Abstract
Purpose
To correlate angiogenic cytokines in the aqueous humor with total retinal blood flow in subjects with Type 2 diabetes with non-proliferative DR (NPDR).
Methods
17 controls and 16 NPDR patients were recruited into the study. Aqueous humor was collected at the beginning of cataract surgery to assess the concentration of 14 angiogenic cytokines. Aqueous humor was analyzed using the suspension array method. Six images were acquired to assess total retinal blood flow (TRBF) using the prototype RTVue™ Doppler FD-OCT (Optovue, Inc., Fremont, CA) using a double circular scan protocol, 1 month post-surgery. At the same visit, forearm blood was collected to determine glycosylated hemoglobin (A1c).
Results
TGF-β1, TGF-β2 and PLGF were increased while FGF-1 was reduced in NPDR compared to controls (Bonferroni corrected, p<0.003 for all). TRBF was significantly reduced in the NPDR group compared to controls (33.1±9.9 vs. 43.3±5.3, p=0.002). Aqueous FGF-1 significantly correlated with TRBF in the NPDR group (r = 0.71, p = 0.01; r2 = 0.51). In a multiple regression analysis, A1c was found to be a significant predictor of aqueous TGFβ1 and FGF-1 (p=0.018 and p=0.020, respectively).
Conclusion
Aqueous angiogenic cytokines (TGF-β1, TGF-β2 and PLGF) were elevated in conjunction with a reduction in TRBF in patients with NPDR. Non-invasive measurement of TRBF may be useful for predicting aqueous FGF-1 levels and severity of vasculopathy in DR.
Keywords: Total retinal blood flow, aqueous humor cytokines, angiogenesis, non-proliferative diabetic retinopathy, optical coherence tomography, retinal blood flow, fibroblast growth factor, type 2 diabetes mellitus, vasoregulatory
INTRODUCTION
Diabetic retinopathy (DR) is a sight threatening complication of diabetes mellitus and is currently the leading cause of blindness in working age adults. Following 20 years of diabetes, nearly all patients with Type 1 and Type 2 diabetes mellitus will suffer from some form of retinopathy.(Klein & Klein 2010, Yau et al. 2012) Using non-invasive measurement techniques, animal and human studies have reported alterations in retinal blood flow before any clinical symptoms of DR.(Bursell et al. 1996, Grunwald et al. 1986, Takagi et al. 1996) Although perturbations in retinal blood flow are hypothesized to be an early hallmark of DR, the roles of diabetes-induced changes that modulate retinal blood flow in early DR are still unknown. To assess the pathogenesis of DR as well as to provide clinical biomarkers of DR severity, we set out to examine the role of angiogenic cytokines during diabetes-induced microvascular disturbances in early DR.
The role of angiogenic cytokines in the progression DR has been reported by many groups. (Clermont et al. 1997a, Clermont et al. 1997a, Noma et al. 2009, Noma et al. 2010) Moreover, previous studies have demonstrated that angiogenic cytokines are elevated in the ocular fluid of eyes affected by DR (Chiang et al. 2012, Dong et al. 2013, Oh et al. 2010) and diabetic macular edema(Jonas et al. 2012, Lee et al. 2012)(Jonas et al. 2012) compared with control eyes. Recently, one study reported that the elevated VEGF levels in the aqueous humor correlates with retinal blood flow in patients with central retinal vein occlusion.(Yamada et al. 2015) However, the use of multiplex bead analysis allows for simultaneous analysis of multiple cytokines in small volumes, provides broader insight into the mechanisms involved in retinal hemodynamic alterations in early DR.
An established technique that has been used to investigate volumetric retinal blood flow in DR is bidirectional laser Doppler velocimetry with simultaneous vessel densitometry. However, this technique is currently limited to large vessels and one single measurement site. Subsequently, Doppler spectral Fourier-domain optical coherence tomography (Doppler FD-OCT) blood flow (i.e. Optovue RTVue) has been developed to evaluate volumetric total retinal blood flow (TRBF). The FD-OCT system uses low coherence light to measure the total retinal blood flow of all vessels around the optic nerve head.(Wang et al. 2008) This system has been proven to reliably and consistently measure retinal blood flow in both healthy (Tayyari et al. 2014) and subjects with DR (Wang et al. 2009a). Thus, the Optovue system is a convenient method for measuring blood flow perturbations in DR and can be used clinically to assess DR severity.
We set out to evaluate the levels of angiogenic factors in the aqueous humor in NPDR to ascertain if changes in these locally produced factors vary in patients with NPDR. Further, we investigate the relationship between angiogenic cytokines and TRBF in patients with NPDR. Non-invasive quantification of TRBF may be a novel biomarker for biochemical alterations in early DR.
METHODS
Sample
All eligibility was determined and confirmed by an ophthalmologist during slit-lamp examination. Participants must be eligible for phacoemulsification. Subjects were excluded from the study if they had any clinical evidence of ocular disease (other than DR) and/or a history of ocular surgery or laser procedure. Subjects with a history of glaucoma in a first-degree relative were excluded from the study. Subjects taking medications with known effects on blood flow (except for well controlled systemic hypertension) and subjects with rheumatologic diseases were excluded. None of the subjects smoked or had any respiratory diseases. This study was approved by the University Health Network Research Ethics Board, University of Toronto Research Ethics Board and Kensington Eye Institute Research Ethics Board. Informed consent was obtained from each participant after thorough explanation of the nature of the study and its possible consequences, according to the tenets of the Declaration of Helsinki.
Aqueous Humor Collection
At the time of surgery, paracentesis was made in the peripheral cornea next to the limbus and undiluted samples of AH (80-120μl) was obtained from all participants. The AH was collected into an attached tuberculin syringe and then subsequently deposited into a 1.5ml micro-tube and placed on dry ice. The AH sample was immediately stored in a −80 degree Celsius refrigerator until analysis was performed. The aqueous samples were thawed at room temperature, vortexed, and then spun at 13,000× g for 5 minutes to remove any precipitates. Aqueous humor samples were then separated into aliquots of 30ul.
Measurement of Angiogenic and Hemodynamic factors using Multiplex Bead Analysis
Multiple cytokines were quantified simultaneously using MILLIPLEX® MAP technology to discover biomarker patterns in small sample volumes (Eve Technologies Corp, Calgary, AB, Canada), using the BioPlex 200 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Capture bead kits (R&D, Minneapolis, MN & Millipore, St. Charles, MO, USA) were used to determine fibroblast growth factor (FGF-1 & FGF-2), angiopoietins (ANG-2), interleukin-8 (IL-8), leptin, stromal-derived factor-1, epidermal growth factor (EGF), transforming growth factor-beta (TGF-β1 and TGF-β2), granulocyte colony stimulating factor (G-CSF) platelet-derived growth factors (PDGFs), vascular endothelial growth factor (VEGF), erythropoietin, endothelin-1 (ET-1), insulin (Millipore, St. Charles, MO, USA). The sensitivities of the markers range from 0.2 – 17.0 pg/ml. Individual analyte values and other assay details are available on Eve Technologies’ website or in the Milliplex protocol. The tests were performed in accordance with the manufacturer’s instructions. An analyte was considered “detectable” if the levels exceeded the minimal detectable levels identified in manual.
Quantifying Total Retinal Blood Flow Using FD-OCT
Four weeks post-surgery, the study eye was dilated with 1% tropicamide and 2.5% phenylephrine eye drops and images were then acquired. Doppler OCT images were taken using the commercially available known as the RTVue Doppler OCT (Optovue Inc, Freemont, CA). The assessment included 6 sets of two concentric circular scans (each 3.4 and 3.75 mm in diameter, centered on the optic nerve head).
Non-invasive OCT technology has applied Doppler FD-OCT to extract information to calculate the volumetric blood flow. A computer algorithm chooses candidate vessels followed by manual refining by human operators, using the Doppler OCT of Retinal Circulation (DOCTORC) grading software. In the post-analysis process, the operator compares the initial automated processing with a colour fundus image to help accurately determine the distribution of vessels. The operator also adjusts the vessel classification and evaluates the Doppler signal size and intensity in order to precisely calculate total blood flow. Since this software is semi-automated, it takes an experienced technician 30-50 minutes per eye for analysis. This method has been previously described and has been recently validated.(Tan et al. 2012, Tayyari et al. 2014, Wang et al. 2008)
Finally, the TRBF is corrected by axial length. In Doppler OCT, the scan is actually based on a model eye with axial length of 24 mm and is found to vary with different eye lengths. Longer axial lengths decrease the magnification of the fundus image, reduce the transverse diameter of the vessel and increase the apparent Doppler angle, making the velocity and flow appear artifactually low. However, this magnification can be corrected. As described by Srinivas et al, (Srinivas et al. 2015) corrected TRBF can be obtained the formula: corrected TRBF= oTRBF*(axial length/default axial length)2. Here, oTRBF is the original total blood flow before eye length correction and the default axial length, which is a value of 24 mm. The axial length–corrected TRBF was used in the statistical analyses.
Briefly, the RTVue system has a light source of 840nm with a bandwidth of 50nm, an axial resolution of 5µm in tissue and a transverse resolution of 20 µm.(Wang et al. 2011) The Doppler OCT is based on the principle that moving particles, such as a red blood cell inside a blood vessel, causes a Doppler frequency shift (∆f) to the scattered light. The Doppler shift (∆f) is proportional to the flow velocity (V) component to the axis of the probe beam. The Doppler shift (∆f) and angle α is measured from the difference between 2 concentric circular OCT scans. The measured Doppler shift, the incident angle and vessel area are used to calculate blood flow in each vessel. Total retinal blood flow was measured by summing the flow of all the detectable veins. Previously, Feke et al confirmed that retinal flow in arterials is equal to the retinal flow in veins due to a steady state system.(Feke & Riva 1978)
Statistical analysis
All analyses were performed with Statistica System 11.0 software (SAS Institute Inc., Cary, North Carolina, USA). All results are presented as the mean ± standard deviation (SD). The mean fold changes of the detected biomarkers were calculated between NPDR and control subjects. Differences in total retinal blood flow and aqueous humor factor components between healthy and NPDR participants were assessed with Student T-test. A Bonferroni correction was applied to each P value according to the number of comparisons in this study (corrected P value of 0.05/14 = 0.003). The correlation between TRBF and aqueous humor cytokines was determined with Pearson Bivariate Correlation test. In all analyses, two-tailed p<0.05 indicated statistical significance.
RESULTS
The demographic data of subjects are shown in Table 1. A total of 18 controls and 16 NPDR patients were initially recruited for the study. NPDR participants had early signs of retinopathy including microaneuryms, as confirmed by the ophthalmologist.
Table 1.
Subject demographics of study population. Sample size per group (n), group mean age (standard deviation, SD), known duration of diabetes (years), systolic and diastolic blood pressure (BP), mean ocular perfusion pressure (MOPP), intraocular pressure (IOP) and glycosylated hemoglobin (A1c) as a function of group.
| Variable | Control | NPDR | P-value |
|---|---|---|---|
| Sample size (n) | 17 | 15 | -- |
| Age (years) | 68.8 ± 6.35 | 67.5 ± 10.1 | 0.658 |
| Duration (years) | 0 | 12.0 | -- |
| Baseline Systolic (mmHg) | 128.1± 17.2 | 134.5 ± 19.1 | 0.329 |
| Baseline Diastolic (mmHg) | 76.9 ± 9.03 | 76.9 ± 8.10 | 0.997 |
| Heart rate (bpm) | 76.7 ± 10.5 | 62.9 ± 18.4 | 0.029 |
| MOPP (mmHg) | 48.1 ± 5.10 | 51.1 ± 5.13 | 0.106 |
| IOP(mmHg) | 14.6 ± 2.03 | 14.9 ± 1.72 | 0.657 |
| A1c (%) | 5.49 ± 0.33 | 7.13 ± 0.74 | <0.001* |
Indicates significant different from controls, p<0.05.
Retinal Blood Flow Findings
Table 2 lists the mean (±standard deviation (SD)) corrected TRBF for the control and NPDR groups. The mean TRBF for controls was 43.3±5.3 ul/min (95% CI: 40.2-46.3 ul/min) and 33.1±9.9 ul/min (95% CI: 27.1-39.1 ul/min) for NPDR patients. NPDR patients had significantly lower TRBF (~22%) than control subjects (p=0.002). Compared to controls, NPDR patients had significantly reduced superior and inferior retinal blood flow rates (~23%; p=0.01 and p=0.02 respectively).
Table 2.
Corrected Total Retinal Blood Flow as a Function of Group. Doppler FD-OCT was used to extract information to calculate the volumetric blood flow using the RTVue Doppler OCT four weeks post-surgery. Total retinal blood flow as investigated in the healthy control group and patients with Type 2 diabetes and non-proliferative diabetic retinopathy.
| Control | NPDR | P-value | |
|---|---|---|---|
| Sample | 17 | 15 | |
|
|
|||
| Total Blood Flow (ul/min) | 43.3±5.3 | 33.1±9.9 | 0.002* |
P < 0.05 compared to controls.
Aqueous Humor Findings
Table 3 lists the results of our analysis of 14 candidate biomarkers in the aqueous humor samples of healthy control and NPDR subjects using suspension array analysis. Due to low aqueous sample volumes, only 11 controls and 8 NPDR samples were analyzed for aqueous TGFβ1 and TGFβ2.
Table 3.
List of Aqueous Humor Cytokines as a Function of Group. Aqueous humor cytokines included fibroblast growth factor (FGF-1 & FGF-2), angiopoietins (ANG-2), interleukin-8 (IL-8), leptin, stromal-derived factor-1, epidermal growth factor (EGF), transforming growth factor-beta (TGF-β1 and TGF-β2), granulocyte colony stimulating factor (G-CSF) platelet-derived growth factors (PDGFs), vascular endothelial growth factor (VEGF), erythropoietin, endothelin-1 (ET-1), insulin. The arrows indicate an increase (↑) or decrease (↓) in aqueous cytokine expression in comparison to healthy controls.
| Control | NPDR | P-Value | ↑/↓ | ||
|---|---|---|---|---|---|
|
| |||||
| N | 17 | 15 | |||
|
| |||||
| ng/ml | ng/ml | ||||
| Angiogenic | |||||
|
| |||||
| 1 | ANG-2 | 12.8±5.7 | 21.3±10.9 | 0.012 | ↑ |
| 2 | EGF | 0.25±0.09 | 0.17±0.06 | 0.011 | ↓ |
| 3 | FGF-1 | 7.49±1.67 | 4.93±1.33 | <0.001* | ↓ |
| 4 | FGF-2 | 22.90±12.37 | 16.20±4.68 | 0.235 | -- |
| 5 | G-CSF | 6.69±19.09 | 0.93±0.78 | 0.287 | -- |
| 6 | HGF | 108.88±41.32 | 236.84±203.40 | 0.023 | ↑ |
| 7 | IL-8 | 1.13±0.54 | 2.25±2.33 | 0.081 | -- |
| 8 | Leptin | 32.23±28.56 | 54.09±35.99 | 0.091 | -- |
| 9 | PLGF | 0.51±0.22 | 0.95±0.45 | 0.002* | ↑ |
| 10 | VEGF-A | 73.1±36.7 | 81.5±35.4 | 0.529 | -- |
| 11 | TGFβ1 | 2.4±1.9 | 14.7±6.6 | <0.001* | ↑ |
| 12 | TGFβ2 | 1977.8±607.1 | 4314.8±768.9 | <0.001* | ↑ |
| Vasogenic | |||||
|
| |||||
| 13 | ET-1 | 2.3±0.8 | 3.1±1.3 | 0.051 | -- |
| 14 | Insulin | 5.9±1.7 | 7.2±2.8 | 0.167 | -- |
Bonferroni corrected, P-value <0.003
Correlations between TRBF and Aqueous Humor Cytokines
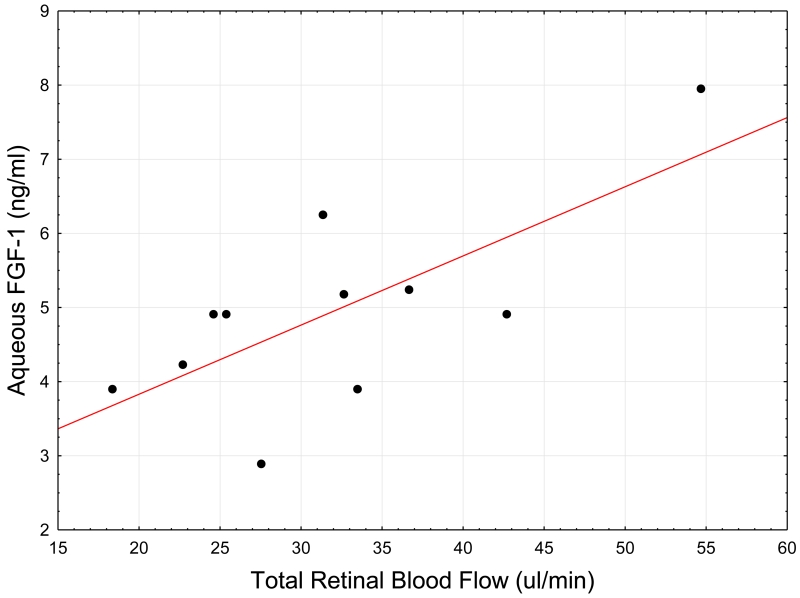
Aqueous FGF-1 was significantly correlated with the TRBF across all participants with a correlation coefficient of r= 0.59, p = 0.001, r2 = 0.34. Within the NPDR group, aqueous FGF-1 remained significantly associated with TRBF with a correlation coefficient of r = 0.71, p = 0.013, r2 = 0.51, Figure 1. The results also revealed a significant association between aqueous TGFβ1 and TRBF with a correlation coefficient of r =−0.64 (p=.005) across all subjects. In the NPDR group, however, aqueous TGFβ1 was not associated with TRBF (r=−0.65, p=0.11).
Figure 1.
Scatter-plot of corrected Total Retinal Blood Flow (TRBF) and Aqueous humor cytokines Fibroblast growth factor-1 (FGF-1) in NPDR subjects. The correlation between TRBF and FGF-1 was found to be r = 0.71, p = 0.01; r2 = 0.51, FGF-1 = 1.9632+0.0933*x. Pearson Bivariate Correlation analysis was used to determine the relationship between aqueous humor cytokines
Aqueous VEGF, ANG-2, ET-1, insulin factors were not significantly correlated with TRBF (p>0.114 for all). Forearm A1c was associated with TRBF across all patients (r=−0.42, p=.023).
Multiple Regression Analysis
A multivariate model was fitted for aqueous TRBF and included predictor variables ANG-2, ET-1, FGF-1 and TGF-β1 in the NPDR group (F=5.37, df=4,7 and p=.027). The only significant predictor for TRBF was aqueous FGF-1 (b*= 0.806, p=0.003). Additionally, a multivariate model was fitted for aqueous FGF-1 (F=5.42, df= 3,21 and p=0.006) and TGF-β1 (F=2.81, df=5,24 and p=0.0387). The only significant predictor for aqueous FGF-1 and TGF-β1 was A1c (p=0.020 and p=0.018, respectively).
DISCUSSION
Although retinal blood flow abnormalities are an early hallmark of DR, the precise mechanism for blood flow dysfunction has not been clear. Using the Doppler OCT, we report a reduction in TRBF in patients with NPDR compared to controls. In patients with NPDR, aqueous TGF-β and PLGF levels were significantly elevated while aqueous FGF-1 levels was decreased compared to controls. Our results reveal a strong association between aqueous FGF-1 with TRBF in patients with NPDR. Although alterations in retinal hemodynamics have previously been found to be associated with increased aqueous humor growth factor expression in advanced retinal ischemic diseases (Noma et al. 2009, Noma et al. 2010, Yamada et al. 2015) this is the first study to identify a role of FGF-1 in impaired retinal blood flow in early DR. Overall, measurement of TRBF using Doppler OCT may be useful for predicting aqueous FGF-1 levels and severity of hemodynamic disturbances in early DR.
We observed a significant increase in TGF-β and PLGF in the aqueous humor of patients with NPDR compared to controls. Although not significant after Bonferroni corrections, additional angiogenic cytokines ANG-2, HGF and EGF tended to increase in the NPDR compared to controls (p<0.023). Similar angiogenic cytokines have been reported in earlier aqueous humor studies of DR.(Chiang et al. 2012, Dong et al. 2013, Oh et al. 2010) Despite the lack of correlation of these angiogenic cytokines with TRBF, the aqueous profile from these eyes showed marked similarity to those with PDR, and was significantly distinct from the controls. These results lend further support that angiogenic growth factors not only have a role in PDR but mediate early vascular changes in early DR.
To the best of our knowledge, this is the first study to demonstrate an association between FGF-1 and retinal blood flow perturbations in DR. As administration of FGF-1 and FGF-2 lowers blood pressure,(Cuevas et al. 1991) both FGF-1 and FGF-2 have shown to play a part in the maintenance of vascular tone. In non-ocular models, reduced plasma FGF levels have been found to correlate with a reduction in peripheral blood flow in Type 2 diabetic patients.(Alrouq et al. 2014) In experimental models, FGF has been shown to stimulate vasodilation and improve blood flow perfusion in animal models of ischemia through NO mediated mechanisms.(Wu et al. 1996) Moreover, basal FGF signaling plays a protective role with ongoing FGF stimulation required for the maintenance of vascular system integrity.(Cuevas et al. 1998, Hatanaka et al. 2012, Murakami et al. 2008) Since diabetes has been found to impair the expression of endogenous FGFs, (Yeboah et al. 2007) low FGF expression may explain the decreased retinal blood flow in early DR. These findings suggest FGF-1 may be a contributing factor for the observed reduction in TRBF and may predisposing for diabetic vascular diseases.
Decreases in retinal blood flow have been found in animal and humans with early diabetes. (Grunwald et al. 1986, Nagaoka et al. 2010, Sinclair et al. 1982) Using FD-OCT, Wang et al report reduced TRBF in in patients with DM without retinopathy compared to normal controls.(Wang et al. 2009a) The range of TRBF values found in this study is within the previously reported TRBF range.(Shahidi et al. 2014, Tayyari et al. 2014, Wang et al. 2009b) This and other corroborating studies (Bursell et al. 1996, Clermont et al. 1997b, Wang et al. 2009a) provide evidence that impaired retinal blood flow is a characteristic in early DR. A potential mechanism for this retinal blood flow change is associated with the vascular endothelial dysfunction which is with abnormal synthesis or action of vasodilators and increased expression and action of vasoconstrictors resulting in a shift of homeostasis to a net vasoconstrictive effect in early diabetes. We have shown that the expression of ET-1 is increased while FGF-1 is decreased in the NPDR group compared to controls. Thus, an increased ET-1 and reduced FGF-1 production in the ocular tissue of diabetic patient may explain the decreased retinal blood flow in patients with early DR.
The simultaneous alteration in retinal hemodynamics and increase in angiogenic cytokine expression found in this study is consistent with the hypothesis that hyperglycemia-induced activation of several activated pathways may mediate diabetes-induced retinal vascular dysfunction in diabetes. There is sufficient evidence in animal and human studies that show that hyperglycemia initiates the development of DR and increase the synthesis and activity angiogenic cytokines (Joussen et al. 2001) and ultimately lead to retinal vascular abnormalities. As shown in our multiple regression analysis, a significant determinant of aqueous FGF-1 and TGF-β1 levels is A1c. A1c is a well-known contributor to metabolic pathways involved in DR progression.(Stratton et al. 2001) Longer studies that will allow the development of retinal blood flow changes and evaluation of the role of cytokines and growth factors, as well as inflammatory factors, will be required to reach firm conclusions.
The present study has its limitations. One limitation is that the cytokines released in the aqueous humor may differ due to the underlying cataract condition. However, we attempted to mitigated this issue as we compared the level of cytokines amongst all patients who underwent the same cataract procedure. This reduced the likelihood of differences in cytokine levels between groups. Another limitation of this study included growth factors and cytokine and did not consist of soluble receptor analysis. Soluble receptors may play an important role in modulating cytokine activity. For instance, a soluble form of the FGFR1 has been found in the vitreous (Hanneken & Baird 1995) and binding of FGF1 and FGF2 may inhibit the biological activity, acting in an agonistic manner. Furthermore, our study was limited to a 4 week follow-up TRBF measurement period in which TRBF may have undergone fluctuations due to the surgical procedure. Measurement of retinal blood flow 4 weeks post-surgery would more accurately reflect the homeostatic condition of the retinal vasculature due to diabetes rather than change secondary to cataract surgery.
Overall, low TRBF was associated with low aqueous FGF-1 levels in patients with NPDR. Additionally, aqueous angiogenic factors (TGF β1, TGF β2 and PLGF) were elevated in conjunction with a reduction in TRBF in patients with NPDR. This study provides evidence of angiogenic factors are involved in early retinal blood flow abnormalities in DR and provides a link between aqueous biomarkers and retinal vascular disorders in diabetes. This study suggests that measurement of TRBF using FD-OCT may be useful for determining aqueous FGF-1 levels and severity of vascular dysfunction in early DR.
ACKNOWLEDGEMENTS
The authors thank Dr. Ayda Shahidi from the University of Waterloo for research assistance, ophthalmology nursing team at the Toronto Western Hospital and Kensington Eye Institute for their surgical assistance.
FUNDING
Supported by the Ontario Research Fund, Research Excellence Program (CH), the Vision Science Research Program at the University of Toronto (LAK), anonymous donor (CH & JGF), NIH UL1TR00128 (DH) and NIH DP3 DK104397 (OT).
References
- Alrouq FA, Al-Masri AA, Al-Dokhi LM, Alregaiey KA, Bayoumy NM, Zakareia FA. Study of the association of adrenomedullin and basic-fibroblast growth factors with the peripheral arterial blood flow and endothelial dysfunction biomarkers in type 2 diabetic patients with peripheral vascular insufficiency. J.Biomed.Sci. 2014;21(1):94. doi: 10.1186/s12929-014-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest.Ophthalmol.Vis.Sci. 1996;37(5):886–897. [PubMed] [Google Scholar]
- Chiang SY, Tsai ML, Wang CY, Chen A, Chou YC, Hsia CW, Wu YF, Chen HM, Huang TH, Chen PH, Liu HT, Shui HA. Proteomic analysis and identification of aqueous humor proteins with a pathophysiological role in diabetic retinopathy. J.Proteomics. 2012;75(10):2950–2959. doi: 10.1016/j.jprot.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Clermont AC, Aiello LP, Mori F, Aiello LM, Bursell SE. Vascular endothelial growth factor and severity of nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: a potential role for vascular endothelial growth factor in the progression of nonproliferative diabetic retinopathy. Am.J.Ophthalmol. 1997a;124(4):433–446. doi: 10.1016/s0002-9394(14)70860-8. [DOI] [PubMed] [Google Scholar]
- Cuevas P, Carceller F, Ortega S, Zazo M, Nieto I, Gimenez-Gallego G. Hypotensive activity of fibroblast growth factor. Science. 1991;254(5035):1208–1210. doi: 10.1126/science.1957172. [DOI] [PubMed] [Google Scholar]
- Cuevas P, Carceller F, Redondo-Horcajo M, Lozano RM, Gimenez-Gallego G. Systemic administration of acidic fibroblast growth factor ameliorates the ischemic injury of the retina in rats. Neurosci.Lett. 1998;255(1):1–4. doi: 10.1016/s0304-3940(98)00672-7. [DOI] [PubMed] [Google Scholar]
- Dong N, Xu B, Wang B, Chu L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol.Vis. 2013;19:1734–1746. [PMC free article] [PubMed] [Google Scholar]
- Feke GT, Riva CE. Laser Doppler measurements of blood velocity in human retinal vessels. J.Opt.Soc.Am. 1978;68(4):526–531. doi: 10.1364/josa.68.000526. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Riva CE, Sinclair SH, Brucker AJ, Petrig BL. Laser Doppler velocimetry study of retinal circulation in diabetes mellitus. Arch.Ophthalmol. 1986;104(7):991–996. doi: 10.1001/archopht.1986.01050190049038. [DOI] [PubMed] [Google Scholar]
- Hanneken A, Baird A. Soluble forms of the high-affinity fibroblast growth factor receptor in human vitreous fluid. Invest.Ophthalmol.Vis.Sci. 1995;36(6):1192–1196. [PubMed] [Google Scholar]
- Hatanaka K, Lanahan AA, Murakami M, Simons M. Fibroblast growth factor signaling potentiates VE-cadherin stability at adherens junctions by regulating SHP2. PLoS One. 2012;7(5):e37600. doi: 10.1371/journal.pone.0037600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JB, Jonas RA, Neumaier M, Findeisen P. Cytokine concentration in aqueous humor of eyes with diabetic macular edema. Retina. 2012;32(10):2150–2157. doi: 10.1097/IAE.0b013e3182576d07. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell S, Adamis AP. Leukocyte-Mediated Endothelial Cell Injury and Death in the Diabetic Retina. Am J Pathol. 2001;158(1):147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BE. Are individuals with diabetes seeing better?: a long-term epidemiological perspective. Diabetes. 2010;59(8):1853–1860. doi: 10.2337/db09-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Kang MH, Seong M, Cho HY. Comparison of aqueous concentrations of angiogenic and inflammatory cytokines in diabetic macular oedema and macular oedema due to branch retinal vein occlusion. Br.J.Ophthalmol. 2012;96(11):1426–1430. doi: 10.1136/bjophthalmol-2012-301913. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M. The FGF system has a key role in regulating vascular integrity. J.Clin.Invest. 2008;118(10):3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T, Sato E, Takahashi A, Yokota H, Sogawa K, Yoshida A. Impaired retinal circulation in patients with type 2 diabetes mellitus: retinal laser Doppler velocimetry study. Invest.Ophthalmol.Vis.Sci. 2010;51(12):6729–6734. doi: 10.1167/iovs.10-5364. [DOI] [PubMed] [Google Scholar]
- Noma H, Funatsu H, Mimura T, Harino S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology. 2009;116(1):87–93. doi: 10.1016/j.ophtha.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Noma H, Funatsu H, Sakata K, Mimura T, Hori S. Association between macular microcirculation and soluble intercellular adhesion molecule-1 in patients with macular edema and retinal vein occlusion. Graefes Arch.Clin.Exp.Ophthalmol. 2010 doi: 10.1007/s00417-010-1350-9. [DOI] [PubMed] [Google Scholar]
- Oh IK, Kim SW, Oh J, Lee TS, Huh K. Inflammatory and angiogenic factors in the aqueous humor and the relationship to diabetic retinopathy. Curr.Eye Res. 2010;35(12):1116–1127. doi: 10.3109/02713683.2010.510257. [DOI] [PubMed] [Google Scholar]
- Shahidi AM, Patel SR, Huang D, Tan O, Flanagan JG, Hudson C. Assessment of total retinal blood flow using Doppler Fourier Domain Optical Coherence Tomography during systemic hypercapnia and hypocapnia. Physiol.Rep. 2014;2(7) doi: 10.14814/phy2.12046. 10.14814/phy2.12046. Print 2014 Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair SH, Grunwald JE, Riva CE, Braunstein SN, Nichols CW, Schwartz SS. Retinal vascular autoregulation in diabetes mellitus. Ophthalmology. 1982;89(7):748–750. doi: 10.1016/s0161-6420(82)34720-x. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Tan O, Wu S, Nittala MG, Huang D, Varma R, Sadda SR. Measurement of retinal blood flow in normal chinese-american subjects by Doppler fourier-domain optical coherence tomography. Invest.Ophthalmol.Vis.Sci. 2015;56(3):1569–1574. doi: 10.1167/iovs.14-15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–163. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- Takagi C, Bursell SE, Lin YW, Takagi H, Duh E, Jiang Z, Clermont AC, King GL. Regulation of retinal hemodynamics in diabetic rats by increased expression and action of endothelin-1. Invest.Ophthalmol.Vis.Sci. 1996;37(12):2504–2518. [PubMed] [Google Scholar]
- Tan O, Wang Y, Konduru RK, Zhang X, Sadda SR, Huang D. Doppler optical coherence tomography of retinal circulation. J.Vis.Exp. 2012;(67):e3524. doi: 10.3791/3524. doi:67: e3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayyari F, Yusof F, Vymyslicky M, Tan O, Huang D, Flanagan JG, Hudson C. Variability and repeatability of quantitative, fourier-domain optical coherence tomography Doppler blood flow in young and elderly healthy subjects. Invest.Ophthalmol.Vis.Sci. 2014;55(12):7716–7725. doi: 10.1167/iovs.14-14430. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bower BA, Izatt JA, Tan O, Huang D. Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence tomography. J.Biomed.Opt. 2008;13(6):064003. doi: 10.1117/1.2998480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fawzi A, Tan O, Gil-Flamer J, Huang D. Retinal blood flow detection in diabetic patients by Doppler Fourier domain optical coherence tomography. Opt.Express. 2009a;17(5):4061–4073. doi: 10.1364/oe.17.004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lu A, Gil-Flamer J, Tan O, Izatt JA, Huang D. Measurement of total blood flow in the normal human retina using Doppler Fourier-domain optical coherence tomography. Br.J.Ophthalmol. 2009b;93(5):634–637. doi: 10.1136/bjo.2008.150276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fawzi AA, Varma R, Sadun AA, Zhang X, Tan O, Izatt JA, Huang D. Pilot Study of Optical Coherence Tomography Measurement of Retinal Blood Flow in Retinal and Optic Nerve Diseases. Investigative Ophthalmology & Visual Science. 2011;52(2):840–845. doi: 10.1167/iovs.10-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HM, Yuan Y, McCarthy M, Granger HJ. Acidic and basic FGFs dilate arterioles of skeletal muscle through a NO-dependent mechanism. Am.J.Physiol. 1996;271(3 Pt 2):H1087–93. doi: 10.1152/ajpheart.1996.271.3.H1087. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Suzuma K, Matsumoto M, Tsuiki E, Fujikawa A, Harada T, Kitaoka T. Retinal Blood Flow Correlates to Aqueous Vascular Endothelial Growth Factor in Central Retinal Vein Occlusion. Retina. 2015 doi: 10.1097/IAE.0000000000000595. [DOI] [PubMed] [Google Scholar]
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY, Meta-Analysis for Eye Disease (META-EYE) Study Group Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeboah J, Sane DC, Crouse JR, Herrington DM, Bowden DW. Low plasma levels of FGF-2 and PDGF-BB are associated with cardiovascular events in type II diabetes mellitus (diabetes heart study) Dis.Markers. 2007;23(3):173–178. doi: 10.1155/2007/962892. [DOI] [PMC free article] [PubMed] [Google Scholar]