Abstract
Members of the nuclear receptor (NR) superfamily of ligand-regulated transcription factors play important roles in reproduction, development, and physiology. In humans, genetic mutations in NRs are causes of rare diseases, while hormones and drugs that target NRs are in widespread therapeutic use. The present issue of the JCI includes a series of Review articles focused on specific NRs and their wide range of biological functions. Here I reflect on the past, present, and potential future highlights of research on the NR superfamily.
Perspective
The nuclear receptor (NR) field was born in the mid 1980s, when the molecular cloning of several hormone receptors led to the realization that they share a common structure. Their cognate hormones, including sex steroids, glucocorticoids, thyroid hormone, and vitamin D metabolites, were previously studied by independent fields that did not interact except under the umbrella of endocrinology. Indeed, the existence and function of these hormones was mainly known only through the gain or loss of function of discrete endocrine glands that make and secrete them into the bloodstream. In humans, excess or deficiency of these hormones constitutes life-threatening conditions, and the ability to cure these diseases by administration of the hormone or ablation of the gland represented one of the first tangible advances of the scientific era of medicine that began at the turn of the 20th century. Indeed, in the 1896 edition of The Principles and Practice of Medicine, Sir William Osler, founder of the Association of American Physicians (the sister organization of the American Society of Clinical Investigation, publisher of the JCI), declared: “That we can restore to life the hopeless victims of myxedema is a triumph of experimental medicine” (1). Such discoveries spurred the proliferation of societies devoted to a specific hormone or hormone-producing gland, each representing a medical specialty or field of its own. Parallel studies of the mechanisms of action of these hormones, particularly estrogen, led to the concept that they acted through specific receptors to regulate the transcription of genes (2–5). This was a huge advance but begged the identification of the molecular receptors for hormones that acted in this way, and there was little recognition that there could be a common mechanism by which multiple hormones functioned in this fashion.
NR gene clones reveal functional similarities
Our understanding of the molecular mechanisms underlying hormone activity changed radically in 1985, when the genes encoding the receptors for glucocorticoids and estrogen were first identified by molecular cloning (6, 7). The predicted structures were very similar and soon thereafter were found to also be related to that of the receptor for thyroid hormone (8, 9). With these findings, the concept of a highly related family of nuclear hormone receptors was established. Soon after, structurally related molecules, termed orphan receptors, were discovered and the NR superfamily was born (10). This superfamily, now established to comprised 48 members in humans, became the subject of intense investigation not just in the silos of fields studying specific hormones, but rather as a class of proteins, with the goal of identifying mechanisms of action common to the group.
For quite a few years, major discoveries in the NR field highlighted conserved features of the receptor molecules. Almost immediately it was realized that the receptors were made up of specific domains that carried out the functions of DNA binding, hormone binding, and transcriptional activation. These domains had been biochemically characterized for the glucocorticoid receptor (11), and molecular cloning not only established their amino acid sequences but also revealed that the domains were modular and self-sufficient, such that swapping the hormone-binding regions of the receptors for glucocorticoids and retinoic acid resulted in chimeric receptors whose hormone and DNA-binding specificity was dictated by the cognate domain controlling each of these functions (12, 13). X-ray crystallographic studies revealed that the DNA-binding domains of different receptors are nearly superimposable in three dimensions (14, 15). Perhaps more surprising, although the ligand-binding domains are less well conserved at the amino acid level and bind their structurally diverse hormones with high specificity, their three-dimensional structures were also remarkably similar, consisting of 12 well-conserved α-helices (16–18). Features common to most NRs extend to the molecules with which they functionally interact, including specific DNA sequences recognized by the DNA-binding domain as well as ligand-binding domain interactions with numerous protein co-regulators (19), several of which underlie ligand-dependent activation (20, 21) and basal repression (22, 23) of gene transcription. Remarkably, although coactivators and corepressors exert opposite effects on transcriptional output, they utilize similar mechanisms to recognize liganded and unliganded NRs, respectively (24).
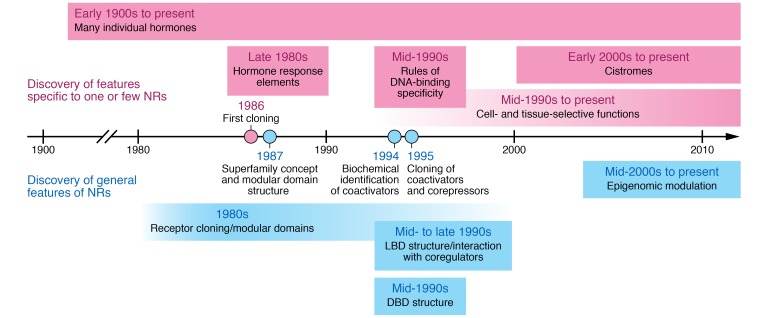
With so much similarity among the NRs, there was much to learn about a given receptor from the properties it shared with other receptors, and investigators with interest in specific NRs increasingly came together at international meetings devoted to the structure and functions of the NR superfamily. It was known that different hormones regulated different genes with different biological consequences, leading to an exciting dialectic in which discoveries of general properties were followed by discoveries of the principles that generate NR specificity (Figure 1). For example, the steroid receptors (except estrogen receptors) recognize different hexameric core sequences in DNA than non-steroid receptors; this characteristic can be attributed to specific regions of the DNA-binding domain (25). Many of the non-steroid NRs heterodimerize with a member of the retinoid X receptor family, with differing specificity based on the spacing of the core DNA sequences (26). Moreover, since a distinguishing feature of NRs is their regulation by hormones and related lipophilic molecules, orphan members of the superfamily that did not bind classical hormones were systematically “adopted” as they were discovered to be receptors for metabolites with previously unknown signaling mechanisms, with exciting implications for regulatory biology and cellular signaling (27).
Figure 1. Progress in the NR field.
Important discoveries are noted relevant to one another in terms of time as well as whether the discovery was a general feature of NRs (blue) or specific to only one or a few NRs (pink). LBD, ligand-binding domain; DBD, DNA-binding domain.
Now that the genome is sequenced we can be fairly confident that there are no more human NRs waiting to be discovered. An increasing number of the 48 we know of have now been assigned to regulatory ligands, and the pace of discovery of new ligands has slowed. The number of potential co-regulators, particularly coactivators, has expanded, and there is less excitement about finding a new co-regulator than there is about understanding the functions and relative importance of the ones we know about. The knowledge of genome sequences, coupled with next-generation DNA sequencing and advances in computational biology, have led to the recognition that NRs bind at thousands of genomic locations in a given cell, together termed the “cistrome” (28). Cistromes are cell type and signal specific and are governed by factors that supercede the inherent DNA-binding specificity of each individual NR; thus, they cannot be predicted simply from the structure and in vitro properties of the NR.
NR tissue and disease specificity reveal functional diversity
The ability to manipulate the mouse genome, in combination with sophisticated phenotyping methodologies, has sparked a return to studying the biological functions of NRs and signaling molecules that regulate them. Different NRs have been discovered to have fundamentally distinct actions in reproduction, development, and physiology, and in disease states, more often than not in a tissue-specific manner that cannot be predicted from encyclopedic knowledge of the genome sequence and/or receptor sequence and structure. Individual NR functions occur in concert with many other molecules that have important regulatory roles in these biological systems. To understand the role of a given NR, it is as important to understand the system as it is to know what the NR can do based on its structure; knowledge of the behavior of another NR is less transitive. As a result, the most exciting current NR biology is less about what the receptors have in common than it is about their specific functions in diverse biological systems. NR investigators focus on specific aspects of a given NR in different tissues and different diseases ranging from cancer to diabetes to neurodegeneration. For example, to understand the hormone dependence of cancer it is as important study the biology of the cancer cell as it is to study the structure and properties of the hormone receptor.
Due to the tissue and disease specificity of NR function, there has been a diaspora, with NR science being brought to meetings devoted to specific diseases, and fewer meetings devoted to the NR superfamily per se. Moreover, there are fewer review articles written about the NR superfamily and more about the complex biological functions of specific NRs, as typified by the special series of articles in this issue of the JCI. I hope that the readers of this exciting series recognize that the remarkable level of depth and scope of the studies reviewed in this series would have been impossible without the NR cloning and structure/function breakthroughs that began three decades ago.
Future directions
What is to become of the field that studies the NR superfamily? There may be a clue from the history of science. The term “vitamine” was coined in 1912 by Casimir Funk as a condensation of “vital” and “amine” (29), and it encompasses the dietary micronutrients required for life, which are divided into water-soluble and fat-soluble categories. The first fat-soluble vitamin was discovered in 1913, by E.V. McCollum and Marguerite Davis, who named it vitamin A with the expectation that many more vitamins would be discovered and named alphabetically (30). Indeed, 12 additional vitamins were discovered in the subsequent three decades. This was an exciting time for this field, but the most recent discovery was that of vitamin B12 in 1948. The functional study of individual vitamins remained an active area of research, and indeed vitamin A (also known as retinol or β-carotene) has turned out to be a precursor of retinoic acid, the powerful morphogen and drug treatment for acne and acute promyelocytic leukemia, which is a ligand for a NR. Science marches on, and the study of NRs will continue to inform us about biology and drive advances in medicine. Indeed, approximately 13% of all drugs approved by the United States Food and Drug Administration target NRs (31). There continue to be major opportunities for the development of tissue-selective NR ligands (analogous to selective estrogen receptor modulators), including improvement of the therapeutic index in targeting inflammation with glucocorticoid receptor ligands, modulation of lipid metabolism with ligands for a variety of NRs, and the use of ligands to treat many other diseases). Members of the NR family may go their separate ways, but their scientific journeys remain exciting.
Acknowledgments
I thank Myles Brown, David Moore, and Ray Soccio for reading the manuscript and helpful discussions. I apologize that a limit to the number of references prevented citation of many important and relevant studies. Work on NRs in my laboratory is funded by NIH grants DK43806, DK45586, and DK49780.
Footnotes
Conflict of interest: M.A. Lazar is on scientific advisory boards for Pfizer, Inc., and Eli Lilly and Company.
Reference information:J Clin Invest. 2017;127(4):1123–1125.https://doi.org/10.1172/JCI92949.
References
- 1. Osler W. The Principles And Practice Of Medicine: Designed For The Use Of Practitioners And Students Of Medicine. 2nd ed. New York, New York, USA: Appleton and Company, 1896. [Google Scholar]
- 2.Hamilton TH, Widnell CC, Tata JR. Sequential stimulations by oestrogen of nuclear RNA synthesis and DNA-dependent RNA polymerase activities in rat uterus. Biochim Biophys Acta. 1965;108(1):168–172. doi: 10.1016/0005-2787(65)90129-2. [DOI] [PubMed] [Google Scholar]
- 3.O’Malley BW, McGuire WL, Middleton PA. Altered gene expression during differentiation: population changes in hybridizable RNA after stimulation of the chick oviduct with oestrogen. Nature. 1968;218(5148):1249–1251. doi: 10.1038/2181249a0. [DOI] [PubMed] [Google Scholar]
- 4.Toft D, Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci U S A. 1966;55(6):1574–1581. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen EV, et al. Estrogen-binding substances of target tissues. Science. 1967;158(3800):529–530. doi: 10.1126/science.158.3800.529-c. [DOI] [PubMed] [Google Scholar]
- 6.Hollenberg SM, et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green S, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- 9.Sap J, et al. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- 10.Mangelsdorf DJ, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlstedt-Duke J, Okret S, Wrange O, Gustafsson JA. Immunochemical analysis of the glucocorticoid receptor: identification of a third domain separate from the steroid-binding and DNA-binding domains. Proc Natl Acad Sci U S A. 1982;79(14):4260–4264. doi: 10.1073/pnas.79.14.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- 13.Green S, Chambon P. Oestradiol induction of a glucocorticoid-responsive gene by a chimaeric receptor. Nature. 1987;325(6099):75–78. doi: 10.1038/325075a0. [DOI] [PubMed] [Google Scholar]
- 14.Freedman LP, Luisi BF, Korszun ZR, Basavappa R, Sigler PB, Yamamoto KR. The function and structure of the metal coordination sites within the glucocorticoid receptor DNA binding domain. Nature. 1988;334(6182):543–546. doi: 10.1038/334543a0. [DOI] [PubMed] [Google Scholar]
- 15.Luisi BF, Xu WX, Otwinowski Z, Freedman LP, Yamamoto KR, Sigler PB. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- 16.Bourguet W, Ruff M, Chambon P, Gronemeyer H, Moras D. Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-alpha. Nature. 1995;375(6530):377–382. doi: 10.1038/375377a0. [DOI] [PubMed] [Google Scholar]
- 17.Wagner RL, et al. A structural role for hormone in the thyroid hormone receptor. Nature. 1995;378(6558):690–697. doi: 10.1038/378690a0. [DOI] [PubMed] [Google Scholar]
- 18.Shiau AK, et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95(7):927–937. doi: 10.1016/S0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 19.Lee JW, Choi HS, Gyuris J, Brent R, Moore DD. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol Baltim. 1995;9(2):243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- 20.Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994;264(5164):1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- 21.Oñate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270(5240):1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 22.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377(6548):454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 23.Hörlein AJ, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377(6548):397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 24.Hu X, Lazar MA. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature. 1999;402(6757):93–96. doi: 10.1038/47069. [DOI] [PubMed] [Google Scholar]
- 25.Green S, Kumar V, Theulaz I, Wahli W, Chambon P. The N-terminal DNA-binding “zinc finger” of the oestrogen and glucocorticoid receptors determines target gene specificity. EMBO J. 1988;7(10):3037–3044. doi: 10.1002/j.1460-2075.1988.tb03168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umesono K, Evans RM. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- 27.Evans RM, Mangelsdorf DJ. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014;157(1):255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krum SA, Miranda-Carboni GA, Lupien M, Eeckhoute J, Carroll JS, Brown M. Unique ERalpha cistromes control cell type-specific gene regulation. Mol Endocrinol. 2008;22(11):2393–2406. doi: 10.1210/me.2008-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semba RD. The discovery of the vitamins. Int J Vitam Nutr Res. 2012;82(5):310–315. doi: 10.1024/0300-9831/a000124. [DOI] [PubMed] [Google Scholar]
- 30.Schneider HA. Rats, fats, and history. Perspect Biol Med. 1986;29(3 pt 1):392–406. doi: 10.1353/pbm.1986.0003. [DOI] [PubMed] [Google Scholar]
- 31.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5(12):993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]