Abstract
Anxiety is common among breast-cancer survivors. This analysis examined the effect of a hypnotic relaxation therapy, developed to reduce hot flashes, on anxiety levels of female breast-cancer survivors. Anxiety was assessed using a numeric analog scale and the Hospital Anxiety and Depression Scale-Anxiety subscale. Significant reductions in anxiety were found from pre- to postintervention for each weekly session and were predictive of overall reductions in anxiety from baseline to after the last intervention. In this analysis, hypnotizability did not significantly predict for anxiety reductions measured before and after each session or from baseline to exit. These data provide initial support for the use of hypnotic relaxation therapy to reduce anxiety among breast-cancer survivors.
Breast cancer is the most common cancer among women in the United States with more than 2.9 million cases reported (American Cancer Society, 2013; U. S. Cancer Statistics Working Group, 2013). The American Cancer Society (2013) estimated that more than 230,000 women were diagnosed with breast cancer in 2013 alone. Due to improvements in cancer detection and treatments, survival rates for breast-cancer patients have increased; thus, women with breast cancer now comprise the largest group of cancer survivors in the United States (American Cancer Society, 2013; Berry et al., 2005; Smith, Singh-Carlson, Downie, Payeur, & Wai, 2011). Therefore, it is imperative to investigate the comorbidities of breast-cancer survivorship, including anxiety, and possible treatments.
Anxiety is a common symptom associated with cancer diagnosis, treatment, and survivorship (Hodgkinson et al., 2007). A recent review found that anxiety is ubiquitous in women receiving treatment for breast cancer and that certain types of treatment, such as chemotherapy and mastectomy, are associated with greater levels of anxiety (Lim, Devi, & Ang, 2011). It has been reported that fear of cancer recurrence affects 33% to 56% of cancer patients and is associated with psychological distress (Lebel, Beattie, Arès, & Bielajew, 2013). In a study examining quality of life in long-term breast-cancer survivors, 71% reported anxiety and fear of cancer recurrence (Gotay & Muraoka, 1998). Boyes, Girgis, D’Este, and Zucca (2011) reported that 30% of breast-cancer survivors experienced clinical levels of anxiety up to 6 months after treatment ended. It has been shown that 80% of women with breast cancer report significant distress (Elkins, 2014b). One source of this distress is hot flashes. Hot flashes are linked to reduced quality of life and increased distress among breast-cancer patients (Carpenter, Johnson, Wagner, & Andrykowski, 2002). The distress caused by hot flashes increases the risk for anxiety in patients already prone to experience high levels of anxiety. It has been noted that among cancer patients, anxiety is particularly difficult to treat with traditional psychopharmacological and therapeutic means due to possible drug interactions and side effects (Schernhammer, Haidinger, Waldhör, & Vutuc, 2009). Therefore, alternatives to pharmacological treatments are needed. Mind-body therapies are increasingly being researched as possible solutions (Schernhammer et al.). The increasing use of complementary and alternative medicine (CAM) for oncology symptoms indicates that interest in nonpharmacological methods of treatment is helpful and desired (Booth-LaForce et al., 2010).
Hypnosis is an intervention that has been shown to be effective in treating anxiety related to various medical conditions, including hemodialysis, atypical cardiac disorder, bone marrow biopsies, colonoscopy, postoperative surgery, emergency dental extraction, and large-core breast biopsy (Chan, 2012; Elkins et al., 2006; Gow, 2006; Lang et al., 2006; Massarini, Rovetto, & Tagliaferri, 2005; Snow et al., 2012; Untas et al., 2013). Studies investigating the use of hypnosis in controlling anxiety in cancer patients found that hypnosis interventions significantly decreased anxiety (Genuis, 1995). Research has shown that hypnosis is likely efficacious in the treatment of both state and trait anxiety; however it is acknowledged that further randomized controlled trials are needed to solidify findings (Coelho, Canter, & Ernst, 2008; Hammond, 2010). Overall, the current literature supports the use of hypnosis as a means of decreasing anxiety in a variety of health conditions. However, there remains a lack of information regarding the effectiveness of a hypnotic intervention on anxiety among breast-cancer survivors.
The current analysis utilizes data from a larger study in which we examined hypnotic relaxation therapy (Elkins, 2014a) as a treatment of hot flashes among breast-cancer survivors (Elkins et al., 2008). The larger study demonstrated hot flashes decreased by an average of 68% over baseline. The full study methods, a description of the baseline sample, and the primary outcomes have been reported elsewhere (Elkins et al., 2008; Elkins et al., 2011). The purpose of this secondary analysis was to investigate the effectiveness of hypnotic relaxation therapy (HRT) on self-reported anxiety among breast-cancer survivors. These data have not been previously analyzed or reported.
Method
Participants
Participants were recruited from the Scott and White Cancer Institute in Temple, Texas. After informed consent, recruited participants were randomly assigned to either the hypnotic relaxation therapy (HRT) intervention or a no-treatment control arm. Participant eligibility was based on age (at least 18 years), a history of primary breast cancer with no evidence of detectable disease, and the presence of at least 14 hot flashes per week for a period of at least 1 month. Further, participants were restricted to females with a life expectancy of greater than 6 months. Participants were screened for medical and psychological conditions that would preclude study participation. Women who had taken antihormonal agents for breast cancer (e.g., tamoxifen or raloxifene) for more than 1 month were deemed eligible to participate. No other concurrent hormonal or putative therapies, chemotherapy, treatments for hot flashes, or the use of hypnosis for any reason were allowed. Full eligibility criteria was reported by Elkins et al. (2008).
Materials
Numeric Analog Scale for Anxiety
A numeric analog scale for anxiety rating consisted of a single-item numeric visual analog scale that consists of numbers (0–10) spaced equally and sequentially along a 100mm horizontal axis on which participants indicate their current level of anxiety. Past research has indicated that this type of scale is an adequate, valid, and reliable measure of anxiety (Davey, Barratt, Butow, & Deeks, 2007; Maxwell, 1978; Williams, Morlock, & Feltner, 2010). A numeric analog scale for anxiety is easy to administer and correlates well with other measures of anxiety (r = 0.64, p < .001, STAI-S; r = 0.46, p < .005, STAI-T; Elkins et al., 2004).
Hospital Anxiety and Depression Scale-Anxiety Subscale (HADS-A)
The HADS-A (Zigmond & Snaith, 1983) consists of seven items. The self-report scale is a measure of participants’ generalized anxiety and fear over the past week. It exhibits high internal consistency (α = 0.84–0.90; Julian, 2011) and concurrent validity with other self-report measures of anxiety (r = 0.80 with the GHQ, BDI, STAI, CAS, and SCL-90; Bjelland et al., 2001).
Stanford Hypnotic Clinical Scale (SHCS)
The SHCS (Morgan & Hilgard, 1978) is a five-item measure of hypnotic responsiveness often used as a brief assessment tool in clinical settings (Woody & Barnier, 2008). Administration takes approximately 25 minutes. The SHCS has been shown to be as reliable and valid as other longer measures of hypnotizability (Elkins, 2014a; Morgan & Hilgard).
Procedure
After obtaining written informed consent, participants randomized to the HRT condition completed a demographic questionnaire and baseline measures (including the HADS-A). A research coordinator scheduled participants for five weekly hypnotic relaxation therapy (HRT) sessions. A numeric analog scale for anxiety was administered before and after each HRT. The HADS-A was completed at baseline and end-of-study. The SCHS was completed by participants at the end-of-study by masters- or doctoral-level professionals who were not involved in delivery of the intervention. The intervention consisted of HRT for the reduction of hot flashes. HRT is a manualized intervention involving both structured and individualized hypnotic inductions and teaching patients self-hypnosis (Elkins, 2014a). Hypnotic suggestions are targeted to symptom reduction. In the present study, suggestions were for a hypnotic state, relaxation, and mental imagery for coolness as well as calmness. The hypnotic intervention was delivered according to a treatment manual by a clinician with a doctoral degree in psychology who had completed training according to the guidelines outlined by Elkins and Hammond (1998). HRT for each session included: a hypnotic induction; mental imagery for coolness; mental imagery and suggestions for relaxation; deepening hypnosis and dissociation from hot flashes; positive suggestions and imagery for the future; self-hypnosis; and the alert, “In a few moments, return to conscious alertness.” A more detailed description of the procedures has been previously published (Elkins et al., 2008).
Statistical analyses were conducted using PASW Statistics v 21.0 (SPSS: An IBM Company). Student’s dependent samples t tests and trend analysis were used to analyze pre- to postintervention changes in anxiety for each HRT session and for overall change in anxiety from baseline to follow-up. Bivariate linear regression was used to determine if changes in self-reported anxiety from pre- to postintervention were predictive of overall declines of anxiety reported on the HADS-A. Regression analysis was also used to determine if hypnotizability (SCHS) scores were predictive of reductions in anxiety.
Results
Twenty-seven breast-cancer survivors were assigned to receive hypnotic relaxation therapy. Mean age for participants was 55.5 years (SD = 7.3), with a range of 42 to 60 years. The ethnicity of participants was Caucasian (n = 25), and Hispanic (n = 1). The majority of participants were married (77%) and reported a college education (69%). See Table 1 for complete demographic information. Data were missing on the current measures for one participant from the original sample and therefore excluded from the analyses (n = 26). Appendix 1 shows the flow of participants from enrollment through study completion.
Table 1.
Demographic Characteristics of HRT Participants
| Characteristic | ||
|---|---|---|
| Mean age, years | 55 years, 6 months | |
| No. | % | |
|
| ||
| Ethnicity | ||
| Caucasian | 25 | 96 |
| Hispanic | 1 | 4 |
| Education | ||
| High School | 8 | 31 |
| Bachelor’s degree | 8 | 31 |
| Master’s degree | 10 | 38 |
| Marital status | ||
| Single | 1 | 4 |
| Married | 20 | 77 |
| Separated | 1 | 4 |
| Other | 3 | 11 |
| Missing data | 1 | 4 |
NOTE: Does not include missing participant demographic; n = 26.
Appendix 1. Flow Diagram of Participant Enrollment1.
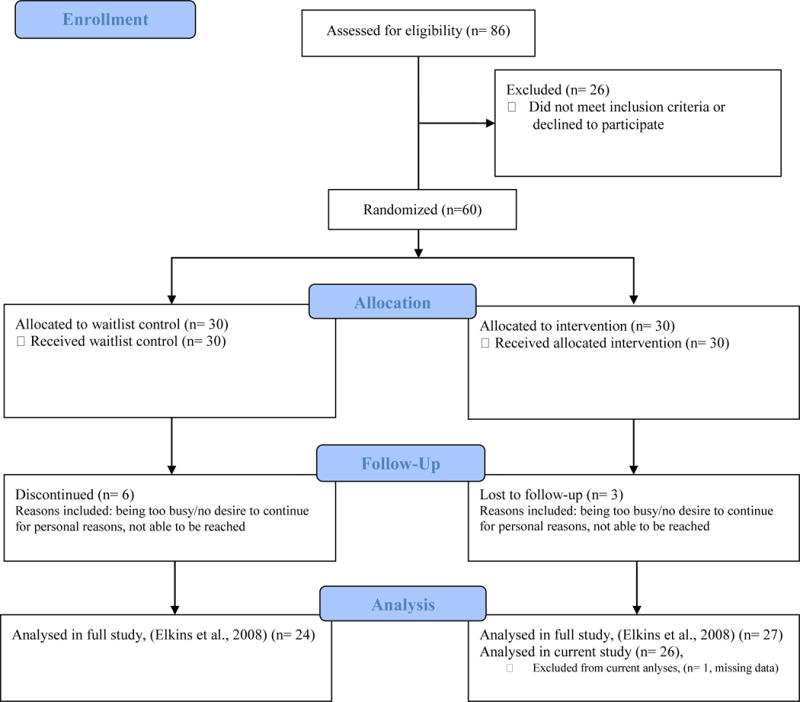
1Flow diagram template courtesy of CONSORT (2010).
The average pretest anxiety score, as reported on a numeric analog scale for anxiety, was 3.83 (SD = 2.11) for the first HRT session. Table 2 summarizes the means and standard deviations of the average numeric analog anxiety scores for all five HRT sessions.
Table 2.
Comparison of a Numeric Rating Scale for Anxiety Ratings for Female Breast-Cancer Survivors Participating in Hypnotic Relaxation Therapy for Hot Flashes
| Preintervention | Postintervention | Analyses | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | Mean | SD | t(25) | 95% CI | Cohen’s d | |
| Session 1 | 3.83 | 2.11 | 0.77 | 1.11 | 8.93* | [2.35, 3.76] | 1.75 |
| Session 2 | 3.27 | 1.89 | 0.65 | 0.94 | 9.98* | [2.08, 3.16] | 1.96 |
| Session 3 | 3.31 | 2.41 | 0.83 | 1.16 | 7.89* | [1.83, 3.13] | 1.55 |
| Session 4 | 3.38 | 2.16 | 0.54 | 0.76 | 7.83* | [2.10, 3.60] | 1.54 |
| Session 5 | 2.44 | 2.00 | 0.40 | 0.72 | 5.92* | [1.33, 2.75] | 1.16 |
Note. Statistics are based on two-tailed dependent-samples t tests using Welch procedure for unequal variances. The sample size for each analysis was n = 26. CI = confidence interval.
p < .001.
Changes in anxiety reported on a numeric analog scale were assessed for normality and skewed distributions and found to be normally distributed. In a sample of 26 women, self-reported anxiety significantly decreased from pre- to postintervention, (p < .05, Table 2). Figure 1 visually illustrates these reductions in self-reported anxiety for each HRT session.
Figure 1.
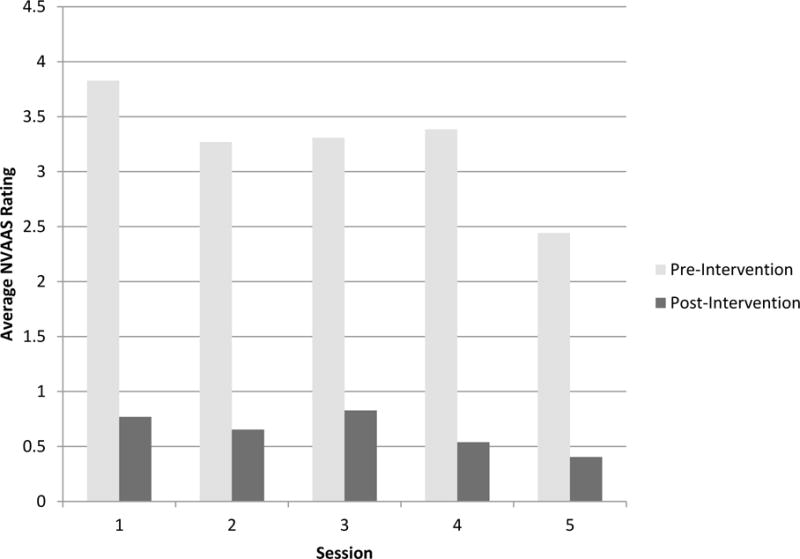
Reductions in Anxiety Following HRT Sessions
As shown in Figure 2, trend analysis indicated significant declines in pretest anxiety from HRT session 1 to HRT session 5, F(1,125) = 4.06, p = .046. A dependent samples t test of pretest anxiety for HRT session 1 and HRT session 5 was statistically significant, t(25) = 2.57, p = .016, d = .50, 95% CI [.277, 2.49]. Trend analysis of posttest anxiety ratings from HRT session 1 to HRT session 5 showed reductions, yet were not found to be statistically significant, F(1,125) = 2.05, p = .099, (see Figure 2).
Figure 2.
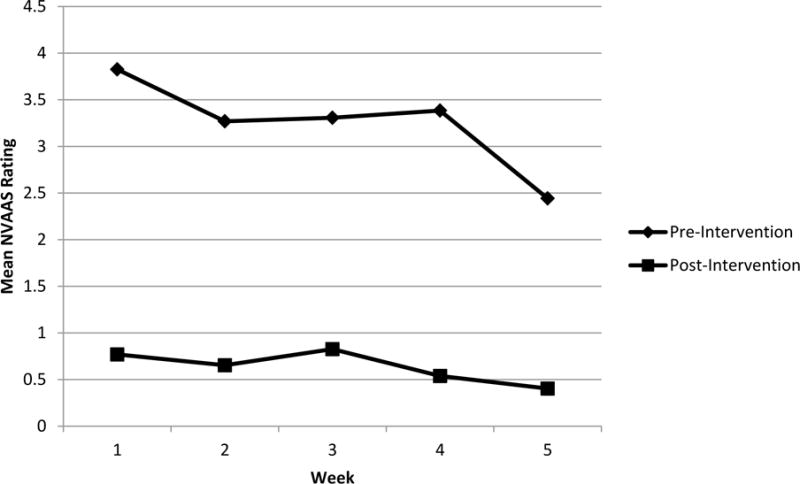
Trends in Anxiety Reduction For Five Weekly Sessions of HRT
As Elkins et al., (2008) previously reported, HADS-A scores reduced significantly among participants receiving the intervention, F(2,44) = 6.083, p < .005, η2 = .217. Findings from the current study provide further support for reductions in HADS-A scores among 26 participants receiving five weekly sessions of HRT (Baseline Mean = 5.89, SD = 4.34; Endpoint Mean = 3.23, SD = 3.19, p < .004). A regression analysis of these participants showed declines in self-reported anxiety pre- to postintervention were predictive of anxiety reductions on the HADS-A from baseline to endpoint, R2 = .15, p < .05.
Hypnotizability as measured by the SCHS was analyzed as a possible moderator of change in self-reported anxiety. A bivariate regression analysis showed hypnotizability did not significantly predict for reductions in self-reported anxiety from pre- to postintervention, R2 = .022, F(1,23) = .51, p = .48, or as reported on the HADS-A, R2 = .004, p = .77. Data were missing for 1 participant on the SHCS and excluded from the analyses (n = 25). The intervention was well tolerated and there were no adverse events (Elkins et al., 2008).
Discussion
Anxiety is common among breast-cancer survivors (Prokop, Bradley, Burish, Anderson, & Fox, 1991). Anxiety disrupts and decreases quality of life, thereby becoming a barrier to complete recovery (Stark & House, 2000). As rates of breast cancer increase, it is imperative that treatments be developed that address the negative sequelae of cancer diagnosis, treatment, and survivorship, including anxiety (Khan, Ward, Watson, & Rose, 2010; Lebel et al., 2013). Research has shown breast-cancer survivors are receptive to complementary therapies, such as mind-body therapies, and seek them out to counteract the accompanying side effects of cancer treatment and recovery (Hann, Baker, Denniston, & Entrekin, 2005; Matthews, Sellergren, Huo, List, & Fleming, 2007).
The present analysis provides data to support that hypnotic relaxation therapy (HRT) may be useful in reducing self-reported anxiety among breast-cancer survivors. Using hypnosis to treat anxiety among breast-cancer patients is an attractive option in that it poses little risk to the patient, as demonstrated by our side-effect data, has low patient burden, and is cost effective. Hypnosis is usually reported as a pleasant experience, has few (if any) known side effects, and does not interact with medications. Additionally, once a patient becomes familiar with hypnosis, it may be generalized to help with other distressing symptoms.
The effect sizes in this study were found to be large, but it has been reported that the use of a pretest-posttest design, such as that used in this study, may inflate effect sizes (Matt & Navarro, 1997). Also, future research utilizing objective physiological measures of anxiety are needed to fully verify the anxiety reducing capabilities of HRT (Vande Vusse, Hanson, Berner, & White Winters, 2010).
Although the effects of HRT show promise for the reduction of anxiety among breast-cancer survivors, larger randomized controlled studies are needed to determine if these results are robust. Also, because the HRT in the current study was specifically intended to address hot flashes, further investigation is warranted to better understand if anxiety reductions were independent of the reductions in hot flashes also experienced by participants receiving HRT. A future study should consider anxiety as the primary measure, both subjectively and objectively, and include an active control group, such as structured attention, thereby providing control for attention, time, self-report bias, and other demand characteristics that could confound results.
Although hypnotizability has been found to moderate the effect of hypnosis on hot flashes (Elkins et al., 2011), in the present study it did not moderate reductions in anxiety among breast-cancer survivors. In the present study, HRT was effective in reducing anxiety regardless of hypnotic ability. Although hypnotizability may play a role in altering a physical symptom (such as hot flashes), reduction of emotional symptoms (such as anxiety) may be largely dependent on relaxation. It is noteworthy that declines in self-reported anxiety pre- to postintervention were predictive of anxiety reductions as measured on the HADS-A. This suggests that the initial response to hypnotic induction with reduction in anxiety may be a positive indicator of later end-point reduction in self-reported anxiety.
There is a need for effective treatments of anxiety among breast-cancer survivors that have low patient burden, are cost effective, and safe. Hypnotic relaxation therapy shows promise as such a treatment; yet more randomized controlled trials are needed before definitive conclusions can be drawn. In order to provide the best care possible to those recovering from the effects of breast cancer, it is imperative that these treatments be researched and developed further so that they may be disseminated to those in need of them.
Acknowledgments
This study was supported in part by grants from the National Center for Complementary and Alternative Medicine (U01AT004634) National Institutes of Health.
Contributor Information
Alisa J. Johnson, Baylor University, Waco, Texas, USA
Joel Marcus, Gayle and Tom Benson Cancer Center, Ochsner Cancer Institute, New Orleans, Louisianan, USA.
Kimberly Hickman, Baylor University, Waco, Texas, USA.
Debra Barton, University of Michigan School of Nursing, Ann Arbor, USA.
Gary Elkins, Baylor University, Waco, Texas, USA.
References
- American Cancer Society. Breast Cancer Facts & Figures, 2013–2014. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Feuer EJ. Effect of screening and adjuvant therapy on mortality from breast cancer. New England Journal of Medicine. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- Booth-LaForce C, Scott CS, Heitkemper MM, Cornman BJ, Lan MC, Bond EF, Swanson KM. Complementary and alternative medicine (CAM) attitudes and competencies of nursing students and faculty: Results of integrating CAM into the nursing curriculum. Journal of Professional Nursing. 2010;26(5):293–300. doi: 10.1016/j.profnurs.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes AW, Girgis A, D’Este C, Zucca AC. Flourishing or floundering? Prevalence and correlates of anxiety and depression among a population-based sample of adult cancer survivors 6 months after diagnosis. Journal of Affective Disorders. 2011;135:184–192. doi: 10.1016/j.jad.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Johnson D, Wagner L, Andrykowki M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncology Nursing Forum. 2002;29(3):E16–25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- Chan IF. The use of hypnosis for the treatment of anxiety in atypical cardiac disorder. Australian Journal of Clinical and Experimental Hypnosis. 2012;40(1):34–42. [Google Scholar]
- Coelho HF, Canter PH, Ernst E. The effectiveness of hypnosis for the treatment of anxiety: A systematic review. Primary Care and Community Psychiatry. 2007;12(2):49–63. doi: 10.1080/17468840701680678. [DOI] [Google Scholar]
- Davey HM, Barratt AL, Butow PN, Deeks JJ. A one-item question with a Likert or visual analog scale adequately measured current anxiety. Journal of Clinical Epidemiology. 2007;60:356–360. doi: 10.1016/j.jclinepi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Elkins GR. Hypnotic Relaxation Therapy: Principles and Applications. NY: Springer; 2014a. [DOI] [PubMed] [Google Scholar]
- Elkins GR. Relief from Hot Flashes: The natural, drug-free program to reduce hot flashes, improve sleep, and ease stress. New York, NY: DemosHEALTH; 2014b. [DOI] [PubMed] [Google Scholar]
- Elkins G, Fisher W, Johnson A, Marcus J, Dove J, Perfect M, Keith T. Moderating effect of hypnotizability on hypnosis for hot flashes in breast cancer survivors. Contemporary Hypnosis and Integrative Therapy. 2011;28(3):21–27. [PMC free article] [PubMed] [Google Scholar]
- Elkins GR, Hammond DC. Standards of training in clinical hypnosis: Preparing professionals for the 21st century. American Journal of Clinical Hypnosis. 1998;41:55–64. doi: 10.1080/00029157.1998.10404185. [DOI] [PubMed] [Google Scholar]
- Elkins G, Marcus J, Stearns V, Perfect M, Rajab MH, Ruud C, Keith T. Randomized trial of a hypnosis intervention for treatment of hot flashes among breast cancer survivors. Journal of Clinical Oncology. 2008;26(31):5022–5026. doi: 10.1200/JCO.2008.16.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins G, Staniunas R, Rajab MH, Marcus J, Snyder T. Use of a numeric visual analog anxiety scale among patients undergoing colorectal surgery. Clinical Nursing Research. 2004;13(3):237–244. doi: 10.1177/1054773803262222. [DOI] [PubMed] [Google Scholar]
- Elkins G, White J, Patel P, Marcus J, Perfect M, Montgomery GH. Hypnosis to manage anxiety and pain associated with colonoscopy for colorectal cancer screening: Case studies and possible benefits. International Journal of Clinical and Experimental Hypnosis. 2006;54:416–431. doi: 10.1080/00207140600856780. [DOI] [PubMed] [Google Scholar]
- Genuis ML. The use of hypnosis in helping cancer patients control anxiety, pain, and emesis: A review of recent empirical studies. American Journal of Clinical Hypnosis. 1995;37(4):316–325. doi: 10.1080/00029157.1995.10403160. [DOI] [PubMed] [Google Scholar]
- Gotay CC, Muraoka MY. Quality of life in long-term survivors of adult-onset cancers. Journal of the National Cancer Institute. 1998;90:656–667. doi: 10.1093/jnci/90.9.656. [DOI] [PubMed] [Google Scholar]
- Gow MA. Hypnosis with a 31-year-old female with dental phobia requiring an emergency extraction. Contemporary Hypnosis. 2006;23(2):83–91. doi: 10.1002/ch.312. [DOI] [Google Scholar]
- Hammond DC. Hypnosis in the treatment of anxiety- and stress-related disorders. Expert Review of Neurotherapeutics. 2010;10(2):263–273. doi: 10.1586/ern.09.140. [DOI] [PubMed] [Google Scholar]
- Hann D, Baker F, Denniston M, Entrekin N. Long-term breast cancer survivors’ use of complementary therapies: Perceived impact on recovery and prevention of recurrence. Integrative Cancer Therapies. 2005;4(1):14–20. doi: 10.1177/1534735404273723. [DOI] [PubMed] [Google Scholar]
- Hodgkinson K, Butow P, Hunt GE, Pendlebury S, Hobbs KM, Wain G. Heightened anxiety among breast cancer survivors: Breast cancer survivors’ supportive care needs 2–10 years after diagnosis. Supportive Care in Cancer. 2007;15(5):515–523. doi: 10.1007/s00520-006-0170-2. [DOI] [PubMed] [Google Scholar]
- Julian LJ. Measures of anxiety. Arthritis Care and Research. 2011;63(S11):S467–S472. doi: 10.1002/acr.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn NF, Ward AM, Watson E, Rose PW. Consulting and prescribing behavior for anxiety and depression in long-term survivors of cancer in the UK. European Journal of Cancer. 2010;46:3339–3344. doi: 10.1016/j.ejca.2010.07.035. [DOI] [PubMed] [Google Scholar]
- Lang EV, Berbaum KS, Faintuch S, Hatsiopoulou O, Halsey N, Li X, Baum J. Adjunctive self-hypnotic relaxation for outpatient medical procedures: A prospective randomized trial with women undergoing large core breast biopsy. Pain. 2006;126:155–164. doi: 10.1016/j.pain.2006.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel S, Beattie S, Arès I, Bielajew C. Young and worried : Age and fear of recurrence in breast cancer survivors. Health Psychology. 2013;32(6):695–705. doi: 10.1037/a0030186. [DOI] [PubMed] [Google Scholar]
- Lim CC, Devi MK, Ang E. Anxiety in women with breast cancer undergoing treatment: A systematic review. International Journal of Evidence-Based Healthcare. 2011;9:215–235. doi: 10.1111/j.1744-1609.2011.00221.x. [DOI] [PubMed] [Google Scholar]
- Massarini M, Rovetto F, Tagliaferri C. Preoperative hypnosis: A controlled study to assess the effects on anxiety and pain in the postoperative period. European Journal of Clinical Hypnosis. 2005;6(1):8–15. [Google Scholar]
- Matt GE, Navarro AM. What meta-analyses have and have not taught us about psychotherapy effects: A review and future directions. Clinical Psychological Review. 1997;17:1–32. doi: 10.1016/S0272-7358(96)00042-6. [DOI] [PubMed] [Google Scholar]
- Matthews AK, Sellergren SA, Huo D, List M, Fleming G. Complementary and alternative medicine use among breast cancer survivors. The Journal of Alternative and Complementary Medicine. 2007;13(5):555–562. doi: 10.1089/acm.2007.03-9040. [DOI] [PubMed] [Google Scholar]
- Maxwell C. Sensitivity and accuracy of the visual analogue scale: A psycho-physical classroom experiment. British Journal of Clinical Pharmacology. 1978;6:15–24. doi: 10.1111/j.1365-2125.1978.tb01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AH, Hilgard JR. The Stanford Hypnotic Clinical Scale for Adults. American Journal of Clinical Hypnosis. 1978;21:134–145. doi: 10.1080/00029157.1978.10403968. [DOI] [PubMed] [Google Scholar]
- Prokop CK, Bradley LA, Burish TG, Anderson KO, Fox JE. Health Psychology: Clinical methods and research. New York, NY: Macmillan; 1991. [Google Scholar]
- Schernhammer ES, Haidinger G, Waldhör T, Vutuc C. Attitudes about the use of complementary and alternative medicine in cancer treatment. The Journal of Alternative and Complementary Medicine. 2009;15(10):1115–1120. doi: 10.1089/acm.2009.0083. [DOI] [PubMed] [Google Scholar]
- Smith SL, Singh-Carlson S, Downie L, Payeur N, Wai ES. Survivors of breast cancer: Patient perspectives on survivorship care planning. Journal of Cancer Survivorship. 2011;5(4):337–344. doi: 10.1007/s11764-011-0185-7. [DOI] [PubMed] [Google Scholar]
- Snow A, Dorfman D, Warbet R, Cammarata M, Eisenman S, Zilberfein F, Navada S. A randomized trial of hypnosis for relief of pain and anxiety in adult cancer patients undergoing bone marrow procedures. Journal of Psychosocial Oncology. 2012;30(3):281–293. doi: 10.1080/07347332.2012.664261. [DOI] [PubMed] [Google Scholar]
- Stark DP, House A. Anxiety in cancer patients. British Journal of Cancer. 2000;83(10):1261–1267. doi: 10.1054/bjoc.2000.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2010 Incidence and Mortality Web-based Report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2013. Retrieved from: http://www.cdc.gov/cancer/dcpc/data/women.htm U.S. [Google Scholar]
- Untas A, Chauveau P, Dupré-Goudable C, Kolko A, Lakdja F, Cazenave N. The effects of hypnosis on anxiety, depression, fatigue, and sleepiness in people undergoing hemodialysis: A clinical report. International Journal of Clinical and Experimental Hypnosis. 2013;61:475–483. doi: 10.1080/00207144.2013.810485. [DOI] [PubMed] [Google Scholar]
- Vande Vusse L, Hanson L, Berner M, White Winters J. Impact of self-hypnosis in women on select physiologic and psychological parameters. JOGNN. 2010;39:159–168. doi: 10.1111/j.1552-6909.2010.01103.x. [DOI] [PubMed] [Google Scholar]
- Williams VS, Morlock RJ, Feltner D. Psychometric evaluation of a visual analog scale for the assessment of anxiety. Health and Quality of Life Outcomes. 2010;8(57):1–8. doi: 10.1186/1477-7525-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody EZ, Barnier AJ. Hypnosis scales for the twenty-first century: What do we need and how should we use them? In: Nash M, Barnier A, editors. The Oxford Handbook of Hypnosis: Theory, Research, and Practice. Oxford: Oxford University Press; 2008. pp. 255–281. [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]


