane and time spent in the cocaine-paired conditioned chamber (r = +0.66, Fig. 2f). The increased localization of cadherin to the synaptic membrane was observed at both pre- and post-synaptic compartments following cocaine CPP (Supplementary Fig. 4), which is consistent with increased trans-synaptic adhesion between cadherins.
Figure 2. Cocaine-induced conditioned place preference (CPP) leads to recruitment of cadherin and GluA1 to excitatory synapses onto dopaminergic neurons in the VTA.
(a) Experimental schedule for CPP and extinction experiments. (b) Cocaine administration produced robust CPP (#p<0.001, significant interaction between treatment and test day, two-way RM ANOVA, F(6,60) = 4.422, **p<0.01, Bonferroni’s test post hoc) that extinguished over 5 days. (c) Electron micrograph of VTA synapse showing immunogold-labelled DAT, PSD-95 and cadherin (Scale bar = 100 nm). (d) Cadherin localization shifted to the synaptic membrane of excitatory synapses following cocaine CPP (20 nm bins). (e) The relative percentage of cadherin at the synaptic membrane at excitatory synapses ([#cadherin beads within 40 nm of the pre and postsynaptic membrane]/[# beads within 500 nm of the synaptic membrane], expressed as a percent relative to saline controls) was significantly increased compared to saline controls following cocaine-induced CPP, (p<0.01, significant interaction, two-way ANOVA, F(4,41) = 4.999, **p< 0.01, Bonferroni’s test post hoc), but not in home cage controls, following extinction of CPP, following return to home cage for 6 days after CPP (CPP + HC), or following food CPP (NF: No food, PF: Palatable food, see also Supplementary Fig. 6). CPP: n = 6 mice saline, 6 mice cocaine; HC: n = 4 mice saline, 3 mice cocaine; CPP+Extinction: n = 5 mice saline, 6 mice cocaine; CPP+HC: n = 6 mice saline, 5 mice cocaine; Food CPP: n = 4 mice NF, 6 mice PF. (f) The percentage of cadherin localized to the synaptic membrane at excitatory synapses was significantly correlated with time spent in conditioned chamber following cocaine CPP (Linear regression, p<0.05, F(1,10) = 7.758), 6 mice per condition. (g) No change in cadherin localization was observed at inhibitory synapses following cocaine CPP. (h) The relative percentage of cadherin at the synaptic membrane was not changed at inhibitory synapses following cocaine CPP, home cage controls, extinction of CPP, or Food CPP. CPP: n = 6 mice saline, 6 mice cocaine; HC: n = 4 mice saline, 3 mice cocaine; CPP+Extinction: n = 4 mice saline, 5 mice cocaine; CPP+HC: n = 6 mice saline, 6 mice cocaine; Food CPP: n = 4 mice NF, 6 mice PF (i) Cadherin localized to synaptic membrane at inhibitory synapses was not correlated with time spent in conditioned chamber following CPP. (j) GluA1 localization shifted towards the PSD (postsynaptic density) membrane following cocaine CPP. (k) The relative percentage of GluA1 at the PSD membrane ([# GluA1 beads within 30nm of the PSD membrane]/[# beads within 500nm of the PSD membrane], expressed as a percent relative to saline controls) was significantly increased at excitatory synapses following cocaine-induced CPP (p<0.01, significant interaction, two-way ANOVA, F(4,44) = 4.049, **p< 0.01, Bonferroni’s test post hoc), but not in home cage controls, following extinction of CPP, following return to home cage for 6 days after CPP, or following food CPP. CPP: n = 6 mice saline, 6 mice cocaine; HC: n = 4 mice saline, 3 mice cocaine; CPP+Extinction: n = 6 mice saline, 6 mice cocaine; CPP+HC: n = 6 mice saline, 6 mice cocaine; Food CPP: n = 6 mice NF, 5 mice PF (l) Percentage of GluA1 localized to the PSD membrane was significantly correlated with time spent in conditioned chamber following cocaine-induced CPP (Linear regression, p<0.05, F(1,10) = 7.848), n = 6 mice per condition. >100 synapses were analyzed per group. Data shown as mean ± SEM with individual mice (circles) overlaid.
Importantly, we saw no significant change in cadherin localization in control mice which received the same schedule of cocaine and saline administration in their home cages rather than in a novel environment (Fig. 2e, Supplementary Fig. 5 or in mice where CPP was induced using palatable food rewards instead of cocaine (Fig. 2e, Supplementary Fig. 6). This suggested that the effects observed after cocaine CPP were not due to general effects of cocaine or non-specific learning-induced plasticity, but were specifically attributable to the formation of drug-associated memories. Together, these findings indicate that insertion of cadherin into the synaptic membrane specifically occurs following the learned association between novel contextual cues and the effects of cocaine during behavioral conditioning. These changes in cadherin localization were also transient, and returned to baseline levels following active extinction of CPP or return of mice to home cages for an equivalent period of time without re-exposure to the CPP apparatus (Fig. 2e). At inhibitory synapses, no changes in cadherin distribution were observed following cocaine CPP, extinction of cocaine CPP, food CPP or in home cage controls (Fig. 2g, h, i, Supplementary Fig. 5, Supplementary Fig. 6). Additionally, no changes in cadherin localization were observed at excitatory or inhibitory synapses onto glutamatergic or GABAergic neurons in the VTA following CPP (Supplementary Fig. 7), indicating the increase in cadherin localization to the synaptic membrane was specific to excitatory synapses onto dopaminergic neurons in the VTA.
GluA1 is recruited to VTA synapses during cocaine CPP
We then used immunogold labelling to identify GluA1-containing AMPARs, and found that they exhibited the same pattern of insertion and removal from the synaptic membrane as cadherin in each of the behavioral conditions (Fig. 2j). The proportion of GluA1-containing AMPARs localized to the post-synaptic density (PSD) membrane was significantly increased following CPP (121% increase, Fig. 2k), though total levels of GluA1 were unchanged at synaptic compartments (Supplementary Fig. 3). Similar to cadherin, there was a strong positive correlation between the amount of GluA1 at the synaptic membrane and time spent in the cocaine-paired chamber for individual mice (r = +0.66, Fig. 2l), and GluA1-containing AMPAR localization was also unchanged compared to saline controls following extinction of CPP, in home cage controls, or following CPP using palatable food (Fig. 2k, Supplementary Fig. 5, Supplementary Fig. 6). These data directly demonstrate that cocaine CPP drives the insertion of GluA1-containing AMPARs to the PSD membrane, which is thought to contribute to the cocaine-mediated increase in AMPA:NMDA ratio at VTA synapses previously observed using electrophysiological techniques1,9,27.
We also examined co-immunolabelling of GluA1 and cadherin together at individual VTA synapses following cocaine CPP and found that individual synapses with a greater proportion of cadherin localized to the synaptic membrane also had significantly more GluA1 localized to the membrane (Supplementary Fig. 8). This correlation between cadherin and GluA1 levels at individual synapses provided further support that cadherin acts to stabilize GluA1 homomers at potentiated synapses, consistent with our electrophysiological data demonstrating that cadherin stability was essential for STD LTP at VTA synapses (Fig. 1b).
Cadherin stabilization at VTA synapses reduces cocaine CPP
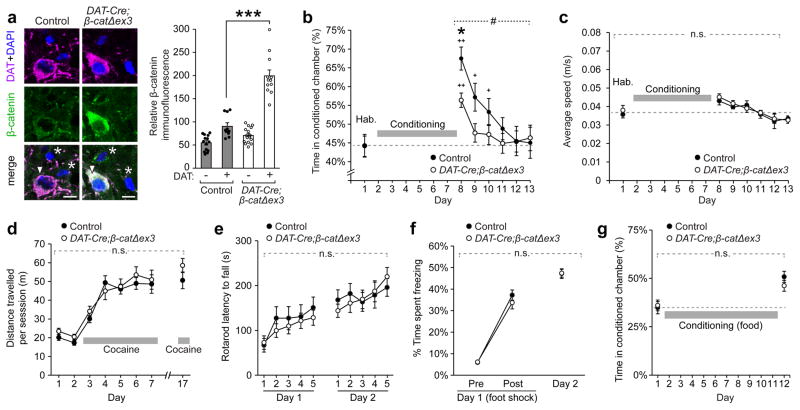
Given the strong correlation between CPP acquisition and cadherin localization at excitatory synaptic membranes in the VTA, we hypothesized that subcellular changes in cadherin localization may regulate cocaine-induced synaptic plasticity and behavioral conditioning. To test this, we increased cadherin at the synaptic membrane using a transgenic mouse line in which β-catenin levels are increased in dopaminergic neurons (‘DAT-Cre;β-catΔex3’ mice, Fig. 3a)28,29. β-catenin is the major intracellular binding partner of all classical cadherins, and we have previously shown that elevating β-catenin levels using this approach significantly increases the stabilization of cadherin and AMPARs at the synaptic membrane in hippocampal neurons in vivo19.
Figure 3. Stabilization of cadherin by β-catenin at synapses in the VTA reduces cocaine-induced CPP.
(a) β-catenin levels were significantly increased in DAT+ neurons (arrowheads) in the VTA of DAT-Cre;β-catΔex3 mice compared to adjacent DAT- cells (asterisks) and DAT+ neurons in control mice (p<0.001, significant interaction between genotype and cell type, two-way ANOVA, F(1,48) = 38.13, ***p<0.001 Bonferroni’s test post hoc, WT: n=14 cells non-DAT, 10 cells DAT+, DAT-Cre;β-catΔex3: 16 cells non-DAT, 12 cells DAT+). (b) Cocaine-induced CPP was significantly reduced in DAT-Cre;β-catΔex3 mice compared to controls (#p<0.05, significant interaction between genotype and test day, two-way RM ANOVA, F(6,264) = 2.194, * p<0.05, Bonferroni’s test post hoc, n=23 mice per genotype). Preference for the cocaine-paired chamber returned to baseline after 3 days of extinction in control mice and 1 day of extinction in DAT-Cre;β-catΔex3 mice (+p<0.01, ++p<0.0001, significantly different from day 1, Dunnett’s test post hoc). DAT-Cre;β-catΔex3 mice showed no differences in average speed (c) in the three-chamber CPP apparatus during habituation or after CPP (n=10 mice control, 14 mice DAT-Cre;β-catΔex3), and no differences in locomotor sensitization to cocaine (d) compared to littermate controls (n = 16 mice control, 16 mice DAT-Cre;β-catΔex3). (e) DAT-Cre;β-catΔex3 mice showed normal coordination and motor learning on an accelerating rotarod task (n=13 mice control, 15 mice DAT-Cre;β-catΔex3). (f) DAT-Cre;β-catΔex3 mice showed no change in the acquisition of contextual fear memory following a foot shock in a novel environment compared to littermate controls, indicating intact spatial memory formation (n=16 mice control, 16 mice DAT-Cre;β-catΔex3) (g) DAT-Cre;β-catΔex3 mice showed no impairments in CPP driven by palatable food rewards (n = 17 mice control, 12 mice DAT-Cre;β-catΔex3). Data shown as mean ± SEM,.
DAT-Cre;β-catΔex3 mice exhibited a 48% reduction in cocaine-induced CPP compared to control mice (Fig. 3b, Day 8). There was no significant difference between groups in the rate of extinction from day 8 to 9 (p>0.05, two-way ANOVA, Bonferroni’s test post hoc). However, due to the decreased magnitude of CPP, DAT-Cre;β-catΔex3 mice returned to baseline levels of preference for the conditioned chamber after 1 day of extinction (day 9), compared to 3 days in controls (day 11). Behavioral changes in these mice were specific to cocaine-mediated CPP; DAT-Cre;β-catΔex3 mice appeared phenotypically normal, exhibited no changes in exploratory behaviour or basal locomotion (Fig. 3c), and showed intact locomotor sensitization to repeated cocaine administration (Fig. 3d). DAT-Cre;β-catΔex3 mice also showed no change in motor learning (Fig. 3e), contextual fear conditioning (Fig. 3f), food consumption (Supplementary Fig. 9), or CPP driven by food rewards (Fig 3g). The lack of change in tasks which require intact recognition of a novel context (contextual fear learning and food CPP) also indicated that impairments in spatial memory were not responsible for the reduction in cocaine CPP observed in DAT-Cre;β-catΔex3 mice. We also verified that, following β-catenin stabilization in DAT-Cre;β-catΔex3 mice, no subsequent changes in Wnt pathway targets were observed in dopaminergic neurons in the VTA, indicating that the observed effects on CPP were not due to alterations in Wnt signalling (Supplementary Fig. 10).
Cadherin stabilization at VTA synapses blocks synaptic plasticity
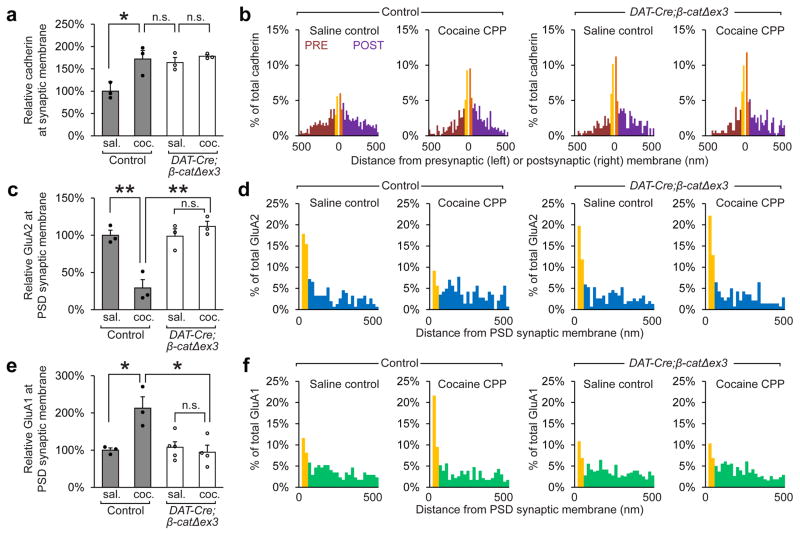
To determine why there was a marked attenuation of cocaine CPP in DAT-Cre;β-catΔex3 mice, we used immunogold EM to examine the distribution of cadherin, GluA1 and GluA2 at excitatory synapses onto VTA dopaminergic neurons after CPP. We found that the proportion of cadherin localized to the synaptic membrane was significantly increased in DAT-Cre;β-catΔex3 mice under basal conditions (~77% increase) (Fig. 4a, b). However, unlike control mice, DAT-Cre;β-catΔex3 mice did not exhibit additional recruitment of cadherin to the synaptic membrane during cocaine-mediated CPP. Additionally, both the removal of GluA2-containing AMPARs and the insertion of GluA1-containing AMPARs driven by cocaine CPP were blocked in DAT-Cre;β-catΔex3 mice (Fig. 4c, d, e, f). There was also no significant change in total levels of cadherin, GluA1 or GluA2 in DAT-Cre;β-catΔex3 mice (Supplementary Fig. 10c, d, e).
Figure 4. Stabilization of cadherin at synapses in the VTA prevents the removal of GluA2-containing AMPARs, and blocks the insertion of GluA1-containing AMPARs.
Immunogold EM was used to identify differences in cadherin, GluA2, and GluA1 localization after cocaine CPP in wildtype and DAT-Cre;β-catΔex3. (a, b) Cadherin localization to the synaptic membrane was increased under basal conditions in DAT-Cre;β-catΔex3 mice, and recruitment of additional cadherin to the synaptic membrane during CPP was blocked (p<0.05, significant interaction between treatment and genotype, two-way ANOVA, F(1,8) = 5.613, n=3 mice per condition. >100 synapses were analyzed per group). (c, d) The removal of GluA2 from the PSD membrane at excitatory synapses following CPP was blocked in DAT-Cre;β-catΔex3 mice (p<0.01, significant interaction between treatment and genotype, two-way ANOVA, F(1,8) = 22.07, n=3 mice per condition). (e, f) The insertion of GluA1 to the PSD membrane at excitatory synapses onto dopaminergic neurons following CPP was blocked in DAT-Cre;β-catΔex3 mice (p<0.01, significant interaction between treatment and genotype, two-way ANOVA, F(1,11) = 10.75, n=3 mice control saline, n=3 mice control cocaine; n=5 DAT-Cre;β-catΔex3 mice saline, 4 DAT-Cre;β-catΔex3 mice cocaine). a, c, e: *p< 0.05, **p< 0.01 Bonferroni’s test post hoc. Data shown as mean ± SEM with individual mice (circles) overlaid.
In addition to interacting with GluA111, cadherins can also interact directly and indirectly with the GluA2 subunit of AMPARs 12,13. Consequently, these data indicate that aberrantly increasing cadherin localization to the synaptic membrane prior to CPP stabilizes GluA1/2 heteromers present at VTA synapses under basal conditions, which blocks cocaine-induced insertion of GluA1 homomers and results in significantly reduced cocaine CPP.
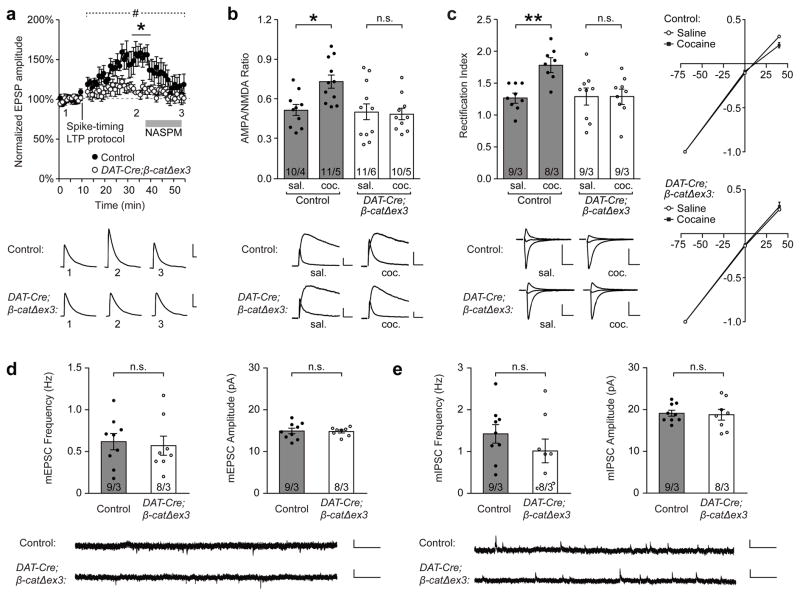
To functionally verify these changes in AMPAR trafficking, we also examined spike-timing-dependent LTP at synapses in the VTA and found that this form of LTP was abolished in DAT-Cre;β-catΔex3 mice (Fig. 5a). Additionally, treatment with NASPM (1-naphthyl acetyl spermine trihydrochloride), a selective antagonist of GluA2-lacking AMPARs, reduced EPSP amplitude back to basal levels in control mice, indicating that the enhanced EPSP amplitude observed following LTP induction was the result of the insertion of GluA2-lacking AMPARs. In contrast, NASPM treatment had no effect on EPSP amplitude in DAT-Cre;β-catΔex3 mice, demonstrating that GluA2-lacking AMPARs were not recruited to these synapses following the spike-timing-dependent LTP protocol (Fig. 5a). These data confirmed that increasing cadherin at the synaptic membrane in DAT-Cre;β-catΔex3 mice results in the retention of GluA2-containing AMPARs at the membrane and prevents the insertion of GluA2-lacking AMPARs which are crucial for the strengthening of these synapses.
Figure 5. Stabilization of cadherin at synapses in the VTA blocks LTP by retaining GluA2-containing AMPARs and preventing the insertion of GluA2-lacking AMPARs.
(a) Spike-timing-dependent LTP in the VTA was abolished in naïve DAT-Cre;β-catΔex3 mice (#p<0.001, significant interaction between genotype and time, two-way RM ANOVA, F(52, 468) = 2.644, * p<0.05, Bonferroni’s test post hoc, n=5 cells/5 mice WT, 6 cells/6 mice DAT-Cre;β-catΔex3). Treatment with NASPM reversed LTP in control mice, but had no effect on EPSP amplitude in DAT-Cre;β-catΔex3 mice. (b) Increased AMPAR/NMDAR ratio 24 h after cocaine administration observed in control mice was abolished in DAT-Cre;β-catΔex3 mice (p<0.05, two-way ANOVA, significant interaction between genotype and drug treatment, F(1,39) = 5.143, *p<0.05, Bonferroni’s test post hoc, n = 10 cells/4 mice control saline, 11 cells/5 mice control cocaine, 12 cells/6 mice DAT-Cre;β-catΔex3 saline, 10 cells/5 mice DAT-Cre;β-catΔex3 cocaine). (c) Increased rectification index of AMPAR EPSCs 24 h after cocaine administration observed in control mice was absent in DAT-Cre;β-catΔex3 mice (p<0.01, two-way ANOVA, significant interaction between genotype and drug treatment, F(1,31) = 7.641, **p<0.01 Bonferroni’s test post hoc, n = 9 cells/3 mice control saline, 8 cells/3 mice control cocaine, 9 cells/3 mice DAT-Cre; β-catΔex3 saline, 9 cells/3 mice DAT-Cre;β-catΔex3 cocaine). The frequency and amplitude of miniature excitatory postsynaptic currents (mEPSCs) (d) and miniature inhibitory postsynaptic currents (mIPSCs) (e) onto dopaminergic neurons in the VTA were unchanged in DAT-Cre;β-catΔex3 mice compared to controls, indicating that basal excitatory and inhibitory synaptic transmission at these synapses was unaltered in the VTA of DAT-Cre;β-catΔex3 mice (n = 9 cells/3 mice control, 8 cells/3 mice DAT-Cre;β-catΔex3, unpaired t-tests, p>0.05). Data shown as mean ± SEM with individual cells (circles) overlaid.
We then examined changes in AMPA/NMDA ratio and inward rectification of AMPA EPSCs 24 hours after cocaine administration in control and DAT:Cre;βcatΔex3 mice. In control mice we found that, consistent with previous studies, cocaine administration caused a significant increase in AMPA/NMDA ratio (Fig. 5b) and inward rectification of AMPA EPSCs (Fig. 5c) indicating increased insertion of GluA2-lacking AMPARs at synapses onto VTA DA neurons30. However, these increases were completely absent in DAT:Cre;βcatΔex3 mice (Fig. 5b, c), consistent with our immuno EM data showing that GluA1 at the synaptic membrane is increased in control mice but not DAT:Cre;βcatΔex3 mice following cocaine CPP. To confirm that these changes were not due to earlier, developmental disruptions, we also examined the morphology, density and function and VTA synapses in DAT-Cre;β-catΔex3 mice. EM analysis demonstrated that the size and density of excitatory and inhibitory synapses in the VTA was unchanged in DAT-Cre;β-catΔex3 mice compared to controls (Supplementary Fig. 11). We also found no differences in the frequency or amplitude of mEPSCs or mIPSCs onto VTA dopaminergic neurons in DAT:Cre;βcatΔex3 mice compared to control mice (Fig. 5d, e). Together, these data demonstrate that increasing cadherin at the synaptic membrane prior to behavioural training results in a significant reduction in cocaine CPP through the aberrant retention of GluA1/2 AMPARs, and the prevention of GluA1 homomer membrane insertion to VTA synapses (Fig. 6).
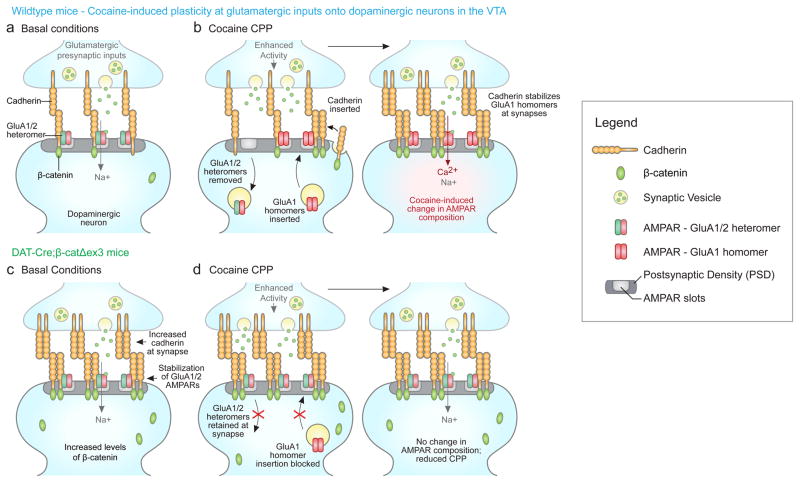
Figure 6. Model of changes in cadherin and AMPAR subunit localization in control and DAT-Cre;β-catΔex3 mice during conditioned place preference (CPP).
(a–b) Wildtype mice. (a) Under basal conditions, the population of AMPARs at excitatory inputs to dopaminergic neurons is composed of GluA1/2 heteromers9. Cadherins regulate the dynamic localization of AMPARs through direct and indirect interactions with GluA1 and GluA2 11–13,44. (b) During cocaine-mediated CPP, activity is enhanced at excitatory inputs to dopaminergic neurons, driving the removal of GluA1/2 heteromers and the insertion of Ca2+-permeable GluA1 homomers into AMPA receptor ‘slots’ within the PSD31,45. Enhanced synaptic activity also leads to increased levels of cadherin at the synaptic membrane. Cadherins are then situated to associate with and stabilize GluA1 homomers at the synaptic membrane, contributing to the potentiation of these synapses underlying behavioral changes in CPP.
(c–d) DAT-Cre;β-catΔex3 mice. (c) Under basal conditions, elevated levels of β-catenin promote the stability of cadherin, resulting in a significant increase in cadherin localized to the synaptic membrane. Cadherins associate with GluA1/2 heteromers11–13, enhancing their stability at the synaptic membrane. (d) During cocaine-mediated CPP, the removal of GluA1/2 heteromers is prevented due to their stabilization by increased synaptic cadherin in DAT-Cre;β-catΔex3 mice. GluA1/2 heteromers are retained in available AMPAR ‘slots’, preventing the insertion of GluA1-containing AMPARs and the potentiation of these synapses. Thus, stabilizing synaptic cadherin in DAT-Cre;β-catΔex3 mice disrupts the cocaine-induced switch in AMPAR composition and significantly reduces CPP.
Discussion
In the present study, we demonstrate that cadherin plays a critical role in synaptic plasticity in the VTA and behavioral conditioning driven by cocaine. We show that cadherins are widely expressed in the VTA, and are essential for the potentiation of excitatory synapses onto dopaminergic neurons. Using immunogold EM, we observed a strong correlation between cocaine-induced CPP in wildtype mice and the insertion of cadherin and GluA1-containing AMPARs into the synaptic membrane of these synapses. These changes in cadherin and AMPAR localization were specific to cocaine-induced CPP, and were not observed when mice were given the same schedule of cocaine and saline administration in their home cage, nor following CPP induced by palatable food rewards. In DAT-Cre;β-catΔex3 mice, we found that stabilization of cadherin at VTA synapses was sufficient to significantly reduce the magnitude of cocaine-induced CPP. This behavioural effect was associated with disruptions in the plasticity of excitatory synapses formed onto dopaminergic neurons; stabilization of cadherin led to the abolishment of LTP induced by both cocaine administration and spike timing-dependent stimulation in these mice. Indeed, our immunogold EM data demonstrate that cocaine-induced internalization of GluA2-containing AMPARs and the subsequent insertion of GluA2-lacking AMPARs into the membrane was blocked in DAT-Cre;β-catΔex3 mice.
Our findings suggest that, in wildtype mice, increased cadherin at the synaptic membrane acts to stabilize newly inserted GluA1 homomers during cocaine CPP. This is supported by evidence that cadherin interacts with the GluA1 subunit and stabilizes GluA1-containing AMPARs11, and by our data showing that intact cadherin adhesion is required for STD LTP at VTA synapses, which is also mediated by insertion of GluA1-homomers similar to potentiation of these synapses by cocaine. Cadherin-mediated stabilization of GluA1 homomers may therefore contribute to increased strength of excitatory synapses onto dopaminergic neurons, as well as prolonged postsynaptic Ca2+ influx through these AMPARs. Potentiation of these synapses is also likely to enhance the activity of dopaminergic neurons and increase dopamine release onto target structures of the mesocortiolimbic system, leading to further downstream changes in synaptic plasticity and circuit activity that contribute to addiction.
Excitatory synapses onto dopaminergic neurons have previously been implicated in contextual conditioning and reward learning7,8, and our data demonstrate a strong relationship between increased cadherin at these synapses and drug-induced behavioural changes in wildtype mice. Increased cadherin at the synaptic membrane was correlated with the magnitude of CPP in individual mice, which suggests that increased cadherin adhesion may be a mechanism that contributes to drug-induced increases in the stability and potentiation of VTA synapses, promoting the ‘hard-wiring’ of synaptic traces of drug-associated memories and behaviours. We speculate that increased strength and stability of VTA synapses mediated by cadherin adhesion may also contribute to sensitization to drug-associated cues, which can trigger relapse both in humans and animal models of addiction even after extended periods of abstinence from drug-taking33.
In DAT-Cre;β-catΔex3 mice, stabilization of cadherin at VTA synapses prior to cocaine administration prevented the removal of GluA2-containing AMPARs and blocked the insertion of GluA1-containing AMPARs. This disruption of cocaine-induced changes in AMPAR localization was associated with significantly attenuated CPP, and the abolishment of STD LTP. These impairments contrast with observations in the hippocampus, where stabilization of cadherin led to impairments in LTD and behavioural flexibility but had no effect on LTP or acquisition of spatial memory19. The key difference in plasticity between these regions appears to be the requirement for removal of GluA2-containing AMPARs for LTP at VTA synapses. It has been proposed that the number of AMPARs at synapses onto dopaminergic neurons is limited by the number of ‘slots’ where receptors can associate with scaffold proteins in the PSD31,32. Indeed, inhibiting the internalization of GluA2-containing AMPARs by disrupting GluA2/PICK1 (protein interacting with C kinase-1) interactions abolishes cocaine-induced increases in AMPA:NMDA ratio that are typically mediated by the insertion of GluA2-lacking AMPARs31. Since cadherin can stabilize AMPARs at synapses through interaction with both the GluA1 and GluA2 subunits11–13, this suggests that in DAT-Cre;β-catΔex3 mice increased cadherin at the synaptic membrane stabilized GluA2-containing AMPARs that occupied available slots in the PSD and prevented the insertion of GluA1 homomers. Our electrophysiological data confirmed the functional identity of AMPARs at VTA synapses in DAT-Cre;β-catΔex3 mice, demonstrating that GluA2-containing AMPARs were indeed retained at these synapses and insertion of GluA2-lacking AMPARs was blocked, leading to abolishment of LTP.
Our findings also show that the timing of cadherin stabilization at the synaptic membrane had a critical effect on AMPAR trafficking and plasticity at VTA synapses. In wildtype mice, increased cadherin localization to the synaptic membrane during CPP resulted in the stabilization of GluA1 homomers that were also inserted during CPP. However, in DAT:Cre;βcatΔex3 mice, increased cadherin localization to the synaptic membrane occurred before CPP, which resulted in the stabilization of GluA1/2 heteromers present under basal conditions. In both cases, the effect of cadherin was consistent: increased cadherin at the synaptic membrane was found to stabilize the AMPARs that were present at that time. However, the differences in timing of when cadherin was stabilized, and the type of AMPAR subunits present at each time, led to different effects in LTP and CPP observed between DAT:Cre;βcatΔex3 mice and controls.
Interestingly, while CPP was significantly reduced in DAT:Cre;βcatΔex3 mice, we observed no changes in cocaine-induced locomotor sensitization in these mice compared with controls. This finding is consistent with studies showing that CPP and sensitization are not necessarily directly correlated, and may be mediated by distinct circuits and neural adaptations in the brain driven by cocaine. Disruption of the dopaminergic projections from the substantia nigra, but not VTA, has been shown to abolish locomotor sensitization to cocaine while leaving CPP intact33. Additionally, an analysis of CPP and locomotor sensitization in different inbred mouse lines found no correlation between the two behaviours, with some strains exhibiting robust CPP but no locomotor sensitization, or vice versa34. Finally, no correlation was found to exist between CPP and sensitization within individual mice in a behavioural task designed to test both parameters simultaneously35.
Our findings also demonstrate interesting differences in CPP driven by cocaine and food rewards. Cocaine CPP, but not food CPP, increased both cadherin and GluA1 localization to the synaptic membrane at excitatory synapses onto dopaminergic neurons in the VTA. Consistent with this, stabilization of cadherin (and GluA1/2 heteromers) at the synaptic membrane of these synapses in DAT-Cre;β-catΔex3 mice markedly decreased the magnitude of cocaine CPP, but had no effect on food CPP. These findings are consistent with a number of studies indicating that changes in dopaminergic neuron activity and behaviour driven by food rewards may be mediated by mechanisms other than increased LTP at VTA synapses. Food-related peptide hormones have been shown to play a major role in mediating behavioural changes driven by food 36, in some cases by acting directly on VTA dopamine neurons to regulate their activity37. Additionally, pharmacological disruption of NMDARs and mGluRs have both been shown to have opposite effects on CPP driven by food rewards and CPP driven by drugs of abuse38,39, which further suggests that major differences exist in the mechanisms underlying these different forms of CPP.
What are the molecular mechanisms responsible for the changes in cadherin localization observed following CPP? In hippocampal neurons, cadherins have been shown to undergo constitutive turnover at the synaptic membrane under basal conditions18. We have previously shown that synaptic activity leads to post-translational changes in the cadherin-binding protein, δ-catenin, increasing its association with cadherin and augmenting cadherin’s retention at the synaptic membrane17. This results in increased trans-synaptic adhesion and the stabilization of postsynaptic AMPARs by cadherin, causing long-lasting increases in synapse strength, size and stability over time. An important direction for future research will be to determine whether these same mechanisms drive changes in cadherin localization during activity- and drug-induced plasticity at synapses in the VTA.
The finding that cadherins regulate AMPAR trafficking at synapses in the VTA supports an emerging view that structural and scaffolding proteins may be of central importance in synaptic plasticity due to their role in mediating the recruitment and removal of postsynaptic receptors40,41. Since the finding that LTP can be induced at synapses as long as extrasynaptic glutamate receptors are present40, there has been a shift of focus to identify the proteins that control the insertion, removal and stabilization of AMPARs during different forms of synaptic plasticity. The present study provides further evidence that cadherins are key molecules that control AMPAR trafficking during plasticity underlying different forms of learning and memory. Additionally, our findings demonstrate that cadherins play a critical role in synaptic plasticity outside of the hippocampus, where they have typically been studied, and likely play specialized roles in mediating different forms of synaptic plasticity throughout the CNS. Another important goal for future studies will be determining the specific cadherin subtypes responsible for mediating different forms of synaptic plasticity throughout the brain. In the VTA, N-cadherin likely plays a major role in activity and cocaine-induced plasticity examined in the present study, as the HAV peptide used to block spike-timing-dependent LTP at VTA synapses has been shown to specifically disrupt N-cadherin trans interactions 42, and N-cadherin plays a critical role in plasticity at excitatory synapses in other brain regions such as the hippocampus15,16,42,43. However, cadherins have highly similar structure and functional redundancy, and other cadherin subtypes expressed in dopaminergic neurons may therefore contribute as well.
Finally, our study provides new mechanistic insight into how mutations in cadherin adhesion complex proteins could contribute to susceptibility or resilience to addiction. Genome-wide association studies in substance abusers have identified increased prevalence of clustered single nucleotide polymorphisms (SNPs) in δ-catenin, which stabilizes cadherin at synapses, and α-catenin, which tethers the cadherin adhesion complex to the actin cytoskeleton21. While the function of these SNPs is currently unknown, we speculate that changes in the function or expression levels of δ-catenin or α-catenin could affect the stabilization of cadherin and AMPARs at the synaptic membrane of VTA synapses, which our findings show is sufficient to alter drug-induced synaptic plasticity and behavioural changes. Cadherins adhesion complex proteins may therefore be targets of interest for future studies investigating genetic risk factors for addiction.
Online Methods
Animals
Male C57BL/6 mice between 6–8 weeks old were used in all experiments, unless stated otherwise. For experiments examining the effects of cadherin stabilization of cocaine-induced plasticity and behavioral conditioning, we used mice Slc6a3:Cre/+;Ctnnb1lox(ex3)/lox(ex3) mice (termed DAT-Cre;β-catΔex3 mice for brevity), which are homozygous for a loxP-flanked exon 3 transgene28 and express Cre recombinase in dopaminergic neurons29. Littermates lacking the Slc6a3:Cre/+ transgene (+/+;Ctnnb1lox(ex3)/lox(ex3) mice) were used as controls. Mice were housed in reverse day/night cycle and given ad libitum access to food and water. Experimental procedures and animal housing conditions were approved by The UBC Animal Care Committee, and were in accordance with Canadian Council on Animal Care (CCAC) guidelines. All mice were housed with littermates in groups of 2–5 and were only used for one behavioral test, unless otherwise noted.
Immunoblot Analysis
Mice were anesthetized by cervical dislocation and their brains quickly removed, then sliced into 300 μm thick horizontal sections by vibratome. The VTA was dissected from the slices, with the VTAs of 5 mice being pooled and homogenized in lysis buffer (20 mM Tris pH 7.4, 137 mM NaCl, 0.5% NP-40, 10% glycerol) with protease and phosphatase inhibitor tablets (Roche) and cleared by centrifugation at 14,000 × g for 40 min at 4°C. VTA lysates were separated by SDS-PAGE and probed with antibodies against N-cadherin (mouse, BD Transduction, CAT# 610920, predicted band size 130 kDa, 1:1000), R-cadherin (rabbit, Novus, CAT# NBP2-27372, predicted band size 130 kDa, 1:1000), cadherin-7 (rabbit, Santa Cruz, CAT# sc-68422, predicted band size 90 kDa, 1:500), cadherin-8 (rabbit, Abcam, CAT# ab97268, predicted band sizes 150 kDa precursor and 90 kDa protein, 1:1000), and cadherin-11 (mouse, Invitrogen, CAT# 321700, predicted band size 110 kDa, 1:1000). Proteins were visualized by chemiluminesence on a Bio-Rad Versadoc 4000 (Bio-Rad Laboratories (Canada) Ltd., Mississauga, ON). Immunoblot experiments were repeated at least twice to ensure reproducibility.
Immunohistochemistry
Mice were anesthetized with sodium pentobarbital (120 mg/kg), transcardially perfused by PBS followed by 4% paraformaldehyde (PFA) in PBS. Brains were removed and post-fixed in 4% PFA for two hours, then cryoprotected by saturation with 30% sucrose, frozen, and sliced into 20 μm thick coronal sections by cryostat. For immunolabelling of target proteins, sections were first placed in a blocking buffer containing 10% goat serum, 0.1% bovine serum albumin and 0.1% Triton-X-100 in PBS. Primary antibodies against DAT (dopamine transporter) (rat, Millipore, CAT# MAB369, 1:500), N-cadherin (mouse, BD Transduction, CAT# 610920, 1:250), R-cadherin (rabbit, Novus, CAT# NBP2-27372, 1:250), cadherin-7 (rabbit, Santa Cruz, CAT# sc-68422, 1:250), cadherin-8 (rabbit, Abcam, CAT# ab97268, 1:250), cadherin-11 (rabbit, Santa Cruz, CAT# sc-28643, 1:250), β-catenin (mouse, BD Transduction, CAT# 610153, 1:250), Axin2, (rabbit, Abcam, CAT# ab109307, 1:250), LEF1 (rabbit, Abcam, CAT# ab137872, 1:250), c-Myc (rabbit, Cell Signaling, CAT# 5605S, 1:250), c-Jun (rabbit, Cell Signaling, CAT# 9165P, 1:250), and GAD67 (chicken, Abcam, CAT# ab75712, 1:250) were diluted in this buffer, added to sections and incubated overnight at 4°C. The following day, samples were washed three times with PBS, and secondary antibodies diluted in the blocking buffer were added to sections and incubated for 1–2 hours at room temperature. Slides were washed again with PBS and stained with DAPI (0.5 μg/mL). Sections were mounted with ProLong Gold (Life Technologies, Carlsbad, CA), and were imaged on an Olympus Fluoview FV1000 confocal microscope using Fluoview software (Olympus, Melville, NY). Immunostaining experiments were repeated at least twice to ensure reproducibility. The brightness and contrast of entire images was moderately adjusted using Photoshop (Adobe Systems Canada, Toronto, ON) following recommended, scientifically acceptable procedures, and no information was obscured or eliminated from the original images. Immunohistochemical experiments were repeated on brain slices from at least 2 mice per genotype to ensure reproducibility.
Electrophysiology
Electrophysiological recordings were taken from dopaminergic cells within the VTA of male and female mice ranging from postnatal day 21 (P21) to P30. Horizontal slices of mouse midbrain were cut with a vibratome (Leica, Nussloch, Germany), and slices (250 μm) were equilibrated in artificial cerebrospinal fluid (aCSF) containing (in mM): 126 NaCl, 1.6 KCl, 1.1 NaH2PO4, 1.4 MgCl2, 2.4 CaCl2, 26 NaHCO3, 11 glucose (32°C–34°C) and saturated with 95% O2/5% CO2. Cells were visualized using infrared differential contrast video microscopy and whole-cell voltage-clamp recordings were made using a MultiClamp 700B amplifier (Axon Instruments, Union City, CA). Putative dopaminergic cells were identified by the presence of a large hyperpolarization-activated cation current, Ih 46,47, fusiform shape and location in the lateral VTA proximal to the medial terminalis of the optic nucleus48. In this VTA subregion, Ih is a reliable predictor of dopamine neurons49,50,51. A subset of dopaminergic neurons was further confirmed by post-hoc immunostaining for tyrosine hydroxylase after recording as described previously52. 99/131 post-hoc stained neurons were identified as TH positive. The remaining 32 were unable to be recovered. In the spike timing-dependent plasticity experiments, neurons were patch clamped in current-clamp mode with electrodes containing, (in mM): K-Methanesulfonate 125mM, KCl 5mM, HEPES 10mM, EGTA 0.2mM, MgCl26H2O 2mM, 2.5 mg/ml MgATP, 0.25 mg/ml GTP, and 0.2% Biocytin, pH 7.2–7.4, 275–285 mOsm and picrotoxin (100 μM) in the external aCSF solution. Slices were preincubated in 200 μM HAV peptide, 200 μM scrambled control peptide, or vehicle. The spike-timing-dependent protocol for LTP induction was carried out as previously described53. Briefly, the protocol consisted of 20 bursts of EPSP-spike pairs, with each burst consisting of 5 paired stimuli at 10 Hz (100 ms intervals), with an interburst interval of 5 s. Postsynaptic spikes were evoked by injection of depolarizing current pulses, with the onset of EPSPs preceding the peak of postsynaptic spikes by 5 ms. Evoked EPSPs were sampled at 0.1 Hz before and after LTP induction. In experiments where NASPM (1-Naphthyl acetyl spermine trihydrochloride, Tocris, Bristol, UK) was used, 100 μM of NASPM in aCSF was added to slices 25 min after STDP LTP induction to inhibit GluA2-lacking AMPA receptors. For the rest of experiments, electrodes (3–5MΩ) contained (in mM) 117 Cesium methanesulfonate, 20 HEPES, 0.4 EGTA, 2.8 NaCl, 5 TEA-Cl, 2.5 Mg-ATP and 0.25 Na-GTP (pH 7.2–7.3, 270–280 mOsm). To calculate AMPAR/NMDAR ratio, neurons were voltage clamped at 40 mV and an average of 30 EPSCs were measured before and after the application of AP-5 (50 μM) for 5 minutes. NMDAR responses were calculated by subtracting the average response in the presence of AP-5 (AMPAR-mediated only) from that recorded in its absence. The peak of the AMPAR EPSC was divided by the peak of the NMDAR EPSC in order to compute the AMPAR/NMDAR ratio. Experiments measuring the I–V relationship were carried out in the presence of picrotoxin (100 μM) and when indicated in the presence of AP-5 (50 μM) to block GABAA and NMDA receptors, respectively. The holding potentials were −70 mV, 0 mV and +40 mV. Synaptic currents were evoked by stimuli at 0.1 Hz, and the rectification index was calculated by dividing the gradient of the slope at negative potentials by the gradient of the slope at positive potentials. Excitatory and inhibitory transmission were recorded in cells voltage-clamped at −67 mV for mEPSCs and +10 for IPSCs in TTX (500 nM). AMPAR mEPSCs were selected based on their amplitude (>12 pA), decay time (<3 ms), and rise time (<1 ms) using the Mini60 MiniAnalysis program (Synaptosoft). Similarly, GABAA mIPSCs were selected for amplitude (>12 pA), rise time (<4 ms), and decay time (<10 ms).
TH Immunocytochemistry
Brain slices from patch-clamp recording were fixed overnight in cold 4% paraformaldehyde, rinsed in phosphate buffer solution (PBS), blocked in 10% normal donkey serum, incubated with monoclonal mouse anti-TH antibody (Sigma, Oakville ON, 1:1000) for 48 hours at 4°C. Secondary donkey anti-mouse fluorescein isothiocyanate antibody (Cedarlane, Burlington, ON, 1:50) was applied for 2 hours at 4°C. DyLight 594 streptavidin (Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania; 1:200) was applied overnight at 4°C. Slices were mounted using Fluoromount (Sigma, Oakville ON).
Conditioned Place Preference (CPP)
CPP was induced using a standard three-chambered apparatus, consisting of two conditioning compartments and a middle, connecting compartment (Stoelting Co., Wood Dale, IL). The two conditioning compartments had distinct wall patterns and floor textures to allow mice to distinguish between them. Naïve mice were first allowed to habituate to the entire apparatus during a 30-minute session on day 1. On day 2, mice in the conditioned group received a 15 mg/kg injection of cocaine, and placed in a ‘conditioned’ chamber for 15 minutes. Mice were assigned to receive cocaine in one compartment or the other using an unbiased design. Individual mice were removed from the experiment if they showed a strong baseline preference (>70%) for the conditioned chamber. The following day, these mice received an equivalent volume of saline, and were placed in the opposite ‘non-conditioned’ compartment. This alternating pattern of conditioning was repeated three times (6 days total). On test day, place preference was assayed by giving each mouse with a priming injection of saline, placing them in the middle connecting compartment, and recording the amount of time spent in the two conditioning compartments over a 30 minute period. If mice underwent the extinction of CPP, the test day protocol was repeated each day until the drug group’s preference for the conditioned chamber had returned to habituation levels. In the CPP + homecage extinction experiment (‘CPP + HC’) mice underwent CPP as described above, and were then returned to their home cages without any re-exposure to the test apparatus for 6 days (the same duration of time as was required for extinction of CPP in the previous experiments). CPP using a palatable food reward (‘Food CPP’) was induced using similar apparatus and methodology. Mice were food restricted for one week before the start of testing, such their weight was maintained at 85% of their baseline weight, and were introduced to the palatable food 5 days before the start of testing so that they were familiar with the food. During conditioning 2–3 grams of palatable food (Bacon Softies™, VWR) was placed in the ‘conditioned’ chamber, and mice were given access to the food in this chamber for 30 minutes. The food pellet was weighed before and after the conditioning sessions so that the amount of food consumed could be determined. Mice received no food reward in the unconditioned chamber during the 30 minute conditioning session. A control group received no food in either conditioning chamber. The alternating pattern of conditioning was repeated for 10 days before place preference was assayed on test day. All experiments were performed in dim lighting conditions, during the dark (wake) cycle. In Figure 2b, we present conditioned place preference data for n = 6 mice per condition. The data presented is from the mice that were used for the subsequent immunoelectron microscopy experiments (specifically the CPP+Extinction experiment). In Supplementary Figure 6a, we present conditioned place preference data for n = 6 mice per condition. The data presented is from the mice that were used for the subsequent immunoelectron microscopy experiments (specifically the Food CPP experiment). Experimenters were always blind to the genotype of the animal during testing, but not to the drug condition or food treatment group during cocaine-induced CPP or food CPP, respectively.
Electron Microscopy Sample Preparation
Mice were anesthetized with sodium pentobarbital (120 mg/kg), transcardially perfused by PBS followed by 4% paraformaldehyde (PFA) as described above, then post-fixed in 4% PFA overnight. Brains were then cut into 250 μm thick horizontal sections by vibratome. Small pieces of VTA tissue (<1mm in any dimension) were dissected from these slices and cryoprotected in 30% glycerol overnight at 4 °C. Samples were then plunge frozen in liquid ethane at −170 °C in an EM cryopreparation chamber (Leica), and transferred to a 1.5% Uranyl Acetate solution in 100% Methanol, kept at −90 °C in a Leica EM AFS for 30 hours. The temperature was increased to −45 °C over 11 hours. Next, samples were rinsed in 100% Methanol, and infiltrated with HM-20 acrylic resin (Electron Microscopy Sciences, Hatfield, PA) by increasing the resin to methanol ratio in 2-hour steps while maintaining the temperature at −45 °C. Samples were set up in capsules containing pure resin and polymerized under UV light for 24 hours at −45 °C, after which the temperature was slowly increased to 0 °C. Tissue sections were cut at 85 nm using a Diatome diamond knife and a Leica Ultramicrotome. Sections were collected on 300-mesh, formvar-coated Nickel grids.
Immunogold Electron Microscopy
Grids were rinsed with distilled water and subsequently immersed in a bead of TTBS with 0.1% Triton-X with 0.1% Sodium Borohydride and 50 mM glycine. The grids were then rinsed with TTBS with 0.1% Triton-X three times. Following this, nonspecific binding was blocked by immersing grids in a bead of 2% BSA in TTBS with 0.1% Triton-X for 10 minutes. Primary antibodies against DAT (dopamine transporter) (rat, Millipore, CAT# MAB369), PSD-95 (rabbit, Frontier Institute, CAT# Af628), pan-cadherin (mouse, Sigma, CAT# C1821), GluA1 (rabbit, Millipore, CAT# ABN241), GluA2 (rabbit, NeuroMab/Antibodies Inc, CAT# 75-002), GAD67 (chicken, Abcam, CAT# ab75712), and VGLUT2 (guinea pig, Synaptic Systems, CAT#124014) were diluted in 2% BSA in TTBS with 0.1% Triton-X. Grids were immersed in 15 μl beads of diluted primary antibody overnight, at room temperature in a humidity chamber. The following day, grids were thoroughly rinsed by immersion in vials of TTBS with 0.1% Triton-X three times. Secondary antibodies were diluted in 2% BSA in TTBS with 0.1% Triton-X, and 0.05% Polyethyleneglycol (PEG) was added to prevent aggregation of gold beads. Grids were immersed in 15 μl beads of secondary antibody for 1.5 hours. Following this step, grids were repeatedly rinsed in TTBS with 0.1% Triton-X, and then rinsed in Milli-Q H2O and dried. Grids were then lightly counterstained with with 2% uranylacetate and Reynold’s lead citrate. Images were collected at 98000X magnification on a Tecnai G2 Spirit transmission electron microscope (FEI Company, Eindhoven, Netherlands). To analyze immunogold labeling, cell types were first identified by the presence of DAT, VGLUT2 or GAD67 markers, and synapse types were identified by the presence or absence of PSD-95 markers. The distance of all immungold-labelled cadherin from the synaptic membrane, or all immungold labelled GluA1 or GluA2 to the postsynaptic active zone membrane was measured. Due to the sizes of proteins and reagents involved54 (see also Results section and Supplementary Fig. 2) the maximum cutoff of immunogold particles considered to be labelling target proteins at the synaptic membrane was 40 nm for cadherin, 30 nm for GluA1, and 35 nm for GluA2. The percentage of immungold particles localized to these regions was determined by [# immungold beads at target membrane]/[total # immunogold beads within 500 nm of target membrane at pre and post-synaptic compartments], and expressed as a percentage relative to saline-only controls which were processed and labelled in parallel. All images were acquired and analyzed blind to the genotype of each mouse. In each experiment, greater than 100 synapses were analyzed for each condition.
Rotarod
Mice were trained on an accelerating rotarod apparatus (Mouse Rota-Rod, Ugo Basile) for five trials per day for two days. The rotarod accelerated from 4 to 400 rotations per minute over 5 minutes. Mice were scored on the latency of time until falling from the rotarod apparatus, with five-minute breaks between trials to allow for recovery. Experiments were performed during the dark (wake) cycle. Experimenters were blind to the genotype of the animal during testing and scoring. One cohort of mice completed the rotarod test, followed by context dependent fear conditioning.
Context-dependent Fear Conditioning
Mice were placed in the conditioning chamber for 5 minutes, and after 3 minutes received an unconditioned foot shock stimulus (1 mA, 50 Hz) lasting 3 seconds. The next day mice were placed in the same chamber for 4 minutes, but did not receive an additional foot shock. Freezing behavior was determined by quantifying laser beam breaks in the conditioning chamber due to mouse activity, and total time % freezing was compared between groups. Experiments were performed during the dark (wake) cycle. Experimenters were blind to the genotype of the animal during testing and video scoring. One cohort of mice performed the context dependent fear conditioning experiment after completing the rotarod test.
Locomotor sensitization to cocaine
During the habituation phase (day 1–2), mice were given an injection of saline (12 uL/g) and were placed in a 20 cm2 open field apparatus for 15 minutes. During cocaine sensitization testing (day 3–7), mice were instead given 15 mg/kg cocaine and placed in the apparatus. After a 10 day rest period, mice were given a 15 mg/kg ‘challenge’ dose of cocaine and re-introduced to the apparatus. During all phases of testing, mouse locomotion in the open field apparatus was recorded and then analyzed using Phenotracker software (TSE Systems, Hamburg, Germany). Experimenters were blind to the genotype of the animal during testing.
Food consumption testing
Mice were given pre-weighed pellets of low-fat (10% fat, Research Diets Inc, CAT# D12450B) or high-fat (60% fat, Research Diets Inc, CAT# D12492) food to consume over a 24-hour period. The food given was in excess of what the mouse could consume in this period, so they were not food restricted. Pellets were weighed after the 24-hour period so that the amount of food consumed could be calculated. Experimenters were blind to the genotype of the animal during testing.
Statistical analysis
Unless otherwise noted, statistical analysis was done using unpaired Student’s t-test (two tailed) and two-way ANOVA. Data distribution was assumed to be normal but this was not formally tested. Correlative data examining the relationship between behavioural data and immunoEM data was analyzed using linear regression. Data from STDP LTP electrophysiology experiments was analyzed by two way repeated measures (RM) ANOVA, and post-hoc analysis was done using Bonferroni’s test. Data from CPP experiments was also analyzed by two-way RM ANOVA with genotype as the between-subjects factor and time as the within-subjects factor. For comparisons between genotypes and within days, Bonferroni’s test was used. For comparisons to baseline within genotypes, Dunnett’s test was used. Correlative data examining the relationship between pre- and postsynaptic cadherin localization and CPP was analyzed using linear regression. No statistical methods were used to pre-determine sample sizes, but our sample sizes are similar to those reported in previous publications19,26,49,52. Results were considered significant when p<0.05. Analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA).
Supplementary Material
Acknowledgments
We thank the UBC Bioimaging Facility for use of shared equipment for sample processing; S.B. Floresco for extensive discussion and comments on the manuscript; C.A. Winstanley, T.P. O’Connor, K. Haas and D.W. Allan for comments on the manuscript, and K. Goodwin for assistance with data analysis. This work was supported by grants from Canadian Institutes of Health Research MOP-130526 to S.X.B. and MOP-102617 to S.L.B.
Footnotes
Author Contributions: F.M. and A.K.G. performed all behavioral, immunogold electron microscopy, and immunohistochemistry experiments. S.L. performed all electrophysiological experiments under S.L.B.’s supervision. C.M.C assisted with EM sample processing and immunoelectron microscopy and performed biochemical experiments. M.M. assisted with data analysis and genotyping of mice. A.G.P. provided experimental reagents. F.M., A.K.G. and S.X.B designed all experiments, interpreted the results and wrote the paper.
Competing Financial Interests Statement: The authors declare no competing financial interests.
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–586. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 2.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of Abuse and Stress Trigger a Common Synaptic Adaptation in Dopamine Neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 3.Mameli M, Lüscher C. Synaptic plasticity and addiction: learning mechanisms gone awry. Neuropharmacology. 2011;61:1052–1059. doi: 10.1016/j.neuropharm.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 4.Luscher C. Cocaine-evoked synaptic plasticity of excitatory transmission in the ventral tegmental area. Cold Spring Harb Perspect Med. 2013;3:a012013. doi: 10.1101/cshperspect.a012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 6.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Stuber GD, et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–2. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003;28:73–6. doi: 10.1038/sj.npp.1300011. [DOI] [PubMed] [Google Scholar]
- 9.Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mameli M, Bellone C, Brown MT, Luscher C. Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat Neurosci. 2011;14:414–6. doi: 10.1038/nn.2763. [DOI] [PubMed] [Google Scholar]
- 11.Nuriya M, Huganir RL. Regulation of AMPA Receptor Trafficking by N-cadherin. J Neurochem. 2006;97:652–661. doi: 10.1111/j.1471-4159.2006.03740.x. [DOI] [PubMed] [Google Scholar]
- 12.Saglietti L, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54:461–77. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Silverman JB, et al. Synaptic anchorage of AMPA receptors by cadherins through neural plakophilin-related arm protein AMPA receptor-binding protein complexes. J Neurosci. 2007;27:8505–16. doi: 10.1523/JNEUROSCI.1395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka H, et al. Molecular modification of N-cadherin in response to synaptic activity. Neuron. 2000;25:93–107. doi: 10.1016/s0896-6273(00)80874-0. [DOI] [PubMed] [Google Scholar]
- 15.Bozdagi O, et al. Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. J Neurosci. 2010;30:9984–9. doi: 10.1523/JNEUROSCI.1223-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendez P, De Roo M, Poglia L, Klauser P, Muller D. N-cadherin mediates plasticity-induced long-term spine stabilization. J Cell Biol. 2010;189:589–600. doi: 10.1083/jcb.201003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brigidi GS, et al. Palmitoylation of delta-catenin by DHHC5 mediates activity-induced synapse plasticity. Nat Neurosci. 2014;17:522–32. doi: 10.1038/nn.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54:771–85. doi: 10.1016/j.neuron.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Mills F, et al. Cognitive flexibility and long-term depression (LTD) are impaired following beta-catenin stabilization in vivo. Proc Natl Acad Sci U A. 2014;111:8631–6. doi: 10.1073/pnas.1404670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrick C, et al. N-cadherin regulates cytoskeletally associated IQGAP1/ERK signaling and memory formation. Neuron. 2007;55:786–98. doi: 10.1016/j.neuron.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu QR, et al. Addiction molecular genetics: 639,401 SNP whole genome association identifies many ‘cell adhesion’ genes. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:918–25. doi: 10.1002/ajmg.b.30436. [DOI] [PubMed] [Google Scholar]
- 22.Obst-Pernberg K, Medina L, Redies C. Expression of R-cadherin and N-cadherin by cell groups and fiber tracts in the developing mouse forebrain: relation to the formation of functional circuits. Neuroscience. 2001;106:505–33. doi: 10.1016/s0306-4522(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 23.Hertel N, Krishna K, Nuernberger M, Redies C. A Cadherin-Based Code for the Divisions of the Mouse Basal Ganglia. J Comp Neurol. 2008;508:511–528. doi: 10.1002/cne.21696. [DOI] [PubMed] [Google Scholar]
- 24.Delva E, Kowalczyk AP. Regulation of Cadherin Trafficking. Traffic. 2009;10:259–267. doi: 10.1111/j.1600-0854.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engblom D, et al. Glutamate Receptors on Dopamine Neurons Control the Persistence of Cocaine Seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 27.Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–90. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bäckman CM, et al. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genes N Y N 2000. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- 30.Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 32.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–50. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beeler JA, Cao ZFH, Kheirbek MA, Zhuang X. Loss of cocaine locomotor response in Pitx3-deficient mice lacking a nigrostriatal pathway. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2009;34:1149–1161. doi: 10.1038/npp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisener-Dorman AF, Grabowski-Boase L, Tarantino LM. Cocaine locomotor activation, sensitization and place preference in six inbred strains of mice. Behav Brain Funct. 2011;7:29. doi: 10.1186/1744-9081-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seymour CM, Wagner JJ. Simultaneous expression of cocaine-induced behavioral sensitization and conditioned place preference in individual rats. Brain Res. 2008;1213:57–68. doi: 10.1016/j.brainres.2008.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meye FJ, Adan RAH. Feelings about food: the ventral tegmental area in food reward and emotional eating. Trends Pharmacol Sci. 2014;35:31–40. doi: 10.1016/j.tips.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Abizaid A, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yonghui L, Xigeng Z, Yunjing B, Xiaoyan Y, Nan S. Opposite effects of MK-801 on the expression of food and morphine-induced conditioned place preference in rats. J Psychopharmacol Oxf Engl. 2006;20:40–46. doi: 10.1177/0269881105057250. [DOI] [PubMed] [Google Scholar]
- 39.Herzig V, Capuani EMI, Kovar KA, Schmidt WJ. Effects of MPEP on expression of food-, MDMA- or amphetamine-conditioned place preference in rats. Addict Biol. 2005;10:243–249. doi: 10.1080/13556210500223272. [DOI] [PubMed] [Google Scholar]
- 40.Granger AJ, Nicoll RA. LTD expression is independent of glutamate receptor subtype. Front Synaptic Neurosci. 2014;6:15. doi: 10.3389/fnsyn.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheng M, Malinow R, Huganir R. Neuroscience: Strength in numbers. Nature. 2013;493:482–3. doi: 10.1038/493482a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang L, Hung CP, Schuman EM. A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron. 1998;20:1165–75. doi: 10.1016/s0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- 43.Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J Neurosci. 1998;18:6892–904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorski JA, Gomez LL, Scott JD, Dell’Acqua ML. Association of an A-kinase-anchoring protein signaling scaffold with cadherin adhesion molecules in neurons and epithelial cells. Mol Biol Cell. 2005;16:3574–90. doi: 10.1091/mbc.E05-02-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mameli M, Balland B, Lujan R, Lüscher C. Rapid Synthesis and Synaptic Insertion of GluR2 for mGluR-LTD in the Ventral Tegmental Area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 46.Lacey MG, Mercuri NB, North RA. Actions of cocaine on rat dopaminergic neurones in vitro. Br J Pharmacol. 1990;99:731–735. doi: 10.1111/j.1476-5381.1990.tb12998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye K, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Liu S, Globa AK, Mills F, Naef L, Qiao M, Bamji SX, Borgland SL. Consumption of palatable food primes food approach behavior by rapidly increasing synaptic density in the VTA. PNAS. 2016;113:2520–2525. doi: 10.1073/pnas.1515724113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu QS, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathiisen T, et al. Postembedding Immunogold Cytochemistry of Membrane Molecules and Amino Acid Transmitters in the Central Nervous System. In: Zaborszky L, Wouterlood F, Lanciego J, editors. Neuroanatomical Tract-Tracing 3. Springer; US: 2006. pp. 72–108. [Google Scholar]
- 55.Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 2001;105:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- 56.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–88. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 57.Kratz JE, et al. Expression of stabilized beta-catenin in differentiated neurons of transgenic mice does not result in tumor formation. BMC Cancer. 2002;2:33. doi: 10.1186/1471-2407-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmeisser MJ, Grabrucker AM, Bockmann J, Boeckers TM. Synaptic cross-talk between N-methyl-D-aspartate receptors and LAPSER1-beta-catenin at excitatory synapses. J Biol Chem. 2009;284:29146–57. doi: 10.1074/jbc.M109.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.