Abstract
The classical pathway of vitamin D activation follows the sequence D3→25(OH)D3→1,25(OH)2D3 with the final product acting on the receptor for vitamin D (VDR). An alternative pathway can be started by the action of CYP11A1 on the side chain of D3, primarily producing 20(OH)D3, 22(OH)D3, 20,23(OH)2D3, 20,22(OH)2D3 and 17,20,23(OH)3D3. Some of these metabolites are hydroxylated by CYP27B1 at C1α, by CYP24A1 at C24 and C25, and by CYP27A1 at C25 and C26. The products of these pathways are biologically active. In the epidermis and/or serum or adrenals we detected 20(OH)D3, 22(OH)D3, 20,22(OH)2D3, 20,23(OH)2D3, 17,20,23(OH)3D3, 1,20(OH)2D3, 1,20,23(OH)3D3, 1,20,22(OH)3D3, 20,24(OH)2D3, 1,20,24(OH)3D3, 20,25(OH)2D3, 1,20,25(OH)3D3, 20,26(OH)2D3 and 1,20,26(OH)3D3. 20(OH)D3 and 20,23(OH)2D3 are non-calcemic, while the addition of an OH at C1α confers some calcemic activity. Molecular modeling and functional assays show that the major products of the pathway can act as “biased” agonists for the VDR with high docking scores to the ligand binding domain (LBD), but lower than that of 1,25(OH)2D3. Importantly, cell based functional receptor studies and molecular modeling have identified the novel secosteroids as inverse agonists of both RORα and RORγ receptors. Specifically, they have high docking scores using crystal structures of RORα and RORγ LBDs. Furthermore, 20(OH)D3 and 20,23(OH)2D3 have been tested in cell model that expresses a Tet-on RORα or RORγ vector and a RORE-LUC reporter (ROR-responsive element), and in a mammalian 2-hybrid model that test interactions between an LBD-interacting LXXLL-peptide and the LBD of RORα/γ. These assays demonstrated that the novel secosteroids have ROR-antagonist activities that were further confirmed by the inhibition of IL17 promoter activity in cells overexpressing RORα/γ. In conclusion, endogenously produced novel D3 hydroxy-derivatives can act both as “biased” agonists of the VDR and/or inverse agonists of RORα/γ. We suggest that the identification of large number of endogenously produced alternative hydroxy-metabolites of D3 that are biologically active, and of possible alternative receptors, may offer an explanation for the pleiotropic and diverse activities of vitamin D, previously assigned solely to 1,25(OH)2D3 and VDR.
Keywords: Hydroxyvitamin D; CYP11A1; RORα; RORγ; VDR; 1,25D3-MARRS
Graphical Abstract
1. Introduction
1.1. Classical pathway of vitamin D activation
Vitamin D is generated from the photochemical transformation of 7-dehydrocholesterol (7DHC) that requires UVB energy (λ=280–320 nm) and represents the most fundamental reaction in photobiology, not requiring any enzyme [1–3]. After exposure to UVB, the B ring of 7DHC absorbs the electromagnetic energy leading to the breakage of the C9–C10 bond, opening the B-ring and thereby producing previtamin D3. The latter subsequently undergoes thermal isomerization to form D3, or with high doses of UVB produces lumisterol (L3) and tachysterol (T3) [1–4]. These reactions are dependent on the temperature and the UVB dose and are reversible.
It is well established that D3 can be activated by two sequential hydroxylations, the first at C25 (catalyzed by CYP2R1 and CYP27A1) and the second at C1α (catalyzed by CYP 27B1) to generate biologically active 1,25(OH)2D3 as a final product [4–7]. In addition, circulating 25(OH)D3 can be activated in target tissues by ubiquitously expressed CYP27B1 (reviewed in [6, 8, 9]). 1,25(OH)2D3 is inactivated by CYP24A1 which initially hydroxylates it at C24 then catalyzes subsequent oxidations leading to shortening of side chain and the production calcitroic acid [10–13].
1.2. Biological activity of 1,25(OH)2D3
In addition to regulating calcium homeostasis, 1,25(OH)2D3 displays a variety pleiotropic activities, which include inhibition of proliferation and stimulation of the differentiation program in cells of different lineage, anticancerogenic effects, and enhancement of innate, and attenuation of adaptive immune activities and inflammation [4, 8, 14–17]. Its effects are mediated via the vitamin D receptor (VDR), which after agonist activation and dimer formation with RXR binds to the VDR responsive element (VDRE) to influence expression of responsive genes [14, 17–19].
In the skin, 1,25(OH)2D3 plays an important role in the regulation of skin barrier functions and in the regulation of hair follicle growth and cycling, and has anti-cancerogenic, anti-proliferative and anti-inflammatory effects [3, 17, 20]. Most recently, it was reported that it can inhibit skin cell death and DNA damage induced by exposure to UVR [20–23]. Because of the toxic effect secondary to calcemia, the pharmacological use of 1,25(OH)2D3 is limited.
1.3 Analogs of 1,25(OH)2D3 with reduced calcemic activity
Many analogs of 1,25(OH)2D3 have been chemically synthesized with the aim of reducing calcemic activity, without the loss of therapeutically useful anticancer activities (reviewed in [14, 24–27]) or immunoregulatory properties (reviewed in [28, 29]). Modification of the A-ring, CD ring and side chain have all produced analogs with reduced calcemic activity. Key changes include replacement of the C1α-hydroxyl group with a 1β-CH2OH, C3-epimerization, removal of C19, epimerization at C20, addition of a second side chain at C20 (Gemini analogs), insertion of a double bond at C16 and a triple bond at C23, and insertion of an oxygen in place of C22. Many side chain modifications between C22 and C26 have also been aimed at reducing metabolism by CYP24A1 rather than reducing calcemic activity. The effects of isomerization of the two hydroxyl groups in the A-ring of 1,25(OH)2D3 was reported by Fleet et al [30], and is of particular interest since one of the resulting diastereomers, 3-epi-25-dihydroxyvitamin D3, is a natural metabolite of 1,25(OH)2D3. 1,25(OH)2D3 (where hydroxyl groups are 1α and 3β) was compared to 1β,3β; 1α,3α and 1β,3α diastereomers. The 1α,3α isomer (3-epi-1,25(OH)2D3) is produced in vivo by the action of vitamin D 3-epimerase on 1,25(OH)2D3 [31–33]. All three of these diastereomers showed reduced binding to the VDR with binding strength only partially correlating with their ability to stimulate calcium transport. The 3-epi-1,25(OH)2D3 diastereomer stimulated calcium transport in excess of its relative ability to bind to the VDR. Other studies on 3-epi-1,25(OH)2D3 indicate that its reduced binding to the VDR does generally correlates with its reduced biological activity [30, 32, 34, 35], but there are notable exceptions such as the maintenance of the ability to suppress parathyroid hormone secretion by cultured parathyroid cells and enhancement of the ability to stimulate HL-60 cell apoptosis, relative to 1,25(OH)2D3 [36, 37].
While several of the low-calcemic synthetic analogs discussed above show some promise for the treatment of hyperproliferative and immunological disorders, hypercalcemia resulting from long-term high therapeutic doses remains a significant problem [24, 26, 29]. The studies with synthetic analogs also illustrate the possibility of designing specific analogs for specific therapeutic applications. It is well established that 1α-hydroxylation of the A-ring of 25(OH) D3 dramatically enhances its binding to the VDR and its calcemic activity. However, until our studies on CYP11A1-derived secosteroids that lack the 1α-hydroxyl group (described below) little was done to explore the possibility that active metabolites might be synthesized without the 1α-hydroxyl group (or equivalent), as it was generally thought to be indispensable for tight binding to the VDR.
1.4. New challenges in vitamin D biology
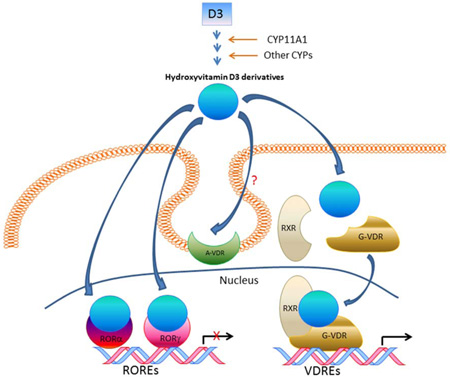
The consensus conveyed by the majority of the literature is that all biologically relevant phenotypic effects of D3 can been assigned to one molecule, 1,25(OH)2D3, and one receptor, VDR [3, 15, 17, 38]. This makes both 1,25(OH)2D3 and VDR a bioregulatory couple, which would regulate vastly unrelated or sometime contradictory effects, which is highly unusual for endogenous ligands and their respective receptor. The existence of an alternative membrane bound receptor for 1,25(OH)2D3, e.g., 1,25D3-membrane-associated, rapid response steroid-binding protein (1,25D3-MARRS), has been proposed by some authors [39, 40]. This review, supplemented by new data and molecular modelling, will offer an additional explanation for the pleiotropic phenotypic effects of D3 by identifying both a family of novel bioactive D3 hydroxy-derivatives and the retinoic acid-related orphan receptors (RORs) α and γ, which function as alternative nuclear receptors for these compounds in addition to the VDR.
2. New pathways of vitamin D activation
Until recently, the traditional role of CYP11A1 was believed to be to initiate steroid synthesis, solely in steroidogenic organs using cholesterol as the substrate. This involved hydroxylations at C22 and C20 followed by oxidative cleavage of the bond between C20 and C22 to produce pregnenolone, a precursor to all steroids [41, 42]. However, the expression of CYP11A1 in peripheral tissues, albeit at low levels, has now been documented [43] and alternative substrates to cholesterol have been identified experimentally, such as 7DHC, vitamins D2 and D3, ergosterol and lumisterol [44–49]. Additionally, the possibility of other sterol/secosteroidal compounds serving as substrates has been predicted theoretically based on molecular modeling [50].
2.1. Hydroxylation of side chain of vitamin D by CYP11A1
An assumed concept that vitamins D3/2 are only activated through 25- and 1-hydroxylations [D3/2→25(OH)D3/2→1,25(OH)2D3/2] has been challenged by recent findings of CYP11A1-initiated metabolism of vitamin D: D3→ 20(OH)D3 +22(OH)D3 + 17(OH)D3→(OH)nD3 and D2→20(OH)D2→17,20(OH)2D2→(OH)nD2 [47, 48, 51–61]. The main metabolite resulting from a single hydroxylation of D3 by CYP11A1 is 20(OH)D3, with 22(OH)D3 and 17(OH)D3 also being produced [47, 51, 61]. In the major pathway, 20(OH)D3 is further hydroxylated by CYP11A1 to 20,23(OH)2D3, 20,22(OH)2D3, 17,20(OH)2D3 and 17,20,23(OH)3D3 [47, 51]. In addition the prodrug, 1α(OH)D3, is metabolized by CYP11A1 to 1,20(OH)2D3 [62] and 1,20,23(OH)3D3 (unpublished observation). A full list of the D3 hydroxymetabolites produced by CYP11A1 and their stereochemistry is presented in Table 1.
Table 1.
Hydroxyvitamin D3 metabolites produced by the action of CYP11A1 on vitamin D3 and further hydroxylated by other vitamin D-metabolizing cytochromes P450.
| CYP11A1 Metabolite | Secondary Metabolite | CYPs Involved | References |
|---|---|---|---|
| 20S(OH)D3 | [44, 47, 51, 58, 60, 61, 67, 71, 72] | ||
| 1α,20S(OH)2D3 | 27B1 | [54, 58, 63, 71, 72] | |
| 20S,24R(OH)2D3 | 24A1 or 3A4 | [55, 57, 65, 66, 72] | |
| 20S,24S(OH)2D3 | 24A1 or 3A4 | [55, 65, 66, 72] | |
| 20S,25(OH)2D3 | 24A1 or 27A1 or 3A4 | [55, 57, 66, 72] | |
| 20S,26(OH)2D3 | 27A1 | [57, 72] | |
| 1α,20S,24R(OH)3D3 | 24A1, 27B1 | [54, 65, 72] | |
| 1α,20S,24S(OH)3D3 | 24A1, 27B1 | [65] | |
| 1α,20S,25(OH)3D3 | 24A1 or 27A1 or 3A4, 27B1 | [54, 72] | |
| 1α,20S,26(OH)3D3 | 27A1, 27B1 | [54, 72] | |
| 17α(OH)D3 | [51, 61] | ||
| 22(OH)D3 | [58, 61, 71, 72] | ||
| 1α,22(OH)2D3 | 27B1 (low activity) | [54] | |
| 20R,22(OH)2D3 | [47, 58, 61, 71, 72] | ||
| 1α,20S,22(OH)3D3 | 27B1 | [54] | |
| 20S,23S(OH)2D3 | [51, 58, 60, 61, 64, 71, 72] | ||
| 1α,20S,23S(OH)3D3 | 27B1 | [54, 56, 58, 64] | |
| 20S,23S,24(OH)3D3 | 24A1 | [55] | |
| 20S,23S,25(OH)3D3 | 24A1 | [55] | |
| 17α,20S(OH)2D3* | [51, 60, 61] | ||
| 17α,20S,23S(OH)3D3* | [51, 58, 60, 61, 72] |
Not acted on by CYP27B1 (9)
2.2. Modification of CYP11A1 derived D3-hydroxymetabolites by CYP27B1, CYP27A1, CYP24A1 and CYP3A4
20(OH)D3, 22(OH)D3, 20,22(OH)2D3 and 20,23(OH)2D3 can be hydroxylated by CYP27B1 at C1α to the corresponding di- or trihydroxyderivatives (Table 1) [54, 56, 58, 63, 64]. The main product of D3 metabolism initiated by CYP11A1, 20(OH)D3, can be hydroxylated on the side chain by CYP27A1, CYP24A1 and CYP3A4 to 20,24(OH)2D3, 20,25(OH)2D3 and/or 20,26(OH)2D3, which are further 1α hydroxylated by CYP27B1 to the corresponding trihydroxyderivatives (Table 1)[55–57, 64–66]. In addition 20,23(OH)2D3 is hydroxylated by CYP24A1 to 20,23,24(OH)3D3 and 20,23,25(OH)3D3 [55].
While originally produced by enzymatic synthesis in amounts enabling identification, chemical routes of synthesis for 20(OH)D3, 20,23(OH)2D3 and 20,24(OH)2D3 have now been established [64, 65, 67–69] which also confirmed that the enzymatically produced 20(OH)D3 is the 20S enantiomer [67]
2.3. In vivo production of novel D3-hydroxyderivatives
Many of the vitamin D3 hydroxymetabolites listed in Table 1, including 20(OH)D3, 22(OH)D3, 20,23(OH)2D3, 20,22(OH)2D3, 1,20(OH)2D3, 1,20,23(OH)3D3, and 17,20,23(OH)3D3 can be made by epidermal keratinocytes, adrenals, placenta, or colorectal adenocarcinoma Caco-2 cells, tissues and cells know to express CYP11A1, following incubation with D3 [58, 70]. Production of 20(OH)D3, 22(OH)D3, 20,23(OH)2D3, 20,22(OH)2D3, 1,20(OH)2D3 also occurs in human dermal fibroblasts incubated with D3 substrate [71]. Similarly, production of 20(OH)D2, 17,20(OH)2D2 and 1,20(OH)2D2 was observed in human placentas ex-utero, adrenal glands ex-vivo, and isolated human epidermal keratinocytes and colorectal Caco-2 cells incubated with D2 [59].
The final proof of the in vivo production of these CYP11A1-derived hydroxyderivatives is provided by our most recent report of the detection of not only 25(OH)D3 and 1,25(OH)2D3, but also of 20(OH)D3, 22(OH)D3, 20,22(OH)2D3, 20,23(OH)2D3, 20,24(OH)2D3, 20,25(OH)2D3, 20,26(OH)2D3, 1,20,23(OH)3D3 and 17,20,23(OH)3D3 in extracts of the human serum and epidermis, and of pig adrenals, with 1,20(OH)2D3 being detected solely in the epidermis [72]. In addition, we detected 1,20,24(OH)3D3, 1,20,25(OH)3D3 and 1,20,26(OH)3D3 in extracts of pig adrenal gland [72]. Interestingly, the serum concentrations of 20(OH)D3 and 22(OH)D3 were, respectively, 30- and 15-times lower than 25(OH)D3, but in the nM range consistent with them displaying biological activity [72]. Contingent upon firmly establishing a physiological role for these secosteroids, future monitoring of human serum samples for CYP11A1-derived D3-hydroxymetabolites may be warranted.
Detection of novel D3 hydroxymetabolites in human serum and epidermis poses new challenges in the field in relation to the biochemistry of the pathway and the attendant physiological consequences. Thus, a relationship between vitamin D levels and production of these compounds in the context of environmental factors and routes of delivery, and in a tissue/organ context, remains to be investigated. For example, since 25(OH)D3 is not metabolized by CYP11A1 [47], we can predict that oral intake of D3 will have a minor effect on the production of the novel hydroxyderivatives. However, D3 derived from skin or delivered through parenteral routes would be metabolized by tissues expressing high levels of CYP11A1 such as adrenals, gonads and placenta, with likely systemic effects. These important considerations should set a background for extensive future investigations.
3. RORs (retinoic acid-related orphan receptors), an overview
There are three members of the ROR subfamily of nuclear receptors, RORα-γ (NR1F1-3) [73, 74]. The ROR transcription factors exhibit a domain structure containing an N-terminal domain, a highly conserved DNA-binding domain (DBD) with two C2-C2 zinc finger motifs, a ligand-binding domain (LBD), and a hinge domain between the DBD and LBD. Transcription regulation by RORs is mediated through monomeric interaction with ROREs (ROR response elements) in the regulatory regions of target genes [73, 75]. RORs are important players in the regulation of many physiological processes. They exhibit critical functions in embryonic development, differentiation, and in many immune and metabolic pathways in the adult [73, 74]. RORs have been implicated a number of pathologies, including cancer, (auto)immune disease and metabolic syndrome.
Evidence has accumulated demonstrating that RORs act as ligand-dependent transcription factors [74, 76–78]. Cholesterol and cholesterol sulfate and small molecules can interact with the LBD of RORs to modulate their transcriptional activities [79]. Intermediates of the pathway of cholesterol biosynthesis can also act as endogenous agonists of RORγ [80, 81], whereas vitamin D derivatives such as 20(OH)D3/2 exhibit RORα and RORγ inverse agonist activity where binding to the receptor reduces the basal activity of the receptor resulting in the opposite pharmacological responses to the agonist [81–83]. Therefore ROR ligands may be of value in the development of novel therapeutic strategies for treatment of different inflammatory and metabolic disorders, and neuropsychiatric diseases and cancer, in which RORs are implicated.
4. Biological activity of CYP11A1-derived vitamin D hydroxyderivatives
4.1. An overview of biological activity
The biological activity of 20S(OH)D3 in the skin was the subject of a recent review [70], therefore the description below is brief. 20(OH)D3 and its hydroxymetabolites exert prodifferentiation, antiproliferative, and antiinflammatory activities on skin cells, comparable or better than that of 1,25(OH)2D3 [53, 64, 65, 67, 70, 83–92]. 20(OH)D3 shows antifibrotic properties both in vitro [87–89] and in an in vivo mouse model of bleomycin induced scleroderma [89]. Most recently it was shown that both 20(OH)D3 and 20,23(OH)2D3 enhance defense mechanisms against UVB-induced oxidative stress and DNA damage in cultured human keratinocytes [23] and murine skin in vivo [22]. 20(OH)D3 and its hydroxymetabolites also show anti-cancer properties that are cell-lineage dependent [53, 54, 64, 68, 69, 88, 90, 92–97].
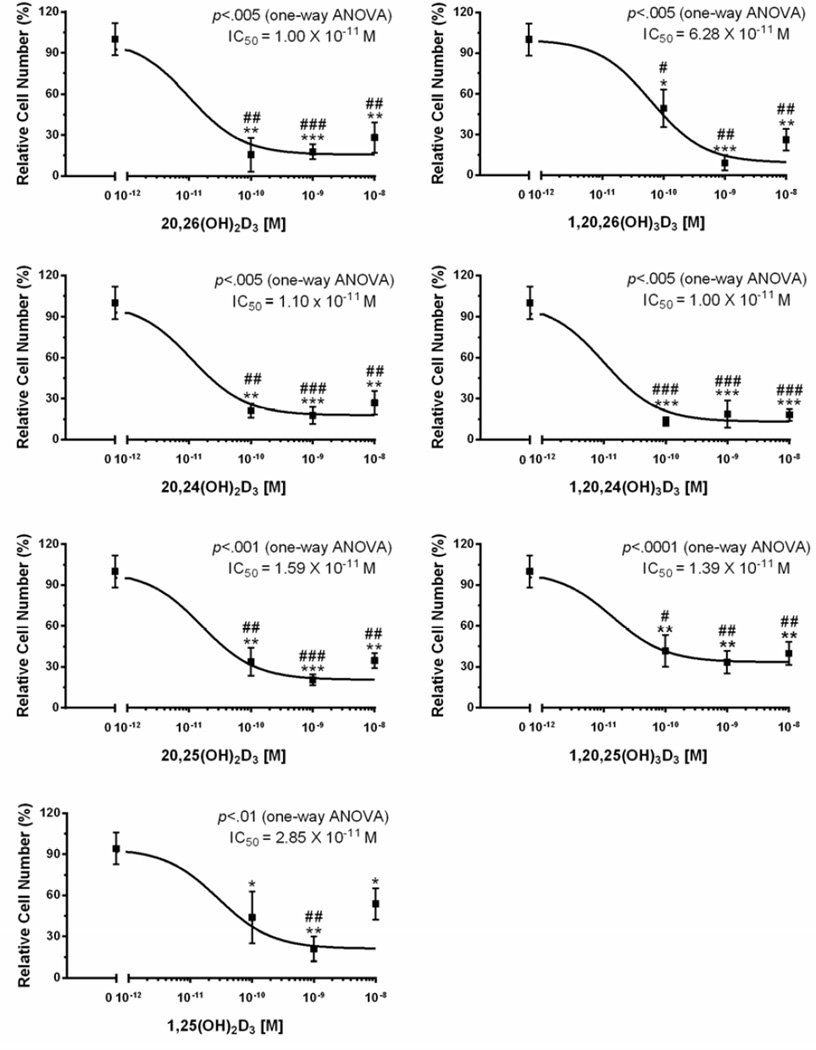
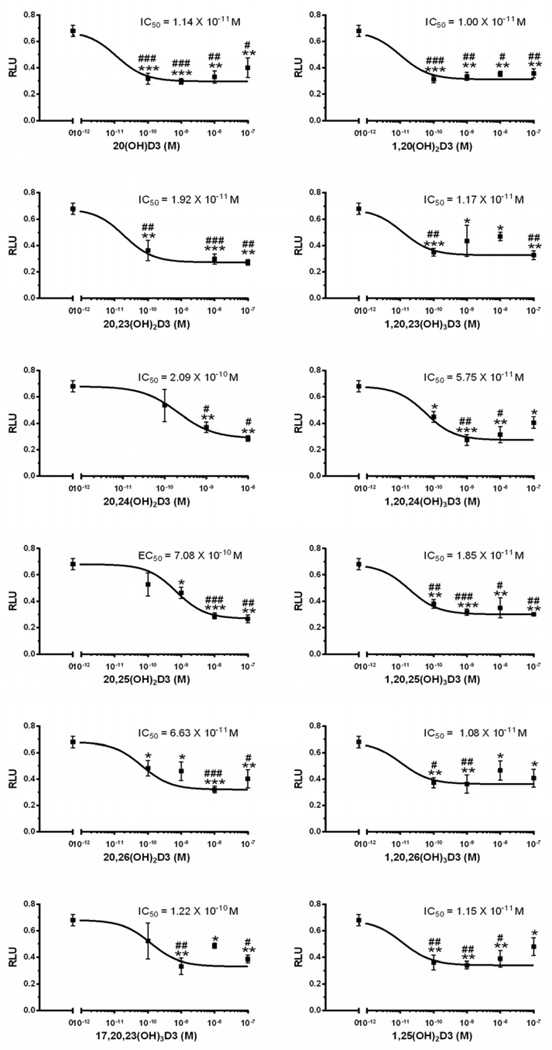
We have now tested the effects of 20,25(OH)2D3, 1,20,25(OH)3D3, 20,26(OH)2D3, 1,20,26(OH)3D3, 20,24(OH)2D3, and 1,20,24(OH)3D3 on keratinocyte proliferation and found that they display similar potency (IC50) and efficacy (maximal inhibition) to 1,25(OH)2D3, both in the presence and absence of the 1α-hydroxyl group, on the inhibition of cell proliferation (Fig. 1).
Figure 1.
Hydroxymetabolites of 20(OH)D3 inhibit keratinocytes proliferation. The cells were cultured for 48 h in the presence or absence of 1α,25(OH)2D3, 20S,25(OH)2D3, 1α,20S,25(OH)3D3, 20S,26(OH)2D3, 1α,20S,26(OH)3D3, 20S,24R(OH)2D3, or 1α,20S,24R(OH)3D3 and cell proliferation was measured by the MTS assay as described in the supplemental file and in [90, 92]. Data represent means ± SE (n=3–4). *, p<0.05; **, p<0.01; ***, p<0.001 at student t-test. #, p<0.05; ##, p<0.01; ###, p<0.001 at one-way ANOVA test.
4.2. CYP11A1-derived D3 hydroxymetabolites are non-calcemic
20S(OH)D3 is noncalcemic at pharmacological doses of 3 µg/kg in rats that is in contrast to 1,25(OH)2D3 and 25(OH)D3 [95]. However, C1α hydroxylation confers limited calcemic activity, similar to that of 25(OH)D3 but still less than for 1,25(OH)2D3 [95]. 20S(OH)D3 and its chemically synthesized epimer 20R(OH)D3 are also noncalcemic at extremely high doses of 30–60 µg/kg in C57BL/6 mice, and do not show any signs of toxicity in serum chemistry, liver and kidney functions, and blood morphology or histopathology of the heart, liver, spleen and kidneys [68, 93]. In addition, 20,23(OH)2D3 is noncalcemic at 3 µg/kg in C57BL/6 mice [89] and 20(OH)D2 is noncalcemic at 4 µg/kg in rats, again showing a lack of histopathological signs of kidney or heart damage [53]. Therefore, we suggest that 20(OH)D3, 20(OH)D2 and 20,23(OH)2D3 can serve as therapeutic or adjuvant agents.
5. Receptors for CYP11A1-derived D3 hydroxymetabolites
5.1. CYP11A1-derived D3 hydroxymetabolites act as “partial/biased” VDR agonists
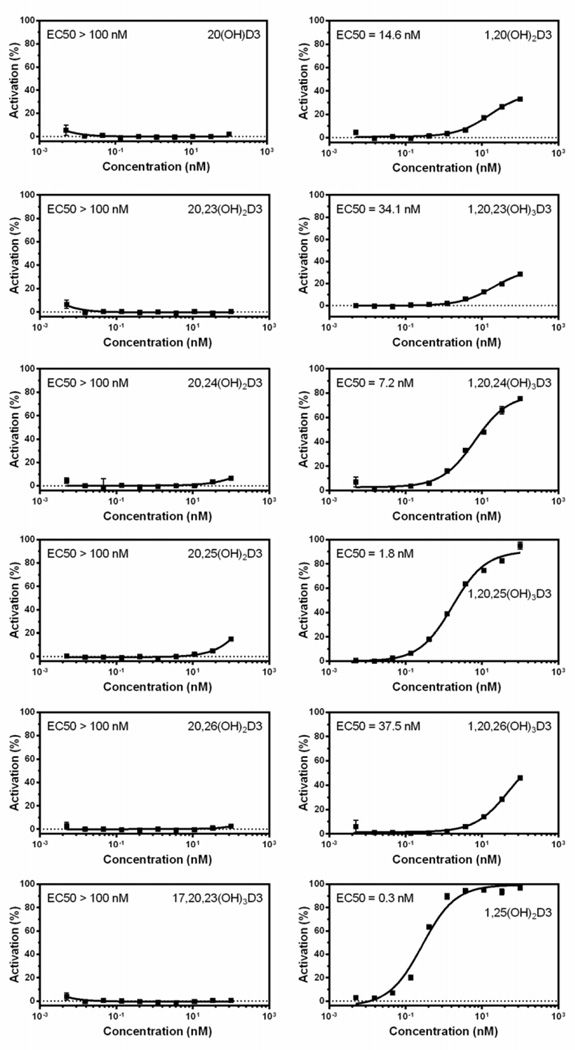
Our previous studies have documented that 20S(OH)D3 and 20,23(OH)2D3 can act as “partial agonists on the VDR (discussed in [70]). They may also be termed biased agonists, a term now commonly applied to some ligands for G-protein coupled receptors which are functionally selective (biased) for certain response pathways from a particular receptor [98, 99]. The involvement of VDR in the regulation of differentiation, proliferation and immune functions of keratinocytes was demonstrated by experiments showing that silencing of the gene encoding the VDR significantly inhibits the phenotypic effects of either 20(OH)D3/2 or 20,23(OH)2D3 [53, 84–86]. This was further substantiated by the ability of CYP11A1-derived D3 hydroxymetabolites to translocate VDR to the nucleus with high affinity [53, 61, 92]. However, they differ substantially from 1,25(OH)2D3 in that these metabolites lack calcemic activity (as described above), act as very poor activators of CYP24A1 expression [53, 84, 86, 95] and have very poor activity on a synthetic VDRE promoter construct that was however improved by hydroxylation in position C1α [64, 65].
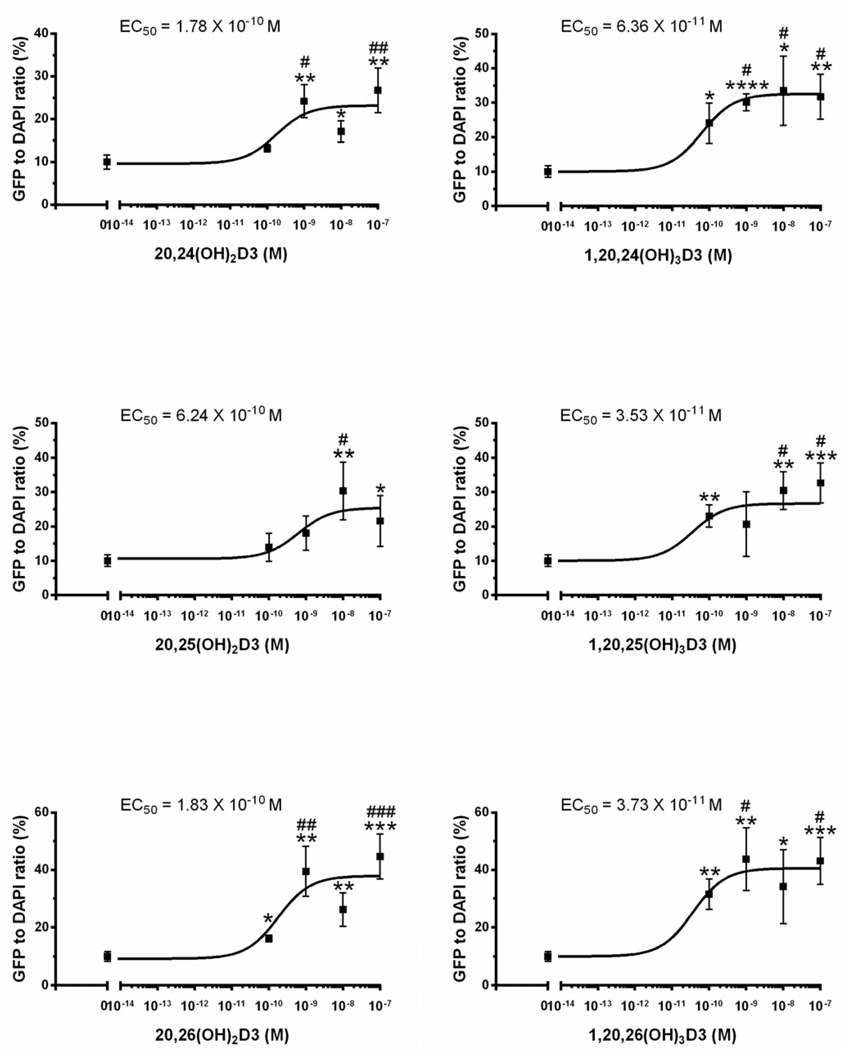
The above findings led us to further test a number of the CYP11A1-derived secosteroids using the commercially available LanthaScreen TR-FRET Vitamin D receptor Coactivator kit assay (Fig. 2). The assay showed that only the 1α(OH) derivatives of CYP11A1-derived D3 hydroxymetabolites increase the affinity of the co-activator peptide to the VDR-LBD suggesting that the conformational change induced by the secosteroids lacking a 1α(OH) is different or less than that induced by 1,25(OH)2D3. Therefore we carried out ligand-induced VDR translocation assays using VDR-GFP (Fig. 3), which showed that CYP11A1-derived hydroxyderivatives, both with and without the 1α(OH) group, cause translocation of the VDR to the nucleus with high potency, however potency was greater with the 1α(OH) group present. This is in agreement with previous data demonstrating the high efficiency of D3 hydroxyderivatives with a full-length side chain for stimulating VDR translocation, with higher potency being seen for the 1α(OH) derivatives and is also consistent with our molecular modeling studies [92]. Thus, our conclusion that novel CYP11A1-derived D3 hydroxymetabolites act as partial or “biased” agonists on the VDR has been correct, however, the nature of the interaction with the receptor requires further analyses with in silico modeling being presented below.
Figure 2.
Only the 1α(OH)-derivatives of 20(OH)D3 hydroxymetabolites increase the affinity of the co-activator peptide to the VDR-LBD in the LanthaScreen TR-FRET Vitamin D receptor Coactivator kit assay (Thermo Fisher Scientific, Inc., Waltham, MA) (for details see supplemental file).
Figure 3.
Hydroxymetabolites of 20(OH)D3 stimulate VDR-GFP translocation from the cytoplasm to the nucleus in the SKMEL-188 melanoma line stably overexpressing VDR-GFP [53]. The cells were incubated for 90 min in the presence of 20S,25(OH)2D3, 1α,20S,25(OH)3D3, 20S,26(OH)2D3,1α,20S,26(OH)3D3, 20S,24R(OH)2D3 or 1α,20S,24R(OH)3D3, or vehicle (ethanol) and translocation to the nucleus was measured as described in [92] with modifications listed in the supplemental file Data represent means ± SE (n=3–4). *, p<0.05; **, p<0.01; ***, p<0.001 at student t-test. #, p<0.05; ##, p<0.01; ###, p<0.001 at one-way ANOVA test.
5.1.1. Molecular modeling utilizing the VDR crystal structure
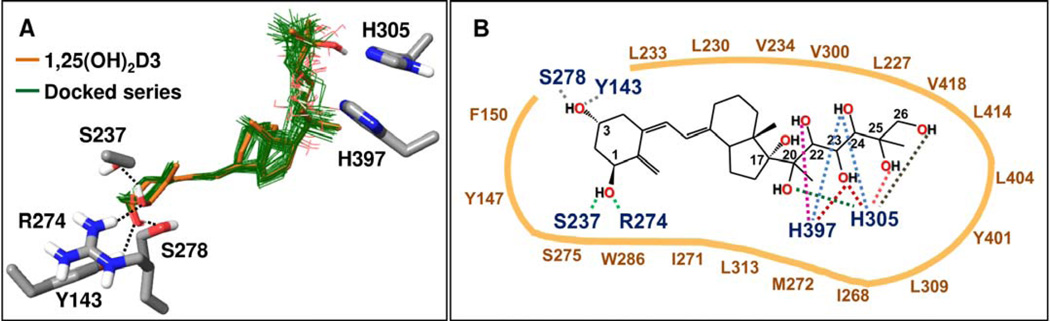
The effects of ligand – VDR interaction can translate into structural changes of functionally relevant regions of the VDR remote to the ligand binding site. To understand better the structural basis of the binding of hydroxyvitamin D3 metabolites to the VDR we performed docking studies into the genomic and non-genomic pockets in the VDR crystal structure (PDB code 1DB1).
Docking results utilizing the genomic site of VDR
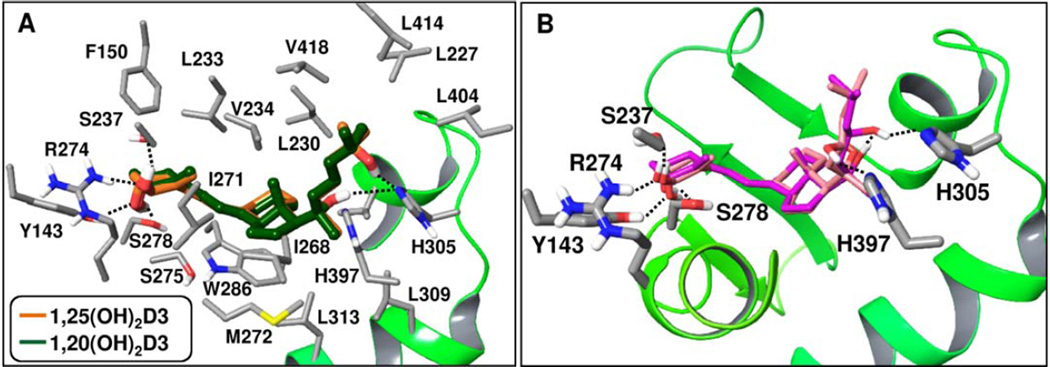
After preparation of the VDR crystal structure (PDB code 1DB1), the series of hydroxy-vitamin D3 metabolites listed in Table 2 were docked into the genomic site of VDR using the Glide XP docking method [84] (Schrödinger software), as detailed in the Supplemental file. Table 2 lists Glide XP docking scores of the resulting poses, which are a measure of the predicted interaction energy between the docked ligand and the receptor. Glide XP scores in the series are generally slightly less favorable compared to that of 1,25(OH)2D3, with five exceptions showing only slightly better scores (Table 2). As expected, docked poses of the hydroxy-D3 series approximately overlap with the co-crystallized 1,25(OH)2D3 (Fig. 4A). Docked poses show excellent complementarity between non-polar ligand atom groups and the large hydrophobic surface of the pocket. Interactions formed by analogs in this series are schematically summarized in Figure 4B. While 23(OH) and 24(OH) groups may interact with either His305 or His397 in the series, hydroxyl groups in other positions show preference for one of the two histidines. Hydrogen bonding interactions formed by 1α(OH) and 3(OH) groups are analogous to those formed by the co-crystallized ligand, 1α,25(OH)2D3. Compounds containing a 1α(OH) group tend to score more favorably than the corresponding analogs lacking this group due to the additional hydrogen bonding of the 1α(OH) group with Arg274 and Ser237. For example, Figure 5A illustrates the docked pose of 1α,20S(OH)2D3, in comparison with the structure of the co-crystallized ligand in VDR (PDB code 1DB1). Figure 5B shows polar interactions predicted by two possible docked poses of 1α,20S,23R(OH)3D3 that mainly differ in the interactions formed with the histidine residues 305 and 397. One of the poses is very similar to that obtained for the parent 20S,23R(OH)2D3 lacking the 1α(OH) group.
Table 2.
Glide XP docking scores of compounds docked into the RORα, RORγ and VDR crystal structures. All R groups not specified are hydrogens. Some of the compounds listed are isomers of naturally produced D3 hydroxyderivatives and their synthesis by human P450s has not been established. NP: not the preferred isomer if the other enantiomer shows a significantly more favorable pose. NI: not included among structures shown. ND: Not successfully docked or only poorly scoring poses obtained.
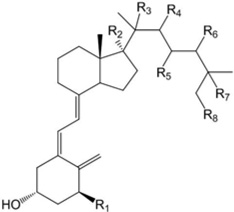 | ||||||
|---|---|---|---|---|---|---|
| Compounds | Structure | RORα | RORγ | VDR (genomic site) |
VDR (non-genomic site) |
1,25D3- MARRS |
| D3 | −10.74 | −11.22 | −12.69* | ND | ND | |
| 1α,25(OH)2D3 | R1=R7=OH | −10.30 | −11.88 | −15.07 | −13.56 | −7.47 |
| 20S(OH)D3 | R3=OH | −10.99 | −12.60 | −12.77 | ND | ND |
| 20R(OH)D3 | R3=OH | NP | −10.99 | −12.85 | ND | ND |
| 20S,22R(OH)2D3 | R3=R4=OH | −10.56 | −11.79 | NP | ND | ND |
| 20S,22S(OH)2D3 | R3=R4=OH | −10.60 | −12.18 | −12.29 | ND | ND |
| 20S,23R(OH)2D3 | R3=R5=OH | −10.60 | −12.87 | −14.05 | ND | −7.98 |
| 20S,23S(OH)2D3 | R3=R5=OH | −10.41 | −12.65 | −13.94 | ND | −7.20 |
| 20S,24R(OH)2D3 | R3=R6=OH | −10.70 | −11.82 | −13.52 | ND | ND |
| 20S,24S(OH)2D3 | R3=R6=OH | −10.81 | −12.29 | −13.87 | ND | ND |
| 20S,25(OH)2D3 | R3=R7=OH | −10.02 | −10.33 | −13.35 | ND | ND |
| 20S,26(OH)2D3 | R3=R8=OH | −10.82 | −11.10 | −13.34 | ND | ND |
| 1α,20S(OH)2D3 | R1=R3=OH | −10.15 | −11.00 | −13.88 | ND | ND |
| 1α,20S,22R(OH)3D3 | R1=R3=R4=OH | −11.47 | −11.64 | NP | ND | ND |
| 1α,20S,22S(OH)3D3 | R1=R3=R4=OH | −11.67 | −12.99 | −14.30 | ND | ND |
| 1α,20S,23R(OH)3D3 | R1=R3=R5=OH | −9.96 | −10.81 | −15.37 −15.27 |
ND | −7.53 |
| 1α,20S,23S(OH)3D3 | R1=R3=R5=OH | −10.36 | −11.22 | −15.03 | ND | −7.90 |
| 1α,20S,24R(OH)3D3 | R1=R3=R6=OH | −10.79 | −11.77 | −14.95 | ND | −8.70 |
| 1α,20S,24S(OH)3D3 | R1=R3=R6=OH | −10.97 | −10.42 | −15.80 | ND | −7.97 |
| 1α,20S,25(OH)3D3 | R1=R3=R7=OH | −11.23 | −11.22 | −14.83 | ND | ND |
| 1α,20S,26(OH)3D3 | R1=R3=R8=OH | −10.54 | −12.15 | −14.92 | ND | ND |
| 22R(OH)D3 | R4=OH | −11.23 | −11.27 | −12.10 | ND | ND |
| 22S(OH)D3 | R4=OH | −11.32 | −12.16 | −13.40 | ND | ND |
| 23R(OH)D3 | R5=OH | −10.19 | −10.82 | −13.00 | NP | ND |
| 23S(OH)D3 | R5=OH | −10.47 | −11.76 | −12.72 | −12.77 | ND |
| 25(OH)D3 | R7=OH | −10.46 | −10.26 | −13.36 | −12.65 | ND |
| 17(OH)D3 | R2=OH | −10.84 | −10.99 | −12.53* | ND | ND |
| 23R,25(OH)2D3 | R5=R7=OH | −10.19 | NP | 14.00 | −14.01 | −7.99 |
| 23S,25(OH)2D3 | R5=R7=OH | NP | −10.74 | −13.21 | −13.26 | −8.66 |
| 1,23R,25(OH)3D3 | R1=R5=R7=OH | −10.35 | NP | −15.01 | −14.54 | −8.40 |
| 1,23S,25(OH)3D3 | R1=R5=R7=OH | −11.08 | −11.34 | −16.02 | −15.05 | −9.11 |
| 24R,25(OH)2D3 | R6=R7=OH | −10.00 | −12.34 | −14.64 | −13.85 | −8.43 |
| 24S,25(OH)2D3 | R6=R7=OH | NP | NP | −14.12 | −14.74 | −8.83 |
| 1α,24R,25(OH)3D3 | R1=R6=R7=OH | −10.07 | −12.32 | −16.21 | NP | −8.34 |
| 1α,24S,25(OH)3D3 | R1=R6=R7=OH | −10.12 | NP | −15.81 | −14.66 | −8.30 |
| 17,20S(OH)2D3 | R2=R3=OH | −10.99 | −12.66 | −13.70 | ND | ND |
| 17,20S,23R(OH)3D3 | R2=R3=R5=OH | −11.06 | −11.84 | −14.67 −14.46 |
ND | −8.11 |
| 17,20S,23S(OH)3D3 | R2=R3=R5=OH | −10.45 | −10.75 | −14.44 | ND | −7.81 |
| Calcitroic acid | NI | −8.72 −8.64 |
−8.96 | −9.96 | −12.11 | |
| 20(OH)-cholesterol | NI | −11.60 | −12.17 | −9.78 | −10.01 | |
| Cholesterol | NI | −11.92 | −12.49 | −9.99 | −10.31 | |
See discussion of potentially unstable poses
Figure 4.
Common binding mode predicted for hydroxy-D3 metabolites in the genomic site of the VDR crystal structure (PDB code 1DB1). (A) Docked poses of hydroxy-D3 metabolites (listed in Table 2) are shown with thin bonds and green colored carbons. For comparison, the co-crystallized 1,25(OH)2D3 is shown with thick bonds and light brown colored carbons. All other atoms are colored by atom type (O: red, N: blue, S: yellow, H: white). Only VDR residues involved in polar interactions with the ligands are included. Hydrogen bonding interactions of 1α(OH) and 3(OH) groups common in docked poses are indicated with dashed lines. (B) Schematic summary of interactions formed by docked analogs listed in Table 2 in the genomic site of VDR. Interactions formed between VDR residues and OH substituents in docked poses of the analog series are indicated with dashed lines, colored distinctly for each OH group. For example, 24(OH) in poses of some analogs interact with H397 while in case of other analogs with H305 (light blue dashed lines). VDR residue labels are color coded: those involved in polar interactions are dark blue; those contributing to non-polar interactions are dark brown.
Figure 5.
Poses of selected hydroxy-D3 metabolites are shown in the genomic site of VDR (PDB code 1DB1). (A) Docked pose of 1α,20S(OH)2D3 shown in comparison with the co-crystallized 1,25(OH)2D3 in the VDR crystal structure (PDB code 1DB1). Carbon atoms in the docked pose are colored dark green, in the co-crystallized ligand light brown; all other atoms are colored by atom types (as in Fig. 4). Hydrogen bonding interactions are shown with dashed lines. In addition to residues involved in hydrogen bonding with shown ligands, all VDR residues that form non-polar contacts with the docked 1α,20S(OH)2D3 are also displayed. (B) Two possible docked poses obtained for 1α,20S,23R(OH)3D3 are shown simultaneously in the VDR genomic pocket, with their carbon atoms colored distinctly. Hydrogen bonding interactions are indicated with dashed lines. Only VDR residues involved in polar interactions are shown.
Docked poses of D3 and 17(OH)D3 differ from those of other secosteroids in having only a single anchor, the 3(OH) group, to participate in hydrogen bonding interaction, while non-specific, hydrophobic contacts constitute the rest of the interactions with VDR. These poses likely would not represent stable, populated conformers or true bound structures although they can be accommodated in the binding site. Compared to D3, alfacalcidol (1α(OH)D3) has an additional hydrogen bonding anchor, the 1α(OH) group and it is a known VDR partial agonist. While calcitroic acid has a lower docking score consistent with low affinity binding, its docked pose suggests that in addition to hydrogen bonding through 1α(OH) and 3(OH) groups it may also form water-mediated polar interactions with His305/His397, and thus may potentially bind to the VDR.
Docking results predict specific ligand – receptor interactions, the effects of which can propagate from local to more distant regions playing a role in modulating VDR functional activity. Hydrogen/Deuterium exchange (HDX) studies demonstrated that ligand interactions in the VDR genomic pocket modulates the flexibility/dynamics of functionally relevant regions in VDR including helix H12, crucial for cofactor interactions, as well as the VDR – retinoid X receptorα (RXRα) heterodimer interface [85]. Further regions remote to the binding site may show altered kinetics as well. A molecular dynamic simulation study of VDR with bound 1,25(OH)2D3 and an antagonist demonstrated distinct effects of these ligands on stabilizing the conformation of key residues in the ligand binding domain of VDR [86]. HDX analysis provides evidence that upon binding of 1α(OH)D3 (alfacalcidol) that lacks a 25(OH) group, helix H12 remains highly dynamic [85]. The lack of the 25(OH) is likely essential for the partial agonist activity of this D3 analog. The agonist 1,25(OH)2D3 co-crystallized in the VDR structure forms hydrogen bonding interaction with His305 through its 25(OH) group. While most analogs in Table 2 interact with His305, it is unclear whether analogs that may bind but lack this interaction would have any functional effects on VDR. Further, C24 enantiomers of the top scoring compound, 1α,24,25(OH)3D3, as well as enantiomers of its analog, 24,25(OH)2D3, are predicted to form hydrogen bonding interactions with both His305 and His397. While docking suggests that these analogs can bind VDR in the genomic site, whether simultaneous interaction with both histidines would result in VDR activation or not is presently not known.
The presented docking results compare predicted poses of the new analog series with the binding mode of the co-crystallized agonist 1,25(OH)2D3. Docked ligand poses and their Glide XP scores (where the more negative the score, the more favorable the binding) suggest that compounds in Table 2 can bind VDR in the genomic pocket analogously to the co-crystallized ligand except for D3 and 17(OH)D3. Non-polar contacts are important for the binding of this series. Analogs containing a 1α(OH) group bind more favorably than their counterparts lacking this group. Docked poses predict interactions common within this series, as well as distinct interactions with His305/His397.
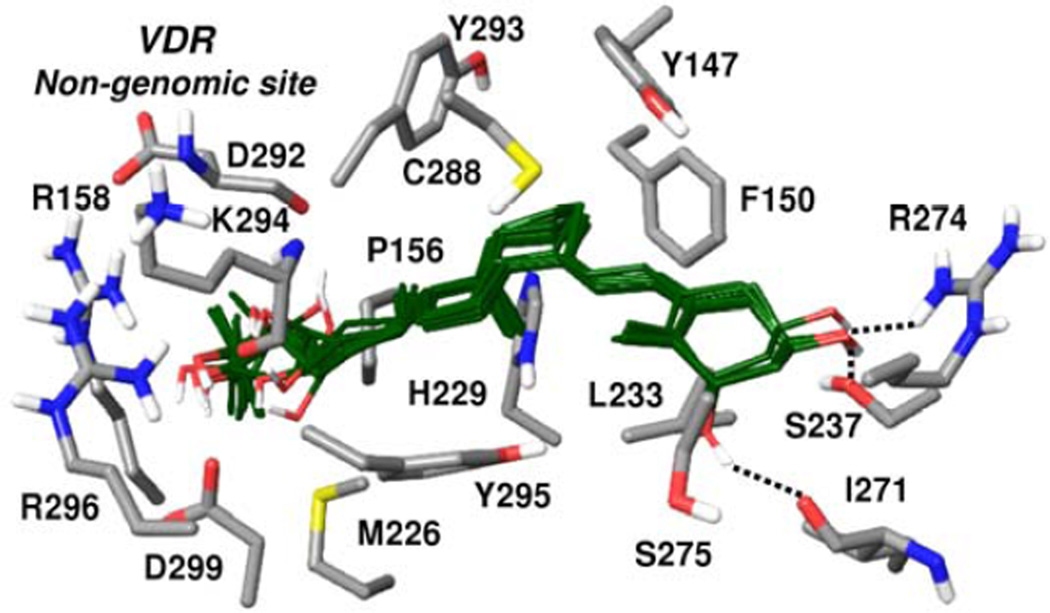
Docking results utilizing the non-genomic site of VDR
Since VDR is also present in the membrane caveolae and has an alternative 1,25(OH)2D3 binding A-pocket where occupation induces non-genomic rapid responses [100–103], we included this site in the analysis. Tyr295 in the non-genomic site of the VDR crystal structure (PDB code 1DB1) penetrates deep into the pocket region and interferes with ligand binding. The side chain of Arg158 is also in a conformation that is not compatible with accommodating ligands into this site. Therefore, prior to docking the rotamer conformations of Tyr295 and Arg158 were changed and the structure was relaxed with the known non-genomic agonist, 1α,25-hydroxylumisterol modelled into the binding site. Preparation of the VDR non-genomic site structure prior to docking is described in Appendix A. Glide XP docking of hydroxy-vitamin D3 compounds listed in Table 2 into the non-genomic site resulted in reasonable poses for some of the analogs with 23(OH), 24(OH) or 25(OH)- substitutions, which form hydrogen bonding interactions in a highly polar region of the pocket. Docked poses are closely overlapping, suggesting a common binding mode, as illustrated in Figure 6. Interactions with non-polar residues mapping the non-genomic pocket contribute significantly to the binding of these metabolites. The 3(OH) group in each docked pose forms hydrogen bonding interactions with Arg274, Ser237 while 1α(OH)-containing analogs interact with the carbonyl backbone of Ile271, in close proximity to Ser275. Superposition of other D3 analogs from Table 2 onto these poses suggests unfavorably close contacts involving hydroxyl groups and VDR residues in this site, although it is possible that some of these analogs can induce further conformational changes of the non-genomic site to adjust to their binding. Calcitroic acid shows a similar orientation to D3 analogs, while its carboxylate group is oriented toward Arg158 and Arg296.
Figure 6.
Docked poses of hydroxy-D3 metabolites in the non-genomic site of VDR, where the VDR crystal structure (PDB code 1DB1) was refined as described under Computational Methods (Supplemental file). VDR residues shown form polar or non-polar interactions with the docked ligands. Carbons in the docked poses are colored dark green; all other atoms are colored by atom type (as in Fig. 4). Hydrogen bonding interactions involving 1α(OH) and 3(OH) groups shared in docked poses are indicated with dashed lines.
Thus, we explored in silico the possible binding of our analog series in the non-genomic site of VDR. The crystal structure of this pocket was slightly modified to simulate conformational changes induced by the binding of the known non-genomic agonist, 1α,25-hydroxylumisterol. Docking results suggest that it is possible to accommodate most hydroxy-D3 analogs with 23-, 24- or 25-(OH) substitutions, as well as calcitroic acid in the ligand-induced conformation of the VDR non-genomic pocket.
5.1.2. Concluding remarks on VDR
Thus, both functional studies and in silico modeling support that the novel CYP11A1-derived D3 hydroxymetabolites with and without a 1α(OH) group act on the VDR, the vast majority at the genomic site. 1α(OH) derivatives generally have better docking scores in agreement with their higher efficiency to translocate VDR to nucleus. Furthermore, interaction with VDRE [53, 64, 65, 84, 86, 95] or with specific coactivators (Fig. 2) is greatly enhanced or requires, respectively, the presence of the 1α(OH) group in the novel secosteroids. On the other hand, VDR silencing [53, 84–86] emphasizes the importance of the VDR for the phenotypic effects in skin cells. It is interesting that antiproliferative and prodifferentiation or immunomodulatory effects of compounds with and without the 1α(OH) group, on human skin cells or immune cells, are either similar or show cell type and phenotypic trait-dependent differences (Fig. 1)[64, 65, 90, 92, 95]. Only a few secosteroids may potentially interact with the non-genomic site including 1α,25(OH)2D3, 23S(OH)D3 (which can potentially be derived from CYP11A1 action on vitamin D3), and the products of 1,25(OH)2D3 catabolism, 24R,25(OH)2D3 and calcitroic acid..
We suggest that the interaction of CYP11A1-derived secosteroids with the VDR and downstrem transcriptional activity is co-activator specifc for VDR in the proper cellular context, with agoinistic activity modified by the presence of a 1α(OH) group. The mechanism may be similar to those proposed for classical vitamin D ligands [16–20, 38, 104]. It must also be noted that 20(OH)D is a relatively poor substrate for CYP27B1, however, its hydroxylation by CYP27A1 or CYP24A1 at either the 24, 25 or 26 position make the resulting dihydroderivative an excellent substrate for 1α hydroxylation [53, 54]. Thus, the challenge posed by the novel hydroxyderivatives on the nature of their activation of the VDR can be properly answered using CYP27B1−/− mice. Furthermore, the nature of their interactions with the VDR-signaling pathway will have to be elucidated using chromatin immunoprecipitation sequencing (ChIP-seq) techniques with a bioinformatics approach. Such experiments represent our future goal and the studies will include testing of membrane bound signaling pathways with selected compounds that may interact with the non-genomic site [100, 103, 105, 106].
5.2. Retinoic acid orphan receptors (ROR)α and γ
5.2.1. Functional studies
Using cultured CHO cells expressing a TET-On RORα or γ vector and RORE-LUC reporter we have shown that 20(OH)D3 and 20,23(OH)2D3 inhibit the transactivation of the reporter by RORα and RORγ, with these secosteroids being significantly more potent than 20(OH)D2, D2 and 1,25(OH)2D3 [83]. The latter showed the lowest effect on transactivation activity. This antagonistic activity was further supported by mammalian two-hybrid analysis examining the agonist-dependent interaction of the LBD with an LBD-interacting LXXLL-peptide [83]. 20(OH)D3 repressed the interaction between the LBD of ROR and the LXXLL-peptide in a dose-dependent fashion. Moreover, 20(OH)D3 and 20,23(OH)2D3 were able to attenuate the promoter activity of RORα and RORγ target genes, including Bmal1 and G6pase, respectively [83]. Again they were more potent than 20(OH)D2 and D2, with 1,25(OH)2D3 having no effect. Finally, 20(OH)D3 and 20,23(OH)2D3 repressed ILl7 promoter activity in cells overexpressing RORγ or RORα, with 20,23(OH)2D3 showing the higher potency [83]. Also, 20(OH)D3, 20,23(OH)2D3, and 1,25(OH)2D3 inhibited RORE-LUC reporter activity in human melanoma cells and keratinocytes in a similar manner [83]. We have repeated these assay with an extended list of CYP11A1-derived secosteroids which demonstrates that they inhibit RORE activity in keratinocytes (Fig. 7), and supports the results of molecular modeling that predicts that they are ligands for RORs (see below).
Figure 7.
Effect of vitamin D3 hydroxyderivatives on ROR-RE activity in HaCaT cells. The cells were grown on 96 well white plates in DMEM media containing 5% charcoal-treated FBS. After transfection with ROR-RE and a Renilla luciferase construct, the cells were treated with the compounds for 48 h followed by luciferase assay. Methodological details are in supplemental file. *, p<0.05; **, p<0.01; ***, p<0.001 at student t-test. #, p<0.05; ##, p<0.01; ###, p<0.001 at one-way ANOVA test.
5.2.2. Molecular modeling utilizing the RORα, RORγ crystal structures
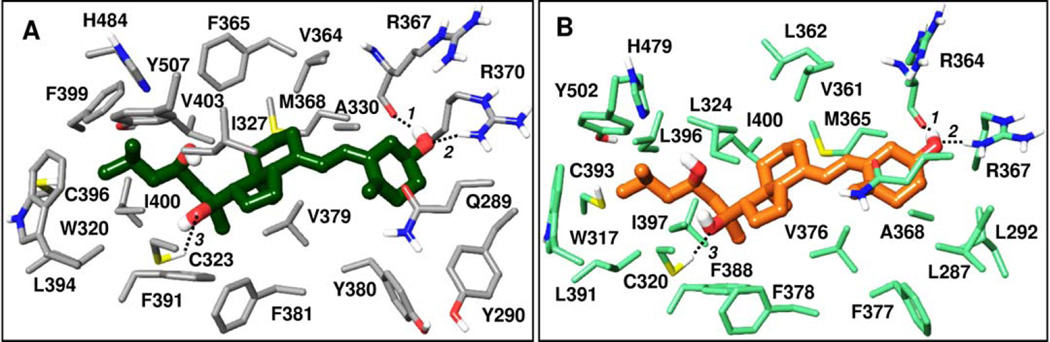
Movement of activation function helix 2 (AF2) is associated with the activation of RORα and RORγ. This helix is stabilized through a network of hydrogen bonding interactions including the His479 – Tyr502 (RORγ) interaction. Inverse agonists can act by disrupting this interaction. Among D3 metabolites the antagonism/inverse agonism of 20S(OH)D3, 20S,23S(OH)2D3 and 1α,25(OH)2D3 has been demonstrated. We therefore performed docking of secosteroids listed in Table 2 to better understand interactions formed by CYP11A1-derived hydroxy-metabolites of D3 with RORα and RORγ and to predict if other natural metabolites in this series may also bind these nuclear receptors. We utilized the Glide XP docking method (implemented in Schrödinger software) to dock the D3 series into refined crystal structures of RORα (PDB code 1N83) and RORγ (PDB code 3KYT), as described in Appendix A. Glide XP docking scores of top ranking poses are listed in Table 2. Most hydroxy-D3 metabolites show comparable or slightly less favorable docking scores compared to cholesterol, 20(OH)-cholesterol and the three confirmed hydroxy-D3 inverse agonists/antagonists listed above. The hydrophobic moiety of D3 is accommodated favorably in the largely non-polar pocket of RORs, approximately overlapping with co-crystallized cholesterol ligands. The 7α-methyl-octahydroindene moiety common in the ring structures between cholesterol and D3 metabolites is closely overlapping between docked poses of the series (Table 2) and the co-crystallized 20(OH)-cholesterol in RORγ and cholesterol in RORα. The aliphatic chains of the docked hydroxylated D3 analogs occupy the same region as the corresponding chain of the co-crystallized cholesterol ligands. The selected docking poses contain cyclohexyl in the α-chair conformation; however, analogous and comparably scoring poses were additionally obtained that mainly differ from those presented in that they adopt a β-chair conformation where the 3(OH) group is hydrogen bonding with (RORα) Gln289 and Arg367 (water-bridged interaction).
20S,23R(OH)2D3 docked into RORα and RORγ, shown in Figures 8A and 8B, respectively, illustrates a binding mode that is common of the hydroxylated vitamin D3 analog series in Table 2. Also, poses docked into RORα and RORγ show similar orientation and analogous interactions. Analogs in this series form favorable non-polar contacts with a number of residues mapping the RORα,γ binding sites as illustrated for 20S,23R(OH)2D3 in Figures 8A, B. Common to all analogs is hydrogen bonding interaction formed by the 3(OH) group with Arg370 and the backbone carbonyl of Arg367 in RORα, and the corresponding residues in RORγ: Arg367 and the backbone carbonyl of Arg364. The 1α(OH) group in most analogs containing this group hydrogen bonds with the backbone carbonyl of Tyr380(RORα)/Phe377(RORγ). In nearly all cases 20S(OH) participates as hydrogen bond acceptor in a hydrogen bond with Cys323(RORα)/Cys320(RORγ). Docked poses at both RORα and RORγ predict that the 22(OH) group can form the analogous interaction with this cysteine. As shown in Figure 8, the 23(OH) is in proximity of His484(RORα)/His479(RORγ) (heavy atom distances: 5.4 Å, 4.5 Å, respectively), which is consistent with possible water-bridged hydrogen bonding with the histidine. Analogously, the position of the 23(OH) as well as the 24(OH) in most docked poses is compatible with potential water-bridged hydrogen bonding with this histidine and/or with Tyr507(RORα)/Tyr502(RORγ). Both 25(OH) and 26(OH) groups tend to form hydrogen bonding interaction with Tyr507(RORα)/Tyr502(RORγ) and in case of several RORγ docked analogs also with His479. Interestingly, the R configuration for 20(OH) is much less favorable than natural S, due to desolvation of the 20R-hydroxyl group in a non-polar environment, which is more pronounced in case of RORα (Glide XP score > 3 kcal/mole less favorable for R than S). Favorable interactions and Glide XP scores comparable to natural ligands suggest that the series of secosteroids listed in Table 2 can fit favorably into the active site of RORα and RORγ.
Figure 8.
20S,23R(OH)2D3 docked in the active site of (A) RORα – dark green colored ligand carbons, and (B) RORγ – light brown colored ligand carbons. Atoms other than carbon are colored by atom type (as in Fig. 4). All residues contributing through polar or non-polar interactions to the binding of the docked ligand are displayed. Distances between hydrogen bonding atoms are as shown via dashed lines between the interacting atoms: (A) Pose at RORα: 1. 2.1 Å, 2. 1.9 Å, 3. 2.5 Å; (B) Pose at RORγ: 1. 1.8 Å, 2. 1.7 Å, 3. 2.0 Å.
Crystal structures of RORγ with small molecule antagonists co-crystallized in a novel allosteric binding pocket has been recently published [92]. In order to explore whether hydroxy-metabolites of D3 may bind this allosteric site we attempted docking our series into the allosteric pocket. Unsuccessful docking suggests that the allosteric site is not suitable to accommodate D3 analogs.
Thus, docking results predict that most of the presented hydroxy-D3 metabolites can bind in the active site of RORα and RORγ in poses that approximately overlap with co-crystallized cholesterol ligands. Docked compounds form extensive non-polar contacts, common in the series, while hydrogen bonding interactions involve specific hydroxyl group substituents in analogs.
5.3. Future directions in identifying receptors for vitamin D metabolites
The functional assays and molecular modeling clearly indicate that novel CYP11A1-derived D3 hydroxymetabolites can act both as partial/biased agonists on VDR and inverse agonists for RORα and RORγ. Their activities on the VDR can be modified by hydroxylation of their side chain and additional hydroxylation at C1α, setting a background for further studies as indicated in 5.1.2 that are necessary to dissect which phenotypic effects, aside of calcemia and CYP24A1 activation, are dependent on the 1α(OH) group. Since it is clear that novel D3 hydroxyderivatives can interact with RORs, it must be clarified which effects are strictly or partially dependent on RORs using RORα/γ−/− and VDR−/− cells. It is interesting that 20(OH)D3 and 20,23(OH)2D3 show higher docking scores on RORγ than previously established natural ligands represented by cholesterol and 20-hydroxycholesterol (Table 2), consistent with their inverse agonist activity reported previously [83]. This suggest a potential beneficial use of these compounds in inflammatory and autoimmune diseases with other secosteroids serving as additional candidates.
The list of compounds potentially acting on the non-genomic site of the VDR is shorter and aside of 1,25(OH)2D3 includes 1α,24S,25(OH)3D3, 23S(OH)D3 and surprisingly calcitroic acid. The in silico modeling with the novel secosteroids indicates that they are not perfect ligands for 1,25D3-MARRS with predicted candidates (1,20,24(OH)3D3, 1,24,25(OH)3D3, 17,20,23(OH)3D3) probably acting as low affinity ligands for this receptor that could be better or similar to the classical 1,25(OH)2D3. A surprising finding is the prediction of cholesterol and 20(OH)cholesterol binding to the genomic VDR pocket, with respective Glide XP scores of −9.99 and −9.78,. It suggests that further careful studies on the role of cholesterol and its metabolites on VDR activity are required, since these are abundant in cells, and related bile acid metabolites can act on the VDR [107–109].
6. Concluding remarks
Over 12 years we have documented the existence of new pathways of vitamin D3 metabolism started by the action of CYP11A1 and further modified by the actions of CYP27B1, CYP27A1, CYP24A1 and CYP3A4, generating at least 21 hydroxymetabolites with additional ones still to be experimentally defined (Table 1) [43, 50]. At least 13 of them are endogenously produced [72]. These metabolites display biological activity by acting both as “biased” agonists of the VDR and/or inverse agonists of RORα and RORγ. A subset of these compounds have the potential to act on the non-genomic VDR site or less likely on 1,25D3-MARS as suggested by molecular modeling.
We propose that the identification of a number of hydroxymetabolites of D3 may offer an explanation for the pleiotropic and diverse activities of vitamin D that have previously been assigned to 1,25(OH)2D3. We suggest that these diverse phenotypic effects are also mediated by interactions with, in additional to the VDR, receptors including RORα and RORγ. Also, for selected compounds one may propose action on the non-genomic site of VDR with the possibility that just a few of the secosteroids act as low affinity agonists on 1,25D3-MARS.
Supplementary Material
Highlights.
-
-
CYP11A1- derived hydroxyvitamin D derivatives are present in human skin and serum
-
-
CYP11A1- derived hydroxyvitamin D derivatives are biologically active
-
-
CYP11A1- derived hydroxyvitamin D derivatives act as partial/biased agonists on VDR
-
-
CYP11A1- derived hydroxyvitamin D derivatives as “inverse” agonists on RORα and RORγ
-
-
20(OH)D3, 20(OH)D2 and 20,23(OH)2D3 are noncalcemic at pharmacological doses
Acknowledgments
We acknowledge the support by NIH grants R21AR066505, 1R01AR056666 and 2R01AR052190 to AS. 1R21AR063242, 1S10OD010678, and RR-026377 to WL, and the University of Western Australia to RCT; and the Intramural Research Program of the NIEHS, NIH (Z01-ES-101586 to AMJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors declare no conflict of interest
References
- 1.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. J Invest Dermatol. 1981;77:51–58. doi: 10.1111/1523-1747.ep12479237. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Biological Effects of Sunlight, Ultraviolet Radiation, Visible Light, Infrared Radiation and Vitamin D for Health. Anticancer Res. 2016;36:1345–1356. [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 5.Bikle DD. Vitamin D: an ancient hormone. Experimental Dermatology. 2011;20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller WL. Genetic disorders of Vitamin D biosynthesis and degradation. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta. 2011;1814:186–199. doi: 10.1016/j.bbapap.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 9.Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21:375–384. doi: 10.1016/j.tem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaki T, Sawada N, Komai K, Shiozawa S, Yamada S, Yamamoto K, Ohyama Y, Inouye K. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur J Biochem. 2000;267:6158–6165. doi: 10.1046/j.1432-1327.2000.01680.x. [DOI] [PubMed] [Google Scholar]
- 11.Beckman MJ, Tadikonda P, Werner E, Prahl J, Yamada S, DeLuca HF. Human 25-hydroxyvitamin D3-24-hydroxylase, a multicatalytic enzyme. Biochemistry. 1996;35:8465–8472. doi: 10.1021/bi960658i. [DOI] [PubMed] [Google Scholar]
- 12.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014;55:13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tieu EW, Tang EK, Tuckey RC. Kinetic analysis of human CYP24A1 metabolism of vitamin D via the C24-oxidation pathway. FEBS J. 2014;281:3280–3296. doi: 10.1111/febs.12862. [DOI] [PubMed] [Google Scholar]
- 14.Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9:941–955. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 15.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikle DD. Vitamin D and cancer: the promise not yet fulfilled. Endocrine. 2014;46:29–38. doi: 10.1007/s12020-013-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlberg C, Molnar F. Vitamin D receptor signaling and its therapeutic implications: Genome-wide and structural view. Can J Physiol Pharmacol. 2015;93:311–318. doi: 10.1139/cjpp-2014-0383. [DOI] [PubMed] [Google Scholar]
- 19.Bikle DD, Oda Y, Tu CL, Jiang Y. Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. J Steroid Biochem Mol Biol. 2015;148:47–51. doi: 10.1016/j.jsbmb.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bikle DD. Vitamin D receptor, a tumor suppressor in skin. Can J Physiol Pharmacol. 2015;93:349–354. doi: 10.1139/cjpp-2014-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon KM, Tongkao-On W, Sequeira VB, Carter SE, Song EJ, Rybchyn MS, Gordon-Thomson C, Mason RS. Vitamin d and death by sunshine. Int J Mol Sci. 2013;14:1964–1977. doi: 10.3390/ijms14011964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tongkao-On W, Carter S, Reeve VE, Dixon KM, Gordon-Thomson C, Halliday GM, Tuckey RC, Mason RS. CYP11A1 in skin: an alternative route to photoprotection by vitamin D compounds. J Steroid Biochem Mol Biol. 2015;148:72–78. doi: 10.1016/j.jsbmb.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Slominski AT, Janjetovic Z, Kim TK, Wasilewski P, Rosas S, Hanna S, Sayre RM, Dowdy JC, Li W, Tuckey RC. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J Steroid Biochem Mol Biol. 2015;148:52–63. doi: 10.1016/j.jsbmb.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyton KZ, Kensler TW, Posner GH. Cancer chemoprevention using natural vitamin D and synthetic analogs. Annu Rev Pharmacol Toxicol. 2001;41:421–442. doi: 10.1146/annurev.pharmtox.41.1.421. [DOI] [PubMed] [Google Scholar]
- 25.Agoston ES, Hatcher MA, Kensler TW, Posner GH. Vitamin D analogs as anti-carcinogenic agents. Anticancer Agents Med Chem. 2006;6:53–71. doi: 10.2174/187152006784111369. [DOI] [PubMed] [Google Scholar]
- 26.Masuda S, Jones G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther. 2006;5:797–808. doi: 10.1158/1535-7163.MCT-05-0539. [DOI] [PubMed] [Google Scholar]
- 27.Ness RA, Miller DD, Li W. The role of vitamin D in cancer prevention. Chin J Nat Med. 2015;13:481–497. doi: 10.1016/S1875-5364(15)30043-1. [DOI] [PubMed] [Google Scholar]
- 28.Adorini L. Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol. 2005;233:115–124. doi: 10.1016/j.cellimm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Adorini L, Penna G. Induction of tolerogenic dendritic cells by vitamin D receptor agonists. Handb Exp Pharmacol. 2009:251–273. doi: 10.1007/978-3-540-71029-5_12. [DOI] [PubMed] [Google Scholar]
- 30.Fleet JC, Bradley J, Reddy GS, Ray R, Wood RJ. 1 alpha,25-(OH)2-vitamin D3 analogs with minimal in vivo calcemic activity can stimulate significant transepithelial calcium transport and mRNA expression in vitro. Arch Biochem Biophys. 1996;329:228–234. doi: 10.1006/abbi.1996.0213. [DOI] [PubMed] [Google Scholar]
- 31.Reddy GS, Muralidharan KR, Okamura WH, Tserng KY, McLane JA. Metabolism of 1alpha,25-dihydroxyvitamin D(3) and its C-3 epimer 1alpha,25-dihydroxy-3-epi-vitamin D(3) in neonatal human keratinocytes. Steroids. 2001;66:441–450. doi: 10.1016/s0039-128x(00)00228-2. [DOI] [PubMed] [Google Scholar]
- 32.Kamao M, Tatematsu S, Hatakeyama S, Sakaki T, Sawada N, Inouye K, Ozono K, Kubodera N, Reddy GS, Okano T. C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1alpha or C-24 hydroxylation. J Biol Chem. 2004;279:15897–15907. doi: 10.1074/jbc.M311473200. [DOI] [PubMed] [Google Scholar]
- 33.Sekimoto H, Siu-Caldera ML, Weiskopf A, Vouros P, Muralidharan KR, Okamura WH, Uskokovic MR, Reddy GS. 1alpha,25-dihydroxy-3-epi-vitamin D3: in vivo metabolite of 1alpha,25-dihydroxyvitamin D3 in rats. FEBS Lett. 1999;448:278–282. doi: 10.1016/s0014-5793(99)00377-4. [DOI] [PubMed] [Google Scholar]
- 34.Masuda S, Kamao M, Schroeder NJ, Makin HL, Jones G, Kremer R, Rhim J, Okano T. Characterization of 3-epi-1alpha,25-dihydroxyvitamin D3 involved in 1alpha,25-dihydroxyvitamin D3 metabolic pathway in cultured cell lines. Biol Pharm Bull. 2000;23:133–139. doi: 10.1248/bpb.23.133. [DOI] [PubMed] [Google Scholar]
- 35.Molnar F, Sigueiro R, Sato Y, Araujo C, Schuster I, Antony P, Peluso J, Muller C, Mourino A, Moras D, Rochel N. 1alpha,25(OH)2-3-epi-vitamin D3, a natural physiological metabolite of vitamin D3: its synthesis, biological activity and crystal structure with its receptor. PLoS One. 2011;6:e18124. doi: 10.1371/journal.pone.0018124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown AJ, Ritter C, Slatopolsky E, Muralidharan KR, Okamura WH, Reddy GS. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxyvitamin D3, is a potent suppressor of parathyroid hormone secretion. J Cell Biochem. 1999;73:106–113. [PubMed] [Google Scholar]
- 37.Nakagawa K, Sowa Y, Kurobe M, Ozono K, Siu-Caldera ML, Reddy GS, Uskokovic MR, Okano T. Differential activities of 1alpha,25-dihydroxy-16-ene-vitamin D(3) analogs and their 3-epimers on human promyelocytic leukemia (HL-60) cell differentiation and apoptosis. Steroids. 2001;66:327–337. doi: 10.1016/s0039-128x(00)00142-2. [DOI] [PubMed] [Google Scholar]
- 38.Carlberg C. What do we learn from the genome-wide perspective on vitamin D3? Anticancer Res. 2015;35:1143–1151. [PubMed] [Google Scholar]
- 39.Khanal R, Nemere I. Membrane receptors for vitamin D metabolites. Crit Rev Eukaryot Gene Expr. 2007;17:31–47. doi: 10.1615/critreveukargeneexpr.v17.i1.30. [DOI] [PubMed] [Google Scholar]
- 40.Sequeira VB, Rybchyn MS, Tongkao-On W, Gordon-Thomson C, Malloy PJ, Nemere I, Norman AW, Reeve VE, Halliday GM, Feldman D, Mason RS. The role of the vitamin D receptor and ERp57 in photoprotection by 1alpha,25-dihydroxyvitamin D3. Mol Endocrinol. 2012;26:574–582. doi: 10.1210/me.2011-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26:273–281. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine Reviews. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015;103:72–88. doi: 10.1016/j.steroids.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci U S A. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, R CT. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slominski A, Semak I, Zjawiony J, Wortsman J, Gandy MN, Li J, Zbytek B, Li W, Tuckey RC. Enzymatic metabolism of ergosterol by cytochrome p450scc to biologically active 17alpha,24-dihydroxyergosterol. Chem Biol. 2005;12:931–939. doi: 10.1016/j.chembiol.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, Tuckey RC. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273:2891–2901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuckey RC, Slominski AT, Cheng CY, Chen J, Kim TK, Xiao M, Li W. Lumisterol is metabolized by CYP11A1: discovery of a new pathway. Int J Biochem Cell Biol. 2014;55:24–34. doi: 10.1016/j.biocel.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, Tuckey RC. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol. 2015;151:25–37. doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, Miller D, Slominski A. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008;275:2585–2596. doi: 10.1111/j.1742-4658.2008.06406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen MN, Slominski A, Li W, Ng YR, Tuckey RC. Metabolism of vitamin D2 to 17,20,24-trihydroxyvitamin D2 by cytochrome p450scc (CYP11A1) Drug Metab Dispos. 2009;37:761–767. doi: 10.1124/dmd.108.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, Li W, Chen J, Nguyen MN, Tang EK, Miller D, Chen TC, Holick M. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011;300:C526–C541. doi: 10.1152/ajpcell.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang EK, Chen J, Janjetovic Z, Tieu EW, Slominski AT, Li W, Tuckey RC. Hydroxylation of CYP11A1-derived products of vitamin D3 metabolism by human and mouse CYP27B1. Drug Metab Dispos. 2013;41:1112–1124. doi: 10.1124/dmd.113.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tieu EW, Li W, Chen J, Kim TK, Ma D, Slominski AT, Tuckey RC. Metabolism of 20-hydroxyvitamin D3 and 20,23-dihydroxyvitamin D3 by rat and human CYP24A1. J Steroid Biochem Mol Biol. 2015;149:153–165. doi: 10.1016/j.jsbmb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang EK, Li W, Janjetovic Z, Nguyen MN, Wang Z, Slominski A, Tuckey RC. Purified mouse CYP27B1 can hydroxylate 20,23-dihydroxyvitamin D3, producing 1alpha,20,23-trihydroxyvitamin D3, which has altered biological activity. Drug Metab Dispos. 2010;38:1553–1559. doi: 10.1124/dmd.110.034389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tieu EW, Li W, Chen J, Baldisseri DM, Slominski AT, Tuckey RC. Metabolism of cholesterol, vitamin D3 and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by human CYP27A1. J Steroid Biochem Mol Biol. 2012;129:163–171. doi: 10.1016/j.jsbmb.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, Tuckey RC. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–3915. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slominski AT, Kim TK, Shehabi HZ, Tang EK, Benson HA, Semak I, Lin Z, Yates CR, Wang J, Li W, Tuckey RC. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol. 2014;383:181–192. doi: 10.1016/j.mce.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tuckey RC, Nguyen MN, Slominski A. Kinetics of vitamin D3 metabolism by cytochrome P450scc (CYP11A1) in phospholipid vesicles and cyclodextrin. Int J Biochem Cell Biol. 2008;40:2619–2626. doi: 10.1016/j.biocel.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, Chen J, Howell DE, Benson HA, Sweatman T, Baldisseri DM, Slominski A. Production of 22-hydroxy metabolites of vitamin D3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab Dispos. 2011;39:1577–1588. doi: 10.1124/dmd.111.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony J, Slominski A. Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2008;112:213–219. doi: 10.1016/j.jsbmb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang EK, Voo KJ, Nguyen MN, Tuckey RC. Metabolism of substrates incorporated into phospholipid vesicles by mouse 25-hydroxyvitamin D3 1alpha-hydroxylase (CYP27B1) J Steroid Biochem Mol Biol. 2010;119:171–179. doi: 10.1016/j.jsbmb.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 64.Lin Z, Marepally SR, Ma D, Kim TK, Oak AS, Myers LK, Tuckey RC, Slominski AT, Miller DD, Li W. Synthesis and Biological Evaluation of Vitamin D3 Metabolite 20S,23S-Dihydroxyvitamin D3 and Its 23R Epimer. J Med Chem. 2016 doi: 10.1021/acs.jmedchem.6b00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin Z, Marepally SR, Ma D, Myers LK, Postlethwaite AE, Tuckey RC, Cheng CY, Kim TK, Yue J, Slominski AT, Miller DD, Li W. Chemical Synthesis and Biological Activities of 20S,24S/R-Dihydroxyvitamin D3 Epimers and Their 1alpha-Hydroxyl Derivatives. J Med Chem. 2015;58:7881–7887. doi: 10.1021/acs.jmedchem.5b00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng CY, Slominski AT, Tuckey RC. Hydroxylation of 20-hydroxyvitamin D3 by human CYP3A4. J Steroid Biochem Mol Biol. 2016;159:131–141. doi: 10.1016/j.jsbmb.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D, Slominski A. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids. 2010;75:926–935. doi: 10.1016/j.steroids.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J, Wang J, Kim TK, Tieu EW, Tang EK, Lin Z, Kovacic D, Miller DD, Postlethwaite A, Tuckey RC, Slominski AT, Li W. Novel vitamin D analogs as potential therapeutics: metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res. 2014;34:2153–2163. [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Q, Lin Z, Kim TK, Slominski AT, Miller DD, Li W. Total synthesis of biologically active 20S-hydroxyvitamin D3. Steroids. 2015;104:153–162. doi: 10.1016/j.steroids.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol. 2014;144PA:28–39. doi: 10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slominski AT, Kim TK, Li W, Tuckey RC. Classical and non-classical metabolic transformation of vitamin D in dermal fibroblasts. Exp Dermatol. 2016;25:231–232. doi: 10.1111/exd.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EK, Tuckey RC. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep. 2015;5:14875. doi: 10.1038/srep14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nuclear receptor signaling. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cook DN, Kang HS, Jetten AM. Retinoic Acid-Related Orphan Receptors (RORs): Regulatory Functions in Immunity, Development, Circadian Rhythm, and Metabolism. Nucl Receptor Res. 2015;2 doi: 10.11131/2015/101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Medvedev A, Yan ZH, Hirose T, Giguere V, Jetten AM. Cloning of a cDNA encoding the murine orphan receptor RZR/ROR gamma and characterization of its response element. Gene. 1996;181:199–206. doi: 10.1016/s0378-1119(96)00504-5. [DOI] [PubMed] [Google Scholar]
- 76.Fauber BP, Magnuson S. Modulators of the nuclear receptor retinoic acid receptor-related orphan receptor-gamma (RORgamma or RORc) J Med Chem. 2014;57:5871–5892. doi: 10.1021/jm401901d. [DOI] [PubMed] [Google Scholar]
- 77.Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov. 2014;13:197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith SH, Peredo CE, Takeda Y, Bui T, Neil J, Rickard D, Millerman E, Therrien JP, Nicodeme E, Brusq JM, Birault V, Viviani F, Hofland H, Jetten AM, Cote-Sierra J. Development of a Topical Treatment for Psoriasis Targeting RORgamma: From Bench to Skin. PLoS One. 2016;11:e0147979. doi: 10.1371/journal.pone.0147979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kallen JA, Schlaeppi JM, Bitsch F, Geisse S, Geiser M, Delhon I, Fournier B. X-ray structure of the hRORalpha LBD at 1.63 A: structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of RORalpha. Structure. 2002;10:1697–1707. doi: 10.1016/s0969-2126(02)00912-7. [DOI] [PubMed] [Google Scholar]
- 80.Hu X, Wang Y, Hao L-Y, Liu X, Lesch CA, Sanchez BM, Wendling JM, Morgan RW, Carter LL, Toogood PL, Glick GD. Sterol metabolism controls TH17 differentiation by generating endogenous RORγ agonists. Nature Chem. Biol. 2015;11:141–147. doi: 10.1038/nchembio.1714. [DOI] [PubMed] [Google Scholar]
- 81.Santori FR, Huang P, van de Pavert SA, Douglass EF, Jr, Leaver DJ, Haubrich BA, Keber R, Lorbek G, Konijn T, Rosales BN, Rozman D, Horvat S, Rahier A, Mebius RE, Rastinejad F, Nes WD, Littman DR. Identification of Natural RORgamma Ligands that Regulate the Development of Lymphoid Cells. Cell Metab. 2015;21:286–297. doi: 10.1016/j.cmet.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu T, Wang X, Zhong B, Nurieva RI, Ding S, Dong C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. The Journal of biological chemistry. 2011;286:22707–22710. doi: 10.1074/jbc.C111.250407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey RC, Jetten AM. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28:2775–2789. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, Nguyen MN, Slominski AT. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128:2271–2280. doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, Slominski AT. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr, Slominski AT. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol. 2010;223:36–48. doi: 10.1002/jcp.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Slominski AT, Li W, Bhattacharya SK, Smith RA, Johnson PL, Chen J, Nelson KE, Tuckey RC, Miller D, Jiao Y, Gu W, Postlethwaite AE. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J Invest Dermatol. 2011;131:1167–1169. doi: 10.1038/jid.2010.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Slominski A, Kim TK, Zmijewski MA, Janjetovic Z, Li W, Chen J, Kusniatsova EI, Semak I, Postlethwaite A, Miller D, Zjawiony J, Tuckey RC. Novel vitamin D photoproducts and their precursors in the skin. Dermato-Endocrinology. 2013;5:1–13. doi: 10.4161/derm.23938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, Li W, Jiao Y, Gu W, Brown M, Postlethwaite AE. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocrinol Metab. 2013;98:E298–E303. doi: 10.1210/jc.2012-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Slominski AT, Janjetovic Z, Kim TK, Wright AC, Grese LN, Riney SJ, Nguyen MN, Tuckey RC. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012;32:3733–3742. [PMC free article] [PubMed] [Google Scholar]
- 91.Piotrowska A, Wierzbicka J, Slebioda T, Wozniak M, Tuckey RC, Slominski AT, Zmijewski MA. Vitamin D derivatives enhance cytotoxic effects of H2O2 or cisplatin on human keratinocytes. Steroids. 2016;110:49–61. doi: 10.1016/j.steroids.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim TK, Wang J, Janjetovic Z, Chen J, Tuckey RC, Nguyen MN, Tang EK, Miller D, Li W, Slominski AT. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol Cell Endocrinol. 2012;361:143–152. doi: 10.1016/j.mce.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, Postlethwaite AE, Miller D, Li W. 20-hydroxyvitamin D(3) inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–746. [PMC free article] [PubMed] [Google Scholar]
- 94.Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, Pfeffer SR, Pfeffer LM, Slominski AT. High basal NF-kappaB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer. 2011;105:1874–1884. doi: 10.1038/bjc.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, Chen TC, Lozanski G, Holick MF. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wasiewicz T, Szyszka P, Cichorek M, Janjetovic Z, Tuckey RC, Slominski AT, Zmijewski MA. Antitumor effects of vitamin D analogs on hamster and mouse melanoma cell lines in relation to melanin pigmentation. Int J Mol Sci. 2015;16:6645–6667. doi: 10.3390/ijms16046645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wierzbicka JM, Binek A, Ahrends T, Nowacka JD, Szydlowska A, Turczyk L, Wasiewicz T, Wierzbicki PM, Sadej R, Tuckey RC, Slominski AT, Chybicki J, Adrych K, Kmiec Z, Zmijewski MA. Differential antitumor effects of vitamin D analogues on colorectal carcinoma in culture. Int J Oncol. 2015;47:1084–1096. doi: 10.3892/ijo.2015.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kenakin T. What is pharmacological 'affinity'? Relevance to biased agonism and antagonism. Trends Pharmacol Sci. 2014;35:434–441. doi: 10.1016/j.tips.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 99.Kenakin T. Biased agonism. F1000 Biol Rep. 2009;1:87. doi: 10.3410/B1-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mizwicki MT, Keidel D, Bula CM, Bishop JE, Zanello LP, Wurtz JM, Moras D, Norman AW. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1alpha,25(OH)2-vitamin D3 signaling. Proc Natl Acad Sci U S A. 2004;101:12876–12881. doi: 10.1073/pnas.0403606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boland RL. VDR activation of intracellular signaling pathways in skeletal muscle. Mol Cell Endocrinol. 2011;347:11–16. doi: 10.1016/j.mce.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 102.Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 103.Mizwicki MT, Norman AW. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal. 2009;2:re4. doi: 10.1126/scisignal.275re4. [DOI] [PubMed] [Google Scholar]
- 104.Carlberg C. Genome-wide (over)view on the actions of vitamin D. Front Physiol. 2014;5:167. doi: 10.3389/fphys.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 106.Bula CM, Huhtakangas J, Olivera C, Bishop JE, Norman AW, Henry HL. Presence of a truncated form of the vitamin D receptor (VDR) in a strain of VDR-knockout mice. Endocrinology. 2005;146:5581–5586. doi: 10.1210/en.2005-0806. [DOI] [PubMed] [Google Scholar]
- 107.Han S, Li T, Ellis E, Strom S, Chiang JY. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Mol Endocrinol. 2010;24:1151–1164. doi: 10.1210/me.2009-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 109.Masuno H, Ikura T, Morizono D, Orita I, Yamada S, Shimizu M, Ito N. Crystal structures of complexes of vitamin D receptor ligand-binding domain with lithocholic acid derivatives. J Lipid Res. 2013;54:2206–2213. doi: 10.1194/jlr.M038307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.