Abstract
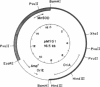
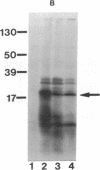
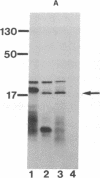
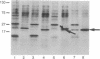
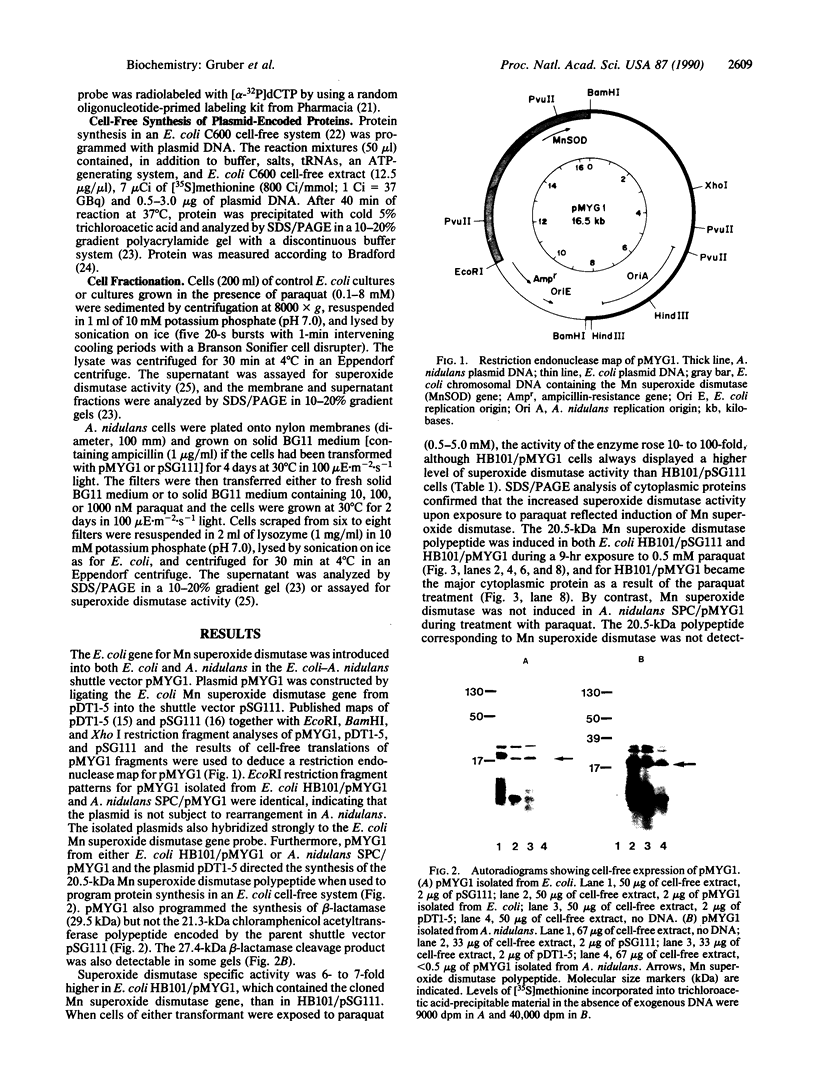
The Mn superoxide dismutase gene of Escherichia coli was subcloned into the E. coli-Anacystis nidulans shuttle vector pSG111 to make the plasmid pMYG1. Transformation of E. coli HB101 with pMYG1 resulted in a 6-fold increase in superoxide dismutase activity. There was also induction of Mn superoxide dismutase in the transformants upon exposure to paraquat, as evidenced by dramatically increased levels of the Mn superoxide dismutase polypeptide in cytoplasmic extracts and a 16-fold further increase in superoxide dismutase activity. As well, the E. coli transformants showed resistance to paraquat-mediated inhibition of growth. Anacystis nidulans, a cyanobacterium that has no detectable Mn superoxide dismutase and is, consequently, very sensitive to oxidative stress, was also transformed with pMYG1. The transformants had detectable levels of Mn superoxide dismutase protein and showed resistance to paraquat-mediated inhibition of growth and photobleaching of pigments. Paraquat is known to promote formation of the superoxide radical anion, O2-., and thus the data have been interpreted as indicating that the cloned Mn superoxide dismutase provides protection in both E. coli and A. nidulans against damage attributable to O2-..
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Kellenberg D., Shilo M. Effect of photooxidative conditions on levels of superoxide dismutase in Anacystis nidulans. Photochem Photobiol. 1974 May;19(5):379–382. doi: 10.1111/j.1751-1097.1974.tb06526.x. [DOI] [PubMed] [Google Scholar]
- Abeliovich A., Shilo M. Photooxidative death in blue-green algae. J Bacteriol. 1972 Sep;111(3):682–689. doi: 10.1128/jb.111.3.682-689.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beyer W. F., Jr, Fridovich I. Effect of hydrogen peroxide on the iron-containing superoxide dismutase of Escherichia coli. Biochemistry. 1987 Mar 10;26(5):1251–1257. doi: 10.1021/bi00379a008. [DOI] [PubMed] [Google Scholar]
- Bittner M., Kupferer P., Morris C. F. Electrophoretic transfer of proteins and nucleic acids from slab gels to diazobenzyloxymethyl cellulose or nitrocellulose sheets. Anal Biochem. 1980 Mar 1;102(2):459–471. doi: 10.1016/0003-2697(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Bloch C. A., Ausubel F. M. Paraquat-mediated selection for mutations in the manganese-superoxide dismutase gene sodA. J Bacteriol. 1986 Nov;168(2):795–798. doi: 10.1128/jb.168.2.795-798.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Expression of the Escherichia coli lacZ gene on a plasmid vector in a cyanobacterium. Science. 1985 Nov 15;230(4727):805–807. doi: 10.1126/science.2997920. [DOI] [PubMed] [Google Scholar]
- Daniell H., Sarojini G., McFadden B. A. Transformation of the cyanobacterium Anacystis nidulans 6301 with the Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2546–2550. doi: 10.1073/pnas.83.8.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloff J. N., Steinitz Y., Shilo M. Photooxidation of cyanobacteria in natural conditions. Appl Environ Microbiol. 1976 Jan;31(1):119–126. doi: 10.1128/aem.31.1.119-126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Bernstein Y., Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J. 1986 Mar;5(3):615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., D'Ari R., Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gendel S., Straus N., Pulleyblank D., Williams J. Shuttle cloning vectors for the cyanobacterium Anacystis nidulans. J Bacteriol. 1983 Oct;156(1):148–154. doi: 10.1128/jb.156.1.148-154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Sherman L. A. A hybrid plasmid is a stable cloning vector for the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1983 Sep;155(3):966–972. doi: 10.1128/jb.155.3.966-972.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977 Nov 10;252(21):7667–7672. [PubMed] [Google Scholar]
- Kuhlemeier C. J., Thomas A. A., van der Ende A., van Leen R. W., Borrias W. E., van den Hondel C. A., van Arkel G. A. A host-vector system for gene cloning in the cyanobacterium Anacystis nidulans R2. Plasmid. 1983 Sep;10(2):156–163. doi: 10.1016/0147-619x(83)90068-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin J. P., Logsdon N. The role of oxygen radicals in dye-mediated photodynamic effects in Escherichia coli B. J Biol Chem. 1987 May 25;262(15):7213–7219. [PubMed] [Google Scholar]
- Porter R. D. Transformation in cyanobacteria. Crit Rev Microbiol. 1986;13(2):111–132. doi: 10.3109/10408418609108736. [DOI] [PubMed] [Google Scholar]
- Robertson P., Jr, Fridovich I. A reaction of the superoxide radical with tetrapyrroles. Arch Biochem Biophys. 1982 Feb;213(2):353–357. doi: 10.1016/0003-9861(82)90560-4. [DOI] [PubMed] [Google Scholar]
- See Y. P., Glick B. R. Analysis of the expression of cloned genes using an Escherichia coli cell-free system. Can J Biochem. 1982 Dec;60(12):1095–1100. doi: 10.1139/o82-140. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Avila H. Structure and gene expression of the E. coli Mn-superoxide dismutase gene. Nucleic Acids Res. 1986 Jun 11;14(11):4577–4589. doi: 10.1093/nar/14.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati D. Cloning and mapping of the manganese superoxide dismutase gene (sodA) of Escherichia coli K-12. J Bacteriol. 1983 Sep;155(3):1078–1087. doi: 10.1128/jb.155.3.1078-1087.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati D. Transcriptional and posttranscriptional regulation of manganese superoxide dismutase biosynthesis in Escherichia coli, studied with operon and protein fusions. J Bacteriol. 1988 Jun;170(6):2511–2520. doi: 10.1128/jb.170.6.2511-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorimier R., Guglielmi G., Bryant D. A., Stevens S. E., Jr Functional expression of plastid allophycocyanin genes in a cyanobacterium. J Bacteriol. 1987 May;169(5):1830–1835. doi: 10.1128/jb.169.5.1830-1835.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]