Abstract
Spontaneous fungal peritonitis (SFP) is an infrequent but severe complication most commonly described in patients with liver cirrhosis. We present the first case of culture-proven SFP occurring in cardiogenic ascites. The diagnosis of SFP was clinically challenging as the initial ascites was consistent with the more common diagnosis of spontaneous bacterial peritonitis (SBP). The patient did not respond to antibacterial therapy, however, and the final diagnosis was only made with positive ascitic cultures that grew Candida glabrata. SFP should be considered in patients with either cardiac or cirrhotic ascites and have a delayed or lack of response to traditional SBP treatment.
Introduction
Spontaneous fungal peritonitis (SFP) is an infection of the peritoneal cavity by fungus without any surgically treatable sources.1 Less well-characterized than spontaneous bacterial peritonitis (SBP), SFP is a potentially fatal complication most commonly described in patients with liver cirrhosis.2 SFP occurring in cardiac ascites is an extremely rare phenomenon, as cardiac ascites has high protein content and is generally accepted as low-risk for spontaneous infection. A timely diagnosis is challenging but critical as SFP is associated with a significantly higher mortality than SBP.3,4
Case Report
A 50-year-old Hispanic man presented with a 3-week history of worsening shortness of breath and diffuse abdominal pain. Medical history was significant for alcohol abuse (daily consumption of 60 g ethanol and frequent binge drinking for 20 years), congestive heart failure (CHF) with reduced ejection fraction secondary to alcoholic cardiomyopathy, and cardiac ascites on oral diuretics. Patient was admitted for a CHF exacerbation and initiated on intravenous (IV) diuretic treatment.
Soon after admission, the patient became febrile (temperature, 102.3°F) and tachycardiac (heart rate > 120 beats/min), and blood pressure was stable at 100/80 mm Hg. The patient reported significantly worsening abdominal pain and was therefore admitted to the intensive care unit for closer monitoring. Physical exam showed positive jugular venous distension, left displaced point of maximal impulse, diffuse abdominal tenderness, and distension.
Initial labs revealed white blood cell (WBC) count 16,300/mm3, absolute neutrophil 13,800/mm3, hemoglobin 13.6 g/dL, platelet count 195 x 103/mm3, serum bicarbonate 20 mEq/L, blood urea nitrogen 61 mg/dL, creatinine 3.1 mg/dL, total bilirubin 2.9 mg/dL, direct bilirubin 1.5 mg/dL, aspartate aminotransferase 329 U/L, alanine aminotransferase 152 U/L, and lipase 17 U/L. Arterial blood gas showed pH 7.315 and lactate 2.08 mmol/L. Abdominal plain film revealed nonobstructive bowel gas pattern. Abdominal computed tomography (CT) without contrast showed a noncirrhotic liver with normal size and texture, a small amount of ascites, and no evidence of pneumoperitoneum or mechanical obstruction. Liver ultrasonography (US) with Doppler and abdominal CT angiography revealed patent mesenteric vessels and portal vessels with no signs of ischemia or thrombus. Hepatobiliary iminodiacetic acid scan was negative for acute cholecystitis. Paracentesis was unsuccessful as no satisfactory fluid pocket could be identified by bedside US.
Empiric piperacillin-tazobactam was initiated for SBP or a possible intraabdominal infection. The patient continued to have fevers daily, with persistent leukocytosis and abdominal pain. A CT scan with contrast on day 9, when renal function improved, revealed worsening ascites with peritoneal enhancement consistent with peritonitis (Figure 1). Diagnostic paracentesis performed on day 10 yielded 60 mL straw-colored fluid, with serum ascites albumin gradient (SAAG) calculated to be 2.0 g/dL, total fluid protein 3.6 g/dL, WBC 1,750/mm3, 66% polymorphonuclear (PMN) leukocytes, 5% lymphocytes, and 32% mononuclear leukocytes. Ascitic fluid was sent for gram stain and bacterial and fungal cultures.
Figure 1.
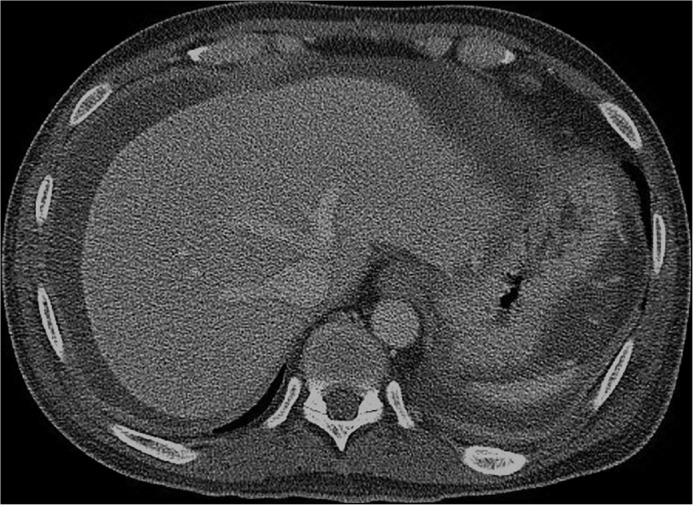
CT scan demonstrating a moderate amount of ascites, with peritoneal thickening and enhancement consistent with peritonitis.
We calculated the ascitic absolute neutrophil count to be 1,155/mm3, which supported a diagnosis of SBP. However, the patient had already been on antibiotics for 10 days and had not clinically improved, suggesting an alternative diagnosis. We also considered bacterial resistance or secondary bacterial peritonitis to explain these discordant findings. On day 12, the preliminary ascitic cultures results were positive for fungus, making the diagnosis of SFP.
The patient was promptly initiated on IV caspofungin, but he had persistent abdominal pain and worsening ascites. A therapeutic paracentesis was performed on day 13 for symptomatic relief. The fluid was also sent for cell count and culture to ensure response to antifungal therapy; the PMN count was 106/mm3. Repeat paracentesis performed on day 15 to ensure downward trend revealed the PMN count was 97/mm3. Speciation was reported on day 17 reflecting Candida glabrata. Antifungals were continued to day 20 to complete a 10-day course. The patient had complete resolution of symptoms and leukocytosis, and he remained clinically well upon follow-up 1 month after discharge.
Discussion
SFP is a potentially fatal infection most commonly described in patients with liver cirrhosis.1 Less well-recognized than SBP, the prevalence of SFP in critically ill patients with cirrhosis is reported to be 10%, which is comparable to that of SBP (14%), but SFP may be underestimated due to low detection rates by routine fungal culture methods.1 In addition, SFP is associated with a 1-month mortality of 73.3% in cirrhotic patients, significantly higher than that of SBP.2,5 Candida albicans and Candida glabrata are the most common pathogens for SFP in a cirrhotic population.6 Fungus has a much larger size (10–12 µm) than bacteria, so the translocation of Candida species from the alimentary tract is limited to patients with a more pronounced cirrhosis-associated immune dysfunction.2 Indeed, risk factors for SFP include a higher Child-Pugh score, a higher MELD score, previous use of prophylactic antibiotics, low ascitic fluid protein (<1 g/dL), and hepatorenal syndrome.2-4,6
In contrast, cardiac ascites is considered low-risk for infection as the opsonic and bactericidal activity of the ascitic fluid is preserved.7 However, the "gut hypothesis" postulated that, in patients with existing CHF, diminished cardiac output would cause chronic intestinal ischemia-reperfusion damage, leading to intestinal hypoxia, hypercapnia, and local pH change, which are all virulence activators for local microorganisms.8 During a CHF exacerbation, intestinal hypoperfusion and congestion can further disrupt the barrier function of the intestine, facilitating bacterial and fungal translocation.8,9 In fact, in a small retrospective study, 41.6% of SFP cases in patients with cirrhosis had concomitant SBP. As the patient in our case was empirically treated with antibiotics prior to his first paracentesis, we considered he might also have concomitant SBP but was adequately treated. Prior cases of SBP in cardiac ascites have reported pathogens including Escherichia coli, Streptococcus pneumoniae, Proteus mirabilis, Staphylococcus aureus, and Klebsiella species,10-13 which would have all been covered by empiric piperacillin-tazobactam.
The delay in diagnosis and treatment of SFP is the most concerning issue in the management of such cases, as seen in the literature.2-4,6 A large retrospective study found that 66% of patients with fungal infections were given antibiotics but were never treated with antifungal agents because of delayed diagnosis.2 Similarly, another study reported 79% of patients with fungus-positive ascites were not treated with antifungal therapy.4 Delay in diagnosis and treatment can be due to low clinical suspicion, insidious clinical symptoms, an indistinguishable ascitic fluid profile from SBP (PMN > 250/mm3), and delayed isolation of fungal species by routine culture methods. Therefore, a non-culture-based rapid diagnostic approach (e.g., polymerase chain reaction, beta-D-glucan) targeted to high-risk populations (advanced cirrhosis with prior antibiotic usage) is encouraged as a possible strategy to ensure timely diagnosis and optimal management of SFP patients.4,5
In conclusion, we report the first case of SFP in cardiac ascites, which was caused by Candida glabrata and was successfully treated with IV caspofungin. Because SFP is extremely rare in cardiac ascites, this diagnosis could be easily delayed or overlooked. We recommend administering empiric antibiotic therapy for suspected SBP in both cardiac and cirrhotic ascites, and maintaining a high clinical suspicion in cases that do not respond to antibacterial therapy and initiating anti-fungal agents in a timely fashion as the key in managing such cases.
Disclosures
Author contributions: Y. Wang wrote and revised the manuscript and is the article guarantor. S. Gandhi and BM Attar revised the manuscript for intellectual content.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Lahmer T, Brandl A, Rasch S, Schmid RM, Huber W. Fungal peritonitis: Underestimated disease in critically ill patients with liver cirrhosis and spontaneous peritonitis. PloS One. 2016;11:e0158389.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang S, Yu S, Lee JH, et al. . Spontaneous fungal peritonitis: A severe complication in patients with advanced liver cirrhosis. Eur J Clin Microbiol Infect Dis. 2014;33:259–64. [DOI] [PubMed] [Google Scholar]
- 3.Hassan E, Abd El-Rehim AS, Hassany SM, Ahmed AO, Elsherbiny NM, Mohammed MH. Fungal infection in patients with end-stage liver disease: Low frequency or low index of suspicion. Int J Infect Dis. 2014;23:69–74. [DOI] [PubMed] [Google Scholar]
- 4.Bucsics T, Schwabl P, Mandorfer M, Peck-Radosavljevic M. Prognosis of cirrhotic patients with fungiascites and spontaneous fungal peritonitis. J Hepatol. 2016;64:1452–4. [DOI] [PubMed] [Google Scholar]
- 5.Navasa M, Follo A, Llovet JM, et al. . Randomized, comparative study of oral ofloxacin versus intravenous cefotaxime in spontaneous bacterial peritonitis. Gastroenterology. 1996;111(4):1011–7. [DOI] [PubMed] [Google Scholar]
- 6.Bremmer DN, Garavaglia JM, Shields RK. Spontaneous fungal peritonitis: A devastating complication of cirrhosis. Mycoses. 2015; 58:387–93. [DOI] [PubMed] [Google Scholar]
- 7.Rossiter JP, Cunningham K, Manley PN. Spontaneous bacterial peritonitis: An unusual life-threatening complication of congestive heart failure. Can J Cardiol. 2015;31: 691.e13–691.e14. [DOI] [PubMed] [Google Scholar]
- 8.Nagatomo Y, Tang W. Intersections between microbiome and heart failure: Revisiting the gut hypothesis. J Card Fail. 2015;21:973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krack A, Richartz BM, Gastmann A, et al. . Studies on intragastric PCO2 at rest and during exercise as a marker of intestinal perfusion in patients with chronic heart failure. Eur J Heart Fail. 2004;6:403–7. [DOI] [PubMed] [Google Scholar]
- 10.Christou L, Economou M, Economou G, Kolettis TM, Tsianos EV. Characteristics of ascitic fluid in cardiac ascites. Scand J Gastroenterol. 2007;42:1102–5. [DOI] [PubMed] [Google Scholar]
- 11.Bulger K, Sugrue D, Crowe J. Spontaneous bacterial peritonitis in cardiac ascites: A case report. Ir J Med Sci. 1987;156:333. [DOI] [PubMed] [Google Scholar]
- 12.Runyon BA. Spontaneous bacterial peritonitis associated with cardiac ascites. Am J Gastroenterol. 1984;79:796. [PubMed] [Google Scholar]
- 13.Shaked Y, Samra Y. Primary pneumococcal peritonitis in patients with cardiac ascites: Report of 2 cases. Cardiology. 1988;75:372–4. [DOI] [PubMed] [Google Scholar]


